Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1314
Peer-review started: April 27, 2023
First decision: May 19, 2023
Revised: May 20, 2023
Accepted: June 19, 2023
Article in press: June 19, 2023
Published online: August 15, 2023
Processing time: 105 Days and 17.1 Hours
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) are commonly prescribed to manage patients with diabetes mellitus. These agents may rarely lead to the development of euglycemic diabetic ketoacidosis (EDKA), which may complicate the disease course of these patients.
To analyze the demographic profile, predisposing factors, symptomology, clinical interventions and outcomes of patients presenting with EDKA secondary to SGLT2i use by reviewing the published case reports and series.
We performed a systematic search of PubMed, Science Direct, Google Scholar and Reference Citation Analysis databases using the terms “canagliflozin” OR “empagliflozin” OR “dapagliflozin” OR “SGLT2 inhibitors” OR “Sodium-glucose cotransporter-2” AND “euglycemia” OR “euglycemic diabetic ketoacidosis” OR “metabolic acidosis”. The inclusion criteria were: (1) Case reports or case series with individual patient details; and (2) Reported EDKA secondary to SGLT2i. Furthermore, the data were filtered from the literature published in the English language and on adults (> 18 years). We excluded: (1) Conference abstracts; and (2) Case reports or series which did not have individual biochemical data. All the case reports and case series were evaluated. The data extracted included patient demographics, clinical symptomatology, clinical interventions, intensive care unit course, need for organ support and outcomes.
Overall, 108 case reports and 17 cases series with 169 unique patients that met all the inclusion criteria were included. The majority of patients were females (54.4%, n = 92), and the commonly reported symptoms were gastrointestinal (nausea/vomiting 65.1%, abdominal pain 37.3%) and respiratory (breathlessness 30.8%). One hundred and forty-nine (88.2%) patients had underlying type II diabetes, and the most commonly involved SGLT-2 inhibitor reported was empagliflozin (46.8%). A triggering factor was reported in most patients (78.7%), the commonest being acute severe infection (37.9%), which included patients with sepsis, coronavirus disease 2019, other viral illnesses, and acute pancreatitis. 61.5% were reported to require intensive unit care, but only a minority of patients required organ support in the form of invasive mechanical ventilation (13%), vasopressors (6.5%) or renal replacement therapy (5.9%). The overall mortality rate was only 2.4%.
Patients on SGLT2i may rarely develop EDKA, especially in the presence of certain predisposing factors, including severe acute infections and following major surgery. The signs and symptoms of EDKA may be similar to that of DKA but with normal blood sugar levels, which may make the diagnosis challenging. Outcomes of EDKA are good if recognized early and corrective actions are taken. Hence, physicians managing such patients must be aware of this potential complication and must educate their patients accordingly to ensure early diagnosis and management.
Core Tip: Sodium-glucose cotransporter-2 inhibitors are a newer class of oral hypoglycemic drugs commonly prescribed for managing patients with diabetes mellitus. Even though these drugs are effective in controlling blood glucose and have favorable cardiac effects, they may rarely lead to the development of euglycemic diabetic ketoacidosis (EDKA), which may complicate the disease course of these patients. Certain risk factors, such as severe acute illness and major surgery, may predispose these patients to develop EDKA. The signs and symptoms of EDKA are similar to classic symptoms of diabetic ketoacidosis, but these patients have normal blood glucose levels, making the diagnosis difficult. Hence, a higher index of suspicion is warranted in such patients, as delay in diagnosis may lead to higher morbidity and mortality.
- Citation: Juneja D, Nasa P, Jain R, Singh O. Sodium-glucose Cotransporter-2 Inhibitors induced euglycemic diabetic ketoacidosis: A meta summary of case reports. World J Diabetes 2023; 14(8): 1314-1322
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1314.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1314
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) are relatively new oral hypoglycemic agents (OHAs), which are increasingly being used to manage patients with diabetes mellitus (DM). The current American Diabetes Association (ADA) guidelines recommend using SGLT2i as one of the second-line agents, along with metformin, in managing patients with type II diabetes mellitus (T2DM). They may also be used as the primary agent in patients with heart failure, chronic kidney disease, risk of atherosclerotic cardiovascular disease, and those who are metformin intolerant or in whom metformin is contraindicated[1].
The mechanism of action of SGLT2i is independent of insulin secretion, making them an appealing choice for combination therapy. By inhibiting the SGLT2 receptors in the proximal tubules of the kidneys, this reduces glucose reabsorption and the renal threshold for glucose, thereby increasing renal excretion and reducing serum glucose levels[2].
SGLT2i have several clinical advantages, including reduced risk of hypoglycemic episodes, improved blood pressure control, weight reduction and positive cardiovascular outcomes[2,3]. However, the use of SGLT2i is also associated with an increased incidence of genitourinary infections and hypovolemia[4]. Within months of Food and Drug Administration (FDA) approval, cases of diabetic ketoacidosis (DKA) were reported among those using SGLT2i. In earlier reports, the incidence of DKA was 0.522 per 1000 patient-years in patients taking canagliflozin 100 mg/d. A higher incidence of 0.763 per 1000 patient-years was reported in patients taking higher doses of 300 mg/d. However, most patients had blood glucose levels higher than 300 mg/dL, and EDKA has been even more rarely reported[5].
DKA is a well-documented complication in patients with T1DM that is often recognized at the time of a new diagnosis of diabetes and is generally precipitated by poor adherence to treatment or acute infection[6]. EDKA is a rare, but mostly missed and under-reported complication of DM management. It is arbitrarily defined as DKA without marked hyperglycemia. The ADA has defined EDKA as the presence of high anion-gap metabolic acidosis and increased plasma ketones in the presence of blood glucose levels below 250 mg/dL (13.9 mmol/L)[7].
The main aim of this meta-summary was to identify the predisposing factors, symptomatology, clinical course and outcomes of the patients on SGLT2i presenting with EDKA. This may aid physicians involved in managing such patients to make an early diagnosis and prevent future events.
For this meta summary, a systematic search of PubMed, Science Direct, Reference Citation Analysis (https://www.refere
All the case reports, and case series were evaluated. The data extracted included patient demographics, clinical symptomatology, clinical interventions, intensive care unit (ICU) course, need for organ support and outcomes. A datasheet for evaluation was also prepared.
The prepared datasheet was analyzed using Excel and Microsoft Office 2019. Categorical variables were presented as frequency and percentage. Mean (SD) or median [interquartile range (IQR)] was used to describe the continuous variables. The statistical analyses were performed using SPSS (version 25.0, IBM SPSS Inc., Chicago, IL, United States). MS Office software (MS Office 2019, Microsoft Corp., WA, United States) was used for tabulation and final docu
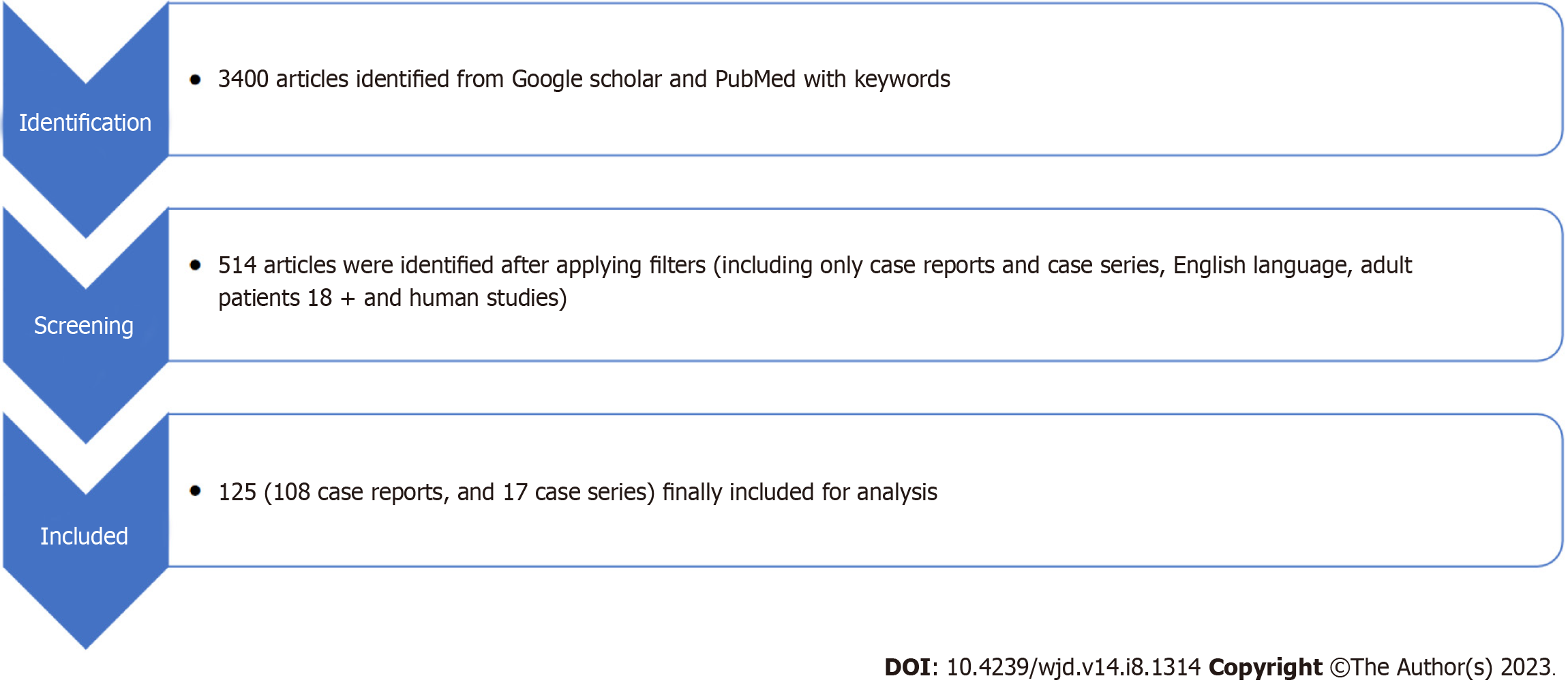
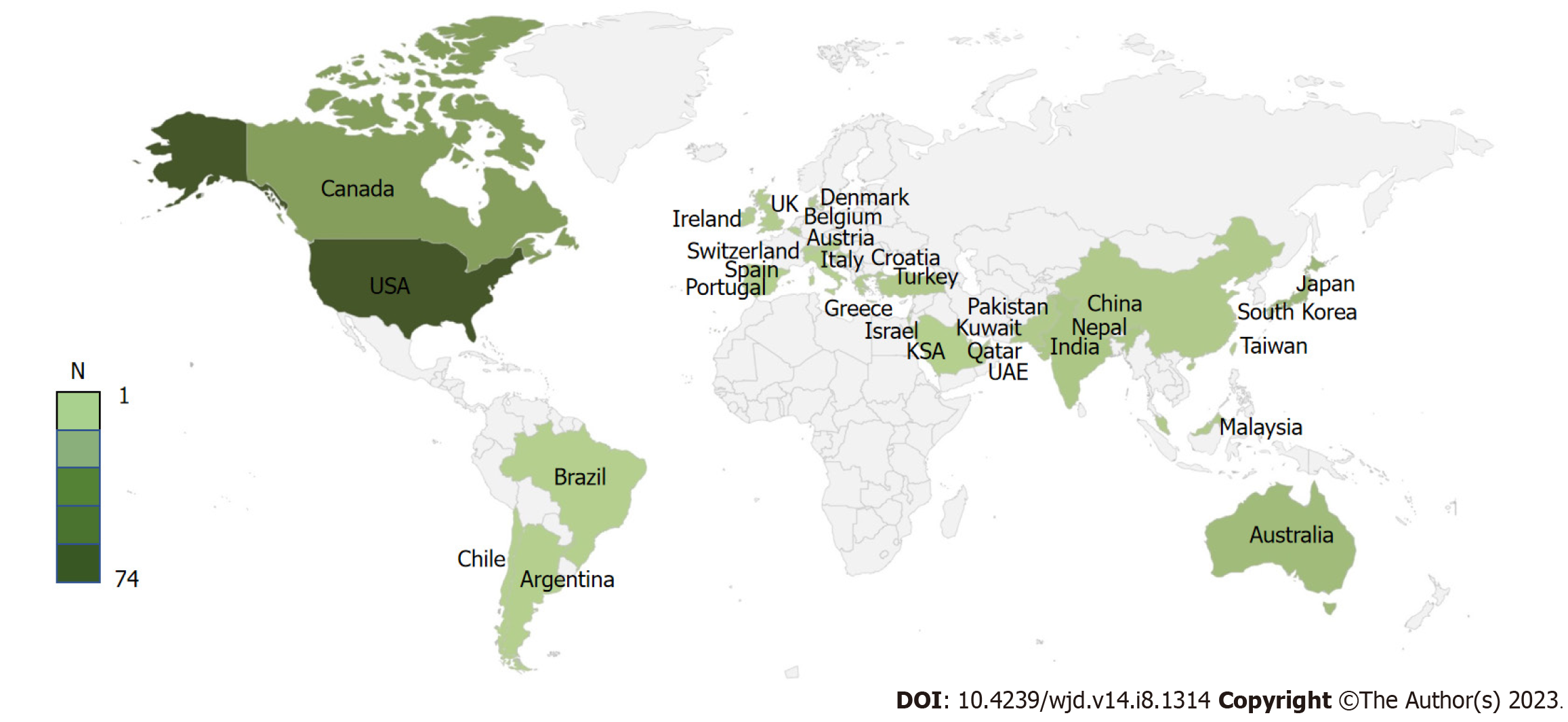
The present review was carried out using the preferred reporting items for systematic reviews and meta-analyses 2009 checklist (Figure 1). Ultimately, 108 case reports and 17 cases series with 169 unique patients meeting the predefined inclusion criteria were included (Supplementary material). The majority of included patients were from the United States of America (74, 43.8%) and Canada (23, 13.6%) (Figure 2). Most of the patients reported were females (54.4%, n = 92), and the commonly reported symptoms were gastrointestinal (nausea/vomiting 65.1%, abdominal pain 37.3%) and respiratory (breathlessness 30.8%). One hundred and forty-nine (88.2%) patients had underlying type II diabetes; the most commonly involved SGLT2i was empagliflozin, 46.8% (Table 1). Most patients (78.7%) reported a triggering factor, the commonest being acute severe infection (37.9%), which included patients with sepsis, coronavirus disease 2019, other viral illnesses, and acute pancreatitis. The second most common triggering factor was a perioperative period (24.3%), which included patients undergoing bariatric surgery, coronary artery bypass grafting, orthopedic surgeries, pancreatectomy and cranial nervous system surgeries. Multiple triggering factors were reported in several patients (Table 1). The median time on SGLT2i before developing EDKA was 30 d (interquartile range 6.5-165 d).
| Parameter | Number of patients, n = 169 |
| Age (± SD), yr | 51.7 (13.8) |
| Gender, n (%) | Females, 92 (54.4) |
| Males, 77 (45.6) | |
| Type of diabetes, n (%) | Type I, 18 (10.7) |
| Type II, 149 (88.2) | |
| Not mentioned, 2 (1.1) | |
| Body mass index (± SD) | 29.6 (6.4) |
| Clinical presentation, n (%) | Nausea/Vomiting, 110 (65.1) |
| Abdominal pain, 63 (37.3) | |
| Breathlessness, 52 (30.8) | |
| Fatigue, 46 (27.2) | |
| Altered mental status, 34 (20.1) | |
| Loss of consciousness, 11 (6.5) | |
| Chest pain, 5 (3) | |
| Shock, 4 (2.4) | |
| Fever, 4 (2.4) | |
| Others, 12 (7.1) | |
| Comorbidities, n (%) | Diabetes, 166 (98.2) |
| Hypertension, 45 (26.6) | |
| Coronary artery disease, 20 (11.8) | |
| Dyslipidemia, 13 (7.7) | |
| Cancer, 4 (2.4) | |
| Others, 24 (14.4) | |
| SGLT-2 inhibitor involved, n (%) | Empagliflozin, 79 (46.8) |
| Canagliflozin, 50 (29.6) | |
| Dapagliflozin, 39 (23.1) | |
| Ipragliflozin, 1 (0.6) | |
| Tofogliflozin, 1 (0.6) | |
| Other OHAs prescribed, n (%) | Yes, 135 (79.9) |
| No, 25 (14.8) | |
| Not mentioned, 9 (5.1) | |
| Other diabetes medications involved, n (%) | Metformin, 119 (70.4) |
| Dipeptidyl peptidase 4 inhibitors, 42 (24.9) | |
| Sulfonylureas, 26 (15.4) | |
| Thiazolidinediones, 10 (5.9) | |
| Meglitinides, 3 (1.8) | |
| α-Glucosidase inhibitors, 2 (1.2) | |
| Insulins, 57 (33.7) | |
| Glucagon-like peptide-1 receptor agonist, 21 (12.4) | |
| Not mentioned, 11 (6.5) | |
| History of alcohol use, n (%) | 6 (3.6) |
| Identifiable triggering factor, n (%) | Present, 133 (78.7) |
| Triggering factor, n (%) | Infection, 64 (37.9) |
| Major surgery, 41 (24.3) | |
| Reduced food intake, 32 (18.9) | |
| Any major illness, 17 (10.1) | |
| Reduced carbohydrate/ketogenic diet 14 (8.3) | |
| Dehydration, 14 (8.3) | |
| Reduced insulin dosages, 10 (5.9) | |
| Prolonged fasting, 9 (5.3) | |
| Trauma, 2 (1.2) | |
| Ketones for diagnosis, n (%) | Only urine, 62 (36.7) |
| Only plasma, 36 (21.3) | |
| Both, 60 (35.5) | |
| Not mentioned, 11 (6.5) | |
| Organ failure, n (%) | Respiratory, 20 (11.8) |
| Cardiac, 11 (6.5) | |
| Renal, 8 (4.7) | |
| Need for organ support, n (%) | Invasive mechanical ventilation, 22 (13) |
| Vasopressors, 11 (6.5) | |
| RRT, 10 (5.9) | |
| Other treatments given, n (%) | Sodium bicarbonate, 21 (12.4) |
| Need for ICU, n (%) | 104 (61.5) |
| Days in ICU (± SD) | 3.4 (5.4) d |
| Days in hospital (± SD) | 9.3 (10.4) d |
| Outcome, n (%) | Alive, 164 (97) |
| Death, 4 (2.4) | |
| Not mentioned, 1 (0.6) |
The median blood glucose level at presentation was 184.5 mg/dL. Most patients had severe metabolic acidosis with a median serum pH of 7.14 and bicarbonate levels of 8.6 mmol/L. Hyperlactatemia was uncommon, with median lactate levels being 1.3 mmol/L (Table 2). The overall mortality rate was only 2.4%.
| Parameter | Median values |
| Blood glucose (IQR) | 184.5 (151.8-219.3) mg/dL |
| Serum osmolality (IQR) | 297 (290.8-312.8) mmol/kg |
| pH (IQR) | 7.14 (7.05-7.24) |
| Bicarbonate (IQR) | 8.6 (6-11) mmol/L |
| Lactates (IQR) | 1.3 (1.1-1.8) mmol/L |
| Anion gap (IQR) | 22.50 (19-28) mmol/L |
In the present meta-summary, data from 169 individual case reports were analyzed. Most patients (88.2%) were suffering from T2DM. Common presenting symptoms included nausea, vomiting, and abdominal pain. Empagliflozin was the commonest SGLT2i involved in 46.8% of cases. At presentation, the median blood glucose levels were 184.5 mg/dL, and the median blood pH was 7.14. Nearly 62% of patients were reported to require ICU admission. Even though patients presented with severe metabolic acidosis, the overall mortality rate was only 2.4%.
DKA is a medical emergency which is diagnosed with hyperglycemia (blood glucose > 250 mg/dL), metabolic acidosis (arterial pH < 7.3, serum bicarbonate < 15 mEq/L), and ketonemia. However, between 2.6% to 3.2% of DKA admissions may present with normal to near-normal blood glucose levels (blood glucose < 250 mg/dL)[8,9]. Even though the association of EDKA with SGLTi is well established, the cause for EDKA secondary to SGLT2i is not well recognized. Several mechanisms have been proposed, including independent action on pancreatic alpha cells, which increases plasma glucagon levels, stimulates hepatic ketogenesis, and reduces renal clearance of ketone bodies (especially beta-hydroxybutyrate and acetoacetate)[2,10]. SGLT2i increase renal excretion and block glucose reabsorption from the proximal convoluted tubule, thereby reducing serum glucose levels[11]. The combined effect of these mechanisms may lead to ketonemia and ketoacidosis without much of an increase in serum glucose levels.
In the present meta-summary, most (88.2%) patients had type II diabetes. This could be explained by the fact that type II diabetes is much more common in adults accounting for almost 90% of cases[12]. SGLT2i are primarily recommended for treating type II diabetes, but they increase the risk of developing DKA 7-fold in these patients[13]. SGLT2i are now being prescribed for type I diabetes also, especially in those who failed to achieve glycemic targets with insulin alone, because their use is associated with improved HbA1c levels, reduction in body weight and better blood pressure control in these patients[14-16]. However, because of their increased potential to precipitate EDKA in TIDM, SGLT2i are generally discouraged in these patients[7,10,17].
A previous meta-summary analyzing data from 77 patients with EDKA associated with SGLT2i also reported a higher incidence among females (67.5%) but reported canagliflozin (44.2%) as the commonest SGLT2 inhibitor involved. However, as our meta-summary shows, empagliflozin (46.8%) was the most commonly implicated agent, followed by canagliflozin (29.6%). This could be explained by the fact that canagliflozin was the first SGLT2 inhibitor commercially available; hence it was more widely prescribed earlier. With changing prescription practices, newer SGLT2i are more widely prescribed, explaining the increase in reporting of side effects. Other findings in the previous meta-summary were similar to our findings, including the age at presentation (51.3 years), presenting symptoms and preponderance of type II diabetics (83.1%)[18]. The risk of developing EDKA is unrelated to the duration of exposure[13,18,19]. In the present study, the median duration of therapy with SGLT2i before the patients developed EDKA was 30 d, but patients developed EDKA even after one day or one dose of therapy[20,21].
The symptoms of EDKA are often non-specific and missed or ignored by patients and even their physicians due to misleadingly normal or near-normal blood glucose levels. This may lead them to maintain or reduce their insulin dose, further exacerbating ketosis and metabolic acidosis.
Testing for urinary ketone bodies remains a standard test for the diagnosis of DKA. However, urine screening of ketones by nitroprusside agents only measures acetone and acetoacetate and does not detect beta-hydroxybutyrate, resulting in missing ketonuria. Hence, testing for blood ketones (b-hydroxybutyrate) is generally recommended[7]. However, urinary ketones remained a standard test in the present meta-summary and was solely relied upon in 36.7% of cases.
Identifying precipitating factors can have significant clinical implications in preventing and managing EDKA. In the present study, acute infection and perioperative stress were found to be common triggering factors. Any major illness, trauma or surgery may result in a stress response associated with an increased release of catecholamines, heightened production of cortisol and reduced secretion and utilization of insulin[22]. If patients continue their SGLT2i, reduced plasma glucose levels may mask the precise insulin requirements, increasing the risk of developing DKA.
As major surgery is an important factor that may precipitate EDKA, taking due precautions before surgery is imperative. Even the current recommendation by FDA and International Consensus Review on SGLT2i is to stop these drugs three days before surgery[23,24]. As these drugs are primarily excreted through the kidneys, it may be prudent to stop them even earlier in patients with renal dysfunction[25].
Other common factors which may predispose patients to develop EDKA include prolonged fasting, low carbohydrate or ketogenic diet, excessive alcohol intake, dehydration and reduction in insulin dosage[18,19,26]. High protein and low carbohydrate diets may increase serum glucagon and reduce serum insulin levels. They may also cause an increase in counterregulatory hormones (epinephrine and cortisol), leading to increased free fatty acids and increased production of ketone bodies. As the reduction in insulin dosage may also precipitate DKA, stopping or drastically reducing the dose abruptly is not recommended. Moreover, the stress response due to a systemic illness or major surgery is a common trigger; hence, timely discontinuation of SGLT2i should be considered in acute stressful conditions such as a major illness or post-operatively[23]. Patient education and pre-operative discontinuation of SGLT2i and switching to insulin may aid in curtailing the risk of EDKA. Furthermore, the European Medicines Agency suggests stopping SGLT2i immediately if symptoms or signs of DKA are suspected and not starting them until EDKA is excluded and an apparent precipitating factor has been identified and resolved[27].
The present meta-analysis compiled 125 global studies involving 169 unique patients who had developed EDKA secondary to the use of SGLT2i. Additionally, we included only those studies which had individual patient details to compare patient demographics, precipitating factors and clinical outcomes. This is the largest such analysis, which adds strength to this review. However, the included studies were only case reports and case series which had no control arm. The studies were heterogeneous, and had a high risk of bias and missing data, which may affect the generalizability of the results. Additionally, because we did not include the case reports or series which did not report individual biochemical data, we may have missed some relevant reports.
Patients on SGLT2i may rarely develop EDKA, primarily due to certain predisposing factors, including severe acute infections and following major surgery. The signs and symptoms may be similar to DKA but with normal blood sugar levels, making the diagnosis challenging. EDKA outcomes are good if recognized timely and corrective actions are taken. Hence, physicians managing such patients must be aware of this potential complication and educate patients accordingly to ensure early diagnosis and management.
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) are commonly prescribed drugs in managing patients with diabetes mellitus (DM). These agents may rarely lead to the development of euglycemic diabetic ketoacidosis (EDKA), which may complicate the disease course of these patients.
EDKA is a rare, but mostly missed and under-reported complication of DM management. The use of SGLT2i may increase the risk of developing EDKA.
The main aim of this meta-summary was to identify the predisposing factors, symptomatology, clinical course and outcomes of the patients on SGLT2i presenting with EDKA.
We performed a systematic search of PubMed, Science Direct, Google Scholar and Reference Citation Analysis (https://www.referencecitationanalysis.com/) databases using the terms “canagliflozin” OR “empagliflozin” OR “dapagliflozin” OR “SGLT2 inhibitors” OR “Sodium-glucose cotransporter-2” AND “euglycemia” OR “euglycemic diabetic ketoacidosis” OR “metabolic acidosis”.
Overall, 108 case reports and 17 cases series with 169 unique patients were included. One hundred and forty-nine (88.2%) patients had underlying type II diabetes, and the most commonly involved SGLT2 inhibitor reported was empagliflozin (46.8%). A triggering factor was reported in most patients (78.7%), the commonest being acute severe infection (37.9%). Sixty-one-point-five percent were reported to require intensive unit care, but only a minority of patients required organ support. The overall mortality rate was only 2.4%.
Patients on SGLT2i may rarely develop EDKA, especially in the presence of certain predisposing factors. The signs and symptoms of EDKA may be similar to those of DKA but with normal blood sugar levels. Outcomes of EDKA are good if recognized early and corrective actions are taken.
Large scale studies must be conducted to find out the true incidence and clinical impact of EDKA in patients using SGLT2i.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He Z, China; Shi J, China S-Editor: Li L L-Editor: Webster JR P-Editor: Xu ZH
| 1. | American Diabetes Association. Disclosures: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46 (Suppl 1):S1-292. [RCA] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 2. | Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors May Predispose to Ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849-2852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 366] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 3. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8298] [Article Influence: 829.8] [Reference Citation Analysis (1)] |
| 4. | Mosley JF 2nd, Smith L, Everton E, Fellner C. Sodium-Glucose Linked Transporter 2 (SGLT2) Inhibitors in the Management Of Type-2 Diabetes: A Drug Class Overview. P T. 2015;40:451-462. [PubMed] |
| 5. | Erondu N, Desai M, Ways K, Meininger G. Diabetic Ketoacidosis and Related Events in the Canagliflozin Type 2 Diabetes Clinical Program. Diabetes Care. 2015;38:1680-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 6. | Umpierrez G, Korytkowski M. Diabetic emergencies - ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol. 2016;12:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 287] [Article Influence: 31.9] [Reference Citation Analysis (1)] |
| 7. | Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care. 2015;38:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 518] [Cited by in RCA: 560] [Article Influence: 56.0] [Reference Citation Analysis (2)] |
| 8. | Yu X, Zhang S, Zhang L. Newer Perspectives of Mechanisms for Euglycemic Diabetic Ketoacidosis. Int J Endocrinol. 2018;2018:7074868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 9. | Jenkins D, Close CF, Krentz AJ, Nattrass M, Wright AD. Euglycaemic diabetic ketoacidosis: does it exist? Acta Diabetol. 1993;30:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Rosenstock J, Ferrannini E. Euglycemic Diabetic Ketoacidosis: A Predictable, Detectable, and Preventable Safety Concern With SGLT2 Inhibitors. Diabetes Care. 2015;38:1638-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 442] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 11. | Pfützner A, Klonoff D, Heinemann L, Ejskjaer N, Pickup J. Euglycemic ketosis in patients with type 2 diabetes on SGLT2-inhibitor therapy-an emerging problem and solutions offered by diabetes technology. Endocrine. 2017;56:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2533] [Article Influence: 281.4] [Reference Citation Analysis (0)] |
| 13. | Blau JE, Tella SH, Taylor SI, Rother KI. Ketoacidosis associated with SGLT2 inhibitor treatment: Analysis of FAERS data. Diabetes Metab Res Rev. 2017;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 14. | Dandona P, Mathieu C, Phillip M, Hansen L, Tschöpe D, Thorén F, Xu J, Langkilde AM; DEPICT-1 Investigators. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study. Diabetes Care. 2018;41:2552-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 15. | Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, Zinman B, Skyler JS, George J, Soleymanlou N, Perkins BA. Empagliflozin as Adjunctive to Insulin Therapy in Type 1 Diabetes: The EASE Trials. Diabetes Care. 2018;41:2560-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 250] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 16. | Snaith JR, Holmes-Walker DJ, Greenfield JR. Reducing Type 1 Diabetes Mortality: Role for Adjunctive Therapies? Trends Endocrinol Metab. 2020;31:150-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and Safety of Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor, as Add-on to Insulin in Patients With Type 1 Diabetes. Diabetes Care. 2015;38:2258-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 18. | Dutta S, Kumar T, Singh S, Ambwani S, Charan J, Varthya SB. Euglycemic diabetic ketoacidosis associated with SGLT2 inhibitors: A systematic review and quantitative analysis. J Family Med Prim Care. 2022;11:927-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Burke KR, Schumacher CA, Harpe SE. SGLT2 Inhibitors: A Systematic Review of Diabetic Ketoacidosis and Related Risk Factors in the Primary Literature. Pharmacotherapy. 2017;37:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 20. | Calçada MB, Fernandes L, Soares Costa R, Montezinho S, Martins Duarte F, Frutuoso L, Freitas AR. Euglycemic Diabetic Ketoacidosis after a Single Dose of Empagliflozin in a Patient with Pancreatitis. Clin Pract. 2021;11:216-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Dizon S, Keely EJ, Malcolm J, Arnaout A. Insights Into the Recognition and Management of SGLT2-Inhibitor-Associated Ketoacidosis: It's Not Just Euglycemic Diabetic Ketoacidosis. Can J Diabetes. 2017;41:499-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 1283] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 23. | Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, Ferrannini E, Fonseca VA, Garber AJ, Grunberger G, LeRoith D, Umpierrez GE, Weir MR. American association of clinical endocrinologists and american college of endocrinology position statement on the association of sglt-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 24. | Danne T, Garg S, Peters AL, Buse JB, Mathieu C, Pettus JH, Alexander CM, Battelino T, Ampudia-Blasco FJ, Bode BW, Cariou B, Close KL, Dandona P, Dutta S, Ferrannini E, Fourlanos S, Grunberger G, Heller SR, Henry RR, Kurian MJ, Kushner JA, Oron T, Parkin CG, Pieber TR, Rodbard HW, Schatz D, Skyler JS, Tamborlane WV, Yokote K, Phillip M. International Consensus on Risk Management of Diabetic Ketoacidosis in Patients With Type 1 Diabetes Treated With Sodium-Glucose Cotransporter (SGLT) Inhibitors. Diabetes Care. 2019;42:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 25. | Pace DJ, Dukleska K, Phillips S, Gleason V, Yeo CJ. Euglycemic Diabetic Ketoacidosis Due to Sodium-Glucose Cotransporter 2 Inhibitor Use in Two Patients Undergoing Pancreatectomy. J Pancreat Cancer. 2018;4:95-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Patel K, Nair A. A Literature Review of the Therapeutic Perspectives of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitor-Induced Euglycemic Diabetic Ketoacidosis. Cureus. 2022;14:e29652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | European Medicines Agency. EMA confirms recommendations to minimise ketoacidosis risk with SGLT2 inhibitors for diabetes. Feb 25, 2016. [cited 23 May 2023]. Available from: https://www.ema.europa.eu/en/medicines/human/referrals/sglt2-inhibitors. |