Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.930
Peer-review started: December 27, 2022
First decision: February 8, 2023
Revised: March 14, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: June 15, 2023
Processing time: 169 Days and 16.5 Hours
Diabetic keratopathy (DK) occurs in 46%-64% of patients with diabetes and requires serious attention. In patients with diabetes, the healing of corneal epithelial defects or ulcers takes longer than in patients without diabetes. Insulin is an effective factor in wound healing. The ability of systemic insulin to rapidly heal burn wounds has been reported for nearly a century, but only a few studies have been performed on the effects of topical insulin (TI) on the eye. Treatment with TI is effective in treating DK.
To review clinical and experimental animal studies providing evidence for the efficacy of TI to heal corneal wounds.
National and international databases, including PubMed and Scopus, were searched using relevant keywords, and additional manual searches were conducted to assess the effectiveness of TI application on corneal wound healing. Journal articles published from January 1, 2000 to December 1, 2022 were examined. The relevancy of the identified citations was checked against pred
A total of eight articles were found relevant to be discussed in this review, including four animal studies and four clinical studies. According to the studies conducted, TI is effective for corneal re-epithelialization in patients with diabetes based on corneal wound size and healing rate.
Available animal and clinical studies have shown that TI promotes corneal wound healing by several mechanisms. The use of TI was not associated with adverse effects in any of the published cases. Further studies are needed to enhance our knowledge and understanding of TI in the healing of DK.
Core Tip: Diabetic keratopathy (DK) is a common complication of diabetes mellitus that is responsible for poor corneal wound healing. It also reduces quality of vision and quality of life. DK is the result of damage resulting from insulin deficiency, hyperglycemia and neuropathy. Topical insulin has been described as an effective and safe new treatment for DK that can normalize the ocular surface and healing rate of epithelial defects. This review examines the available evidence.
- Citation: Leong CY, Naffi AA, Wan Abdul Halim WH, Bastion MLC. Usage of topical insulin for the treatment of diabetic keratopathy, including corneal epithelial defects. World J Diabetes 2023; 14(6): 930-938
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/930.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.930
Diabetes mellitus is an international public health concern with many complications, both microvascular and macrovascular. The International Diabetes Federation states that 451 million adults worldwide had diabetes in 2017, and this number is expected to increase to 693 million by 2045[1].
Diabetic keratopathy (DK) or diabetic corneal epitheliopathy is one of the complications of diabetes mellitus. It is a degenerative corneal disease that requires serious attention. DK occurs in 46%-64% of patients with diabetes, which affects their quality of life[2]. Ocular surgery, such as corneal tran
Insulin is a biologically active peptide closely related to insulin-like growth factor (IGF) that can stimulate the haptotactic migration of human epidermal keratinocytes and is involved in cell growth, proliferation, metabolism and wound healing[9]. The mechanism by which TI improves corneal wound healing is not yet fully understood. Insulin is found in the tear film of the eye. Insulin receptors are found in the corneal epithelium and ocular surface tissue[10]. The presence of insulin and insulin receptors on the cornea and lacrimal glands suggests that insulin may contribute to corneal wound healing[11]. Rocha et al[12] also detected insulin in tears and the expression of the insulin receptor and IGF-1 receptor (IGF-1R) on the human ocular surface. IGF-1 promotes corneal epithelial healing by increasing cell proliferation. The topical application of insulin can stimulate IGF-1R and treat DK[12].
A literature search was conducted and completed on 10 December 2022. Two databases, namely, PubMed and Scopus, were used to identify all studies concerning topical insulin (TI) treatment for DK. Articles were limited to journal articles with the keywords “topical insulin”, diabetes, and keratopathy in the field of the search. The following string was used: TITLE-ABS-KEY [“topical insulin” OR (“local” AND “insulin”) OR (“topical” AND “insulin”)] AND TITLE-ABS-KEY (“diabetes” OR “diabetic” OR “diabetes mellitus” OR “diabetics”) AND TITLE-ABS-KEY (“cornea” OR “corneal” OR “cornea wound healing” OR “corneal wound healing” OR “keratopathy” OR “diabetic keratopathy” OR “cornea wound” OR “corneal wound” OR “eye” OR “eyes”). The search was further supplemented by manual searching for relevant references and using reference citation analysis to find the latest research results. We only examined journal articles published from January 1, 2000 to December 1, 2022.
Studies that fulfilled the following criteria were included: (1) The experimental group (adults and animals) was diabetic; (2) The experimental group with DK was treated with TI or insulin-growth-factor; and (3) The effects on the cornea, such as the corneal epithelial defect healing rate, healing size, time to heal, ocular surface disease index score or tear break-up time, were compared between the experimental and control groups. Publications from case reports, letters, and studies without raw data were excluded. We would select either the article with the most recent publication date or with the largest sample size if multiple articles were published based on the same population and were based on one study. In addition, exclusion criteria included articles not published in English, nondiabetic experimental populations, reported effects that were not on the cornea and inadequate information in the article’s text.
In the first phase of the search, the first reviewer (Leong CY) reviewed the articles and studies that were duplicated and overlapping were excluded. Subsequently, two reviewers (Leong CY and Naffi AA) independently screened the titles and abstracts, and irrelevant abstract articles were excluded. The full texts of the remaining publications were reviewed by three reviewers (Leong CY, Naffi AA, and Wan Abdul Halim WH), and studies meeting the exclusion criteria were eliminated. Finally, the fourth reviewer (Bastion MLC) reviewed the articles for comprehensiveness and accuracy.
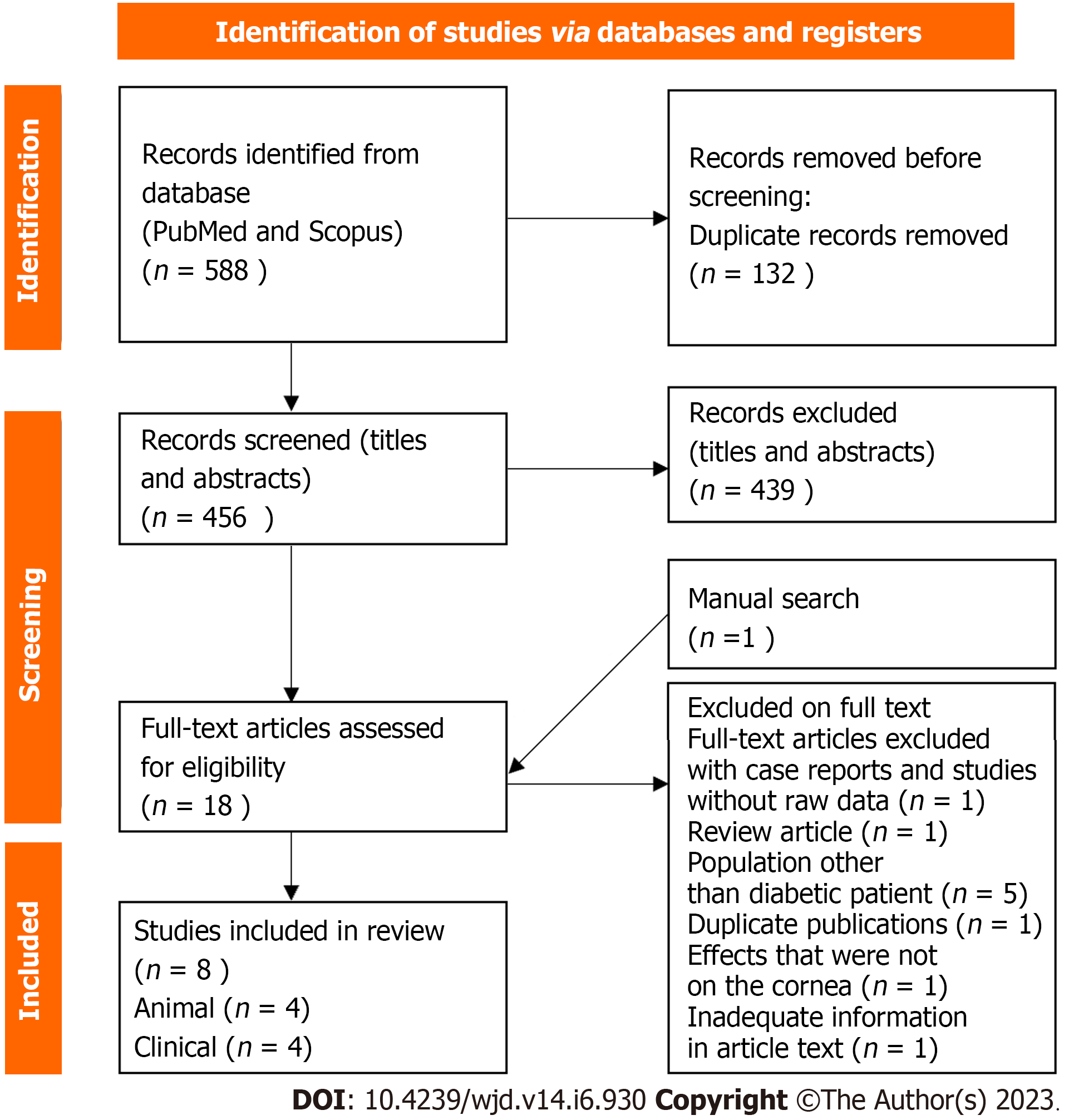
In the first phase of the search, a total of 588 related articles were found with the above strategy. A total of eight articles were found relevant to be discussed in this review, including four animal studies and four clinical studies. The flow chart is presented in Figure 1. All articles in this review are listed in the reference sources. Table 1 contains a list of characteristics of each animal study, and Table 2 illustrates the characteristics of human clinical studies.
| Ref. | Country | Aim | Study design | Subject groups (number) | Insulin type and dose | Results |
| Nakamura et al[13], 2003 | Japan | To study the effect of the combination of FGLM-NH2 and IGF-1 on corneal epithelial wound healing in rats with diabetes | Animal | 4-wk-old male Sprague-Dawley Streptozocin-induced diabetic rats; 100 g (n = 20) | FGLM-NH2 (1 mmol/L) and IGF-1 (1 μg/mL) 6 times per day | Similar wound healing processes were observed in normal rats and diabetic rats treated with FGLM-NH2 and IGF-1. Wound closure was significantly faster in diabetic rats treated with FGLM-NH2 and IGF-1 than in those treated with vehicle |
| Zagon et al[14], 2007 | United States | To determine TI normalizes delayed corneal wound healing in rats with diabetes | Animal | Male Sprague-Dawley Streptozocin-induced diabetic rats; 165 g (38 diabetic rats; 11 nondiabetic rats) | Bovine insulin 1, 2, or 5 U. Single drop (20 μL) | TI normalizes corneal re-epithelialization in diabetic rats. No difference in efficacy of insulin dose of 1, 2, or 5 U and safe for cornea |
| Chen et al[15], 2013 | United States | To determine corneal nerve depletion in type 1 diabetes rats using corneal confocal microscopy and its relationship with TI | Animal | Female Swiss Webster Streptozocin-induced diabetic mice; 25-30 g (8 diabetic mice; 8 control) | 0.1 IU of regular U-100 Humulin (Lilly, Indianapolis, IN, United States) in 10 μL saline | TI prevent depletion of nerve occupancy in the subbasal nerve plexus of the cornea without affecting systemic glycemic control |
| Yang et al[10], 2020 | China | To investigate the relationship between TI and WnT/β-catenin signaling pathway in corneal epithelial healing and corneal nerve repair in diabetic mice | Animal | Streptozocin-induced diabetic mice (6 to 8-year-old-male C57BL/6J mice) | Human neural insulin (Lilly France S.A., Fegersheim, France). 3 μL QID (1 IU/mL) | Insulin contributes to diabetic corneal epithelial wound healing and nerve injury healing via Wnt signaling, making it a potential protective factor for diabetic corneal epithelial wounds and nerve injuries |
| Ref. | Country | Aim | Study design | Subject groups (number) | Insulin type and dose | Results |
| Bastion and Ling[16], 2003 | Malaysia | To determine whether TI improve healing rate of corneal epithelial erosion during vitreoretinal surgery | Retrospective review | Human (15 eyes of 14 patients underwent corneal debridement during vitreoretinal surgery) | Actrapid HM, Novo Nordisk 1 U QID (50 UI/mL) | Delayed epithelial healing in diabetic eyes compared with normal eyes. Diabetic eyes treated with TI had significantly smaller defect size than diabetic eyes treated with conventional therapy |
| Fai et al[3], 2017 | Malaysia | To investigate the effect of 3 concentration of TI in corneal epithelial wound healing in postoperative patient with diabetes | Double blind randomized controlled | Human (32 eyes of 32 diabetic patient underwent corneal debridement during vitreoretinal surgery) | Actrapid HM, Novonordisk 0.5, 1, 2 U QID | TI 0.5 U QID is most effective for corneal re-epithelialization in patients with diabetes after vitrectomy surgery as compared with placebo and higher concentrations. TI is safe for human ocular use |
| Aniah Azmi and Bastion[17], 2020 | Malaysia | To determine the short-term effects of TI on symptoms and signs of dry eye disease in patients with diabetes | Randomized, double-blind interventional study | Human (320 eyes of 160 patients with diabetes for treatment of dry eyes) | Actrapid HM, Novo Nordisk 1 U QID (25 UL/mL) | Similar improvement in the Ocular Surface Disease Index score for TI 1 U QID and standard artificial tears in the treatment of dry eye in patients with diabetes |
| Quiroz-Mendoza et al[18], 2021 | Mexico | To compare the effect of TI and sodium hyaluronate in epithelial defects postoperative in patients with diabetes | Controlled human clinical trial | Human (36 eyes of 36 patients with diabetes who underwent corneal debridement during vitreoretinal surgery) | Recombinant human insulin (Humulin® R, Eli Lilly and Company, Indiana, United States) 0.5 IU/drop QID (25 IU/mL) | TI 0.5 IU/drops monotherapy and combined treatment with 0.15% sodium hyaluronate is effective in healing corneal epithelial defects after intraoperative corneal debridement in patients with diabetes. Adding sodium hyaluronate to TI did not provide additional benefit |
Nakamura et al[13] studied the effects of combining IGF-1 and a substance P-derived tetrapeptide (phenylalanine-glycine-leucine-methionine-amide, or FGLM-NH2) on corneal epithelial wound healing in diabetic rats. The corneal epithelium was removed in both diabetic and nondiabetic rats from limbus to limbus and treated with eye drops containing 1 mmol/L FGLM-NH2 (Peptide Institute, Osaka, Japan) and IGF-1 (1 μg/mL-1) (Becton Dickinson, Bedford, Mass., United States) 6 times daily for 3 d or vehicle alone as a control. The area of the corneal epithelial wound was measured several times for up to 72 h after treatment onset. A delay in wound closure was observed in diabetic rats compared with nondiabetic rats. Similar wound healing processes were observed in normal rats and diabetic rats treated with FGLM-NH2 and IGF-1. However, wound closure was significantly faster in diabetic rats treated with FGLM-NH2 and IGF-1 than in those treated with vehicle[13].
Zagon et al[14] performed an animal study and reported that the remaining corneal epithelial defects were 35% larger in rats with diabetes than in healthy animals. In diabetic rats that received TI, corneal healing was significantly enhanced compared to diabetic rats without TI. This study also compared 1, 2, or 5 U insulin in healthy and diabetic rats. Insulin concentrations with more than a 5-fold difference showed no difference in efficacy and safety for the cornea, as determined by corneal thickness, intraocular pressure and ocular surface morphological characteristics[14].
Chen et al[15] studied corneal nerve density depletion in patients with diabetes using corneal confocal microscopy and its relationship with TI. The effects of type 1 diabetes on corneal nerves were then studied over time using female Sprague-Dawley rats, whereas the impact of TI on corneal nerves was investigated using female Swiss Webster mice. In rats with diabetes, nerve occupancy in the subbasal plexus was significantly reduced at week 40. TI was applied (0.1 IU daily) to the eyes of diabetes mellitus rats for 4 wk prevented the depletion of nerves of the subbasal plexus without any effect on systemic glycemic control[15].
Yang et al[10] investigated the relationship between TI and the WnT/β-catenin signaling pathway in corneal epithelial healing and corneal nerve repair in diabetic mice. Type 1 diabetes was induced in 6- to 8-year-old male C57BL/6J mice. TI (3 μL) was administered four times daily one week before and one week after corneal scraping. This study showed that TI stimulated the accumulation of β-catenin in the cell, activated the Wnt/β-catenin signaling pathway, and finally stimulated cell proliferation. In addition, a preliminary study of this research showed that TI also promoted epithelial healing in mice with type 2 diabetes after corneal injury[10].
Bastion and Ling[16] retrospectively reviewed 15 eyes of 14 patients who underwent corneal epithelial debridement during vitreoretinal surgery to improve the surgeon’s view in 2010 over a 10-mo period. This study compared three groups: Patients with diabetes treated with TI 1 U/drop four times daily in addition to conventional postoperative therapy, patients with diabetes treated with conventional therapy, namely, topical antibiotics and steroids only, and nondiabetic patients treated with conventional therapy. TI (1 U) was prepared using Actrapid HM, Novo Nordisk, Denmark to provide 50 U/mL insulin at approximately 1 U per drop 4 times per day. Patients with diabetes treated with TI had significantly smaller defect sizes at 24, 36 and 48 h than patients with diabetes treated with conventional therapy. In addition, insulin-treated diabetic eyes re-epithelialized within 48 h, whereas conventionally treated eyes re-epithelialized within 72 h[16].
Fai et al[3] prospectively studied the effect of TI at three concentrations (0.5, 1, and 2 U per drop) vs placebo four times daily on the postoperative wound healing of corneal epithelium in patients with diabetes after vitreoretinal surgery. This work was a randomized, controlled, double-blind study. Thirty-two eyes of 32 patients with diabetes who underwent intraoperative corneal debridement with a Tookes knife with resulting epithelial defects of various sizes were randomized into 3 different concentrations of TI or placebo. The insulin used was Actrapid HM 100 U/mL, as in the study by Bastion and Ling[16]. The results of this study showed that TI (0.5 U) was superior to the other insulin concentrations in achieving a 100% healing rate within 72 h. TI (0.5 U) 4 times a day (QID) was found to be most effective for healing corneal epithelial defects in patients with diabetes in this study compared to placebo and insulin at higher concentrations after vitrectomy. TI was also shown to be safe for use in the human eye[3].
Aniah Azmi and Bastion[17] evaluated the short-term effects of TI (1 U per drop) four times daily for one month on patients with diabetic dry eye disease (DDED). This work was a randomized, double-blind intervention study involving patients with diabetes with dry eye who were randomly assigned to be treated with TI or artificial tears (AT). The insulin used was actrapid HM (Novo Nordisk, Bagsvaerd, Denmark). Patients were assessed at baseline, week 2, and week 4 of treatment. This study showed that TI and AT produced similar improvements in the Ocular Surface Disease Index in the treatment of dry eye in patients with diabetes, whose symptoms had improved after both therapies. However, TI worsened tear break-up time compared with baseline, but this did not differ from that of the AT group. Nevertheless, after one month of treatment, symptoms or clinical signs of DDED did not significantly differ between TI and AT[17].
Quiroz-Mendoza et al[18] compared the effect of TI and sodium hyaluronate on the healing of corneal epithelial defects in patients with diabetes after corneal epithelial debridement during pars plana vitrectomy. This study was a controlled clinical trial in which patients were randomly assigned to groups treated with TI 0.5 (IU/drops), 0.15% topical sodium hyaluronate (Hyabak®, Laboratorios Théa® México), or combined treatment with 0.5 IU/drop TI and 0.15% sodium hyaluronate. Insulin was prepared using recombinant human insulin (Humulin® R, Eli Lilly and Company, Indiana, United States). Patients were required to instill TI 4 times per day. Both treatments, i.e., 0.5 IU/drop TI as monotherapy and TI combined with 0.15% sodium hyaluronate, were effective in treating corneal epithelial defects resulting from intraoperative corneal debridement during pars plana vitrectomy in patients with diabetes. The addition of sodium hyaluronate to TI did not provide a greater benefit than TI alone. No adverse effects were noted in this study[18].
Wang et al[19] reviewed 6 patients with refractory neurotropic ulcers treated with TI. This study was a retrospective study of 6 patients with neurotropic corneal ulcers who did not respond to conventional medical and surgical treatments. The addition of TI resulted in rapid and complete corneal re-epithelialization after the initiation of treatment[19].
Diaz-Valle et al[20] evaluated treatment with TI for persistent epithelial defects (PED) refractory to conventional treatment. This study was a prospective, nonrandomized study that enrolled patients with refractory PEDs who did not respond to usual treatment. Patients were treated with insulin eye drops four times daily. This study demonstrated that TI accelerates corneal re-epithelialization and improves and safety promotes healing in PED patients who are not responsive to standard treatment[20].
Tong et al[21] reported a case of bilateral neurotropic keratitis that was unresponsive to conventional therapy and was successfully treated with 25 IU/mL TI six times daily in each eye. The neurotropic ulcers dramatically re-epithelialized within 1 wk. In this instance, TI was evidently successful in promoting re-epithelialization where other forms of treatment had failed[21].
Galvis et al[22] discussed a case diagnosed with exposure keratopathy after acoustic neuroma resection with involvement of the facial nerve and trigeminal nerve that developed into infectious keratitis 2 wks after surgery. The patient had a persistent epithelial defect despite topical antibiotics, steroids, autologous serum drops, and bandage contact lenses. TI (1 UI/mL) was administered as adjuvant therapy four times daily, and the epithelial defect closed completely after 2 wks[22].
Bartlett et al[23] conducted a prospective, randomized, single-masked study in 8 healthy volunteers on the safety of TI. Subjects were administered different concentrations: 0, 0.1, 1.0, 10.0, and 100 IU/mL TI in one eye and placebo in the other eye. They were evaluated immediately after instillation and 2 h after instillation. Several parameters were measured: Stinging, burning, tearing, itching, foreign body sensation, visual acuity and slit lamp examination. The results showed no significant difference in toxicity between the eyes receiving TI and those receiving placebo[23]. No adverse effect was observed in any of the published cases with the use of TI at concentrations up to 100 IU/mL.
Although all clinical data support the safety of TI, a stable formulation of TI is currently not commercially available. Le Nguyen et al[24] first introduced information on the stability of 1 UI/mL insulin eye drops. This study utilized the concentration reported for the effective treatment of refractory epithelial defects in both diabetic and nondiabetic eyes. The physicochemical and microbiological stability of the formulation of TI eye drops were evaluated. TI was prepared by diluting commercial Humalog insulin Lispro solution (100 UI/mL) with polyethylene and propylene glycol-based artificial eye drops to a concentration of 1 UI/mL. The resultant solution was stored in a multidose eyedropper made of low-density polyethylene. The stability of this TI formulation was studied at 4 °C for 12 mo in unopened eyedroppers and under stimulated use conditions at 4 °C and 25 °C for 30 d. The parameters studied for physicochemical stability were visual inspection, pH, turbidity, ultraviolet spectral absorption and osmolality.
In addition, insulin and m-cresol concentrations were tested using a new size-exclusion chromatographic method. The results showed that all tested parameters were favorable, and unopened eye droppers were physicochemically and microbiologically stable at 4 °C for 12 mo. Under stimulated eye conditions, these parameters also remained stable at 4 °C for one month. Furthermore, a similar result was observed when solutions were stored at 25 °C under stimulated eye conditions, with no effect of potential temperature increases on the insulin and m-cresol concentrations in the insulin eyedropper[24]. Studies on the stability of studies utilizing higher concentrations of insulin, such as those described in the various clinical studies on diabetic eyes with epithelial defects or DDED mentioned earlier in this review, are currently lacking. Studies on the stability of TI in various types of AT are also lacking.
Artificial intelligence (AI) has been developed and used in the field of ophthalmology. The majority of AI research in the past has focused on posterior segment diseases, such glaucoma, retinopathy of prematurity, and optic neuropathy[25]. In recent years, an increasing number of studies have employed AI to recognize different keratopathies. The use of AI in DED, particularly automatic DED detection and categorization, has tremendous potential[26]. The study of DED using machine learning may aid in the diagnosis and monitoring of treatments, such as TI.
This study was subject to limitations. The literature included in this study used a variety of types, dosages and methods of dilution of TI. The methodology to assess outcomes in each study, such as cornea wound size and rate, also varied. For animal studies, different types of rats and ages were used, and the sample sizes were small. In addition, the sample sizes of clinical studies were also small, and the types of diabetic keratopathies were different.
Treatment with TI is effective in treating DK, including DDED, epithelial defects after corneal debridement and refractory epithelial defects. It offers many advantages, including excellent tolerability, availability, cost-effectiveness and, most importantly, safety when applied to the human eye, without adverse events. More studies are needed to determine its stability in normal saline and in AT of various types, and the advantage of combining TI with AT to increase its contact time and reduce the need for frequent dosing warrants further study.
Diabetic keratopathy (DK) is one of the complications of diabetes mellitus. In diabetic patients, any corneal epithelial defect or ulcer takes longer to heal and persists longer. Treatment with topical insulin (TI) is effective in treating DK.
Insulin is an effective factor in wound healing. The ability of systemic insulin to rapidly heal burn wounds has been reported for nearly a century, but only a few studies have been performed on the effects of TI on the eye.
The aim of the study is to review clinical and experimental animal studies providing evidence for the efficacy of TI to heal corneal wounds.
To evaluate the efficacy of TI application on corneal wound healing, the published literature was reviewed systematically for publication. The available data was then thoroughly reviewed.
Eight articles in total, comprising four animal studies and four clinical studies, were identified and discussed. According to the studies conducted, TI is effective for corneal re-epithelialization in patients with diabetes based on corneal wound size and healing rate.
Treatment with TI is effective in treating DK. It offers many advantages, including excellent tolerability, availability, cost-effectiveness and, most importantly, safety when applied to the human eye, without adverse events. Further studies are needed to enhance our knowledge and understanding of TI in the healing of DK.
TI promotes corneal wound healing and was not associated with adverse effects in any of the published cases. More studies are needed to determine its stability in normal saline and in artificial tear (AT) of various types, and the advantage of combining TI with AT to increase its contact time and reduce the need for frequent dosing warrants further study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lee KS, South Korea; Nassar M, United States; Setiyorini E, Indonesia; Horowitz M, Australia S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4389] [Article Influence: 627.0] [Reference Citation Analysis (0)] |
| 2. | Priyadarsini S, Whelchel A, Nicholas S, Sharif R, Riaz K, Karamichos D. Diabetic keratopathy: Insights and challenges. Surv Ophthalmol. 2020;65:513-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 3. | Fai S, Ahem A, Mustapha M, Mohd Noh UK, Bastion MC. Randomized Controlled Trial of Topical Insulin for Healing Corneal Epithelial Defects Induced During Vitreoretinal Surgery in Diabetics. Asia Pac J Ophthalmol (Phila). 2017;6:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Milner MS, Beckman KA, Luchs JI, Allen QB, Awdeh RM, Berdahl J, Boland TS, Buznego C, Gira JP, Goldberg DF, Goldman D, Goyal RK, Jackson MA, Katz J, Kim T, Majmudar PA, Malhotra RP, McDonald MB, Rajpal RK, Raviv T, Rowen S, Shamie N, Solomon JD, Stonecipher K, Tauber S, Trattler W, Walter KA, Waring GO 4th, Weinstock RJ, Wiley WF, Yeu E. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27 Suppl 1:3-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Dogru M, Okada N, Asano-Kato N, Tanaka M, Igarashi A, Takano Y, Fukagawa K, Shimazaki J, Tsubota K, Fujishima H. Atopic ocular surface disease: implications on tear function and ocular surface mucins. Cornea. 2005;24:S18-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Zhang X, Zhao L, Deng S, Sun X, Wang N. Dry Eye Syndrome in Patients with Diabetes Mellitus: Prevalence, Etiology, and Clinical Characteristics. J Ophthalmol. 2016;2016:8201053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Georgakopoulos CD, Makri OE, Pagoulatos D, Vasilakis P, Peristeropoulou P, Kouli V, Eliopoulou MI, Psachoulia C. Effect of Omega-3 Fatty Acids Dietary Supplementation on Ocular Surface and Tear Film in Diabetic Patients with Dry Eye. J Am Coll Nutr. 2017;36:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Midena E, Brugin E, Ghirlando A, Sommavilla M, Avogaro A. Corneal diabetic neuropathy: a confocal microscopy study. J Refract Surg. 2006;22:S1047-S1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Shanley LJ, McCaig CD, Forrester JV, Zhao M. Insulin, not leptin, promotes in vitro cell migration to heal monolayer wounds in human corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Yang S, Zhang Y, Zhang Z, Dan J, Zhou Q, Wang X, Li W, Zhou L, Yang L, Xie L. Insulin Promotes Corneal Nerve Repair and Wound Healing in Type 1 Diabetic Mice by Enhancing Wnt/β-Catenin Signaling. Am J Pathol. 2020;190:2237-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Cruz-Cazarim ELC, Cazarim MS, Ogunjimi AT, Petrilli R, Rocha EM, Lopez RFV. Prospective insulin-based ophthalmic delivery systems for the treatment of dry eye syndrome and corneal injuries. Eur J Pharm Biopharm. 2019;140:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Rocha EM, Cunha DA, Carneiro EM, Boschero AC, Saad MJ, Velloso LA. Identification of insulin in the tear film and insulin receptor and IGF-1 receptor on the human ocular surface. Invest Ophthalmol Vis Sci. 2002;43:963-967. [PubMed] |
| 13. | Nakamura M, Kawahara M, Morishige N, Chikama T, Nakata K, Nishida T. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1. Diabetologia. 2003;46:839-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol. 2007;125:1082-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Chen DK, Frizzi KE, Guernsey LS, Ladt K, Mizisin AP, Calcutt NA. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. J Peripher Nerv Syst. 2013;18:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Bastion ML, Ling KP. Topical insulin for healing of diabetic epithelial defects? Med J Malaysia. 2013;68:208-216. [PubMed] |
| 17. | Aniah Azmi N, Bastion MC. Short-Term Results of Trial of Topical Insulin for Treatment of Dry Eyes in Diabetics. Eye Contact Lens. 2020;46 Suppl 1:S25-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Quiroz-Mendoza JL, Garcia Roa MR, Marlon VR, Valera-Cornejo DA, Vazquez Membrillo MA, Ramírez Neria P, Villalpando-Gomez Y, García-Franco Y. Clinical trial of topical insulin and sodium hyaluronate in the treatment of epithelial defects produced by intraoperative corneal epithelial debridement during pars plana vitrectomy in diabetics. Revista Mexicana de Oftalmología (English Edition). 2021;95. [DOI] [Full Text] |
| 19. | Wang AL, Weinlander E, Metcalf BM, Barney NP, Gamm DM, Nehls SM, Struck MC. Use of Topical Insulin to Treat Refractory Neurotrophic Corneal Ulcers. Cornea. 2017;36:1426-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Diaz-Valle D, Burgos-Blasco B, Gegundez-Fernandez JA, Garcia-Caride S, Puebla-Garcia V, Peña-Urbina P, Benitez-Del-Castillo JM. Topical insulin for refractory persistent corneal epithelial defects. Eur J Ophthalmol. 2021;31:2280-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Tong CM, Iovieno A, Yeung SN. Topical insulin for neurotrophic corneal ulcers. Can J Ophthalmol. 2020;55:e170-e172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Galvis V, Niño CA, Tello A, Grice JM, Gómez MA. Topical insulin in neurotrophic keratopathy after resection of acoustic neuroma. Arch Soc Esp Oftalmol (Engl Ed). 2019;94:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Bartlett JD, Turner-Henson A, Atchison JA, Woolley TW, Pillion DJ. Insulin administration to the eyes of normoglycemic human volunteers. J Ocul Pharmacol. 1994;10:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Le Nguyen MH, Naoum MS, Andre C, Lethier L, Limat S, Fagnoni-Legat C, Guillaume Y, Gauthier AS. Physicochemical and microbiological stability of insulin eye drops in an artificial tear vehicle used in the treatment of refractory neurotrophic keratopathy. J Fr Ophtalmol. 2022;45:860-871. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Ji Y, Liu S, Hong X, Lu Y, Wu X, Li K, Liu Y. Advances in artificial intelligence applications for ocular surface diseases diagnosis. Front Cell Dev Biol. 2022;10:1107689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 26. | Storås AM, Strümke I, Riegler MA, Grauslund J, Hammer HL, Yazidi A, Halvorsen P, Gundersen KG, Utheim TP, Jackson CJ. Artificial intelligence in dry eye disease. Ocul Surf. 2022;23:74-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |