Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.680
Peer-review started: December 26, 2022
First decision: February 20, 2023
Revised: February 20, 2023
Accepted: April 10, 2023
Article in press: April 10, 2023
Published online: June 15, 2023
Processing time: 170 Days and 14.1 Hours
Diabetes is a chronic disease that is considered one of the most stubborn global health problems that continues to defy the efforts of scientists and physicians. The prevalence of diabetes in the global population continues to grow to alarming levels year after year, causing an increase in the incidence of diabetes complications and health care costs all over the world. One major complication of diabetes is the high susceptibility to infections especially in the lower limbs due to the immunocompromised state of diabetic patients, which is considered a definitive factor in all cases. Diabetic foot infections continue to be one of the most common infections in diabetic patients that are associated with a high risk of serious complications such as bone infection, limb amputations, and life-threatening systemic infections. In this review, we discussed the circumstances associated with the high risk of infection in diabetic patients as well as some of the most commonly isolated pathogens from diabetic foot infections and the related virulence behavior. In addition, we shed light on the different treatment strategies that aim at eradicating the infection.
Core Tip: Diabetic foot infection is a common complication of diabetes that can lead to serious consequences, such as amputations and even death. The microbiome of the wound plays a crucial role in the development and progression of diabetic foot ulcer. The current review shed light on the most prevalent bacterial infections and their related virulence factors that are associated with diabetic foot complications. Additionally, various approaches for treatment were explored.
- Citation: Rajab AAH, Hegazy WAH. What’s old is new again: Insights into diabetic foot microbiome. World J Diabetes 2023; 14(6): 680-704
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/680.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.680
Diabetes is a chronic metabolic disorder that is characterized by the failure of the body to regulate blood glucose levels. The worldwide prevalence of diabetes has increased to epidemic levels in the last decade; the latest report from the International Diabetes Federation Diabetes Atlas stated a global diabetes prevalence of 10.5% in 2021 with the expected incidence to reach 12.2% in 2045. By comparing to the 2019 report, which stated a 9.3% global incidence of diabetes with a 2045 rate projection of 10.9%, the data suggest an exaggerated increase in diabetes prevalence worldwide[1,2]. Diabetes is associated with many complications that are commonly encountered in health care facilities, especially cardiovascular disease, retinopathy, neuropathy, nephropathy, and lower limb infections in addition to the high risk of amputations and systemic infections that are linked to high mortality rate[3,4]. Diabetic foot ulcer is a serious condition characterized by chronic lower limb wound that is often complicated by disseminating polymicrobial infections that can affect the underlying bone tissues. Diabetic foot infection (DFIs) require careful attention from health care providers regarding the proper diagnosis of the wound level and prompt management including debridement procedures, antimicrobial treatments, and follow-up of the wound healing process[5-7].
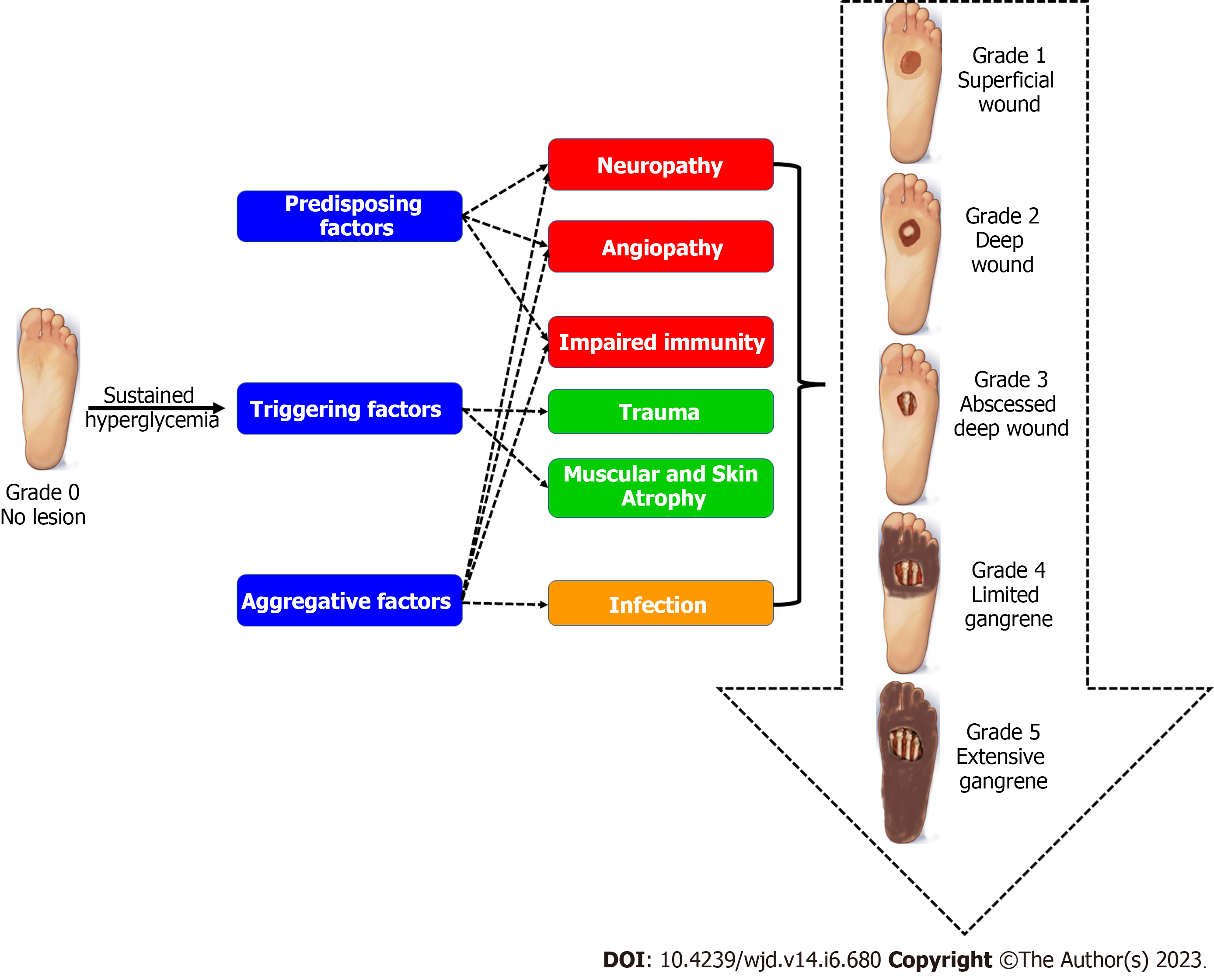
During the examination of the diabetic foot wound, the accurate evaluation of the wound plays a pivotal role in the proper management selection. Usually, the wound examination should include specimen collection from the deepest parts of the wound in order to identify the associated etiologic pathogens, accompanied by inspection of the underlying vascular and bone tissues. The Meggitt-Wagner guide is a commonly used system for classification of the DFI based on three parameters: the depth of the ulcer; the infection level; and the degree of necrosis. The guide classifies the DFI into five main categories, which are outlined in Figure 1. A progressive DFI needs immediate management in order to minimize the risk of bone infection and osteomyelitis, which are common complications in 50%-60% of severe infections and associated with a high risk of limb amputations[8,9]. In this review, we discussed the most common pathogens related to DFIs along with the associated virulence factors and possible treatment options for eradication of the infection and subsequent minimization of comorbidities and mortality rates.
Impaired immune functions represent a defining element in diabetes that impacts both innate and adaptive immunity. The innate immunity is the first line defense against pathogens and foreign particles. The response is mediated through phagocytes, natural killer cells, and inflammation[10]. Diabetes is associated with elevated levels of tumor necrosis factor α, macrophages, and inflammatory cytokine release that predisposes patients to chronic inflammation and increased pathogenicity of infections[11]. Additionally, diabetes is associated with an impaired number and functioning of natural killer cells with high connectivity to autoimmune diseases and increased risk of cardiovascular disease, malignancy, and susceptibility to infection[12]. On the other hand, the decreased number and function of dendritic cells results in impaired antigen presenting function and subsequently deterioration of the function of adaptive immunity[12]. Likewise, diabetes is associated with marked suppression in release of interleukin 6, decreased antibody production, decreased effector T cell development, and impaired leukocyte recruitment, all of which are considered important mediators of the adaptive immune response against pathogens[10,13].
Elevated blood glucose level is the main symptom of diabetes; failing to control blood glucose levels in diabetic patients will cause serious complications as a result of alterations in multiple metabolic pathways[14]. The high blood glucose level results in activation of the polyol pathway, increased glycation of end products, and eventually boosted release of reactive oxygen species and nitric oxide that contribute to oxidative stress and inflammation[15]. Hyperglycemia also contributes to immuno
As mentioned earlier, persistent hyperglycemia results in overproduction of reactive oxygen species and superoxides especially peroxynitrite leading to increased nitrosylation and eventually causing endothelial dysfunction, vasoconstriction, and platelet aggregation. In addition, the diabetic proinflammatory environment results in vascular inflammation and proliferation of vascular smooth muscles predisposing to atherosclerosis and atherothrombosis[20]. Some of the common vasculopathy presentations in diabetic patients involve peripheral artery diseases giving way to peripheral cramps, numbness, discoloration of limbs, weak pulse in the affected limb, and critical limb ischemia[21]. Peripheral ischemia results in delayed wound healing and tissue necrosis as a result of a decreased supply of oxygen, nutrients, and immune cells; in addition, the reduced tissue perfusion would limit the delivery of antibodies and antibiotics. A combination of all the preceding factors would result in an environment that favors microbial proliferation at the injured tissues, which supports the development of chronic diabetic foot ulcers[22].
Diabetic neuropathy is a neurodegenerative disorder that affects the peripheral sensory nervous system in 50% of cases. The condition is characterized by pain, numbness, and loss of sensory function that begins in the lower extremities[23]. Again, hyperglycemia along with the associated inflammation and oxidative stress play the lead role in the mechanisms predisposing to diabetic neuropathy, where Schwan cells and the myelin sheath are the first affected resulting in delayed signal transmission and eventually neuron dysfunction especially in distal terminals of motor nerve axons[23,24]. Diabetic neuropathy contributes to increased risk of infection in diabetic patients through inhibition of local vasodilation of the microcirculation at the affected tissues, which is a normal response to injury or inflammation; the reduced vasodilation results in reduced local blood flow and further promotes local ischemia[25]. On top of that, the loss of sensory nervous function will impair pain sensation, thus diminishing the ability of the patient to sense or detect wounds and injuries in peripheral tissues especially toes and foot soles, which in turn leads to delayed response and management of the condition and increasing the risk of amputation[26]. Peripheral neuropathy is a common manifestation in 90% of hospital admissions of diabetic foot ulcers; in addition, 14%–24% of people with a diabetic foot ulcer will ultimately undergo an amputation procedure with subsequent high mortality rate[24].
Adhesins are fine protein extensions expressed on the bacterial cell surface usually represented by a small protein subunit at the tip of the fimbriae. Their primary function is to facilitate the attachment or adherence of bacteria to host cells, which is the first step in initiation of an infection[27,28]. Adhesins also play a pivotal role in establishment of biofilms. This fact was proven by many studies that reported that biofilm formation can be completely blocked by downregulation of pili expression or by using adhesins antibodies that can drastically inhibit bacterial attachment to the target tissues, hence inhibiting subsequent initiation of infection and biofilm formation[29,30]. Some adhesins are called hemaglutinins due to their ability to induce the agglutination and hemolysis of red blood cells. Hemaglutinins contribute to localized destruction of red blood cell (RBCs) and release of iron, which is an essential nutrient requirement for most pathogenic bacteria[31]. Additionally, bacterial adhesins play an important role in intracellular bone invasion as observed in the ability of S. aureus to invade osteoblasts and fibroblasts, which contribute to serious complications of diabetic foot ulcer as well as increased risk of amputation[32].
Biofilm formation represents an important virulence factor that plays a leading role in the persistence and recurrence of diabetic foot ulcers. Biofilms are closed microbial communities embedded in a mucoid extracellular polymer matrix consisting of a wide range of molecules including polysaccharides, proteins, glycoproteins, glycolipids, cell debris, wastes, and surfactants[33,34]. These molecules provide high viscosity to the biofilm matrix acting as a physical protective barrier that prevents penetration of host immune defenses as well as antimicrobial treatments[35]. In addition, diabetic patients suffer from reduced peripheral blood supply, which makes the it even harder for the immune system and antibiotic treatments to eradicate biofilms in DFIs[36].
Within the biofilm, bacteria can coordinate their behavior using a communication system called quorum sensing (QS). This system is activated once the bacterial population reaches a certain threshold level beyond which the members of the biofilm initiate a coordinated group response that favors the public interests of the biofilm community; this coordinated activity aims at conserving energy and nutrients by reducing the metabolic activity of biofilm inhabitants[37-39]. Additionally, bacterial gene expression is directed towards increased expression of virulence factors especially extracellular toxins, which initiate extensive tissue destruction at the biofilm site; this ensures generous release of nutrients from the damaged tissues as well as facilitating the spread of infection to adjacent tissues, which further cements the biofilm and increases its persistence[40,41].
Another important feature of biofilms is the shift in bacterial phenotypes within the biofilm community towards the formation of persister cells that are inherently resistant to eradication by antimicrobial agents. Persister cells are dormant slow-growing cells with altered metabolic pathways that result in loss of the target site of most antibiotic treatments hence contributing to persistence and recurrence of biofilm ulcers[42]. At the same time, the high bacterial population within the biofilm results in an increased rate of horizontal gene transfer (HGT) between biofilm inhabitants, creating a rich pool of characteristics that eventually lead to natural selection of virulence genes and antimicrobial resistance genes[43]. Indeed, it was reported by many studies that biofilm formation is highly linked to an increased rate of antimicrobial resistance in DFIs, which contributes to a high incidence of chronic recurrent ulcers and higher risk of amputations[36,44].
Enzymes like proteases, collagenase, hyaluronidase, lipases, fibrinolysin, gelatinase, and elastase are all upregulated in diabetic foot biofilms under control of QS[45-48]. Such enzymes play an important role in inducing tissue damage, which helps in the release of nutrients that are required by the pathogens for growth[49-51]. Additionally, the vascular tissue damage would diminish tissue perfusion and contribute to the reduced ability of the immune system and antibiotic treatments to reach the site of the infection[52]. At the same time, the destroyed physical integrity of the tissues facilitate invasion of adjacent tissues and spread of the infection. Moreover, proteases result in delayed healing of the affected tissues, which further contributes to the chronic nature of diabetic foot ulcers[16,49]. Immunoglobulin proteases represent a different category of proteases that target humoral components of immune defense (mainly immunoglobulin A, immunoglobulin M, and immunoglobulin G) rather than inducing generalized tissue damage[53,54]. Immunoglobulin proteases represent an important virulence factor in many pathogens that allows them to evade the host immune response[55,56]. Local therapy with protease inhibitors is an essential element in control of diabetic foot ulcer in order to improve wound healing and minimize the complications accompanying chronic wounds[49,57].
Hemolysins and leukocidins belong to a group of pore-forming toxins that destroy blood cells by inducing perforation in the cell membrane and subsequent cell lysis[58-60]. Hemolysins are important virulence factors in pathogenic infections since they induce RBC lysis and release of iron, which is an essential nutrient requirement for pathogens. Iron is an important element for life since it is required for making important enzymes in all living cells[36,59,61,62]. However, iron is never found in a free form in biological tissues or in the extracellular fluids; the ability of most pathogens to survive in an iron-free environment highly depends on its iron acquisition talents including hemolysin and siderophore production[63].
S. aureus is one of the most common causative agents of DFIs. S. aureus is equipped with an arsenal of toxins including four hemolysins targeting a wide range of host cells: α-hemolysin (mainly targeting lymphocytes and monocytes); β-hemolysin (targeting human monocytes and sheep erythrocytes with no effect on human erythrocytes); γ-hemolysin (highly toxic to neutrophils ); and δ-hemolysin (toxic to erythrocytes). The combined actions of these toxins result in RBC hemolysis as well as inhibition of leukocyte function and subsequent evasion of host immune defenses[64,65].
Antimicrobial resistance is an escalating worldwide problem with increased prevalence among diabetic patients. As discussed previously, diabetic patients are at high risk of contracting microbial infections especially due to their immunocompromised status, which leads to higher rates of persistent difficult to treat infections, and such circumstances usually predispose to higher probability of development of antimicrobial resistance[66-68]. This relationship can be explained based on many factors: (1) The development of bacterial biofilms in chronic infections, like in cases of diabetic foot ulcers, is associated with activation of QS communication systems, which in turn induces upregulation of virulence gene expression including antimicrobial resistance genes[69-71]; (2) Bacterial biofilms are also associated with an increased rate of HGT between members of the biofilm community, which means increased rate of transfer of antibiotic resistance genes between different species within polymicrobial biofilm communities[72]; and (3) Chronic infections are usually associated with prolonged antimicrobial treatment courses, especially with broad spectrum antibiotics that exert stress pressure on pathogenic bacteria leading to natural selection of resistant strains[73,74].
Similarly, antibiotic self-administration and empirical prescription of broad-spectrum antibiotics by general practitioners are considered predisposing factors for higher rates of development of antibiotic resistance in diabetic patients[75-77]. One interesting observation was discussed in a previous study that reported a three-fold higher incidence of antibiotic resistance in diabetic foot patients in 2020 as compared with individuals admitted with the same diagnosis in 2019. The authors linked this observation to the circumstances that surrounded the coronavirus disease 2019 pandemic with increased administration of antibiotics for control of the infection complications, bearing in mind the fact that diabetic patients were among the high-risk categories at that point[78,79].
Additionally, some diabetic foot ulcers can result from impaired healing of wound tissues rather than the presence of wound infection. Therefore, it is highly recommended to avoid empirical antibiotic treatments before confirming the presence of an infection in diabetic foot ulcers. In addition, antibiotic therapy should not be given for uninfected foot wounds as prophylaxis against infection or as a method to improve wound healing[50,80]. Instead, it is advised to collect wound samples or swabs for microbiological examination in order to confirm the presence or absence of infection. This also allows for identification of the causative pathogen in cases of confirmed infection as well as performing antimicrobial susceptibility testing in order to identify the optimum antimicrobial treatment for every individual case[81,82].
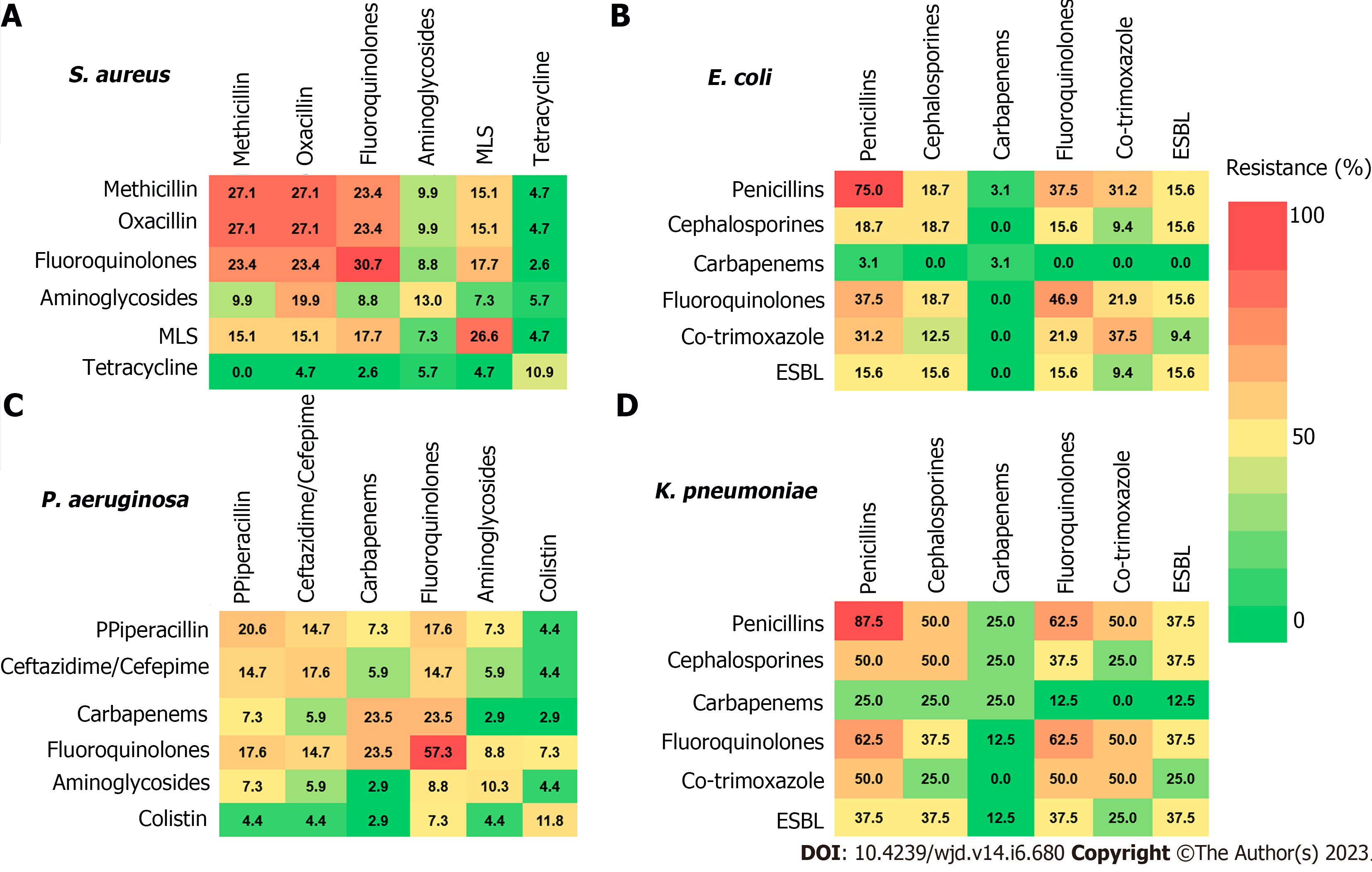
On a similar basis, the administration of broad-spectrum antibiotics for recurrent episodes of diabetic foot ulcers is not required, as recommended by a recent study that concluded that a patient history of previous DFIs does not necessarily reflect a higher risk of antibiotic resistance in subsequent episodes[83]. Boschetti et al[84] documented the resistance patterns of the most prevalent bacterial species isolated from DFIs to different classes of antibiotics when administered as a monotherapy or as a combination treatment. The results presented in Figure 2 provide an alarming outlook at the dangerous growing levels of antimicrobial resistance in many antimicrobial groups[84].
The dwindled immunity of the diabetic patients paves the way for easy contraction of opportunistic pathogens from the patient’s environment, leading to high risk of the progression of minor foot injuries into life-threatening infections[85,86]. The Meggit-Wagner system is the most commonly used classification guide of DFIs that assesses the ulcer depth, the presence of osteomyelitis, and/or gangrene using an ascending level from 0 to 5[87,88]. The more aggressive pathogenic bacterial infections are usually denoted by a higher level number[85,89,90]. There are multiple variables contributing to the establishment and progression of the infection, mainly: (1) Host response; (2) Ulcer location; (3) Tissue perfusion; and (4) Ulcer depth[87,91,92]. Upon trying to identify the etiologic agents behind DFIs, it is hard to name one exclusive pathogenic agent since DFIs are always caused by polymicrobial infections[90,93,94].
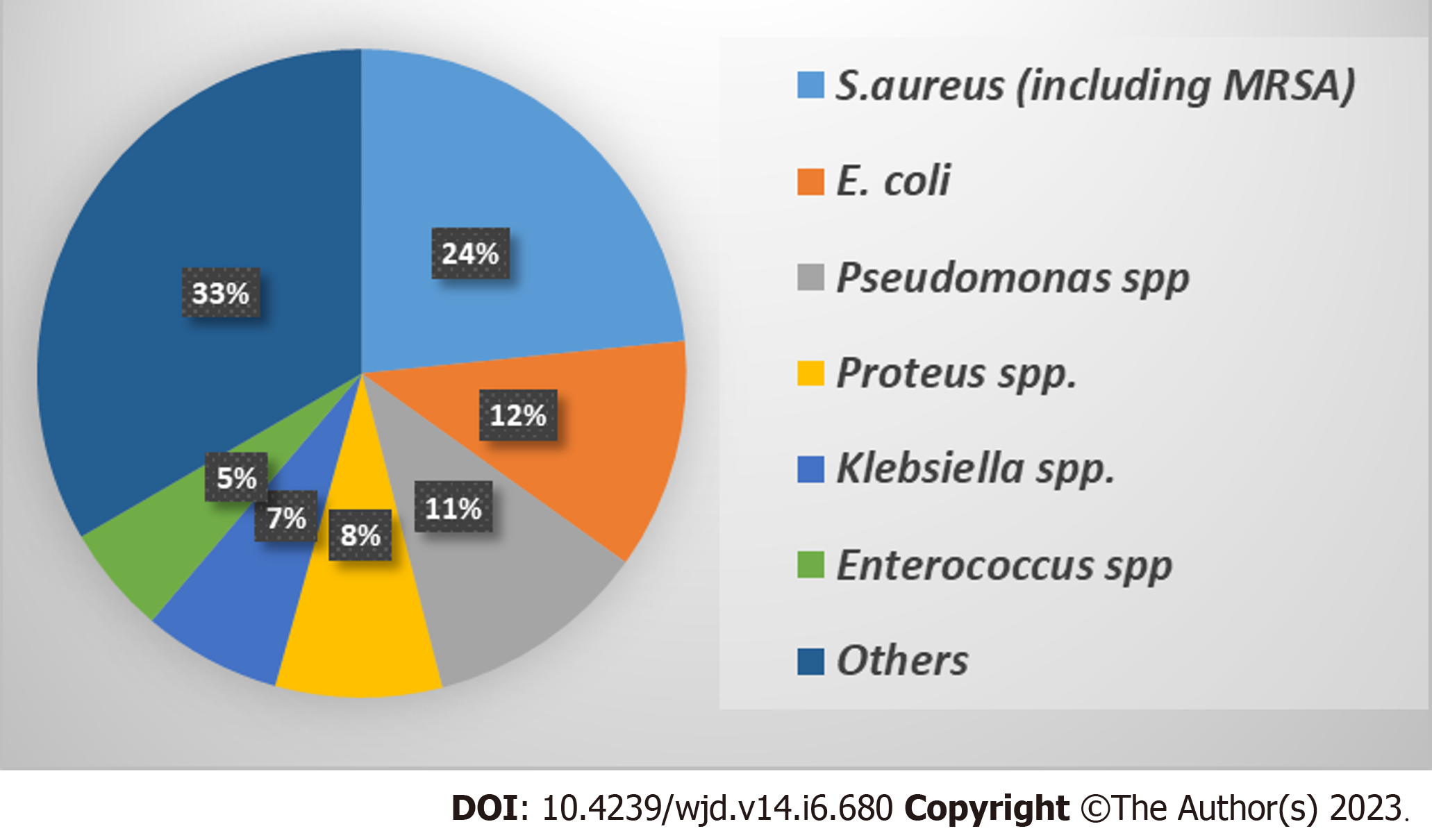
It is noteworthy that the polybacterial nature of DFIs makes the identification of different bacterial species a difficult task and mandates the application of both phenotypic and genotypic detection methods[91,95]. Several studies documented that the most prevalent bacterial species isolated from DFIs are S. aureus, Escherichia coli (E. coli), P. aeruginosa, Proteus spp., Klebsiella spp., and Enterococcus spp. with variable prevalence rates that are presented in Figure 3[32,96]. The following section shed light on the most prevalent Gram-negative and Gram-positive bacterial DFIs especially those isolated from deep wounds with higher Wagner grades.
Staphylococcus spp. are Gram-positive cocci that are ubiquitous in the environment. They are divided into pathogenic S. aureus and opportunistic coagulase negative Staphylococcus spp.[97-99]. However, the coagulase negative Staphylococcus spp. (S. epidermidis, S. saprophyticus, and others) are prevalent in the normal skin flora and could cause aggressive opportunistic infections in diabetic foot wounds[97,100]. S. aureus is considered by far the most commonly isolated species from macerated DFI especially in wounds of higher Wagner grade. It accounts for 20%-25% of all isolated bacteria[86,88-90,92]. The predominance of S. aureus in diabetic foot wounds can be attributed to: (1) Their ubiquitous presence in the environment; (2) The high ability of S. aureus to survive and resist bactericidal agents especially in healthcare settings giving rise to nosocomial infections; (3) A robust arsenal of virulence factors that facilitates anchoring of S. aureus infection; (4) The significantly high biofilm forming ability of S. aureus; and (5) The especially high rate of HGT between S. aureus and other members of a polymicrobial population leading to an increased ability of S. aureus to gain antibiotic resistant genes[85,86,88,90,91,94,95,101-103]. S. aureus has a collection of different virulence factors including the production of diverse extracellular enzymes such as coagulase, gelatinase, hemolysins, and proteases in addition to a cocktail of toxins, such as pore-forming toxins, α-toxin, exfoliative toxin, enterotoxin, toxic shock syndrome toxin, and the virulent pigment staphylolysin[32,95,97,98].
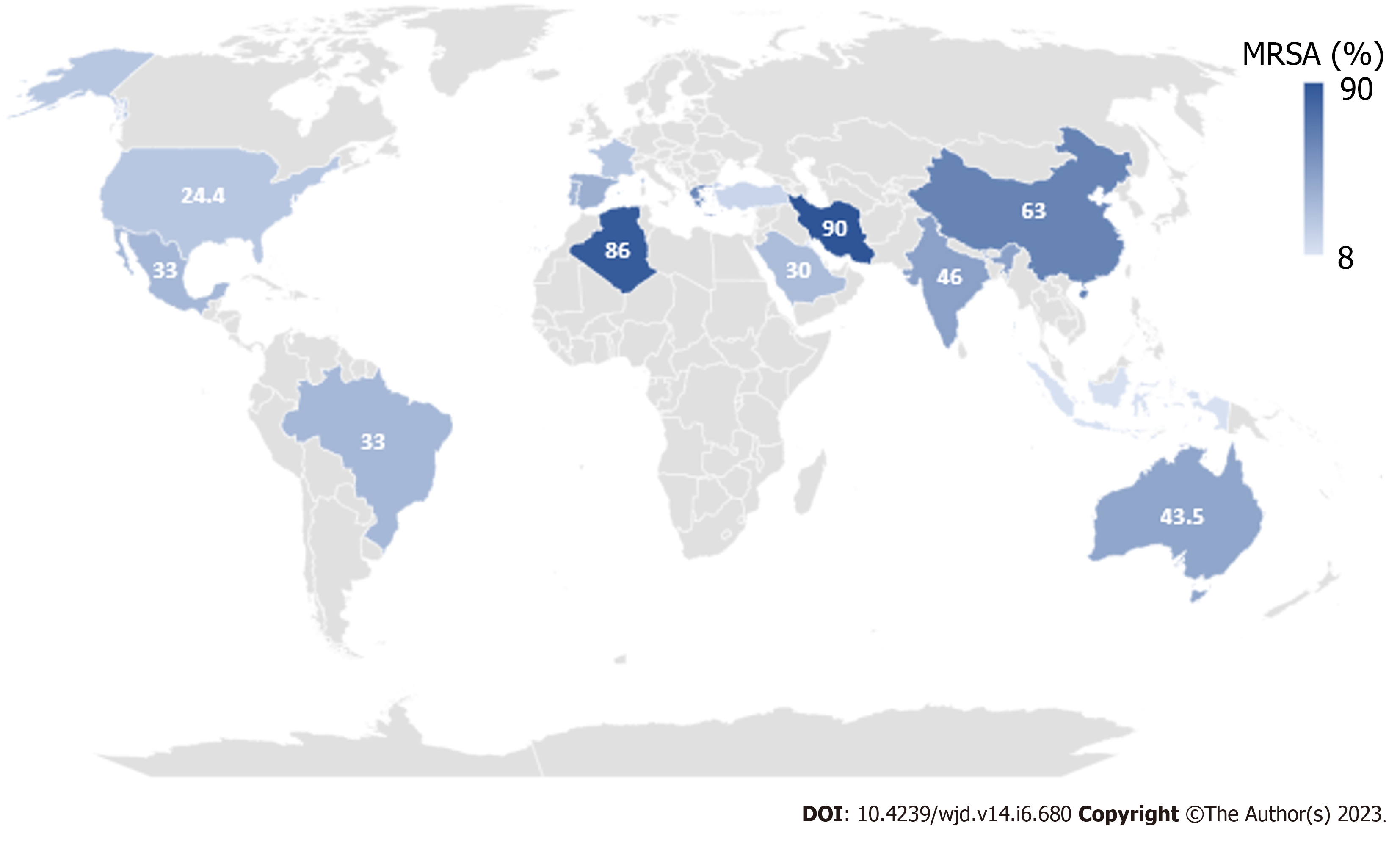
The recent increase in the rates of antibiotic resistance patterns requires careful attention during the choice of a proper antimicrobial treatment. Methicillin-resistant S. aureus (MRSA) is a problematic pathogen that continues to grow as a public health concern[95,101,102]. Unfortunately, several studies have reported an increased rate of MRSA in polymicrobial DFIs as demonstrated in Figure 4[85,88,94,95,102-135]. Although the complete identification of the full bacterial spectrum in a DFI is sometimes difficult, the detection of MRSA can be easily confirmed using the Kirby-Bauer antibiotic disk method in addition to genotypic detection methods[91,95]. Generally, vancomycin has been and still is the pillar therapy for MRSA. However, there is a growing mass of evidence that the minimum inhibitory concentrations of vancomycin to MRSA are increasing globally[106].
E. coli is one of the most common causative pathogens of DFIs with a high incidence of biofilm formation[96] E. coli is also considered one of the most common causes of Gram-negative bacteremia in hospitalized patients[34,136]. E. coli is an opportunistic pathogen that is a common member of the human skin and colon flora[137]. The initiation of a pathogenic lifestyle in E. coli infection benefits from multiple virulence factors that allow for colonization and tissue destruction at different body organs especially in immunocompromised individuals. E. coli adhesins, mainly type 1 fimbriae and P fimbriae, are important virulence factors that are essential for adhesion and initiation of the infection[138,139]. Additionally, adhesins play an important role in diabetic foot pathogenesis due to their role in cytokine induction, tissue inflammation, and biofilm initiation[138]. E. coli also secretes hemolysin and siderophores that induce RBC damage and subsequent iron acquisition from the damaged tissues[140]. Importantly, many studies have confirmed a positive correlation between the hemolytic activity, biofilm formation, and high levels of antimicrobial resistance in E. coli infections[141,142].
P. aeruginosa is a Gram-negative bacillus that is characterized with an armory of virulence factors including multiple bacterial surface structures such as pili and flagella in addition to a diverse array of extracellular toxins[143-145]. The observed prevalence of P. aeruginosa in DFIs is fluctuating from high to moderate levels, yet it is still among the most prevalent bacterial infections in DFIs[83,86,90,93,96,107,125,131,146-149]. P. aeruginosa employs five secretion systems (T1SS, T2SS, T3SS, T5SS, and T6SS) that are used to regulate bacterial survival and utilized in establishment of infection[143,145]. Additionally, P. aeruginosa has at least three types of QS communication systems that orchestrate the expression of several virulence factors such as biofilm formation, motility, resistance to host immunity, and production of extracellular toxins such as protease, lipase, hemolysin, elastase, and pyocyanin pigments[150].
Furthermore, P. aeruginosa has a remarkable ability to acquire antibiotic resistance against most of the commonly used antibiotics, making its eradication a difficult task[143,146,149]. P. aeruginosa can easily establish an infection on intact healthy skin[147,148] and even more so on already vulnerable tissues in immunocompromised patients such as in diabetic foot wounds[146,148,149]. The guidelines provided by the American Infectious Diseases Society for DFIs state that empiric therapy directed against P. aeruginosa is usually not recommended[147,149]. However, once the infection is identified, it is recommended to perform antibiotic susceptibility tests of the bacterial isolates[151-153]. There are several classes of antibiotics that are proposed as a monotherapy or as a combination therapy for eradication of P. aeruginosa in DFIs, including fluroquinolones, aminoglycosides, and colistin[83,84,143,147,151,152].
Proteus mirabilis (P. mirabilis) is a Gram-negative bacterium that is famous for its swarming motility and its remarkable survival in challenging environmental conditions[154,155]. The ability of P. mirabilis to initiate a pathogenic infection depends on multiple virulence factors such as multiple types of fimbriae and adhesins that allow attachment to different surfaces, giving rise to the remarkable stickiness and biofilm-forming ability of the bacterium onto many surfaces and at different conditions[156]. Additionally, P. mirabilis secretes a lethal cocktail of extracellular toxins including proteases, hemolysin, and urease, which all contribute to the extensive tissue damage and inflammation at the infection site[157]. Another significant feature of P. mirabilis is the formation of robust biofilms that are highly adhesive and persistent. Moreover, the biofilm formation in P. mirabilis is highly associated with increased rates of antimicrobial resistance and increased expression of toxins[155]. The combination of the aforementioned factors makes P. mirabilis a problematic pathogen in DFIs especially chronic ulcers.
Klebsiella pneumonia (K. pneumonia) is a Gram-negative bacterium that is commonly isolated from chronic wound infections especially in immunocompromised individuals[158,159]. K. pneumonia is known for its high adhesiveness as a result of its thick polysaccharide capsule that is enriched with type 1 and type 3 pili. The polysaccharide capsule in K. pneumoniae consists of two fibrous layers: An inner thick densely packed fibrous layer and an outer layer in which the fibers are loosely packed and become finer outwards, forming a fluffy network on the capsule surface[160,161]. This structure plays a leading role in the remarkable adhesiveness of the bacterium onto mucus membranes and inanimate surfaces followed by fast accumulation of bacteria as a result of entangled fibrous polysaccharide capsules of adjacent bacterial cells and subsequently rapid biofilm formation[161,162]. The thickness of the fibrous capsule of K. pneumonia is known to be one of the thickest protective bacterial coats, which imparts extra protection against host immune responses such as phagocytosis and serum complement deposition. In addition, its thick compact nature reduces the penetration of antibiotics and bacteriophages[163,164]. The overall result of the aforementioned factors is the formation of a highly adhesive biofilm that is resistant to immune defenses and antibiotic treatments and makes K. pneumonia challenging to eradicate in healthcare facilities, contributing to the high incidence of nosocomial infection associated with this pathogen especially in immunocompromised individuals and diabetic patients[165,166]. It is noteworthy that both K. pneumonia and P. mirabilis are linked to an increased risk of ascending urinary tract infections in diabetic foot patients as a result of self-infection[167,168].
Enterococci are facultative anaerobic Gram-positive cocci; there are two species considered the most common commensal organisms in the intestines of humans: Enterococcus faecalis and Enterococcus faecium[169,170]. Enterococci are opportunistic pathogens, commonly responsible for surgical wound infections, urinary tract infections, endocarditis, and intra-abdominal and pelvic infections among many others[171,172]. Enterococci are well adapted for withstanding harsh environmental conditions. This enables them to survive routine disinfection methods resulting in high persistence of these bacteria on inanimate surfaces in healthcare settings making them common causative agents of nosocomial infections[172]. It is widely documented that Enterococci are among the most prevalent bacterial infections in DFIs[96,117,121,122,124,125,173,174]. Interestingly, Enterococci are not considered true pathogens; their abundance in the gut flora provides them the opportunity to interact with other bacteria increasing the possibility of acquiring virulence genes and antimicrobial resistance genes[171,172]. Lately, there has been an alarming increase in antimicrobial resistance patterns of Enterococci, especially associated with hospital-acquired infections affecting immunocompromised patients including DFIs[174]. Unfortunately, many studies reported an increase in the mortality rates related to the emergence of vancomycin-resistant Enterococci that are usually linked to hospital-acquired infections[170,171,173]. The current antibiotic choice regimen for control of stubborn multidrug resistant enterococcal DFIs includes antibiotic combinations of β-lactams, aminoglycosides, and fluoroquinolones[171,174].
As explained previously, antibiotic treatment should only commence after the confirmation of the presence of an infected wound. However, broad-spectrum antibiotics are typically used during routine care of progressive diabetic foot wounds as an empiric treatment until microbiology culture results are available. Then the treatment should be switched to targeted antimicrobial therapy[175]. Ideally, narrow spectrum antibiotic treatment is preferred in order to avoid antibacterial resistance. Additionally, the treatment should be used for the shortest duration possible in cases of mild and medium diabetic wound infections: For 2-4 wk for progressive wounds and up to 6 wk in cases of osteomyelitis. If the treatment is not effective then the case should be re-evaluated regarding the antibiotic choice[176,177].
The Infectious Diseases Society of America provides a detailed description of antibiotic choices regarding DFIs. However, the report highlights the absence of a single recommended antimicrobial regimen. Instead an appropriate regimen should be designed based on the results of antibiotic susceptibility testing, severity of the infection, possible side effects, price, interactions with other drugs, and other patient related factors. The report recommends including suitable coverage of Gram-positive cocci (mainly S. aureus and Streptococcus spp.) in empiric treatment protocols. For mild DFIs, the choices include: clindamycin, levofloxacin, and β-lactamase inhibitor combinations. For moderate to severe infections the antibiotic options are extended to include ertapenem, tigecycline, piperacillin-tazobactam combination, and imipenem-cilastatinb combination with the latter showing especially broad spectrum activity. An anti-MRSA agent should be included in the regimen choice in cases of severe infections or previously confirmed MRSA infection. The suggested anti-MRSA choices include: Vancomycin, linezolid, and daptomycin. However, these options are considered narrow spectrum activity, and they should be combined with other agents such as a fluoroquinolone, carpabenem, aztreonam, or piperacillin-tazobactam to increase the activity spectrum especially in severe progressive infections[50,176].
The fierce increase in antibiotic resistance rates continues to be a growing worldwide crisis, which results in gradual erosion of the list of treatment options available for eradication of multidrug resistant infections, especially DFIs. For example, vancomycin, which is one of the last resort antibiotics that should be conserved for treatment of MRSA, has shown an alarming increase in resistance rates in the last decade[178,179]. Linezolid is considered an effective vancomycin alternative acting against both vancomycin-resistant S. aureus and MRSA. Linezolid showed good tissue and bone penetration and sufficient in vivo anti-MRSA activity in DFIs, even in cases of blood flow impairment[180,181]. However, linezolid suffers from serious side effects and high toxicity in cases of prolonged treatments. In addition it is not acknowledged by the United States Food and Drug Administration (FDA) for treatment of osteomyelitis[50,182]. Daptomycin, on the other hand, is approved for intravenous treatment for MRSA in DFIs[106,183]. Additionally, it has a lower side effect profile and promising activity against both MRSA and vancomycin-resistant S. aureus that is accompanied by low rates of bacterial resistance development[184,185].
Streptogramins combination of quinopristin and dalfopristin represent another promising alternative treatment of MRSA, which inhibits both the early and the late protein synthesis stages showing significant activity against nosocomial MRSA isolates[186,187]. Tigecycline is a tetracycline derivative that has potent in vitro activity against MRSA[186]. However, a Phase III randomized, double-blinded clinical trial showed that tigecycline is significantly less effective and associated with more adverse effects than ertapenem in achieving clinical resolution of DFIs even in presence of osteomyelitis[188]. Ceftobiprole is a fifth generation cephalosporin that is approved for intravenous administration. Ceftobiprole was compared to vancomycin in a multicenter, multinational, double‐blind, randomized trial concerning DFIs caused by Gram‐positive bacteria. The rates for complete eradication of MRSA in infected patients using ceftobiprole and vancomycin as antimicrobial treatment were 92% and 90%, respectively. In DFI patients, the clinical recovery rate with ceftobiprole monotherapy was 86%, which is as effective as the combination of vancomycin plus ceftazidime[189].
Ceftaroline is another novel cephalosporine that showed significant activity against MRSA. In two randomized, observer‐blinded studies to evaluate the efficacy of ceftaroline vs standard therapy with vancomycin in combination with aztreonam in adults, the clinical cure rates were comparable (about 86% in both treatments). Importantly, the adverse effects were similar in different treatment groups with a safety similar to that of the cephalosporins[190]. That being said, it is important to bear in mind that any novel antimicrobial treatment, no matter how effective it is against multidrug resistant pathogens, will eventually join the list of ineffective treatments as a result of the continuous evolution of bacterial resistance patterns, which is faster than our ability to develop and approve new alternative treatments.
Topical antimicrobial treatments of medium to severe DFI wounds are generally considered ineffective[191,192]. Antiseptics are generally applied during surgical debridement procedures and wound dressing changes. This is important to diminish further wound contamination that usually thrives on polymicrobial infections[193]. However, it should be noted that most antiseptics that affect the wound tissues subsequently leave a negative impact on the wound healing process. Furthermore, improper and excessive application of antiseptics can encourage antimicrobial resistance within the wound microenvironment, especially those containing polymicrobial biofilms, thus giving rise to delayed resolution of the infection and increased risk of complications[194]. Based on these considerations, international guidelines do not suggest antiseptics as in the management of DFI wounds[193]. However, several studies documented the in vitro effectiveness of iodine-based preparations and dressings containing polyhexamethylene biguanide or silver in controlling DFI wounds[195].
It is reported that biofilm formation within DFIs is likely to increase the incidence of antimicrobial resistance 100 to 1000 times[196], which mandates employment of efficient drug delivery systems to ensure better penetration of the biofilm matrix and higher recovery rates. Some drug delivery suggestions include calcium sulfate beads and antimicrobials immobilized on collagen sponges[196]. Some studies reported a new generation of anti-biofilm hydro-fiber dressings containing carboxymethylcellulose silver, which showed efficient disruption and removal the bacterial biofilms[197].
Another promising dressing was suggested by Yang et al[198]. It is a surfactant-based gel dressing that showed promising recovery rates when applied in vivo on wounds infected with P. aeruginosa. The results showed significant reduction in bacterial growth and disruption in the formed biofilms[198]. Another surfactant-based dressing containing Pluronic F127 in combination with melatonin and chitosan was used to diminish the bacterial growth and biofilm formation in S. aureus wound infection[199]. On a similar basis, other studies reported promising in vitro antibacterial, anti-biofilm, and healing results upon using wound dressings coated with Chitlac-silver nanoparticles combined with alginate and hyaluronic acid[200].
Other studies went as far as using dressings loaded with mesenchymal stem cells that also showed improved wound healing rates especially in chronic ulcers[201]. The combination of wound dressings with natural products have also been reported in some studies that showed the use of honey[202,203], cranberry extracts[204], tannic acid[205], tea-tree oil[206], and cinnamon oils[207] were linked to improved resolution and healing of DFIs.
Surgical debridement is classically used to remove necrotized and infected tissues from DFI wounds. This surgical intervention is routinely used in combination with antibiotics, to control the spread of infection allowing early closure of the wound[208]. The proper removal of infected tissues and bacterial biofilms optimizes the healing and regeneration of the wound tissues, which in turn improves blood flow and improves the effectiveness of the treatment[206]. In association with surgical debridement, negative pressure therapy is commonly employed to promote wound healing in DFIs[209]. Negative pressure is generated using a vacuum source connected to the wound, resulting in suction of cellular debris, diffuse toxins, and infected extracellular fluids that eventually reflects a positive impact on the resolution of the infection as well as wound healing progress[210].
Photodynamic therapy is a novel technology that is mainly used in oncology. The therapy depends on the use of a photosensitive agent that is activated by illumination to produce lethal oxygen species at the infection site. In a clinical trial, this method was employed for patients suffering from DFIs. The results showed that all the non-treated cases suffered from deterioration of the wound and eventually underwent amputation procedures in comparison to the treated group that showed only 1 case of amputation out of 18 patients who received the photodynamic therapy[211].
Hyperbaric oxygen therapy is another oxygenation-based approach in which pure oxygen is inhaled in a special compression chamber and increases oxygen supply all over the body, including the wound tissues. However, this therapy did not show beneficial results regarding short-term healing of DFI wounds[212].
The risk of amputation remains significantly high in progressive severe DFIs; such procedures are considered extreme treatment options that usually result in a drastic negative impact on the patient’s psychology and productivity in real life. There are numerous new approaches that address this problem by minimizing the need for amputations in severe DFIs. Some of these approaches are discussed in the following points.
Stem cell therapy: One method describes the use of stem cell technology to regenerate the vascular tissues in an ischemic limb, hence increasing blood supply and healing rates in severe DFIs and minimizing the risk of amputations. Additionally, stem cells can be directed towards the release of cytokines, which enhance immunity, cell recruitment, and regeneration of neurons. Similarly, progenitor stem cells can be employed since they have the potential to differentiate into various cell types such as endothelial cells, keratinocytes, pericytes, and myofibroblasts all of which play an effective role in DFI wound healing[213,214]. Stem cell-based therapy has been approved by the FDA as an effective interventional treatment strategy to treat DFI macerated wounds[213]. Secretome stem cells are derived from undifferentiated human mesenchymal endothelial stem cells; they have been successfully deployed for the treatment of the DFIs. It was shown that secretomes enhanced in vivo wound healing and increased the proliferation of endothelial cells via promotion of the production of a cocktail of vascular endothelial and fibroblast growth factors in addition to angiopoietins[215].
Growth factors: Other approaches are based on the fact that chronic wounds are associated with decreased levels of epidermal growth factor. Hence the application of hormonal growth factors will promote the proliferation and differentiation of fibroblasts, gliocytes, and neo-epidermal cells leading to improved healing rates[213,214]. Other growth factors that modulate signal transduction and replication of epidermal cells were also reported to improve wound healing in DFIs[213,216]. Similar results were obtained upon using granulocyte colony-stimulating factors and human platelet-derived growth factors, which are frequently used for the treatment of DFI wounds and neuropathic ulcers[213].
Skin substitute matrices: One example involves the use of keratinocytes and fibroblasts that are immobilized onto an extracellular matrix that functions as scaffold supports for the wound healing process[217]. Another example is shown by the use of neonatal foreskin equivalent to allogeneic cultured skin apligraf/graftskin. It was shown that this supportive tissue significantly improved the healing of chronic wound ulcers[218]. Dermagraft is an isolated neonatal human dermal fibroblast. Its application significantly improved the healing rates up to 30% in DFI wounds[219]. Furthermore, the allogeneic membranes obtained from human placenta have been employed successfully in the treatment of DFI wounds; such membranes provide growth factors, cytokines, and structural collagen support, which improved the repair of deteriorated tissues[220]. Furthermore, allografts from human skin such as GraftJacket were also reported as successful scaffolds for support of vascular and cellular growth in severe wounds[213].
Phage therapy: Phage therapy is an old method that is starting to gain renewed worldwide attention. The method is based on the use of bacteriophages, which are viruses that infect bacteria. Bacteriophages are considered the natural predator of bacteria that are abundant in nature[221,222]. Phage therapy usually uses a cocktail of bacteriophages to increase the host spectrum range[223]. In one in vitro study, a phage cocktail was designed to target S. aureus, P. aeruginosa, and Acinetobacter baumannii isolated from DFIs. The results showed significant antimicrobial and anti-biofilm activity of the tested bacteriophages[224]. These results were supported by case reports that encourage phage therapy for DFIs[225,226]. Examples of in vitro tested bacteriophages against the most prevalent bacterial species in DFIs are listed in Table 1.
| Agent | Target microbe | Ref. |
| Bacteriophages | ||
| vB_SauM_ME18 vB_SauM_ME126 | S. aureus | [246] |
| Bacteriophage K | S. aureus | [247] |
| pSp-J and pSp-S | Staphylococcus spp. | [248] |
| Staphylococcus bacteriophage K | S. epidermidis | [249] |
| Bacteriophage cocktail | P. aeruginosa | [250] |
| Pseudomonas Phage | P. aeruginosa | [251] |
| vB_EcoS-Golestan | E. coli | [252] |
| Lytic bacteriophage cocktail | P. mirabilis, E. coli | [253] |
| Bacteriophage cocktail | P. mirabilis | [254] |
| vB_PmiS-TH | P. mirabilis | [255] |
| PhiS1 | P. aeruginosa | [256] |
| PhiE2005-A | P. aeruginosa | [257] |
| Lytic bacteriophage | K. pneumonia | [258] |
| Anti-biofilm and Anti-virulence agents | ||
| Sitagliptin (anti-diabetic) | P. aeruginosa | [46,235,259] |
| S. aureus | [46] | |
| Linagliptin | P. aeruginosa | [238] |
| Metformin (anti-diabetic) | P. aeruginosa | [45,236] |
| Diclofenac (analgesic) | P. mirabilis | [239] |
| Metronidazole (antibacterial) | P. mirabilis | [260] |
| Fluoxetine (antipsychotics) | P. mirabilis | [261,262] |
| Thioridazine (antipsychotics) | ||
| Penfluridol (antipsychotics) | E. faecalis | [263] |
| Terazosin (adrenoreceptor blockers) | P. aeruginosa | [231,264] |
| Prazosin (adrenoreceptor blockers) | P. aeruginosa, P. mirabilis | [48,265,266] |
| Metoprolol (adrenoreceptor blockers) | P. aeruginosa, S. enterica | [233,267] |
| Atenolol (adrenoreceptor blockers) | P. aeruginosa, P. mirabilis | |
| Allopurinol (anti-gout) | P. aeruginosa | [47] |
| Azithromycin (antibiotic) | P. aeruginosa | [268] |
| Ciprofloxacin (antibiotic) | S. enterica | [269] |
| Resveratrol (anticancer) | S. aureus | [270] |
| E. coli | [271] | |
| Ribavirin (antiviral) | C. albicans | [272] |
| Theophylline (bronchodilator) | C. albicans | [273] |
| Stolon (fenugreek) | P. aeruginosa | [232] |
| Garlic extract | P. aeruginosa | [274] |
| Allicin (garlic) | P. mirabilis | [275] |
| Carvacrol (oregano) | P. aeruginosa | [276] |
| Emodin (Polygonum cuspidatum) | S. aureus | [277] |
| C. albicans | [278] | |
| Curcumin (curcuma) | Acinetobacter baumannii | [279] |
| C. albicans, P. mirabilis | ||
| Tannic acid | E. coli | [280] |
| Sodium citrate | P. aeruginosa | [281] |
| Isolimonic acid (citrus fruits) | E. coli | [282] |
| Zingerone (ginger) | P. aeruginosa | [283] |
The use of bacteriophages for treatment of pathogenic bacterial infections offers many advantages: (1) High specificity of action because bacteriophages are highly specific in selection of their host, which is usually limited to one species or even one specific strain within a species; (2) Can be used against multidrug resistant bacteria because bacteriophages use a pathway that is different from all antimicrobial treatments. Therefore, most resistance mechanisms will not affect the phage pathway; (3) Phages will only attack the target bacterial host leaving no effect on eukaryotic cells, which means localized activity at the infected tissues with minimal side effects; (4) Self-amplification of phages means that minimal doses will replicate exponentially at the infection site in relation to the wound infection burden; (5) High ability to penetrate deep tissues and bacterial biofilms, which further results in complete eradication of the infection; and (6) Minimal effect on the normal host flora[227]. On the other hand, there are limitations, mainly the lack of approval from the FDA and the need to formulate a phage cocktail that is based on accurate identification of polymicrobial infection members[227]. Moreover, it was observed that biofilm formation was induced by exposure to some phages[228,229].
Anti-biofilm and anti-virulence agents: Bacterial biofilms and bacterial virulence play major roles in the establishment and spread of DFIs. Anti-biofilm and anti-virulence agents are promising adjuvants to be used in combination with conventional antibiotic treatment of DFI wounds[206]. Bacteria employ several interplaying systems to control the expression of their virulence factors, most importantly the QS system. QS is used in both Gram-positive and Gram-negative bacteria to communicate between each other in an inducer-receptor manner[37,40,46]. Several approaches have been suggested to diminish the bacterial biofilm formation and virulence factor production based on targeting the QS systems[47,69,71]. QS inhibitors are known to reflect a significant reduction in bacterial virulence as well as reduced resistance development[230-234].
There are several chemical structures that have been screened for their anti-QS, anti-biofilm, and anti-virulence activities, with maximum attention given to the screening of already used and approved medications with the aim of using them for other applications than their originally intended use (Table 1). Some of the screened drug groups included several anti-diabetic agents. Fortunately, some anti-diabetics showed promising anti-QS, anti-virulence, and anti-biofilm activities. One promising example is the group of gliptins, which are dipeptidase inhibitors that are widely used as hypoglycemic agents. A detailed virtual study was performed to assess the anti-QS activity of some gliptins, mainly sitagliptin and linagliptin[46,235-238]. The results showed a significant ability to diminish biofilm formation is S. aureus and P. aeruginosa in addition to significant reduction in the expression of virulence factors such as protease, hemolysins, and other toxins[45,46,238]. There is a growing list of drug groups that are screened for their antibacterial and anti-QS activities, including analgesics and anti-inflammatory agents that are commonly used for symptomatic treatment of DFIs. Diclofenac is a commonly used anti-inflammatory agent that showed promising in vitro results regarding biofilm inhibition and downregulation of virulence factors in P. mirabilis isolates collected from deep DFIs[239]. There are many other drug groups and natural products that were screened for their anti-QS, anti-biofilm, and anti-virulence activities. Some of these agents are presented in Table 1.
There are other approaches that aim at inhibition of bacterial biofilm formation, for example chelation of essential metals, ethylene diamine tetra-acetic, and citrate[240]. Another approach is the use of enzymes for dispersion of bacterial biofilm, e.g., α-amylase[241], proteinase K, trypsin[206], deoxyribonuclease I, hydrolases, and DNase[241-243]. In addition, some synthetic chemical agents such as 2-aminoimidazole showed powerful anti-biofilm activity against S. aureus[244].
In another study published by Barki et al[245], wireless electroceutical dressings were used successfully for the eradication of P. aeruginosa and Acinetobacter baumannii biofilms in vivo. It was shown that the dressing disrupted the formed biofilms and accelerated wound healing. Furthermore, this treatment was found to downregulate the QS-encoding genes and restore the skin barrier function by silencing the proteins required for skin barrier function (E-cadherin)[245].
Diabetes and its complications represent a growing public concern worldwide. DFIs are considered one of the most commonly encountered problems at healthcare facilities. The management of DFIs are usually problematic due to many factors, including the reduced immunity in diabetic patients, the delayed wound healing, and the high incidence of a multidrug resistant polymicrobial infection. The delay or failure of treatment of DFIs will increase the risk of serious life-threatening complications such as amputations and systemic infections. There has been a global increase in the levels of bacterial resistance to antibiotics that reached a catastrophic level, especially with more and more antibiotics being added to the list of ineffective treatments. This has caused increased rates of mortalities caused by multidrug resistant infections. The proper selection of the antibiotic treatment course for DFI is crucial to avoid microbial resistance. Additionally, it is important to combine antimicrobial treatment with supportive therapy such as anti-biofilm agents, drug delivery systems, and rejuvenating dressings to ensure maximum outcomes of the treatment. In addition, the use of QS inhibitors will decrease the severity of the infection by downregulation of bacterial virulence factors, biofilm formation, and reduction of the incidence of antimicrobial resistance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mostafavinia A, Iran; Portillo R, Czech Republic S-Editor: Li L L-Editor: Filipodia P-Editor: Cai YX
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [PubMed] [DOI] [Full Text] |
| 2. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [PubMed] [DOI] [Full Text] |
| 3. | Alonso-Morán E, Orueta JF, Fraile Esteban JI, Arteagoitia Axpe JM, Marqués González ML, Toro Polanco N, Ezkurra Loiola P, Gaztambide S, Nuño-Solinis R. The prevalence of diabetes-related complications and multimorbidity in the population with type 2 diabetes mellitus in the Basque Country. BMC Public Health. 2014;14:1059. [PubMed] [DOI] [Full Text] |
| 4. | Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3-16. [PubMed] [DOI] [Full Text] |
| 5. | Amin N, Doupis J. Diabetic foot disease: From the evaluation of the "foot at risk" to the novel diabetic ulcer treatment modalities. World J Diabetes. 2016;7:153-164. [PubMed] [DOI] [Full Text] |
| 6. | Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017;110:104-109. [PubMed] [DOI] [Full Text] |
| 7. | Pourkazemi A, Ghanbari A, Khojamli M, Balo H, Hemmati H, Jafaryparvar Z, Motamed B. Diabetic foot care: knowledge and practice. BMC Endocr Disord. 2020;20:40. [PubMed] [DOI] [Full Text] |
| 8. | Giurato L, Meloni M, Izzo V, Uccioli L. Osteomyelitis in diabetic foot: A comprehensive overview. World J Diabetes. 2017;8:135-142. [PubMed] [DOI] [Full Text] |
| 9. | Lázaro Martínez JL, García Álvarez Y, Tardáguila-García A, García Morales E. Optimal management of diabetic foot osteomyelitis: challenges and solutions. Diabetes Metab Syndr Obes. 2019;12:947-959. [PubMed] [DOI] [Full Text] |
| 10. | Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125:S3-23. [PubMed] [DOI] [Full Text] |
| 11. | Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Full Text] |
| 12. | Lu ZH, Yu WL, Sun Y. Multiple immune function impairments in diabetic patients and their effects on COVID-19. World J Clin Cases. 2021;9:6969-6978. [PubMed] [DOI] [Full Text] |
| 13. | Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16:442-449. [PubMed] [DOI] [Full Text] |
| 14. | Giri B, Dey S, Das T, Sarkar M, Banerjee J, Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed Pharmacother. 2018;107:306-328. [PubMed] [DOI] [Full Text] |
| 15. | Graves DT, Kayal RA. Diabetic complications and dysregulated innate immunity. Front Biosci. 2008;13:1227-1239. [PubMed] [DOI] [Full Text] |
| 16. | Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals (Basel). 2020;13. [PubMed] [DOI] [Full Text] |
| 17. | Subbaram K, Ali PSS, Ali S. Enhanced endocytosis elevated virulence and severity of SARS-CoV-2 due to hyperglycemia in type 2 diabetic patients. Gene Rep. 2022;26:101495. [PubMed] [DOI] [Full Text] |
| 18. | Wei R, Wang X, Wang Q, Qiang G, Zhang L, Hu HY. Hyperglycemia in Diabetic Skin Infections Promotes Staphylococcus aureus Virulence Factor Aureolysin: Visualization by Molecular Imaging. ACS Sens. 2022;7:3416-3421. [PubMed] [DOI] [Full Text] |
| 19. | Thurlow LR, Stephens AC, Hurley KE, Richardson AR. Lack of nutritional immunity in diabetic skin infections promotes Staphylococcus aureus virulence. Sci Adv. 2020;6. [PubMed] [DOI] [Full Text] |
| 20. | Rahman S, Rahman T, Ismail AA, Rashid AR. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab. 2007;9:767-780. [PubMed] [DOI] [Full Text] |
| 21. | Mota RI, Morgan SE, Bahnson EM. Diabetic vasculopathy: macro and microvascular injury. Curr Pathobiol Rep. 2020;8:1-14. [PubMed] [DOI] [Full Text] |
| 22. | Güley O, Pati S, Bakas S. Classification of Infection and Ischemia in Diabetic Foot Ulcers Using VGG Architectures. Diabet Foot Ulcers Grand Chall (2021). 2022;13183:76-89. [PubMed] [DOI] [Full Text] |
| 23. | Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41. [PubMed] [DOI] [Full Text] |
| 24. | Hicks CW, Selvin E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr Diab Rep. 2019;19:86. [PubMed] [DOI] [Full Text] |
| 25. | Wukich DK, Crim BE, Frykberg RG, Rosario BL. Neuropathy and poorly controlled diabetes increase the rate of surgical site infection after foot and ankle surgery. J Bone Joint Surg Am. 2014;96:832-839. [PubMed] [DOI] [Full Text] |
| 26. | Shatnawi NJ, Al-Zoubi NA, Hawamdeh HM, Khader YS, Garaibeh K, Heis HA. Predictors of major lower limb amputation in type 2 diabetic patients referred for hospital care with diabetic foot syndrome. Diabetes Metab Syndr Obes. 2018;11:313-319. [PubMed] [DOI] [Full Text] |
| 27. | Chahales P, Thanassi DG. Structure, Function, and Assembly of Adhesive Organelles by Uropathogenic Bacteria. Microbiol Spectr. 2015;3. [PubMed] [DOI] [Full Text] |
| 28. | Askoura M, Almalki AJ, Lila ASA, Almansour K, Alshammari F, Khafagy ES, Ibrahim TS, Hegazy WAH. Alteration of Salmonella enterica Virulence and Host Pathogenesis through Targeting sdiA by Using the CRISPR-Cas9 System. Microorganisms. 2021;9. [PubMed] [DOI] [Full Text] |
| 29. | Chen Q, Xie S, Lou X, Cheng S, Liu X, Zheng W, Zheng Z, Wang H. Biofilm formation and prevalence of adhesion genes among Staphylococcus aureus isolates from different food sources. Microbiologyopen. 2020;9:e00946. [PubMed] [DOI] [Full Text] |
| 30. | Paharik AE, Horswill AR. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol Spectr. 2016;4. [PubMed] [DOI] [Full Text] |
| 31. | Hrv R, Devaki R, Kandi V. Comparison of Hemagglutination and Hemolytic Activity of Various Bacterial Clinical Isolates Against Different Human Blood Groups. Cureus. 2016;8:e489. [PubMed] [DOI] [Full Text] |
| 32. | Dunyach-Remy C, Ngba Essebe C, Sotto A, Lavigne JP. Staphylococcus aureus Toxins and Diabetic Foot Ulcers: Role in Pathogenesis and Interest in Diagnosis. Toxins (Basel). 2016;8. [PubMed] [DOI] [Full Text] |
| 33. | Vestby LK, Grønseth T, Simm R, Nesse LL. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics (Basel). 2020;9. [PubMed] [DOI] [Full Text] |
| 34. | Delcaru C, Alexandru I, Podgoreanu P, Grosu M, Stavropoulos E, Chifiriuc MC, Lazar V. Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies. Pathogens. 2016;5. [PubMed] [DOI] [Full Text] |
| 35. | Di Martino P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018;4:274-288. [PubMed] [DOI] [Full Text] |
| 36. | Pouget C, Dunyach-Remy C, Pantel A, Schuldiner S, Sotto A, Lavigne JP. Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms. 2020;8. [PubMed] [DOI] [Full Text] |
| 37. | Abisado RG, Benomar S, Klaus JR, Dandekar AA, Chandler JR. Erratum for Abisado et al., "Bacterial Quorum Sensing and Microbial Community Interactions". mBio. 2018;9. [PubMed] [DOI] [Full Text] |
| 38. | Mukherjee S, Bassler BL. Bacterial quorum sensing in complex and dynamically changing environments. Nat Rev Microbiol. 2019;17:371-382. [PubMed] [DOI] [Full Text] |
| 39. | Abbas HA, Hegazy WAH. Targeting the virulence factors of Serratia marcescens by ambroxol. Roumanian Archives of Microbiology and Immunology. 2017;76:27-32. |
| 40. | Jiang Q, Chen J, Yang C, Yin Y, Yao K. Quorum Sensing: A Prospective Therapeutic Target for Bacterial Diseases. Biomed Res Int. 2019;2019:2015978. [PubMed] [DOI] [Full Text] |
| 41. | Popoff MR. Bacterial Toxins, Current Perspectives. Toxins (Basel). 2020;12. [PubMed] [DOI] [Full Text] |
| 42. | Wood TK, Knabel SJ, Kwan BW. Bacterial persister cell formation and dormancy. Appl Environ Microbiol. 2013;79:7116-7121. [PubMed] [DOI] [Full Text] |
| 43. | Abe K, Nomura N, Suzuki S. Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol Ecol. 2020;96. [PubMed] [DOI] [Full Text] |
| 44. | Afonso AC, Oliveira D, Saavedra MJ, Borges A, Simões M. Biofilms in Diabetic Foot Ulcers: Impact, Risk Factors and Control Strategies. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Full Text] |
| 45. | Hegazy WAH, Khayat MT, Ibrahim TS, Nassar MS, Bakhrebah MA, Abdulaal WH, Alhakamy NA, Bendary MM. Repurposing Anti-diabetic Drugs to Cripple Quorum Sensing in Pseudomonas aeruginosa. Microorganisms. 2020;8. [PubMed] [DOI] [Full Text] |
| 46. | Khayat MT, Abbas HA, Ibrahim TS, Khayyat AN, Alharbi M, Darwish KM, Elhady SS, Khafagy ES, Safo MK, Hegazy WAH. Anti-Quorum Sensing Activities of Gliptins against Pseudomonas aeruginosa and Staphylococcus aureus. Biomedicines. 2022;10. [PubMed] [DOI] [Full Text] |
| 47. | Saqr AA, Aldawsari MF, Khafagy ES, Shaldam MA, Hegazy WAH, Abbas HA. A Novel Use of Allopurinol as A Quorum-Sensing Inhibitor in Pseudomonas aeruginosa. Antibiotics (Basel). 2021;10. [PubMed] [DOI] [Full Text] |
| 48. | Thabit AK, Eljaaly K, Zawawi A, Ibrahim TS, Eissa AG, Elbaramawi SS, Hegazy WAH, Elfaky MA. Muting Bacterial Communication: Evaluation of Prazosin Anti-Quorum Sensing Activities against Gram-Negative Bacteria Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens. Biology (Basel). 2022;11. [PubMed] [DOI] [Full Text] |
| 49. | McCarty SM, Percival SL. Proteases and Delayed Wound Healing. Adv Wound Care (New Rochelle). 2013;2:438-447. [PubMed] [DOI] [Full Text] |
| 50. | Ramirez-Acuña JM, Cardenas-Cadena SA, Marquez-Salas PA, Garza-Veloz I, Perez-Favila A, Cid-Baez MA, Flores-Morales V, Martinez-Fierro ML. Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics (Basel). 2019;8. [PubMed] [DOI] [Full Text] |
| 51. | Khayyat AN, Abbas HA, Khayat MT, Shaldam MA, Askoura M, Asfour HZ, Khafagy ES, Abu Lila AS, Allam AN, Hegazy WAH. Secnidazole Is a Promising Imidazole Mitigator of Serratia marcescens Virulence. Microorganisms. 2021;9. [PubMed] [DOI] [Full Text] |
| 52. | Małecki R, Klimas K, Kujawa A. Different Patterns of Bacterial Species and Antibiotic Susceptibility in Diabetic Foot Syndrome with and without Coexistent Ischemia. J Diabetes Res. 2021;2021:9947233. [PubMed] [DOI] [Full Text] |
| 53. | Askoura M, Abbas HA, Al Sadoun H, Abdulaal WH, Abu Lila AS, Almansour K, Alshammari F, Khafagy ES, Ibrahim TS, Hegazy WAH. Elevated Levels of IL-33, IL-17 and IL-25 Indicate the Progression from Chronicity to Hepatocellular Carcinoma in Hepatitis C Virus Patients. Pathogens. 2022;11. [PubMed] [DOI] [Full Text] |
| 54. | Hegazy WAH, Henaway M. Hepatitis C virus pathogenesis: Serum IL-33 Level indicates liver damage. Afr. J. Microbiol. Res.. 2015;9:1386-1393. [DOI] [Full Text] |
| 55. | Spoerry C, Hessle P, Lewis MJ, Paton L, Woof JM, von Pawel-Rammingen U. Novel IgG-Degrading Enzymes of the IgdE Protease Family Link Substrate Specificity to Host Tropism of Streptococcus Species. PLoS One. 2016;11:e0164809. [PubMed] [DOI] [Full Text] |
| 56. | Aldawsari MF, Alalaiwe A, Khafagy ES, Al Saqr A, Alshahrani SM, Alsulays BB, Alshehri S, Abu Lila AS, Danish Rizvi SM, Hegazy WAH. Efficacy of SPG-ODN 1826 Nanovehicles in Inducing M1 Phenotype through TLR-9 Activation in Murine Alveolar J774A.1 Cells: Plausible Nano-Immunotherapy for Lung Carcinoma. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Full Text] |
| 57. | Perez-Favila A, Martinez-Fierro ML, Rodriguez-Lazalde JG, Cid-Baez MA, Zamudio-Osuna MJ, Martinez-Blanco MDR, Mollinedo-Montaño FE, Rodriguez-Sanchez IP, Castañeda-Miranda R, Garza-Veloz I. Current Therapeutic Strategies in Diabetic Foot Ulcers. Medicina (Kaunas). 2019;55. [PubMed] [DOI] [Full Text] |
| 58. | Spaan AN, van Strijp JAG, Torres VJ. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol. 2017;15:435-447. [PubMed] [DOI] [Full Text] |
| 59. | Hu H, Liu M, Sun S. Pore-Forming Toxins During Bacterial Infection: Molecular Mechanisms and Potential Therapeutic Targets. Drug Des Devel Ther. 2021;15:3773-3781. [PubMed] [DOI] [Full Text] |
| 60. | Khayyat AN, Hegazy WAH, Shaldam MA, Mosbah R, Almalki AJ, Ibrahim TS, Khayat MT, Khafagy ES, Soliman WE, Abbas HA. Xylitol Inhibits Growth and Blocks Virulence in Serratia marcescens. Microorganisms. 2021;9. [PubMed] [DOI] [Full Text] |
| 61. | Askoura M, Youns M, Halim Hegazy WA. Investigating the influence of iron on Campylobacter jejuni transcriptome in response to acid stress. Microb Pathog. 2020;138:103777. [PubMed] [DOI] [Full Text] |
| 62. | Youns M, Askoura M, Abbas HA, Attia GH, Khayyat AN, Goda RM, Almalki AJ, Khafagy ES, Hegazy WAH. Celastrol Modulates Multiple Signaling Pathways to Inhibit Proliferation of Pancreatic Cancer via DDIT3 and ATF3 Up-Regulation and RRM2 and MCM4 Down-Regulation. Onco Targets Ther. 2021;14:3849-3860. [PubMed] [DOI] [Full Text] |
| 63. | Sheldon JR, Laakso HA, Heinrichs DE. Iron Acquisition Strategies of Bacterial Pathogens. Microbiol Spectr. 2016;4. [PubMed] [DOI] [Full Text] |
| 64. | Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol. 2012;2:12. [PubMed] [DOI] [Full Text] |
| 65. | Zhang H, Zheng Y, Gao H, Xu P, Wang M, Li A, Miao M, Xie X, Deng Y, Zhou H, Du H. Identification and Characterization of Staphylococcus aureus Strains with an Incomplete Hemolytic Phenotype. Front Cell Infect Microbiol. 2016;6:146. [PubMed] [DOI] [Full Text] |
| 66. | Ferlita S, Yegiazaryan A, Noori N, Lal G, Nguyen T, To K, Venketaraman V. Type 2 Diabetes Mellitus and Altered Immune System Leading to Susceptibility to Pathogens, Especially Mycobacterium tuberculosis. J Clin Med. 2019;8. [PubMed] [DOI] [Full Text] |
| 67. | Agha KA, Abo-Dya NE, Ibrahim TS, Abdel-Aal EH, Hegazy WA. Benzotriazole-Mediated Synthesis and Antibacterial Activity of Novel N-Acylcephalexins. Sci Pharm. 2016;84:484-496. [PubMed] [DOI] [Full Text] |
| 68. | Alshahrani SM, Khafagy ES, Riadi Y, Al Saqr A, Alfadhel MM, Hegazy WAH. Amphotericin B-PEG Conjugates of ZnO Nanoparticles: Enhancement Antifungal Activity with Minimal Toxicity. Pharmaceutics. 2022;14. [PubMed] [DOI] [Full Text] |
| 69. | Zhao X, Yu Z, Ding T. Quorum-Sensing Regulation of Antimicrobial Resistance in Bacteria. Microorganisms. 2020;8. [PubMed] [DOI] [Full Text] |
| 70. | Preda VG, Săndulescu O. Communication is the key: biofilms, quorum sensing, formation and prevention. Discoveries (Craiova). 2019;7:e100. [PubMed] [DOI] [Full Text] |
| 71. | Sionov RV, Steinberg D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms. 2022;10. [PubMed] [DOI] [Full Text] |
| 72. | Uruén C, Chopo-Escuin G, Tommassen J, Mainar-Jaime RC, Arenas J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics (Basel). 2020;10. [PubMed] [DOI] [Full Text] |
| 73. | Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisar MA, Alvi RF, Aslam MA, Qamar MU, Salamat MKF, Baloch Z. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645-1658. [PubMed] [DOI] [Full Text] |
| 74. | Dadgostar P. Antimicrobial Resistance: Implications and Costs. Infect Drug Resist. 2019;12:3903-3910. [PubMed] [DOI] [Full Text] |
| 75. | Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit Care. 2016;20:221. [PubMed] [DOI] [Full Text] |
| 76. | Strich JR, Heil EL, Masur H. Considerations for Empiric Antimicrobial Therapy in Sepsis and Septic Shock in an Era of Antimicrobial Resistance. J Infect Dis. 2020;222:S119-S131. [PubMed] [DOI] [Full Text] |
| 77. | Vishwa B, Moin A, Gowda DV, Rizvi SMD, Hegazy WAH, Abu Lila AS, Khafagy ES, Allam AN. Pulmonary Targeting of Inhalable Moxifloxacin Microspheres for Effective Management of Tuberculosis. Pharmaceutics. 2021;13. [PubMed] [DOI] [Full Text] |
| 78. | Caruso P, Maiorino MI, Macera M, Signoriello G, Castellano L, Scappaticcio L, Longo M, Gicchino M, Campitiello F, Bellastella G, Coppola N, Esposito K. Antibiotic resistance in diabetic foot infection: how it changed with COVID-19 pandemic in a tertiary care center. Diabetes Res Clin Pract. 2021;175:108797. [PubMed] [DOI] [Full Text] |
| 79. | Bendala Estrada AD, Calderón Parra J, Fernández Carracedo E, Muiño Míguez A, Ramos Martínez A, Muñez Rubio E, Rubio-Rivas M, Agudo P, Arnalich Fernández F, Estrada Perez V, Taboada Martínez ML, Crestelo Vieitez A, Pesqueira Fontan PM, Bustamante M, Freire SJ, Oriol-Bermúdez I, Artero A, Olalla Sierra J, Areses Manrique M, Carrasco-Sánchez HFJ, Vento VC, García García GM, Cubero-Morais P, Casas-Rojo JM, Núñez-Cortés JM. Inadequate use of antibiotics in the covid-19 era: effectiveness of antibiotic therapy. BMC Infect Dis. 2021;21:1144. [PubMed] [DOI] [Full Text] |
| 80. | Abbas M, Uçkay I, Lipsky BA. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin Pharmacother. 2015;16:821-832. [PubMed] [DOI] [Full Text] |
| 81. | Huang Y, Cao Y, Zou M, Luo X, Jiang Y, Xue Y, Gao F. A Comparison of Tissue versus Swab Culturing of Infected Diabetic Foot Wounds. Int J Endocrinol. 2016;2016:8198714. [PubMed] [DOI] [Full Text] |
| 82. | Nelson EA, Backhouse MR, Bhogal MS, Wright-Hughes A, Lipsky BA, Nixon J, Brown S, Gray J. Concordance in diabetic foot ulcer infection. BMJ Open. 2013;3. [PubMed] [DOI] [Full Text] |
| 83. | Lebowitz D, Gariani K, Kressmann B, Dach EV, Huttner B, Bartolone P, Lê N, Mohamad M, Lipsky BA, Uçkay I. Are antibiotic-resistant pathogens more common in subsequent episodes of diabetic foot infection? Int J Infect Dis. 2017;59:61-64. [PubMed] [DOI] [Full Text] |
| 84. | Boschetti G, Sgarabotto D, Meloni M, Bruseghin M, Whisstock C, Marin M, Ninkovic S, Pinfi M, Brocco E. Antimicrobial Resistance Patterns in Diabetic Foot Infections, an Epidemiological Study in Northeastern Italy. Antibiotics (Basel). 2021;10. [PubMed] [DOI] [Full Text] |
| 85. | Ambrosch A, Haefner S, Jude E, Lobmann R. Diabetic foot infections: microbiological aspects, current and future antibiotic therapy focusing on methicillin-resistant Staphylococcus aureus. Int Wound J. 2011;8:567-577. [PubMed] [DOI] [Full Text] |
| 86. | Miyan Z, Fawwad A, Sabir R, Basit A. Microbiological pattern of diabetic foot infections at a tertiary care center in a developing country. J Pak Med Assoc. 2017;67:665-669. [PubMed] |
| 87. | Han P, Ezquerro R. Diabetic foot wound care algorithms. J Am Podiatr Med Assoc. 2002;92:336-349. [PubMed] [DOI] [Full Text] |
| 88. | Radji M, Putri CS, Fauziyah S. Antibiotic therapy for diabetic foot infections in a tertiary care hospital in Jakarta, Indonesia. Diabetes Metab Syndr. 2014;8:221-224. [PubMed] [DOI] [Full Text] |
| 89. | Mutonga DM, Mureithi MW, Ngugi NN, Otieno FCF. Bacterial isolation and antibiotic susceptibility from diabetic foot ulcers in Kenya using microbiological tests and comparison with RT-PCR in detection of S. aureus and MRSA. BMC Res Notes. 2019;12:244. [PubMed] [DOI] [Full Text] |
| 90. | Shahrokh S, Aliye T, Yazdi M, Siavash M, Aminorroaya A. Bacterial Profile and Antimicrobial Resistance Patterns of Infected Diabetic Foot Ulcers in Iran: A Systematic Review and Meta-Analysis of Cross-Sectional Studies. Int J Low Extrem Wounds. 2022;21:364-373. [PubMed] [DOI] [Full Text] |
| 91. | Miller AO, Henry M. Update in diagnosis and treatment of diabetic foot infections. Phys Med Rehabil Clin N Am. 2009;20:611-625. [PubMed] [DOI] [Full Text] |
| 92. | Nageen A. The Most Prevalent Organism in Diabetic Foot Ulcers and Its Drug Sensitivity and Resistance to Different Standard Antibiotics. J Coll Physicians Surg Pak. 2016;26:293-296. [PubMed] |
| 93. | Kosinski MA, Joseph WS. Update on the treatment of diabetic foot infections. Clin Podiatr Med Surg. 2007;24:383-396, vii. [PubMed] [DOI] [Full Text] |
| 94. | Reveles KR, Duhon BM, Moore RJ, Hand EO, Howell CK. Epidemiology of Methicillin-Resistant Staphylococcus aureus Diabetic Foot Infections in a Large Academic Hospital: Implications for Antimicrobial Stewardship. PLoS One. 2016;11:e0161658. [PubMed] [DOI] [Full Text] |
| 95. | Abalkhail A, Elbehiry A. Methicillin-Resistant Staphylococcus aureus in Diabetic Foot Infections: Protein Profiling, Virulence Determinants, and Antimicrobial Resistance. Appl. Sci. 2022. 12:10803. [DOI] [Full Text] |
| 96. | Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis. 2021;21:770. [PubMed] [DOI] [Full Text] |
| 97. | Otto M. Staphylococcus epidermidis--the 'accidental' pathogen. Nat Rev Microbiol. 2009;7:555-567. [PubMed] [DOI] [Full Text] |
| 98. | Kwiecinski JM, Horswill AR. Staphylococcus aureus bloodstream infections: pathogenesis and regulatory mechanisms. Curr Opin Microbiol. 2020;53:51-60. [PubMed] [DOI] [Full Text] |
| 99. | Abd El-Hamid MI, Sewid AH, Samir M, Hegazy WAH, Bahnass MM, Mosbah RA, Ghaith DM, Khalifa E, Ramadan H, Alshareef WA, Alshareef HM, Ghoneim MM, Al-Sanea MM, Bendary MM. Clonal Diversity and Epidemiological Characteristics of ST239-MRSA Strains. Front Cell Infect Microbiol. 2022;12:782045. [PubMed] [DOI] [Full Text] |
| 100. | Almalki AJ, Ibrahim TS, Taher ES, Mohamed MFA, Youns M, Hegazy WAH, Al-Mahmoudy AMM. Synthesis, Antimicrobial, Anti-Virulence and Anticancer Evaluation of New 5(4H)-Oxazolone-Based Sulfonamides. Molecules. 2022;27. [PubMed] [DOI] [Full Text] |
| 101. | Bowling FL, Jude EB, Boulton AJ. MRSA and diabetic foot wounds: contaminating or infecting organisms? Curr Diab Rep. 2009;9:440-444. [PubMed] [DOI] [Full Text] |
| 102. | Couret G, Desbiez F, Thieblot P, Tauveron I, Bonnet R, Beytout J, Laurichesse H, Lesens O. [Emergence of monomicrobial methicillin-resistant Staphylococcus aureus infections in diabetic foot osteomyelitis (retrospective study of 48 cases)]. Presse Med. 2007;36:851-858. [PubMed] [DOI] [Full Text] |
| 103. | Lavery LA, Fontaine JL, Bhavan K, Kim PJ, Williams JR, Hunt NA. Risk factors for methicillin-resistant Staphylococcus aureus in diabetic foot infections. Diabet Foot Ankle. 2014;5. [PubMed] [DOI] [Full Text] |
| 104. | Eleftheriadou I, Tentolouris N, Argiana V, Jude E, Boulton AJ. Methicillin-resistant Staphylococcus aureus in diabetic foot infections. Drugs. 2010;70:1785-1797. [PubMed] [DOI] [Full Text] |
| 105. | Tentolouris N, Petrikkos G, Vallianou N, Zachos C, Daikos GL, Tsapogas P, Markou G, Katsilambros N. Prevalence of methicillin-resistant Staphylococcus aureus in infected and uninfected diabetic foot ulcers. Clin Microbiol Infect. 2006;12:186-189. [PubMed] [DOI] [Full Text] |
| 106. | Sader HS, Fey PD, Limaye AP, Madinger N, Pankey G, Rahal J, Rybak MJ, Snydman DR, Steed LL, Waites K, Jones RN. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother. 2009;53:4127-4132. [PubMed] [DOI] [Full Text] |
| 107. | Hatipoglu M, Mutluoglu M, Turhan V, Uzun G, Lipsky BA; Turk-Day Study Group, Sevim E, Demiraslan H, Eryilmaz E, Ozuguz C, Memis A, Ay H, Arda B, Uysal S, Motor VK, Kader C, Erturk A, Coskun O, Duygu F, Guler S, Altay FA, Ogutlu A, Bolukcu S, Yildiz S, Kandemir O, Aslaner H, Polat A, Karahocagil MK, Yasar KK, Sehmen E, Kilic S, Sunbul M, Gencer S, Bozkurt F, Yanik T, Oztoprak N, Batirel A, Sozen H, Kilic I, Celik I, Ay B, Tosun S, Kadanali A, Çomoglu S, Denk A, Hosoglu S, Aydin O, Elaldi N, Akalin S, Kandemir B, Akbulut A, Demirdal T, Balik R, Azak E, Sengoz G. Causative pathogens and antibiotic resistance in diabetic foot infections: A prospective multi-center study. J Diabetes Complications. 2016;30:910-916. [PubMed] [DOI] [Full Text] |
| 108. | Newman LG, Waller J, Palestro CJ, Schwartz M, Klein MJ, Hermann G, Harrington E, Harrington M, Roman SH, Stagnaro-Green A. Unsuspected osteomyelitis in diabetic foot ulcers. Diagnosis and monitoring by leukocyte scanning with indium in 111 oxyquinoline. JAMA. 1991;266:1246-1251. [PubMed] [DOI] [Full Text] |
| 109. | Commons RJ, Robinson CH, Gawler D, Davis JS, Price RN. High burden of diabetic foot infections in the top end of Australia: An emerging health crisis (DEFINE study). Diabetes Res Clin Pract. 2015;110:147-157. [PubMed] [DOI] [Full Text] |
| 110. | Markanday A. Diagnosing diabetic foot osteomyelitis: narrative review and a suggested 2-step score-based diagnostic pathway for clinicians. Open Forum Infect Dis. 2014;1:ofu060. [PubMed] [DOI] [Full Text] |
| 111. | Game F, Jeffcoate W. MRSA and osteomyelitis of the foot in diabetes. Diabet Med. 2004;21 Suppl 4:16-19. [PubMed] [DOI] [Full Text] |
| 112. | Lavery LA, Harkless LB, Felder-Johnson K, Mundine S. Bacterial pathogens in infected puncture wounds in adults with diabetes. J Foot Ankle Surg. 1994;33:91-97. [PubMed] |
| 113. | Dang CN, Prasad YD, Boulton AJ, Jude EB. Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: a worsening problem. Diabet Med. 2003;20:159-161. [PubMed] [DOI] [Full Text] |
| 114. | Barshes NR, Rodriguez-Barradas MC, Bechara CF, Pisimisis G, Young EJ, Kougias P. Microbial isolates and their antimicrobial susceptibilities in inframalleolar foot infections. Surg Infect (Larchmt). 2014;15:585-591. [PubMed] [DOI] [Full Text] |
| 115. | Citron DM, Goldstein EJ, Merriam CV, Lipsky BA, Abramson MA. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J Clin Microbiol. 2007;45:2819-2828. [PubMed] [DOI] [Full Text] |
| 116. | Richard JL, Lavigne JP, Got I, Hartemann A, Malgrange D, Tsirtsikolou D, Baleydier A, Senneville E. Management of patients hospitalized for diabetic foot infection: results of the French OPIDIA study. Diabetes Metab. 2011;37:208-215. [PubMed] [DOI] [Full Text] |
| 117. | Lavery LA, Sariaya M, Ashry H, Harkless LB. Microbiology of osteomyelitis in diabetic foot infections. J Foot Ankle Surg. 1995;34:61-64. [PubMed] [DOI] [Full Text] |
| 118. | Al Benwan K, Al Mulla A, Rotimi VO. A study of the microbiology of diabetic foot infections in a teaching hospital in Kuwait. J Infect Public Health. 2012;5:1-8. [PubMed] [DOI] [Full Text] |
| 119. | Djahmi N, Messad N, Nedjai S, Moussaoui A, Mazouz D, Richard JL, Sotto A, Lavigne JP. Molecular epidemiology of Staphylococcus aureus strains isolated from inpatients with infected diabetic foot ulcers in an Algerian University Hospital. Clin Microbiol Infect. 2013;19:E398-E404. [PubMed] [DOI] [Full Text] |
| 120. | Akhi MT, Ghotaslou R, Asgharzadeh M, Varshochi M, Pirzadeh T, Memar MY, Zahedi Bialvaei A, Seifi Yarijan Sofla H, Alizadeh N. Bacterial etiology and antibiotic susceptibility pattern of diabetic foot infections in Tabriz, Iran. GMS Hyg Infect Control. 2015;10:Doc02. [PubMed] [DOI] [Full Text] |
| 121. | Raja NS. Microbiology of diabetic foot infections in a teaching hospital in Malaysia: a retrospective study of 194 cases. J Microbiol Immunol Infect. 2007;40:39-44. [PubMed] |
| 122. | Anvarinejad M, Pouladfar G, Japoni A, Bolandparvaz S, Satiary Z, Abbasi P, Mardaneh J. Isolation and Antibiotic Susceptibility of the Microorganisms Isolated from Diabetic Foot Infections in Nemazee Hospital, Southern Iran. J Pathog. 2015;2015:328796. [PubMed] [DOI] [Full Text] |
| 123. | Yoga R, Khairul A, Sunita K, Suresh C. Bacteriology of diabetic foot lesions. Med J Malaysia. 2006;61 Suppl A:14-16. [PubMed] |
| 124. | Martínez-Gómez Dde A, Ramírez-Almagro C, Campillo-Soto A, Morales-Cuenca G, Pagán-Ortiz J, Aguayo-Albasini JL. [Diabetic foot infections. Prevalence and antibiotic sensitivity of the causative microorganisms]. Enferm Infecc Microbiol Clin. 2009;27:317-321. [PubMed] [DOI] [Full Text] |
| 125. | Perim MC, Borges Jda C, Celeste SR, Orsolin Ede F, Mendes RR, Mendes GO, Ferreira RL, Carreiro SC, Pranchevicius MC. Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev Soc Bras Med Trop. 2015;48:546-554. [PubMed] [DOI] [Full Text] |
| 126. | Shanmugam P, M J, Susan S L. The bacteriology of diabetic foot ulcers, with a special reference to multidrug resistant strains. J Clin Diagn Res. 2013;7:441-445. [PubMed] [DOI] [Full Text] |
| 127. | Sugandhi P, Prasanth DA. Microbiological profile of bacterial pathogens from diabetic foot infections in tertiary care hospitals, Salem. Diabetes Metab Syndr. 2014;8:129-132. [PubMed] [DOI] [Full Text] |
| 128. | Sekhar S, Vyas N, Unnikrishnan M, Rodrigues G, Mukhopadhyay C. Antimicrobial susceptibility pattern in diabetic foot ulcer: a pilot study. Ann Med Health Sci Res. 2014;4:742-745. [PubMed] [DOI] [Full Text] |
| 129. | Wang SH, Sun ZL, Guo YJ, Yang BQ, Yuan Y, Wei Q, Ye KP. Meticillin-resistant Staphylococcus aureus isolated from foot ulcers in diabetic patients in a Chinese care hospital: risk factors for infection and prevalence. J Med Microbiol. 2010;59:1219-1224. [PubMed] [DOI] [Full Text] |
| 130. | Ding Q, Li DQ, Wang PH, Chu YJ, Meng SY, Sun Q. [Risk factors for infections of methicillin-resistant Staphylococci in diabetic foot patients]. Zhonghua Yi Xue Za Zhi. 2012;92:228-231. [PubMed] |
| 131. | Sharma VK, Khadka PB, Joshi A, Sharma R. Common pathogens isolated in diabetic foot infection in Bir Hospital. Kathmandu Univ Med J (KUMJ). 2006;4:295-301. [PubMed] |
| 132. | Islam S, Cawich SO, Budhooram S, Harnarayan P, Mahabir V, Ramsewak S, Naraynsingh V. Microbial profile of diabetic foot infections in Trinidad and Tobago. Prim Care Diabetes. 2013;7:303-308. [PubMed] [DOI] [Full Text] |
| 133. | Mendes JJ, Marques-Costa A, Vilela C, Neves J, Candeias N, Cavaco-Silva P, Melo-Cristino J. Clinical and bacteriological survey of diabetic foot infections in Lisbon. Diabetes Res Clin Pract. 2012;95:153-161. [PubMed] [DOI] [Full Text] |
| 134. | El-Tahawy AT. Bacteriology of diabetic foot. Saudi Med J. 2000;21:344-347. [PubMed] |
| 135. | Guira O, Tiéno H, Traoré S, Diallo I, Ouangré E, Sagna Y, Zabsonré J, Yanogo D, Traoré SS, Drabo YJ. [The bacterial microflora of diabetic foot infection and factors determining its spectrum in Ouagadougou (Burkina Faso)]. Bull Soc Pathol Exot. 2015;108:307-311. [PubMed] [DOI] [Full Text] |
| 136. | Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269-284. [PubMed] [DOI] [Full Text] |
| 137. | Dieterich W, Schink M, Zopf Y. Microbiota in the Gastrointestinal Tract. Med Sci (Basel). 2018;6. [PubMed] [DOI] [Full Text] |
| 138. | Petkovsek Z, Elersic K, Gubina M, Zgur-Bertok D, Starcic Erjavec M. Virulence potential of Escherichia coli isolates from skin and soft tissue infections. J Clin Microbiol. 2009;47:1811-1817. [PubMed] [DOI] [Full Text] |
| 139. | Hegazy WAH, Abbas HA. Evaluation of the role of SsaV 'Salmonella pathogenicity island-2 dependent type III secretion system components on the virulence behavior of Salmonella enterica serovar Typhimurium. Afr. J. Biotechnol. 2017;16:718-726. [DOI] [Full Text] |
| 140. | Sarowska J, Futoma-Koloch B, Jama-Kmiecik A, Frej-Madrzak M, Ksiazczyk M, Bugla-Ploskonska G, Choroszy-Krol I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog. 2019;11:10. [PubMed] [DOI] [Full Text] |
| 141. | Shah C, Baral R, Bartaula B, Shrestha LB. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 2019;19:204. [PubMed] [DOI] [Full Text] |
| 142. | Shang Y, Guo J, Zhao Y, Chen J, Meng Q, Qu D, Zheng J, Yu Z, Wu Y, Deng Q. Clemastine Inhibits the Biofilm and Hemolytic of Staphylococcus aureus through the GdpP Protein. Microbiol Spectr. 2022;10:e0054121. [PubMed] [DOI] [Full Text] |
| 143. | Liao C, Huang X, Wang Q, Yao D, Lu W. Virulence Factors of Pseudomonas Aeruginosa and Antivirulence Strategies to Combat Its Drug Resistance. Front Cell Infect Microbiol. 2022;12:926758. [PubMed] [DOI] [Full Text] |
| 144. | Antonic V, Stojadinovic A, Zhang B, Izadjoo MJ, Alavi M. Pseudomonas aeruginosa induces pigment production and enhances virulence in a white phenotypic variant of Staphylococcus aureus. Infect Drug Resist. 2013;6:175-186. [PubMed] [DOI] [Full Text] |
| 145. | Bottomley MJ, Muraglia E, Bazzo R, Carfì A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J Biol Chem. 2007;282:13592-13600. [PubMed] [DOI] [Full Text] |
| 146. | Ertugrul BM, Lipsky BA, Ture M, Sakarya S. Risk Factors for Infection with Pseudomonas aeruginosa in Diabetic Foot Infections. J Am Podiatr Med Assoc. 2017;107:483-489. [PubMed] [DOI] [Full Text] |
| 147. | Uçkay I, Lebowitz D, Kressmann B, von Dach E, Lipsky BA, Gariani K. Pseudomonal Diabetic Foot Infections: Vive la Différence? Mayo Clin Proc Innov Qual Outcomes. 2022;6:250-256. [PubMed] [DOI] [Full Text] |
| 148. | Young H, Knepper B, Hernandez W, Shor A, Bruntz M, Berg C, Price CS. Pseudomonas aeruginosa: an uncommon cause of diabetic foot infection. J Am Podiatr Med Assoc. 2015;105:125-129. [PubMed] [DOI] [Full Text] |
| 149. | Uçkay I, Holy D, Schöni M, Waibel FWA, Trache T, Burkhard J, Böni T, Lipsky BA, Berli MC. How good are clinicians in predicting the presence of Pseudomonas spp. in diabetic foot infections? Endocrinol Diabetes Metab. 2021;4:e00225. [PubMed] [DOI] [Full Text] |
| 150. | Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159-173. [PubMed] [DOI] [Full Text] |
| 151. | Sivanmaliappan TS, Sevanan M. Antimicrobial Susceptibility Patterns of Pseudomonas aeruginosa from Diabetes Patients with Foot Ulcers. Int J Microbiol. 2011;2011:605195. [PubMed] [DOI] [Full Text] |
| 152. | Chai W, Wang Y, Jiao F, Wu Y, Wang S. A Severe Diabetic Foot Ulcer With Intermediate Cuneiform Displacement and Multidrug-Resistant Pseudomonas aeruginosa Infection: A Rare Case Report. Front Med (Lausanne). 2020;7:131. [PubMed] [DOI] [Full Text] |
| 153. | Askoura M, Hegazy WAH. Ciprofloxacin interferes with Salmonella Typhimurium intracellular survival and host virulence through repression of Salmonella pathogenicity island-2 (SPI-2) genes expression. Pathog Dis. 2020;78. [PubMed] [DOI] [Full Text] |
| 154. | Pearson MM, Rasko DA, Smith SN, Mobley HL. Transcriptome of swarming Proteus mirabilis. Infect Immun. 2010;78:2834-2845. [PubMed] [DOI] [Full Text] |
| 155. | Wasfi R, Hamed SM, Amer MA, Fahmy LI. Proteus mirabilis Biofilm: Development and Therapeutic Strategies. Front Cell Infect Microbiol. 2020;10:414. [PubMed] [DOI] [Full Text] |
| 156. | Scavone P, Iribarnegaray V, Caetano AL, Schlapp G, Härtel S, Zunino P. Fimbriae have distinguishable roles in Proteus mirabilis biofilm formation. Pathog Dis. 2016;74. [PubMed] [DOI] [Full Text] |
| 157. | Armbruster CE, Mobley HLT, Pearson MM. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus. 2018;8. [PubMed] [DOI] [Full Text] |
| 158. | Effah CY, Sun T, Liu S, Wu Y. Klebsiella pneumoniae: an increasing threat to public health. Ann Clin Microbiol Antimicrob. 2020;19:1. [PubMed] [DOI] [Full Text] |
| 159. | Akash MSH, Rehman K, Fiayyaz F, Sabir S, Khurshid M. Diabetes-associated infections: development of antimicrobial resistance and possible treatment strategies. Arch Microbiol. 2020;202:953-965. [PubMed] [DOI] [Full Text] |
| 160. | Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev. 2016;80:629-661. [PubMed] [DOI] [Full Text] |
| 161. | Alcántar-Curiel MD, Blackburn D, Saldaña Z, Gayosso-Vázquez C, Iovine NM, De la Cruz MA, Girón JA. Multi-functional analysis of Klebsiella pneumoniae fimbrial types in adherence and biofilm formation. Virulence. 2013;4:129-138. [PubMed] [DOI] [Full Text] |
| 162. | Wang H, Yan Y, Rong D, Wang J, Wang H, Liu Z, Yang R, Han Y. Increased biofilm formation ability in Klebsiella pneumoniae after short-term exposure to a simulated microgravity environment. Microbiologyopen. 2016;5:793-801. [PubMed] [DOI] [Full Text] |
| 163. | Huang X, Li X, An H, Wang J, Ding M, Wang L, Li L, Ji Q, Qu F, Wang H, Xu Y, Lu X, He Y, Zhang JR. Capsule type defines the capability of Klebsiella pneumoniae in evading Kupffer cell capture in the liver. PLoS Pathog. 2022;18:e1010693. [PubMed] [DOI] [Full Text] |
| 164. | Vuotto C, Longo F, Balice MP, Donelli G, Varaldo PE. Antibiotic Resistance Related to Biofilm Formation in Klebsiella pneumoniae. Pathogens. 2014;3:743-758. [PubMed] [DOI] [Full Text] |
| 165. | Mohd Asri NA, Ahmad S, Mohamud R, Mohd Hanafi N, Mohd Zaidi NF, Irekeola AA, Shueb RH, Yee LC, Mohd Noor N, Mustafa FH, Yean CY, Yusof NY. Global Prevalence of Nosocomial Multidrug-Resistant Klebsiella pneumoniae: A Systematic Review and Meta-Analysis. Antibiotics (Basel). 2021;10. [PubMed] [DOI] [Full Text] |
| 166. | Bengoechea JA, Sa Pessoa J. Klebsiella pneumoniae infection biology: living to counteract host defences. FEMS Microbiol Rev. 2019;43:123-144. [PubMed] [DOI] [Full Text] |
| 167. | Sharma S, Govind B, Naidu SK, Kinjarapu S, Rasool M. Clinical and Laboratory Profile of Urinary Tract Infections in Type 2 Diabetics Aged over 60 Years. J Clin Diagn Res. 2017;11:OC25-OC28. [PubMed] [DOI] [Full Text] |
| 168. | Biswas D, Pawar N, Patro SK, Krishna NS, Parida D, Bhagtana PK. Clinical profile and spectrum of bacteriuria in patients with diabetes: An analytical study. J Family Med Prim Care. 2022;11:3190-3195. [PubMed] [DOI] [Full Text] |
| 169. | Gaca AO, Lemos JA. Adaptation to Adversity: the Intermingling of Stress Tolerance and Pathogenesis in Enterococci. Microbiol Mol Biol Rev. 2019;83. [PubMed] [DOI] [Full Text] |
| 170. | Grenda A, Grenda T, Domaradzki P, Kwiatek K. Enterococci-Involvement in Pathogenesis and Therapeutic Potential in Cancer Treatment: A Mini-Review. Pathogens. 2022;11. [PubMed] [DOI] [Full Text] |
| 171. | Raza T, Ullah SR, Mehmood K, Andleeb S. Vancomycin resistant Enterococci: A brief review. J Pak Med Assoc. 2018;68:768-772. [PubMed] |
| 172. | Fiore E, Van Tyne D, Gilmore MS. Pathogenicity of Enterococci. Microbiol Spectr. 2019;7. [PubMed] [DOI] [Full Text] |
| 173. | Seputiene V, Bogdaite A, Ruzauskas M, Suziedeliene E. Antibiotic resistance genes and virulence factors in Enterococcus faecium and Enterococcus faecalis from diseased farm animals: pigs, cattle and poultry. Pol J Vet Sci. 2012;15:431-438. [PubMed] [DOI] [Full Text] |
| 174. | Shettigar K, Bhat DV, Satyamoorthy K, Murali TS. Severity of drug resistance and co-existence of Enterococcus faecalis in diabetic foot ulcer infections. Folia Microbiol (Praha). 2018;63:115-122. [PubMed] [DOI] [Full Text] |
| 175. | Boulton AJM, Armstrong DG, Hardman MJ, Malone M, Embil JM, Attinger CE, Lipsky BA, Aragón-Sánchez J, Li HK, Schultz G, Kirsner RS. Diagnosis and Management of Diabetic Foot Infections. Arlington (VA): American Diabetes Association; 2020 Jan. [PubMed] [DOI] [Full Text] |
| 176. | Kwon KT, Armstrong DG. Microbiology and Antimicrobial Therapy for Diabetic Foot Infections. Infect Chemother. 2018;50:11-20. [PubMed] [DOI] [Full Text] |
| 177. | Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411:153-165. [PubMed] [DOI] [Full Text] |
| 178. | Ayobami O, Willrich N, Reuss A, Eckmanns T, Markwart R. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: an epidemiological analysis of bloodstream infections. Emerg Microbes Infect. 2020;9:1180-1193. [PubMed] [DOI] [Full Text] |
| 179. | Piezzi V, Gasser M, Atkinson A, Kronenberg A, Vuichard-Gysin D, Harbarth S, Marschall J, Buetti N; Swiss Centre for Antibiotic Resistance (ANRESIS); National Centre for Infection Control (Swissnoso). Increasing proportion of vancomycin-resistance among enterococcal bacteraemias in Switzerland: a 6-year nation-wide surveillance, 2013 to 2018. Euro Surveill. 2020;25. [PubMed] [DOI] [Full Text] |
| 180. | Stein GE, Schooley S, Peloquin CA, Missavage A, Havlichek DH. Linezolid tissue penetration and serum activity against strains of methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility in diabetic patients with foot infections. J Antimicrob Chemother. 2007;60:819-823. [PubMed] [DOI] [Full Text] |
| 181. | Goldstein EJ, Citron DM, Warren YA, Tyrrell KL, Merriam CV, Fernandez HT. In vitro activities of dalbavancin and 12 other agents against 329 aerobic and anaerobic gram-positive isolates recovered from diabetic foot infections. Antimicrob Agents Chemother. 2006;50:2875-2879. [PubMed] [DOI] [Full Text] |
| 182. | Abou Hassan OK, Karnib M, El-Khoury R, Nemer G, Ahdab-Barmada M, BouKhalil P. Linezolid Toxicity and Mitochondrial Susceptibility: A Novel Neurological Complication in a Lebanese Patient. Front Pharmacol. 2016;7:325. [PubMed] [DOI] [Full Text] |
| 183. | Joseph WS, Quast T, Cogo A, Crompton MG, Yoon MJ, Lamp KC, Culshaw D, Chaves RL. Daptomycin for methicillin-resistant Staphylococcus aureus diabetic foot infections. J Am Podiatr Med Assoc. 2014;104:159-168. [PubMed] [DOI] [Full Text] |
| 184. | Porter KB, Lynch B, Mani CS. The Use of Daptomycin and Linezolid to Treat Vancomycin-Intermediate Staphylococcus haemolyticus Infection in a Premature Infant. J Pediatr Pharmacol Ther. 2010;15:297-300. [PubMed] |
| 185. | Maraolo AE, Giaccone A, Gentile I, Saracino A, Bavaro DF. Daptomycin versus Vancomycin for the Treatment of Methicillin-Resistant Staphylococcus aureus Bloodstream Infection with or without Endocarditis: A Systematic Review and Meta-Analysis. Antibiotics (Basel). 2021;10. [PubMed] [DOI] [Full Text] |
| 186. | Mendes RE, Sader HS, Deshpande L, Jones RN. Antimicrobial activity of tigecycline against community-acquired methicillin-resistant Staphylococcus aureus isolates recovered from North American medical centers. Diagn Microbiol Infect Dis. 2008;60:433-436. [PubMed] [DOI] [Full Text] |
| 187. | Nichols RL, Graham DR, Barriere SL, Rodgers A, Wilson SE, Zervos M, Dunn DL, Kreter B. Treatment of hospitalized patients with complicated gram-positive skin and skin structure infections: two randomized, multicentre studies of quinupristin/dalfopristin versus cefazolin, oxacillin or vancomycin. Synercid Skin and Skin Structure Infection Group. J Antimicrob Chemother. 1999;44:263-273. [PubMed] [DOI] [Full Text] |
| 188. | Lauf L, Ozsvár Z, Mitha I, Regöly-Mérei J, Embil JM, Cooper A, Sabol MB, Castaing N, Dartois N, Yan J, Dukart G, Maroko R. Phase 3 study comparing tigecycline and ertapenem in patients with diabetic foot infections with and without osteomyelitis. Diagn Microbiol Infect Dis. 2014;78:469-480. [PubMed] [DOI] [Full Text] |
| 189. | Noel GJ, Bush K, Bagchi P, Ianus J, Strauss RS. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin Infect Dis. 2008;46:647-655. [PubMed] [DOI] [Full Text] |
| 190. | Corey GR, Wilcox M, Talbot GH, Friedland HD, Baculik T, Witherell GW, Critchley I, Das AF, Thye D. Integrated analysis of CANVAS 1 and 2: phase 3, multicenter, randomized, double-blind studies to evaluate the safety and efficacy of ceftaroline versus vancomycin plus aztreonam in complicated skin and skin-structure infection. Clin Infect Dis. 2010;51:641-650. [PubMed] [DOI] [Full Text] |
| 191. | Johani K, Malone M, Jensen SO, Dickson HG, Gosbell IB, Hu H, Yang Q, Schultz G, Vickery K. Evaluation of short exposure times of antimicrobial wound solutions against microbial biofilms: from in vitro to in vivo. J Antimicrob Chemother. 2018;73:494-502. [PubMed] [DOI] [Full Text] |
| 192. | Uçkay I, Kressmann B, Malacarne S, Toumanova A, Jaafar J, Lew D, Lipsky BA. A randomized, controlled study to investigate the efficacy and safety of a topical gentamicin-collagen sponge in combination with systemic antibiotic therapy in diabetic patients with a moderate or severe foot ulcer infection. BMC Infect Dis. 2018;18:361. [PubMed] [DOI] [Full Text] |
| 193. | Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M, van Asten SA, Urbančič-Rovan V, Peters EJG; International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3280. [PubMed] [DOI] [Full Text] |
| 194. | Stewart PS. Antimicrobial Tolerance in Biofilms. Microbiol Spectr. 2015;3. [PubMed] [DOI] [Full Text] |
| 195. | Pavlík V, Sojka M, Mazúrová M, Velebný V. Dual role of iodine, silver, chlorhexidine and octenidine as antimicrobial and antiprotease agents. PLoS One. 2019;14:e0211055. [PubMed] [DOI] [Full Text] |
| 196. | Markakis K, Faris AR, Sharaf H, Faris B, Rees S, Bowling FL. Local Antibiotic Delivery Systems: Current and Future Applications for Diabetic Foot Infections. Int J Low Extrem Wounds. 2018;17:14-21. [PubMed] [DOI] [Full Text] |
| 197. | Parsons D, Meredith K, Rowlands VJ, Short D, Metcalf DG, Bowler PG. Enhanced Performance and Mode of Action of a Novel Antibiofilm Hydrofiber® Wound Dressing. Biomed Res Int. 2016;2016:7616471. [PubMed] [DOI] [Full Text] |
| 198. | Yang Q, Larose C, Della Porta AC, Schultz GS, Gibson DJ. A surfactant-based wound dressing can reduce bacterial biofilms in a porcine skin explant model. Int Wound J. 2017;14:408-413. [PubMed] [DOI] [Full Text] |
| 199. | Percival SL, Chen R, Mayer D, Salisbury AM. Mode of action of poloxamer-based surfactants in wound care and efficacy on biofilms. Int Wound J. 2018;15:749-755. [PubMed] [DOI] [Full Text] |
| 200. | Tarusha L, Paoletti S, Travan A, Marsich E. Alginate membranes loaded with hyaluronic acid and silver nanoparticles to foster tissue healing and to control bacterial contamination of non-healing wounds. J Mater Sci Mater Med. 2018;29:22. [PubMed] [DOI] [Full Text] |
| 201. | Pérez-Díaz MA, Silva-Bermudez P, Jiménez-López B, Martínez-López V, Melgarejo-Ramírez Y, Brena-Molina A, Ibarra C, Baeza I, Martínez-Pardo ME, Reyes-Frías ML, Márquez-Gutiérrez E, Velasquillo C, Martínez-Castañon G, Martinez-Gutierrez F, Sánchez-Sánchez R. Silver-pig skin nanocomposites and mesenchymal stem cells: suitable antibiofilm cellular dressings for wound healing. J Nanobiotechnology. 2018;16:2. [PubMed] [DOI] [Full Text] |
| 202. | Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev. 2015;2015:CD005083. [PubMed] [DOI] [Full Text] |
| 203. | Minden-Birkenmaier BA, Bowlin GL. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering (Basel). 2018;5. [PubMed] [DOI] [Full Text] |
| 204. | LaPlante KL, Sarkisian SA, Woodmansee S, Rowley DC, Seeram NP. Effects of cranberry extracts on growth and biofilm production of Escherichia coli and Staphylococcus species. Phytother Res. 2012;26:1371-1374. [PubMed] [DOI] [Full Text] |
| 205. | Chen Y, Tian L, Yang F, Tong W, Jia R, Zou Y, Yin L, Li L, He C, Liang X, Ye G, Lv C, Song X, Yin Z. Tannic Acid Accelerates Cutaneous Wound Healing in Rats Via Activation of the ERK 1/2 Signaling Pathways. Adv Wound Care (New Rochelle). 2019;8:341-354. [PubMed] [DOI] [Full Text] |
| 206. | Pouget C, Dunyach-Remy C, Pantel A, Boutet-Dubois A, Schuldiner S, Sotto A, Lavigne JP, Loubet P. Alternative Approaches for the Management of Diabetic Foot Ulcers. Front Microbiol. 2021;12:747618. [PubMed] [DOI] [Full Text] |
| 207. | Cui H, Li W, Li C, Vittayapadung S, Lin L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling. 2016;32:215-225. [PubMed] [DOI] [Full Text] |
| 208. | Wolcott RD, Kennedy JP, Dowd SE. Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds. J Wound Care. 2009;18:54-56. [PubMed] [DOI] [Full Text] |
| 209. | Liu Z, Dumville JC, Hinchliffe RJ, Cullum N, Game F, Stubbs N, Sweeting M, Peinemann F. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev. 2018;10:CD010318. [PubMed] [DOI] [Full Text] |
| 210. | Apelqvist J, Willy C, Fagerdahl AM, Fraccalvieri M, Malmsjö M, Piaggesi A, Probst A, Vowden P. EWMA Document: Negative Pressure Wound Therapy. J Wound Care. 2017;26:S1-S154. [PubMed] [DOI] [Full Text] |
| 211. | Tardivo JP, Adami F, Correa JA, Pinhal MA, Baptista MS. A clinical trial testing the efficacy of PDT in preventing amputation in diabetic patients. Photodiagnosis Photodyn Ther. 2014;11:342-350. [PubMed] [DOI] [Full Text] |
| 212. | Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;2015:CD004123. [PubMed] [DOI] [Full Text] |
| 213. | Sethuram L, Thomas J, Mukherjee A, Chandrasekaran N. A review on contemporary nanomaterial-based therapeutics for the treatment of diabetic foot ulcers (DFUs) with special reference to the Indian scenario. Nanoscale Adv. 2022;4:2367-2398. [PubMed] [DOI] [Full Text] |
| 214. | Lv J, Yang S, Lv M, Lv J, Sui Y, Guo S. Protective roles of mesenchymal stem cells on skin photoaging: A narrative review. Tissue Cell. 2022;76:101746. [PubMed] [DOI] [Full Text] |
| 215. | Ormazabal V, Nova-Lampeti E, Rojas D, Zúñiga FA, Escudero C, Lagos P, Moreno A, Pavez Y, Reyes C, Yáñez M, Vidal M, Cabrera-Vives G, Oporto K, Aguayo C. Secretome from Human Mesenchymal Stem Cells-Derived Endothelial Cells Promotes Wound Healing in a Type-2 Diabetes Mouse Model. Int J Mol Sci. 2022;23. [PubMed] [DOI] [Full Text] |
| 216. | Zhang J, Wang C, An Q, Quan Q, Li M, Zhao D. Gene Expression Profile Analyses of the Skin Response of Balb/c-Nu Mice Model Injected by Staphylococcus aureus. Clin Cosmet Investig Dermatol. 2022;15:217-235. [PubMed] [DOI] [Full Text] |
| 217. | Noh HK, Lee SW, Kim JM, Oh JE, Kim KH, Chung CP, Choi SC, Park WH, Min BM. Electrospinning of chitin nanofibers: degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials. 2006;27:3934-3944. [PubMed] [DOI] [Full Text] |
| 218. | Dixon D, Edmonds M. Managing Diabetic Foot Ulcers: Pharmacotherapy for Wound Healing. Drugs. 2021;81:29-56. [PubMed] [DOI] [Full Text] |
| 219. | Lantis JC, Snyder R, Reyzelman AM, Van Gils CC, Sigal F, Vayser D, Caporusso JM, Cazzell S, Lavery LA; PriMatrix Study Group. Fetal bovine acellular dermal matrix for the closure of diabetic foot ulcers: a prospective randomised controlled trial. J Wound Care. 2021;30:S18-S27. [PubMed] [DOI] [Full Text] |
| 220. | Huang F, Thokerunga E, He F, Zhu X, Wang Z, Tu J. Research progress of the application of mesenchymal stem cells in chronic inflammatory systemic diseases. Stem Cell Res Ther. 2022;13:1. [PubMed] [DOI] [Full Text] |
| 221. | Knezevic P, Hoyle NS, Matsuzaki S, Gorski A. Editorial: Advances in Phage Therapy: Present Challenges and Future Perspectives. Front Microbiol. 2021;12:701898. [PubMed] [DOI] [Full Text] |
| 222. | Clokie MR, Millard AD, Letarov AV, Heaphy S. Phages in nature. Bacteriophage. 2011;1:31-45. [PubMed] [DOI] [Full Text] |
| 223. | Lin DM, Koskella B, Lin HC. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. 2017;8:162-173. [PubMed] [DOI] [Full Text] |
| 224. | Mendes JJ, Leandro C, Mottola C, Barbosa R, Silva FA, Oliveira M, Vilela CL, Melo-Cristino J, Górski A, Pimentel M, São-José C, Cavaco-Silva P, Garcia M. In vitro design of a novel lytic bacteriophage cocktail with therapeutic potential against organisms causing diabetic foot infections. J Med Microbiol. 2014;63:1055-1065. [PubMed] [DOI] [Full Text] |
| 225. | Taha OA, Connerton PL, Connerton IF, El-Shibiny A. Bacteriophage ZCKP1: A Potential Treatment for Klebsiella pneumoniae Isolated From Diabetic Foot Patients. Front Microbiol. 2018;9:2127. [PubMed] [DOI] [Full Text] |
| 226. | Fish R, Kutter E, Bryan D, Wheat G, Kuhl S. Resolving Digital Staphylococcal Osteomyelitis Using Bacteriophage-A Case Report. Antibiotics (Basel). 2018;7. [PubMed] [DOI] [Full Text] |
| 227. | Morozova VV, Vlassov VV, Tikunova NV. Applications of Bacteriophages in the Treatment of Localized Infections in Humans. Front Microbiol. 2018;9:1696. [PubMed] [DOI] [Full Text] |
| 228. | Tan D, Dahl A, Middelboe M. Vibriophages Differentially Influence Biofilm Formation by Vibrio anguillarum Strains. Appl Environ Microbiol. 2015;81:4489-4497. [PubMed] [DOI] [Full Text] |
| 229. | Henriksen K, Rørbo N, Rybtke ML, Martinet MG, Tolker-Nielsen T, Høiby N, Middelboe M, Ciofu O. P. aeruginosa flow-cell biofilms are enhanced by repeated phage treatments but can be eradicated by phage-ciprofloxacin combination. Pathog Dis. 2019;77. [PubMed] [DOI] [Full Text] |
| 230. | Abd El-Hamid MI, Y El-Naenaeey ES, M Kandeel T, Hegazy WAH, Mosbah RA, Nassar MS, Bakhrebah MA, Abdulaal WH, Alhakamy NA, Bendary MM. Promising Antibiofilm Agents: Recent Breakthrough against Biofilm Producing Methicillin-Resistant Staphylococcus aureus. Antibiotics (Basel). 2020;9. [PubMed] [DOI] [Full Text] |
| 231. | Almalki AJ, Ibrahim TS, Elhady SS, Darwish KM, Hegazy WAH. Repurposing α-Adrenoreceptor Blockers as Promising Anti-Virulence Agents in Gram-Negative Bacteria. Antibiotics (Basel). 2022;11. [PubMed] [DOI] [Full Text] |
| 232. | Aldawsari MF, Khafagy ES, Saqr AA, Alalaiwe A, Abbas HA, Shaldam MA, Hegazy WAH, Goda RM. Tackling Virulence of Pseudomonas aeruginosa by the Natural Furanone Sotolon. Antibiotics (Basel). 2021;10. [PubMed] [DOI] [Full Text] |
| 233. | Almalki AJ, Ibrahim TS, Elhady SS, Hegazy WAH, Darwish KM. Computational and Biological Evaluation of β-Adrenoreceptor Blockers as Promising Bacterial Anti-Virulence Agents. Pharmaceuticals (Basel). 2022;15. [PubMed] [DOI] [Full Text] |
| 234. | Lila ASA, Rajab AAH, Abdallah MH, Rizvi SMD, Moin A, Khafagy ES, Tabrez S, Hegazy WAH. Biofilm Lifestyle in Recurrent Urinary Tract Infections. Life (Basel). 2023;13. [PubMed] [DOI] [Full Text] |
| 235. | Abbas HA, Hegazy WAH. Repurposing anti-diabetic drug "Sitagliptin" as a novel virulence attenuating agent in Serratia marcescens. PLoS One. 2020;15:e0231625. [PubMed] [DOI] [Full Text] |
| 236. | Hegazy WAH, Khayat MT, Ibrahim TS, Youns M, Mosbah R, Soliman WE. Repurposing of antidiabetics as Serratia marcescens virulence inhibitors. Braz J Microbiol. 2021;52:627-638. [PubMed] [DOI] [Full Text] |
| 237. | Hegazy WAH, Rajab AAH, Abu Lila AS, Abbas HA. Anti-diabetics and antimicrobials: Harmony of mutual interplay. World J Diabetes. 2021;12:1832-1855. [PubMed] [DOI] [Full Text] |
| 238. | Khayat MT, Ibrahim TS, Darwish KM, Khayyat AN, Alharbi M, Khafagy ES, Ali MAM, Hegazy WAH, Abbas HA. Hiring of the Anti-Quorum Sensing Activities of Hypoglycemic Agent Linagliptin to Alleviate the Pseudomonas aeruginosa Pathogenesis. Microorganisms. 2022;10. [PubMed] [DOI] [Full Text] |
| 239. | Hegazy WAH. Diclofenac inhibits virulence of Proteus mirabilis isolated from diabetic foot ulcer. Afr. J. Microbiol. Res.. 2016;10:733-743. [DOI] [Full Text] |
| 240. | Abraham NM, Lamlertthon S, Fowler VG, Jefferson KK. Chelating agents exert distinct effects on biofilm formation in Staphylococcus aureus depending on strain background: role for clumping factor B. J Med Microbiol. 2012;61:1062-1070. [PubMed] [DOI] [Full Text] |
| 241. | Fleming D, Chahin L, Rumbaugh K. Glycoside Hydrolases Degrade Polymicrobial Bacterial Biofilms in Wounds. Antimicrob Agents Chemother. 2017;61. [PubMed] [DOI] [Full Text] |
| 242. | Chen KJ, Lee CK. Twofold enhanced dispersin B activity by N-terminal fusion to silver-binding peptide for biofilm eradication. Int J Biol Macromol. 2018;118:419-426. [PubMed] [DOI] [Full Text] |
| 243. | Sharma K, Pagedar Singh A. Antibiofilm Effect of DNase against Single and Mixed Species Biofilm. Foods. 2018;7. [PubMed] [DOI] [Full Text] |
| 244. | Rogers SA, Huigens RW 3rd, Cavanagh J, Melander C. Synergistic effects between conventional antibiotics and 2-aminoimidazole-derived antibiofilm agents. Antimicrob Agents Chemother. 2010;54:2112-2118. [PubMed] [DOI] [Full Text] |
| 245. | Barki KG, Das A, Dixith S, Ghatak PD, Mathew-Steiner S, Schwab E, Khanna S, Wozniak DJ, Roy S, Sen CK. Electric Field Based Dressing Disrupts Mixed-Species Bacterial Biofilm Infection and Restores Functional Wound Healing. Ann Surg. 2019;269:756-766. [PubMed] [DOI] [Full Text] |
| 246. | Mishra R, Panda AK, De Mandal S, Shakeel M, Bisht SS, Khan J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front Microbiol. 2020;11:566325. [PubMed] [DOI] [Full Text] |
| 247. | Alves DR, Gaudion A, Bean JE, Perez Esteban P, Arnot TC, Harper DR, Kot W, Hansen LH, Enright MC, Jenkins AT. Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation. Appl Environ Microbiol. 2014;80:6694-6703. [PubMed] [DOI] [Full Text] |
| 248. | Kim SG, Giri SS, Yun S, Kim SW, Han SJ, Kwon J, Oh WT, Lee SB, Park YH, Park SC. Two Novel Bacteriophages Control Multidrug- and Methicillin-Resistant Staphylococcus pseudintermedius Biofilm. Front Med (Lausanne). 2021;8:524059. [PubMed] [DOI] [Full Text] |
| 249. | Cerca N, Oliveira R, Azeredo J. Susceptibility of Staphylococcus epidermidis planktonic cells and biofilms to the lytic action of staphylococcus bacteriophage K. Lett Appl Microbiol. 2007;45:313-317. [PubMed] [DOI] [Full Text] |
| 250. | Alves DR, Perez-Esteban P, Kot W, Bean JE, Arnot T, Hansen LH, Enright MC, Jenkins AT. A novel bacteriophage cocktail reduces and disperses Pseudomonas aeruginosa biofilms under static and flow conditions. Microb Biotechnol. 2016;9:61-74. [PubMed] [DOI] [Full Text] |
| 251. | Hanlon GW, Denyer SP, Olliff CJ, Ibrahim LJ. Reduction in exopolysaccharide viscosity as an aid to bacteriophage penetration through Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2001;67:2746-2753. [PubMed] [DOI] [Full Text] |
| 252. | Yazdi M, Bouzari M, Ghaemi EA, Shahin K. Isolation, Characterization and Genomic Analysis of a Novel Bacteriophage VB_EcoS-Golestan Infecting Multidrug-Resistant Escherichia coli Isolated from Urinary Tract Infection. Sci Rep. 2020;10:7690. [PubMed] [DOI] [Full Text] |
| 253. | Carson L, Gorman SP, Gilmore BF. The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol Med Microbiol. 2010;59:447-455. [PubMed] [DOI] [Full Text] |
| 254. | Nzakizwanayo J, Hanin A, Alves DR, McCutcheon B, Dedi C, Salvage J, Knox K, Stewart B, Metcalfe A, Clark J, Gilmore BF, Gahan CG, Jenkins AT, Jones BV. Bacteriophage Can Prevent Encrustation and Blockage of Urinary Catheters by Proteus mirabilis. Antimicrob Agents Chemother. 2015;60:1530-1536. [PubMed] [DOI] [Full Text] |
| 255. | Yazdi M, Bouzari M, Ghaemi EA. Isolation and Characterization of a Lytic Bacteriophage (vB_PmiS-TH) and Its Application in Combination with Ampicillin against Planktonic and Biofilm Forms of Proteus mirabilis Isolated from Urinary Tract Infection. J Mol Microbiol Biotechnol. 2018;28:37-46. [PubMed] [DOI] [Full Text] |
| 256. | Sillankorva S, Oliveira R, Vieira MJ, Sutherland I, Azeredo J. Pseudomonas fluorescens infection by bacteriophage PhiS1: the influence of temperature, host growth phase and media. FEMS Microbiol Lett. 2004;241:13-20. [PubMed] [DOI] [Full Text] |
| 257. | Liao KS, Lehman SM, Tweardy DJ, Donlan RM, Trautner BW. Bacteriophages are synergistic with bacterial interference for the prevention of Pseudomonas aeruginosa biofilm formation on urinary catheters. J Appl Microbiol. 2012;113:1530-1539. [PubMed] [DOI] [Full Text] |
| 258. | Verma V, Harjai K, Chhibber S. Restricting ciprofloxacin-induced resistant variant formation in biofilm of Klebsiella pneumoniae B5055 by complementary bacteriophage treatment. J Antimicrob Chemother. 2009;64:1212-1218. [PubMed] [DOI] [Full Text] |
| 259. | Abbas HA, Shaldam MA, Eldamasi D. Curtailing Quorum Sensing in Pseudomonas aeruginosa by Sitagliptin. Curr Microbiol. 2020;77:1051-1060. [PubMed] [DOI] [Full Text] |
| 260. | Khayyat AN, Abbas HA, Mohamed MFA, Asfour HZ, Khayat MT, Ibrahim TS, Youns M, Khafagy E-S, Abu Lila AS, Safo MK, Hegazy WAH. Not Only Antimicrobial: Metronidazole Mitigates the Virulence of Proteus mirabilis Isolated from Macerated Diabetic Foot Ulcer. Appl. Sci.. 2021;11:6847. [DOI] [Full Text] |
| 261. | Holling N, Lednor D, Tsang S, Bissell A, Campbell L, Nzakizwanayo J, Dedi C, Hawthorne JA, Hanlon G, Ogilvie LA, Salvage JP, Patel BA, Barnes LM, Jones BV. Elucidating the genetic basis of crystalline biofilm formation in Proteus mirabilis. Infect Immun. 2014;82:1616-1626. [PubMed] [DOI] [Full Text] |
| 262. | Nzakizwanayo J, Scavone P, Jamshidi S, Hawthorne JA, Pelling H, Dedi C, Salvage JP, Hind CK, Guppy FM, Barnes LM, Patel BA, Rahman KM, Sutton MJ, Jones BV. Fluoxetine and thioridazine inhibit efflux and attenuate crystalline biofilm formation by Proteus mirabilis. Sci Rep. 2017;7:12222. [PubMed] [DOI] [Full Text] |
| 263. | Zeng X, She P, Zhou L, Li S, Hussain Z, Chen L, Wu Y. Drug repurposing: Antimicrobial and antibiofilm effects of penfluridol against Enterococcus faecalis. Microbiologyopen. 2021;10:e1148. [PubMed] [DOI] [Full Text] |
| 264. | Hegazy WAH, Salem IM, Alotaibi HF, Khafagy ES, Ibrahim D. Terazosin Interferes with Quorum Sensing and Type Three Secretion System and Diminishes the Bacterial Espionage to Mitigate the Salmonella Typhimurium Pathogenesis. Antibiotics (Basel). 2022;11. [PubMed] [DOI] [Full Text] |
| 265. | Thabit AK, Eljaaly K, Zawawi A, Ibrahim TS, Eissa AG, Elbaramawi SS, Hegazy WAH, Elfaky MA. Silencing of Salmonella typhimurium Pathogenesis: Atenolol Acquires Efficient Anti-Virulence Activities. Microorganisms. 2022;10. [PubMed] [DOI] [Full Text] |
| 266. | Elfaky MA, Thabit AK, Eljaaly K, Zawawi A, Abdelkhalek AS, Almalki AJ, Ibrahim TS, Hegazy WAH. Controlling of Bacterial Virulence: Evaluation of Anti-Virulence Activities of Prazosin against Salmonella enterica. Antibiotics (Basel). 2022;11. [PubMed] [DOI] [Full Text] |
| 267. | Cavalu S, Elbaramawi SS, Eissa AG, Radwan MF, S Ibrahim T, Khafagy ES, Lopes BS, Ali MAM, Hegazy WAH, Elfaky MA. Characterization of the Anti-Biofilm and Anti-Quorum Sensing Activities of the β-Adrenoreceptor Antagonist Atenolol against Gram-Negative Bacterial Pathogens. Int J Mol Sci. 2022;23. [PubMed] [DOI] [Full Text] |
| 268. | Nalca Y, Jänsch L, Bredenbruch F, Geffers R, Buer J, Häussler S. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother. 2006;50:1680-1688. [PubMed] [DOI] [Full Text] |
| 269. | Alandiyjany MN, Abdelaziz AS, Abdelfattah-Hassan A, Hegazy WAH, Hassan AA, Elazab ST, Mohamed EAA, El-Shetry ES, Saleh AA, ElSawy NA, Ibrahim D. Novel In Vivo Assessment of Antimicrobial Efficacy of Ciprofloxacin Loaded Mesoporous Silica Nanoparticles against Salmonella typhimurium Infection. Pharmaceuticals (Basel). 2022;15. [PubMed] [DOI] [Full Text] |
| 270. | Qin N, Tan X, Jiao Y, Liu L, Zhao W, Yang S, Jia A. RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci Rep. 2014;4:5467. [PubMed] [DOI] [Full Text] |
| 271. | Lee JH, Cho HS, Joo SW, Chandra Regmi S, Kim JA, Ryu CM, Ryu SY, Cho MH, Lee J. Diverse plant extracts and trans-resveratrol inhibit biofilm formation and swarming of Escherichia coli O157:H7. Biofouling. 2013;29:1189-1203. [PubMed] [DOI] [Full Text] |
| 272. | Yousfi H, Cassagne C, Ranque S, Rolain JM, Bittar F. Repurposing of Ribavirin as an Adjunct Therapy against Invasive Candida Strains in an In Vitro Study. Antimicrob Agents Chemother. 2019;63. [PubMed] [DOI] [Full Text] |
| 273. | Singh S, Fatima Z, Ahmad K, Hameed S. Repurposing of respiratory drug theophylline against Candida albicans: mechanistic insights unveil alterations in membrane properties and metabolic fitness. J Appl Microbiol. 2020;129:860-875. [PubMed] [DOI] [Full Text] |
| 274. | Harjai K, Kumar R, Singh S. Garlic blocks quorum sensing and attenuates the virulence of Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2010;58:161-168. [PubMed] [DOI] [Full Text] |
| 275. | Ranjbar-Omid M, Arzanlou M, Amani M, Shokri Al-Hashem SK, Amir Mozafari N, Peeri Doghaheh H. Allicin from garlic inhibits the biofilm formation and urease activity of Proteus mirabilis in vitro. FEMS Microbiol Lett. 2015;362. [PubMed] [DOI] [Full Text] |
| 276. | Tapia-Rodriguez MR, Bernal-Mercado AT, Gutierrez-Pacheco MM, Vazquez-Armenta FJ, Hernandez-Mendoza A, Gonzalez-Aguilar GA, Martinez-Tellez MA, Nazzaro F, Ayala-Zavala JF. Virulence of Pseudomonas aeruginosa exposed to carvacrol: alterations of the Quorum sensing at enzymatic and gene levels. J Cell Commun Signal. 2019;13:531-537. [PubMed] [DOI] [Full Text] |
| 277. | Yan X, Gu S, Shi Y, Cui X, Wen S, Ge J. The effect of emodin on Staphylococcus aureus strains in planktonic form and biofilm formation in vitro. Arch Microbiol. 2017;199:1267-1275. [PubMed] [DOI] [Full Text] |
| 278. | Janeczko M, Masłyk M, Kubiński K, Golczyk H. Emodin, a natural inhibitor of protein kinase CK2, suppresses growth, hyphal development, and biofilm formation of Candida albicans. Yeast. 2017;34:253-265. [PubMed] [DOI] [Full Text] |
| 279. | Packiavathy IA, Priya S, Pandian SK, Ravi AV. Inhibition of biofilm development of uropathogens by curcumin - an anti-quorum sensing agent from Curcuma longa. Food Chem. 2014;148:453-460. [PubMed] [DOI] [Full Text] |
| 280. | Samoilova Z, Tyulenev A, Muzyka N, Smirnova G, Oktyabrsky O. Tannic and gallic acids alter redox-parameters of the medium and modulate biofilm formation. AIMS Microbiol. 2019;5:379-392. [PubMed] [DOI] [Full Text] |
| 281. | Khayat MT, Ibrahim TS, Khayyat AN, Alharbi M, Shaldam MA, Mohammad KA, Khafagy ES, El-Damasy DA, Hegazy WAH, Abbas HA. Sodium Citrate Alleviates Virulence in Pseudomonas aeruginosa. Microorganisms. 2022;10. [PubMed] [DOI] [Full Text] |
| 282. | Vikram A, Jesudhasan PR, Pillai SD, Patil BS. Isolimonic acid interferes with Escherichia coli O157:H7 biofilm and TTSS in QseBC and QseA dependent fashion. BMC Microbiol. 2012;12:261. [PubMed] [DOI] [Full Text] |
| 283. | Kumar L, Chhibber S, Kumar R, Kumar M, Harjai K. Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia. 2015;102:84-95. [PubMed] [DOI] [Full Text] |