Published online Apr 15, 2023. doi: 10.4239/wjd.v14.i4.424
Peer-review started: December 15, 2022
First decision: January 20, 2023
Revised: February 8, 2023
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: April 15, 2023
Processing time: 117 Days and 19.1 Hours
Semaglutide is a glucagon-like peptide-1 receptor agonist used either orally every day or subcutaneously once a week for the treatment of type 2 diabetes mellitus and, more recently, at higher doses, for the treatment of obesity. Both diseases are reaching epidemic proportions and often coexist, posing patients with a high risk for cardiovascular disease and death. Therefore, an agent such as semaglutide, which offers clinically significant weight loss and cardiovascular benefits, is essential and will be increasingly used in high-risk patients. However, during the SUSTAIN clinical trial program (Semaglutide Unabated Sustainability in treat-ment of type 2 diabetes), a safety issue concerning the progression and worsening of diabetic retinopathy emerged. The existing explanation so far mainly supports the role of the magnitude and speed of HbA1c reduction, a phenomenon also associated with insulin treatment and bariatric surgery. Whether and to which extent the effect is direct is still a matter of debate and an intriguing topic to investigate for suitable preventative and rehabilitative purposes. In this minireview, we will summarize the available data and suggest guidelines for a comprehensive semaglutide clinical utilization until new evidence becomes available.
Core Tip: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) benefit patients with type 2 diabetes mellitus in terms of glucose management, weight control, oxidative damage, and prevention of adverse renal and cardiovascular events without increasing the of risk of hypoglycemia. After reviewing the literature to investigate upon such a clinically relevant issue, the authors found that: Semaglutide per se seems to cause no direct damage to the retina, and the reported adverse effects might even be ascribed to a bias in the trial design. Special attention to the retinopathy status should be paid at present when using semaglutide in older patients with longstanding diabetes.
- Citation: Cigrovski Berkovic M, Strollo F. Semaglutide-eye-catching results. World J Diabetes 2023; 14(4): 424-434
- URL: https://www.wjgnet.com/1948-9358/full/v14/i4/424.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i4.424
Glucagon-like peptide-1 receptor agonists (GLP-1RA) are a class of drugs increasingly used for the treatment of patients with type 2 diabetes mellitus (T2DM), enhancing glucose management while promoting weight loss and exposing patients to no significant hypoglycemic risk[1]. In addition to the abovementioned advantages, most agents within the class also provide cardiovascular benefits to diabetic patients, who are well known to be at high risk for cardiovascular atherosclerotic disease. Specifically, seven dedicated cardiovascular outcome trials (CVOTs), i.e., LEADER, SUSTAIN-6, EXSCEL, HARMONY, REWIND, ELIXA, and PIONEER-6, included patients on liraglutide, once weekly sc semaglutide, exenatide, albiglutide, dulaglutide, lixisenatide, and daily oral semaglutide respectively. Data obtained from those CVOTs suggested benefits for T2DM patients with a longstanding history of diabetes, obesity, or overweight in terms of primary and secondary prevention of adverse cardiovascular (CV) events for liraglutide, semaglutide, albiglutide, and dulaglutide, while neutrality was proven in the ELIXA trial[2-8]. Other benefits were also noticed, including reduced albuminuria and improved kidney function in terms of hindered diabetic nephropathy progression and lower need for kidney replacement therapy[9]. However, conflicting data were reported concerning another relevant microvascular complication, i.e., diabetic retinopathy (DR)[10].
DR is the main microvascular complication of diabetes, currently affecting approximately 100 million people worldwide. It increases the likelihood of visual impairment and blindness by 64% and 27%, respectively. Data from the US suggests that almost a third of patients with diabetes over 40 years of age have DR[11,12]. According to the meta-analysis performed for patients with type 1 diabetes, the long-term risk of developing DR can be reduced by intensive glucose control, granting the achievement of near-normal glucose levels[13]. Despite United Kingdom Prospective Diabetes Study results showing that each 1% reduction in HbA1c was associated with a 37% reduction in the development of retinopathy in patients with T2DM[14], conflicting results were also reported concerning the association of tight glucose control with the worsening of previously identified DR signs[15]. However, along with persistent hyperglycemia, risk factors for DR onset include the patient’s age, diabetes duration, and high blood pressure, together with genetic predisposition, smoking habits, anemia, non-Caucasian ethnicity, and hyperlipidemia[16-19]. Indeed, pre-existing DR progression can also be related to the magnitude of HbA1c reduction, where data support the evidence of temporary, paradoxical DR worsening during the first three to 36 mo of initiation of intensive glucose lowering before the long-term benefits of glucose optimization become apparent[13,20,21]. The abovementioned deterioration in DR has been described in patients with T1DM and T2DM and during pregnancy. It was initially noticed in patients intensively treated with continuous subcutaneous insulin infusion, but afterward with various diabetic pharmacotherapy, as well as bariatric surgery and pancreatic transplantation[22].
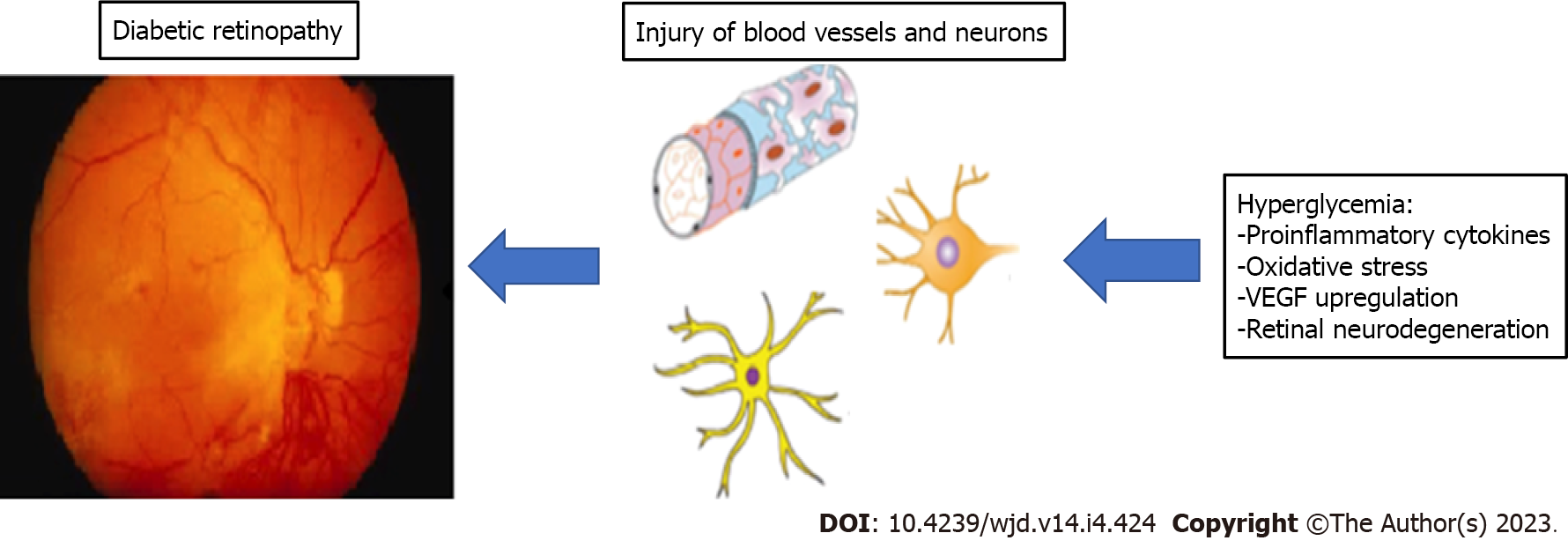
The pathophysiology of DR includes persistent hyperglycemia-related retinal microvessel dilatation, abnormal permeability, and ischemic occlusion with subsequent neovascularization resulting from up-regulation of vascular endothelial growth factor (VEGF) expression[14,23-25]. The evidence available from in vivo and in vitro models suggests a significant role of longstanding and generalized low-grade chronic inflammation inducing neurodegeneration and oxidative stress at the retinal level[26-29]. Also, according to Zhou et al[30], visual function maintenance requires special attention to any available methods, including GLP-1 analogs, to protect retinal ganglion cells from their high intrinsic vulnerability to any sources of damage.
In addition, what is often forgotten is that the metabolic memory, widely recognized as a significant factor in diabetes complications onset and progression, involves the whole body, including the eye, where it acts by increasing the cytosolic protein Drp1 translocation inside the mitochondria with enhanced GTPase activity in response to various stimuli, including hyperglycemia[31,32]. Such changes consistently impair mitochondrial dynamics even after getting back to normoglycemia. Because of that, retinal capillary cells undergo accelerated apoptosis due to cytochrome C leakage into the cytosol due to functional and structural mitochondrial changes, including the DNA. This phenomenon impairs perfusion of acellular capillaries with subsequent neovascularization[33,34].
Although various theories exist trying to explain changes in retinal blood flow and hemodynamics associated with early worsening of DR, the mechanism has not been fully elucidated, while optimal in vitro conditions and animal models are still lacking[35]. For example, the osmotic theory suggests blood glucose changes to alter lens hydration through altered osmotic pressure, thus causing hyperopic/myopic changes[36,37]. Indeed, insulin has been mostly associated with early DR worsening so far. The mechanisms behind that phenomenon seem to be blood-retinal barrier disruption in the presence of hyperglycemia, consequent to microvascular changes induced by enhanced VEGF production and expression through increased endothelial cell reactive oxygen species (ROS) concentrations, and the often associated hypoglycemic events, which also trigger oxidative stress thus further building up ROS concentrations over time[35,38-40]. In addition, persistently increased IGF-1 levels accompanying fast-improved glucose control might also have a role in initiating or worsening DR[41]. The classification and reporting of this phenomenon are further complicated by the highly variable definition of “DR worsening” by different authors, including cotton-wool exudates, hemorrhages, microaneurysms, intraretinal microvascular abnormalities, and capillary-free areas[42]. Nonetheless, we feel reassured enough by some reports concerning improved DR in the follow-up of patients maintaining tight glucose control over time[43].
As already pointed out, among GLP-1RAs, semaglutide, dulaglutide, and liraglutide proved to prevent major cardiovascular events in the large CVOTs published so far[44]. However, the mechanism behind this observation is still unclear but might include the already mentioned antihyperglycemic and weight loss effects, the potential to decrease systolic and diastolic blood pressure, and more direct endothelial protective mechanisms[45]. In addition to that, GLP-1RAs offer benefits in terms of lowering unfavorable renal outcomes[9]. Such relevant effects also depend on their ability to rapidly and consistently grant quite good glycemic control with significant reductions in HbA1c levels[46]. Their effect on DR is poorly understood and somewhat conflicting, as experimental data even suggests for GLP-1RAs protective effects through hindered blood-retinal barrier disruption and retinal neuron apoptosis[47].
On the other hand, one of the published retrospective studies with exenatide showed a significant proportion of treated patients with fast-improved HbA1c to be at risk of development and progression of DR, in addition to other potentially identified risk factors such as duration of diabetes and presence of preexistent DR[48]. However, the follow-up study published by the same authors reported improved DR in 62% of patients, with no documented DR status changes in a further 18% of patients and DR progression in the remaining 20% (n = 8). Moreover, maculopathy verified in the initial study either regressed or kept stable. In the case of patients with new-onset DR occurring after exenatide initiation, there was evidence of some improvement in 71% (n = 10), and stabilization in three more patients (21%)[49]. An additional interventional case study with exenatide showed complete regression of diabetes-related macular edema and improved visual acuity within one month[50]. On the other hand, multivariate analysis in the AngioSafe study, which included a cohort of 3154 patients with T2DM, found no association between the exposure to GLP-1RAs (liraglutide and exendin-4) and severe DR (P = 0.47)[51]. Similarly, cohort studies from registries did not show any additional risk of DR complications with GLP-1RAs as compared to oral incretin therapy with dipeptidyl peptidase-4 (DPP-4) inhibitors[52], while the results of Brooks and Lissett’s investigation suggested a dramatic deterioration of DR be associated with significantly improved HbA1c levels achieved within four months of exenatide treatment[53].
Further on, the LEADER, SUSTAIN-6, and REWIND CVOTs, including liraglutide, subcutaneous semaglutide, and dulaglutide and having retinopathy as a secondary outcome measure (quite broadly pre-specified as vitreous hemorrhage, diabetes-related blindness defined as Snellen visual acuity equal or less than 20/200 or a visual field lower than 20 degrees, or requirement for retinal photocoagulation, intra-vitreal anti- VEGF treatment, or vitrectomy) reported no decrease in DR rate[3,4,7]. Instead, DR complications tended to increase in LEADER and REWIND [hazard ratios 1.15 (95%CI: 0.87-1.52) and 1.24 (95%CI: 0.92-1.68), and significantly increased in the SUSTAIN-6 trial [50 events/1648 people in the semaglutide group vs 29 events/1649 people in the placebo group; hazard ratio 1.76 (95%CI: 1.11-2.78)]. In the meta-analysis by Bethel and coauthors[54] including patients treated with semaglutide (oral and subcutaneous), dulaglutide, exenatide, and albiglutide, with similar age (range 62-66 years), and BMI (32-33 kg/m2), 31%-46% were women, and the mean duration of diabetes ranged from 10 to 15 years. Mean HbA1c ranged from 7.3% to 8.7% (56.3-71.6 mmol/mol), and previous CVD was established in all patients included in the HARMONY trial (albiglutide), while 70% of patients in the REWIND trial (dulaglutide) only had CV risk factors. The prevalence of a differently defined among trials DR ranged from 9.0% to 28.2% at baseline in the REWIND, HARMONY, and PIONEER-6 trials. Moreover, PIONEER-6 excluded patients with already established DR, while only SUSTAIN-6 and PIONEER-6 evaluated retinopathy outcomes with fundus photography or fundoscopy as a scheduled study protocol. The authors did not report a significant association between DR risk and overall GLP-1RA use. However, the data suggested higher differences in the HbA1c levels during the first three months of GLP-1RA initiation in SUSTAIN-6 to represent the most significant risk for DR, while trials EXSCEL, HARMONY, and PIONEER-6, which were associated with negligible impact on HbA1c during the first three months of treatment initiation, did not point out any significant DR risk. Moreover, in line with other evidence, DR risk was not related to blood pressure or weight changes[55]. So, the most critical limitation in DR retinopathy data interpretation is the non-homogeneity of all GLP-1RAs CVOTs in the method of adjudication of DR events and the assessment of DR through widely used scores[10,56,57].
Semaglutide had an extensive efficacy and safety program (SUSTAIN). In studies SUSTAIN 1-5 and SUSTAIN 7, the DR status was annotated within the medical history as not present, present, or unknown, and if present, designated as proliferative, nonproliferative, or unknown and considered an adverse event; patients with proliferative DR and maculopathy requiring acute treatment or HbA1c > 10% and > 10.5%, respectively, did not enter the study. In the SUSTAIN-6, instead, patient exclusion criteria did not take into consideration upper HbA1c limits or the presence of DR, which was indeed adjudicated as a composite endpoint as already described[58,59]. When compared to the overall SUSTAIN-6 study population, those patients developing eye complications had the more severe disease as defined by longer diabetes duration (17.5 years vs 13.9 years), higher initial HbA1c (9.4% vs 8.7%), and higher insulin-treatment rate (75.9% vs 58.0%). Moreover, those patients had a higher proportion of more advanced DR complications at baseline and achieved a more relevant HbA1c reduction during the first 16 wk of semaglutide treatment[58].
On the other hand, a recent meta-analysis of GLP-1RA CVOTs failed to find any association between the drug class with retinopathy. However, after stressing that the abovementioned trials had a median follow-up of 3.4 years (i.e., much shorter than needed for retinopathy onset), used different diagnostic criteria, and were not even powered enough to assess the incidence of retinopathy, the authors reported on a significant association of such complication with HbA1c downward slope (i.e., 0.77, P < 0.01), quantifiable as a 6%, 14%, or 8% increased Ln (OR) at 3-mo, 1-year, and overall follow-up, respectively, every 0.1% (1.09 mmol/mol) increase in HbA1c reduction[54].
On the whole, according to a post hoc analysis, the degree of HbA1c decrease and pre-existing retinopathy stood out as the main retinopathy worsening factors[58], yet SUSTAIN-7, i.e., a head-to-head dulaglutide-semaglutide comparison study, showed DR onset or worsening in only two patients (1%) on semaglutide 0.5 mg, two (1%) on dulaglutide 0.75 mg, two (1%) on semaglutide 1.0 mg, and three (1%) on dulaglutide 1.5 mg[59]. Indeed, as no one expects any drugs to develop advanced DR signs in such a short period as the one provided by SUSTAIN-6, a randomization bias might have occurred regarding DR severity assessment at study entry due to the focus on cardiovascular disease monitoring. Once again, the above consideration and the dramatic drop in blood glucose levels might better explain DR results[60].
Contrary to what we reported above, when turning to GLP1-RAs utilization in obesity, retinopathy was not reported as a complication in long-duration trials assessing weekly semaglutide 2.4 mg injection vs placebo for weight loss in obese patients[61-63], and based on a recent revision of the literature, no definite conclusions can be drawn on the role of semaglutide in the incidence or worsening of retinopathy[64], especially when taking into account a systematic review and meta-analysis of all trials, which ruled out any increased DR risk compared to placebo (RR: 1.14, 95%CI: 0.98-1.33), despite suggesting caution in the case of older patients with the long-standing disease (age ≥ 60 years and diabetes duration ≥ 10 years) due to a higher DR risk (RR: 1.27, 95%CI: 1.02-1.59; RR: 1.28, 95%CI: 1.04-1.58, respectively)[65].
The Consortium for the Early Treatment of DR (EUROCONDOR) study failed to find any signs of neurodegeneration in a significant percentage of patients with microangiopathic retinal lesions[66,67]. However, as already mentioned before, neurodegeneration has been proposed as a hallmark of DR[68-70]. Indeed, the American Diabetes Association (ADA) has classified DR as a microvascular and neurovascular complication[29]. In Figure 1 we present the schematics of development of DR. So, at present, drugs like GLP-1RAs, expected to protect both neurons and microvessels, are suggested for the management of early DR stages[71,72].
In such a perspective, a protocol recently set up to rule out a direct role of the drug in retinal damage evaluated the effect of a semaglutide eye-drop solution on retinal neurodegeneration, neuroinflammation, and early vascular leakage in mice. Study results suggested that the drug prevents exactly those three features of diabetes-related retinal damage. The mechanism behind this phenomenon seemed to rely on the decisive anti-inflammatory action linked to decreased expression of NF-kB, proinflammatory cytokines (IL-1, IL-6, and IL-18), and ICAM-1, as well as on the prevention of neuroretinal cell apoptosis promoted by the activation of Akt pathway, which is essential for neuron survival[70]. Moreover, as blood glucose levels were not affected by topical administration, no confounding factors were present due to eventually occurring drops in glucose concentrations. Interestingly, recent results from mice experiments also suggested that semaglutide is unable to cross the blood-brain barrier[73] and has beneficial rather than deleterious effects, as already reported with other GLP-1RAs in the experimental animals[71,72,74,75], possibly due to improved blood-retina barrier function and neuronal apoptosis[47], reduced glutamate levels obtained through upregulated glutamate-aspartate transporter (GLAST)[75], and decreased placental growth factor and intercellular adhesion molecule-1 expression[71,72].
The potential direct mechanism of action of GLP-1RA on the retinal cells is still elusive and debated. Although the GLP-1 receptor is present in the human eye, no GLP-1R expression was found in the eyes of people with long-standing proliferative DR[76].
However, the effects of a newly identified confounding factor, i.e., subtle differences in individual gut microbiota composition, cannot be easily ruled out. Indeed, despite the relationship between intestinal microbiota and host still requiring complete elucidation, gut dysbiosis, i.e., the chronic disequilibrium within the many different microbial colonies, seems associated with several inflammatory/metabolic diseases and central nervous system disorders, including retinopathy as an expression of the emerging concept of the so-called “microbiota-retina axis”. Indeed, as longstanding diabetes is associated not only with retinopathy but also with significant intestinal dysbiosis[77,78], relevant changes in the bacterial population might trigger the onset of retinopathy via their influence on the lipid content of both retinal and CNS tissues, eventually responsible for decreased intraocular succinate concentrations or increased trimethylamine-N-oxide (TMAO) plasma levels[79-86].
Retinopathy is a disabling disease, so rehabilitation should start as soon as possible when prevention fails. Based on the abovementioned neuroinflammatory mechanisms, several anti-inflammatory substances may help prevent DR progression, including nutritional supplements like resveratrol. However, the latter, despite looking promising enough, has a too low level of bioavailability and is contraindicated in the frequently occurring case of iron-deficiency-dependent anemia[87].
Semaglutide has a 94% amino acid sequence homology to native GLP-1. However, structural modifications from endogenous GLP-1, i.e., alanine residue substitution at the 8th position with Aib, make it less susceptible to degradation by DPP-4 while acylation of Lysine residue at the 26th position and attachment of a C18 fatty-diacid increase its binding affinity to albumin. The above changes result in a half-life of approximately one week, making it appropriate for once-weekly use in clinical practice. In the phase-3 SUSTAIN trials semaglutide showed superiority to different comparators and during different stages of diabetes in reducing HbA1c and body weight. In a SUSTAIN-6 trial investigating cardiovascular outcomes, semaglutide led to a 26% reduction in risk of the primary 3-point MACE (a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) when compared to placebo. The pharmacokinetic properties of semaglutide are not significantly affected by impaired hepatic or renal function. Therefore, no dose adjustments are required in that case. It achieves a steady state concentration in 4 wk to 5 wk (both subcutaneous and oral form). Indeed, in the case of subcutaneous semaglutide, 1 d to 3 d are needed to achieve the maximum concentration, and in the case of oral semaglutide, one hour is needed following intake. Moreover, during clinical pharmacology studies, no relevant impact of semaglutide on concomitant orally administered medications was observed, making its use safe in a broad population[88]. Semaglutide improves the efficiency of incretin function by activating GLP-1 receptors and enhancing GLP-1 to supraphysiological levels. It reduces fasting and postprandial glucose levels by promoting insulin secretion in a glucose-dependent manner and suppressing hepatic gluconeogenesis through blunted glucagon release. Moreover, it improves both proinsulin to insulin ratio, which suggests improved β-cell function and insulin sensitivity through body weight and fat loss consequent to reduced energy intake and gastric motility[89].
A cardiovascular risk (3-P MACE) reduction effect compared to placebo was shown for injectable and oral semaglutide in SUSTAIN-6 and PIONEER-6 trials, respectively. Animal studies have shown antiatherosclerotic effects mediated by regulating multiple inflammatory pathways and antiapoptotic effects in cardiac cells[90]. In addition, three ongoing trials, i.e., SOUL (NCT03914326), SELECT (Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity; NCT03574597), and STRIDE (NCT04560998), will give further insight into different cardiovascular effects of both oral and subcutaneous semaglutide in patients with and without T2DM, and overweight/obesity[91-93].
The semaglutide mechanism of action is glucose-dependent and, therefore, associated per se with a shallow risk of hypoglycemia. Nevertheless, semaglutide cannot avoid the risk of hypoglycemia due to add-on sulfonylureas or insulin. Such drug association might initially cause acute glucose level drops and probably transient worsening of DR, as observed in the first four weeks of treatment[58].
Nowadays, the most robust relationship between semaglutide treatment and early worsening of DR might find its sizable initial impact on HbA1c as a suitable explanation. Indeed, both semaglutide formulations (subcutaneous and oral) have been investigated in two phase-3 clinical programs, i.e., the SUSTAIN and PIONEER program. Combining all individual studies within these two programs, over 21500 patients participated in the studies with a treatment duration of at least 26 wk, giving a plethora of safety evidence[94]. Data insinuating a connection between semaglutide and DR emerged during the phase-3 trials. However, the answer to whether that phenomenon depended on the drug itself or the magnitude of fast-occurring glucose lowering will come from the presently underway FOCUS trial on approximately 1500 patients with T2DM, a bilateral 10 to 75 Early Treatment DR Study (ETDRS) score and no need for ophthalmologic therapy. Such a study will analyze once-weekly subcutaneous semaglutide 1.0 mg compared with placebo for up to 5 years, with the primary endpoint of at least three-digit ETDRS score progression[95]. Until FOCUS results become available, caution is mandatory in patients with DR. It may be wise to perform fundoscopy prior to semaglutide therapy, and existing DR should be treated concomitantly. In addition, given the strong effects of semaglutide on glucose levels, down-titrating basal insulin therapy or stopping sulphonylurea will prevent rapid decreases in glucose concentrations, thereby reducing the risk of acute DR worsening. Guidelines on DR management do not differ for patients receiving other antidiabetic agents. Therefore, the same treatment, including anti-VEGF agents, should be applied.
Both current and future research on GLP-1RA is quite exciting and targets different metabolic conditions and health aspects associated with both T2DM and obesity, such as cognitive health and dementia, PCO, and NAFLD.
However, the scientific community must shed light on the topic and draw definite conclusions concerning a direct mechanism of one (or more) GLP-1RA class drug(s) in retinopathy development and worsening.
Care required by T2DM patients initiating GLP-1RA treatment does not differ from that expected with other types of intensive glucose-lowering medication, i.e., early detection of DR onset/progression and, eventually, specific treatment. Screening for DR is recommended for patients at the time of T2DM diagnosis and then annually while also taking into account individual metabolic control and baseline DR conditions[96]. In the case of severe, proliferative DR, treatment of retinopathy should start before or with intensive glucose-lowering therapy, expecting a typically transient worsening though[97]. While increasingly more overweight and obese T2DM patients at high CV risk start on GLP-1RAs, clinicians should consider their DR status upon initiation and adopt the best available treatment for DR when present.
When choosing semaglutide in the intensification of T2DM treatment, a cautious attitude is mandatory, at the moment, especially in the case of coexisting insulin treatment and advanced disease stages.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alsaidan A, Saudi Arabia; Morya AK, India S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, Ceriello A, Chiodini P, Esposito K. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 2. | Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC; ELIXA Investigators. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 1733] [Article Influence: 173.3] [Reference Citation Analysis (0)] |
| 3. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 4918] [Article Influence: 546.4] [Reference Citation Analysis (0)] |
| 4. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3025] [Cited by in RCA: 4087] [Article Influence: 454.1] [Reference Citation Analysis (1)] |
| 5. | Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1599] [Cited by in RCA: 1495] [Article Influence: 186.9] [Reference Citation Analysis (0)] |
| 6. | Green JB, Hernandez AF, D'Agostino RB, Granger CB, Janmohamed S, Jones NP, Leiter LA, Noronha D, Russell R, Sigmon K, Del Prato S, McMurray JJV. Harmony Outcomes: A randomized, double-blind, placebo-controlled trial of the effect of albiglutide on major cardiovascular events in patients with type 2 diabetes mellitus-Rationale, design, and baseline characteristics. Am Heart J. 2018;203:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1921] [Cited by in RCA: 1799] [Article Influence: 299.8] [Reference Citation Analysis (1)] |
| 8. | Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC; PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 1184] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 9. | Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 1065] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 10. | Saw M, Wong VW, Ho IV, Liew G. New anti-hyperglycaemic agents for type 2 diabetes and their effects on diabetic retinopathy. Eye (Lond). 2019;33:1842-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Leasher JL, Bourne RR, Flaxman SR, Jonas JB, Keeffe J, Naidoo K, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR; Vision Loss Expert Group of the Global Burden of Disease Study. Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-analysis From 1990 to 2010. Diabetes Care. 2016;39:1643-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 415] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 12. | Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 879] [Cited by in RCA: 805] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 13. | Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group; Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015;64:631-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 14. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [PubMed] |
| 15. | Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;2014:CD009122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 16. | Scanlon PH. Improving the screening of risk factors in diabetic retinopathy. Expert Rev Endocrinol Metab. 2022;17:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Bryl A, Mrugacz M, Falkowski M, Zorena K. The Effect of Hyperlipidemia on the Course of Diabetic Retinopathy-Literature Review. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Bryl A, Mrugacz M, Falkowski M, Zorena K. The Effect of Diet and Lifestyle on the Course of Diabetic Retinopathy-A Review of the Literature. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 19. | Milluzzo A, Barchitta M, Maugeri A, Magnano San Lio R, Favara G, Mazzone MG, Sciacca L, Agodi A. Do Nutrients and Nutraceuticals Play a Role in Diabetic Retinopathy? Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116:874-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 352] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Bain SC, Klufas MA, Ho A, Matthews DR. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes Metab. 2019;21:454-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 22. | Feldman-Billard S, Larger É, Massin P; Standards for screeningand surveillance of ocular complications in people with diabetes SFD study group. Early worsening of diabetic retinopathy after rapid improvement of blood glucose control in patients with diabetes. Diabetes Metab. 2018;44:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Beltramo E, Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem. 2013;20:3218-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 24. | Lupo G, Motta C, Giurdanella G, Anfuso CD, Alberghina M, Drago F, Salomone S, Bucolo C. Role of phospholipases A2 in diabetic retinopathy: in vitro and in vivo studies. Biochem Pharmacol. 2013;86:1603-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16280] [Article Influence: 508.8] [Reference Citation Analysis (3)] |
| 26. | Abcouwer SF. Müller Cell-Microglia Cross Talk Drives Neuroinflammation in Diabetic Retinopathy. Diabetes. 2017;66:261-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53:971-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, Juzych MS, Abrams GW, Kumar A. Assessment of Neurotrophins and Inflammatory Mediators in Vitreous of Patients With Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2017;58:5594-5603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 29. | Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:412-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 445] [Cited by in RCA: 600] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 30. | Zhou HR, Ma XF, Lin WJ, Hao M, Yu XY, Li HX, Xu CY, Kuang HY. Neuroprotective Role of GLP-1 Analog for Retinal Ganglion Cells via PINK1/Parkin-Mediated Mitophagy in Diabetic Retinopathy. Front Pharmacol. 2020;11:589114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Mohammad G, Kowluru RA. Mitochondrial Dynamics in the Metabolic Memory of Diabetic Retinopathy. J Diabetes Res. 2022;2022:3555889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Duraisamy AJ, Mohammad G, Kowluru RA. Mitochondrial fusion and maintenance of mitochondrial homeostasis in diabetic retinopathy. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1617-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 33. | Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805-3811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 34. | Kowluru RA. Mitochondrial Stability in Diabetic Retinopathy: Lessons Learned From Epigenetics. Diabetes. 2019;68:241-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Jingi AM, Tankeu AT, Ateba NA, Noubiap JJ. Mechanism of worsening diabetic retinopathy with rapid lowering of blood glucose: the synergistic hypothesis. BMC Endocr Disord. 2017;17:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Okamoto F, Sone H, Nonoyama T, Hommura S. Refractive changes in diabetic patients during intensive glycaemic control. Br J Ophthalmol. 2000;84:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Casson RJ, Wood JP, Osborne NN. Hypoglycaemia exacerbates ischaemic retinal injury in rats. Br J Ophthalmol. 2004;88:816-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Wu H, Jiang C, Gan D, Liao Y, Ren H, Sun Z, Zhang M, Xu G. Different effects of low- and high-dose insulin on ROS production and VEGF expression in bovine retinal microvascular endothelial cells in the presence of high glucose. Graefes Arch Clin Exp Ophthalmol. 2011;249:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Poulaki V, Qin W, Joussen AM, Hurlbut P, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. 2002;109:805-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Kennedy A, Frank RN. The influence of glucose concentration and hypoxia on VEGF secretion by cultured retinal cells. Curr Eye Res. 2011;36:168-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Henricsson M, Berntorp K, Fernlund P, Sundkvist G. Progression of retinopathy in insulin-treated type 2 diabetic patients. Diabetes Care. 2002;25:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Wong TY, Cheung CM, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 699] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 43. | Kohner EM, Sleightholm M. Does microaneurysm count reflect severity of early diabetic retinopathy? Ophthalmology. 1986;93:586-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S125-S150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 319] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 45. | Ma X, Liu Z, Ilyas I, Little PJ, Kamato D, Sahebka A, Chen Z, Luo S, Zheng X, Weng J, Xu S. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci. 2021;17:2050-2068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 46. | Dungan KM, Povedano ST, Forst T, González JG, Atisso C, Sealls W, Fahrbach JL. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 47. | Pang B, Zhou H, Kuang H. The potential benefits of glucagon-like peptide-1 receptor agonists for diabetic retinopathy. Peptides. 2018;100:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Varadhan L, Humphreys T, Hariman C, Walker AB, Varughese GI. GLP-1 agonist treatment: implications for diabetic retinopathy screening. Diabetes Res Clin Pract. 2011;94:e68-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Varadhan L, Humphreys T, Walker AB, Varughese GI. The impact of improved glycaemic control with GLP-1 receptor agonist therapy on diabetic retinopathy. Diabetes Res Clin Pract. 2014;103:e37-e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Sarao V, Veritti D, Lanzetta P. Regression of diabetic macular edema after subcutaneous exenatide. Acta Diabetol. 2014;51:505-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Gaborit B, Julla JB, Besbes S, Proust M, Vincentelli C, Alos B, Ancel P, Alzaid F, Garcia R, Mailly P, Sabatier F, Righini M, Gascon P, Matonti F, Houssays M, Goumidi L, Vignaud L, Guillonneau X, Erginay A, Dupas B, Marie-Louise J, Autié M, Vidal-Trecan T, Riveline JP, Venteclef N, Massin P, Muller L, Dutour A, Gautier JF, Germain S. Glucagon-like Peptide 1 Receptor Agonists, Diabetic Retinopathy and Angiogenesis: The AngioSafe Type 2 Diabetes Study. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 52. | Ueda P, Pasternak B, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, Hveem K, Jonasson C, Melbye M, Svanström H. Glucagon-Like Peptide 1 Receptor Agonists and Risk of Diabetic Retinopathy Complications: Cohort Study in Nationwide Registers From Two Countries. Diabetes Care. 2019;42:e92-e94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Brooks AM, Lissett CA. A dramatic deterioration in diabetic retinopathy with improvement in glycated haemoglobin (HbA(1c)) on exenatide treatment. Diabet Med. 2009;26:190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Bethel MA, Diaz R, Castellana N, Bhattacharya I, Gerstein HC, Lakshmanan MC. HbA(1c) Change and Diabetic Retinopathy During GLP-1 Receptor Agonist Cardiovascular Outcome Trials: A Meta-analysis and Meta-regression. Diabetes Care. 2021;44:290-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 55. | Zhu W, Wu Y, Meng YF, Xing Q, Tao JJ, Lu J. Association of obesity and risk of diabetic retinopathy in diabetes patients: A meta-analysis of prospective cohort studies. Medicine (Baltimore). 2018;97:e11807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 56. | Do DV, Wang X, Vedula SS, Marrone M, Sleilati G, Hawkins BS, Frank RN. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD006127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT; Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1980] [Cited by in RCA: 2292] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 58. | Vilsbøll T, Bain SC, Leiter LA, Lingvay I, Matthews D, Simó R, Helmark IC, Wijayasinghe N, Larsen M. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 59. | Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, Viljoen A; SUSTAIN 7 investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 519] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 60. | Simó R, Bogdanov P, Ramos H, Huerta J, Simó-Servat O, Hernández C. Effects of the Topical Administration of Semaglutide on Retinal Neuroinflammation and Vascular Leakage in Experimental Diabetes. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, Lingvay I, O'Neil PM, Rubino DM, Skovgaard D, Wallenstein SOR, Garvey WT; STEP 3 Investigators. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA. 2021;325:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 594] [Article Influence: 148.5] [Reference Citation Analysis (0)] |
| 62. | Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 2216] [Article Influence: 554.0] [Reference Citation Analysis (0)] |
| 63. | Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, Lingvay I, Mosenzon O, Rosenstock J, Rubio MA, Rudofsky G, Tadayon S, Wadden TA, Dicker D; STEP 4 Investigators. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021;325:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 688] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 64. | Gallwitz B, Giorgino F. Clinical Perspectives on the Use of Subcutaneous and Oral Formulations of Semaglutide. Front Endocrinol (Lausanne). 2021;12:645507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 65. | Wang F, Mao Y, Wang H, Liu Y, Huang P. Semaglutide and Diabetic Retinopathy Risk in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Clin Drug Investig. 2022;42:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 66. | Santos AR, Ribeiro L, Bandello F, Lattanzio R, Egan C, Frydkjaer-Olsen U, García-Arumí J, Gibson J, Grauslund J, Harding SP, Lang GE, Massin P, Midena E, Scanlon P, Aldington SJ, Simão S, Schwartz C, Ponsati B, Porta M, Costa MÂ, Hernández C, Cunha-Vaz J, Simó R; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Functional and Structural Findings of Neurodegeneration in Early Stages of Diabetic Retinopathy: Cross-sectional Analyses of Baseline Data of the EUROCONDOR Project. Diabetes. 2017;66:2503-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 67. | Hernández C, Porta M, Bandello F, Grauslund J, Harding SP, Aldington SJ, Egan C, Frydkjaer-Olsen U, García-Arumí J, Gibson J, Lang GE, Lattanzio R, Massin P, Midena E, Ponsati B, Ribeiro L, Scanlon P, Cunha-Vaz J, Simó R. The Usefulness of Serum Biomarkers in the Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Simó R, Hernández C; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration is an early event in diabetic retinopathy: therapeutic implications. Br J Ophthalmol. 2012;96:1285-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 69. | Simó R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61:1902-1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 390] [Article Influence: 55.7] [Reference Citation Analysis (1)] |
| 70. | Simó R, Simó-Servat O, Bogdanov P, Hernández C. Diabetic Retinopathy: Role of Neurodegeneration and Therapeutic Perspectives. Asia Pac J Ophthalmol (Phila). 2022;11:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 71. | Fan Y, Liu K, Wang Q, Ruan Y, Ye W, Zhang Y. Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic Goto-Kakizaki rats. Exp Eye Res. 2014;127:104-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 72. | Hernández C, Bogdanov P, Corraliza L, García-Ramírez M, Solà-Adell C, Arranz JA, Arroba AI, Valverde AM, Simó R. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes. 2016;65:172-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 73. | Salameh TS, Rhea EM, Talbot K, Banks WA. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer's and Parkinson's disease therapeutics. Biochem Pharmacol. 2020;180:114187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 74. | Zhang Y, Wang Q, Zhang J, Lei X, Xu GT, Ye W. Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Zhang Y, Zhang J, Wang Q, Lei X, Chu Q, Xu GT, Ye W. Intravitreal injection of exendin-4 analogue protects retinal cells in early diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 76. | Hebsgaard JB, Pyke C, Yildirim E, Knudsen LB, Heegaard S, Kvist PH. Glucagon-like peptide-1 receptor expression in the human eye. Diabetes Obes Metab. 2018;20:2304-2308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 77. | Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 1076] [Article Influence: 215.2] [Reference Citation Analysis (0)] |
| 78. | Liu K, Zou J, Fan H, Hu H, You Z. Causal effects of gut microbiota on diabetic retinopathy: A Mendelian randomization study. Front Immunol. 2022;13:930318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 79. | Oresic M, Seppänen-Laakso T, Yetukuri L, Bäckhed F, Hänninen V. Gut microbiota affects lens and retinal lipid composition. Exp Eye Res. 2009;89:604-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Albouery M, Buteau B, Grégoire S, Cherbuy C, Pais de Barros JP, Martine L, Chain F, Cabaret S, Berdeaux O, Bron AM, Acar N, Langella P, Bringer MA. Age-Related Changes in the Gut Microbiota Modify Brain Lipid Composition. Front Cell Infect Microbiol. 2019;9:444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 81. | Barba I, Garcia-Ramírez M, Hernández C, Alonso MA, Masmiquel L, García-Dorado D, Simó R. Metabolic fingerprints of proliferative diabetic retinopathy: an 1H-NMR-based metabonomic approach using vitreous humor. Invest Ophthalmol Vis Sci. 2010;51:4416-4421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 82. | Jin H, Zhu B, Liu X, Jin J, Zou H. Metabolic characterization of diabetic retinopathy: An (1)H-NMR-based metabolomic approach using human aqueous humor. J Pharm Biomed Anal. 2019;174:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 83. | Liu W, Wang C, Xia Y, Xia W, Liu G, Ren C, Gu Y, Li X, Lu P. Elevated plasma trimethylamine-N-oxide levels are associated with diabetic retinopathy. Acta Diabetol. 2021;58:221-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 84. | Nowiński A, Ufnal M. Trimethylamine N-oxide: A harmful, protective or diagnostic marker in lifestyle diseases? Nutrition. 2018;46:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 85. | Shan Z, Sun T, Huang H, Chen S, Chen L, Luo C, Yang W, Yang X, Yao P, Cheng J, Hu FB, Liu L. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017;106:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 86. | Bringer MA, Gabrielle PH, Bron AM, Creuzot-Garcher C, Acar N. The gut microbiota in retinal diseases. Exp Eye Res. 2022;214:108867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 87. | Bryl A, Falkowski M, Zorena K, Mrugacz M. The Role of Resveratrol in Eye Diseases-A Review of the Literature. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 88. | Ikushima I, Jensen L, Flint A, Nishida T, Zacho J, Irie S. A Randomized Trial Investigating the Pharmacokinetics, Pharmacodynamics, and Safety of Subcutaneous Semaglutide Once-Weekly in Healthy Male Japanese and Caucasian Subjects. Adv Ther. 2018;35:531-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Mahapatra MK, Karuppasamy M, Sahoo BM. Semaglutide, a glucagon like peptide-1 receptor agonist with cardiovascular benefits for management of type 2 diabetes. Rev Endocr Metab Disord. 2022;23:521-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 90. | Iorga RA, Bacalbasa N, Carsote M, Bratu OG, Stanescu AMA, Bungau S, Pantis C, Diaconu CC. Metabolic and cardiovascular benefits of GLP-1 agonists, besides the hypoglycemic effect (Review). Exp Ther Med. 2020;20:2396-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 91. | ClinicalTrials. A Heart Disease Study of Semaglutide in Patients With Type 2 Diabetes (SOUL). 2019. [cited 4 December 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT03914326. |
| 92. | ClinicalTrials. Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT). 2018. [cited 4 December 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT03574597. |
| 93. | ClinicalTrials. A Research Study to Compare a Medicine Called Semaglutide Against Placebo in People with Peripheral Arterial Disease and Type 2 Diabetes (STRIDE). 2020. [cited 4 December 2022]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04560998. |
| 94. | Smits MM, Van Raalte DH. Safety of Semaglutide. Front Endocrinol (Lausanne). 2021;12:645563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 95. | ClinicalTrials. US National Library of Medicine. A research study to look at how semaglutide compared to placebo affects diabetic eye disease in people with type 2 diabetes (FOCUS). [cited 4 December 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT03811561. |
| 96. | Introduction: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S1-S2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 97. | Aiello LP; DCCT/EDIC Research Group. Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |