Published online Apr 15, 2023. doi: 10.4239/wjd.v14.i4.364
Peer-review started: December 23, 2022
First decision: January 9, 2023
Revised: January 16, 2023
Accepted: March 21, 2023
Article in press: March 21, 2023
Published online: April 15, 2023
Processing time: 109 Days and 21.6 Hours
Wounds in diabetic patients, especially diabetic foot ulcers, are more difficult to heal compared with normal wounds and can easily deteriorate, leading to amputation. Common treatments cannot heal diabetic wounds or control their many complications. Growth factors are found to play important roles in regulating complex diabetic wound healing. Different growth factors such as transforming growth factor beta 1, insulin-like growth factor, and vascular endothelial growth factor play different roles in diabetic wound healing. This implies that a therapeutic modality modulating different growth factors to suit wound healing can significantly improve the treatment of diabetic wounds. Further, some current treatments have been shown to promote the healing of diabetic wounds by modulating specific growth factors. The purpose of this study was to discuss the role played by each growth factor in therapeutic approaches so as to stimulate further therapeutic thinking.
Core Tip: This review summarizes the main causes of poor wound healing in diabetes and the role of various therapeutically available growth factors in wound healing. In terms of treatment, it summarizes the treatment methods and drug delivery systems of various growth factors, and discusses the therapeutic effects of different methods and the special properties of drug delivery systems. We hope these discussions will provide the basis for more effective treatments, advance growth factor research, and help more people with diabetes heal their wounds.
- Citation: Zheng SY, Wan XX, Kambey PA, Luo Y, Hu XM, Liu YF, Shan JQ, Chen YW, Xiong K. Therapeutic role of growth factors in treating diabetic wound. World J Diabetes 2023; 14(4): 364-395
- URL: https://www.wjgnet.com/1948-9358/full/v14/i4/364.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i4.364
The prevalence of diabetes continues to increase at an alarming rate worldwide[1,2]. According to a recent analysis, the global prevalence of diabetes among adults aged 20-79 years is currently at 536.6 million and is projected to rise to 783.2 million by 2045[3]. As of 2015, diabetes was a direct cause of death for about 1.5 million people worldwide (WHO. Accessible at http://www.who.int/diabetes/en, accessed on 26 November 2022). Complications such as cardiovascular disease, nephropathy, retinopathy, neuropathy, and diabetic wounds occur in patients with diabetes. Diabetic wounds are one of the consequences having a lasting impact on patients with diabetes. Diabetic foot ulcer (DFU) is the most common type of diabetic wounds, which has a recurrence rate of 30%-50%[4,5]. Currently, no effective means of foreseeing the development of diabetic sores exist. Thus, the primary goals of treating diabetic wounds include identifying them early, performing a thorough examination, debriding and cleansing the wounds, and preventing or controlling the spread of infection.
One of the trickiest aspects of managing diabetes is dealing with wounds. Normal wound care is insufficient for diabetic wounds due to the differences in blood composition, vascular development, nerve survival, and inflammatory processes[6,7]. Many therapies have been developed for diabetic wounds in recent years[4,8], but those involving growth factors (GFs) have gained the maximum attention. GFs govern most of the processes involved in wound healing[9]. Although several GFs have been shown to be useful in treating diabetic wounds, only a few have been authorized for use in clinical practice. Among the three GF products available, only Regal Maltose has been approved by the Food and Drug Administration for treating neuropathic diabetic ulcers. The limitations in trial design, poor patient compliance, risk of immunogenicity, protein degradation, and variation in the responsiveness and healing support supplied by surrounding tissues are only a few of the many obstacles that stand in the way of the therapeutic application of GF products. It is essential, therefore, to have a firm grasp on the specifics of diabetic wounds, the healing effects of various GFs, and the provision of a dependable and efficient GF delivery system to propose a GF therapy.
In this review, we summarized the major factors contributing to impaired wound healing in patients with diabetes, and the significance of several GFs currently available for therapeutic use. We also conducted illustrative GF treatment experiments to explore various delivery mechanisms and facilitate an understanding about the therapeutic effects of various strategies.
Intermittent claudication, ischemia-induced rest discomfort, skin ulcers, and avascular necrosis are all symptoms of peripheral arterial disease (PAD), a group of disorders caused by arterial stenosis distal to the aortic arch. A strong correlation exists between diabetes and PAD; however, determining the true frequency of PAD in patients with diabetes is challenging due to the many complicating factors[10]. One cross-sectional study indicated that 43.87% of patients with DFU also had peripheral artery disease[11]. The most immediate effect of PAD, whether it affects local micro or macro vessels, is a disruption in blood flow or even ischemia[12]. Further, a number of negative consequences occur due to microcirculatory dysfunction. The microcirculation of patients with diabetes differs from that of patients without diabetes in several important ways, including increased vascular permeability, poor autoregulation, and unresponsiveness to vasodilatory stimuli[10,13]. The endothelial dysfunction due to hyperinsulinemia or hyperglycemia is the primary cause of these characteristics[14,15]. Capillary damage and oxidative stress are two complications of diabetes that can be exacerbated by microcirculatory perfusion problems[16].
Tissues surrounding diabetic wounds may suffer from hypoxia and anemia due to circulatory abnormalities triggered by PAD. The activity and gene expression in cells of injured tissues may be affected by hypoxia[17]. The combination of vascular damage and increased tissue oxygen consumption can lead to hypoxia in diabetic wounds, just as it does in regular wounds. However, hypoxia triggers hypoxia-inducible factor-1 (HIF-1) to enhance wound repair in healthy individuals, but hyperglycemia inhibits HIF-1 and hence slows wound healing in patients with diabetes[18-20]. Since hypoxia is detrimental to healing rather than serving as positive feedback that accelerates diabetic wound closure, it is no longer a factor in promoting wound closure. Lower oxygen levels in DFU were linked to slower wound healing in a study on flow-mediated skin fluorescence monitoring[21]. Furthermore, anemia can inhibit the healing process by inhibiting the metabolic pathways in injured tissues. Patients with diabetes often suffer from anemia, and those with severe foot ulcers are particularly at risk[22-24]. Patients with severe anemia have a higher likelihood of experiencing adverse malignant outcomes, according to a number of studies. These outcomes include a more severe disease-free interval, a more severe infection, and even death[23,25-27]. The impact of anemia on DFU is still debatable, as some studies have shown that it is not significantly linked to the severity or prognosis of DFU[28,29].
Moreover, diabetic wounds frequently exhibit impaired angiogenesis, which results in reduced vascularity and capillary density[30]. Inhibiting the death of important cells in damaged tissue, providing proliferative support activity, facilitating the remodeling phase of repair, and promoting healing growth are all facilitated by oxygen and nutrients provided by angiogenesis[31-33]. However, in diabetic wounds, many factors that promote angiogenesis are disrupted due to hyperglycemia and chronic inflammation, for example, the release of vascular endothelial growth factor (VEGF)[34] and platelet-derived growth factor (PDGF)[35] and the composition ratio of Ang1/Ang2/Tie2 complex[36,37]. Moreover, the unique internal environment of diabetes may also contribute to the effects of various anti-angiogenic factors, such as the anti-angiogenic factor pigment epithelium-derived factor[38]. These factors interfere with inflammation-mediated angiogenesis and delay the transition from inflammation to proliferative remodeling in wound healing.
Hyperglycemia can delay the healing of diabetic wounds and even exacerbate DFU through the impaired function of various skin cells and peripheral neuropathy. In patients with diabetes, hyperglycemia is an important factor causing dysfunction or reduction of endothelial cells[39-44], which are essential for the healing of diabetic wounds[45,46]. Further, hyperglycemia affects protein synthesis and migration and proliferation of keratinocytes and fibroblasts, which disrupts important processes of re-epithelialization[47-49], for instance, the altered expression of cytoskeletal keratin proteins (K2/K6/K10) and a laminin-5 α3 chain precursor protein (LM-3A32) in DFU keratinocytes[50]. Also, the fibroblasts from DFU exhibit morphological changes, GF energy, extracellular matrix (ECM) deposition, and reduced proliferation and migration of fibroblasts[51-54]. In the pathogenesis of neuropathy, hyperglycemia can damage nerves through the polyol pathway, hexosamine pathway, oxidative stress, advanced glycation end-products (AGEs) pathway, PARP pathway, NF-κB pathway, and so forth[55].
Hyperglycemia can also induce a delay in diabetic wound healing via free radicals or reactive oxygen species (ROS). In patients with diabetes, hyperglycemia can induce excessive ROS production through several pathways[56]: (1) Reactions in mitochondria[57,58]; (2) impairment of intracellular antioxidative defense systems[59,60]; (3) glycosylation and subsequent signal transduction[61,62]; (4) lipid peroxidation[55,63]; (5) activation of free radical generator enzymes[64,65]; (6) polyol pathway[66]; (7) protein kinase C pathway[67,68]; and (8) hexosamine pathway[69]. These pathways have been verified in patients with diabetes and are abnormally active and hence disrupt the metabolism of ROS in hyperglycemia. Although the presence of ROS can, sometimes, promote wound healing (e.g., bacterially infected wounds)[70,71], excessive release of ROS can lead to cell and tissue damage and delayed wound healing in DFU[72].
More than 90% of patients with DFU also have diabetic neuropathy[73]. The most common types of neuropathy are sensory, motor, and autonomic neuropathies of the periphery[74]. Diabetes can cause neuropathy in numerous ways, the most common of which are: (1) Autoimmunity; (2) microvascular dysfunction; and (3) various humoral variables (hyperglycemia, hyperinsulinemia, and so on)[75-79].
An important risk factor for wound formation in neuropathy is the deterioration of subjective sensation[80-82]. The selective targeting of C and Aδ fibers by neuropathy in diabetes can lead to neuropathic pain and/or sensory loss[83]. Studies have shown reduced cutaneous innervation in the biopsies of patients with diabetes based on reduced immunoreactivity of protein gene product 9.5 (PGP9.5) (detecting sensory neurons) and various neuropeptides, specifically calcitonin gene-related peptide, substance P (SP), and neuropeptide Y[84,85]. Reduced nerve density, a more fragmented distribution across the dermis[86,87], and reduced nerve afferents in the epidermis[88-91] and dermal papillae[92] are found in the skin of patients with diabetes, even in the absence of clinically detectable sensory neuropathy[93]. Moreover, patients with diabetes may show a significant reduction in amplitude and nerve conduction velocity associated with nerve fiber loss[94]. Patients may have trouble deciphering the severity of their sores or ulcers in the limbs, especially if their pain threshold has been drastically lowered[95]. These variables increase the likelihood of diabetic wound development and may also contribute to the progression of existing wounds. The upper-body paralysis from autonomic neuropathy reduces perspiration production, leading to dry, cracked skin that can increase susceptibility to irritation and infection while slowing the healing process[72,78]. In dull pain, the increased pressure on the plantar surface of the foot caused by motor neuropathy can cause ischemia and possibly death of tissues in the affected area[78].
Skin innervation is important for normal wound healing and can impact wound healing processes such as keratinocyte proliferation[96], wound re-epithelialization[97], wound contraction[98], and production of granulation tissue[99]. However, in diabetic wounds, neuropathy can impede these steps, delaying the healing of wounds[100]. Further, SP stimulates leukocyte chemotaxis to promote wound healing during the inflammatory phase of a wound[101]. However, reduced SP levels in denervated tissues in patients with diabetes may lead to delayed wound healing[102,103]. Denervation leads to delayed protein extravasation and cell migration[104,105]. Animals exposed to capsaicin have no vasodilation and plasma protein extravasation at the time of injury[106]. A similar delay in inflammatory cell migration was observed in mice with diabetes[107-109]. Chemical and surgical denervation can reduce small nerve fibers in the skin by at least 70%, which leads to poor wound repair[110]. The reduction in skin sensory nerves by subcutaneous injection of capsaicin in mice and rats without diabetes delayed re-epithelialization, reduced epidermal stem cell migration, and inhibited angiogenesis and VEGF expression[111-113].
In fact, many types of neuropathy are complicated by diabetes, and not every neuropathy affects the efficiency of wound healing[78,114]. The neuropathy discussed in this study refers to the ubiquitous disorders of the cutaneous nerves and dysregulation of neuropeptide secretion.
The wounds in patients with diabetes are highly susceptible to microbial invasion, often leading to life-threatening infections that delay wound closure. The damage of the skin barrier, such as increased trans epidermal water loss and decreased secretion of antimicrobial peptides[115,116], has been linked to an increased risk of infection. Neuropathy and chronic inflammation have been proposed as possible causes of this damage[72,117]. Furthermore, the microbial composition of patients with diabetes differs from that of healthy individuals[118-120]. Staphylococcus aureus and S. epidermidis, for example, are more likely to colonize the skin of patients with diabetes[119,120]. Diabetic wounds have a more restricted microbiome than healthy skin and are home to bacteria such as Klebsiella sp., Abiotrophia sp., Escherichia coli, and Peptoniphilus sp.[121-123]. Notably, S. aureus and Streptococcus genera predominate among the harmful microorganisms in infected wounds[124,125]. The increased release of pro-inflammatory cytokines and a prolonged inflammatory phase due to bacteria and endotoxins in an infected wound are two factors that prevent the wounds from healing[126]. Additionally, the pathogenic bacteria or their secretions continuously damage the wound’s tissue and cells, slowing the healing process.
Unlike nondiabetic acute wounds, DFUs have a nonlytic inflammatory phase, with numerous neutrophils and macrophages identified in the wound[127-129] and persistent release of pro-inflammatory cytokines such as leukocyte interleukin (IL)-1, tumor necrosis factor-α (TNF-α), plasma C-reactive protein, and others[128,130,131]. Poor phagocytic activity and dysfunctional leukocytes are also common in patients with diabetes[132-134]. However, in DFU, M1 macrophages continue to dominate the wound milieu and perpetuate inflammation, whereas, in normal wounds, M2 macrophages (promote tissue repair) progressively replace M1 macrophages (promote inflammation)[135-137]. Because of the ongoing inflammatory response, neutrophils remain activated and secrete proteases, which indiscriminately destroy the wound microenvironment[138]. However, inflammation can stifle angiogenesis by limiting VEGF production[139,140]. Diabetic wounds cannot heal properly because chronic inflammation continues to cause harm to tissue cells even after the remodeling phase has begun[109].
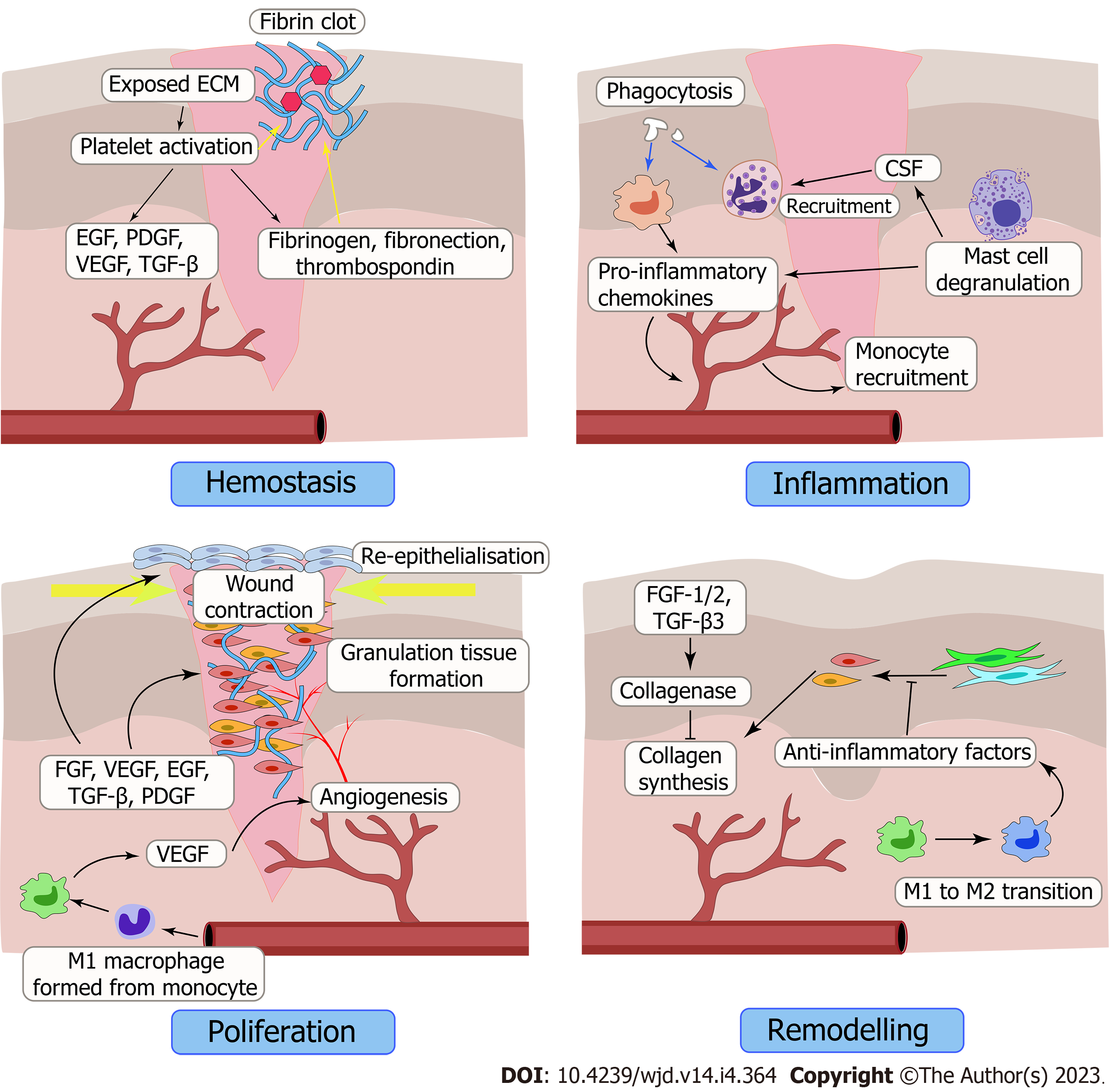
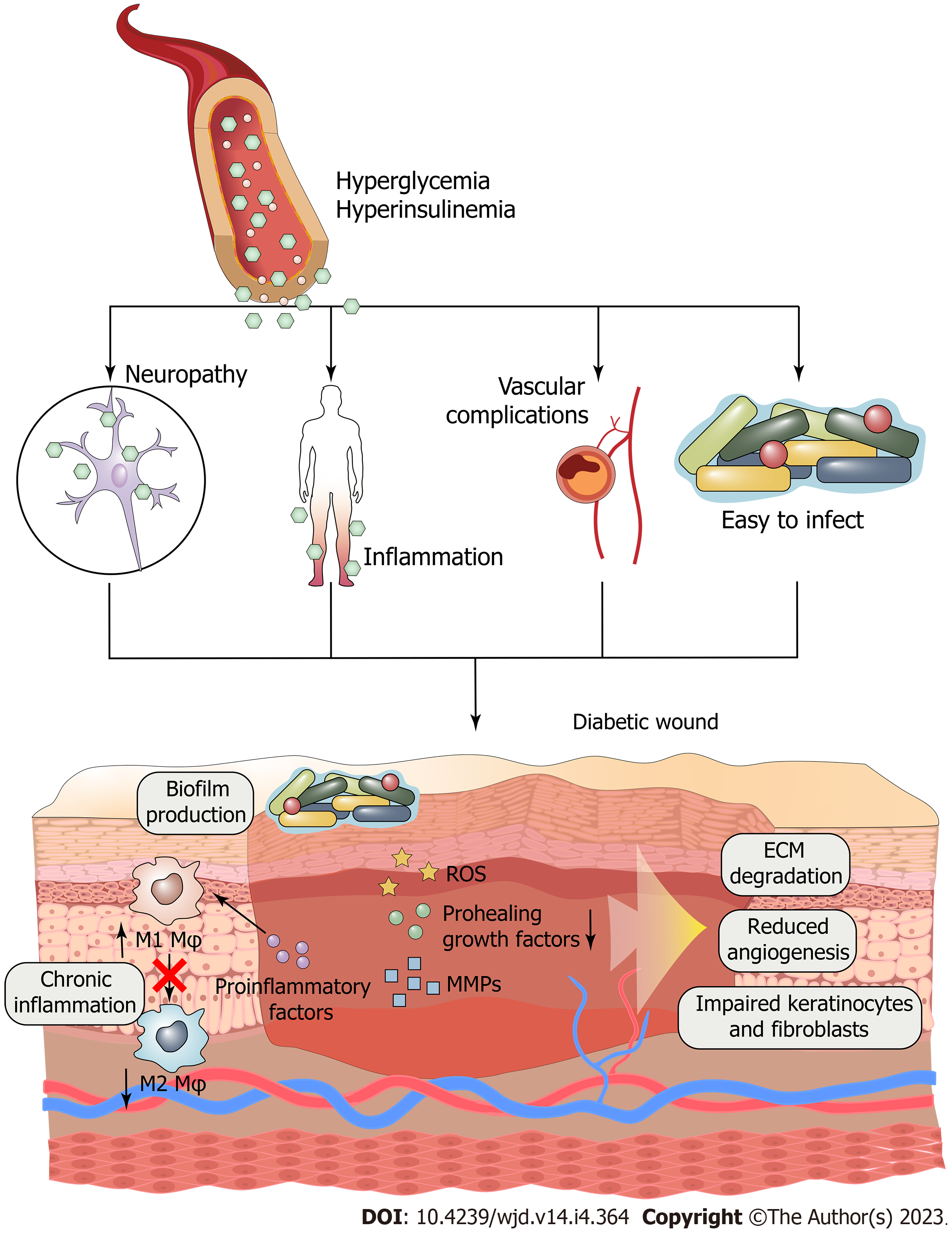
Normal wound healing can be divided into four stages: hemostasis, inflammation, hyperplasia and remodeling (Figure 1). But for diabetic wounds, the injuries, infections, and other consequences can all be exacerbated by diabetes, which slows recovery time[141] (Figure 2). Excessive formation of AGEs, insufficient neovascularization, insufficient concentration of GFs, imbalance between metabolism and nutrient delivery, abnormal regulation of gene expression, and impaired vascularization are just some of the factors making the healing of diabetic wounds difficult[142]. Therefore, common treatment measures cannot effectively improve the condition of diabetic wounds. Currently, many treatments exist for diabetic wounds, such as oxygen therapy, negative-pressure wound therapy, platelet-rich plasma, stem cells, and cell- and tissue-based products[4,143-150]. Among these, GF therapy has been regarded as an important means to treat diabetic wounds due to its ability to participate in promoting various stages of healing.
GFs execute an important role in impaired wound healing, especially in diabetic wounds. They affect many processes, such as the growth and movement of different types of cells, endothelial cell stimulation, angiogenesis, fibroblast chemotaxis, and changes in inflammatory cells. GFs that accelerate and promote wound healing through their physiological effects mainly include VEGF, PDGF, epidermal growth factor (EGF), fibroblast growth factor (FGF), transforming growth factor β (TGF-β), hepatocyte growth factor (HGF), and so forth (Table 1)[108,151-197].
| Type of GF | Source cells | Target cells | Receptor⁄signaling protein | Involved wound healing process | Acute wound | Chronic wound | Ref. |
| VEGF | Keratinocytes, fibroblasts, macrophages, endothelial cells | Endothelial cells, macrophages | ICAM-1, VCAM-1, PLCγ/PKC/Ras/Raf/MEK/ERK | Inflammation, angiogenesis | Increased | Decreased | [151-158] |
| TGF-β | Fibroblasts, keratinocytes, macrophages, platelets | Fibroblasts, keratinocytes, macrophages, leukocytes, endothelial cells | TGF-βRI-II, Smad 2-4, α-SMA, MAPK, integrins | Inflammation, angiogenesis, granulation tissue formation, collagen synthesis, matrix formation and remodeling, leukocyte chemotactic function | Increased | Decreased | [157,159-174] |
| PDGF | Platelets | Leukocytes, macrophages, fibroblasts | PDGFR, Ras/Erk1/2/MAPK, PI3K | Inflammation, re-epithelialization, collagen deposition, tissue remodelling | Increased | Decreased | [108,157,175,176] |
| HGF | Fibroblasts | Endothelial cells, keratinocytes | c-Met, ERK1/2, Akt, PAK-1/2, Gab1 | Suppression of inflammation, granulation tissue formation, angiogenesis, re-epithelialization | - | - | [155,176-180] |
| bFGF | Keratinocytes, fibroblasts, endothelial cells | Keratinocytes, fibroblasts, endothelial cells | ERK2 | Angiogenesis, granulation tissue formation | Increased | Decreased | [108,175,181-184] |
| FGF-7, FGF-10 | Fibroblasts, keratinocytes | Keratinocytes | Peroxiredoxin-6, Nrf2, Nrf3 | Re-epithelialization, detoxification of ROS | - | - | [185-188] |
| EGF, HB-EGF, TGF-α | Platelets, macrophages, keratinocytes | Fibroblasts, endothelial cells, keratinocytes | EGFR, STAT3, AP1, PI3K, ERK | Tissue formation, re-epithelialization | Increased | Decreased | [189-197] |
The VEGF family consists of a variety of GFs, among which VEGF-A and VEGF-C are mainly involved in wound healing[161]. VEGF-A is produced by endothelial cells, keratinocytes, fibroblasts, smooth muscle cells, platelets, neutrophils, and macrophages[198-200]. It binds to the tyrosine kinase surface receptors Flt-1 (VEGF receptor 1) and kinase insert region receptor (KDR) (VEGF receptor 2) located on the endothelial surface of blood vessels[201-203]. By acting on these receptors, VEGF-A can participate in the chemotaxis of endothelial cells and promote endothelial cell proliferation, differentiation, and regulation of vascular permeability[204-206]. VEGF-A levels are elevated in nondiabetic wounds[152,207]. Other GFs that can enhance VEGF-A expression include TGF-β1, EGF, TGF-α, KGF, bFGF, and PDGF-BB[208,209]. VEGF can promote angiogenesis to restore tissue perfusion, re-establish microcirculation, and increase oxygen tension in the wound[153]. In diabetic wounds, VEGF-A can promote early angiogenesis, especially the migration of endothelial cells, and improve the re-epithelialization and granulation tissue formation of diabetic wounds[210,211]. In vivo, the wounds in mice with diabetes exhibited accelerated re-epithelialization and contraction of wound area after treatment with VEGF mRNA delivery[212,213]. Many drugs and stem cells promote diabetic wound healing through VEGF[214-216].
VEGF-C is released primarily by macrophages and acts through VEGF receptor 3, which is expressed on lymphatic endothelial cells, pore endothelial cells, and monocytes/macrophages[217-219]. The proteolytically processed mature form of VEGF-C can also bind to KDR in the vascular endothelium to increase vascular permeability[220]. The proteolytically processed mature form of VEGF-C can also bind to KDR in the vascular endothelium to increase vascular permeability[220,221]. The administration of VEGF-C via an adenoviral vector to diabetic wounds accelerated healing in animal models of diabetes[222].
Many different homologous and heterodimeric GFs exist in the PDGF family. Platelets, macrophages, vascular endothelium, fibroblasts, and keratinocytes are the primary cell types responsible for PDGF production in wounds[223-225]. PDGF is required for the majority of wound healing processes. It is found in wound fluid and secreted from the degranulated plate following injury[176,226]. It promotes the proliferation and migration of inflammatory cells such as neutrophils, fibroblasts, macrophages, and smooth muscle cells[227-229]. Furthermore, it promotes tissue debridement and granulation tissue development via macrophages by increasing the production and secretion of GFs such as TGF-β[223]. Further, PDGF plays a crucial role in developing mature blood vessels[230]. It promotes myofibroblast differentiation to rescue delayed wound healing after a diabetic wound penetrates subcutaneously and causes muscle damage[231]. The combination of PDGF-BB, VEGF, and EGF has been shown to increase cell composition and promote wound healing in diabetic wounds, and the use of PDGF-BB alone has been approved for treating diabetic wounds[232]. PDGF-D was also found to be highly effective when applied to wounds in patients with diabetes and ischemia[233]. PDGF is often used in combination with VEGF, which has a significant positive effect on angiogenesis and recovery in diabetic wounds[234-236]. In vivo, PDGF-BB can improve the healing quality of full-thickness excision wounds in rats with diabetes; promote angiogenesis, cell proliferation, and epithelialization; and led to thicker and more organized collagen fiber deposition[237]. For clinical trials, the application of PDGF can significantly reduce the healing rate of diabetic wounds and improve the probability of complete healing[238-241].
Many members of the EGF family aid in wound healing, such as heparin-binding EGF (HB-EGF) and TGF-α[161]. The binding of these ligands to the EGF receptor (EGFR) causes the receptor to dimerize and autophosphorylate, which in turn triggers the tyrosine phosphorylation of downstream proteins inside the cell[191]. Studies in vitro demonstrated that EGFR activation facilitated re-epithelialization by increasing keratinocyte proliferation and migration in wounds[242-246].
The paracrine action of EGF on keratinocytes is primarily mediated by its secretion from platelets, macrophages, and fibroblasts[247]. Wound re-epithelialization and tensile strength were both remarkably improved by post-injury EGF upregulation in nondiabetic patients[195]. However, EGF levels were found to be lower in diabetic wounds, and a majority of EGFRs were found to be trans-located to the cytoplasm rather than localized on the cell membrane[161,197]. The addition of topical EGF to diabetic wounds has been shown in clinical trials to improve epithelialization and speed up healing[248,249]. Various attempts have been made to load EGF into various delivery systems for treating diabetic wounds[250,251]. In vivo, the application of EGF balances collagen distribution, increases granulation formation, and accelerates wound healing[251-253]. In a clinical trial of 68 patients treated with combined EGF and dressings, 52 had diabetic wounds, which healed completely within 2-14 wk with a low recurrence rate[254].
TGF-α is mainly secreted by platelets, keratinocytes, macrophages, fibroblasts, and lymphocytes[255-258]. It has been shown to increase keratinocyte migration and proliferation and induce the expression of K6 and K16[194,259,260]. In in vivo studies, TGF-α played a role in early stimulation and maintenance of wound epithelialization in partial-thickness wounds[261]. In vivo, TGF-α can be combined with PDGF-BB to make the wound healing speed in mice with diabetes close to that of nondiabetic mice[262]. However, TGF-α has not been applied to the clinical treatment of wounds so far.
HB-EGF is also upregulated in nondiabetic wounds and secreted by keratinocytes[199,263]. HB-EGF can promote re-epithelialization by binding to the EGFR subtypes HER1 and HER472[264,265]. In vivo, HB-EGF is thought to play a role in promoting keratinocyte migration, showing its importance in the early stages of re-epithelialization[189]. At present, HB-EGF has been widely regarded as one of the targets for treating skin wounds and carried in various delivery systems[266]. In a rodent diabetic wound model, HB-EGF improved re-epithelialization and increased collagen content and wound contraction via a heparin-based cohesive delivery system[267]. In vivo, HB-EGF can promote the proliferation and migration of epidermal keratinocytes in full-thickness excision wounds of mice with diabetes and accelerate epithelialization[267].
FGF family is a cell signaling protein family comprising 23 members. The members of this family mainly involved in skin wound healing are FGF2, FGF7 (or KGF1), and FGF10 (or KGF2). FGF2, the basic FGF, is mainly involved in granulation tissue formation, re-epithelialization, and matrix formation and remodeling in wounds[161,182]. FGF7 and FGF10 stimulate keratinocyte proliferation and migration, promote re-epithelialization, and increase the transcription of factors involved in ROS detoxification[268,269]. FGF2 is deficient in diabetic wounds, and wound closure is accelerated following the topical application of FGF2[183,184].
The TGF-β superfamily comprises many members playing essential roles in development and repair. TGF-β1 and TGF-β2 are significant players in wound repair, and can be potent stimulators of extracellular matrix protein and integrin expression[168,270,271]. TGF-β1 is abundantly released from platelets immediately after injury[272]. Latent TGF-βs in the wound matrix also allow the sustained release of proteolytic enzymes. This combination of different cell sources ensures a continuous supply of TGF-β throughout the repair process[273]. Additionally, some researchers have reported the presence of TGF-βs in wound fluid[274,275]. At the cellular regulatory level, TGF-β has many cellular regulatory functions, such as attracting macrophages and fibroblasts to the wound area to improve healing[276,277]. Also, TGF-β can promote re-epithelialization mainly by enhancing keratinocyte migration via regulatory factor forkhead box-1 after binding to receptors on the cell surface[277,278]. Moreover, studies show the involvement of TGF-β in scar formation, later wound repair, angiogenesis, and granulation tissue formation[163,169,172,273,279]. In diabetic wounds, TGF-β also promotes wound healing[162]. Compared with other reduced GFs, TGF-β showed a lack of upregulation in diabetic wounds, which might be a factor delaying the healing[279,280]. In vivo, the TGF-β/Small mothers against decapentaplegic (Smad) pathway is often activated as an important factor in promoting diabetic wound healing, for example, WDR74 and Baicalin[281,282].
HGF is a GF capable of regulating the growth, motility, and morphogenesis of various types of cells[283-285]. In wounds, HGF is mainly derived from fibroblasts, acts on epithelial cells, keratinocytes, and endothelial cells, and participates in healing processes such as suppression of inflammation, granulation tissue formation, angiogenesis, and re-epithelialization[155,177,179,180]. Although no changes in HGF levels have been reported in diabetic wounds, the delayed healing process appears to be associated with an imbalance in the activation and inactivation of the HGF/c-Met pathway[9,178,286]. Moreover, HGF can assist other GFs in promoting healing in diabetic wounds[287].
Nerve growth factor (NGF) is a neurotrophic factor essential for the development and survival of some sympathetic and sensory neurons in the central and peripheral nervous systems[288]. NGF levels increase when wounds appear. NGF mRNA is detected in newly formed epithelial cells and granulation tissue fibroblasts at the wound edge[289], with exceptionally high expression in granulation tissue myofibroblasts[290]. Additionally, NGF in wounds can also originate from salivary gland secretion and be transported via serum[289]. In wound healing, NGF mainly involves keratinocyte proliferation; proliferation, differentiation, and migration of epidermal stem cells; angiogenesis; fibroplasia; and peripheral nerve regeneration[291-297]. NGF levels are much lower in diabetic wounds and surrounding tissue than in normal skin wounds[298,299]. When NGF was applied explicitly to diabetic wounds, the healing and efficacy rate significantly improved[300,301].
Insulin-like growth factors (IGFs) are anti-catabolic and anabolic drugs having two isoforms: IGF-1 and IGF-2[302]. They can regulate the growth and differentiation of cells throughout the body[303]. In normal skin, only a few cells express this protein. However, all epidermal cells and some inflammatory cells were found to produce IGF in the initial 1-3 d after injury[304]. IGF-1 may be involved in granulation formation in wounds, inhibit apoptotic pathways, and attenuate pro-inflammatory cytokine production[142]. In diabetic wounds, the expression of IGFs is markedly decreased and is absent in the basal layer of the epidermis and fibroblasts[305-307].
The connective tissue growth factor (CTGF) is a member of the cellular communication network family[308], also known as CCN2. It can stimulate the proliferation and differentiation of fibroblasts in the skin[309]. Further, CTGF is involved in promoting cell adhesion, inflammatory cell chemotaxis, and cell differentiation[310,311]. The specific role of CTGF in nondiabetic wound healing has yet to be definitively concluded. However, the application of recombinant human CTGF in diabetic wounds did show better collagen IV accumulation and macrophage infiltration. Also, it increased α-smooth muscle actin level and healing rate in diabetic wounds compared with nontreated diabetic wounds[312]. In rats with diabetes, individuals treated with CTGF exhibited increased aggregation of type IV collagen, α-smooth muscle actin level, and macrophage infiltration; the rate of diabetic wound healing was also significantly accelerated in these individuals[312].
Colony-stimulating factor (CSF) family has many isoforms, but the CSFs involved in wound healing are mainly granulocyte-macrophage CSF (GM-CSF) and granulocyte CSF (G-CSF). GM-CSF primarily stimulates cell proliferation and differentiation in wound healing and stimulates stem cells to produce granulocytes and monocytes[7]. However, the effectiveness of GM-CSF in promoting wound healing appears to be only somewhat recognized at present[313]. G-CSF is mainly involved in the inflammatory process of wounds and is related to the formation of neutrophils[314]. The ability of G-CSF to promote healing and resist infection has been verified in randomized clinical trials of diabetic wounds[315,316].
GFs are essential for healing diabetic wounds, as outlined earlier, because of their pro-healing effects. However, it is challenging to observe normal GF-regulated healing events in nontreated diabetic wounds due to the absence of GFs[157,161,192]. This prevents the healing process of hemostasis, inflammation, granulation tissue formation, wound contraction and re-epithelialization, and remodeling from functioning correctly. Moreover, it further leads to prolonged inflammation, tissue hypoxia, wound infection, and chronic healing[141]. Although this does not imply that the missing GF is the source of the lack of healing function, supplementing the wound with the required GFs has an excellent healing-promoting effect.
The application of exogenous GFs is considered a promising approach for treating diabetic wounds. The reason for using GFs is their ability to stimulate and regulate complex cellular and molecular events to alleviate the specific adverse effects of vascular complications, neuropathy, and inflammation in diabetic wounds, which are essential for good and rapid diabetic wound healing[142,317]. So far, a series of GFs, including PDGF, VEGF, EGF, FGF, TGF-β, KGF, and IGF, has shown the potential to accelerate diabetic wound healing[225,257]. Therefore, introducing appropriate GFs into diabetic wounds effectively promotes chronic healing (Table 2)[237,262,280,318-348].
| Therapeutic agents | Delivery system and route | Animal type | Wound size | Response on wound closure | Ref. |
| PDGF/TGF-α | Gel/topical spraying to wound bed | C57BL/KsJ-db/db mouse | Full-thickness wound measuring 1.5 cm × 1.5 cm | Accelerated wound closure at 15-21 d | [262] |
| bFGF | Chitosan film/topical using | C57BL/KsJ-db/db mice | Full-thickness wound (1.6 cm diameter) | Reduced wound area and increased ECM formation | [318] |
| bFGF | Chitosan/hydrogels implant | C57BL/KsJ-db/db mice | Full-thickness wounds (about 100 mm2) | 80% wound closure by 12 d | [319] |
| pDNA TGF-β1 | PEG-PLGA-PEG/hydrogels implant | C57BKS.Cg-m +/+ Leprdb female mice | Full-thickness wounds (7 mm × 7 mm) | Accelerated wound closure at 5 d | [280] |
| bFGF | Chitosan, hydrogel/topical using | C57BL/KsJ-db/db mice | Full-thickness circular wounds (about 100 mm2) | Accelerated tissue filling rate of wounds and increased number of CD-34-positive vessels | [320] |
| PDGF-BB | Carboxymethyl cellulose hydrogel/topical using | C57/Bl6 wild-type mice and lep/r db/db homozygous diabetic mice | Either a 0.6 cm2, 1.0 cm2, or 1.5 cm2 full-thickness area of skin | Accelerated healing by enhanced granulation tissue formationand angiogenesis | [321] |
| bFGF | Collagen, PGA/porous scaffolds implant | C57BLKS/J Iar- + Leprdb/ + Leprdb | Full-thickness wounds (6 mm diameter | NA | [322] |
| rhPDGF | Gel/topical spraying to wound bed | Wistar diabetic rats | Full-thickness dermal wounds of 2.54 cm2 (1.8 cm diameter) | Outstanding re-epithelialization within the first 7 d | [323] |
| bFGF | Chondroitin-6-sulfate, heparin/hydrogels implant | C57BLKs/J-m1/db, db/db mice, heterozygous (m1/db) | Full-thickness wounds (1.6 cm diameter) | 89% wound closure by 2 wk | [324] |
| rhEGF | PCL, PCL-PEG/non-woven mesh (electrospun) implant | Ful-thickness wounds (0.8 cm diameter) | Accelerated wound closure at 7 d | [325] | |
| PDGF | 5% polyethylene glycol gel/intradermal injection | Wistar rats | Full-thickness wounds (1.8 cm diameter) | Significant wound improvement within 14 d | [237] |
| aFGF | Collagen, chitosan/porous scaffolds implant | SD rats | Whole skin layer round wounds (1.8 cm diameter) | Complete healing at 14 d | [326] |
| rhEGF | PLGA microspheres | SD rats | Full-thickness dermal wounds (2.54 cm2, 1.9 cm diameter) | 90% healing rate on the 14th day | [327] |
| Collagen-binding domain (CBD)-VEGF | Collagen domain/praye onto the traumatic surface | SD rats | Full-thickness wounds (2 cm × 2.5 cm) | 95% healing rate basically reached after 21 d | [328] |
| rhEGF | PLGA nanoparticles/topical spraying to wound bed | SD rats | Full-thickness dermal wounds (1.8 cm in diameter) | Complete wound closure by 21 d | [329] |
| bFGF | PELA/non-woven mesh (electrospun) implant | SD rats | Full-thickness circular wounds (about 250 mm2 each) | Complete wound closure by 3 wk | [330] |
| rhEGF | Dextrin conjugated/topical using | BKS.Cg-m a/a +/+ Leprdb/J db/db mice | Full-thickness wounds (10 mm × 10 mm) | Accelerated wound closure, neo-dermal tissue formation, increased granulation tissue deposition and angiogenesis | [331] |
| EGF | Collagen, hyaluronic acid/porous scaffolds implant | BKS.Cg-+Leprdb/+Leprdb (db/db) mice | Full-thickness wounds (15 mm × 20 mm) | N/A | [332] |
| pDNA bFGF | PELA/electrospun mesh implant | Male SD rats | Full-thickness wounds (about 250 mm2) | Complete wound closure by 3 wk | [333] |
| bFGF | Collagen, gelatine/porous scaffolds implant | BKS.Cg- + Leprdb/+ Leprdb/Jcl | 8 mm diameter and 3 mm thickness | NA | [334] |
| VEGF/bFGF | PLGA nps, fibrin/porous scaffolds implant | BKS.Cg-m+/+ Lepr, db/db | Full-thickness dermal wound (0.8 cm in diameter) | 85% wound closure at 15 d | [335] |
| rhEGF | PLGA-alginate microspheres/intralesional injection | Wistar rats | Full-thickness dermal wound (1 cm in diameter) | 90% wound closure at 11 d | [336] |
| rhEGF | Lipid nanocarriers/topical application to wound bed | BKS.Cg-m+/+Lepr 286 db/J | Full-thickness wounds 0.8 cm in diameter | 95% wound closure at 15 d | [337] |
| VEGF, bFGF, EGF, PDGF | Collagen, hyaluronic acid, gelatine nps/non-woven mesh (electrospun) implant | SD rats | Full thickness wound (diameter of 15 mm) | Complete wound closure by 4 wk | [338] |
| pDNA VEGF | Hyaluronic acid/hydrogels implant | db/db mice | Full-thickness wounds were then generated using a 6 mm biopsy punch (4 mm for wounds on smaller balb/c mice) | Induction of wound closure by day 8-10 | [339] |
| VEGF | PLGA nanoparticles/intradermal injection | db/db mice | Full thickness excisional wounds, two (8 mm diameter) and four (6 mm diameter) | Complete wound closure by 19 d | [340] |
| VEGF, PDGF | Poly (β-amino esters), poly (acrylic acid), heparan sulfate/woven nylon mesh implant | db/db mice | Full-thickness skin wound | Accelerated wound closure at 14 d | [341] |
| rhEGF | NaCMCh-rhEGF/hydrogels implant | SD rats | Full-thickness wounds (2 cm diameter, 3.14 cm2 circular area) | Wound healed in day 15 | [342] |
| rhEGF | PU/porous scaffolds implant | SD rats | Full-thickness wounds (dimensions of 2 cm × 2 cm) | 97% wound closure at 21 d | [343] |
| VEGF | PEG, heparin/hydrogels implant | Cg-m +/+ Leprdb/J (db/db) mice | Full-thickness punch biopsy wound | - | [344] |
| rhPDGF | PLGA/Non-woven mesh (electrospun) implant | SD rats | Full-thickness excision (8.0 mm in diameter) | Complete wound closure by 14 d | [345] |
| bFGF | Chitosan, hydrogel + heparin/topical using | C57BL/KsJ-db/db mice | - | Significant angiogenesis and collateral circulation construction | [346] |
| bFGF | Gelatin hydrogel microspheres/topical injection | C57BL/KsJ-db/db mice | Full-thickness wounds (10 mm in diameter) | Accelerated diabetic skin wound healing and reduced scarring | [347] |
| bFGF | Acidic gelatin sheet/topical coverage | C57BL/KsJ-db/db mice | Full-thickness wound (1.5 cm × 1.5 cm) | Promoted neoepithelialization, granulation, neovascularization, and wound healing | [348] |
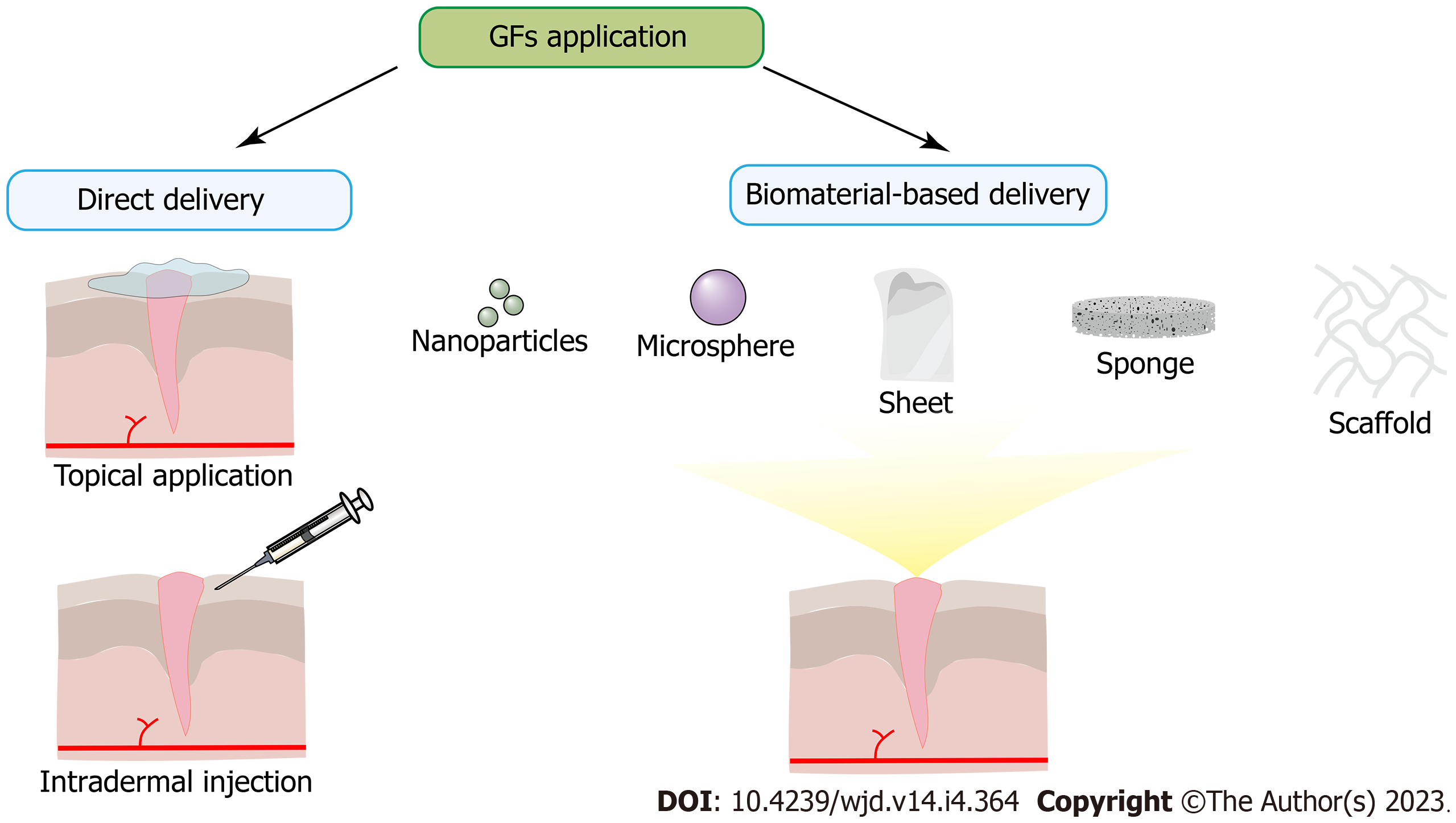
The standard methods of introducing GFs can be divided into direct or biomaterial-based delivery (Figure 3). Direct delivery is achieved via topical application or intradermal injection but with only short-term bioactivity due to proteolysis and destabilizing support. For example, a large injection of multiple GFs is insufficient to maintain angiogenesis[349]. Biomaterial-based delivery is achieved by incorporating GFs into ECM-like hydrogels, scaffolds, or particles, which can provide proteolytic protection and structural support to maintain the bioactivity of GFs[141]. Furthermore, the increase in the levels of GFs in diabetic wounds can also be achieved by gene-mediated delivery methods, which can be divided into plasmid DNA delivery, transfection with nonintegrating viral vectors, chemical carrier delivery, and gene-eluting biomaterial constructs[317].
This treatment method requires suitable biological materials that maintain the structure and biological activity of GFs, high encapsulation efficiency, and bioavailability, ensuring the complete release of GFs. Moreover, it is necessary to consider the biocompatibility, degradation, and absorption characteristics of the delivery system. Typically, synthetic polymers of the polyester family, such as polyglycolic acid, poly (lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), or polymers of natural origins, such as collagen, gelatin, fibrin, hyaluronic acid, dextran, alginate, and chitosan, are common delivery materials[141] (Table 3)[318,342,346-348,350-370]. Attempts to improve the feasibility of clinical treatment through innovative delivery systems have attracted much attention (Table 4)[240,241,371-378].
| Biomaterial | Forms | GFs | Effects | Ref. |
| Chitosan | Film | rhEGF | Sustained release in vitro for 24 h and extended therapeutic effect | [350] |
| Chitosan | Film | bFGF | The activity of bFGF remained stable for 21 d at 5 °C, and 86.2% of the activity was maintained at 25 °C | [318] |
| Chitosan | Hydrogel | EGF | 97.3% release after 24 h in an in vitro study and sustained therapeutic effect | [351] |
| Chitosan | Hydrogel | bFGF | Significant angiogenesis and collateral circulation construction after addition of heparin in chitosan-bFGF system | [346] |
| CMC-Chitosan | Hydrogel (as the carrier of NaCMCh-rhEGF nanoparticle) | rhEGF | In vitro results indicated that the conjugated form exhibited greater stability to proteolysis and also retained EGF therapeutic activity | [342] |
| CNC-HA-chitosan | Nanoparticle + scaffold | GM-CSF | Proper mechanical properties, high swelling capacity (swelling ratio: 2622.1% ± 35.2%) and controlled release of GM-CSF up to 48 h | [352] |
| PVA-gelatin-chitosan | Hydrogel | bFGF | In vitro release-cumulative over 25 d, non-toxic to fibroblasts | [353] |
| Chitosan-nanodiamond | Hydrogel | VEGF | 3-d sustained release, improved hydrogel mechanical properties and better biocompatibility | [354] |
| N-carboxymethyl chitosan-alginate | Hydrogel | EGF | 12 h sustained release, non-toxic | [355] |
| CMCS-poly (vinyl alcohol) (PVA)-alginate microspheres | Hydrogel | bFGF | 48 h sustained release, high activity for two weeks | [356] |
| Hyaluronic acid-sulfated glycosaminoglycan-heparin | Hydrogel | bFGF | Remain highly active for 14 d | [357] |
| Heparin + PEGDA | Hydrogel | bFGF | Remain active over 35 d | [358] |
| Collagen-transglutaminase | Hydrogel | bFGF | Suitable mechanical properties and biocompatibility, sustained release up to 48 h | [359] |
| CBD | Collagen membrane | PDGF | Maintain a higher concentration and stronger biological activity of PDGF | [360] |
| Collagen | Scaffold | VEGF-A | Cross-linking slows the degradation rate of collagen scaffolds and improves the persistent activity of VEGF | [361] |
| Extracellular matrix protein (INSUREGRAF®) | Scaffold | EGF | 8 h sustained release and active | [362] |
| GTA-collagen sponge | Sponge | rhEGF | Sustained release and activity for about 10 d, no cytotoxicity | [363] |
| TFA-denatured collagen | Sponge | bFGF | Sustained release for 18 d and remains largely active | [364] |
| PCL nanofibers (surface coating with collagen type I) | Hydrogel | G-CSF | Accumulative in vitro release for 15 d, no cytotoxicity | [365] |
| Gelatin | Microspheres | VEGF | Sustained release over 14 d | [347] |
| Gelatin | Sheet | bFGF | Sustained release for about 14 d | [348] |
| Gelatin | Sponge | EGF | Increased tensile strength | [366] |
| Gelatin | TEECM + Gelatin hydrogel microspheres | EGF | Cumulative in vitro release over 14 d | [367] |
| EUP polysaccharide, gelatin | Electrospun hydrogel sponge | PDGF-BB | In vitro release lasts 48 h | [368] |
| Fibrin | Hydrogel | VEGF | In vitro release lasts 7 d | [369] |
| Fibrin | Hydrogel | VEGF | Sustained release of VEGF for 15 d | [370] |
| Therapeutic agents | Delivery system and route | Response on wound closure | Ref. |
| EGF | Cream | Significantly improve wound healing rates and reduced the risk of amputation | [371] |
| bFGF | CGS/suture to surrounding skin | Significant wound improvement within 14 d | [372] |
| PDGF | Topical gel wound dressing | Reduce healing time by 30% | [373] |
| PDGF | Topical becaplermin gel | Improve wound healing by 35% | [240] |
| bFGF | 0.0005% benzalkonium chloride in saline/spray on the wound | Significantly reduce wound area | [374] |
| rhVEGF | Methylcellulose gel/apply evenly to wounds and edges | Significantly increase incidence of complete wound healing | [375] |
| PDGF | Becaplermin gel/topical apply | The incidence of complete closure was significantly increased by 43% | [241] |
| EGF | Intralesional injection | Reduced wound area and increased re-epithelialization rate | [376] |
| EGF | Topical spray | Faster healing velocity and higher complete healing rate | [377] |
| EGF | Topical hydrogel | 78% of wounds healed after 30 d | [378] |
Biodegradable polymer particles are site-specific controlled-release therapeutic systems. Chu et al[329] used a double-emulsion method to develop recombinant human EGF (rhEGF)-loaded PLGA nanoparticles and applied them to diabetic wound management. The results showed that the group treated with rhEGF PLGA nanoparticles had the best sustained GF release and the fastest wound healing compared with the group treated with rhEGF or PLGA alone[329]. Another study that used the intradermal route to deliver VEGF-loaded PLGA nanoparticles found that PLGA nanoparticles could sustain VEGF release for 30 d and showed a promoted healing response[340]. Further, another study pointed out that using PLGA-alginate microspheres as the GF carriers could significantly reduce the frequency of administration while maintaining the therapeutic effect[336].
Therapeutic polymer nanofiber mats are new carriers for GF-loaded dressings. This material has the characteristics of high porosity and large surface area to facilitate the penetration of GFs and the circulation of body fluids. rhPDGF-mixed PLGA nanofiber could release rhPDGF for 21 d and significantly induce the complete closure of diabetic wounds in rats with diabetes[345]. The PCL or PCL-polyethylene glycol (PEG) nanofibers implanted with rhEGF also exhibited effective promotion of wound re-epithelialization via increasing keratinocyte proliferation and phenotypic expression in diabetic wounds[325]. Hence, the production of carrier materials and loading of various GFs can be adjusted to achieve better wound healing ability[338]. Moreover, a research group experimented with a polymeric fiber mat different from electrospinning, namely a hydrolytically degradable four-layer structure consisting of polyacrylic acid, poly-β-amino acid ester, VEGF or PDGF, and heparan sulfate. In this structure, GFs could be stacked between each layer of materials to promote the complementary effects on wound healing[341].
The three-dimensional biomaterial is a typical structure used to make dressings with GFs. Both sponges and foams are used as standard wound dressings due to their high absorbency and permeability to moisture and oxygen[379]. Collagen is a new material that can be used to develop biomimetic scaffolds. The collagen base can act as a scaffold and bind other natural polymers, such as gelatin, hyaluronic acid, and chitosan, or other synthetic materials[322,36,332,334,380]. Collagen combined with other polymers to generate composite scaffolds can provide resistance to collagenase digestion and sustained slow release of GFs[326]. Further, a study showed that hydrophilic polyurethane (PU) formed by the copolymerization of polyethylene glycol can serve as a dressing material with good moisture conditions in the wound bed [water vapor transmission rate of approximately 3000 g/(m2 × day)]. The PU dressing loaded with rhEGF sustained the release of rhEGF for 7 d and eventually promoted re-epithelialization and complete recovery of diabetic wounds in rats[343].
Gene-mediated therapeutic delivery at diabetic wounds is primarily the local transfection of therapeutic transgenes or complementary DNA into cells to increase the transcription of GF messenger RNAs and maintain high concentrations of local GFs[381] (Table 5)[162,222,382-393].
| Therapeutic agents | Delivery system and route | Response on wound closure | Ref. |
| Plasmid KGF-1 | Intradermal injection | Enhanced wound closure at day 9 | [382] |
| Plasmid TGF-β1 | Intradermal injection | Complete wound closure by 7 d | [162] |
| Plasmid TGF-β1 | Intradermal injection, Electroporation | Early induction of closure by day 5 | [383] |
| Plasmid KGF-1 | Intradermal injection, Electroporation | Enhanced wound closure at day 12 | [384] |
| Minicircle-VEGF | Subcutaneous injection, Sonoporation | Complete wound closure by 12 d | [385] |
| Adenovirus encoding VEGF | Topical application to wound bed | Complete wound closure by 13 d | [386] |
| Adenovirus encoding VEGF | Intradermal injection | Complete wound closure by 27 d | [387] |
| Adenovirus encoding PDGF | Intralesional injection | Residual epithelial gap of 3 mm at day 7 | [388] |
| Adenovirus encoding VEGF‐C | Intradermal injection | Complete wound closure by 21 d | [222] |
| Lentivirus encoding PDGF | Injected into base and wound margin | No effect | [389] |
| Adeno-associated virus encoding VEGF | Intradermal injection | Complete re-epithelialization at 28 d | [390] |
| Bicistronic Adeno-associated virus encoding VEGF-A and FGF4 | Intradermal injection | Complete wound closure by 17 d | [391] |
| RGDK‐lipopeptide:rhPDGF-B lipoplex | Subcutaneous injection | Complete wound closure by 12 d | [392] |
| Minicircle VEGF | Subcutaneous injection | Complete wound closure by 12 d | [393] |
Naked plasmids are the most basic vector form that can accommodate large amounts of genomic DNA. Early research on diabetic wound therapy focused on forcing pDNA into cells by intradermal injection, with higher pDNA infusions achievable with the aid of electroporation[162,382-384,394]. Furthermore, Yoon et al[385] used an ultrasonic microbubble agent (SonoVue) to assist in the ultrasonic puncture delivery of VEGF165-encoded microplastics in diabetic wounds of mice. Their results showed significantly increased cutaneous blood perfusion, accelerated wound closure, and complete recovery of normal wound tissue in mice undergoing ultrasonic portion[385].
Viral vector transfection has excellent freedom of improvement and can efficiently integrate GF genes into wound cells for expression, thus having significant advantages in delivering therapeutic genes. The most commonly used viral vectors during diabetic wound care include lentivirus (LV), adenovirus (AV), and adeno-associated virus (AAV). The transfection of the VEGF165 gene with replication-defective AV has been reported to induce and accelerate early wound healing responses, including angiogenesis and granulation tissue formation, in mice with diabetes[387,386]. Furthermore, Galeano et al[390] found that viral transfection could release VEGF in diabetic wounds for 4 mo (even after wound healing). Furthermore, de Felipe proposed that a single viral vector capable of transfecting multiple genes could be used for treatment to address the issue that the transfection of a single GF or GF isoform gene only activated a single corresponding signaling pathway rather than promoting multiple stages of wound healing[395]. Jazwa et al[391] demonstrated the simultaneous delivery of VEGF-A and FGF4 genes via bicistronic AAV, and the results showed that the therapeutic effect of multiple gene delivery was better than that of single GF.
Substances such as cationic polymers and lipids are emerging chemical carriers due to their ability to form electrostatic complexes with anionic biomolecules such as pDNA[396]. The advantage of this type of chemical carrier is that it can avoid the use of potentially immunogenic viruses, improve the biostability of pDNA, and facilitate cellular uptake[397]. For example, a single subcutaneous injection of rhPDGF-B loaded with complexing integrin receptor ligand-conjugated lipopeptide or a complex consisting of arginine-grafted dendrimers loaded with minicyclic VEGF can accelerate the induction of complete wound closure in mice with diabetes[392,393].
Gene-eluting biomaterial scaffolds are similar to GF-loaded scaffolds and focus on improving the stability of the vector. Lee et al[280] developed a thermosensitive hydrogel synthesized from PEG, PLGA, and PEG that enabled the controlled release of encapsulated plasmids (containing the TGF-β1 gene) and the acquisition of accelerated re-epithelialization. The customizable properties of hydrogels bridge the gap between conventional gel-based systems and solid scaffolds, and the porosity of hydrogels provides a large area for released polymers to come into contact with infiltrating cells. However, the contribution of angiogenesis in transfected cells to wound closure is insignificant as the release of polymers is thought to be extremely slow[339]. Nevertheless, this characteristic based on the electrostatic interaction of positively charged polymers in an anionic hyaluronic acid hydrogel matrix provides the basis for developing more controllable polymer delivery systems. For instance, Yang et al[333] showed in 2012 that the molecular weight and content of PEG in the copolymer matrix could be changed to regulate the release of polymers (plasmid bFGF/polyethyleneimine) from the core of core-sheath emulsion electrospun fibers. However, such an approach appears to be flawed in diabetic wounds. The problem of low cell availability at the wound edge and reduced cell migration may increase the difficulty of regulating transfection efficiency in vivo with this system[398-400].
Diabetic wounds are encompassed by various factors (e.g., vascular system abnormalities, neuropathy, and inflammatory process stagnation) induced by the underlying disease and various concomitant diseases that impede normal wound healing. Furthermore, GFs that govern numerous healing processes are rarely detected in diabetic wounds compared with normal healing. The effects of GFs are particularly specific and have been shown to be beneficial in addressing the discussed diabetic wound features. As a result, GFs can be regarded as a direct and effective agent in managing and treating diabetic wounds. Nonetheless, it is disheartening that only a handful of products have entered clinical trials thus far[141]. We discussed the peculiarity of diabetic wounds and provided a theoretical basis and potential of GFs in treating diabetic wounds and optimizing therapeutic techniques. Combining GF with other therapies such as stem cell transplant, cytokine therapy, and anti-inflammatory drugs can be a promising treatment for diabetic wounds, albeit extensive studies are warranted to further examine the efficacy of this combination treatment strategy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khan NR, Pakistan; Li Y, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9565] [Article Influence: 735.8] [Reference Citation Analysis (0)] |
| 2. | Thomas CC, Philipson LH. Update on diabetes classification. Med Clin North Am. 2015;99:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4692] [Article Influence: 1564.0] [Reference Citation Analysis (36)] |
| 4. | Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic Wound-Healing Science. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 326] [Article Influence: 81.5] [Reference Citation Analysis (1)] |
| 5. | Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7148] [Cited by in RCA: 6601] [Article Influence: 275.0] [Reference Citation Analysis (0)] |
| 6. | Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 567] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 7. | Sharma P, Kumar A, Dey AD, Behl T, Chadha S. Stem cells and growth factors-based delivery approaches for chronic wound repair and regeneration: A promise to heal from within. Life Sci. 2021;268:118932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Okur ME, Bülbül EÖ, Mutlu G, Eleftherıadou K, Karantas ID, Okur NÜ, Siafaka PI. An Updated Review for the Diabetic Wound Healing Systems. Curr Drug Targets. 2022;23:393-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2012;26:812-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 10. | Yang SL, Zhu LY, Han R, Sun LL, Li JX, Dou JT. Pathophysiology of peripheral arterial disease in diabetes mellitus. J Diabetes. 2017;9:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Azhar A, Basheer M, Abdelgawad MS, Roshdi H, Kamel MF. Prevalence of Peripheral Arterial Disease in Diabetic Foot Ulcer Patients and its Impact in Limb Salvage. Int J Low Extrem Wounds. 2021;15347346211027063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Forsythe RO, Brownrigg J, Hinchliffe RJ. Peripheral arterial disease and revascularization of the diabetic foot. Diabetes Obes Metab. 2015;17:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Wyss CR, Matsen FA 3rd, Simmons CW, Burgess EM. Transcutaneous oxygen tension measurements on limbs of diabetic and nondiabetic patients with peripheral vascular disease. Surgery. 1984;95:339-346. [PubMed] |
| 14. | Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009;335:165-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 15. | Hayden MR. Endothelial activation and dysfunction in metabolic syndrome, type 2 diabetes and coronavirus disease 2019. J Int Med Res. 2020;48:300060520939746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Dos Santos JM, Tewari S, Mendes RH. The Role of Oxidative Stress in the Development of Diabetes Mellitus and Its Complications. J Diabetes Res. 2019;2019:4189813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | D'Alessandro S, Magnavacca A, Perego F, Fumagalli M, Sangiovanni E, Prato M, Dell'Agli M, Basilico N. Effect of Hypoxia on Gene Expression in Cell Populations Involved in Wound Healing. Biomed Res Int. 2019;2019:2626374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53:3226-3232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Gao W, Ferguson G, Connell P, Walshe T, Murphy R, Birney YA, O'Brien C, Cahill PA. High glucose concentrations alter hypoxia-induced control of vascular smooth muscle cell growth via a HIF-1alpha-dependent pathway. J Mol Cell Cardiol. 2007;42:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Ruthenborg RJ, Ban JJ, Wazir A, Takeda N, Kim JW. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol Cells. 2014;37:637-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Los-Stegienta A, Katarzynska J, Borkowska A, Marcinek A, Cypryk K, Gebicki J. Differentiation of Diabetic Foot Ulcers Based on Stimulation of Myogenic Oscillations by Transient Ischemia. Vasc Health Risk Manag. 2021;17:145-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 22. | Aborajooh E, Alqaisi TM, Yassin M, Alqpelat E, Abofaraj A, Alrawajih T, Alzoubi H, Abu Lubad M. Diabetic foot ulcer in Southern Jordan: A cross-sectional Study of Clinical and Microbiological Aspects. Ann Med Surg (Lond). 2022;76:103552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Gezawa ID, Ugwu ET, Ezeani I, Adeleye O, Okpe I, Enamino M. Anemia in patients with diabetic foot ulcer and its impact on disease outcome among Nigerians: Results from the MEDFUN study. PLoS One. 2019;14:e0226226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Shareef AM, Ahmedani MY, Waris N. Strong association of anemia in people with diabetic foot ulcers (DFUs): Study from a specialist foot care center. Pak J Med Sci. 2019;35:1216-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Costa RHR, Cardoso NA, Procópio RJ, Navarro TP, Dardik A, de Loiola Cisneros L. Diabetic foot ulcer carries high amputation and mortality rates, particularly in the presence of advanced age, peripheral artery disease and anemia. Diabetes Metab Syndr. 2017;11 Suppl 2:S583-S587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Ezeani IU, Ugwu ET, Adeleye FO, Gezawa ID, Okpe IO, Enamino MI. Determinants of wound healing in patients hospitalized for diabetic foot ulcer: results from the MEDFUN study. Endocr Regul. 2020;54:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Yammine K, Hayek F, Assi C. Is there an association between anemia and diabetic foot ulcers? Wound Repair Regen. 2021;29:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Kow RY, Low CL, Ruben JK, Zaharul-Azri MZ, Lim BC. Predictive Factors of Major Lower Extremity Amputations in Diabetic Foot Infections: A Cross-sectional Study at District Hospital in Malaysia. Malays Orthop J. 2019;13:45-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Shabhay A, Horumpende P, Shabhay Z, Mganga A, Van Baal J, Msuya D, Chilonga K, Chugulu S. Clinical profiles of diabetic foot ulcer patients undergoing major limb amputation at a tertiary care center in North-eastern Tanzania. BMC Surg. 2021;21:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des. 2005;11:2301-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 495] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 32. | Okonkwo UA, DiPietro LA. Diabetes and Wound Angiogenesis. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 616] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 33. | Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1369] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 34. | Seitz O, Schürmann C, Hermes N, Müller E, Pfeilschifter J, Frank S, Goren I. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res. 2010;2010:476969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Beer HD, Longaker MT, Werner S. Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol. 1997;109:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 177] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Isidori AM, Venneri MA, Fiore D. Angiopoietin-1 and Angiopoietin-2 in metabolic disorders: therapeutic strategies to restore the highs and lows of angiogenesis in diabetes. J Endocrinol Invest. 2016;39:1235-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Kämpfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and -2 and the tie-1 and -2 receptor tyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Lab Invest. 2001;81:361-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Qi W, Yang C, Dai Z, Che D, Feng J, Mao Y, Cheng R, Wang Z, He X, Zhou T, Gu X, Yan L, Yang X, Ma JX, Gao G. High levels of pigment epithelium-derived factor in diabetes impair wound healing through suppression of Wnt signaling. Diabetes. 2015;64:1407-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, Liu PL, Chen YL, Chen JW. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 40. | Hu L, Dai SC, Luan X, Chen J, Cannavicci A. Dysfunction and Therapeutic Potential of Endothelial Progenitor Cells in Diabetes Mellitus. J Clin Med Res. 2018;10:752-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Kränkel N, Adams V, Linke A, Gielen S, Erbs S, Lenk K, Schuler G, Hambrecht R. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol. 2005;25:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 664] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 43. | Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1111] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 44. | van Ark J, Moser J, Lexis CP, Bekkema F, Pop I, van der Horst IC, Zeebregts CJ, van Goor H, Wolffenbuttel BH, Hillebrands JL. Type 2 diabetes mellitus is associated with an imbalance in circulating endothelial and smooth muscle progenitor cell numbers. Diabetologia. 2012;55:2501-2512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Pyšná A, Bém R, Němcová A, Fejfarová V, Jirkovská A, Hazdrová J, Jude EB, Dubský M. Endothelial Progenitor Cells Biology in Diabetes Mellitus and Peripheral Arterial Disease and their Therapeutic Potential. Stem Cell Rev Rep. 2019;15:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Yu JQ, Liu XF, Chin LK, Liu AQ, Luo KQ. Study of endothelial cell apoptosis using fluorescence resonance energy transfer (FRET) biosensor cell line with hemodynamic microfluidic chip system. Lab Chip. 2013;13:2693-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Andrade TAM, Masson-Meyers DS, Caetano GF, Terra VA, Ovidio PP, Jordão-Júnior AA, Frade MAC. Skin changes in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun. 2017;490:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Kim JH, Yoon NY, Kim DH, Jung M, Jun M, Park HY, Chung CH, Lee K, Kim S, Park CS, Liu KH, Choi EH. Impaired permeability and antimicrobial barriers in type 2 diabetes skin are linked to increased serum levels of advanced glycation end-product. Exp Dermatol. 2018;27:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Lima AL, Illing T, Schliemann S, Elsner P. Cutaneous Manifestations of Diabetes Mellitus: A Review. Am J Clin Dermatol. 2017;18:541-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 50. | Blakytny R, Jude EB. Altered molecular mechanisms of diabetic foot ulcers. Int J Low Extrem Wounds. 2009;8:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 51. | Berlanga-Acosta J, Mendoza-Mari Y, Martínez MD, Valdés-Perez C, Ojalvo AG, Armstrong DG. Expression of cell proliferation cycle negative regulators in fibroblasts of an ischemic diabetic foot ulcer. A clinical case report. Int Wound J. 2013;10:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Liang L, Stone RC, Stojadinovic O, Ramirez H, Pastar I, Maione AG, Smith A, Yanez V, Veves A, Kirsner RS, Garlick JA, Tomic-Canic M. Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions. Wound Repair Regen. 2016;24:943-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Maione AG, Brudno Y, Stojadinovic O, Park LK, Smith A, Tellechea A, Leal EC, Kearney CJ, Veves A, Tomic-Canic M, Mooney DJ, Garlick JA. Three-dimensional human tissue models that incorporate diabetic foot ulcer-derived fibroblasts mimic in vivo features of chronic wounds. Tissue Eng Part C Methods. 2015;21:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Maione AG, Smith A, Kashpur O, Yanez V, Knight E, Mooney DJ, Veves A, Tomic-Canic M, Garlick JA. Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair. Wound Repair Regen. 2016;24:630-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 55. | Dewanjee S, Das S, Das AK, Bhattacharjee N, Dihingia A, Dua TK, Kalita J, Manna P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur J Pharmacol. 2018;833:472-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 56. | Yaribeygi H, Atkin SL, Sahebkar A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J Cell Physiol. 2019;234:1300-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 57. | Fakhruddin S, Alanazi W, Jackson KE. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. J Diabetes Res. 2017;2017:8379327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 58. | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3956] [Cited by in RCA: 3609] [Article Influence: 240.6] [Reference Citation Analysis (0)] |
| 59. | Shailey S, Basir SF. Strengthening of antioxidant defense by Azadirachta indica in alloxan-diabetic rat tissues. J Ayurveda Integr Med. 2012;3:130-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Sindhu RK, Koo JR, Roberts CK, Vaziri ND. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: response to insulin and antioxidant therapies. Clin Exp Hypertens. 2004;26:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1597] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 62. | Park HY, Kim JH, Jung M, Chung CH, Hasham R, Park CS, Choi EH. A long-standing hyperglycaemic condition impairs skin barrier by accelerating skin ageing process. Exp Dermatol. 2011;20:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3370] [Cited by in RCA: 3830] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 64. | Lee HJ, Lee DY, Mariappan MM, Feliers D, Ghosh-Choudhury G, Abboud HE, Gorin Y, Kasinath BS. Hydrogen sulfide inhibits high glucose-induced NADPH oxidase 4 expression and matrix increase by recruiting inducible nitric oxide synthase in kidney proximal tubular epithelial cells. J Biol Chem. 2017;292:5665-5675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Liu J, Wang C, Liu F, Lu Y, Cheng J. Metabonomics revealed xanthine oxidase-induced oxidative stress and inflammation in the pathogenesis of diabetic nephropathy. Anal Bioanal Chem. 2015;407:2569-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 66. | Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003;14:S233-S236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 391] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 67. | Chen F, Yu Y, Haigh S, Johnson J, Lucas R, Stepp DW, Fulton DJ. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PLoS One. 2014;9:e88405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Xu S, Zhao Y, Jin C, Yu L, Ding F, Fu G, Zhu J. PKC/NADPH oxidase are involved in the protective effect of pioglitazone in high homocysteine-induced paracrine dyfunction in endothelial progenitor cells. Am J Transl Res. 2017;9:1037-1048. [PubMed] |
| 69. | Horal M, Zhang Z, Stanton R, Virkamäki A, Loeken MR. Activation of the hexosamine pathway causes oxidative stress and abnormal embryo gene expression: involvement in diabetic teratogenesis. Birth Defects Res A Clin Mol Teratol. 2004;70:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg. 2008;34:1159-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 71. | Xu S, Chisholm AD. C. elegans epidermal wounding induces a mitochondrial ROS burst that promotes wound repair. Dev Cell. 2014;31:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 72. | Deng L, Du C, Song P, Chen T, Rui S, Armstrong DG, Deng W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid Med Cell Longev. 2021;2021:8852759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 301] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 73. | Kumar S, Ashe HA, Parnell LN, Fernando DJ, Tsigos C, Young RJ, Ward JD, Boulton AJ. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population-based study. Diabet Med. 1994;11:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 209] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 74. | Gilbey SG. Neuropathy and foot problems in diabetes. Clin Med (Lond). 2004;4:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 75. | Baum P, Toyka KV, Blüher M, Kosacka J, Nowicki M. Inflammatory Mechanisms in the Pathophysiology of Diabetic Peripheral Neuropathy (DN)-New Aspects. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 76. | Mengstie MA, Chekol Abebe E, Behaile Teklemariam A, Tilahun Mulu A, Agidew MM, Teshome Azezew M, Zewde EA, Agegnehu Teshome A. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front Mol Biosci. 2022;9:1002710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 77. | Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol. 2014;2014:674987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 78. | Volmer-Thole M, Lobmann R. Neuropathy and Diabetic Foot Syndrome. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 79. | Zenker J, Ziegler D, Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci. 2013;36:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 80. | Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004;351:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 330] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 81. | Peltier A, Goutman SA, Callaghan BC. Painful diabetic neuropathy. BMJ. 2014;348:g1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 82. | Walczak A, Szczepankiewicz AA, Ruszczycki B, Magalska A, Zamlynska K, Dzwonek J, Wilczek E, Zybura-Broda K, Rylski M, Malinowska M, Dabrowski M, Szczepinska T, Pawlowski K, Pyskaty M, Wlodarczyk J, Szczerbal I, Switonski M, Cremer M, Wilczynski GM. Novel higher-order epigenetic regulation of the Bdnf gene upon seizures. J Neurosci. 2013;33:2507-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 83. | Barker AR, Rosson GD, Dellon AL. Wound healing in denervated tissue. Ann Plast Surg. 2006;57:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Levy DM, Karanth SS, Springall DR, Polak JM. Depletion of cutaneous nerves and neuropeptides in diabetes mellitus: an immunocytochemical study. Diabetologia. 1989;32:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Lindberger M, Schröder HD, Schultzberg M, Kristensson K, Persson A, Ostman J, Link H. Nerve fibre studies in skin biopsies in peripheral neuropathies. I. Immunohistochemical analysis of neuropeptides in diabetes mellitus. J Neurol Sci. 1989;93:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Hsieh ST, Chiang HY, Lin WM. Pathology of nerve terminal degeneration in the skin. J Neuropathol Exp Neurol. 2000;59:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 87. | Krishnan ST, Quattrini C, Jeziorska M, Malik RA, Rayman G. Neurovascular factors in wound healing in the foot skin of type 2 diabetic subjects. Diabetes Care. 2007;30:3058-3062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Bönhof GJ, Strom A, Püttgen S, Ringel B, Brüggemann J, Bódis K, Müssig K, Szendroedi J, Roden M, Ziegler D. Patterns of cutaneous nerve fibre loss and regeneration in type 2 diabetes with painful and painless polyneuropathy. Diabetologia. 2017;60:2495-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 89. | Luo KR, Chao CC, Chen YT, Huang CM, Yang NC, Kan HW, Wang SH, Yang WS, Hsieh ST. Quantitation of sudomotor innervation in skin biopsies of patients with diabetic neuropathy. J Neuropathol Exp Neurol. 2011;70:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Luo KR, Chao CC, Hsieh PC, Lue JH, Hsieh ST. Effect of glycemic control on sudomotor denervation in type 2 diabetes. Diabetes Care. 2012;35:612-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, Brüggemann J, Strom A, Peschel S, Köhler B, Stachs O, Guthoff RF, Roden M; German Diabetes Study (GDS) Group. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63:2454-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 92. | Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Galkowska H, Olszewski WL, Wojewodzka U, Rosinski G, Karnafel W. Neurogenic factors in the impaired healing of diabetic foot ulcers. J Surg Res. 2006;134:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 94. | Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, Tai TY, Hsieh ST. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. 2004;127:1593-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 95. | Boulton AJ. What you can't feel can hurt you. J Vasc Surg. 2010;52:28S-30S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Hsieh ST, Choi S, Lin WM, Chang YC, Mcarthur JC, Griffin JW. Epidermal denervation and its effects on keratinocytes and Langerhans cells. J Neurocytol. 1996;25:513-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Buckley G, Wong J, Metcalfe AD, Ferguson MW. Denervation affects regenerative responses in MRL/MpJ and repair in C57BL/6 ear wounds. J Anat. 2012;220:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Engin C, Demirkan F, Ayhan S, Atabay K, Baran NK. Delayed effect of denervation on wound contraction in rat skin. Plast Reconstr Surg. 1996;98:1063-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Alapure BV, Lu Y, Peng H, Hong S. Surgical Denervation of Specific Cutaneous Nerves Impedes Excisional Wound Healing of Small Animal Ear Pinnae. Mol Neurobiol. 2018;55:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Ebenezer GJ, O'Donnell R, Hauer P, Cimino NP, McArthur JC, Polydefkis M. Impaired neurovascular repair in subjects with diabetes following experimental intracutaneous axotomy. Brain. 2011;134:1853-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 101. | Richards AM, Floyd DC, Terenghi G, McGrouther DA. Cellular changes in denervated tissue during wound healing in a rat model. Br J Dermatol. 1999;140:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 102. | Chiang HY, Chen CT, Chien HF, Hsieh ST. Skin denervation, neuropathology, and neuropathic pain in a laser-induced focal neuropathy. Neurobiol Dis. 2005;18:40-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Fitzgerald M. Capsaicin and sensory neurones--a review. Pain. 1983;15:109-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 415] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 104. | Gamse R, Holzer P, Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol. 1980;68:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 485] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 105. | Helme RD, Andrews PV. The effect of nerve lesions on the inflammatory response to injury. J Neurosci Res. 1985;13:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Jancsó N, Jancsó-Gábor A, Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967;31:138-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 546] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 107. | Fahey TJ 3rd, Sadaty A, Jones WG 2nd, Barber A, Smoller B, Shires GT. Diabetes impairs the late inflammatory response to wound healing. J Surg Res. 1991;50:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 201] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 108. | Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235-1246. [PubMed] |
| 109. | Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 434] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 110. | Wallengren J, Chen D, Sundler F. Neuropeptide-containing C-fibres and wound healing in rat skin. Neither capsaicin nor peripheral neurotomy affect the rate of healing. Br J Dermatol. 1999;140:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 111. | Martínez-Martínez E, Galván-Hernández CI, Toscano-Márquez B, Gutiérrez-Ospina G. Modulatory role of sensory innervation on hair follicle stem cell progeny during wound healing of the rat skin. PLoS One. 2012;7:e36421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 112. | Smith PG, Liu M. Impaired cutaneous wound healing after sensory denervation in developing rats: effects on cell proliferation and apoptosis. Cell Tissue Res. 2002;307:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 113. | Toda M, Suzuki T, Hosono K, Kurihara Y, Kurihara H, Hayashi I, Kitasato H, Hoka S, Majima M. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother. 2008;62:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 114. | Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 115. | Ibuki A, Kuriyama S, Toyosaki Y, Aiba M, Hidaka M, Horie Y, Fujimoto C, Isami F, Shibata E, Terauchi Y, Akase T. Aging-like physiological changes in the skin of Japanese obese diabetic patients. SAGE Open Med. 2018;6:2050312118756662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 116. | Rivas-Santiago B, Trujillo V, Montoya A, Gonzalez-Curiel I, Castañeda-Delgado J, Cardenas A, Rincon K, Hernandez ML, Hernández-Pando R. Expression of antimicrobial peptides in diabetic foot ulcer. J Dermatol Sci. 2012;65:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 117. | Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, Liao H, Evans AT, Goudie DR, Lewis-Jones S, Arseculeratne G, Munro CS, Sergeant A, O'Regan G, Bale SJ, Compton JG, DiGiovanna JJ, Presland RB, Fleckman P, McLean WH. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 687] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 118. | Gardiner M, Vicaretti M, Sparks J, Bansal S, Bush S, Liu M, Darling A, Harry E, Burke CM. A longitudinal study of the diabetic skin and wound microbiome. PeerJ. 2017;5:e3543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 119. | Redel H, Gao Z, Li H, Alekseyenko AV, Zhou Y, Perez-Perez GI, Weinstock G, Sodergren E, Blaser MJ. Quantitation and composition of cutaneous microbiota in diabetic and nondiabetic men. J Infect Dis. 2013;207:1105-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 120. | Thimmappaiah Jagadeesh A, Prakash PY, Karthik Rao N, Ramya V. Culture characterization of the skin microbiome in Type 2 diabetes mellitus: A focus on the role of innate immunity. Diabetes Res Clin Pract. 2017;134:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 121. | Gontcharova V, Youn E, Sun Y, Wolcott RD, Dowd SE. A comparison of bacterial composition in diabetic ulcers and contralateral intact skin. Open Microbiol J. 2010;4:8-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 122. | Oates A, Bowling FL, Boulton AJ, McBain AJ. Molecular and culture-based assessment of the microbial diversity of diabetic chronic foot wounds and contralateral skin sites. J Clin Microbiol. 2012;50:2263-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 123. | Park JU, Oh B, Lee JP, Choi MH, Lee MJ, Kim BS. Influence of Microbiota on Diabetic Foot Wound in Comparison with Adjacent Normal Skin Based on the Clinical Features. Biomed Res Int. 2019;2019:7459236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 124. | Messad N, Landraud L, Canivet B, Lina G, Richard JL, Sotto A, Lavigne JP, Lemichez E; French Study Group on the Diabetic Foot. Distribution of edin in Staphylococcus aureus isolated from diabetic foot ulcers. Clin Microbiol Infect. 2013;19:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 125. | Radzieta M, Sadeghpour-Heravi F, Peters TJ, Hu H, Vickery K, Jeffries T, Dickson HG, Schwarzer S, Jensen SO, Malone M. A multiomics approach to identify host-microbe alterations associated with infection severity in diabetic foot infections: a pilot study. NPJ Biofilms Microbiomes. 2021;7:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 126. | Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3887] [Cited by in RCA: 3173] [Article Influence: 211.5] [Reference Citation Analysis (0)] |
| 127. | Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2114] [Cited by in RCA: 2131] [Article Influence: 193.7] [Reference Citation Analysis (0)] |
| 128. | MacLeod AS, Mansbridge JN. The Innate Immune System in Acute and Chronic Wounds. Adv Wound Care (New Rochelle). 2016;5:65-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 129. | Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkötter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 859] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 130. | Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg. 2009;49:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 131. | Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94:2157-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 132. | Bagdade JD, Root RK, Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974;23:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 350] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 133. | Bybee JD, Rogers DE. The Phagocytic Activity of Polymorphonuclear Leukocytes Obtained from Patients with Diabetes Mellitus. J Lab Clin Med. 1964;64:1-13. [PubMed] |
| 134. | Richard C, Wadowski M, Goruk S, Cameron L, Sharma AM, Field CJ. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res Care. 2017;5:e000379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 135. | Montanaro M, Meloni M, Anemona L, Giurato L, Scimeca M, Izzo V, Servadei F, Smirnov A, Candi E, Mauriello A, Uccioli L. Macrophage Activation and M2 Polarization in Wound Bed of Diabetic Patients Treated by Dermal/Epidermal Substitute Nevelia. Int J Low Extrem Wounds. 2022;21:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 136. | Morey M, O'Gaora P, Pandit A, Hélary C. Hyperglycemia acts in synergy with hypoxia to maintain the pro-inflammatory phenotype of macrophages. PLoS One. 2019;14:e0220577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 137. | Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the Culprits: Macrophages-Versatile Regulators of Wound Healing. Adv Wound Care (New Rochelle). 2013;2:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 138. | Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 461] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 139. | Jayasuriya R, Dhamodharan U, Karan AN, Anandharaj A, Rajesh K, Ramkumar KM. Role of Nrf2 in MALAT1/ HIF-1α loop on the regulation of angiogenesis in diabetic foot ulcer. Free Radic Biol Med. 2020;156:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 140. | Zhu L, Zhong Q, Yang T, Xiao X. Improved therapeutic effects on diabetic foot by human mesenchymal stem cells expressing MALAT1 as a sponge for microRNA-205-5p. Aging (Albany NY). 2019;11:12236-12245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 141. | Berry-Kilgour C, Cabral J, Wise L. Advancements in the Delivery of Growth Factors and Cytokines for the Treatment of Cutaneous Wound Indications. Adv Wound Care (New Rochelle). 2021;10:596-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 142. | Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019;20:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 143. | Armstrong DG, Orgill DP, Galiano RD, Glat PM, Kaufman JP, Carter MJ, Zelen CM. An observational pilot study using a purified reconstituted bilayer matrix to treat non-healing diabetic foot ulcers. Int Wound J. 2020;17:966-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 144. | Bini Antunes M, Costa L, Carneiro M, Santos F, Oliveira R, Ferreira A, Sampaio M, Guimarães R, Pereira J, Neto H, Amil M, Coutinho J, Carvalho R. Topic platelet gel application in chronic diabetic foot ulcers. Diabetes Metab Syndr. 2019;13:644-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 145. | Erdoğan A, Düzgün AP, Erdoğan K, Özkan MB, Coşkun F. Efficacy of Hyperbaric Oxygen Therapy in Diabetic Foot Ulcers Based on Wagner Classification. J Foot Ankle Surg. 2018;57:1115-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 146. | Gonzalez IG, Angel MA, Baez MV, Ruiz Flores B, de Los Angeles Martinez Ferretiz M, Woolf SV, López I, Sandoval-Jurado L, Pat-Espadas FG, Cruz AA, Delgado AT. Handcrafted Vacuum-Assisted Device for Skin Ulcers Treatment Versus Traditional Therapy, Randomized Controlled Trial. World J Surg. 2017;41:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 147. | He M, Guo X, Li T, Jiang X, Chen Y, Yuan Y, Chen B, Yang G, Fan Y, Liang Z, Armstrong DG, Deng W. Comparison of Allogeneic Platelet-rich Plasma With Autologous Platelet-rich Plasma for the Treatment of Diabetic Lower Extremity Ulcers. Cell Transplant. 2020;29:963689720931428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 148. | Irawan H, Semadi IN, Widiana IGR. A Pilot Study of Short-Duration Hyperbaric Oxygen Therapy to Improve HbA1c, Leukocyte, and Serum Creatinine in Patients with Diabetic Foot Ulcer Wagner 3-4. ScientificWorldJournal. 2018;2018:6425857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 149. | Shi R, Lian W, Jin Y, Cao C, Han S, Yang X, Zhao S, Li M, Zhao H. Role and effect of vein-transplanted human umbilical cord mesenchymal stem cells in the repair of diabetic foot ulcers in rats. Acta Biochim Biophys Sin (Shanghai). 2020;52:620-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 150. | Viezzer C, Mazzuca R, Machado DC, de Camargo Forte MM, Gómez Ribelles JL. A new waterborne chitosan-based polyurethane hydrogel as a vehicle to transplant bone marrow mesenchymal cells improved wound healing of ulcers in a diabetic rat model. Carbohydr Polym. 2020;231:115734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 151. | Chen CC, Mo FE, Lau LF. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem. 2001;276:47329-47337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 152. | Frank S, Hübner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607-12613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 510] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 153. | Johnson KE, Wilgus TA. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv Wound Care (New Rochelle). 2014;3:647-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 632] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 154. | Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol. 2007;171:1774-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 155. | Min JK, Lee YM, Kim JH, Kim YM, Kim SW, Lee SY, Gho YS, Oh GT, Kwon YG. Hepatocyte growth factor suppresses vascular endothelial growth factor-induced expression of endothelial ICAM-1 and VCAM-1 by inhibiting the nuclear factor-kappaB pathway. Circ Res. 2005;96:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 156. | Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 476] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 157. | Robson MC. The role of growth factors in the healing of chronic wounds. Wound Repair Regen. 1997;5:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 158. | Zhang Z, Schluesener HJ. Mammalian toll-like receptors: from endogenous ligands to tissue regeneration. Cell Mol Life Sci. 2006;63:2901-2907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 159. | Amendt C, Mann A, Schirmacher P, Blessing M. Resistance of keratinocytes to TGFbeta-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J Cell Sci. 2002;115:2189-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 160. | Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 687] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 161. | Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2237] [Cited by in RCA: 2531] [Article Influence: 148.9] [Reference Citation Analysis (2)] |
| 162. | Chesnoy S, Lee PY, Huang L. Intradermal injection of transforming growth factor-beta1 gene enhances wound healing in genetically diabetic mice. Pharm Res. 2003;20:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 163. | Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1625] [Cited by in RCA: 1712] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 164. | Gailit J, Welch MP, Clark RA. TGF-beta 1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Invest Dermatol. 1994;103:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 165. | Klass BR, Grobbelaar AO, Rolfe KJ. Transforming growth factor beta1 signalling, wound healing and repair: a multifunctional cytokine with clinical implications for wound repair, a delicate balance. Postgrad Med J. 2009;85:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 166. | Kopecki Z, Luchetti MM, Adams DH, Strudwick X, Mantamadiotis T, Stoppacciaro A, Gabrielli A, Ramsay RG, Cowin AJ. Collagen loss and impaired wound healing is associated with c-Myb deficiency. J Pathol. 2007;211:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 167. | Lamar JM, Iyer V, DiPersio CM. Integrin alpha3beta1 potentiates TGFbeta-mediated induction of MMP-9 in immortalized keratinocytes. J Invest Dermatol. 2008;128:575-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 168. | Munger JS, Sheppard D. Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3:a005017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 169. | Pastar I, Stojadinovic O, Krzyzanowska A, Barrientos S, Stuelten C, Zimmerman K, Blumenberg M, Brem H, Tomic-Canic M. Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol Med. 2010;16:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 170. | Philipp K, Riedel F, Germann G, Hörmann K, Sauerbier M. TGF-beta antisense oligonucleotides reduce mRNA expression of matrix metalloproteinases in cultured wound-healing-related cells. Int J Mol Med. 2005;15:299-303. [PubMed] |
| 171. | Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987;165:251-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 570] [Cited by in RCA: 603] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 172. | Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167-4171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1935] [Cited by in RCA: 2021] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 173. | Stuelten CH, DaCosta Byfield S, Arany PR, Karpova TS, Stetler-Stevenson WG, Roberts AB. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-alpha and TGF-beta. J Cell Sci. 2005;118:2143-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 174. | Yuan W, Varga J. Transforming growth factor-beta repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J Biol Chem. 2001;276:38502-38510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 206] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 175. | Pierce GF, Tarpley JE, Yanagihara D, Mustoe TA, Fox GM, Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-beta 1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am J Pathol. 1992;140:1375-1388. [PubMed] |
| 176. | Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 284] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 177. | Buchstein N, Hoffmann D, Smola H, Lang S, Paulsson M, Niemann C, Krieg T, Eming SA. Alternative proteolytic processing of hepatocyte growth factor during wound repair. Am J Pathol. 2009;174:2116-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 178. | Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, Wehland J, Birchmeier C, Birchmeier W. c-Met is essential for wound healing in the skin. J Cell Biol. 2007;177:151-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 179. | Toyoda M, Takayama H, Horiguchi N, Otsuka T, Fukusato T, Merlino G, Takagi H, Mori M. Overexpression of hepatocyte growth factor/scatter factor promotes vascularization and granulation tissue formation in vivo. FEBS Lett. 2001;509:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 180. | Yoshida S, Yamaguchi Y, Itami S, Yoshikawa K, Tabata Y, Matsumoto K, Nakamura T. Neutralization of hepatocyte growth factor leads to retarded cutaneous wound healing associated with decreased neovascularization and granulation tissue formation. J Invest Dermatol. 2003;120:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 181. | Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18:26-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 228] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 182. | Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 972] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 183. | Robson MC, Hill DP, Smith PD, Wang X, Meyer-Siegler K, Ko F, VandeBerg JS, Payne WG, Ochs D, Robson LE. Sequential cytokine therapy for pressure ulcers: clinical and mechanistic response. Ann Surg. 2000;231:600-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 184. | Sogabe Y, Abe M, Yokoyama Y, Ishikawa O. Basic fibroblast growth factor stimulates human keratinocyte motility by Rac activation. Wound Repair Regen. 2006;14:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 185. | Braun S, auf dem Keller U, Steiling H, Werner S. Fibroblast growth factors in epithelial repair and cytoprotection. Philos Trans R Soc Lond B Biol Sci. 2004;359:753-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 186. | Davidson JM. First-class delivery: getting growth factors to their destination. J Invest Dermatol. 2008;128:1360-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 187. | Marti GP, Mohebi P, Liu L, Wang J, Miyashita T, Harmon JW. KGF-1 for wound healing in animal models. Methods Mol Biol. 2008;423:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 188. | Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, Williams LT. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994;266:819-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 445] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 189. | Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, Yokota K, Nakamura M, Sayama K, Mekada E, Higashiyama S, Hashimoto K. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118:2363-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 190. | Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, Yoshikawa K, Akira S, Takeda J. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18:4657-4668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 413] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 191. | Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;1:2005.0010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 771] [Cited by in RCA: 755] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 192. | Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 374] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 193. | Li G, Gustafson-Brown C, Hanks SK, Nason K, Arbeit JM, Pogliano K, Wisdom RM, Johnson RS. c-Jun is essential for organization of the epidermal leading edge. Dev Cell. 2003;4:865-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 194. | Jiang CK, Magnaldo T, Ohtsuki M, Freedberg IM, Bernerd F, Blumenberg M. Epidermal growth factor and transforming growth factor alpha specifically induce the activation- and hyperproliferation-associated keratins 6 and 16. Proc Natl Acad Sci USA. 1993;90:6786-6790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 195. | Brown GL, Curtsinger LJ, White M, Mitchell RO, Pietsch J, Nordquist R, von Fraunhofer A, Schultz GS. Acceleration of tensile strength of incisions treated with EGF and TGF-beta. Ann Surg. 1988;208:788-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 202] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 196. | Brown GL, Curtsinger L 3rd, Brightwell JR, Ackerman DM, Tobin GR, Polk HC Jr, George-Nascimento C, Valenzuela P, Schultz GS. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986;163:1319-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 225] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 197. | Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, Golinko M, Rosenberg H, Tomic-Canic M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 198. | Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445-1452. [PubMed] |
| 199. | Namiki A, Brogi E, Kearney M, Kim EA, Wu T, Couffinhal T, Varticovski L, Isner JM. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem. 1995;270:31189-31195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 347] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 200. | Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, Selby PJ. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998;77:956-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 434] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 201. | Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Böhlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1053] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 202. | de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1469] [Cited by in RCA: 1459] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 203. | Breier G, Damert A, Plate KH, Risau W. Angiogenesis in embryos and ischemic diseases. Thromb Haemost. 1997;78:678-683. [PubMed] |
| 204. | Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988-26995. [PubMed] |
| 205. | Malavaud B, Tack I, Jonca F, Praddaude F, Moro F, Ader JL, Plouët J. Activation of Flk-1/KDR mediates angiogenesis but not hypotension. Cardiovasc Res. 1997;36:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 206. | Clauss M. Functions of the VEGF receptor-1 (FLT-1) in the vasculature. Trends Cardiovasc Med. 1998;8:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 207. | Shukla A, Dubey MP, Srivastava R, Srivastava BS. Differential expression of proteins during healing of cutaneous wounds in experimental normal and chronic models. Biochem Biophys Res Commun. 1998;244:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 208. | Stavri GT, Zachary IC, Baskerville PA, Martin JF, Erusalimsky JD. Basic fibroblast growth factor upregulates the expression of vascular endothelial growth factor in vascular smooth muscle cells. Synergistic interaction with hypoxia. Circulation. 1995;92:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 244] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 209. | Brogi E, Wu T, Namiki A, Isner JM. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994;90:649-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 342] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 210. | Song SH, Lee MO, Lee JS, Jeong HC, Kim HG, Kim WS, Hur M, Cha HJ. Genetic modification of human adipose-derived stem cells for promoting wound healing. J Dermatol Sci. 2012;66:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 211. | Huang SP, Hsu CC, Chang SC, Wang CH, Deng SC, Dai NT, Chen TM, Chan JY, Chen SG, Huang SM. Adipose-derived stem cells seeded on acellular dermal matrix grafts enhance wound healing in a murine model of a full-thickness defect. Ann Plast Surg. 2012;69:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 212. | Sun N, Ning B, Hansson KM, Bruce AC, Seaman SA, Zhang C, Rikard M, DeRosa CA, Fraser CL, Wågberg M, Fritsche-Danielson R, Wikström J, Chien KR, Lundahl A, Hölttä M, Carlsson LG, Peirce SM, Hu S. Modified VEGF-A mRNA induces sustained multifaceted microvascular response and accelerates diabetic wound healing. Sci Rep. 2018;8:17509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 213. | Zha W, Wang J, Guo Z, Zhang Y, Wang Y, Dong S, Liu C, Xing H, Li X. Efficient delivery of VEGF-A mRNA for promoting diabetic wound healing via ionizable lipid nanoparticles. Int J Pharm. 2022;632:122565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 214. | Chen L, Zheng Q, Liu Y, Li L, Chen X, Wang L. Adipose-derived stem cells promote diabetic wound healing via the recruitment and differentiation of endothelial progenitor cells into endothelial cells mediated by the VEGF-PLCγ-ERK pathway. Arch Biochem Biophys. 2020;692:108531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 215. | Zafari F, Shirian S, Sadeghi M, Teimourian S, Bakhtiyari M. CD93 hematopoietic stem cells improve diabetic wound healing by VEGF activation and downregulation of DAPK-1. J Cell Physiol. 2020;235:2366-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 216. | Zhou J, Ni M, Liu X, Ren Z, Zheng Z. Curcumol Promotes Vascular Endothelial Growth Factor (VEGF)-Mediated Diabetic Wound Healing in Streptozotocin-Induced Hyperglycemic Rats. Med Sci Monit. 2017;23:555-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 217. | Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 295] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 218. | Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R, Birner P. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery. 2006;139:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 219. | Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, Stacker SA, Achen MG, Alitalo K. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14:2087-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 241] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 220. | McColl BK, Baldwin ME, Roufail S, Freeman C, Moritz RL, Simpson RJ, Alitalo K, Stacker SA, Achen MG. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J Exp Med. 2003;198:863-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 221. | Güç E, Briquez PS, Foretay D, Fankhauser MA, Hubbell JA, Kilarski WW, Swartz MA. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials. 2017;131:160-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 222. | Saaristo A, Tammela T, Farkkilā A, Kärkkäinen M, Suominen E, Yla-Herttuala S, Alitalo K. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol. 2006;169:1080-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 223. | Uutela M, Wirzenius M, Paavonen K, Rajantie I, He Y, Karpanen T, Lohela M, Wiig H, Salven P, Pajusola K, Eriksson U, Alitalo K. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood. 2004;104:3198-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 224. | Niessen FB, Andriessen MP, Schalkwijk J, Visser L, Timens W. Keratinocyte-derived growth factors play a role in the formation of hypertrophic scars. J Pathol. 2001;194:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 225. | Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003;90:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 226. | Vogt PM, Lehnhardt M, Wagner D, Jansen V, Krieg M, Steinau HU. Determination of endogenous growth factors in human wound fluid: temporal presence and profiles of secretion. Plast Reconstr Surg. 1998;102:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 227. | Pontén A, Folestad EB, Pietras K, Eriksson U. Platelet-derived growth factor D induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice. Circ Res. 2005;97:1036-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 228. | Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1721] [Cited by in RCA: 1798] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 229. | Bowen-Pope DF, Raines EW. History of discovery: platelet-derived growth factor. Arterioscler Thromb Vasc Biol. 2011;31:2397-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 230. | Folestad E, Kunath A, Wågsäter D. PDGF-C and PDGF-D signaling in vascular diseases and animal models. Mol Aspects Med. 2018;62:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 231. | Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1008] [Cited by in RCA: 1435] [Article Influence: 130.5] [Reference Citation Analysis (2)] |
| 232. | White MJV, Briquez PS, White DAV, Hubbell JA. VEGF-A, PDGF-BB and HB-EGF engineered for promiscuous super affinity to the extracellular matrix improve wound healing in a model of type 1 diabetes. NPJ Regen Med. 2021;6:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 233. | Moriya J, Wu X, Zavala-Solorio J, Ross J, Liang XH, Ferrara N. Platelet-derived growth factor C promotes revascularization in ischemic limbs of diabetic mice. J Vasc Surg. 2014;59:1402-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 234. | Drela E, Kulwas A, Jundziłł W, Góralczyk B, Boinska J, Drewniak W, Gadomska G, Rość D. VEGF-A and PDGF-BB--angiogenic factors and the stage of diabetic foot syndrome advancement. Endokrynol Pol. 2014;65:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 235. | Kartika RW, Alwi I, Suyatna FD, Yunir E, Waspadji S, Immanuel S, Silalahi T, Sungkar S, Rachmat J, Reksodiputro MH, Bardosono S. The role of VEGF, PDGF and IL-6 on diabetic foot ulcer after Platelet Rich Fibrin + hyaluronic therapy. Heliyon. 2021;7:e07934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 236. | Shi R, Lian W, Han S, Cao C, Jin Y, Yuan Y, Zhao H, Li M. Nanosphere-mediated co-delivery of VEGF-A and PDGF-B genes for accelerating diabetic foot ulcers healing in rats. Gene Ther. 2018;25:425-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 237. | Li H, Fu X, Zhang L, Huang Q, Wu Z, Sun T. Research of PDGF-BB gel on the wound healing of diabetic rats and its pharmacodynamics. J Surg Res. 2008;145:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 238. | Embil JM, Papp K, Sibbald G, Tousignant J, Smiell JM, Wong B, Lau CY. Recombinant human platelet-derived growth factor-BB (becaplermin) for healing chronic lower extremity diabetic ulcers: an open-label clinical evaluation of efficacy. Wound Repair Regen. 2000;8:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 239. | Ma C, Hernandez MA, Kirkpatrick VE, Liang LJ, Nouvong AL, Gordon II. Topical platelet-derived growth factor vs placebo therapy of diabetic foot ulcers offloaded with windowed casts: a randomized, controlled trial. Wounds. 2015;27:83-91. [PubMed] |
| 240. | Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999;7:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 313] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 241. | Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 459] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 242. | Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 787] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 243. | McCawley LJ, O'Brien P, Hudson LG. Epidermal growth factor (EGF)- and scatter factor/hepatocyte growth factor (SF/HGF)- mediated keratinocyte migration is coincident with induction of matrix metalloproteinase (MMP)-9. J Cell Physiol. 1998;176:255-265. [PubMed] [DOI] [Full Text] |
| 244. | Massagué J, Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 474] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 245. | Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3466] [Cited by in RCA: 3333] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 246. | Hudson LG, McCawley LJ. Contributions of the epidermal growth factor receptor to keratinocyte motility. Microsc Res Tech. 1998;43:444-455. [PubMed] [DOI] [Full Text] |
| 247. | Schultz G, Rotatori DS, Clark W. EGF and TGF-alpha in wound healing and repair. J Cell Biochem. 1991;45:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 255] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 248. | Wei Y, Li J, Huang Y, Lei X, Zhang L, Yin M, Deng J, Wang X, Fu X, Wu J. The clinical effectiveness and safety of using epidermal growth factor, fibroblast growth factor and granulocyte-macrophage colony stimulating factor as therapeutics in acute skin wound healing: a systematic review and meta-analysis. Burns Trauma. 2022;10:tkac002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (1)] |
| 249. | Brown GL, Nanney LB, Griffen J, Cramer AB, Yancey JM, Curtsinger LJ 3rd, Holtzin L, Schultz GS, Jurkiewicz MJ, Lynch JB. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989;321:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 469] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 250. | Zhu J, Jiang G, Hong W, Zhang Y, Xu B, Song G, Liu T, Hong C, Ruan L. Rapid gelation of oxidized hyaluronic acid and succinyl chitosan for integration with insulin-loaded micelles and epidermal growth factor on diabetic wound healing. Mater Sci Eng C Mater Biol Appl. 2020;117:111273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 251. | Jeong S, Kim B, Park M, Ban E, Lee SH, Kim A. Improved Diabetic Wound Healing by EGF Encapsulation in Gelatin-Alginate Coacervates. Pharmaceutics. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 252. | Hu P, Lei Q, Duan S, Fu Y, Pan H, Chang C, Zheng Z, Wu Y, Zhang Z, Li R, Li YY, Ao N. In-situ formable dextran/chitosan-based hydrogels functionalized with collagen and EGF for diabetic wounds healing. Biomater Adv. 2022;136:212773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 253. | Lee HJ, Jeong M, Na YG, Kim SJ, Lee HK, Cho CW. An EGF- and Curcumin-Co-Encapsulated Nanostructured Lipid Carrier Accelerates Chronic-Wound Healing in Diabetic Rats. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 254. | Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg. 2006;56:394-8; discussion 399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 255. | Rappolee DA, Mark D, Banda MJ, Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988;241:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 760] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 256. | Pittelkow MR, Cook PW, Shipley GD, Derynck R, Coffey RJ Jr. Autonomous growth of human keratinocytes requires epidermal growth factor receptor occupancy. Cell Growth Differ. 1993;4:513-521. [PubMed] |
| 257. | Papanas N, Maltezos E. Growth factors in the treatment of diabetic foot ulcers: new technologies, any promises? Int J Low Extrem Wounds. 2007;6:37-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 258. | Coffey RJ Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, Moses HL, Pittelkow MR. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature. 1987;328:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 613] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 259. | Li Y, Fan J, Chen M, Li W, Woodley DT. Transforming growth factor-alpha: a major human serum factor that promotes human keratinocyte migration. J Invest Dermatol. 2006;126:2096-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 260. | Cha D, O'Brien P, O'Toole EA, Woodley DT, Hudson LG. Enhanced modulation of keratinocyte motility by transforming growth factor-alpha (TGF-alpha) relative to epidermal growth factor (EGF). J Invest Dermatol. 1996;106:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 261. | Kim I, Mogford JE, Chao JD, Mustoe TA. Wound epithelialization deficits in the transforming growth factor-alpha knockout mouse. Wound Repair Regen. 2001;9:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 262. | Brown RL, Breeden MP, Greenhalgh DG. PDGF and TGF-alpha act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res. 1994;56:562-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 263. | Marikovsky M, Breuing K, Liu PY, Eriksson E, Higashiyama S, Farber P, Abraham J, Klagsbrun M. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci USA. 1993;90:3889-3893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 260] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 264. | Taylor SR, Markesbery MG, Harding PA. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) and proteolytic processing by a disintegrin and metalloproteinases (ADAM): a regulator of several pathways. Semin Cell Dev Biol. 2014;28:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 265. | Hashimoto K, Higashiyama S, Asada H, Hashimura E, Kobayashi T, Sudo K, Nakagawa T, Damm D, Yoshikawa K, Taniguchi N. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem. 1994;269:20060-20066. [PubMed] |
| 266. | Dao DT, Anez-Bustillos L, Adam RM, Puder M, Bielenberg DR. Heparin-Binding Epidermal Growth Factor-Like Growth Factor as a Critical Mediator of Tissue Repair and Regeneration. Am J Pathol. 2018;188:2446-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 267. | Johnson NR, Wang Y. Coacervate delivery of HB-EGF accelerates healing of type 2 diabetic wounds. Wound Repair Regen. 2015;23:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 268. | Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694-15700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 951] [Cited by in RCA: 925] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 269. | Raja, Sivamani K, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 354] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 270. | Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 271. | Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2322] [Cited by in RCA: 2349] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 272. | Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155-7160. [PubMed] |
| 273. | Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2305] [Cited by in RCA: 2403] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 274. | Watters JM, Blakslee JM, March RJ, Redmond ML. The influence of age on the severity of peritonitis. Can J Surg. 1996;39:142-146. [PubMed] |
| 275. | Breuing K, Andree C, Helo G, Slama J, Liu PY, Eriksson E. Growth factors in the repair of partial thickness porcine skin wounds. Plast Reconstr Surg. 1997;100:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 276. | Roberts AB. Transforming growth factor-beta: activity and efficacy in animal models of wound healing. Wound Repair Regen. 1995;3:408-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 277. | Martinez-Ferrer M, Afshar-Sherif AR, Uwamariya C, de Crombrugghe B, Davidson JM, Bhowmick NA. Dermal transforming growth factor-beta responsiveness mediates wound contraction and epithelial closure. Am J Pathol. 2010;176:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 278. | Peplow PV, Chatterjee MP. A review of the influence of growth factors and cytokines in in vitro human keratinocyte migration. Cytokine. 2013;62:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 279. | Jude EB, Blakytny R, Bulmer J, Boulton AJ, Ferguson MW. Transforming growth factor-beta 1, 2, 3 and receptor type I and II in diabetic foot ulcers. Diabet Med. 2002;19:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 280. | Lee PY, Li Z, Huang L. Thermosensitive hydrogel as a Tgf-beta1 gene delivery vehicle enhances diabetic wound healing. Pharm Res. 2003;20:1995-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 281. | Geng K, Ma X, Jiang Z, Gu J, Huang W, Wang W, Xu Y. WDR74 facilitates TGF-β/Smad pathway activation to promote M2 macrophage polarization and diabetic foot ulcer wound healing in mice. Cell Biol Toxicol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 282. | Mao X, Li Z, Li B, Wang H. Baicalin regulates mRNA expression of VEGF-c, Ang-1/Tie2, TGF-β and Smad2/3 to inhibit wound healing in streptozotocin-induced diabetic foot ulcer rats. J Biochem Mol Toxicol. 2021;35:e22893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 283. | Nakanishi K, Uenoyama M, Tomita N, Morishita R, Kaneda Y, Ogihara T, Matsumoto K, Nakamura T, Maruta A, Matsuyama S, Kawai T, Aurues T, Hayashi T, Ikeda T. Gene transfer of human hepatocyte growth factor into rat skin wounds mediated by liposomes coated with the sendai virus (hemagglutinating virus of Japan). Am J Pathol. 2002;161:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 284. | Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem Biophys Res Commun. 1997;239:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 269] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 285. | Conway K, Price P, Harding KG, Jiang WG. The molecular and clinical impact of hepatocyte growth factor, its receptor, activators, and inhibitors in wound healing. Wound Repair Regen. 2006;14:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 286. | Conway K, Ruge F, Price P, Harding KG, Jiang WG. Hepatocyte growth factor regulation: an integral part of why wounds become chronic. Wound Repair Regen. 2007;15:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 287. | Barć P, Antkiewicz M, Frączkowska-Sioma K, Kupczyńska D, Lubieniecki P, Witkiewicz W, Małodobra-Mazur M, Baczyńska D, Janczak D, Skóra JP. Two-Stage Gene Therapy (VEGF, HGF and ANG1 Plasmids) as Adjunctive Therapy in the Treatment of Critical Lower Limb Ischemia in Diabetic Foot Syndrome. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 288. | Wise BL, Seidel MF, Lane NE. The evolution of nerve growth factor inhibition in clinical medicine. Nat Rev Rheumatol. 2021;17:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 289. | Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, Matsumoto M, Konno K, Ushio H, Matsuda K. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998;187:297-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 166] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 290. | Hasan W, Zhang R, Liu M, Warn JD, Smith PG. Coordinate expression of NGF and alpha-smooth muscle actin mRNA and protein in cutaneous wound tissue of developing and adult rats. Cell Tissue Res. 2000;300:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 291. | Tanigawa T, Ahluwalia A, Watanabe T, Arakawa T, Tarnawski AS. Nerve growth factor injected into the gastric ulcer base incorporates into endothelial, neuronal, glial and epithelial cells: implications for angiogenesis, mucosal regeneration and ulcer healing. J Physiol Pharmacol. 2015;66:617-621. [PubMed] |
| 292. | Marconi A, Terracina M, Fila C, Franchi J, Bonté F, Romagnoli G, Maurelli R, Failla CM, Dumas M, Pincelli C. Expression and function of neurotrophins and their receptors in cultured human keratinocytes. J Invest Dermatol. 2003;121:1515-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 293. | Landi F, Aloe L, Russo A, Cesari M, Onder G, Bonini S, Carbonin PU, Bernabei R. Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Ann Intern Med. 2003;139:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 294. | Gibran NS, Tamura R, Tsou R, Isik FF. Human dermal microvascular endothelial cells produce nerve growth factor: implications for wound repair. Shock. 2003;19:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 295. | Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5:1131-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 450] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 296. | Bernabei R, Landi F, Bonini S, Onder G, Lambiase A, Pola R, Aloe L. Effect of topical application of nerve-growth factor on pressure ulcers. Lancet. 1999;354:307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 297. | Ashrafi M, Baguneid M, Bayat A. The Role of Neuromediators and Innervation in Cutaneous Wound Healing. Acta Derm Venereol. 2016;96:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 298. | Shi CM, Qu JF, Cheng TM. Effects of the nerve growth factor on the survival and wound healing in mice with combined radiation and wound injury. J Radiat Res. 2003;44:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 299. | Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996;2:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 213] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 300. | Rocco ML, Soligo M, Manni L, Aloe L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr Neuropharmacol. 2018;16:1455-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 301. | Generini S, Tuveri MA, Matucci Cerinic M, Mastinu F, Manni L, Aloe L. Topical application of nerve growth factor in human diabetic foot ulcers. A study of three cases. Exp Clin Endocrinol Diabetes. 2004;112:542-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 302. | Stuard WL, Titone R, Robertson DM. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front Endocrinol (Lausanne). 2020;11:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 303. | Baxter RC, Martin JL. Radioimmunoassay of growth hormone-dependent insulinlike growth factor binding protein in human plasma. J Clin Invest. 1986;78:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 283] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 304. | Gartner MH, Benson JD, Caldwell MD. Insulin-like growth factors I and II expression in the healing wound. J Surg Res. 1992;52:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 305. | Brown DL, Kane CD, Chernausek SD, Greenhalgh DG. Differential expression and localization of insulin-like growth factors I and II in cutaneous wounds of diabetic and nondiabetic mice. Am J Pathol. 1997;151:715-724. [PubMed] |
| 306. | Blakytny R, Jude EB, Martin Gibson J, Boulton AJ, Ferguson MW. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J Pathol. 2000;190:589-594. [PubMed] [DOI] [Full Text] |
| 307. | Bitar MS, Labbad ZN. Transforming growth factor-beta and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res. 1996;61:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 308. | Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 501] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 309. | Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, van Nieuwenhoven FA. Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxf). 2009;195:321-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 310. | Yosimichi G, Nakanishi T, Nishida T, Hattori T, Takano-Yamamoto T, Takigawa M. CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK). Eur J Biochem. 2001;268:6058-6065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 311. | Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285-1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 679] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 312. | Henshaw FR, Boughton P, Lo L, McLennan SV, Twigg SM. Topically applied connective tissue growth factor/CCN2 improves diabetic preclinical cutaneous wound healing: potential role for CTGF in human diabetic foot ulcer healing. J Diabetes Res. 2015;2015:236238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 313. | Hu X, Sun H, Han C, Wang X, Yu W. Topically applied rhGM-CSF for the wound healing: a systematic review. Burns. 2011;37:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 314. | Zarei F, Negahdari B, Eatemadi A. Diabetic ulcer regeneration: stem cells, biomaterials, growth factors. Artif Cells Nanomed Biotechnol. 2018;46:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 315. | Gough A, Clapperton M, Rolando N, Foster AV, Philpott-Howard J, Edmonds ME. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet. 1997;350:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 316. | de Lalla F, Pellizzer G, Strazzabosco M, Martini Z, Du Jardin G, Lora L, Fabris P, Benedetti P, Erle G. Randomized prospective controlled trial of recombinant granulocyte colony-stimulating factor as adjunctive therapy for limb-threatening diabetic foot infection. Antimicrob Agents Chemother. 2001;45:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 317. | Laiva AL, O'Brien FJ, Keogh MB. Innovations in gene and growth factor delivery systems for diabetic wound healing. J Tissue Eng Regen Med. 2018;12:e296-e312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 318. | Mizuno K, Yamamura K, Yano K, Osada T, Saeki S, Takimoto N, Sakurai T, Nimura Y. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J Biomed Mater Res A. 2003;64:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 319. | Obara K, Ishihara M, Ishizuka T, Fujita M, Ozeki Y, Maehara T, Saito Y, Yura H, Matsui T, Hattori H, Kikuchi M, Kurita A. Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials. 2003;24:3437-3444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 210] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 320. | Obara K, Ishihara M, Fujita M, Kanatani Y, Hattori H, Matsui T, Takase B, Ozeki Y, Nakamura S, Ishizuka T, Tominaga S, Hiroi S, Kawai T, Maehara T. Acceleration of wound healing in healing-impaired db/db mice with a photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2. Wound Repair Regen. 2005;13:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 321. | Chan RK, Liu PH, Pietramaggiori G, Ibrahim SI, Hechtman HB, Orgill DP. Effect of recombinant platelet-derived growth factor (Regranex) on wound closure in genetically diabetic mice. J Burn Care Res. 2006;27:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 322. | Nagato H, Umebayashi Y, Wako M, Tabata Y, Manabe M. Collagen-poly glycolic acid hybrid matrix with basic fibroblast growth factor accelerated angiogenesis and granulation tissue formation in diabetic mice. J Dermatol. 2006;33:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 323. | Cheng B, Liu HW, Fu XB, Sun TZ, Sheng ZY. Recombinant human platelet-derived growth factor enhanced dermal wound healing by a pathway involving ERK and c-fos in diabetic rats. J Dermatol Sci. 2007;45:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 324. | Liu Y, Cai S, Shu XZ, Shelby J, Prestwich GD. Release of basic fibroblast growth factor from a crosslinked glycosaminoglycan hydrogel promotes wound healing. Wound Repair Regen. 2007;15:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 325. | Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials. 2008;29:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 337] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 326. | Wang W, Lin S, Xiao Y, Huang Y, Tan Y, Cai L, Li X. Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci. 2008;82:190-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 327. | Dong X, Xu J, Wang W, Luo H, Liang X, Zhang L, Wang H, Wang P, Chang J. Repair effect of diabetic ulcers with recombinant human epidermal growth factor loaded by sustained-release microspheres. Sci China C Life Sci. 2008;51:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 328. | Yan X, Chen B, Lin Y, Li Y, Xiao Z, Hou X, Tan Q, Dai J. Acceleration of diabetic wound healing by collagen-binding vascular endothelial growth factor in diabetic rat model. Diabetes Res Clin Pract. 2010;90:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 329. | Chu Y, Yu D, Wang P, Xu J, Li D, Ding M. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010;18:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 330. | Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang C, Li X. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials. 2011;32:4243-4254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 331. | Hardwicke JT, Hart J, Bell A, Duncan R, Thomas DW, Moseley R. The effect of dextrin-rhEGF on the healing of full-thickness, excisional wounds in the (db/db) diabetic mouse. J Control Release. 2011;152:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 332. | Kondo S, Niiyama H, Yu A, Kuroyanagi Y. Evaluation of a wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor in diabetic mice. J Biomater Sci Polym Ed. 2012;23:1729-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 333. | Yang Y, Xia T, Chen F, Wei W, Liu C, He S, Li X. Electrospun fibers with plasmid bFGF polyplex loadings promote skin wound healing in diabetic rats. Mol Pharm. 2012;9:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 334. | Kanda N, Morimoto N, Ayvazyan AA, Takemoto S, Kawai K, Nakamura Y, Sakamoto Y, Taira T, Suzuki S. Evaluation of a novel collagen-gelatin scaffold for achieving the sustained release of basic fibroblast growth factor in a diabetic mouse model. J Tissue Eng Regen Med. 2014;8:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 335. | Losi P, Briganti E, Errico C, Lisella A, Sanguinetti E, Chiellini F, Soldani G. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013;9:7814-7821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 336. | Gainza G, Aguirre JJ, Pedraz JL, Hernández RM, Igartua M. rhEGF-loaded PLGA-Alginate microspheres enhance the healing of full-thickness excisional wounds in diabetised Wistar rats. Eur J Pharm Sci. 2013;50:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 337. | Gainza G, Pastor M, Aguirre JJ, Villullas S, Pedraz JL, Hernandez RM, Igartua M. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J Control Release. 2014;185:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 338. | Lai HJ, Kuan CH, Wu HC, Tsai JC, Chen TM, Hsieh DJ, Wang TW. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014;10:4156-4166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 339. | Tokatlian T, Cam C, Segura T. Porous hyaluronic acid hydrogels for localized nonviral DNA delivery in a diabetic wound healing model. Adv Healthc Mater. 2015;4:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 340. | Chereddy KK, Lopes A, Koussoroplis S, Payen V, Moia C, Zhu H, Sonveaux P, Carmeliet P, des Rieux A, Vandermeulen G, Préat V. Combined effects of PLGA and vascular endothelial growth factor promote the healing of non-diabetic and diabetic wounds. Nanomedicine. 2015;11:1975-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 341. | Almquist BD, Castleberry SA, Sun JB, Lu AY, Hammond PT. Combination Growth Factor Therapy via Electrostatically Assembled Wound Dressings Improves Diabetic Ulcer Healing In Vivo. Adv Healthc Mater. 2015;4:2090-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 342. | Hajimiri M, Shahverdi S, Esfandiari MA, Larijani B, Atyabi F, Rajabiani A, Dehpour AR, Amini M, Dinarvand R. Preparation of hydrogel embedded polymer-growth factor conjugated nanoparticles as a diabetic wound dressing. Drug Dev Ind Pharm. 2016;42:707-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 343. | Pyun DG, Choi HJ, Yoon HS, Thambi T, Lee DS. Polyurethane foam containing rhEGF as a dressing material for healing diabetic wounds: Synthesis, characterization, in vitro and in vivo studies. Colloids Surf B Biointerfaces. 2015;135:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 344. | Freudenberg U, Zieris A, Chwalek K, Tsurkan MV, Maitz MF, Atallah P, Levental KR, Eming SA, Werner C. Heparin desulfation modulates VEGF release and angiogenesis in diabetic wounds. J Control Release. 2015;220:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 345. | Lee CH, Liu KS, Chang SH, Chen WJ, Hung KC, Liu SJ, Pang JS, Juang JH, Chou CC, Chang PC, Chen YT, Wang FS. Promoting Diabetic Wound Therapy Using Biodegradable rhPDGF-Loaded Nanofibrous Membranes: CONSORT-Compliant Article. Medicine (Baltimore). 2015;94:e1873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 346. | Fujita M, Ishihara M, Shimizu M, Obara K, Nakamura S, Kanatani Y, Morimoto Y, Takase B, Matsui T, Kikuchi M, Maehara T. Therapeutic angiogenesis induced by controlled release of fibroblast growth factor-2 from injectable chitosan/non-anticoagulant heparin hydrogel in a rat hindlimb ischemia model. Wound Repair Regen. 2007;15:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 347. | Huang C, Orbay H, Tobita M, Miyamoto M, Tabata Y, Hyakusoku H, Mizuno H. Proapoptotic effect of control-released basic fibroblast growth factor on skin wound healing in a diabetic mouse model. Wound Repair Regen. 2016;24:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 348. | Miyoshi M, Kawazoe T, Igawa HH, Tabata Y, Ikada Y, Suzuki S. Effects of bFGF incorporated into a gelatin sheet on wound healing. J Biomater Sci Polym Ed. 2005;16:893-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 349. | Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1302] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 350. | Hong JP, Kim YW, Lee SK, Kim SH, Min KH. The effect of continuous release of recombinant human epidermal growth factor (rh-EGF) in chitosan film on full thickness excisional porcine wounds. Ann Plast Surg. 2008;61:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 351. | Alemdaroğlu C, Değim Z, Celebi N, Zor F, Oztürk S, Erdoğan D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns. 2006;32:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 352. | Karimi Dehkordi N, Minaiyan M, Talebi A, Akbari V, Taheri A. Nanocrystalline cellulose-hyaluronic acid composite enriched with GM-CSF loaded chitosan nanoparticles for enhanced wound healing. Biomed Mater. 2019;14:035003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 353. | Shamloo A, Sarmadi M, Aghababaie Z, Vossoughi M. Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int J Pharm. 2018;537:278-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 354. | Pacelli S, Acosta F, Chakravarti AR, Samanta SG, Whitlow J, Modaresi S, Ahmed RPH, Rajasingh J, Paul A. Nanodiamond-based injectable hydrogel for sustained growth factor release: Preparation, characterization and in vitro analysis. Acta Biomater. 2017;58:479-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 355. | Hu Y, Zhang Z, Li Y, Ding X, Li D, Shen C, Xu FJ. Dual-Crosslinked Amorphous Polysaccharide Hydrogels Based on Chitosan/Alginate for Wound Healing Applications. Macromol Rapid Commun. 2018;39:e1800069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 356. | Liu Q, Huang Y, Lan Y, Zuo Q, Li C, Zhang Y, Guo R, Xue W. Acceleration of skin regeneration in full-thickness burns by incorporation of bFGF-loaded alginate microspheres into a CMCS-PVA hydrogel. J Tissue Eng Regen Med. 2017;11:1562-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 357. | Liu LS, Ng CK, Thompson AY, Poser JW, Spiro RC. Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor (FGF-2). J Biomed Mater Res. 2002;62:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 358. | Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054-6067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 301] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 359. | Guo Y, Xu B, Wang Y, Li Y, Si H, Zheng X, Chen Z, Chen F, Fan D. Dramatic promotion of wound healing using a recombinant human-like collagen and bFGF cross-linked hydrogel by transglutaminase. J Biomater Sci Polym Ed. 2019;30:1591-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 360. | Sun W, Lin H, Xie H, Chen B, Zhao W, Han Q, Zhao Y, Xiao Z, Dai J. Collagen membranes loaded with collagen-binding human PDGF-BB accelerate wound healing in a rabbit dermal ischemic ulcer model. Growth Factors. 2007;25:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 361. | He Q, Zhao Y, Chen B, Xiao Z, Zhang J, Chen L, Chen W, Deng F, Dai J. Improved cellularization and angiogenesis using collagen scaffolds chemically conjugated with vascular endothelial growth factor. Acta Biomater. 2011;7:1084-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 362. | Yoon D, Yoon D, Cha HJ, Lee JS, Chun W. Enhancement of wound healing efficiency mediated by artificial dermis functionalized with EGF or NRG1. Biomed Mater. 2018;13:045007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 363. | Yang CH. Evaluation of the release rate of bioactive recombinant human epidermal growth factor from crosslinking collagen sponges. J Mater Sci Mater Med. 2008;19:1433-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 364. | Côté MF, Laroche G, Gagnon E, Chevallier P, Doillon CJ. Denatured collagen as support for a FGF-2 delivery system: physicochemical characterizations and in vitro release kinetics and bioactivity. Biomaterials. 2004;25:3761-3772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 365. | Tanha S, Rafiee-Tehrani M, Abdollahi M, Vakilian S, Esmaili Z, Naraghi ZS, Seyedjafari E, Javar HA. G-CSF loaded nanofiber/nanoparticle composite coated with collagen promotes wound healing in vivo. J Biomed Mater Res A. 2017;105:2830-2842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 366. | Dogan S, Demirer S, Kepenekci I, Erkek B, Kiziltay A, Hasirci N, Müftüoglu S, Nazikoglu A, Renda N, Dincer UD, Elhan A, Kuterdem E. Epidermal growth factor-containing wound closure enhances wound healing in non-diabetic and diabetic rats. Int Wound J. 2009;6:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 367. | Huang S, Zhang Y, Tang L, Deng Z, Lu W, Feng F, Xu X, Jin Y. Functional bilayered skin substitute constructed by tissue-engineered extracellular matrix and microsphere-incorporated gelatin hydrogel for wound repair. Tissue Eng Part A. 2009;15:2617-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 368. | Li Q, Niu Y, Diao H, Wang L, Chen X, Wang Y, Dong L, Wang C. In situ sequestration of endogenous PDGF-BB with an ECM-mimetic sponge for accelerated wound healing. Biomaterials. 2017;148:54-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 369. | Sacchi V, Mittermayr R, Hartinger J, Martino MM, Lorentz KM, Wolbank S, Hofmann A, Largo RA, Marschall JS, Groppa E, Gianni-Barrera R, Ehrbar M, Hubbell JA, Redl H, Banfi A. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc Natl Acad Sci USA. 2014;111:6952-6957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 370. | Zhao N, Coyne J, Xu M, Zhang X, Suzuki A, Shi P, Lai J, Fong GH, Xiong N, Wang Y. Assembly of Bifunctional Aptamer-Fibrinogen Macromer for VEGF Delivery and Skin Wound Healing. Chem Mater. 2019;31:1006-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 371. | Tsang MW, Wong WK, Hung CS, Lai KM, Tang W, Cheung EY, Kam G, Leung L, Chan CW, Chu CM, Lam EK. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care. 2003;26:1856-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 372. | Morimoto N, Yoshimura K, Niimi M, Ito T, Aya R, Fujitaka J, Tada H, Teramukai S, Murayama T, Toyooka C, Miura K, Takemoto S, Kanda N, Kawai K, Yokode M, Shimizu A, Suzuki S. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: clinical trial for chronic skin ulcers. Tissue Eng Part A. 2013;19:1931-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 373. | Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg. 2006;117:143S-149S; discussion 150S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 374. | UCHI H, IGARASHI A, URABE K, KOGA T, NAKAYAMA J, KAWAMORI R, TAMAKI K, HIRAKATA H, OHURA T, FURUE M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol. 2009;19:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 375. | Hanft JR, Pollak RA, Barbul A, van Gils C, Kwon PS, Gray SM, Lynch CJ, Semba CP, Breen TJ. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care. 2008;17:30-32, 34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 376. | Gomez-Villa R, Aguilar-Rebolledo F, Lozano-Platonoff A, Teran-Soto JM, Fabian-Victoriano MR, Kresch-Tronik NS, Garrido-Espíndola X, Garcia-Solis A, Bondani-Guasti A, Bierzwinsky-Sneider G, Contreras-Ruiz J. Efficacy of intralesional recombinant human epidermal growth factor in diabetic foot ulcers in Mexican patients: a randomized double-blinded controlled trial. Wound Repair Regen. 2014;22:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 377. | Park KH, Han SH, Hong JP, Han SK, Lee DH, Kim BS, Ahn JH, Lee JW. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: A phase III multicenter, double-blind, randomized, placebo-controlled trial. Diabetes Res Clin Pract. 2018;142:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 378. | Viswanathan V, Juttada U, Babu M. Efficacy of Recombinant Human Epidermal Growth Factor (Regen-D 150) in Healing Diabetic Foot Ulcers: A Hospital-Based Randomized Controlled Trial. Int J Low Extrem Wounds. 2020;19:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 379. | Moura LI, Dias AM, Carvalho E, de Sousa HC. Recent advances on the development of wound dressings for diabetic foot ulcer treatment--a review. Acta Biomater. 2013;9:7093-7114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 499] [Article Influence: 41.6] [Reference Citation Analysis (3)] |
| 380. | Moura LI, Dias AM, Suesca E, Casadiegos S, Leal EC, Fontanilla MR, Carvalho L, de Sousa HC, Carvalho E. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim Biophys Acta. 2014;1842:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 381. | Raftery RM, Walsh DP, Castaño IM, Heise A, Duffy GP, Cryan SA, O'Brien FJ. Delivering Nucleic-Acid Based Nanomedicines on Biomaterial Scaffolds for Orthopedic Tissue Repair: Challenges, Progress and Future Perspectives. Adv Mater. 2016;28:5447-5469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 382. | Byrnes CK, Khan FH, Nass PH, Hatoum C, Duncan MD, Harmon JW. Success and limitations of a naked plasmid transfection protocol for keratinocyte growth factor-1 to enhance cutaneous wound healing. Wound Repair Regen. 2001;9:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 383. | Lee PY, Chesnoy S, Huang L. Electroporatic delivery of TGF-beta1 gene works synergistically with electric therapy to enhance diabetic wound healing in db/db mice. J Invest Dermatol. 2004;123:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 384. | Marti G, Ferguson M, Wang J, Byrnes C, Dieb R, Qaiser R, Bonde P, Duncan MD, Harmon JW. Electroporative transfection with KGF-1 DNA improves wound healing in a diabetic mouse model. Gene Ther. 2004;11:1780-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 385. | Yoon CS, Jung HS, Kwon MJ, Lee SH, Kim CW, Kim MK, Lee M, Park JH. Sonoporation of the minicircle-VEGF(165) for wound healing of diabetic mice. Pharm Res. 2009;26:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 386. | Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, Capogrossi MC. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 2002;9:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 387. | Brem H, Kodra A, Golinko MS, Entero H, Stojadinovic O, Wang VM, Sheahan CM, Weinberg AD, Woo SL, Ehrlich HP, Tomic-Canic M. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol. 2009;129:2275-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 388. | Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D, Herlyn M, Crombleholme TM. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen. 2004;12:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 389. | Lee JA, Conejero JA, Mason JM, Parrett BM, Wear-Maggitti KD, Grant RT, Breitbart AS. Lentiviral transfection with the PDGF-B gene improves diabetic wound healing. Plast Reconstr Surg. 2005;116:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 390. | Galeano M, Deodato B, Altavilla D, Cucinotta D, Arsic N, Marini H, Torre V, Giacca M, Squadrito F. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003;46:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 391. | Jazwa A, Kucharzewska P, Leja J, Zagorska A, Sierpniowska A, Stepniewski J, Kozakowska M, Taha H, Ochiya T, Derlacz R, Vahakangas E, Yla-Herttuala S, Jozkowicz A, Dulak J. Combined vascular endothelial growth factor-A and fibroblast growth factor 4 gene transfer improves wound healing in diabetic mice. Genet Vaccines Ther. 2010;8:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 392. | Bhattacharyya J, Mondal G, Madhusudana K, Agawane SB, Ramakrishna S, Gangireddy SR, Madhavi RD, Reddy PK, Konda VR, Rao SR, Udaykumar P, Chaudhuri A. Single subcutaneous administration of RGDK-lipopeptide:rhPDGF-B gene complex heals wounds in streptozotocin-induced diabetic rats. Mol Pharm. 2009;6:918-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 393. | Kwon MJ, An S, Choi S, Nam K, Jung HS, Yoon CS, Ko JH, Jun HJ, Kim TK, Jung SJ, Park JH, Lee Y, Park JS. Effective healing of diabetic skin wounds by using nonviral gene therapy based on minicircle vascular endothelial growth factor DNA and a cationic dendrimer. J Gene Med. 2012;14:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 394. | Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, Nastai M, Semenza GL, Harmon JW. Age-dependent impairment of HIF-1alpha expression in diabetic mice: Correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008;217:319-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 395. | de Felipe P. Polycistronic viral vectors. Curr Gene Ther. 2002;2:355-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 396. | Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1513] [Cited by in RCA: 1690] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 397. | Samal SK, Dash M, Van Vlierberghe S, Kaplan DL, Chiellini E, van Blitterswijk C, Moroni L, Dubruel P. Cationic polymers and their therapeutic potential. Chem Soc Rev. 2012;41:7147-7194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 491] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 398. | Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1167] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 399. | Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 366] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 400. | Xuan YH, Huang BB, Tian HS, Chi LS, Duan YM, Wang X, Zhu ZX, Cai WH, Zhu YT, Wei TM, Ye HB, Cong WT, Jin LT. High-glucose inhibits human fibroblast cell migration in wound healing via repression of bFGF-regulating JNK phosphorylation. PLoS One. 2014;9:e108182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |