Published online Jan 15, 2024. doi: 10.4239/wjd.v15.i1.105
Peer-review started: October 6, 2023
First decision: November 14, 2023
Revised: November 28, 2023
Accepted: December 15, 2023
Article in press: December 15, 2023
Published online: January 15, 2024
Processing time: 97 Days and 21.1 Hours
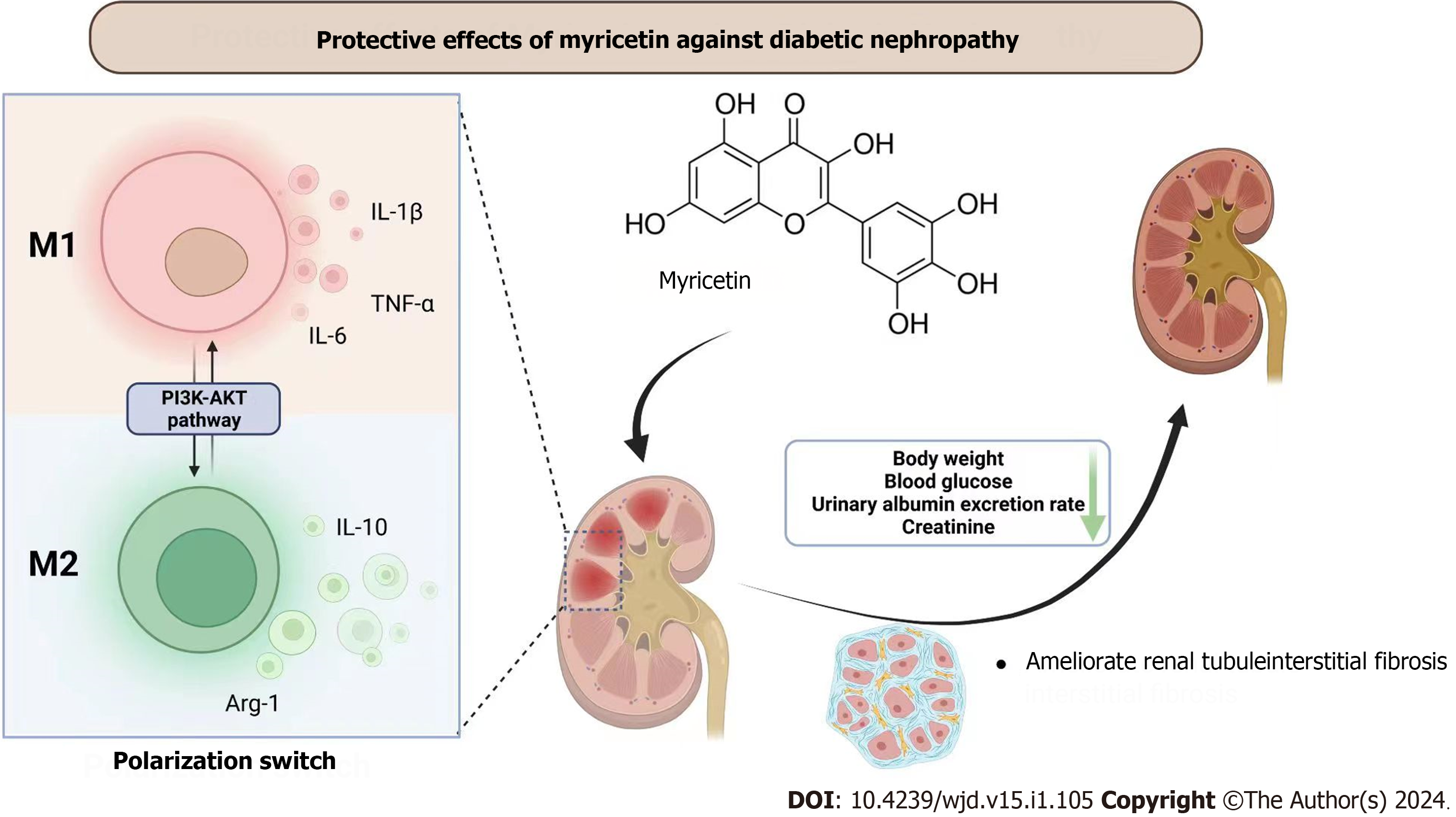
Development of end-stage renal disease is predominantly attributed to diabetic nephropathy (DN). Previous studies have indicated that myricetin possesses the potential to mitigate the pathological alterations observed in renal tissue. Never
To investigate the effects of myricetin on DN and explore its potential therapeutic mechanism.
Db/db mice were administered myricetin intragastrically on a daily basis at doses of 50 mg/kg or 100 mg/kg for a duration of 12 wk. Subsequently, blood and urine indexes were assessed, along with examination of renal tissue pathology. Kidney morphology and fibrosis were evaluated using various staining techniques including hematoxylin and eosin, periodic acid–Schiff, Masson’s trichrome, and Sirius-red. Additionally, high-glucose culturing was conducted on the RAW 264.7 cell line, treated with 25 mM myricetin or co-administered with the PI3K/Akt inhibitor LY294002 for a period of 24 h. In both in vivo and in vitro settings, quantification of inflammation factor levels was conducted using western blotting, real-time qPCR and ELISA.
In db/db mice, administration of myricetin led to a mitigating effect on DN-induced renal dysfunction and fibrosis. Notably, we observed a significant reduction in expressions of the kidney injury markers kidney injury molecule-1 and neutrophil gelatinase associated lipocalin, along with a decrease in expressions of inflammatory cytokine-related factors. Furthermore, myricetin treatment effectively inhibited the up-regulation of tumor necrosis factor-alpha, interleukin-6, and interluekin-1β induced by high glucose in RAW 264.7 cells. Additionally, myricetin modulated the M1-type polarization of the RAW 264.7 cells. Molecular docking and bioinformatic analyses revealed Akt as the target of myricetin. The protective effect of myricetin was nullified upon blocking the polarization of RAW 264.7 via inhibition of PI3K/Akt activation using LY294002.
This study demonstrated that myricetin effectively mitigates kidney injury in DN mice through the regulation of macrophage polarization via the PI3K/Akt signaling pathway.
Core Tip: Myricetin, a flavonoid, has been extensively utilized in the domains of anti-inflammatory and anti-cancer research. However, the precise mechanism by which myricetin influences the onset and development of diabetic nephropathy (DN) remains a mystery. To address this knowledge gap, we conducted a study wherein we induced DN in db/db mice and subsequently administered myricetin as a treatment. Our findings demonstrated that high concentrations of myricetin effectively mitigated renal injury, inflammation, fibrosis, and other related factors in DN mice. Furthermore, we conducted in vitro experiments to confirm that myricetin activated the PI3K/Akt signaling pathway, thereby inhibiting the polarity shift of macrophages.
- Citation: Xu WL, Zhou PP, Yu X, Tian T, Bao JJ, Ni CR, Zha M, Wu X, Yu JY. Myricetin induces M2 macrophage polarization to alleviate renal tubulointerstitial fibrosis in diabetic nephropathy via PI3K/Akt pathway. World J Diabetes 2024; 15(1): 105-125
- URL: https://www.wjgnet.com/1948-9358/full/v15/i1/105.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i1.105
Diabetes mellitus (DM) is a major global public health concern that continues to increase in prevalence. Reportedly, there is an estimated 12.8% prevalence of diabetes in China, with a concerning trend of affecting younger generations[1,2]. DM ranks as the third most detrimental chronic non-communicable disease to human health, trailing behind cancer and cardiovascular diseases. Moreover, DM patients commonly experience chronic hyperglycemia, a metabolic disorder that adversely affects multiple kidney cell types, ultimately leading to progressive kidney failure[3,4]. Diabetic nephropathy (DN) is frequently observed as a chronic microvascular complication linked to end-stage renal disease (ESRD), and it constitutes a significant contributor to both disability and mortality[5]. The clinical characteristics of DN include persistent proteinuria and the gradual decline of glomerular filtration rate. Additionally, DN patients commonly exhibit pathological alterations such as tubulointerstitial fibrosis and progressive glomerular damage[6,7].
The precise pathogenesis and progression of DN remain unclear. Existing evidence suggests that the metabolic disorder induced by hyperglycemia may initiate the excessive activation of multiple pathways, potentially leading to mechanical damage of renal tissue[4,8,9]. Notably, inflammatory responses and the immune system play a crucial role in the progression of DN[10-12]. Macrophages, famous for their pluripotency and plasticity, differentiate into classically activated (M1) cells and alternatively activated (M2) cells, playing opposing roles in the regulation of inflammation[13]. M1 cells are closely associated with the proinflammatory response, and increased expressions of CD86, tumor necrosis factor-alpha (TNF-α) and inducible nitric oxide synthase (iNOS) represent the phenotype transformation of M1; whereas, M2 cells have increased expression of CD206, arginase-1 (Arg-1) and interleukin (IL)-10[14,15]. More importantly, the above two distinct cell subsets exist in a dynamic balanced state. Indeed, one study has indicated that the regulation of macrophage polarization could inhibit renal inflammation in mice with DM[16].
The PI3K-Akt pathway serves a crucial function in the advancement of DN, not only in regulating cell survival and proliferation but also in facilitating the progression of DN. Recent findings have indicated that, in the presence of diabetic conditions, the inhibitory effect of the transcription coregulator YAP on PTEN leads to activation of the PI3K/Akt pathway. Consequently, this activation results in accumulation of nuclear YAP, thereby enhancing the proliferation of glomerular mesangial cells and contributing to the formation of DN[17]. It has been reported that the wogonin flavonoid exhibited inhibitory effects on tubulointerstitial fibrosis and renal tubular cell injury in mouse models of streptozotocin (STZ)-induced diabetes via PI3K/Akt/NF-κB signaling[18].
Currently, there is a limited availability of curative therapies for DN, and the effective prevention of renal failure progression caused by DN remains challenging. A significant proportion of ESRD patients who undergo long-term dialysis experience a high mortality rate, with approximately 20% of the population succumbing to this condition annually[19]. The primary strategies for DN patients to delay renal injury involve the control of blood glucose levels, blood pressure, and lifestyle modifications. However, the efficacy of these interventions is notably restricted[20,21]. As a result, there is an urgent need for new effective treatments to counter DM-associated kidney damage.
Abelmoschus Manihot capsule, an important Chinese patent medicine and widely used in treating kidney diseases, consists of seven flavonoids, including rutin, hyperoside, quercetin, myricetin, hibifolin, isoquercetin, and quercetin-3-o-robinobioside[22]. Our studies have been devoted to examining the roles of the total flavones of Abelmoschus manihot[23], hyperoside[24] and quercetin[25] in animal models of DN. Myricetin, present in dicotyledonous plants, has demonstrated a wide range of medicinal properties including anti-inflammatory, anti-cancer, and hepatoprotective[26,27]. For example, Park et al[28] found that 30 Μm of myricetin suppresses NF-κB activation and attenuates the secretion of TNF-α and IL-6. Liao et al[29] found that myricetin prevented diabetic-associated cardiac injury in STZ-induced mice and in high glucose-challenged neonatal rat cardiomyocytes. These investigators also found that myricetin possesses a potential protective effect by inhibiting IκBα/NF-κB pathways and enhancing Nrf2/HO-1. Kandasamy and Ashokkumar[30] found that STZ-induced diabetic nephrotoxic rats treated with myricetin were protected from glomerular injury, further suggesting its potential as an anti-hyperglycemic agent. These data collectively indicate that myricetin has effects on inhibiting the secretion of inflammatory factors and its potential therapeutic functions on diabetic-related disease as well. However, the mechanism underlying how myricetin inhibits the progress of DN remains a mystery.
For the current study, db/db mice were used to explore the effects of myricetin in the progression of DN. The mouse RAW 264.7 cell line was then used to study the mechanism by which myricetin intervenes in high glucose-induced macrophage injury. Our results indicate that myricetin regulates the polarization of macrophages through mediating the phosphorylation of Akt, and thus participates in the progression of DN.
In vitro studies were carried out in the mouse RAW 264.7 cell line (American Type Culture Collection, Manassas, VA, United States), which was maintained in low glucose-Dulbecco's modified Eagle media supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% fetal bovine serum inactivated by heat. The cells were grown at 37°C in a humidified atmosphere of 5% CO2. For experimentation, the cells were plated on a dish or in a microplate after trypsinization and incubated for 24 h before use. For the high-glucose treatments, glucose concentrations were adjusted to 5.5 mM, 25 mM or 33.3 mM (the latter designated as ‘HG’). For the myricetin treatments, 12.5 μM, 25 μM or 50 μM were applied for 48 h before further analysis. LY294002 (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), an Akt phosphorylation inhibitor, was administered for selective inhibition of Akt activation.
Mice (6 wk of age) of the db/m and db/db genotypes were obtained from the Hangzhou Ziyuan experimental animal facility [SCXK (Zhe) 2019–0004]. For the study period, the Experimental Animal Center of Jiangsu Provincial Hospital of Traditional Chinese Medicine (Jiangsu, China) housed all mice at 22°C with 12 h/12 h light/dark cycle, without restrictions on food or water intake. A 2-wk adaptation period with normal diet was allowed to all mice before experimental procedures were initiated. After another 4 wk of adaptive feeding, myricetin was administered to the db/db mice intragastrically at dosages of 50 mg/kg and 100 mg/kg every day. Positive controls included six db/db mice given the angiotensin II receptor blocker irbesartan (Sigma-Aldrich, Merck KGaA). All animals were subjected to weighing and serum and urine collection every 4 wk. At week 24, the mice were sacrificed for renal tissue collection.
In all experiments, guidelines provided by the National Institutes of Health (NIH, Bethesda, MD, United States) were followed. The study was carried out with approval by the Ethical Committee of Jiangsu Provincial Hospital of Traditional Chinese Medicine, in compliance with the guidelines for the Care and Use of Laboratory Animals [QK-20200408-001].
Collected renal tissue was initially fixed with a 4% paraformaldehyde solution for a duration of 24 h, after which it was embedded in paraffin. Subsequently, the tissue samples were sliced into sections with a thickness of 4 mm and subjected to staining using hematoxylin and eosin (HE), periodic acid-Schiff (PAS), Masson’s trichrome, and Sirius-red, respectively. Ten sections were chosen from every mouse and each was examined under light microscope at 100 × optical magnification. Histological changes were assessed at 200 × optical magnification. Brightfield images were acquired using an IX83 microscope (Olympus, Tokyo, Japan), and these images were subsequently analyzed using Image-Pro Plus software (Media Cybernetics, Rockville, MD, United States). Semi-quantitative analysis was performed to compare the samples from each group and a histogram was made. Representative renal images are presented from each group.
The cultured cells were washed and subsequently treated with CD16/CD32 antibodies to inhibit the activity of cell surface Fc receptors. Following this, the cells were stained using fluorescence-conjugated monoclonal antibodies, specifically APC-anti-CD206, PE-anti-CD86, and FITC-anti-F4/80 respectively (BioLegend, San Diego, CA, United States). The staining procedure was conducted for a duration of 20 min at room temperature in a dark environment, after which the samples were washed and analyzed using flow cytometry equipment from BD Biosciences (Franklin Lakes, NJ, United States). The data were analyzed using the FlowJo v10.8 software (Ashland, OR, United States).
Urinary albumin and creatinine levels were determined utilizing urinary albumin and creatinine testing kits, following the manufacturer's instructions (Jiancheng Bioengineering, Nanjing, Jiangsu, China). Urinary albumin was measured by immunoturbidimetry and creatinine by the sarcosine oxidase method. The urine albumin-to-creatinine ratio (uACR) measurement was calculated by dividing urinary albumin by urinary creatinine (μg/mg), which could be applied for detection, diagnosis and monitoring. The calibration of urine albumin by creatinine can effectively avoid the interference of other baseline factors such as body weight (BW) and food intake, and ensure comparability of the results.
A 30-min incubation of 5% bovine serum albumin was conducted on 5-mm sections of the kidney. Subsequently, the sections were incubated with primary antibodies overnight at 4°C. After washing, the slides were incubated with goat anti-rabbit IgG H&L antibody (Abcam, Cambridge, United Kingdom) for 60 min. Following this, a 1-min hematoxylin staining procedure was performed. Finally, the slides were mounted and observed under a microscope.
The concentration of each predicted mediator was quantified using commercially available ELISA kits obtained from Beyotime Biotech (Beijing, China), strictly following the manufacturer’s instructions.
Cell lysates were extracted and separated by SDS-PAGE, and then transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, United States) following protein concentration measurement. The blotted membranes were first blocked using fat-free milk and incubated overnight at 4°C with primary antibodies. Afterward, the membranes were washed with Tris-buffered saline-Tween and incubated with secondary antibodies. Results were detected via enhanced chemiluminescence (Applygen Technologies Inc, Beijing, China), and the densitometric data (based on the immunoreactive signals) were analyzed using open-source ImageJ software (https://imagej.net/ij/download.html). Protein levels were determined by calculating induction folds using the density ratio of the target protein to β-actin. The antibodies targeting kidney injury molecule-1 (Kim-1; Catalog No. sc-518008), neutrophil gelatinase associated lipocalin (NGAL; sc-515876), collagen-1a1 (Col1a1; sc-59772), alpha-smooth muscle actin (α-SMA; sc-53142), iNOS (sc-7271), Arg-1 (sc-166920), and others were purchased from Santa Cruz Biotechnology (Dallas, TX, United States).
To determine the concentration of total RNA, Trizol reagent (Sigma-Aldrich, St Louis, MO, United States) was used following the manufacturer's instructions. The first strand of cDNA was synthesized using a reverse transcriptase enzyme (Life Technologies, Waltham, MA, United States). The relative levels of target genes were determined through reverse transcription-qPCR, as previously reported[31]. Normalization to β-actin was carried out, and the 2-∆∆CT method was utilized to calculate the relative levels of target genes. The primers used for real-time PCR (5’-3’) can be found in Table 1.
| Gene | Forward primer | Reverse primer |
| Kim-1 | ACATATCGTGGAATCACAACGAC | ACAAGCAGAAGATGGGCATTG |
| Ngal | TGGCCCTGAGTGTCATGTG | CTCTTGTAGCTCATAGATGGTGC |
| Col1a1 | GCTCCTCTTAGGGGCCACT | CCACGTCTCACCATTGGGG |
| α-Sma | GTCCCAGACATCAGGGAGTAA | TCGGATACTTCAGCGTCAGGA |
| iNos | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| Tnf-a | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| Il-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| Il-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| Il-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| Arg-1 | CTCCAAGCCAAAGTCCTTAGAG | AGGAGCTGTCATTAGGGACATC |
SwissTargetPrediction (http://www.SwissTargetPrediction.ch) was utilized for the purpose of predicting the structural similarity of anticipated targets based on the acquired formula. Concurrently, our disease-associated targets were obtained from GeneCards (https://www.genecards.org/) and Online Mendelian Inheritance in Man compendium (OMIM; https://www.OMIM.org/), with a specific focus on identifying targets related to "diabetic nephropathy". The target gene's name was matched by configuring the subject as "human" and employing the "Vlookup" function to filter genes that intersect with drugs and diseases.
DN and myricetin share numerous common targets, as evidenced by a Venn diagram generated using an online bioinformatics tool (http://www.bioinformatics.com.cn). To identify protein-protein interactions (PPIs), potential targets were entered into the STRING database (https://cn.string-db.org/), resulting in a visual PPI network constructed using Cytoscape 3.7.2. The degree value was then calculated to determine the key protein within the PPI network. Nodes within the network were color-coded and sized proportionally to their respective degree values, with larger and darker nodes indicating higher degree values.
The clusterProfiler, Stringin, DOSE, and Pathview programs were executed in the R language utilizing the bioinformatics open source platform Bioconductor (http://www.Bioconductor.org/). The visualization display was conducted through the WeChat letter platform. Gene Ontology (GO) bioinformatic analysis was used to elucidate the role of target proteins in drug therapy gene function, encompassing molecular function, cellular component, and biological process. In addition, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was conducted as a component of signal pathway enrichment analysis to identify drug therapy targets.
Myricetin was subjected to docking with Akt and PI3K, respectively. The crystal structure file of the protein target was obtained from the Protein Data Bank (PDB) database in PDB format. In order to facilitate docking, the compounds of myricetin in SDF format were downloaded from PubChem (https://PubChem.ncbi.nlm.nih.gov/). Subsequently, virtual docking experiments were conducted using Autodock 4.2.6. It is important to note that docking solely alters the conformation of the ligand, while preserving the conformational changes of the protein and leaving all other parameters unchanged. The outcomes of the docking process were visualized using PyMOL 2.2.0 software (https://pymol.org/2/) and Discovery Studio Client v19.1.0 (https://discover.3ds.com/discovery-studio-visualizer-download).
A total of three repetitions of each experiment were performed, and the results were summarized as means and standard deviations. The statistical software SPSS 22.0 (IBM Corp, Armonk, NY, United States) was utilized to conduct a one-way ANOVA with the aim of assessing between-group variation. A probability level of P < 0.05 was deemed as statistically significant.
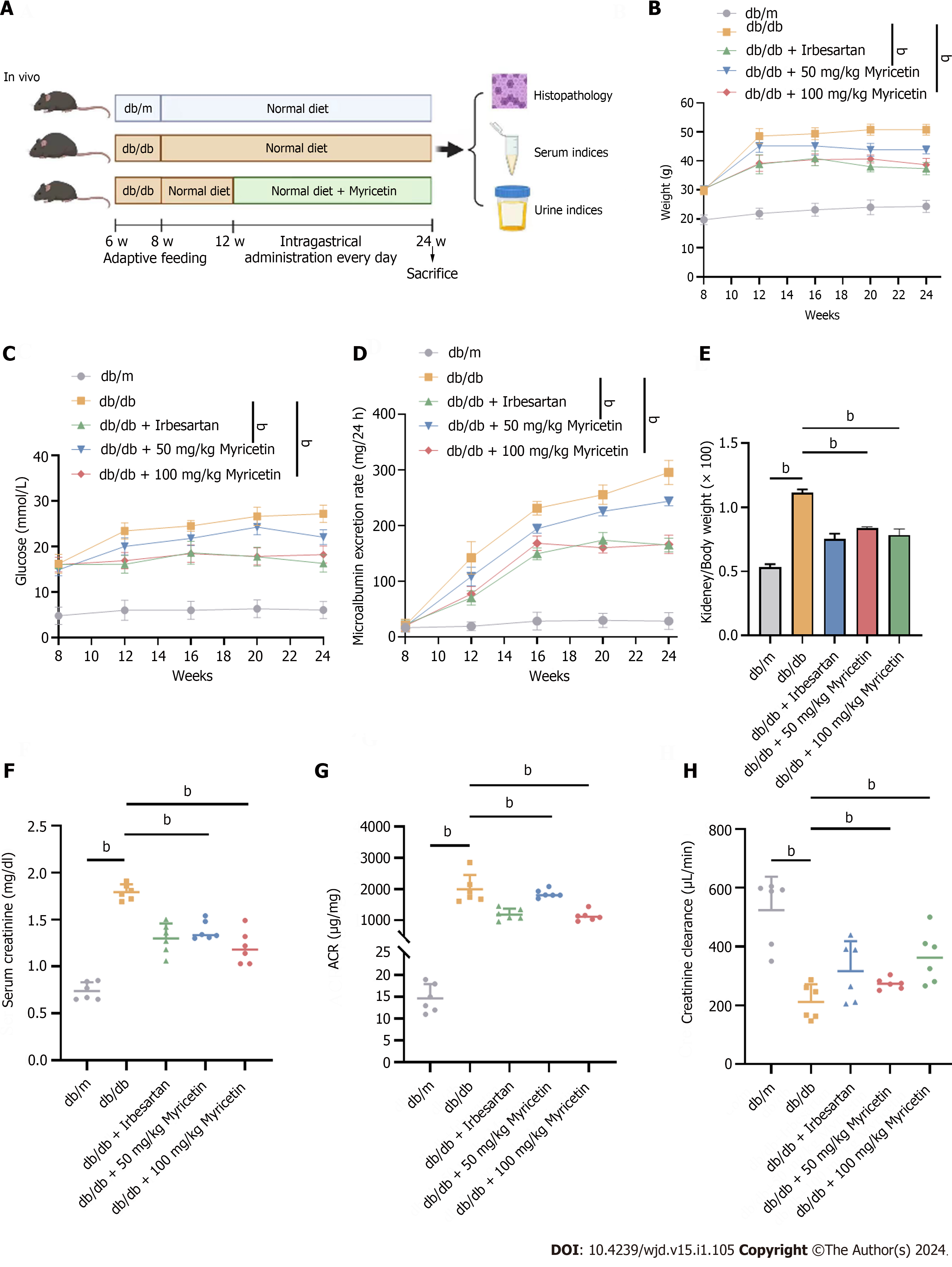
We first assessed the protective effects of myricetin in DN mice, with irbesartan serving as a positive control. As shown in Figure 1A, DN (db/db) mice exhibited significantly elevated BWs, blood glucose levels, and 24-h microalbumin concentrations (P < 0.01) compared to control (db/m) mice at week 12, confirming the successful establishment of the diabetic mouse model. During the entirety of the treatment period, the db/db + myricetin group (both 100 mg/kg and 50 mg/kg subgroups) exhibited a significant decrease in BW, blood glucose level, and 24-h microalbumin compared to the db/db group (P < 0.01) (Figure 1B-D).
Microalbuminuria is recognized as the earliest clinical indicator in the initial stages of DN. Therefore, we assessed the kidney/BW index, serum creatinine, creatinine clearance, and uACR following 12 wk of myricetin treatment. In comparison to db/m diabetic mice, the db/db diabetic mice exhibited significantly higher levels of serum creatinine, kidney/BW index, and uACR at 24 wk (P < 0.01; Figure 1E-G). The db/db mice demonstrated a reduced clearance of creatinine compared to the control mice (P < 0.01; Figure 1H), indicating further evidence of diabetic renal pathological damages. Following a 12-wk treatment with either 100 mg/kg or 50 mg/kg myricetin, the db/db + myricetin group displayed significant decreases in kidney/BW, serum creatinine and uACR, and a notable enhancement in the clearance of creatinine compared to the db/db group (P < 0.01; Figure 1E-H). Thus, these data indicated that myricetin treatment alleviated the kidney injuries of DN mice.
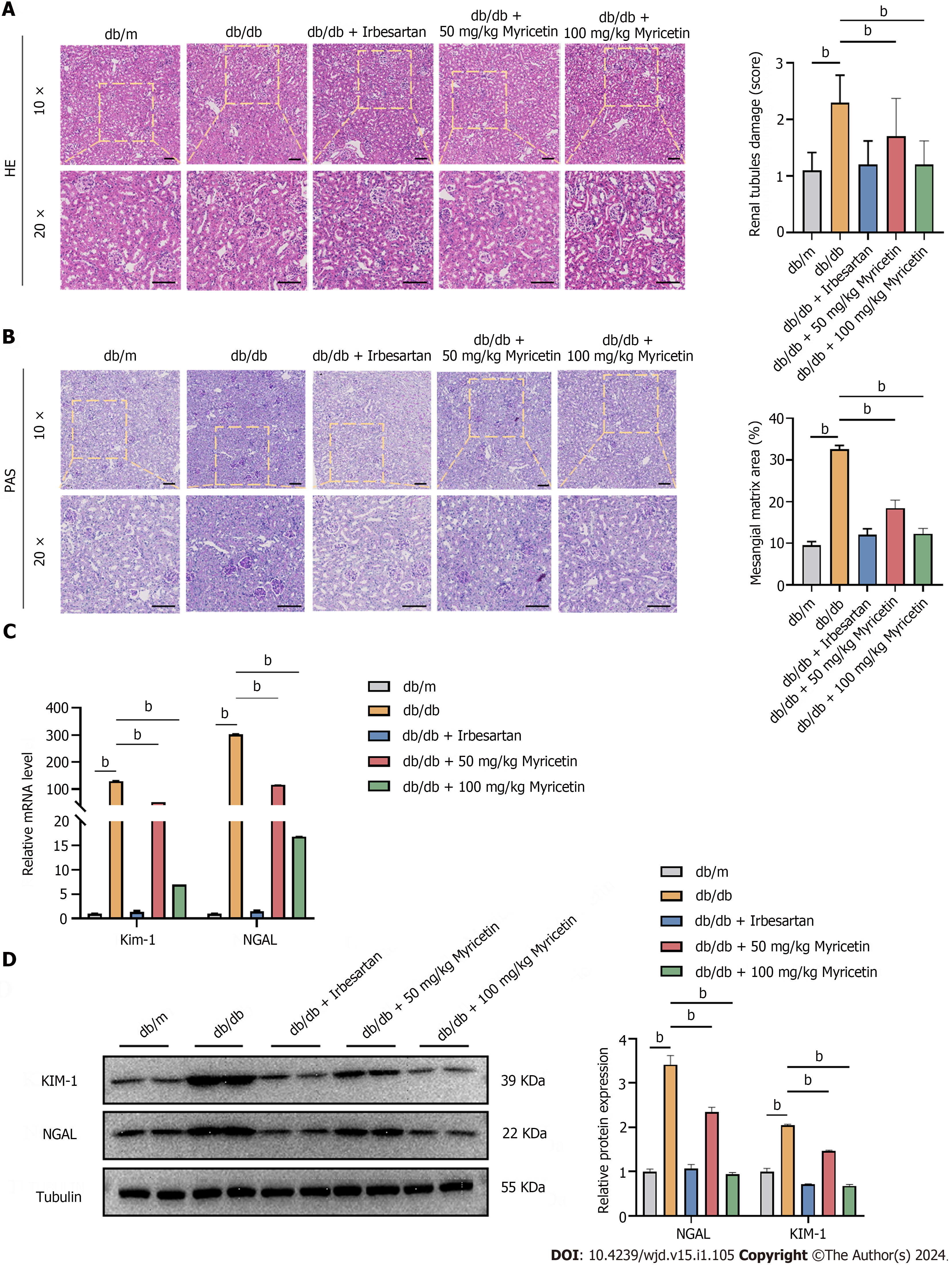
We conducted histological analyses of the kidney using HE staining and PAS staining to further evaluate the therapeutic effect of myricetin in DN mice. Notably, DN mice exhibited distinct pathological kidney alterations, including glomerular mesangial cell proliferation, thickening of capillary basement membranes, and increased vacuolation of renal tubules (Figure 2A and B). Importantly, the renal tubular damage and expansion of mesangial matrix were significantly more serious in DN mice than in the control group (P < 0.001). The administration of myricetin resulted in a significant decrease in the renal tubular damage score and a reduction in the area of the mesangial matrix in db/db mice compared to the control group. Additionally, there was a notable increase in the expressions of KIM-1 and NGAL in the kidney tissues of db/db mice, which was reversed in the db/db + myricetin mice, particularly in the high-dose subgroup (Figure 2C and D). Taken together, these findings suggested that myricetin treatment effectively ameliorated the renal histopathological alterations in db/db mice.
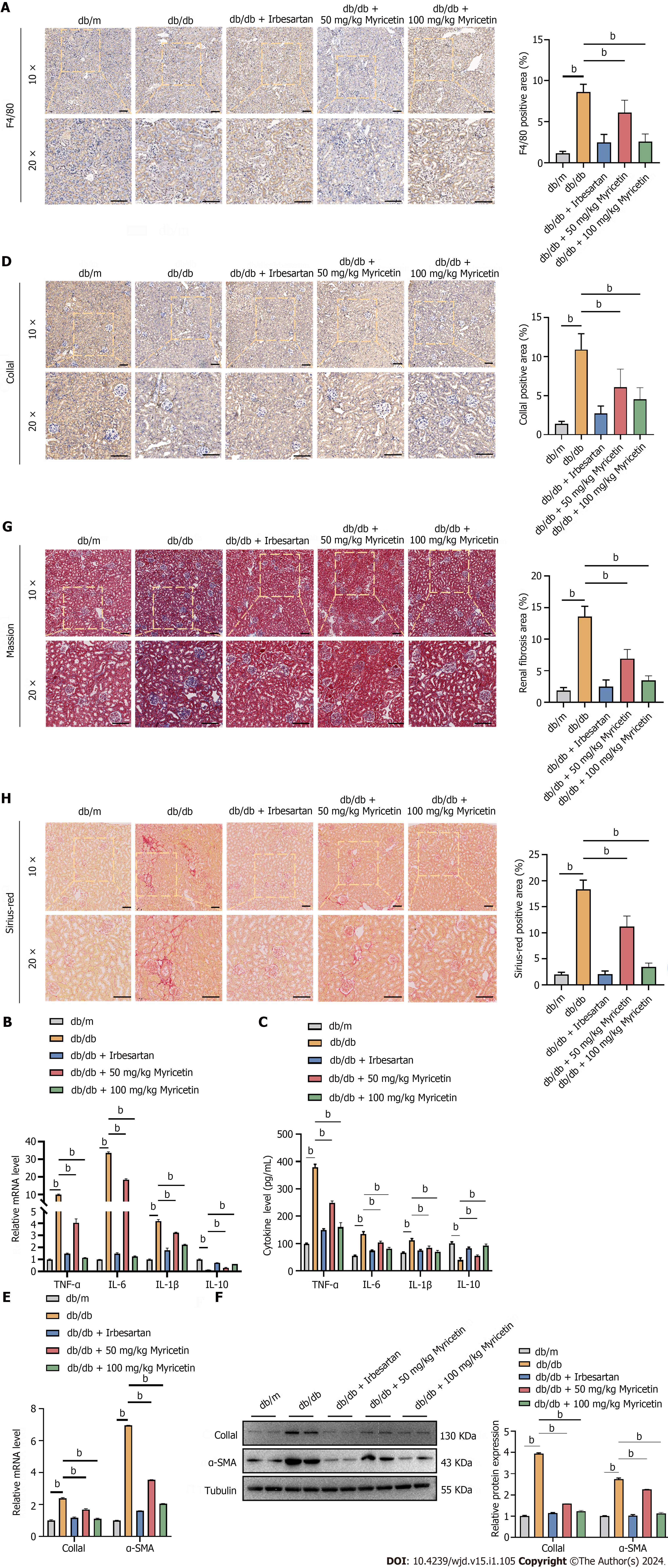
We used F4/80, a surface marker of M1-type macrophages, to stain the renal tissues to investigate the potential of myricetin in regulating renal function. Notably, an obvious infiltration of macrophage inflammation was observed in the kidney tissues of db/db mice when compared to the control group. However, the administration of myricetin significantly mitigated the infiltration of inflammation in the renal tissues (Figure 3A). Furthermore, the serum levels of M1 inflammatory factors were found to be up-regulated in db/db mice in comparison to db/m mice. Conversely, there was a significant decrease in the levels of IL-10, an M2 inflammatory factor, in db/db mice (Figure 3B and C). The administration of myricetin, particularly at a dosage of 100 mg/kg, resulted in a significant reduction in M1 inflammatory factors at both the mRNA and protein levels. Additionally, there was a notable increase in the expression of IL-10 following the administration of myricetin.
Given that prolonged inflammatory infiltration triggers the activation of immune cells such as fibroblasts and myofibroblasts, which are involved in collagen synthesis and deposition and ultimately lead to fibrosis, we assessed the extent of renal fibrosis by using Col1a1 antibody staining of renal tissues. It was observed that db/db mice exhibited a higher percentage of Col1a1-positive region compared to db/m mice. Furthermore, the administration of myricetin resulted in a significant decrease in the percentage of the Col1a1-positive region (P < 0.001; Figure 3D). Additionally, we measured the levels of Col1a1 and α-SMA in the renal tissues. It was found that the fibrosis marker genes Col1a1 and α-SMA were significantly up-regulated in db/db mice, but not in those treated with myricetin, particularly in the high-dose subgroup (Figure 3E and F). Compared with db/m mice, db/db mice had significantly more fibrosis based on Masson’s trichrome staining and Sirius-red staining, while db/db mice treated with myricetin showed reduced fibrosis in their renal tissues (Figure 3G and H). Collectively, these data indicated that the myricetin treatment efficiently reduced inflammatory factor infiltration and occurrence of renal fibrosis in the db/db mice.
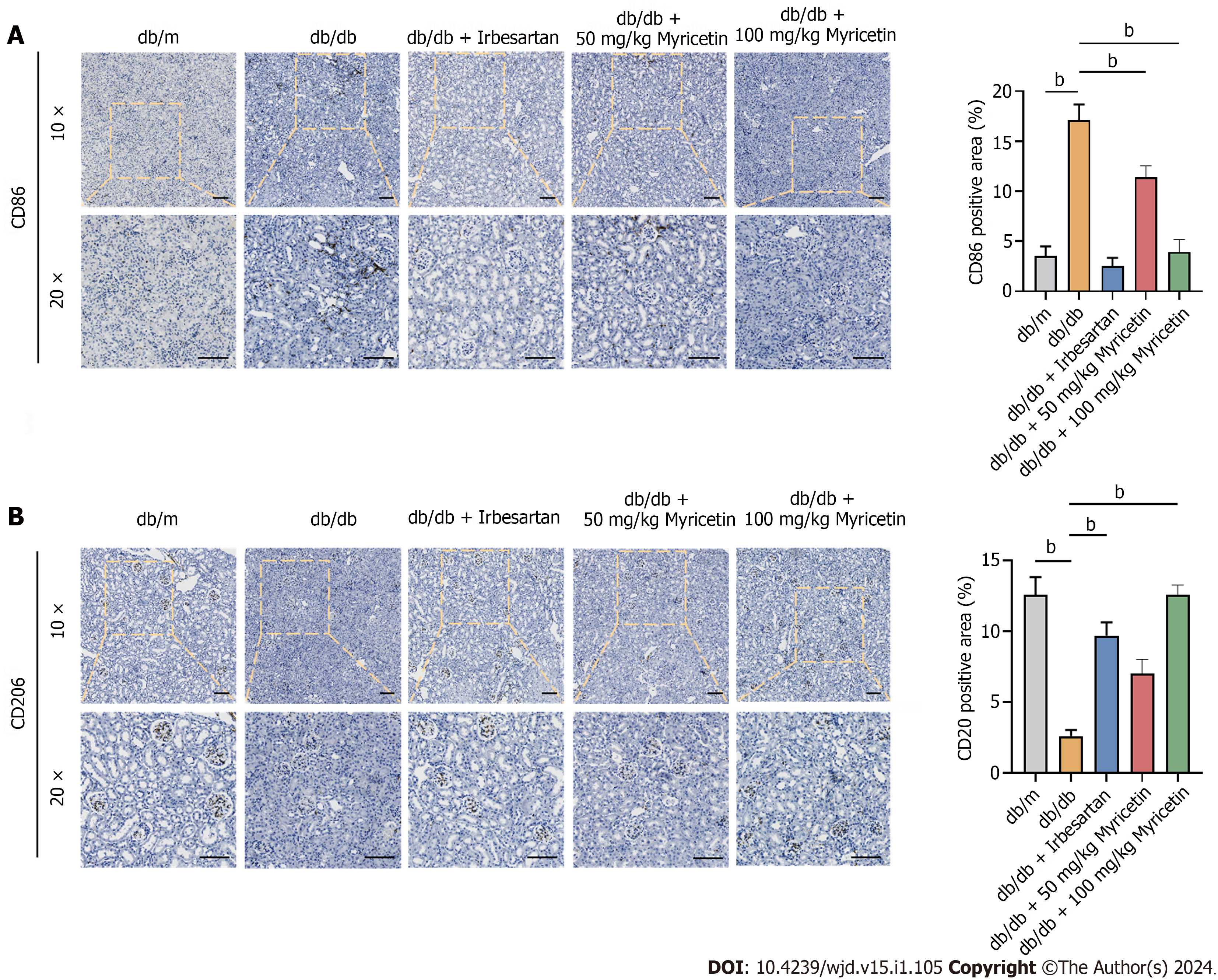
As shown in Figure 4, CD86 and CD206 were used as surface markers to identify the distinct polarized phenotypes of macrophages. The analysis of the polarized phenotypes of renal macrophages across the various groups of mice revealed a significant increase in CD86+ macrophages in the kidneys of db/db mice compared to the control mice. Conversely, the number of CD206+ macrophages was significantly reduced, indicating a bias towards M1 macrophage polarization in the kidneys of DN mice fed a high-fat diet. Following the administration of myricetin, a notable decrease in macrophage polarization of CD86+ and an increase in macrophage polarization of CD206+ were observed. This effect was particularly significant at higher doses and indicated that myricetin promoted the transition of macrophages from the M1 to the M2 phenotype, thereby mitigating the inflammatory effects. Notably, no significant disparity in the expressions of CD86+ and CD206+ macrophages was observed between the db/m group and the db/db + myricetin group. Thus, our results suggested that myricetin may have switched the phenotypes of macrophages in the renal tissue of db/db mice.
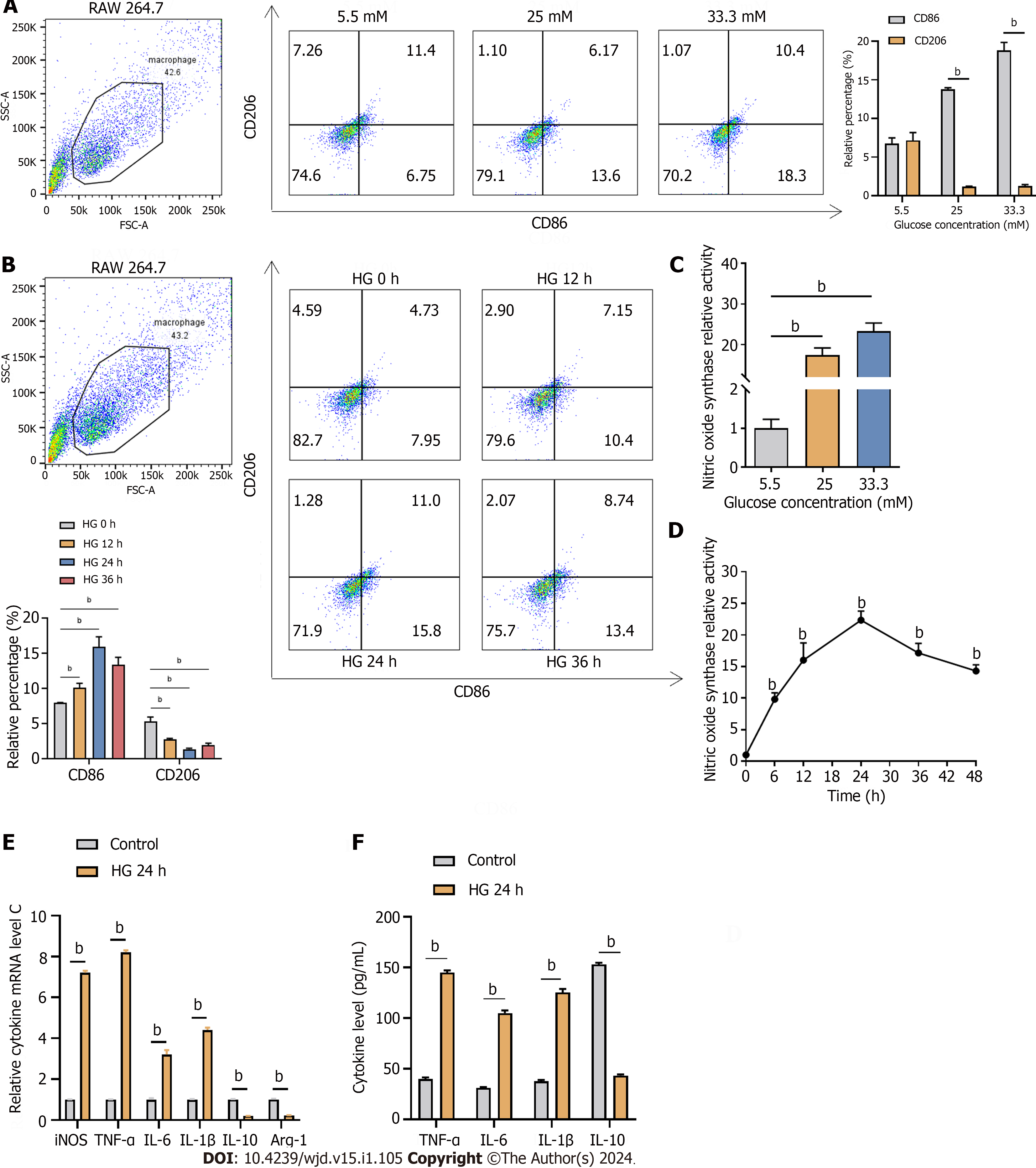
In order to validate the regulation of myricetin against kidney injury of DN, we built a cell model using RAW 264.7 macrophages exposed to high glucose concentrations. Flow cytometric analysis was conducted to assess the expression of CD86 and CD206. The results revealed a higher prevalence of M1 macrophages in cultures with high glucose concentrations (25 mM and HG) as compared to M2 macrophages, while no significant difference was observed in the low-glucose (5.5 mM) culture condition (Figure 5A). Under the stimulation of HG, it was observed that the abundance of M1 macrophages peaked at 24 h, and their polarization decreased with prolonged exposure time (Figure 5B). Similarly, when RAW 264.7 cells were induced with HG, the highest level of nitric oxide synthase (NOS) activity was observed at 24 h (Figure 5C and D). Furthermore, a significant increase in the expression and secretion of M1 inflammatory factors was observed in cells stimulated with HG, while the levels of IL-10 and Arg-1 were significantly reduced (Figure 5E and F).
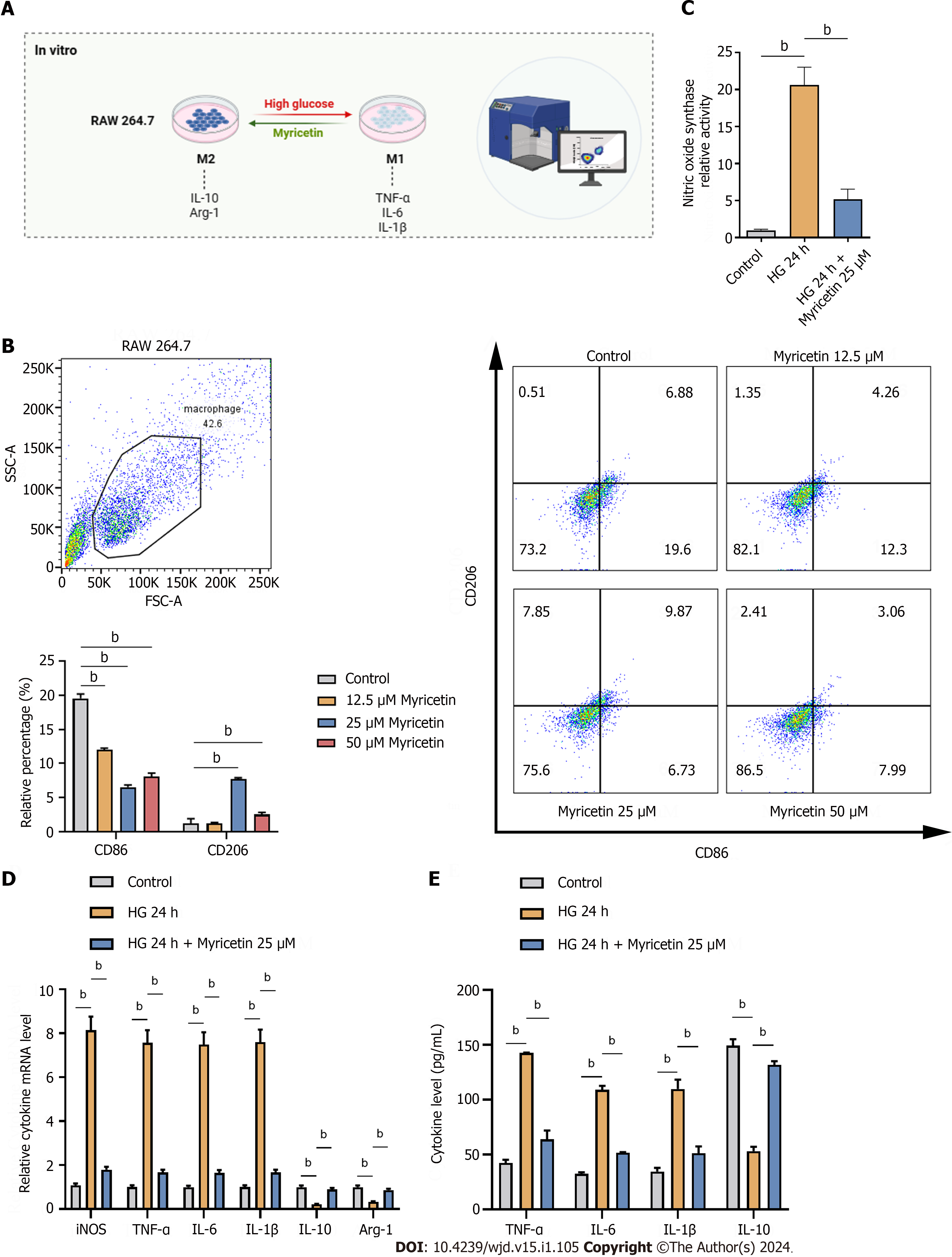
As shown in Figure 6A, a concentration gradient of myricetin at 12.5 μM, 25 μM, and 50 μM was administered to the cells for 24 h. Flow cytometry analysis revealed that 25 μM myricetin exhibited the most potent inhibitory effect on M1-type polarization of the RAW 264.7 cells. The average percentage of M1-type macrophages was 6.56%, whereas the control group exhibited a percentage of 7.72% (Figure 6B). Furthermore, 25 μM myricetin significantly suppressed the NOS activity of RAW 264.7 induced by HG (P < 0.001; Figure 6C). Furthermore, our results revealed that treatment with 25 μM myricetin significantly decreased the expressions of iNOS, TNF-α, IL-6, and IL-1β and increased the levels of IL-10 and Arg-1 (Figure 6D and E). Collectively, these data demonstrated that myricetin effectively modulated the polarization of RAW 264.7 macrophages from M1 to M2 under HG stimulation.
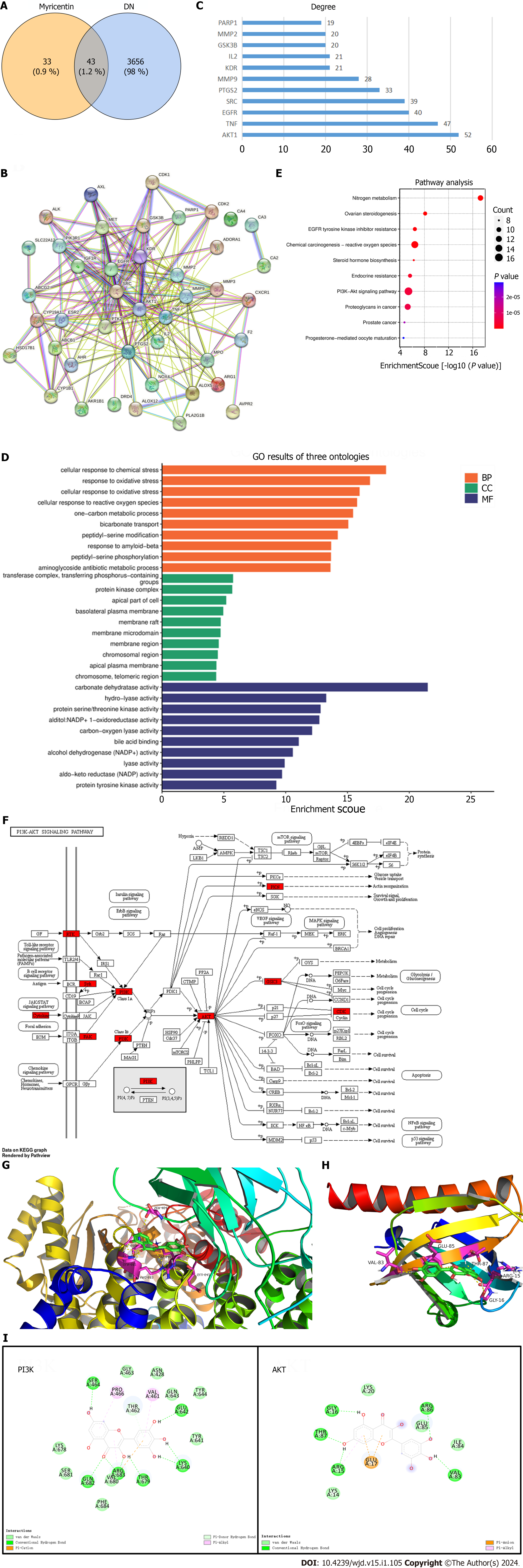
As shown in Figure 7A, a total of 3699 genes associated with DN were obtained from GeneCards and OMIM. The 43 genes that were linked to both myricetin and DN are presented in Venn diagram (Figure 7A). Subsequently, a PPI network comprised of these 43 potential targets was generated using the STRING database and visualized using Cytoscape 3.7.2 (Figure 7B). The PPI network revealed that Akt, TNF, and EGFR were the top three protein targets in immune and inflammatory signaling (Figure 7C). GO enrichment analysis revealed that myricetin treatment was associated with cellular response to oxidative stress, chemical stimulation, and protein kinases (Figure 7D). Additionally, KEGG pathway analysis indicated that myricetin primarily improved DN through the PI3K-Akt signaling pathway (Figure 7E and F). To further investigate this interaction, we used Autodock 4.2.6 to dock myricetin with Akt and PI3K, and subsequently performed a docking simulation using PyMOL 2.2.0 and Discovery Studio Client v19.1.0 (Figure 7G-H). Binding energy analysis indicated that myricetin exhibited a robust binding ability with Akt (-6.31 kcal/mol) and PI3K (-8.31 kcal/mol) (Figure 7I). Taken together, these results implied that the PI3K-Akt pathway served as a potential target in the protective mechanism of myricetin against kidney injury of DN mice.
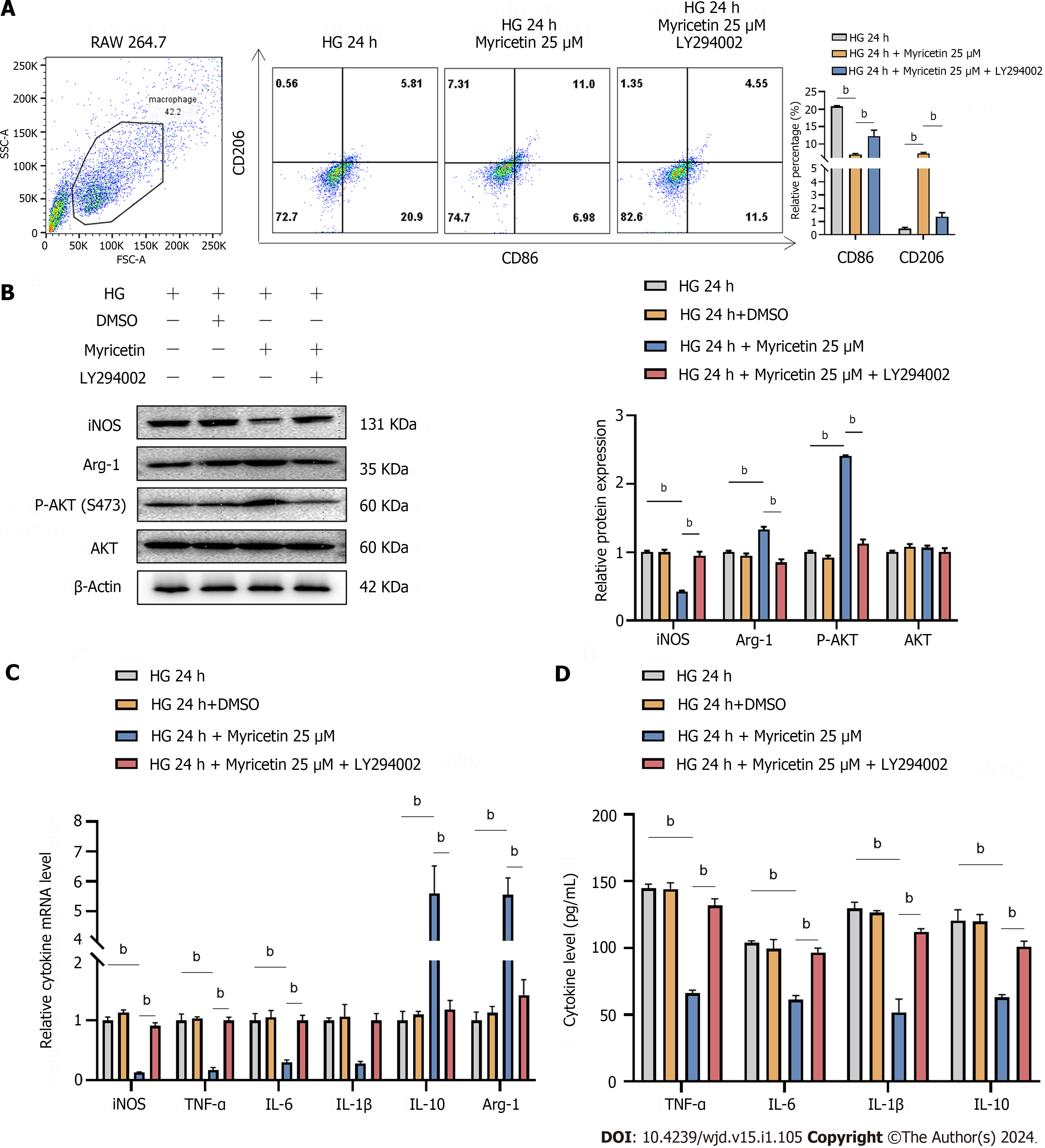
We conducted additional experiments to validate the role of myricetin in regulating Akt kinase activity, thereby influencing macrophage polarization and cytokine secretion. Expression of the M1-type marker CD86 was significantly increased while the expression of the M2-type marker CD206 was significantly decreased compared to RAW 264.7 cells treated with myricetin alone (P < 0.001; Figure 8A). Our results indicated that myricetin induced a significant increase in Akt phosphorylation, while both LY294002 treatment and the HG condition inhibited Akt phosphorylation (Figure 8B). In accordance with prior data, we observed the up-regulation of M1 inflammatory factor-related genes in response to the HG condition and the administration of myricetin induced a notable decrease in the expression of these factors. However, the co-administration of LY294002 and myricetin to RAW 264.7 cells did not trigger the down-regulation of M1-related inflammation factors' expression (Figure 8C and D). Thus, our data indicated that myricetin may have regulated the macrophage polarization via the PI3K-Akt signaling pathway.
The worldwide prevalence of DM constitutes a pervasive metabolic disorder that is accompanied by a wide range of public health challenges. Projections indicate that by the year 2035, the number of individuals affected by this condition will reach 600 million[32,33]. The sustained elevation of blood glucose levels over an extended period of time gives rise to various well-established chronic complications affecting multiple organs, such as the heart, kidneys, nerves, and retinas. Among these complications, DN is the most frequently occurring chronic microvascular complication, and it has been identified as the primary cause of ESRD, disability, and mortality[5,19]. According to research, chronic hyperglycemia has been found to impact various kidney cell types and lead to progressive renal failure.
Currently, the only effective treatments available for ESRD patients are dialysis and transplantation. However, dialysis does not halt progression of the disease and the availability of donor kidneys is limited. Strategies for DN primarily focus on the control of blood glucose, with few effective therapies available for DN patients. Thus, there is an urgent need to develop novel and efficacious therapies. The molecular mechanism underlying DN is highly intricate, involving various metabolic disorders and pathways such as ferroptosis[34], oxidative stress[35], apoptosis[35], immune response and inflammatory-related pathways[36-38]. It is widely acknowledged that inflammation plays a pivotal role in the progression of DN. Notably, renal inflammation has been demonstrated to contribute to the development of DN. Additionally, abnormal levels of IL-6, IL-18, and IL-1 have been identified as significant points to the development of DN[39,40]. Thus, the investigation of novel therapeutic approaches for DN now places significant emphasis on the pharmaceutical agents that specifically target inflammation.
The observed anti-diabetic activity of flavonoids has boosted their potential as therapeutic agents for DM and its complications. Indeed, it has been demonstrated that application of quercetin led to a reduction in blood glucose levels in a diabetes animal model induced by STZ[41]. More recently, it has been reported that kaempferol and myricetin combination treatment is promising in diabetes rats, due to their modulation of levels of glucose, inflammation, lipids and liver enzymes[42]. Another study has further demonstrated that DM could be alleviated by myricetin alone via its effects on normalizing the profile of intestinal flora[43]. The application of compounds in these contexts has collectively demonstrated the ability of a natural product to improve glucose levels and inflammatory cytokine levels in diabetic rats.
Our current findings indicate that the administration of myricetin at doses of 50 mg/kg or 100 mg/kg partially improved glucose levels, kidney/BW index, serum creatinine, creatinine clearance, and uACR in db/db mice. Furthermore, histopathological analysis revealed that myricetin significantly alleviated the DN pathological injury of renal tissue in mice. The administration of myricetin resulted in a reduction in inflammatory factors’ infiltration of kidney tissues and a decrease in the proportion of type M1 macrophages. Additionally, the renal fibrosis of DN mice was improved, as evidenced by a significant decrease in the accumulation of Col1a1 and α-SMA in DN mice treated with myricetin. These findings align with previous research and provide further validation of the role of myricetin in inhibiting the pathological progression of DN mice.
Importantly, myricetin not only plays a vital role in DN but also decreases migration of retinal pericytes[44], restores impaired motor and sensory functions[45], and enhances diabetic wound repair[46]. To our knowledge, only one cross-sectional population clinical study (consisting of 24138 subjects, among which 1357 had type 2 DM) has shown that myricetin intake might lower the prevalence type 2 DM and extend the period until other clinical treatments become necessary[47]. Other studies have shown that Abelmoschus manihot capsule containing myricetin could be useful in decreasing proteinuria, blood creatinine and blood urea nitrogen in kidney patients[48,49].
Given the emphasis on the role of inflammation in the development of DM and its complications, our study aimed to investigate the mechanism by which myricetin regulates the serum levels of TNF-α, IL-6, and IL-1β in DN mice. We observed that myricetin exhibited anti-inflammatory effects by reducing inflammatory factors associated with M1. To further understand this mechanism, we performed experiments using RAW 264.7 cells and found that treatment with 25 μM myricetin effectively mitigated cell injury induced by the HG condition (specifically 33.3 mM glucose). The results indicated that myricetin exerted an inhibitory effect on the polarization of RAW 264.7 macrophages towards the M1-type and significantly suppressed the iNOS activity induced by HG. Furthermore, treatment with myricetin led to a significant downregulation of M1-dependent inflammatory factors, as evidenced by decreased IL-10 and Arg-1 expression and secretion. These findings suggested that myricetin modulated the polarization of RAW 264.7 macrophages towards the M2-type, which was implicated in the progression of DN.
Several proteins and pathways have been documented as capable of inducing alterations in the polarization of RAW 264.7 macrophages, such as the PI3K-Akt, Notch1, NF-κB, MAPKs, and JNK/STAT3 signaling pathways[50-53]. In the context of a diet-induced non-alcoholic steatohepatitis model in mice, myricetin was able to mitigate inflammatory hepatitis and fibrosis by modulating macrophage polarization. This was achieved through the inhibition of NF-κB signaling and STAT3 activation, as well as the phosphorylation of the signal transducer[54]. Furthermore, the administration of flavonoids showed ability to mitigate inflammation in lipopolysaccharide-stimulated RAW 264.7 cells through involvement of the NF-κB and MAPK pathways[55]. These data in the literature stimulated our interest to investigate the mechanism by which myricetin modulates macrophage polarization.
Our bioinformatics analysis revealed that Akt was the primary target protein associated with immune response and inflammatory signaling pathways in DN mice and cultured cells. Additionally, our results indicated that myricetin had significant binding affinity with both Akt and PI3K. It has been reported that the PI3K/Akt pathway activates NF-κB through Akt phosphorylation, leading to the transcription of numerous inflammatory genes and receptors for advanced glycation products, and thereby promoting the production of cytokines that induce inflammation[56]. The association between up-regulated cytokines, including TNF-α, IL-1β, IL-1, IL-18, and DN and other diseases has been extensively documented[11,12]. Furthermore, the activation of the PI3K/Akt pathway has been identified as a critical factor in the polarization, migration, proliferation, and survival of macrophages[57]. The PI3K/Akt signaling pathway, through mTORC1, can regulate a macrophage's effector response, thereby modulating innate immune responses and macrophage polarization[58]. Genes related to PI3K/Akt signaling pathway deletion, including SHIP and PTEN, significantly inhibit the production of pro-inflammatory cytokines by enhancing the M2 macrophage phenotype[59]. Activation of the PTEN/PI3k/Akt pathway was reported to mediate the polarization of M2 macrophages among RAW 264.7 cells and in emphy-sematous mice[60].
In this study, we specifically inhibited the phosphorylation of Akt in myricetin-treated HG-induced RAW 264.7 cells, and in agreement with previous findings we found that the polarization of RAW 264.7 cells to M2-type was blocked; this indicated that myricetin can regulate the polarization of macrophages through the PI3K/Akt pathway and thus affect the secretion of cytokines (Figure 9). However, the PI3K/Akt pathway is well known for its ability to modulate various proteins and pathways, prompting the next question of interest: whether or not the function of myricetin in DN is due to its activation of Akt or other yet-unidentified proteins?
The results from this study suggest that high concentrations of myricetin have the potential to impede M1-type polarization of macrophages through the PI3K-AKT signaling pathway. Simultaneously, it exhibits promising efficacy in the treatment of renal injury, inflammation, and fibrosis in mice with DN. Our study provides a proof-of-concept of the function of myricetin against the progress of kidney injury induced by DM and provides more fundamental data for the development of myricetin as a bona fide treatment for diabetics.
Diabetic nephropathy (DN) is frequently observed as a chronic microvascular complication linked to end-stage renal disease, and it constitutes a significant contributor to both disability and mortality worldwide. Current therapies merely delay renal injury by controlling metabolic disturbances that occur in the early stage and, as such, there remains an urgent need to seek out and develop new drugs for clinical use. To this end, we have performed focused research on the Chinese patent medicine Abelmoschus manihot for its ability to decrease proteinuria in patients with DN.
Previous studies have indicated that myricetin possesses the potential to mitigate the pathological alterations observed in renal tissues of DN patients and models. Nevertheless, the precise molecular mechanism through which myricetin influences the progression of DN remains uncertain.
To investigate the effects of myricetin on DN and explore the underlying mechanisms of its potential therapeutic effects.
Db/db diabetic mice were administered myricetin and effects on blood and urine indexes and renal tissue pathology were assessed. Additionally, the RAW 264.7 cell line was cultured in high glucose conditions and then exposed to the PI3K/Akt inhibitor LY294002. In both the in vivo and in vitro settings, quantification of various inflammation factors’ levels was conducted using western blotting, real-time qPCR and ELISA.
In the db/db mice, myricetin had a mitigating effect on renal dysfunction and fibrosis, including kidney injury markers kidney injury molecule-1 and neutrophil gelatinase associated lipocalin and inflammatory cytokine-related factors. In the RAW 264.7 cells, myricetin treatment effectively inhibited the up-regulation of tumor necrosis factor-alpha, interleukin (IL)-6, and IL-1β and modulated M1-type polarization. Molecular docking and bioinformatic analyses revealed that Akt was the target of myricetin. The protective effect of myricetin was nullified upon blocking the polarization of RAW 264.7 via inhibition of PI3K/Akt activation using LY294002.
Myricetin effectively mitigates kidney injury in DN mice through the regulation of macrophage polarization via the PI3K/Akt signaling pathway.
Myricetin represents a promising therapy in treating DN.
We acknowledge the SwissTargetPrediction, GeneCards, OMIM, STRING and PubChem databases for providing platforms for uploading their datasets with meaningful information, and thank all animals involved in this study who gave their lives for the advancement of human health.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Horowitz M, Australia; Morya AK, India; Nagoba B, India S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, Shi B, Sun H, Ba J, Chen B, Du J, He L, Lai X, Li Y, Chi H, Liao E, Liu C, Liu L, Tang X, Tong N, Wang G, Zhang JA, Wang Y, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Ning G, Mu Y, Zhao J, Teng W, Shan Z. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1030] [Cited by in RCA: 999] [Article Influence: 199.8] [Reference Citation Analysis (1)] |
| 2. | Komorowsky CV, Brosius FC 3rd, Pennathur S, Kretzler M. Perspectives on systems biology applications in diabetic kidney disease. J Cardiovasc Transl Res. 2012;5:491-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Nakamura K, Miyoshi T, Yoshida M, Akagi S, Saito Y, Ejiri K, Matsuo N, Ichikawa K, Iwasaki K, Naito T, Namba Y, Sugiyama H, Ito H. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 139] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 4. | Naaman SC, Bakris GL. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. Diabetes Care. 2023;46:1574-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 106] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 5. | Samsu N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res Int. 2021;2021:1497449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 492] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 6. | Chang DY, Li MR, Yu XJ, Wang SX, Chen M, Zhao MH. Clinical and Pathological Characteristics of Patients With Nonproteinuric Diabetic Nephropathy. Front Endocrinol (Lausanne). 2021;12:761386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Shen Z, Fang Y, Xing T, Wang F. Diabetic Nephropathy: From Pathophysiology to Treatment. J Diabetes Res. 2017;2017:2379432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Iacobini C, Vitale M, Pesce C, Pugliese G, Menini S. Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Yaribeygi H, Atkin SL, Sahebkar A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J Cell Physiol. 2019;234:1300-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 10. | Li X, Wen J, Dong Y, Zhang Q, Guan J, Liu F, Zhou T, Li Z, Fan Y, Wang N. Wnt5a promotes renal tubular inflammation in diabetic nephropathy by binding to CD146 through noncanonical Wnt signaling. Cell Death Dis. 2021;12:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Wu L, Liu C, Chang DY, Zhan R, Sun J, Cui SH, Eddy S, Nair V, Tanner E, Brosius FC, Looker HC, Nelson RG, Kretzler M, Wang JC, Xu M, Ju W, Zhao MH, Chen M, Zheng L. Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 2021;100:107-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 12. | Rayego-Mateos S, Rodrigues-Diez RR, Fernandez-Fernandez B, Mora-Fernández C, Marchant V, Donate-Correa J, Navarro-González JF, Ortiz A, Ruiz-Ortega M. Targeting inflammation to treat diabetic kidney disease: the road to 2030. Kidney Int. 2023;103:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 144] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 13. | Minton K. Macrophages: a transcription factor to call their own. Nat Rev Immunol. 2011;11:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kianoush F, Nematollahi M, Waterfield JD, Brunette DM. Regulation of RAW264.7 macrophage polarization on smooth and rough surface topographies by galectin-3. J Biomed Mater Res A. 2017;105:2499-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1401] [Cited by in RCA: 1449] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 16. | Liu J, Zhang Y, Sheng H, Liang C, Liu H, Moran Guerrero JA, Lu Z, Mao W, Dai Z, Liu X, Zhang L. Hyperoside Suppresses Renal Inflammation by Regulating Macrophage Polarization in Mice With Type 2 Diabetes Mellitus. Front Immunol. 2021;12:733808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 17. | Qian X, He L, Hao M, Li Y, Li X, Liu Y, Jiang H, Xu L, Li C, Wu W, Du L, Yin X, Lu Q. YAP mediates the interaction between the Hippo and PI3K/Akt pathways in mesangial cell proliferation in diabetic nephropathy. Acta Diabetol. 2021;58:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Lei L, Zhao J, Liu XQ, Chen J, Qi XM, Xia LL, Wu YG. Wogonin Alleviates Kidney Tubular Epithelial Injury in Diabetic Nephropathy by Inhibiting PI3K/Akt/NF-κB Signaling Pathways. Drug Des Devel Ther. 2021;15:3131-3150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 388] [Article Influence: 43.1] [Reference Citation Analysis (1)] |
| 20. | Lv M, Chen Z, Hu G, Li Q. Therapeutic strategies of diabetic nephropathy: recent progress and future perspectives. Drug Discov Today. 2015;20:332-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Warren AM, Knudsen ST, Cooper ME. Diabetic nephropathy: an insight into molecular mechanisms and emerging therapies. Expert Opin Ther Targets. 2019;23:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 22. | Li N, Tang H, Wu L, Ge H, Wang Y, Yu H, Zhang X, Ma J, Gu HF. Chemical constituents, clinical efficacy and molecular mechanisms of the ethanol extract of Abelmoschus manihot flowers in treatment of kidney diseases. Phytother Res. 2021;35:198-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Liu S, Ye L, Tao J, Ge C, Huang L, Yu J. Total flavones of Abelmoschus manihot improve diabetic nephropathy by inhibiting the iRhom2/TACE signalling pathway activity in rats. Pharm Biol. 2017;56:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Zhou J, Zhang S, Sun X, Lou Y, Bao J, Yu J. Hyperoside ameliorates diabetic nephropathy induced by STZ via targeting the miR-499-5p/APC axis. J Pharmacol Sci. 2021;146:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Jiang X, Yu J, Wang X, Ge J, Li N. Quercetin improves lipid metabolism via SCAP-SREBP2-LDLr signaling pathway in early stage diabetic nephropathy. Diabetes Metab Syndr Obes. 2019;12:827-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Song X, Tan L, Wang M, Ren C, Guo C, Yang B, Ren Y, Cao Z, Li Y, Pei J. Myricetin: A review of the most recent research. Biomed Pharmacother. 2021;134:111017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 27. | Sun J, Sun J, Zhou X. Protective functions of myricetin in LPS-induced cardiomyocytes H9c2 cells injury by regulation of MALAT1. Eur J Med Res. 2019;24:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Park HH, Lee S, Son HY, Park SB, Kim MS, Choi EJ, Singh TS, Ha JH, Lee MG, Kim JE, Hyun MC, Kwon TK, Kim YH, Kim SH. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res. 2008;31:1303-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 29. | Liao HH, Zhu JX, Feng H, Ni J, Zhang N, Chen S, Liu HJ, Yang Z, Deng W, Tang QZ. Myricetin Possesses Potential Protective Effects on Diabetic Cardiomyopathy through Inhibiting IκBα/NFκB and Enhancing Nrf2/HO-1. Oxid Med Cell Longev. 2017;2017:8370593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Kandasamy N, Ashokkumar N. Myricetin, a natural flavonoid, normalizes hyperglycemia in streptozotocin-cadmium-induced experimental diabetic nephrotoxic rats. Biomed Prevent Nutrit. 2012;2:246-251. |
| 31. | Miranda-Díaz AG, Pazarín-Villaseñor L, Yanowsky-Escatell FG, Andrade-Sierra J. Oxidative Stress in Diabetic Nephropathy with Early Chronic Kidney Disease. J Diabetes Res. 2016;2016:7047238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 32. | Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The Growing Epidemic of Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 33. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 2956] [Article Influence: 268.7] [Reference Citation Analysis (1)] |
| 34. | Wang Y, Bi R, Quan F, Cao Q, Lin Y, Yue C, Cui X, Yang H, Gao X, Zhang D. Ferroptosis involves in renal tubular cell death in diabetic nephropathy. Eur J Pharmacol. 2020;888:173574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 35. | Sifuentes-Franco S, Padilla-Tejeda DE, Carrillo-Ibarra S, Miranda-Díaz AG. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int J Endocrinol. 2018;2018:1875870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 36. | Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol. 2016;12:13-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 324] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 37. | Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 38. | Chen J, Liu Q, He J, Li Y. Immune responses in diabetic nephropathy: Pathogenic mechanisms and therapeutic target. Front Immunol. 2022;13:958790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 39. | Wong CK, Ho AW, Tong PC, Yeung CY, Kong AP, Lun SW, Chan JC, Lam CW. Aberrant activation profile of cytokines and mitogen-activated protein kinases in type 2 diabetic patients with nephropathy. Clin Exp Immunol. 2007;149:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Navarro JF, Mora C. Role of inflammation in diabetic complications. Nephrol Dial Transplant. 2005;20:2601-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 41. | Roshanravan N, Askari SF, Fazelian S, Ayati MH, Namazi N. The roles of quercetin in diabetes mellitus and related metabolic disorders; special focus on the modulation of gut microbiota: A comprehensive review. Crit Rev Food Sci Nutr. 2023;63:2990-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Al-Abbasi FA, Kazmi I. Therapeutic role of kaempferol and myricetin in streptozotocin-induced diabetes synergistically via modulation in pancreatic amylase, glycogen storage and insulin secretion. Mol Cell Biochem. 2023;478:1927-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 43. | Zhao Z, Chen Y, Li X, Zhu L, Wang X, Li L, Sun H, Han X, Li J. Myricetin relieves the symptoms of type 2 diabetes mice and regulates intestinal microflora. Biomed Pharmacother. 2022;153:113530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 44. | Kim YS, Kim J, Kim KM, Jung DH, Choi S, Kim CS, Kim JS. Myricetin inhibits advanced glycation end product (AGE)-induced migration of retinal pericytes through phosphorylation of ERK1/2, FAK-1, and paxillin in vitro and in vivo. Biochem Pharmacol. 2015;93:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Niisato N, Marunaka Y. Therapeutic potential of multifunctional myricetin for treatment of type 2 diabetes mellitus. Front Nutr. 2023;10:1175660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Xu Z, Liu G, Huang J, Wu J. Novel Glucose-Responsive Antioxidant Hybrid Hydrogel for Enhanced Diabetic Wound Repair. ACS Appl Mater Interfaces. 2022;14:7680-7689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 139] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 47. | Yao Z, Li C, Gu Y, Zhang Q, Liu L, Meng G, Wu H, Bao X, Zhang S, Sun S, Wang X, Zhou M, Jia Q, Song K, Li Z, Gao W, Niu K, Guo C. Dietary myricetin intake is inversely associated with the prevalence of type 2 diabetes mellitus in a Chinese population. Nutr Res. 2019;68:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Zhang L, Li P, Xing CY, Zhao JY, He YN, Wang JQ, Wu XF, Liu ZS, Zhang AP, Lin HL, Ding XQ, Yin AP, Yuan FH, Fu P, Hao L, Miao LN, Xie RJ, Wang R, Zhou CH, Guan GJ, Hu Z, Lin S, Chang M, Zhang M, He LQ, Mei CL, Wang L, Chen X. Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. Am J Kidney Dis. 2014;64:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 49. | Wei LI, Ping X, Wei S, Jing Z, Qiong L, Lianyi G, Yao Z, Kun G. Effects of the Huangkui capsule on chronic kidney disease: a systematic review and Meta-analysis. J Tradit Chin Med. 2023;43:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Huang C, Liu XJ, QunZhou, Xie J, Ma TT, Meng XM, Li J. MiR-146a modulates macrophage polarization by inhibiting Notch1 pathway in RAW264.7 macrophages. Int Immunopharmacol. 2016;32:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 51. | Sun JM, Ho CK, Gao Y, Chong CH, Liu YD, Liu YX, Zheng DN, Zhang YF, Yu L. Salvianolic Acid B Reduces the Inflammation of Fat Grafts by Inhibiting the NF-Kb Signalling Pathway in Macrophages. Aesthet Surg J. 2023;43:NP372-NP390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 52. | Li Y, Feng L, Li G, An J, Zhang S, Li J, Liu J, Ren J, Yang L, Qi Z. Resveratrol prevents ISO-induced myocardial remodeling associated with regulating polarization of macrophages through VEGF-B/AMPK/NF-kB pathway. Int Immunopharmacol. 2020;84:106508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Saranyutanon S, Acharya S, Deshmukh SK, Khan MA, Singh S, Singh AP. Nicotine causes alternative polarization of macrophages via Src-mediated STAT3 activation: Potential pathobiological implications. J Cell Physiol. 2022;237:1486-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 54. | Yao Q, Li S, Li X, Wang F, Tu C. Myricetin Modulates Macrophage Polarization and Mitigates Liver Inflammation and Fibrosis in a Murine Model of Nonalcoholic Steatohepatitis. Front Med (Lausanne). 2020;7:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 55. | Jiang F, Guan H, Liu D, Wu X, Fan M, Han J. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct. 2017;8:1313-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 56. | Chen J, Crawford R, Xiao Y. Vertical inhibition of the PI3K/Akt/mTOR pathway for the treatment of osteoarthritis. J Cell Biochem. 2013;114:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Linton MF, Moslehi JJ, Babaev VR. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 58. | Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015;25:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 617] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 59. | Weisser SB, McLarren KW, Voglmaier N, van Netten-Thomas CJ, Antov A, Flavell RA, Sly LM. Alternative activation of macrophages by IL-4 requires SHIP degradation. Eur J Immunol. 2011;41:1742-1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Lu J, Xie L, Liu C, Zhang Q, Sun S. PTEN/PI3k/AKT Regulates Macrophage Polarization in Emphysematous mice. Scand J Immunol. 2017;85:395-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |