Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.892
Peer-review started: December 23, 2022
First decision: March 28, 2023
Revised: April 5, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: June 15, 2023
Processing time: 174 Days and 2.7 Hours
Coronavirus disease 2019 (COVID-19) is one of the current global public health threats and vaccination is the most effective tool to reduce the spread and decrease the severity of COVID-19. Diabetes is one of the important chronic diseases threatening human health and is a common comorbidity of COVID-19. What is the impact of diabetes on the immunization effect of COVID-19 vaccination? Conversely, does vaccination against COVID-19 exacerbate the severity of pre-existing diseases in patients with diabetes? There are limited and conflicting data on the interrelationship between diabetes and COVID-19 vaccination.
To explore the clinical factors and possible mechanisms underlying the interaction between COVID-19 vaccination and diabetes.
We conducted a comprehensive search of PubMed, MEDLINE, EMBASE, and Reference Citation Analysis (https://www.referencecitationanalysis.com) online datab
A total of 54 studies were included, from 17 countries. There were no randomized controlled studies. The largest sample size was 350963. The youngest of the included samples was 5 years old and the oldest was 98 years old. The included population included the general population and also some special populations with pediatric diabetes, hemodialysis, solid organ transplantation, and autoimmune diseases. The earliest study began in November 2020. Thirty studies discussed the effect of diabetes on vaccination, with the majority indicating that diabetes reduces the response to COVID-19 vaccination. The other 24 studies were on the effect of vaccination on diabetes, which included 18 case reports/series. Most of the studies concluded that COVID-19 vaccination had a risk of causing elevated blood glucose. A total of 12 of the 54 included studies indicated a "no effect" relationship between diabetes and vaccination.
There is a complex relationship between vaccination and diabetes with a bidirectional effect. Vaccination may contribute to the risk of worsening blood glucose in diabetic patients and diabetic patients may have a lower antibody response after vaccination than the general population.
Core Tip: Coronavirus disease 2019 (COVID-19) is one of the current global public health threats and vaccination is the most effective tool to reduce the spread and decrease the severity of COVID-19. Diabetes is one of the important chronic diseases threatening human health and is a common comorbidity of COVID-19. There are limited and conflicting data on the interrelationship between diabetes and COVID-19 vaccination. Vaccination may be at risk of worsening glycemia in diabetic patients, and diabetic patients may have a lower immune response after vaccination than the general population, and there is a bidirectional relationship between vaccination and diabetes.
- Citation: He YF, Ouyang J, Hu XD, Wu N, Jiang ZG, Bian N, Wang J. Correlation between COVID-19 vaccination and diabetes mellitus: A systematic review. World J Diabetes 2023; 14(6): 892-918
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/892.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.892
The coronavirus disease 2019 (COVID-19) pandemic is one of the greatest public health threats to huma
Reassuringly, the vaccine has demonstrated efficacy and safety in the prevention of severe COVID-19 in both phase III trials and real-world data[12-14]. The vaccine also plays a key role in protecting vulnerable populations associated with an increased risk of morbidity and mortality, including patients with diabetes[12]. However, there is evidence of multiple immunodeficiencies in patients with DM that affect the innate and acquired immune system[15]. Therefore, it can be expected that the protective effect of vaccination may be weaker compared to the general population. Previous studies have shown reduced immunogenicity to the hepatitis B vaccine in patients with DM, while results are less consistent for influenza, pneumococcal, and varicella zoster[16]. In several recent studies using real-world data, vaccine efficacy was found to be lower in patients with DM than in the total population[17,18], while another Japanese study reported no significant association between vaccine efficacy and DM[19]. There are conflicting results regarding the immune efficacy of the COVID-19 vaccine in patients with DM. Furthermore, hyperglycemic crisis, acute myocardial injury[20], Guillain-Barre syndrome[21], and herpes zoster[22] are some of the very rare vaccine-related adverse events that have been reported occasionally. In patients with pre-existing DM, does the COVID-19 vaccination cause perturbations in blood glucose levels or even alter the natural history of the disease? There are very limited data on the interrelationship between DM and COVID-19 vaccination.
Therefore it seems important and interesting to understand the interrelationship between COVID-19 vaccination and diabetes. To elucidate this complexity, we summarized almost all current clinical studies and systematically analyzed various factors regarding the interconnection between DM and COVID-19 vaccination in order to inform diabetic patients of the optimal vaccination strategy and clinical management.
What is the effect of DM on the immunization effect of COVID-19 vaccination? Conversely, does vaccination against COVID-19 disrupt blood glucose? Or accelerate the progression of pre-existing diabetic complications?
An experienced information specialist conducted a comprehensive search of PubMed, MEDLINE, and EMBASE online databases with no time limit, and the last data update was December 2, 2022. We used the keywords "SARS-CoV-2", "COVID-19", "vaccine", "vaccination", "antibody", and "diabetes" individually or in combination to achieve a comprehensive literature search. We also searched the gray literature of medRxiv and bioRxiv as well as the most recent literature of the Reference Citation Analysis (https://www.referencecitationanalysis.com). Finally, we manually searched the references cited in the original articles included in the study in order to avoid missing any relevant and important literature. Inclusion criteria were all studies conducted in humans that discussed the relationship between DM and vaccination against COVID-19. Studies that included the same population but reported different data and outcomes were also included. Exclusion criteria were: Non-human (animal), non-English, only exploring willingness to vaccinate, and participants who were not diabetic or who received a vaccine other than the COVID-19 vaccine. The type of diabetes, the type of vaccine, the age of participants, and the type of literature were not restricted. A detailed search strategy is available in the Supplementary Material.
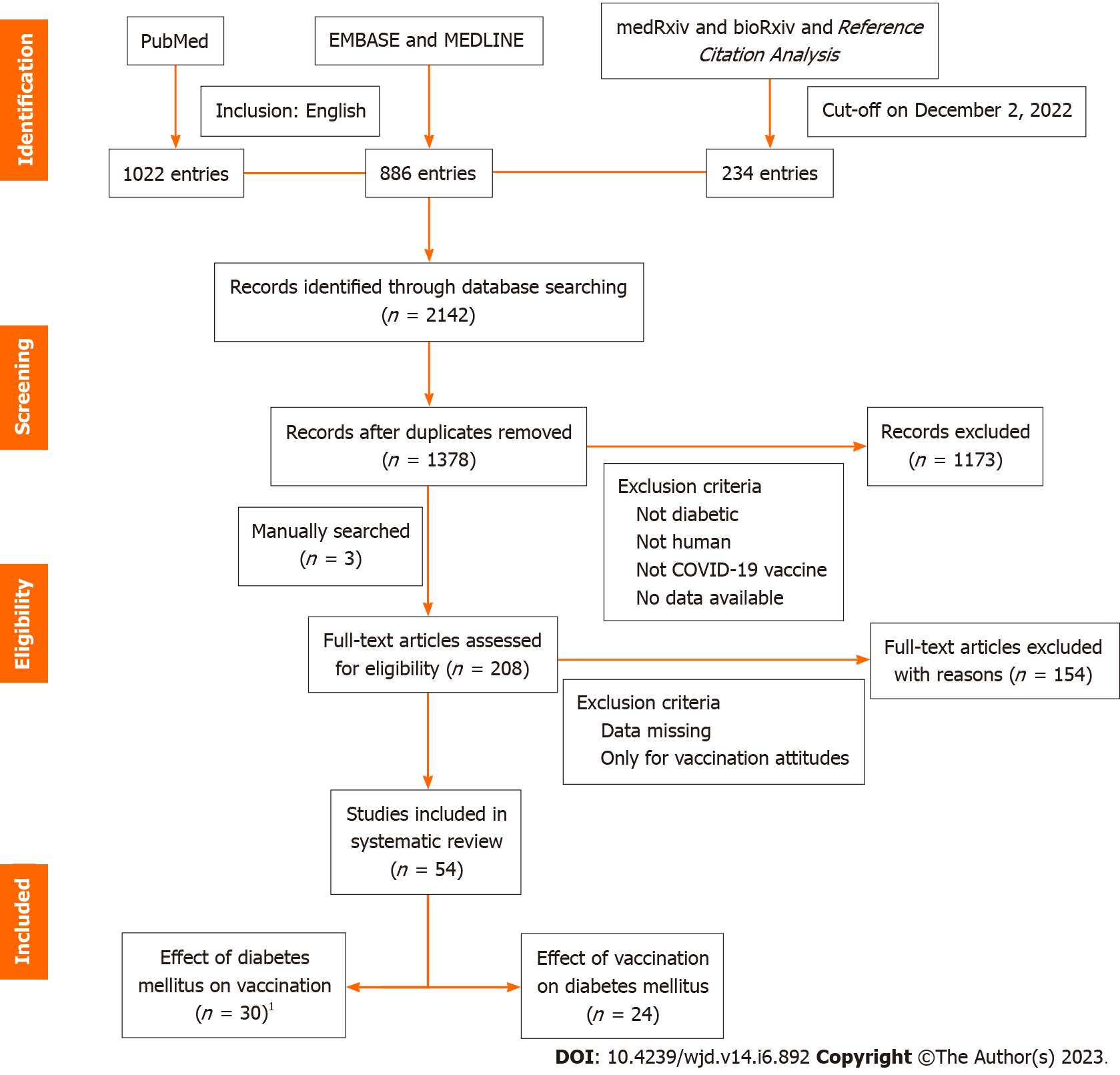
After completing the initial search, two independent reviewers conducted a screening process, and literature with quantifiable evidence was included in our review, including case reports, qualitative analyses, and other gray literature. We excluded repetitive publications and articles without relevant data. One reviewer reviewed the selected articles in their entirety, and studies containing full data descriptions were used for data graphs. Any conflicts that arose during the data extraction process were discussed or consulted and resolved by third-party experts. All seven authors were involved in the discussions. Figure 1 shows a visual representation of the inclusion workflow.
A total of 2142 publications were retrieved as of December 2, 2022, and after screening by the inclusion criteria described above, we reviewed 208 full-text papers for eligibility, plus three manually retrieved papers, resulting in 54 papers included in this review (Figure 1). We extracted data for each paper regarding the first author's name, country, study design, basic demographic characteristics of participants, the type of vaccination, vaccination regimen, and blood glucose for tabulation and discussion. We did not perform any meta-analysis of the data obtained because, as expected, there was substantial heterogeneity among the designs, methods, populations, and vaccines used in the studies we encountered, making meaningful comparisons between studies impossible. A summary of information on the included studies is presented in Table 1.
| Ref. | Country | Study design | Study time span | Population | Sample size (n) | No. of patients with DM (n) T1DM T2DM | Sex (F/M) | Age, median (min-max), yr | Type and name of vaccine | Dose schedule | Related findings | |
| Zhang et al[23] | China | Observational study | Between October 2021 and January 2022 | The population is aged ≥ 60 yr with hypertension or (/and) DM | 1413 | 620 | 661/752 | 67.6 | Vero cell (19nCov-CDC-Tan-HB02) | Two doses (day 0, day 28) | After vaccination, there was no significant abnormal fluctuation in blood glucose in diabetic patients | |
| Marfella et al[24] | Italy | Prospective observational study | December 2020 | Healthcare and educator workers | 478 | 201 | 212/266 | 18-60 | mRNA-BNT162b2 (Pfizer-BioNTech) or ChAdOx1-S (Astra-Zeneca) or mRNA-1273 (Moderna) | One (day 0, day 21) or two (day 52) doses | Significant decrease in the immune response in people with poorly controlled blood glucose | |
| Kılınç-Toker et al[25] | Turkey | Retrospective study | Between August 1, 2021 and October 31, 2021 | Hospitalized patients with COVID-19 | 541 | 195 | 282/259 | 70.2 (21-98) | (CoronaVac) and/or BNT162b2 mRNA (Pfizer-BioNTech) | 14 d after dose 2 | For hospitalized patients after the second dose, diabetes was not associated with their ICU stay and mortality | |
| Barocci et al[26] | Italy | Observational study | Between December 2020 and June 2021 | Healthcare workers and university staff | 2845 | 8 | 155/129 | 43-61 | ChAdOx1-S and (BNT162b2/BNT162b2 and ChAdOx1-S/ChAdOx1-S) | 2 mo after dose 2 | DM does not affect antibody levels | |
| Singh et al[27]1 | India | Cross-sectional study | Between January 16, 2021 and May 15, 2021 | Healthcare workers | 5154 | 0 | 52 | 210/305 | 44.8 ± 13.19 | CovishieldTM (ChAdOx1-nCOV) or CovaxinTM (BBV-152) | One (day 21) and two (day 21-28, day 83-97, and day 173-187) doses | People with T2DM had a significantly lower seropositivity rate compared to those without |
| Singh et al[28]1 | India | Longitudinal study | Between January 16, 2021 and November 15, 2021 | Healthcare workers | 481 | 0 | 51 | 195/286 | ≤ 60 years, n = 411; > 60 years, n = 70 | CovishieldTM (ChAdOx1-nCOV) or CovaxinTM (BBV-152) | 3 wk, 3 mo, and 6 mo after dose 2 | Participants with T2DM have a lower seropositivity rate at all time points |
| Shim et al[29] | Korea | Retrospective study | February2021 | Vaccination participants | 736 | 48 | 433/303 | 51.5 (20-80) | AZD1222, BNT162b2, mRNA-1273 and Ad26.COV2.S | 2 wk before and 6 mo after dose 2 | Diabetics had a lower rate of neutralizing antibodies after vaccination | |
| Alqassieh et al[30] | Jordan | Prospective observational cohort | Between March and April 2021 | Jordanian adults | 288 | 76 | 189/151 | 20-60 years, n = 137, > 60 years, n = 151 | Pfizer-BioNTech or Sinopharm | 6 wk after dose 2 | Although DM negatively affected IgG titer, it was not statistically significant | |
| Wan et al[31] | China (Hong Kong) | Population-based study | Between February 23, 2021 and January 31, 2022 | Patients with T2DM in Hong Kong electronic case records | 350963 | 0 | 350963 | 167073/183890 | 64.7 ± 1.37/68.1 ± 0.747 | BNT162b2 or CoronaVac | Complete at least one dose of vaccination | Patients with T2DM do not appear to have higher risks of AESI and acute diabetic complications after vaccination |
| Lee et al[32] | South Korea | Questionnaire study | Between March 8, 2021 and March 11, 2021 | Healthcare workers | 1603 | 27 | 1261/342 | 37.7 ± 10.89 | ChAdOx1 | 7 d after dose 1 | DM is associated with an increased risk of grade 3 to 4 adverse reactions after the first dose | |
| Rangsrisaeneepitak et al[33] | Thailand | PSM observational study | Between June 8, 2021 and July 12, 2021 | Healthcare workers and T2DM patients | 282 | 94 | 129/153 | 30-83 | ChAdOx1 nCoV-19 (AZD1222) | 56 d after dose 1 | People with T2DM had weaker antibody responses than those without diabetes after the first dose | |
| Sourij et al[34] | Austria | Multicentre prospective cohort study | Between April and June 2021 | T1DM, T2DM, and healthy participants | 150 | 75 | 75 | 68/82 | 49.2 ± 14.59 | BioNTech-Pfizer, Moderna, or AstraZeneca | 7 to 14 d after dose 1 and 14 to 21 dafter dose 2 | The antibody levels after the second vaccination were comparable in healthy controls and DM patients, irrespective of glycaemic control |
| Tawinprai et al[35] | Thailand | Prospective cohort study | Between March 31, 2021 and May 5, 2021 | Healthcare workers | 796 | 11 | 517/279 | 40 (30-57)3 | ChAdOx1 (AZD1222) | At least 21 d after dose 1 and before dose 2 | DM reduces the immune response to vaccination | |
| Ali et al[18] | Kuwait | Case-control study | August 2021 | Non-diabetics and patients with T2DM | 262 | 0 | 81 | 126/136 | 49.3 ± 14.59 | BNT162b2 (Pfizer-BioNTech) | At least 3 wk after dose 2 | Both neutralizing antibody and IgG antibody titers were significantly lower in the T2DM group than in the non-diabetic group |
| Karamese et al[36] | Turkey | Descriptive study | March 2021 | Participants over 65 years of age who have received two doses of vaccine | 235 | 49 | 111/124 | 70.4 ± 4.89 | CoronaVac | 4 wk after dose 1 and 4 wk after dose 2 | Lower rates of antibody response were detected in participants with DM | |
| Lustig et al[37] | Israel | Single-centre, prospective, longitudinal cohort study | Between December 19, 2020 and January 30, 2021 | Health-care workers | 2607 | 139 | 1883/724 | 47.7 ± 12.59 | Pfizer-BioNTech BNT162b2 | 1-2 wk after dose 1 and 1-2 wk after dose 2 | Decreased antibody response in diabetic patients after vaccination | |
| Islam et al[38] | Japan | Cross-sectional study | June 2021 | Workers | 953 | 21 | 654/299 | 21-75 | BNT162b2 (Pfizer-BioNTech) | 15 to 71 d after dose 2 | Spike IgG antibody titers were lower in the presence of hyperglycemia | |
| Parthymou et al[39] | Greece | Longitudinal observational cohort study | September 2021 | Healthcare units participants | 712 | 50 | 444/268 | 50.8 ± 11.49 | BNT162b2 (BioNTech-Pfizer) | 3 wk and 3 mo after Dose2 | DM is not an independent factor affecting antibody titers | |
| Priddy et al[40] | New Zealand | Prospective cohort study | Between June 10, 2021 and September 18, 2021 | Participants in two centers | 285 | 28 | 156/1296 | 52 (16-92) | BNT162b2 (BioNTech-Pfizer) | 28 d after dose 2 | Participants with diabetes had lower anti-S IgG antibodies compared to those without DM | |
| Naschitz et al[41] | Israel | Retrospective study | May 2021 | Residents in long-term geriatric and palliative care and assisted living facilities | 304 | 103 | 208/96 | ≥ 60 | BNT162b2 (Pfizer-BioNTech) | 3-4 mo after dose 2 | DM is associated with negative serological results | |
| Güzel et al[42] | Turkey | Prospective study | May 20212 | Volunteers, outpatient clinic people, and COVID-19 patients | 183 | 80 | 98/85 | 21-60 | CoronaVac-SinoVac | 21 d after dose 2 | IgG antibody levels were significantly lower in patients with DM than in those without DM | |
| Virgilio et al[43] | Italy | Multicenter prospective study | Between June 2021 and December 2021 | Residents of long-term care facilities | 555 | 0 | 140 | 378/177 | 82.1 | BNT162b2 (Cominarty) Moderna (mRNA-1273) | Before the vaccination, 2 mo, and 6 mo after dose 1 | Vaccination in elderly residents with T2DM is associated with a reduced humoral immune response |
| Patalon et al[44] | Israel | Retrospective cohort study | Between February and May 2021 | A large patient cohort from Maccabi Healthcare Services | 4740 | 377 | 1914/2826 | 16-59 years, n = 3355; ≥ 60 years, n = 1385 | BNT162b2 (BioNTech-Pfizer) | Two vaccinations at intervals of 21 to 27 d | DM is not a relevant factor affecting antibody levels | |
| Mitsunaga et al[45] | Japan | Prospective study | Between April 15, 2021 and June 9, 2021 | Hospital’s workers | 374 | 6 | 264/110 | 36 | BNT162b2 vaccine (COMIRNATY (Tozinameran) | Before vaccination, 7 to 20 d after dose 1, and 7 to 20 d after dose 2 | HbA1c higher than 6.5% was a significant suppressor of antibody responses | |
| Papadokostaki et al[46] | Greece | Prospective observational study | Between May and September 2021. | Participants attended the vaccination center | 174 | 14 | 44 | 107/67 | 52.6 ± 10.6 | BNT162b2 (BioNTech-Pfizer) | 21 d after dose 1, 7-15 d after dose 2, and 70-75 d after dose 2 but before dose 3 | It was high and similar after the second dose in both participants with and without DM |
| Zhao et al[47] | United States | Prospective longitudinal study | Between December 2020 and December 2021 | Veterans and healthcare workers | 124 | 39 | 33/91 | 20-95 | BNT162b2 (Pfizer-BioNTech) | 48 h before dose 1 and dose 2, 1 mo, 3 mo, 6 mo, 12 mo after dose 2, and 1 mo after dose 3 | DM was significantly associated with a decrease in response intensity after completion of the primary vaccine series, but responses to the third dose were generally robust | |
| Santotoribio et al[48] | Spain | Descriptive, retrospective, observational, and cross-sectional study | Between November 1, 2020 and March 31, 2021 | Infected patients and vaccinated subjects | 175 | 17 | 112/63 | 51.0 (19-89) | Pfizer-BioNTech | At least 21 d after dose 2 | Serum antibody levels did not decrease significantly in patients with DM | |
| Mehta et al[49] | India | Observational cohort study | Between March 2021 and October 2021 | Vaccinated patients with AIRDs | 495 | 63 | 416/79 | 56.5 | AZD1222 (AstraZeneca) | 4 wk and10-14 wk after dose 2 | DM was significantly associated with lower anti-RBD antibodies | |
| Ajlan et al[50] | Saudi Arabia | PSM prospective study | June 14, 20222 | Patients from a large hospital | 431 | 191 | 136/295 | 51.3 ± 16.29 | BNT162b2 or ChAdOx1 | 7 d after dose 1 and dose 2, and 2 wk after dose 1 and dose 2 | There was no difference in the primary outcome between the two vaccine platforms. Unresponsiveness was mainly linked to DM | |
| Billany et al[51] | United Kingdom | Prospective observational study | March 2021 | Maintenance hemodialysis patients | 94 | 43 | 38/56 | 62.1 ± 12.29 | BNT162b2 or AZD1222 | 28 d after dose 1 | There was no difference in antibody testing with or without DM | |
| Aberer et al[52] | Austria | Multicenter prospective study | Between April and June 2021 | DM patients | 74 | 58 | 16 | NR | T1DM: 39.5 ± 14.1; T2DM: 60.6 ± 6.2 | BioNTech-Pfizer and Moderna and AstraZeneca | First dose | No change in insulin dose before and after vaccination. Vaccination significantly reduced TIR in T1DM patients, but had no effect on TIR in T2DM patients |
| Piccini et al[53] | Italy | Observational cohort study | Between March and June 2021 | T1DM patients | 39 | 39 | 0 | 17/22 | 18.7 ± 2.19 | mRNA-BNT162b1 (Pfizer-BioNTech) and Moderna (mRNA-1273) | One (day 7, day 14) and two (day 7, day 14) doses and 14 d after dose 1 and dose 2 | COVID-19 vaccination was safe and not associated with significant perturbation of glycemic control in patients with T1DM |
| Heald et al[54]1 | United Kingdom | Observational cohort study | Between January 14, and March 7, 2021 | T1DM patients | 20 | 20 | 0 | 11/9 | 53 (26-70) | mRNA-BNT162b2 (Pfizer-BioNTech) and Oxford /AstraZeneca | 7 d before and 7 d after dose 1 | COVID-19 vaccination can cause temporary relative hyperglycemia in people with T1DM. No relationship between vaccine type and blood glucose perturbation |
| D'Onofrio et al[55] | Italy | Observational cohort study | July 13, 20212 | T1DM (AD) patients | 35 | 35 | 14/21 | 36 (27-51)3 | mRNA-BNT162b2 (Comirnaty) | 14 d before and 3 d after dose 1 and dose 2 | No significant differences in TIR, TAR, TBR, and CV between, after, and before the COVID-19 vaccination in T1DM patients | |
| Heald et al[56]1 | United Kingdom | Survey and evaluation study | Between January 5, 2021 and April 4, 2021 | Adults (18 years of age or more) with T1DM | 97 | 97 | 0 | 51/46 | 44 (18-70) | Pfizer-BioNTech or Oxford-AstraZeneca | 7 d before and 7 dafter dose 1 | In T1DM, vaccination can cause a temporary perturbation of interstitial glucose. There is no difference between vaccines |
| Gouda et al[57] | Greece | Observational study | March 2022 | T1DM patients | 1358 | 135 | 0 | 72/63 | 11.7 (5-18) | BNT162b2 (Pfizer-BioNTech), Moderna (mRNA-1273), or AstraZeneca | 7 d before and 7 d after dose 1, dose 2, and dose 3 | SARS-CoV-2 vaccination in children and adolescents with T1DM is safe and is not associated with immediate glucose imbalance |
| Sakurai et al[58] | Japan | Case report | December 11, 20212 | Healthy woman | 1 | 1/0 | 36 | mRNA-BNT162b2 (Pfizer-BioNTech) | First dose | mRNA vaccine is associated with new-onset T1DM | ||
| Patrizio et al[59] | Italy | Case report | September 15, 20212 | T2DM patient | 1 | 0 | 1 | 0/1 | 52 | mRNA-BNT162b2 (Pfizer-BioNTech) | Second dose | T1DM may be triggered after SARS-CoV-2 vaccination |
| Aydoğan et al[60] | Turkey | Case series | Between May 2021 and October 2021 | One had Hashimoto's thyroiditis, and the other 3 were healthy | 4 | 1/3 | 27-56 | mRNA-BNT162b2 (Pfizer-BioNTech) or CoronaVac | Second dose | Vaccination with BNT162b2 may trigger T1DM | ||
| Sato et al[61] | Japan | Case report | April 19, 20222 | Malignant melanoma patient | 1 | 0/1 | 43 | mRNA-based SARS-CoV-2 vaccination | Second dose | mRNA vaccine may trigger T1DM | ||
| Yakou et al[62] | Japan | Case series | December 21, 20212 | T1DM patients | 2 | 2 | 0 | 2/0 | 52-71 | mRNA-BNT162b2 (Pfizer-BioNTech) | Second dose | A temporary decrease in insulin secretion after vaccination |
| Mishra et al[63] | India | Case series | Between January 18, 2021 and March 4, 2021 | T2DM patients | 3 | 0 | 3 | 1/2 | 58-65 | Covishield™ (ChAdOx1-nCOV) (AstraZeneca) | First dose | Vaccination may result in a mild and temporary increase in blood glucose levels |
| Abu-Rumaileh et al[64] | Jordan | Case report | January 14, 2021 | Hypertension patient | 1 | 0/1 | 58 | mRNA-BNT162b1 (Pfizer-BioNTech) | Second dose | COVID-19 vaccine has a risk of causing new-onset T2DM | ||
| Sasaki et al[65] | Japan | Case report | December 13, 20212 | Osteoporosis, mild glucose intolerance | 1 | 0 | 0 | 1/0 | 73 | Moderna (Spikevax, mRNA-1273) | Second dose | The development of T1DM is attributable to the COVID-19 vaccination |
| Lee et al[66] | United States | Case Series | June 30, 20212 | T2DM and hypertension patients | 3 | 0 | 2 | 1/2 | 52-87 | mRNA-BNT162b1 (Pfizer-BioNTech) and Moderna (Spikevax, mRNA-1273) | First dose | Vaccination may trigger a hyperglycemic episode and DKA |
| Edwards et al[67] | United Kingdom | Case Series | April 2021 | Hypertension, hypothyroidism, and pre-diabetes | 3 | 0/3 | 53-68 | Covishield™ (ChAdOx1-nCOV) | First dose | The first administration of the COVID-19 vaccine can trigger an acute hyperglycemic crisis | ||
| Ganakumar et al[68] | India | Case series | November 2021 | T1DM | 2 | 2 | 0 | 1/1 | 20-25 | COVISHIELD (ChAdOx1 nCoV-19) or COVAXIN (BBV152) | 1 to 4 d after dose 2 | COVID-19 Vaccination has the potential to induce DKA |
| Zilbermint et al[69] | United States | Case report | September 11, 20212 | T1DM | 1 | 1 | 0 | 1/0 | 24 | Moderna (mRNA-1273) | 15 h after dose 2 | A plausible mechanism exists between COVID-19 vaccination and DKA |
| Yaturu et al[70] | United States | Case report | May 2021 | Hypertension, primary hyperparathyroidism, and obesity patient | 1 | 0 | 1 | 0/1 | 56 | BNT162b2 (Pfizer-BioNTech) | Right after the second dose | COVID-19 Vaccination has the potential to induce HHS |
| Kshetree et al[71] | United States | Case report | NR | Hypertension and pre-diabetes | 1 | 1 | 0 | 0/1 | 69 | mRNA vaccine | 2 mo after dose 3 | COVID-19 mRNA vaccine has the potential to induce DKA |
| Prasad[72] | India | Case report | March 2021 | Patient with T2DM | 1 | 0 | 1 | 1/0 | 73 | Covishield | 6 d after dose 1 | Vaccination may cause glycaemic disturbances |
| Sasaki et al[73] | Japan | Case report | January 4, 20222 | Healthy person | 1 | 1 | 0 | 1/0 | 45 | BNT162b2 (Pfizer-BioNTech) | 1 d after dose 1 | COVID-19 vaccine might trigger the onset of fulminant T1DM in susceptible individuals |
| Yano et al[74] | Japan | Case report | November 11, 20212 | Healthy person | 1 | 1 | 0 | 1/0 | 51 | Moderna (mRNA-1273) | 28 d after dose 1 | COVID-19 vaccination can induce T1DM in some individuals |
| Ohuchi et al[75] | Japan | Case report | November 20212 | Cutaneous malignant melanoma with axillary lymph node metastasis | 1 | 1 | 0 | 0/1 | 45 | BNT162b2 (Pfizer-BioNTech) | 3 d after dose 2 | There is a highly suspicious causal relationship between fulminant T1DM and COVID-19 vaccination |
A total of 54 studies were included[18,23-75], from 17 countries, including 9 from Japan. The earliest date of the studies was November 2020[48]. There were no randomized controlled studies, but two studies applied propensity score matching (PSM) methods. What was surprising was that one study analyzed the bidirectional relationship between vaccination and blood glucose[23]. There were 30 studies that discussed the effect of diabetes on vaccination[18,23-51], two of which were specifically about whether DM increased adverse effects after vaccination[31,32], and three of which had participants with autoimmune rheumatic disease[49], organ transplantation[50], and a special group on blood pressure dialysis[51]. The other 24 studies were on the effect of vaccination on DM[52-75] and included 18 case reports or case series[58-75]. The largest sample size was 350,963, a population-based study from Hong Kong, China, which evaluated the risk of adverse events of special concern and acute diabetic complications after COVID-19 vaccination in the type 2 DM (T2DM) population[31]. Of the sample included in the 54 studies, the youngest age was five years[57] and the oldest was 98 years[25]. Only one study analyzed the effects of glycemia on both cellular and humoral responses after vaccination[24]. Only one study performed a comparative analysis between type 1 diabetes and type 2 diabetes[34]. The authors of some studies claim that they are reporting for the first time, trying to fill a gap in the literature regarding certain relationships between COVID-19 vaccination and DM.
From the current studies, the effect of vaccination on diabetes is mainly manifested in the effect on blood glucose after vaccination, with a total of 24 studies describing this relationship, including 18 case reports or case series. To make the various characteristics of these case series readily apparent, we have additionally tabulated a total of 29 cases from these 18 case reports or case series (Table 2). Of these 29 cases, 12 were new-onset type 1 DM (T1DM) and three were new-onset T2DM. Fourteen cases were vaccinated with two doses, 14 with only one dose, and one with a third dose. mRNA vaccines were used in 19 cases (13 cases of mRNA-BNT162b2 (Pfizer-BioNTech) and 6 cases of Moderna (mRNA- 1273)) and eight cases used the adenoviral vector vaccine Covishield™ (ChAdOx1-nCOV or AstraZeneca). Most events occurred within days of vaccination, with the longest being a diagnosis of new-onset T1DM two months after the third dose[71]. No deaths were reported. Of these 24 studies, only three indicated that vaccination had no effect on blood glucose[53,55,57], while the rest indicated that it may cause an increase in blood glucose. No vaccinated individuals with episodes of hypoglycemia were identified. Of course, it cannot be ruled out that some patients develop mild or self-limiting hypoglycemia after vaccination, which may not cause certain subjective symptoms in patients and therefore may go undocumented by clinical diagnosis.
| Ref. | Age (yr) | Gender | Type and name of vaccine | Blood glucose (mg/dL)/HbA1c (%) pre-vaccination post-vaccination | Onset after vaccination | Pre-existing condition | Final diagnosis | C-peptide (ng/mL) | GAD65Ab (IU/mL) | Treatment | Outcomes | Conclusion | |
| Sakurai et al[58] | 36 | Female | mRNA-BNT162b2 (Pfizer-BioNTech) | Normal | 501/7.0 | 3 d after dose 1 | None | Fulminant T1DM | 0.13 | NA | Insulin infusion | Discharged | mRNA vaccine is associated with new-onset T1DM |
| Patrizio et al[59] | 52 | Male | mRNA-BNT162b2 (Pfizer-BioNTech) | 531 | 871 | 4 wk after dose 2 | Vitiligo vulgaris and T2DM | Graves’ disease and T1DM | 1 | 61.2 | Insulin analogues | NR | T1DM may be triggered after SARS-CoV-2 vaccination |
| Aydoğan et al[60] | 56 | Male | mRNA-BNT162b1 (Pfizer-BioNTech) | Normal | 440/8.2 | 15 d after dose 2 | Vitiligo vulgaris and Hashimoto's thyroiditis | T1DM | 1.5 | > 2000 | Insulin infusion | Recovery | Vaccination with BNT162b2 may trigger T1DM |
| 48 | Male | mRNA-BNT162b2 (Pfizer-BioNTech) | Normal | 352/10.1 | 8 wk after dose 2 | None | T1DM | 0.97 | 94 | Low-carbohydrate diet | Recovery | ||
| 27 | Male | mRNA-BNT162b2 (Pfizer-BioNTech) | Normal | 320/12.5 | 3 wk after dose 2 | None | T1DM | 0.87 | 725 | Basal insulin | Recovery | ||
| 36 | Male | mRNA-BNT162b2 (Pfizer-BioNTech) and CoronaVac | Normal | 526/12.6 | 3 wk after dose 2 | None | T1DM | 0.38 | 234 | Insulin infusion | Recovery | ||
| Sato et al[61] | 43 | Male | mRNA-based SARS-CoV-2 vaccination | 94/5.6 | 655/8.0 | 14 d after dose 2 | Malignant melanoma | Fulminant T1DM | 0.33 | Insulin infusion | Discharged | mRNA vaccine may trigger T1DM | |
| Yakou et al[62] | 71 | Female | mRNA-BNT162b1 (Pfizer-BioNTech) | 93/8.1 | 944/8.0 | 1 d after dose 2 | T1DM | Diabetic ketoacidosis | < 0.03 | > 2000 | Insulin infusion | Discharged | Risk of inducing ketoacidosis after vaccination in T1DM patients |
| 52 | Female | mRNA-BNT162b1 (Pfizer-BioNTech) | 106 | 494/11.6 | 1 d after dose 2 | T1DM | Diabetic ketoacidosis | ND | 123 | Insulin infusion | Discharged | ||
| Mishra et al[63] | 58 | Female | Covishield™ (ChAdOx1-nCOV) (AstraZeneca) | 110 | 183 | 1 d after dose 1 | T2DM | T2DM | NR | NR | Increased dose of metformin. | Discharged | Vaccination may result in a mild and temporary increase in blood glucose levels |
| 64 | Male | Covishield™ (ChAdOx1-nCOV) (AstraZeneca) | 95 | 150 | 1 d after dose 1 | T2DM | T2DM | NR | NR | Without additional intervention | Discharged | ||
| 65 | Male | Covishield™ (ChAdOx1-nCOV) (AstraZeneca) | 107 | 186 | 6 d after dose 1 | T2DM | T2DM | NR | NR | Without additional intervention | Discharged | ||
| Abu-Rumaileh et al[64] | 58 | Male | mRNA-BNT162b1 (Pfizer-BioNTech) | 80 | 1253/13 | 26 d after dose 1 | Hypertension | T2DM | 1.1 | NR | Insulin infusion | Discharged | COVID-19 vaccine has a risk of causing new-onset T2DM |
| Sasaki et al[65] | 73 | Female | Moderna (Spikevax, mRNA-1273) | 7.3 | 318/9.3 | 8 wk after dose 2 | Osteoporosis, mild glucose intolerance | T1DM | 0.48 | > 2000 | Intensive insulin therapy | NR | COVID-19 Vaccination may lead to the new-onset T1DM |
| Lee et al[66] | 52 | Female | mRNA-BNT162b2 (Pfizer-BioNTech) | 5.5-6.2 | 1062/12.0 | 3 d after dose 1 | Hypertension | T2DM and nonketotic HHS | NR | NR | Insulin infusion. | Discharged | Vaccination may trigger HHS |
| 60 | Male | Moderna (mRNA-1273) | 7.5 | 847/13.2 | 2 d after dose 1 | T2DM | T2DM and HHS | NR | NR | Insulin infusion | Discharged | Vaccination may trigger a hyperglycemic episode | |
| 87 | Male | Moderna (mRNA-1273) | 7 | 923 | 10 d after dose 1 | T2DM | T2DM and HHS and DKA | NR | NR | Insulin infusion | Discharged | Vaccination may trigger HHS and DKA | |
| Edwards et al[67] | 59 | Male | Covishield™ (ChAdOx1-nCOV) | 5.6 | 594/14.1 | 21 d after dose 1 | Obesity | Hyperglycemic ketosis | 2352 | NR | NA | Discharged | The first administration of the adenovirus-vectored COVID-19 vaccine can trigger an acute hyperglycemic crisis |
| 68 | Male | Covishield™ (ChAdOx1-nCOV) | 6.5 | 918/14.7 | 36 d after dose 1 | Pre-diabetes | Mixed HHS/DKA | 5612 | NR | ICU admission | Discharged | ||
| 53 | Male | Covishield™ (ChAdOx1-nCOV) | 6.2 | 576/17.1 | 20 d after dose 1 | Pre-diabetes | DKA | 3772 | NR | ICU admission | Discharged | ||
| Ganakumar et al[68] | 20 | Male | COVISHIELD (ChAdOx1 nCoV-19) | NR | 14.1 | 1 d after dose 2. | None | Severe DKA | NR | NR | Insulin infusion | Discharged | COVID-19 vaccination has the potential to induce DKA |
| 25 | Female | COVAXIN (BBV152) | NR | 16.3 | 4 d after dose 2 | None | Severe DKA | NR | NR | Insulin infusion | Discharged | ||
| Zilbermint et al[69] | 24 | Female | Moderna (mRNA-1273) | NR | 505/12.0 | 15 h after dose 2 | T1DM | Severe DKA | NR | NR | Insulin infusion | NR | A plausible mechanism exists between COVID-19 vaccination and DKA |
| Yaturu et al[70] | 56 | Male | BNT162b2 (Pfizer-BioNTech) | 5.6 | 997/14 | Right after the second dose. | Hypertension, primary hyperparathyroidism, and obesity | T2DM and HHS | NR | NR | Insulin infusion | Discharged | COVID-19 vaccination has the potential to induce HHS |
| Kshetree et al[71] | 69 | Male | mRNA vaccine | 5.8 | 13.7 | Two months after dose 3 | Hypertension and pre-diabetes | T1DM and DKA | 0.4 | 0.33 | Insulin infusion | Discharged | COVID-19 mRNA vaccine has the potential to induce DKA |
| Prasad[72] | 73 | Male | Covishield | 92/7.1 | 215/8 | 6 d after dose 1 | T2DM | T2DM | NR | NR | Insulin infusion | Discharged | Vaccination may cause glycaemic disturbances |
| Sasaki et al[73] | 45 | Female | BNT162b2 (Pfizer-BioNTech) | Normal | 344/7.6 | 1 d after dose 1 | None | Fulminant T1DM and DKA | NR | NA | Insulin infusion | Discharged | COVID-19 vaccine might trigger the onset of fulminant T1DM in susceptible individuals |
| Yano et al[74] | 51 | Female | Moderna (mRNA-1273) | Normal | 648/10.3 | 28 d after dose 1 | None | Fulminant T1DM and DKA | 1.72 | NA | Insulin infusion | Discharged | COVID-19 vaccination can induce T1DM in some individuals |
| Ohuchi et al[75] | 45 | Male | BNT162b2 (Pfizer-BioNTech) | NR | 655 | 3 d after dose 2 | Cutaneous malignant melanoma | Fulminant T1DM | 0.99 | Negative | NR | NR | There is a highly suspicious causal relationship between fulminant T1DM and vaccination, especially in patients treated with ICI |
Of the 30 studies on the effect of DM on vaccination, only one study analyzed the correlation between blood glucose levels and the humoral and cellular immunity of the organism after immunization[24]. Most of the studies examined whether blood glucose levels as an indicator of effect or DM as comorbidity negatively affected the immune response to vaccination. Twenty-one of the studies showed that DM reduced response to vaccination, while the other nine indicated that DM had no effect on vaccine efficiency[23,25,26,30,34,44,46,48,51]. Some studies also quantified the association with vaccine biological effects in terms of patient-specific attributes. Fifteen studies expressed a negative correlation between age and immune response, with older individuals having a weaker immune response than their younger individuals[25,28-30,32-34,36,37,40,42,45,47,51]. Seven studies showed a correlation between gender and immune response after vaccination, with women having a more positive immune effect than men[25,27,32,33,35,39,44]. Eight studies analyzed the effect of vaccine type on the immune response after vaccination in patients with DM, and four of these studies showed an effect[26,27,30,50]. There were also studies that concluded that mixed or heterologous vaccination produced better vaccine efficiency[25,26]. Three studies suggested that participants with previous SARS-CoV-2 infection would have a better antibody response than SARS-CoV-2-naive individuals[28,47,51]. We attempted to systematize the variables in the literature regarding the interrelationship between diabetes and vaccination and summarized the important findings of the studies related to these variables in Table 3. Ten studies mentioned adverse effects of vaccination[23,26-29,33-35,50,53] and only one study manifested that it would have an effect on antibody production[29]. Regarding the effect of BMI on vaccination, one study stated that a lower BMI increased the risk of grade 3 to 4 adverse reactions compared to normal-weight individuals[32], while another study showed that a higher BMI decreased the immune response after vaccination[42].
| Ref. | Assessed variables | Findings related to variables | Conclusion | Limitations |
| Zhang et al[23] | Hypertension, Comorbidity, Side effects | None | After vaccination, no significant abnormal fluctuations in blood glucose values were observed in the DM patients | Lack of data on the duration of antibodies after vaccination in the study population |
| Marfella et al[24] | HbA1c, Time since vaccination, type of vaccine | On Day 21 after the second vaccine dose, T2DM patients with HbA1c > 7% showed significantly reduced virus-neutralizing antibody capacity than normoglycemic subjects and T2DM patients with good glycaemic control. At 21 d after the first vaccine dose, neutralizing antibody titers and CD4 cytokine responses involving type 1 helper T cells were lower in T2DM patients with HbA1c levels > 7% than in individuals with HbA1c levels ≤ 7%. The reduction of HbA1c levels 52 d after vaccination was associated with neutralizing antibody titers and CD4 cytokine increases | Hyperglycemia at the time of vaccination can worsen the immune response, and proper glycemic control can improve the immune response | The statistical significance of the relevant indicators was relatively low |
| Kılınç-Toker et al[25] | Age, sex, mixed vaccination, delta variant, BMI, Diabetes, hypertension, COPD, cardiovascular diseases, chronic kidney disease, cancer | Age, male gender, delta variant, and mixed vaccination (CoronaVac plus BioNTech) were associated with death. The delta variant had higher ICU admission and mortality rate | For hospitalized patients who received two doses of the vaccine, diabetes was not associated with their ICU stay and mortality | Retrospective design, short follow-up, and assessment of inpatients only |
| Barocci et al[26] | Homologous vaccination, heterologous vaccination, type of vaccine, vaccine schedule, sex, age, BMI, smoking, DM, cardiovascular diseases, respiratory tract diseases, previous SARS-CoV-2 infection, side effects | Heterologous vaccination induced a significantly higher humoral response than homologous vaccination. The type of vaccine influenced antibody titers | DM does not affect antibody levels | Results were influenced by anti-S IgG levels in asymptomatic subjects |
| Singh et al[27]1 | Sex, T2DM, age, BMI, side effects, type of vaccine, dose 1, dose 2 | Gender, presence of comorbidities, and vaccine type were independent predictors of antibody seropositivity and anti-spike antibody titer levels. Patients with T2DM had a significantly lower seropositivity rate compared to those without the comorbid disease. Seropositivity rates were lower in those with T2DM compared to those without T2DM. Both vaccine recipients had similar mild to moderate adverse events, and none had serious side effects | T2DM is associated with lower seropositivity rates and anti-spike antibody titers | No assessment of the cell-mediated immune response |
| Singh et al[28]1 | Age, previous SARS-CoV-2 infection, sex, BMI, side effects, type of vaccine, dose 1, dose 2, T2DM, blood group, dyslipidemia, ischemic heart disease | The seropositivity rate was significantly higher in the ≤ 60 years age group than in the > 60 years age group at all time points. GMT was significantly higher in participants with past SARS-CoV-2 infection than in SARS-CoV-2-naiveindividuals. | Participants with T2DM had a lower rate of seropositivity at all time points | The sample was drawn from a healthy population with few comorbidities |
| Shim et al[29] | Age, DM, type of vaccine, side effects, vaccination interval, hypertension, BMI, sex | There were significant differences in general and neutralizing antibodies based on age, vaccine type, vaccination interval, pain score, diabetes, and hypertension | For all vaccines, subjects with diabetes showed lower rates of neutralizing antibody production after vaccination | Vaccination priority policies bring heterogeneity across age groups |
| Alqassieh et al[30] | Age, type of vaccine, hypertension, cardiovascular disease, DM, sex, BMI | Old people (> 60) had lower IgG titers than their younger counterparts. The use of the Pfizer-Biotech vaccine was positively associated with positive IgG titers, while cardiovascular disease had a negative effect on IgG titers. Although diabetes had a negative impact on positive IgG titers, it was not statistically significant | Although DM negatively affected IgG titer positivity, it was not statistically significant | Samples were collected only once at a specific period (6 wk) after vaccination |
| Wan et al[31] | Dose 1, dose 2, HbA1c, side effects | None | Patients with T2DM do not appear to have higher risks of AESI and acute diabetic complications after vaccination | Adverse events are defined using diagnosis codes and may be biased by underdiagnosis or misclassification |
| Lee et al[32] | Sex, age, DM, type of vaccine, BMI | Being young, female or underweight, and having diabetes were associated with an increased risk of developing grade 3 to 4 adverse reactions after the first dose of the ChAdOx1nCoV-19 vaccine | DM is associated with an increased risk of grade 3 to 4 adverse reactions after the first dose of vaccine, especially in women | Sample from relatively healthy subjects working in hospitals |
| Rangsrisaeneepitak et al[33] | T2DM, age, sex, BMI, side effects | After the first dose of AZD1222, the antibody response was weaker in T2DM patients than in non-diabetic patients. The seroconversion rate was higher in the control group than in the diabetic group. Older age was associated with a weaker antibody response in older diabetic patients. The GMC of SARS-CoV-2 IgG antibodies at 56 d was significantly lower in diabetic patients than in age- and sex-matched controls. In the age- and sex-matched controls, SARS-CoV-2 IgG antibody levels were significantly higher in women than in men. During the first 24 h, injection site reactions were more common in diabetic patients than in healthy controls | After the first dose of AZD1222, the antibody response was weaker in T2DM patients than in non-diabetic patients | Participants in the control group were healthcare workers, so natural immunity may have been a confounding factor |
| Sourij et al[34] | T2DM, eGFR, HbA1c, side effects, T1D | Age and renal function were significantly associated with the extent of antibody levels. The most common side effect was injection site reactions, with a significantly lower rate in patients with T2DM | The antibody levels after the second vaccination were comparable in healthy controls and in DM patients, irrespective of glycaemic control | Focused only on the humoral immune response after vaccination, but did not investigate the cellular immune response |
| Tawinprai et al[35] | DM, hematologic disease, sex, age, time since the first dose of vaccination, BMI, side effects, cardiovascular disease, hypertension, dyslipidemia, end-stage kidney disease | Participants with diabetes or hematologic comorbidities had lower concentrations of anti-RBD antibodies. Anti-RBD antibody concentrations were significantly higher in female participants than in male participants. The immune response was lower in older participants. Anti-RBD antibody concentrations were significantly higher at 2 and 3 mo post-vaccination than at 1-mo post-vaccination | Participants with diabetes or hematologic comorbidities had lower concentrations of anti-RBD antibodies | The presence of participants who did not complete two anti-RBD antibody assays withdrew from the study |
| Ali et al[18] | T2DM, age, sex, BMI, comorbidity, previous SARS-CoV-2 infection, hypertension | T2DM is associated with lower titers of neutralizing and IgG antibodies | Both neutralizing antibody and IgG antibody titers were significantly lower in the T2DM group than in the non-diabetic group | Participants in the study were self-selected verbally and through job advertisements |
| Karamese et al[36] | T2DM, age, hypertension, COPD, dose 1, dose 2 | Lower antibody response rates were detected in participants with T2DM and in those aged 65 years and older | DM patients have lower antibody levels | The study population was an advanced age group with a high number of comorbidities |
| Lustig et al[37] | Age, sex, DM, immunosuppression, hypertension, heart disease, autoimmune disorders, BMI | Lower antibody concentrations are consistently associated with males, older age, immunosuppression, diabetes, hypertension, heart disease, and autoimmune disorders | Lower IgG concentrations and lower detectable IgA antibodies were observed in DM patients, indicating a reduced antibody response to vaccination in these patients | The sample was drawn from a healthy population with few comorbidities |
| Islam et al[38] | Hyperglycemia, FPG, age, sex, BMI, hypertension, smoking, alcohol consumption | Spike IgG antibody titers were lower in the presence of hyperglycemia and IFG | Vaccine recipients with diabetes and IFG had lower concentrations of SARS-CoV-2 spike IgG antibodies than the vaccine recipients with normoglycemia did | Associations observed in cross-sectional studies do not necessarily indicate causality |
| Parthymou et al[39] | Sex, age, smoking, BMI, DM, hypertension, statin use, vitamin D levels | Age, male gender, and tobacco use are negatively associated with antibody titers after COVID-19 vaccination | Antibody titers were numerically lower in diabetic patients, but this association was not statistically significant | Reliance on questionnaires to record anthropometric parameters and medical history affects reliability |
| Priddy et al[40] | Age, DM, sex, BMI, race | IgG and neutralization responses decreased with age. Lower responses were associated with age ≥ 75 and DM | Lower responses were associated with DM | Most of the IgG and neutralization tests used are not standardized |
| Naschitz et al[41] | Cancer, DM, congestive heart failure, sex, age, hypertension, COPD, cerebrovascular disease, chronic liver disease, cognitive disability | Cancer, DM, or congestive heart failure were all associated with having a negative serology result | DM is associated with negative serological results | There was a large age difference between the two sample groups |
| Güzel et al[42] | Cardiovascular diseases, DM, age, BMI, sex, smoking, vitamin use, viral load, comorbidities | Cardiovascular disease and diabetes were associated with lower IgG antibody levels. In the healthcare workers group, IgG antibody response values were negatively correlated with BMI and age | IgG antibody levels were significantly lower in patients with DM than in those without DM | ELISA test may lead to false positive results |
| Virgilio et al[43] | Sex, T2DM, insulin therapy | The negative impact of diabetes in determining a steeper antibody decline was greater in female residents than in male residents. T2DM is associated with a reduced humoral immune response after SARS-CoV-2 vaccination. Antibody kinetics in diabetic patients receiving insulin therapy are similar to those in patients without diabetes | Vaccination in elderly residents with type 2 diabetes is associated with a reduced humoral immune response | Data on blood glucose or glycated hemoglobin levels were not specifically collected to assess the control or severity of diabetes |
| Patalon et al[44] | Sex, age, BMI, COPD, DM, congestive heart failure, inflammatory bowel disease | Females were associated with higher levels of antibodies. Lower antibody levels were observed in higher age groups | DM is not a relevant factor affecting antibody levels | The study population was older and had more comorbidities |
| Mitsunaga et al[45] | Age, Hypertension, HbA1c, Outdoor exercises, Vaccination interval, BMI, COPD, Dyslipidemia, DM, Autoimmune diseases, Cancer, dose 1, dose 2, BG | Older than 60 years, hypertension, HbA1c higher than 6.5%, and lack of outdoor exercises were significant suppressors of antibody responses, whereas the length of days from the first to the second vaccination longer than 25 d promoted a significant antibody response | HbA1c higher than 6.5% was a significant suppressor of antibody responses | The sample was relatively healthy health workers but did not include participants with serious comorbidities |
| Papadokostaki et al[46] | Age, DM, dose 1, dose 2, sample testing time, HbA1c, BMI, duration of diabetes, HbA1c | In the diabetic group, Abs-RBD-IgG was significantly correlated with age and time, and dose after vaccination | The humoral immune responses after the second dose were high and similar in participants with and without DM | No comparison between type 1 and type 2 diabetes |
| Zhao et al[47] | DM, dose 1, dose 2, dose 3, age, end-stage kidney disease, cancer, steroid use, previous SARS-CoV-2 infection, time since vaccination | DM was significantly associated with a decrease in response intensity after completion of the primary vaccine series, but responses to the third dose were generally robust. Age and malignancy had a negative effect on the initial strength of the humoral immune response. Being over 65 years, end-stage renal disease, diabetes, and clinical comorbidities of steroid use had a negative effect on the humoral immune response. SARS-CoV-2 infection enhanced the neutralization antibody response to the third dose | DM was significantly associated with a decrease in response intensity after completion of the primary vaccine series, but responses to the third dose were generally robust | Small sample size |
| Santotoribio et al[48] | Age, sex, DM, hypertension, heart disease | None | Serum antibody levels were not significantly reduced in patients with common conditions such as arterial hypertension, diabetes, heart disease, or chronic respiratory disease | No assessment of the cell-mediated immune response |
| Mehta et al[49] | DM, immunosuppression, vaccination interval, sex, comorbidity | DM, immunosuppression, and vaccination interval were all significantly associated with anti-RBD antibodies | DM patients had significantly lower titers of anti-spiking antibodies than patients without diabetes | The sample group was patients with autoimmune rheumatic diseases with a high proportion of comorbidities |
| Ajlan et al[50] | DM, type of vaccine, age, triple immunosuppressive therapy, side effects, sex, time since transplantation | Diabetes and triple immunosuppressive therapy appear to significantly affect the immune response. Triple immunosuppressive therapy and age were identified as significant factors in the lack of response to the vaccine after the second dose. Response rates after the first dose of vaccine with the Pfizer vaccine were higher than those with the AstraZeneca vaccine | Diabetes mellitus and triple immunosuppressive therapy appear to significantly affect response | Lack of immunocompetence control group |
| Billany et al[51] | Age, immunosuppression, previous SARS-CoV-2 infection, sex, race, DM | Patients with detectable antibodies were younger than patients without detectable antibodies. Patients who were immunosuppressed were less likely to have detectable antibodies than patients who were not immunosuppressed. Patients previously infected with COVID-19 were more likely to have detectable antibodies than those with no history of SARS-CoV-2 infection | There was no difference in antibody testing with or without DM | Small sample size |
| Aberer et al[52] | TIR, TBR, TAR, T1DM, T2DM, carbohydrate intake, CV | None | At the time of side effects, T1DM patients had significantly less TIR and significantly more TAR, while there was no effect on T2DM patients | Short assessment time and small sample size |
| Piccini et al[53] | Side effects, dose 1, dose 2, TIR, time in different glucose ranges, mean glucose levels, TDD of insulin, bolus proportion, type of vaccine | Side effects after the vaccination were mild and more frequent after the second dose. No severe adverse reactions were reported | No significant differences in glycemic control and glycemic indices were observed at different times throughout the vaccination cycle and were independent of the vaccine type | Small sample size |
| Heald et al[54]1 | Age, BMI, mode of treatment, sex, HbA1c, type of vaccine, duration of diagnosed T1DM | The fall in the percentage BG on target was also greater for those with a median BMI of 28.1 kg/m2 or more. The fall in the percentage BG on target categorized by additional Metformin/Dapagliflozin was greater than no oral hypoglycemic agents, and the median age ≥ 53 yr was greater than < 53 yr | In T1DM, COVID-19 vaccination can cause a temporary BG disturbance, and this effect is more pronounced in patients taking oral hypoglycemic drugs plus insulin and in the elderly | No analysis of changes in insulin dose in the week following the COVID-19 vaccination |
| D'Onofrio et al[55] | TIR, TBR, TAR, CV, dose 1, dose 2, insulin dosage, SD | None | Pre- and post-CGM data collected during the two vaccine doses did not show any significant differences between the two groups in terms of TIR, TAR, TBR, CV, and SD | Small sample size |
| Heald et al[56]1 | Medication, HbA1c, oral hypoglycemic drugs plus insulin therapy, age, sex, type of vaccine, duration with diabetes, BMI | COVID-19 vaccination can cause a temporary perturbation of interstitial glucose, an effect that is more pronounced in patients taking oral hypoglycemic agents plus insulin. This effect was more pronounced in those with lower HbA1c | In T1DM, vaccination can cause a temporary perturbation of interstitial glucose. There is no difference between the AstraZeneca and the Pfizer vaccines | The effects of the first and second vaccination on interstitial glucose regulation could not be compared |
| Gouda et al[57] | TIR, TDD of insulin, dose 1, dose 2, type of vaccine, insulin dosage, average glucose level, bolus insulin, automated bolus | One week after vaccination, there was a slight decrease in TIR along with an increase in mean blood glucose levels, but both were statistically insignificant | No differences in blood glucose or glycemic perturbations were shown before and after vaccination in patients with T1DM. There was no correlation between vaccine side effects and TIR | The effects of the first and second vaccination on interstitial glucose regulation could not be compared |
Of the 54 studies included, a total of 12 studies indicated a "no effect" relationship between DM and vaccination. Nine of them concluded that DM had no effect on the immune response to the vaccine[23,25,26,30,34,44,46,48,51]. Similarly, three studies showed no effect of vaccination on DM or blood glucose[53,55,57]. Of the two studies that specifically investigated DM and adverse reactions to vaccination[31,32], one suggested that patients with T2DM did not appear to have a higher risk of adverse reactions after vaccination[31].
Does COVID-19 vaccination lead to dysglycemia or even a hyperglycemic crisis with serious adverse consequences in patients? Of the 54 studies included, most suggested that there may be some association between vaccination and blood glucose, mainly in the form of elevated blood glucose or even induction of new-onset DM. Table 2 Lists 12 cases of new-onset DM. In addition, Heald et al[54] also implied that COVID-19 vaccination can cause temporary relative hyperglycemia in patients with T1DM. SARS-CoV-2 infection is known to cause an immune stress response and dysglycemia. The worsening of blood glucose that occurs after vaccination is thought to have a possible common pathophysiology with the hyperglycemia associated with SARS-CoV-2 infection. Possible mechanisms here include islet cell injury and acute insulin reduction following entry through the islet angiotensin-converting enzyme 2 (ACE2) receptor[76], cytokine storm[77], oxidative stress, over-activation of the renin-angiotensin-aldosterone system[78], and dysregulation of stress hormone release such as cortisol and catecholamines leading to increased insulin resistance[79]. The vaccine can activate the immune system and inflammatory factors leading to a cytokine storm that reduces pancreatic blood flow or directly impairs β-cell function via ACE2 receptors, or the inflammatory response increases the cellular oxidative stress and causes pancreatic fibrosis, resulting in decreased insulin synthesis and secretion and reduced insulin sensitivity in target tissues, thereby elevating blood glucose levels[80]. Pancreatic injury has been reported in individuals following the COVID-19 vaccination, which may be a possible cause of hyperglycemia in individuals following vaccination[81,82]. Of these new-onset diabetic patients listed in Table 2, many exhibited low c-peptide levels, suggesting pancreatic damage. Another possible explanation comes from vaccine excipients, adenoviral vectors, and vaccine SARS-CoV-2 spike protein immunogens that trigger similar mechanisms leading to pancreatic damage and inducing subsequent hyperglycemic crises. mRNA vaccine was used in 19 of 29 patients and the adenoviral vector vaccine was used in eight. It appears that the mRNA-COVID-19 vaccine was associated with more reports of elevated blood glucose compared to the viral vector vaccine. Although the mRNA-COVID-19 vaccine does not contain an adjuvant, mRNA appears to have self-adjuvant properties that induce autoimmune/inflammatory syndromes and trigger new-onset DM, especially the new-onset T1DM[83].
Vaccination elicits different levels of immune responses within and between individuals and is determined by a range of factors either present within the vaccine, such as the type of adjuvant, or within the host, such as the immune response genes, one or more of which combine to act together. It is important to note that clinicians should remain vigilant for these events, especially for diabetic patients, who require strict glucose monitoring and adequate diabetic treatment in the days following vaccination.
Does vaccination of diabetic patients affect the inherent efficiency of the vaccine? If so, what factors can contribute to these effects?
The efficiency of the vaccine is mainly demonstrated by immunogenicity, neutralizing antibodies, and cellular immunity. Twenty-one of the studies included in this review showed that diabetes decreases the response after vaccination. Marfella et al[24] compared the neutralizing antibody titers and antigen-specific CD4 cell responses after the COVID-19 vaccine in a non-diabetic population, a diabetic population with well-regulated glucose (HbA1c ≤ 7%), and a diabetic population with poor regulation (glycosylated hemoglobin > 7%) capacity, the results showed that the rate of neutralizing antibody production and the immune response was significantly reduced in the poorly controlled glycemic population, but that T2DM patients with initially poor glycemic control had improved the immune responses after achieving good glycemic control. Their data underscore the notion that hyperglycemia worsens the immune response and that adequate glycemic control improves the immune response.
The underlying cause of the impaired immune response exhibited by diabetic patients after COVID-19 vaccination is not fully understood and may be related to the dysfunction of the adaptive immune response in diabetic patients. The adaptive immune system can be compromised by poor proliferation in response to antigenic stimuli, impaired production of CD4+ T follicular helper cells, and a reduced ability to produce effector lymphokines. Diabetic patients have reduced numbers of circulating CD4+ cells, reduced CD4+ to CD8+ lymphocyte ratios, reduced lymphocyte proliferative responses, impaired monocytes or macrophages, and defective antigen presentation[84]. Intriguingly, some authors have found that patients with T2DM present with an increased white blood cell counts, but they are more likely to have decreased lymphocytes and more senescent CD4+ and CD8+ T cells[85]. These cells are characterized by overexpression of chemokines (particularly C-X-C motif chemokine receptor type 2) and exhibit altered migratory capacity, resulting in poorer vaccine responses in diabetic patients. In addition, the hyperglycemic environment at the time of vaccination worsens the immunological response and also leads to a decreased immune system response to the vaccine.
Age: Age is one of the most critical factors affecting the production of immunoglobulins and neutralizing antibodies. In general, younger people have a stronger immune response to the COVID-19 vaccine and older people have a reduced immune response to vaccination. B-cell activation is critical for the effectiveness of antibody production, but there are several age-related changes in B-cell function and phenotype. Older adults are usually marked by immune senescence, which may reduce the effectiveness of vaccines[86,87]. The immune response to vaccination is controlled by a delicate balance between effector T cells and follicular T cells, and the aging process disrupts this balance, leading to age-related defects in post-transcriptional regulation, T cell receptor signaling, and metabolic function[88]. The age-related immune responses may be heterogeneous, and co-morbidities and their treatment may also affect the immune response[89]. Therefore, booster vaccines for the elderly may be considered.
Gender: Seven studies observed a stronger immune response after vaccination in women compared to men. Genetic differences as well as sex hormone differences can influence vaccine-induced immunity. X chromosomes express 10 times more genes than Y chromosomes, and differences in gene expression between X and Y chromosomes promote sex differences in vaccine-induced immunity[90]. Testosterone suppresses anti-inflammatory immune cells and promotes a more aggressive T helper cell-type immune response, thereby reducing the immune response to vaccines. In contrast, estrogen has a suppressive effect on pro-inflammatory T cells[91]. In addition, ACE2 receptor expression is influenced by estrogen and correlates with the strength of the immune response[92]. Whether diabetes may interact with gender to influence the extent and persistence of vaccine response is unclear. We found that five of the six studies that observed stronger immune responses in women than in men had study populations from healthcare workers[27,32,35,39,44], and, unquestionably, these studies included a higher proportion of women in their samples, potentially biasing the results.
Type of vaccine and method of vaccination: Surprisingly, Kılınç-Toker et al[25] observed that mixed vaccination (CoronaVac plus BioNTech) produced better vaccine efficiency, and similarly, Barocci et al[26] found that heterologous vaccination also produced better vaccine efficiency. Wan et al[93] observed that two doses of CoronaVac followed by a BNT162b2 heterologous booster may be more effective than three doses of CoronaVac in a diabetic population. A study comparing the immune responses generated by mRNA-based vaccines and inactivated whole virus particle vaccines found that mRNA-based vaccines induced stronger humoral immune responses and higher levels of cellular responses than inactivated whole virus particle vaccines[94]. Adenoviral vectors carry antigens that can persist for long periods of time. Anti-glycoprotein IgG antibodies persist until day 180 after single-dose vaccination with ChAd3-EBO-Z in phase 1/2a clinics[95], and antibody responses to a single dose of ChAdOx1 (AZD1222) vaccine have a long half-life[96]. The mixed vaccination may combine the respective advantages of the different vaccine types, while the robust humoral response induced by the heterologous booster may be attributed to the extended interval between the primary and booster doses. Extended intervals between booster doses may result in higher neutralizing activity and a more extensive humoral response through germinal center responses, including somatic cell hypermutation and affinity maturation[97]. Evidence from several studies suggests that heterologous inoculation is safe and effective and induces a robust humoral response to SARS-CoV-2, allowing for faster protection of the target population[98-100].
Obesity: Adipose tissue is another metabolic organ with high ACE2 Levels that may exhibit a propensity for SARS-CoV-2 and is also a source of inflammatory adipokines and cytokines that regulate glucose and insulin resistance. A previous study suggested that excess adipose tissue may impede nutrient supply to immune cells[101]. Obesity leads to adipocyte hypertrophy, which induces low levels of inflammation and insulin resistance[102]. In addition, the hyperleptinemia and hyperinsulinemia that accompany the obese state contribute to T-cell dysfunction, leading to impaired immune responses[103]. These mechanisms of immune cell suppression can reduce antibody production after vaccination.
Special Populations: Patients with autoimmune rheumatic diseases, hemodialysis patients, and organ transplant patients, a special group with high comorbidity and impaired immune response, have significantly lower antibody titers established after vaccination, and the persistence of IgG titers may follow different kinetics. Billany et al[51] described 94 patients on maintenance hemodialysis (including 43 diabetic patients) at the first dose of vaccine antibody response 28 d after vaccination. The results showed that neutralizing antibodies were detectable in 75 patients (79.8%), and there was no difference in the presence or absence of diabetes on antibody detection in the cohort. Reassuringly, Agur et al[104] expressed the same notion. Ajlan et al[50] evaluated the efficacy and safety of two different vaccine platforms in 431 patients with liver or kidney solid organ transplants (191 of whom were diabetic patients), and they found no difference in efficacy between the two vaccine platforms in solid organ transplant patients, with response unresponsiveness primarily related to DM. Bieber et al[105] also reached similar conclusions. These findings seem to support the notion that both vaccination and booster use in immunodeficient populations are associated with better COVID-19-related outcomes, and therefore, regardless of the presence of diabetes, they should be encouraged to receive booster vaccinations to obtain vaccine protection that may be close to that obtained in the general population after two doses, and that combination or allogenic vaccination is a vaccination strategy worth considering for them.
Adverse reactions: Of the 54 studies included, the earliest study began in November 2020, only two years ago so far. SARS-CoV-2 is a novel virus in the history of human viruses, and the COVID-19 vaccine is even more novel for the human being as a whole, given the incredible speed with which many vaccines were developed during the period of COVID-19. It is too early to observe from just two years how the vaccine affects the life cycle of patients with pre-existing DM, so the effect of the COVID-19 vaccine on the natural course of diabetes is more in the form of observed adverse effects. Ten studies mentioned adverse reactions after vaccination, and only Lee et al[32] claimed that diabetes had an increased risk of grade 3 to 4 adverse reactions, while most studies expressed that people with DM were less likely to experience significant side effects after COVID-19 vaccination compared to healthy individuals. The most common systemic side effects are headache, chills, fever, and fatigue, and local effects are pain, redness, and swelling at the injection site. Most side effects are mild and disappear within a few days after vaccination and do not interfere with daily activities. Even for those patients diagnosed with new-onset DM or hyperglycemic crisis, their symptoms resolved rapidly with reasonable treatment, and there was not a single case of death. Although some very rare and serious vaccine-related adverse events have also been reported in myocarditis[106], myocardial infarction[107], and Green-Barre syndrome[21], the vast majority of studies have concluded that vaccination is safe in patients with DM.
Understanding the factors associated with the strength of the immune response to these vaccines and the adverse effects associated with vaccine safety is necessary to optimize vaccination programs. These findings support prioritizing vaccination of vulnerable populations such as diabetes and completing the vaccination cycle, and in countries where conditions permit, promoting the use of booster doses, especially for those special groups with impaired immune responses.
Of the 54 studies included, a total of 12 studies indicated a "no effect" relationship between DM and vaccination. Piccini et al[53] used two types of vaccines in 39 patients over 16 years of age with T1DM who were vaccinated for the entire cycle and showed that no significant differences were observed in time in range, time in different glucose ranges, mean glucose levels, total daily dose of insulin, or bolus ratios before and after any dose or before and after the entire vaccination cycle. They used a hybrid closed-loop system to exclude the effect on glucose brought about by automatic insulin correction of the treatment system. No serious adverse reactions were reported, although minor post-vaccination side effects were observed. Similarly, another study expressed the same opinion[55]. In a prospective multicenter cohort study analyzing T1DM and T2DM patients as well as healthy controls, it was found that anti-SARS-CoV-2 S receptor binding domain antibody levels after the second vaccination were comparable in healthy controls and in patients with T1DM and T2DM, independent of glycemic control. Papadokostaki et al[46] also confirmed this notion. These studies suggest that vaccination has no effect on glycemia in patients with DM, regardless of the vaccine type and before and after vaccination; also, DM has no effect on vaccine efficacy or safety. We analyzed the possible reasons for the differences in the results of these 12 studies compared to other studies: First, when the effect of blood glucose on vaccination was studied, it was done in healthy or special populations and not specifically designed for diabetic populations, for example, Billany et al's study[51] was from a hemodialysis population. In addition, the number of diabetic patients included in these studies was very low. The number of diabetic patients in these two studies was 39 and 35, respectively. Therefore, the results cannot be extrapolated to all diabetic patients. Second, the clinical characteristics of the diabetic subgroups in these studies were not sufficient to explain the heterogeneity of the immune response. The confounding factors of diabetics such as age, type of diabetes, severity of the disease, course of the disease, and therapeutic schedule may affect the results to some extent. Third, heterogeneity in assay methods, differences in the timing of antibody detection (whether it coincides with the lowest value of antibody titers), and differences in the period studied (whether it is affected by a mutant strain that exhibits antibody unresponsiveness) can lead to differences in the immune response to vaccination among vaccinated individuals. Although these differences were faced in other studies as well, it is possible that in these 12 studies, it happened to intersect with more factors and showed inconsistent results with other studies.
Combining the findings of these studies, we can infer that although vaccination gives diabetic patients more possible risk of causing elevated blood glucose than the general population, after vaccination, there is a lower antibody response in diabetic patients compared with healthy subjects, but there is still a considerable amount and intensity of the vaccine immune response, and overall the second dose immune response is higher than the first dose, and diabetic patients with good glycemic control and vaccination with the second dose, the immune response can be significantly improved, and booster vaccination is advocated in special populations subject to immunosuppression, the immune response from mixed vaccination is better than that from a single vaccine type, and heterologous vaccination is better than homologous vaccination.
This is the first systematic review to date to comprehensively analyze the bidirectional effects of COVID-19 vaccination and DM. First, the question about the interaction of DM and vaccination is a novel one, and our review addresses a very clinically relevant question that both physicians and patients are eager to answer. Second, the studies included in this review include a variety of special populations, including pediatric diabetes, hemodialysis, solid organ transplantation, and autoimmune disease populations, as well as a broad representation of patients with two major types of diabetes, which can inform vaccination strategies for patients with DM on a larger scale. Finally, our study data are from real-world sources, providing real and reliable information for optimizing vaccination in this vulnerable population with DM and providing objective and qualitative evidence for future public policy formulation and optimal vaccine strategies.
Of course, there are some limitations to this systematic review. First, as described in Strengths, the wide representation of the included populations also implies large heterogeneity. Population heterogeneity includes, in addition to the common heterogeneity in demographic characteristics, the health-seeking behavior of these populations and the geographic distribution of the population, and these heterogeneities can introduce bias into the interpretation of the overall results. Second, the small sample size of some studies, with a total of 18 cases (series) reported, and the small proportion of people with DM in some studies limit the ability to test for possible differential effects between subgroups. Third, possibly because of ethical challenges in clinical practice, no randomized controlled studies were found among the included studies, although some authors made their best efforts to reduce potential bias from selection by using PSM methods. Finally, important reports not published in English may have been omitted from this review, or the search strategy failed to capture them.
In the world of the COVID-19 vaccine and DM, many questions remain: How frequent is the new-onset of DM after COVID-19 vaccination? Which component of the vaccine is more likely to cause dysglycemia and will COVID-19 vaccine heterologous vaccination reduce adverse events in patients with diabetes? Our systematic review implies some gaps in the literature that could be addressed in the future. Studies on the effects of COVID-19 vaccination on DM in type 1 and type 2 for comparative analysis and studies on changes in the effects of vaccination on the cellular immunity in patients with DM and the effects of vaccination on the natural course of pre-existing DM are scarce, and there is a need for longer follow-up or well-designed large-scale studies in the future to further provide an updated and more comprehensive evidence-based basis for the relationship between DM and COVID-19.
In conclusion, there is a complex relationship between vaccination and DM with bidirectional effects. Vaccination may contribute to the risk of worsening glycemia in diabetic patients, and diabetic patients may have a lower antibody response after vaccination than the general population, but good glycemic control can significantly improve the immune response.
Both coronavirus disease 2019 (COVID-19) and diabetes pose a serious threat to human health. Vaccination is an effective way to prevent the spread of COVID-19. There are few and conflicting data on the interaction between COVID-19 vaccination and diabetes mellitus.
We searched all current clinical studies to explore the complex relationship between COVID-19 vaccination and diabetes.
We analyzed various factors and possible mechanisms of the interaction between COVID-19 vaccination and diabetes in order to inform the optimal vaccination strategy and clinical management of patients with diabetes.
We comprehensively searched PubMed, MEDLINE, and EMBASE online databases and the grey literature of medRxiv and bioRxiv using keywords individually or in combination, with a cut-off date of December 2, 2022. We followed the inclusion and exclusion criteria and studies with quantifiable evidence were included in the full-text review. We also manually searched for important references cited by the included studies.
A total of 54 studies were included. The earliest study began in November 2020. Thirty studies discussed the effect of diabetes on COVID-19 vaccination, with the majority indicating that diabetes decreases the response to vaccination. Of the other 24 studies on the effect of vaccination on diabetes, most concluded that vaccination was associated with a risk of elevated blood glucose. Twelve of the 54 studies expressed a "no effect" relationship between diabetes and vaccination.
There is a bidirectional relationship between vaccination and diabetes. Vaccination may contribute to the risk of elevated blood glucose in diabetic patients, and diabetes may have a lower antibody response after vaccination than in the general population, but good glycemic control can significantly improve the immune response.
Our review reveals a complex relationship between diabetes and vaccination and suggests some gaps in the literature that can be addressed in the future, necessitating well-designed large-scale studies to further provide a more comprehensive basis for the relationship between diabetes and COVID-19.
Many thanks to Mr. Han Boning for editing the manuscript, polishing the English, and providing the audio for the core tip of the manuscript. Many thanks to Mr. Zhao Kai for his help in English correction during the manuscript revision process.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Nooripour R, Iran S-Editor: Li L L-Editor: A P-Editor: Fan JR
| 1. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10698] [Article Influence: 2139.6] [Reference Citation Analysis (1)] |
| 2. | Yek C, Warner S, Wiltz JL, Sun J, Adjei S, Mancera A, Silk BJ, Gundlapalli AV, Harris AM, Boehmer TK, Kadri SS. Risk Factors for Severe COVID-19 Outcomes Among Persons Aged ≥18 Years Who Completed a Primary COVID-19 Vaccination Series - 465 Health Care Facilities, United States, December 2020-October 2021. MMWR Morb Mortal Wkly Rep. 2022;71:19-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 3. | Johns Hopkins. Johns Hopkins Coronavirus research center. [cited 17 December 2022]. Available from: https://coronavirus.jhu.edu/map.html. |
| 4. | Cleveland Clinic. Cleveland Clinics Diabetes: Types, Risk Factors, Symptoms, Tests, Treatments & Prevention. [cited 13 November 2022]. Available from: https://my.clevelandclinic.org/health/diseases/7104-diabetes-mellitus-an-overview. |
| 5. | Erener S. Diabetes, infection risk and COVID-19. Mol Metab. 2020;39:101044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 6. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4794] [Article Influence: 1598.0] [Reference Citation Analysis (36)] |
| 7. | Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1758] [Cited by in RCA: 1951] [Article Influence: 390.2] [Reference Citation Analysis (0)] |
| 8. | Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes MetabSyndr. 2020;14:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 480] [Article Influence: 96.0] [Reference Citation Analysis (1)] |
| 9. | Targher G, Mantovani A, Wang XB, Yan HD, Sun QF, Pan KH, Byrne CD, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020;46:335-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 10. | Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of Infection in Type 1 and Type 2 Diabetes Compared With the General Population: A Matched Cohort Study. Diabetes Care. 2018;41:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 419] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 11. | Al-Kuraishy HM, Al-Gareeb AI, Alblihed M, Guerreiro SG, Cruz-Martins N, Batiha GE. COVID-19 in Relation to Hyperglycemia and Diabetes Mellitus. Front Cardiovasc Med. 2021;8:644095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 12. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7565] [Article Influence: 1891.3] [Reference Citation Analysis (1)] |
| 13. | Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1772] [Article Influence: 354.4] [Reference Citation Analysis (0)] |
| 14. | Abu-Raddad LJ, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Malek JA, Coyle P, Ayoub HH, Al Kanaani Z, Al Kuwari E, Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Abdul Rahim HF, Nasrallah GK, Al Kuwari MG, Al Romaihi HE, Al-Thani MH, Al Khal A, Butt AA, Bertollini R. Pfizer-BioNTech mRNA BNT162b2 Covid-19 vaccine protection against variants of concern after one vs two doses. J Travel Med. 2021;28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, Koup RA, Picker LJ. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983-11991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 563] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 16. | Verstraeten T, Fletcher MA, Suaya JA, Jackson S, Hall-Murray CK, Scott DA, Schmöle-Thoma B, Isturiz RE, Gessner BD. Diabetes mellitus as a vaccine-effect modifier: a review. Expert Rev Vaccines. 2020;19:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Saciuk Y, Kertes J, Mandel M, Hemo B, Shamir Stein N, Ekka Zohar A. Pfizer-BioNTech vaccine effectiveness against Sars-Cov-2 infection: Findings from a large observational study in Israel. Prev Med. 2022;155:106947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Ali H, Alterki A, Sindhu S, Alahmad B, Hammad M, Al-Sabah S, Alghounaim M, Jamal MH, Aldei A, Mairza MJ, Husain M, Deverajan S, Ahmad R, Cherian P, Alkhairi I, Alkandari A, Abubaker J, Abu-Farha M, Al-Mulla F. Robust Antibody Levels in Both Diabetic and Non-Diabetic Individuals After BNT162b2 mRNA COVID-19 Vaccination. Front Immunol. 2021;12:752233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 19. | Kageyama T, Ikeda K, Tanaka S, Taniguchi T, Igari H, Onouchi Y, Kaneda A, Matsushita K, Hanaoka H, Nakada TA, Ohtori S, Yoshino I, Matsubara H, Nakayama T, Yokote K, Nakajima H. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect. 2021;27:1861.e1-1861.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 20. | Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19:75-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 180] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 21. | Rosenblum HG, Hadler SC, Moulia D, Shimabukuro TT, Su JR, Tepper NK, Ess KC, Woo EJ, Mba-Jonas A, Alimchandani M, Nair N, Klein NP, Hanson KE, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF, Mbaeyi SA, Oliver SE. Use of COVID-19 Vaccines After Reports of Adverse Events Among Adult Recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices - United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1094-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 22. | Lee C, Cotter D, Basa J, Greenberg HL. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Chen H, Lv J, Huang T, Zhang R, Zhang D, Luo L, Wei S, Liu X, Zhang S, Mu Q, Huang R, Huang J, Xiao Y, Yang Y, Han Y, Gong H, Guan Q, Xie F, Wang H, Li L, Yang X. Evaluation of Immunogenicity and Safety of Vero Cell-Derived Inactivated COVID-19 Vaccine in Older Patients with Hypertension and Diabetes Mellitus. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 24. | Marfella R, D'Onofrio N, Sardu C, Scisciola L, Maggi P, Coppola N, Romano C, Messina V, Turriziani F, Siniscalchi M, Maniscalco M, Boccalatte M, Napolitano G, Salemme L, Marfella LV, Basile E, Montemurro MV, Papa C, Frascaria F, Papa A, Russo F, Tirino V, Papaccio G, Galdiero M, Sasso FC, Barbieri M, Rizzo MR, Balestrieri ML, Angelillo IF, Napoli C, Paolisso G. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: The CAVEAT study. Diabetes ObesMetab. 2022;24:160-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 25. | Kılınç-Toker A, Turunç-Özdemir A, Civan-Yüksel R, Eryilmaz-Eren E, Toker İ, Çelik İ. Clinical characteristics of patients hospitalized for COVID-19 vaccinated with at least two doses in a tertiary care hospital in Turkey Microbes Infect. Chemother. 2022;2:e1465. [DOI] [Full Text] |
| 26. | Barocci S, Orlandi C, Diotallevi A, Buffi G, Ceccarelli M, Vandini D, Carlotti E, Galluzzi L, Rocchi MBL, Magnani M, Casabianca A. Evaluation of Two-Month Antibody Levels after Heterologous ChAdOx1-S/BNT162b2 Vaccination Compared to Homologous ChAdOx1-S or BNT162b2 Vaccination. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Singh AK, Phatak SR, Singh R, Bhattacharjee K, Singh NK, Gupta A, Sharma A. Antibody response after first and second-dose of ChAdOx1-nCOV (Covishield(TM)®) and BBV-152 (Covaxin(TM)®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine. 2021;39:6492-6509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 28. | Singh AK, Phatak SR, Singh R, Bhattacharjee K, Singh NK, Gupta A, Sharma A. Humoral antibody kinetics with ChAdOx1-nCOV (Covishield™) and BBV-152 (Covaxin™) vaccine among Indian Healthcare workers: A 6-month longitudinal cross-sectional Coronavirus Vaccine-induced antibody titre (COVAT) study. Diabetes MetabSyndr. 2022;16:102424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Shim HW, Shin JH, Shin SC, Lee HJ, So KS, Lee SY, Jun JW, Seo JK, Lee HS, Kim SH, Kim SJ, Kim KC, Ryu GH. Analysis of Factors Affecting Neutralizing Antibody Production after COVID-19 Vaccination Using Newly Developed Rapid Point-of-Care Test. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Alqassieh R, Suleiman A, Abu-Halaweh S, Santarisi A, Shatnawi O, Shdaifat L, Tarifi A, Al-Tamimi M, Al-Shudifat AE, Alsmadi H, Al Sharqawi A, Alnawaiseh H, Anasweh Y, Domaidah FA, Jaber HA, Al-Zarir MR, Bsisu I. Pfizer-BioNTech and Sinopharm: A Comparative Study on Post-Vaccination Antibody Titers. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Wan EYF, Chui CSL, Mok AHY, Xu W, Yan VKC, Lai FTT, Li X, Wong CKH, Chan EWY, Lui DTW, Tan KCB, Hung IFN, Lam CLK, Leung GM, Wong ICK. mRNA (BNT162b2) and Inactivated (CoronaVac) COVID-19 Vaccination and Risk of Adverse Events and Acute Diabetic Complications in Patients with Type 2 Diabetes Mellitus: A Population-Based Study. Drug Saf. 2022;45:1477-1490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Lee SW, Lee H, Lee SK, Moon JY, Moon S, Chung SJ, Yeo Y, Park TS, Won Park D, Kim TH, Sohn JW, Yoon HJ, Kim SH. Risk Factors for Grade 3 to Grade 4 Adverse Reactions to the ChAdOx1 nCoV-19 Vaccine (AZD1222) Against SARS-CoV-2. Front Med (Lausanne). 2021;8:738049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Rangsrisaeneepitak V, Porntharukchareon T, Dechates B, Sirisreetreerux S, Tawinprai K. Antibody levels in people with diabetes after one dose of the ChAdOx1 nCoV-19 (AZD1222) vaccine. Diabetol Int. 2022;13:637-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Sourij C, Tripolt NJ, Aziz F, Aberer F, Forstner P, Obermayer AM, Kojzar H, Kleinhappl B, Pferschy PN, Mader JK, Cvirn G, Goswami N, Wachsmuth N, Eckstein ML, Müller A, Abbas F, Lenz J, Steinberger M, Knoll L, Krause R, Stradner M, Schlenke P, Sareban N, Prietl B, Kaser S, Moser O, Steinmetz I, Sourij H; COVAC-DM study group. Humoral immune response to COVID-19 vaccination in diabetes is age-dependent but independent of type of diabetes and glycaemic control: The prospective COVAC-DM cohort study. Diabetes ObesMetab. 2022;24:849-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 35. | Tawinprai K, Siripongboonsitti T, Porntharukchareon T, Dechates B, Monprach H, Sornsamdang G, Wittayasak K, Soonklang K, Mahanonda N. Persistence of immunogenicity, contributing factors of an immune response, and reactogenicities after a single dose of the ChAdOx1 (AZD1222) COVID-19 vaccine in the Thai population. Hum VaccinImmunother. 2022;18:2035573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Karamese M, Tutuncu EE. The effectiveness of inactivated SARS-CoV-2 vaccine (CoronaVac) on antibody response in participants aged 65 years and older. J Med Virol. 2022;94:173-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 37. | Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, Indenbaum V, Mandelboim M, Doolman R, Amit S, Mendelson E, Ziv A, Huppert A, Rubin C, Freedman L, Kreiss Y. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 267] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 38. | Islam Z, Yamamoto S, Mizoue T, Tanaka A, Oshiro Y, Inamura N, Konishi M, Ozeki M, Sugiura W, Ohmagari N. Association of Impaired Fasting Glucose and Diabetes with SARS-CoV-2 Spike Antibody Titers after the BNT162b2 Vaccine among Health Care Workers in a Tertiary Hospital in Japan. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Parthymou A, Habeos EE, Habeos GI, Deligakis A, Livieratos E, Marangos M, Chartoumpekis DV. Factors associated with anti-SARS-CoV-2 antibody titres 3 mo post-vaccination with the second dose of BNT162b2 vaccine: a longitudinal observational cohort study in western Greece. BMJ Open. 2022;12:e057084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 40. | Priddy FH, Williams M, Carson S, Lavender B, Mathieson J, Frampton C, Moreland NJ, McGregor R, Williams G, Brewerton M, Gell K, Ussher J, Le Gros G. Immunogenicity of BNT162b2 COVID-19 vaccine in New Zealand adults. Vaccine. 2022;40:5050-5059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Naschitz JE, Kertes J, Pinto G, Zaigraykin N, Oz D, Goland E, Nasser S, Supino-Rosin L, Lazar R, Ekka-Zohar A. Comparison of Covid-19 antibody status after vaccination between residents in long-term geriatric care and residents assisted-living facilities. Infect Dis (Lond). 2022;54:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Güzel EÇ, Çelikkol A, Erdal B, Sedef N. Immunogenicity after CoronaVac vaccination. Rev Assoc Med Bras (1992). 2021;67:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Virgilio E, Trevisan C, Abbatecola A, Malara A, Palmieri A, Fedele G, Stefanelli P, Leone P, Schiavoni I, Maggi S, Volpato S, Antonelli Incalzi R, Onder G; GeroCovid Vax Working Group. Diabetes Affects Antibody Response to SARS-CoV-2 Vaccination in Older Residents of Long-term Care Facilities: Data From the GeroCovid Vax Study. Diabetes Care. 2022;45:2935-2942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 44. | Patalon T, Ben Moshe S, Peretz A, Neuberger A, Schreiber L, Lazar R, Supino-Rosin L, Perez G, Mizrahi-Reuveni M, Gazit S. SARS-CoV-2 spike IgG titres up to 137 days following Comirnaty mRNA COVID-19 vaccination, Israel, February to May 2021. Euro Surveill. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 45. | Mitsunaga T, Ohtaki Y, Seki Y, Yoshioka M, Mori H, Suzuka M, Mashiko S, Takeda S, Mashiko K. The evaluation of factors affecting antibody response after administration of the BNT162b2 vaccine: a prospective study in Japan. PeerJ. 2021;9:e12316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Papadokostaki E, Tentolouris A, Anastasiou IA, Psichogiou M, Iliaki E, Eleftheriadou I, Hatzakis A, Tentolouris N. Immunogenicity of SARS-CoV-2 BNT162b2 Vaccine in People with Diabetes: A Prospective Observational Study. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Zhao M, Slotkin R, Sheth AH, Pischel L, Kyriakides TC, Emu B, McNamara C, Shi Q, Delgobbo J, Xu J, Marhoffer E, Mercer-Falkoff A, Holleck J, Ardito D, Sutton RE, Gupta S. Serum Neutralizing Antibody Titers 12 Months After Coronavirus Disease 2019 Messenger RNA Vaccination: Correlation to Clinical Variables in an Adult, US Population. Clin Infect Dis. 2023;76:e391-e399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 48. | Santotoribio JD, Franco-Garcia C, Mondejar R, Virto-Pena I, Mayor-Reyes M, Garcia-Martin S, Canavate-Solano C, Rodriguez-Garcia M, Diez-Herran L, Cebada-Romero C, Rubia-Martin F, Jordan-Chaves J, Martinez-Rubio C, Freyre-Carrillo C. Clinical Evaluation of Serum Levels of SARS-CoV-2 Anti-Spike Protein IgG Antibodies in Infected Patients and Vaccinated Subjects. Clin Lab. 2022;68. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 49. | Mehta P, Paul A, Ahmed S, Cherian S, Panthak A, Benny J, Shenoy P. Effectiveness of delayed second dose of AZD1222 vaccine in patients with autoimmune rheumatic disease. Clin Rheumatol. 2022;41:3537-3542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Ajlan AA, Ali T, Aleid H, Almeshari K, DeVol E, Alkaff MA, Fajji L, Alali A, Halabi D, Althuwaidi S, Alghamdi S, Ullah A, Alrajhi A, Bzeizi K, Almaghrabi R, Marquez KAH, Elmikkaoui B, Albogumi E, Aldakhil H, Al-Awwami M, Broering DC. Comparison of the safety and immunogenicity of the BNT-162b2 vaccine and the ChAdOx1 vaccine for solid organ transplant recipients: a prospective study. BMC Infect Dis. 2022;22:786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Billany RE, Selvaskandan H, Adenwalla SF, Hull KL, March DS, Burton JO, Bishop NC, Carr EJ, Beale R, Tang JW, Bird PW, Holmes CW, Baines R, Brunskill NJ, Graham-Brown MPM. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: a call to arms. Kidney Int. 2021;99:1492-1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 52. | Aberer F, Moser O, Aziz F, Sourij C, Ziko H, Lenz J, Abbas F, Obermayer AM, Kojzar H, Pferschy PN, Müller A, Unteregger C, Leitner M, Banfic T, Eckstein ML, Wachsmuth N, Kaser S, Mader JK, Tripolt NJ, Sourij H. Impact of COVID-19 Vaccination on Glycemia in Individuals With Type 1 and Type 2 Diabetes: Substudy of the COVAC-DM Study. Diabetes Care. 2022;45:e24-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 53. | Piccini B, Pessina B, Pezzoli F, Casalini E, Toni S. COVID-19 vaccination in adolescents and young adults with type 1 diabetes: Glycemic control and side effects. Pediatr Diabetes. 2022;23:469-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Heald AH, Rea R, Horne L, Metters A, Steele T, Leivesley K, Whyte MB, Stedman M, Ollier W. Analysis of continuous glucose tracking data in people with type 1 diabetes after COVID-19 vaccination reveals unexpected link between immune and metabolic response, augmented by adjunctive oral medication. Int J Clin Pract. 2021;75:e14714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | D'Onofrio L, Coraggio L, Zurru A, Carlone A, Mignogna C, Moretti C, Maddaloni E, Buzzetti R. Short-term safety profile of Sars-Cov2 vaccination on glucose control: Continuous glucose monitoring data in people with autoimmune diabetes. Diabetes Res Clin Pract. 2021;179:109022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Heald AH, Stedman M, Horne L, Rea R, Whyte M, Gibson JM, Anderson SG, Ollier W. The change in glycaemic control immediately after COVID-19 vaccination in people with type 1 diabetes. Diabet Med. 2022;39:e14774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Gouda N, Dimitriadou M, Sotiriou G, Christoforidis A. The impact of COVID-19 vaccination on glycaemic control in children and adolescents with type 1 diabetes mellitus on continuous glucose monitoring. Acta Diabetol. 2022;59:1609-1614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Sakurai K, Narita D, Saito N, Ueno T, Sato R, Niitsuma S, Takahashi K, Arihara Z. Type 1 diabetes mellitus following COVID-19 RNA-based vaccine. J Diabetes Investig. 2022;13:1290-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 59. | Patrizio A, Ferrari SM, Antonelli A, Fallahi P. A case of Graves' disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun. 2021;125:102738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 60. | Aydoğan Bİ, Ünlütürk U, Cesur M. Type 1 diabetes mellitus following SARS-CoV-2 mRNA vaccination. Endocrine. 2022;78:42-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 61. | Sato T, Kodama S, Kaneko K, Imai J, Katagiri H. Type 1 Diabetes Mellitus Associated with Nivolumab after Second SARS-CoV-2 Vaccination, Japan. Emerg Infect Dis. 2022;28:1518-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 62. | Yakou F, Saburi M, Hirose A, Akaoka H, Hirota Y, Kobayashi T, Awane N, Asahi N, Amagawa T, Ozawa S, Ohno A, Matsushita T. A Case Series of Ketoacidosis After Coronavirus Disease 2019 Vaccination in Patients With Type 1 Diabetes. Front Endocrinol (Lausanne). 2022;13:840580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Mishra A, Ghosh A, Dutta K, Tyagi K, Misra A. Exacerbation of hyperglycemia in patients with type 2 diabetes after vaccination for COVID19: Report of three cases. Diabetes MetabSyndr. 2021;15:102151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 64. | Abu-Rumaileh MA, Gharaibeh AM, Gharaibeh NE. COVID-19 Vaccine and Hyperosmolar Hyperglycemic State. Cureus. 2021;13:e14125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 65. | Sasaki H, Itoh A, Watanabe Y, Nakajima Y, Saisho Y, Irie J, Meguro S, Itoh H. Newly developed type 1 diabetes after coronavirus disease 2019 vaccination: A case report. J Diabetes Investig. 2022;13:1105-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 66. | Lee HJ, Sajan A, Tomer Y. Hyperglycemic Emergencies Associated With COVID-19 Vaccination: A Case Series and Discussion. J Endocr Soc. 2021;5:bvab141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 67. | Edwards AE, Vathenen R, Henson SM, Finer S, Gunganah K. Acute hyperglycaemic crisis after vaccination against COVID-19: A case series. Diabet Med. 2021;38:e14631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 68. | Ganakumar V, Jethwani P, Roy A, Shukla R, Mittal M, Garg MK. Diabetic ketoacidosis (DKA) in type 1 diabetes mellitus (T1DM) temporally related to COVID-19 vaccination. Diabetes MetabSyndr. 2022;16:102371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Zilbermint M, Demidowich AP. Severe Diabetic Ketoacidosis After the Second Dose of mRNA-1273 COVID-19 Vaccine. J Diabetes Sci Technol. 2022;16:248-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Yaturu S, Azimi S, Allen A, Atkins J. COVID-19 Vaccine Related Hyperosmolar Hyperglycemic State and Normalized Glycemia within 2 Months. J Diabetes Mellitus. 2022;12:12-17. [DOI] [Full Text] |
| 71. | Kshetree B, Lee J, Acharya S. COVID-19 Vaccine-Induced Rapid Progression of Prediabetes to Ketosis-Prone Diabetes Mellitus in an Elderly Male. Cureus. 2022;14:e28830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 72. | PrasadASV. COVID 19 vaccine induced glycaemic disturbances in DM2-A Case Report. World J Adv Res Rev. 2021;10:149-156. [DOI] [Full Text] |
| 73. | Sasaki K, Morioka T, Okada N, Natsuki Y, Kakutani Y, Ochi A, Yamazaki Y, Shoji T, Ohmura T, Emoto M. New-onset fulminant type 1 diabetes after severe acute respiratory syndrome coronavirus 2 vaccination: A case report. J Diabetes Investig. 2022;13:1286-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 74. | Yano M, Morioka T, Natsuki Y, Sasaki K, Kakutani Y, Ochi A, Yamazaki Y, Shoji T, Emoto M. New-onset Type 1 Diabetes after COVID-19 mRNA Vaccination. Intern Med. 2022;61:1197-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 75. | Ohuchi K, Amagai R, Tamabuchi E, Kambayashi Y, Fujimura T. Fulminant type 1 diabetes mellitus triggered by coronavirus disease 2019 vaccination in an advanced melanoma patient given adjuvant nivolumab therapy. J Dermatol. 2022;49:e167-e168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 77. | Kazakou P, Paschou SA, Psaltopoulou T, Gavriatopoulou M, Korompoki E, Stefanaki K, Kanouta F, Kassi GN, Dimopoulos MA, Mitrakou A. Early and late endocrine complications of COVID-19. Endocr Connect. 2021;10:R229-R239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 78. | Kalupahana NS, Moustaid-Moussa N. The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev. 2012;13:136-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 79. | Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 539] [Cited by in RCA: 604] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 80. | Varghese E, Samuel SM, Liskova A, Kubatka P, Büsselberg D. Diabetes and coronavirus (SARS-CoV-2): Molecular mechanism of Metformin intervention and the scientific basis of drug repurposing. PLoS Pathog. 2021;17:e1009634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 81. | Cieślewicz A, Dudek M, Krela-Kaźmierczak I, Jabłecka A, Lesiak M, Korzeniowska K. Pancreatic Injury after COVID-19 Vaccine-A Case Report. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 82. | Parkash O, Sharko A, Farooqi A, Ying GW, Sura P. Acute Pancreatitis: A Possible Side Effect of COVID-19 Vaccine. Cureus. 2021;13:e14741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 83. | Xu S, Yang K, Li R, Zhang L. mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 84. | Marseglia G, Alibrandi A, d'Annunzio G, Gulminetti R, Avanzini MA, Marconi M, Tinelli C, Lorini R. Long term persistence of anti-HBs protective levels in young patients with type 1 diabetes after recombinant hepatitis B vaccine. Vaccine. 2000;19:680-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Lau EYM, Carroll EC, Callender LA, Hood GA, Berryman V, Pattrick M, Finer S, Hitman GA, Ackland GL, Henson SM. Type 2 diabetes is associated with the accumulation of senescent T cells. Clin Exp Immunol. 2019;197:205-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 86. | Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, Rabl D, Ostermann PN, Robitzsch R, Hauka S, Walker A, Menne C, Grutza R, Timm J, Adams O, Schaal H. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin Infect Dis. 2021;73:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 347] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 87. | Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019;19:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 88. | Gustafson CE, Kim C, Weyand CM, Goronzy JJ. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 2020;145:1309-1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 89. | Collier DA, Ferreira IATM, Kotagiri P, Datir RP, Lim EY, Touizer E, Meng B, Abdullahi A; CITIID-NIHR BioResource COVID-19 Collaboration, Elmer A, Kingston N, Graves B, Le Gresley E, Caputo D, Bergamaschi L, Smith KGC, Bradley JR, Ceron-Gutierrez L, Cortes-Acevedo P, Barcenas-Morales G, Linterman MA, McCoy LE, Davis C, Thomson E, Lyons PA, McKinney E, Doffinger R, Wills M, Gupta RK. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 517] [Cited by in RCA: 521] [Article Influence: 130.3] [Reference Citation Analysis (0)] |
| 90. | Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 91. | Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 507] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 92. | Viveiros A, Rasmuson J, Vu J, Mulvagh SL, Yip CYY, Norris CM, Oudit GY. Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. Am J Physiol Heart Circ Physiol. 2021;320:H296-H304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 93. | Wan EYF, Mok AHY, Yan VKC, Wang B, Zhang R, Hong SN, Chui CSL, Li X, Wong CKH, Lai FTT, Tan KCB, Lau CS, Wong ICK, Chan EWY. Vaccine effectiveness of BNT162b2 and CoronaVac against SARS-CoV-2 Omicron BA.2 infection, hospitalisation, severe complications, cardiovascular disease and mortality in patients with diabetes mellitus: A case control study. J Infect. 2022;85:e140-e144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 94. | Mok CKP, Cohen CA, Cheng SMS, Chen C, Kwok KO, Yiu K, Chan TO, Bull M, Ling KC, Dai Z, Ng SS, Lui GC, Wu C, Amarasinghe GK, Leung DW, Wong SYS, Valkenburg SA, Peiris M, Hui DS. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology. 2022;27:301-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 95. | De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, Cochet C, Estoppey D, Steiner-Monard V, Lonchampt S, Thierry AC, Mayor C, Bailer RT, Mbaya OT, Zhou Y, Ploquin A, Sullivan NJ, Graham BS, Roman F, De Ryck I, Ballou WR, Kieny MP, Moorthy V, Spertini F, Genton B. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis. 2016;16:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 96. | Flaxman A, Marchevsky NG, Jenkin D, Aboagye J, Aley PK, Angus B, Belij-Rammerstorfer S, Bibi S, Bittaye M, Cappuccini F, Cicconi P, Clutterbuck EA, Davies S, Dejnirattisai W, Dold C, Ewer KJ, Folegatti PM, Fowler J, Hill AVS, Kerridge S, Minassian AM, Mongkolsapaya J, Mujadidi YF, Plested E, Ramasamy MN, Robinson H, Sanders H, Sheehan E, Smith H, Snape MD, Song R, Woods D, Screaton G, Gilbert SC, Voysey M, Pollard AJ, Lambe T; Oxford COVID Vaccine Trial group. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398:981-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 182] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 97. | Laidlaw BJ, Ellebedy AH. The germinal centre B cell response to SARS-CoV-2. Nat Rev Immunol. 2022;22:7-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 176] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 98. | Stuart ASV, Shaw RH, Liu X, Greenland M, Aley PK, Andrews NJ, Cameron JC, Charlton S, Clutterbuck EA, Collins AM, Darton T, Dinesh T, Duncan CJA, England A, Faust SN, Ferreira DM, Finn A, Goodman AL, Green CA, Hallis B, Heath PT, Hill H, Horsington BM, Lambe T, Lazarus R, Libri V, Lillie PJ, Mujadidi YF, Payne R, Plested EL, Provstgaard-Morys S, Ramasamy MN, Ramsay M, Read RC, Robinson H, Screaton GR, Singh N, Turner DPJ, Turner PJ, Vichos I, White R, Nguyen-Van-Tam JS, Snape MD; Com-COV2 Study Group. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399:36-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 99. | Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, Cameron JC, Charlton S, Clutterbuck EA, Collins AM, Dinesh T, England A, Faust SN, Ferreira DM, Finn A, Green CA, Hallis B, Heath PT, Hill H, Lambe T, Lazarus R, Libri V, Long F, Mujadidi YF, Plested EL, Provstgaard-Morys S, Ramasamy MN, Ramsay M, Read RC, Robinson H, Singh N, Turner DPJ, Turner PJ, Walker LL, White R, Nguyen-Van-Tam JS, Snape MD; Com-COV Study Group. Safety and immunogenicity of heterologous vs homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 364] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 100. | Normark J, Vikström L, Gwon YD, Persson IL, Edin A, Björsell T, Dernstedt A, Christ W, Tevell S, Evander M, Klingström J, Ahlm C, Forsell M. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N Engl J Med. 2021;385:1049-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 101. | Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 102. | Manna P, Jain SK. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. MetabSyndrRelatDisord. 2015;13:423-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 709] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 103. | Green WD, Beck MA. Obesity Impairs the Adaptive Immune Response to Influenza Virus. Ann Am Thorac Soc. 2017;14:S406-S409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 104. | Agur T, Ben-Dor N, Goldman S, Lichtenberg S, Herman-Edelstein M, Yahav D, Rozen-Zvi B, Zingerman B. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients - a prospectivecohort study. Nephrol Dial Transplant. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 105. | Bieber A, Sagy I, Novack L, Brikman S, Abuhasira R, Ayalon S, Novofastovski I, Abu-Shakra M, Mader R. BNT162b2 mRNA COVID-19 vaccine and booster in patients with autoimmune rheumatic diseases: a national cohort study. Ann Rheum Dis. 2022;81:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 106. | Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, Scobie HM, Moulia D, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF, Oliver SE. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 400] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 107. | Boivin Z, Martin J. Untimely Myocardial Infarction or COVID-19 Vaccine Side Effect. Cureus. 2021;13:e13651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |