Published online Feb 15, 2023. doi: 10.4239/wjd.v14.i2.76
Peer-review started: October 6, 2022
First decision: November 18, 2022
Revised: December 13, 2022
Accepted: January 16, 2023
Article in press: January 16, 2023
Published online: February 15, 2023
Processing time: 131 Days and 9.1 Hours
Insulin is a hormone secreted by pancreatic β cells. The concentration of glucose in circulation is proportional to the secretion of insulin by these cells. In target cells, insulin binds to its receptors and activates phosphatidylinositol-3-kinase/protein kinase B, inducing different mechanisms depending on the cell type. In the liver it activates the synthesis of glycogen, in adipose tissue and muscle it allows the capture of glucose, and in the hypothalamus, it regulates thermogenesis and appetite. Defects in insulin function [insulin resistance (IR)] are related to the development of neurodegenerative diseases in obese people. Furthermore, in obesity and diabetes, its role as an anorexigenic hormone in the hypothalamus is diminished during IR. Therefore, hyperphagia prevails, which aggravates hyper-glycemia and IR further, becoming a vicious circle in which the patient cannot regulate their need to eat. Uncontrolled calorie intake induces an increase in reactive oxygen species, overcoming cellular antioxidant defenses (oxidative stress). Reactive oxygen species activate stress-sensitive kinases, such as c-Jun N-terminal kinase and p38 mitogen-activated protein kinase, that induce phos-phorylation in serine residues in the insulin receptor, which blocks the insulin signaling pathway, continuing the mechanism of IR. The brain and pancreas are organs mainly affected by oxidative stress. The use of drugs that regulate food intake and improve glucose metabolism is the conventional therapy to improve the quality of life of these patients. Currently, the use of antioxidants that regulate oxidative stress has given good results because they reduce oxidative stress and inflammatory processes, and they also have fewer side effects than synthetic drugs.
Core Tip: Insulin is the connection between the β cells of the pancreas and the hypothalamus. Insulin reaches the arcuate nucleus of the hypothalamus and represses the expression of orexigenic neuropeptides to suppress appetite. However, its function decreases when there is damage to the β cells of the pancreas. Its anorexigenic effect decreases and thus increases appetite. The excess of nutrients, specifically carbohydrates, aggravates the damage to β cells and induces obesity and/or diabetes and oxidative damage. The use of antioxidants constitutes a therapeutic approach that has been approached experimentally to regulate the negative effects of alterations in insulin secretion and function.
- Citation: De la Cruz-Concepción B, Flores-Cortez YA, Barragán-Bonilla MI, Mendoza-Bello JM, Espinoza-Rojo M. Insulin: A connection between pancreatic β cells and the hypothalamus. World J Diabetes 2023; 14(2): 76-91
- URL: https://www.wjgnet.com/1948-9358/full/v14/i2/76.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i2.76
Insulin is a peptide hormone that plays an important role in glucose homeostasis, cell growth and metabolism[1]. This hormone is synthesized in the β cells of the pancreatic islets; its transcription and translation is regulated in part by nutrients, specifically in response to glucose concentrations[2,3]. The active structure of this hormone is formed by two chains named “chain A” with 21 amino acid residues and “chain B” with 30 amino acid residues linked by three disulfide bonds between both chains[2,4]. The insulin is stored in vesicles to be released into the bloodstream when β cells take up glucose from the extracellular medium[1]; through the bloodstream it will reach all peripheral organs and the brain[5].
Insulin will bind to its receptor in the cell membrane and allow activation of the phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) insulin signaling pathway[6]. The effects of the activation of this pathway will depend on the cell lineage. It has an anti-atherogenic effect in the vascular system. In the liver, it promotes energy utilization. In the muscle, insulin promotes glucose metabolism and par-ticipates in protein synthesis. In adipose tissue, insulin induces lipogenesis[7,8], and finally in the brain it will activate thermogenesis and regulate appetite, glucose homeostasis and metabolism[8-10]. When there are alterations in the secretion or function of insulin, chronic-degenerative pathologies are produced such as hyperphagia, hyperglycemia, insulin resistance (IR) and diabetes mellitus (DM)[11]. A common feature of these pathologies is the formation of reactive oxygen species (ROS), which alter signaling pathways activated by insulin[12-14]. Currently, the use of nutraceuticals has been reported with highly positive effects on the control of ROS and alterations in the secretion and function of insulin at the pancreatic[15] and cerebral levels[16].
The human pancreas is a retroperitoneal organ in the upper abdomen weighing between 100-150 g and measuring between 15-25 cm in length. It is connected to other abdominal organs such as the spleen, stomach, duodenum and colon[17]. This organ is surrounded by a fibrous capsule that divides its parenchyma into distinct lobes and lobules[18] separated by connective tissue that divides the pancreas into two structurally distinct components: The exocrine pancreas, which consists mainly of acinar cells and duct cells; and the endocrine pancreas, which is the site of islet cells[17,19].
The endocrine portion is composed of groups of cells known as islets of Langerhans, which are attributed with the secretion of several pancreatic peptide hormones for glucose homeostasis, including insulin. There are five major cell types that constitute the islet: α cells; β cells; δ cells; PP cells; and ε cells. They are responsible for producing glucagon, insulin, somatostatin, pancreatic polypeptide and ghrelin, respectively[1,17,20]. The most numerous are the β cells that synthesize and secrete insulin. Insulin is a peptide hormone that was discovered in 1922 by surgeon Frederick Grant Banting and physician Charles Herbert Best and purified by biochemist James Bertam Collip[21,22]. This hormone plays an important role in glucose homeostasis, cell growth and metabolism[1]. In humans, it is encoded by the INS gene on chromosome 11, in rats (Rattus norvegicus) by the ins1/2 gene on chromosome 1 and in mice (Mus musculus) by the Ins1 (chromosome 19) and Ins2 (chromosome 7) genes[23].
The human INS gene (1425 bp) is composed of three exons and two introns, as is the rodent Ins2 gene. However, the rodent Ins2 gene is composed of only two exons, with the entire coding sequence contained in the second exon[24,25]. In the insulin gene promoter, there are response elements such as the A element, GG box, C1 [rat insulin promoter element (RIPE)3b1/C2 (RIPE3b2) element (RIPE 3b1/2), cyclic 3´5´-adenosinemonophosphate (cAMP) response element, E element, insulin-linked polymorphic region], enhancer core and Z region where the negative regulatory element is located. These regulatory elements within the promoter region of the insulin gene either enhance or inhibit transcription of the gene and are located between positions -340 and -91 bp relative to the transcription start site.
Several transcription factors bind in these regions including pancreatic-duodenal homeobox-1 protein 1, pair box protein 4 and 6, transcription factor A, hepatocyte nuclear factor-1 alpha and neurogenic differentiation factor 1[2,26,27]. The signal transducer and activator of transcription (STAT) protein also has a very important role in the activation of insulin gene transcription. It has been reported that elevated Ca2+ levels activate calpain-1, a protease that cleaves a cytosolic fragment of islet cell autoantigen 512, which promotes transient fusion of the cell membrane with the membrane of insulin-containing granules to release insulin into the extracellular milieu. The free fragment of islet cell autoantigen 512 targets the nucleus and binds to STAT5, which in turn promotes increased transcription of the insulin gene, thus maintaining optimal levels of stored insulin[3].
In addition, there are polypyrimidine tract-binding proteins that positively regulate mRNA translation. Cytosolic polypyrimidine tract-binding protein 1 can bind to pyrimidines, i.e. cytosine-uracil-rich sequences in the 3’ untranslated regions of insulin mRNA, thereby stabilizing the insulin mRNA strand and increasing its translation[28].
Insulin translation in pancreatic β cells is regulated in part by nutrients, specifically in response to glucose concentrations[2]. Levels between approximately 2 mmol/L and 4 mmol/L glucose are required to promote insulin biosynthesis and levels greater than 5 mmol/L to promote insulin release[29]. Increased glucose concentrations contribute to the activation of protein phosphatase 1, which dephosphorylates eukaryotic translation initiation factor 2a promoting insulin translation. However, pancreatic endoplasmic reticulum (ER) kinase decreases insulin synthesis through phosphorylation of eukaryotic translation initiation factor 2a[2].
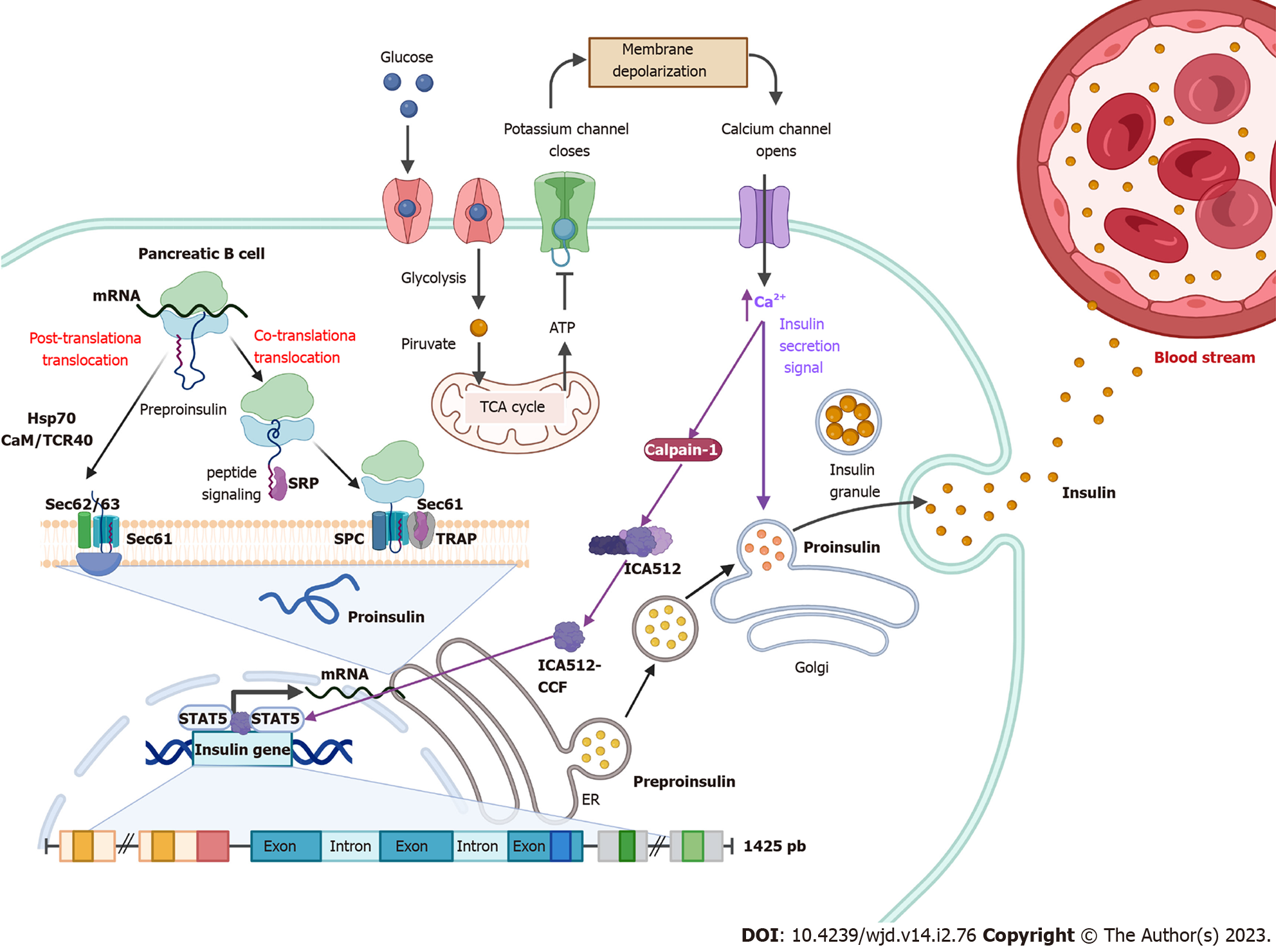
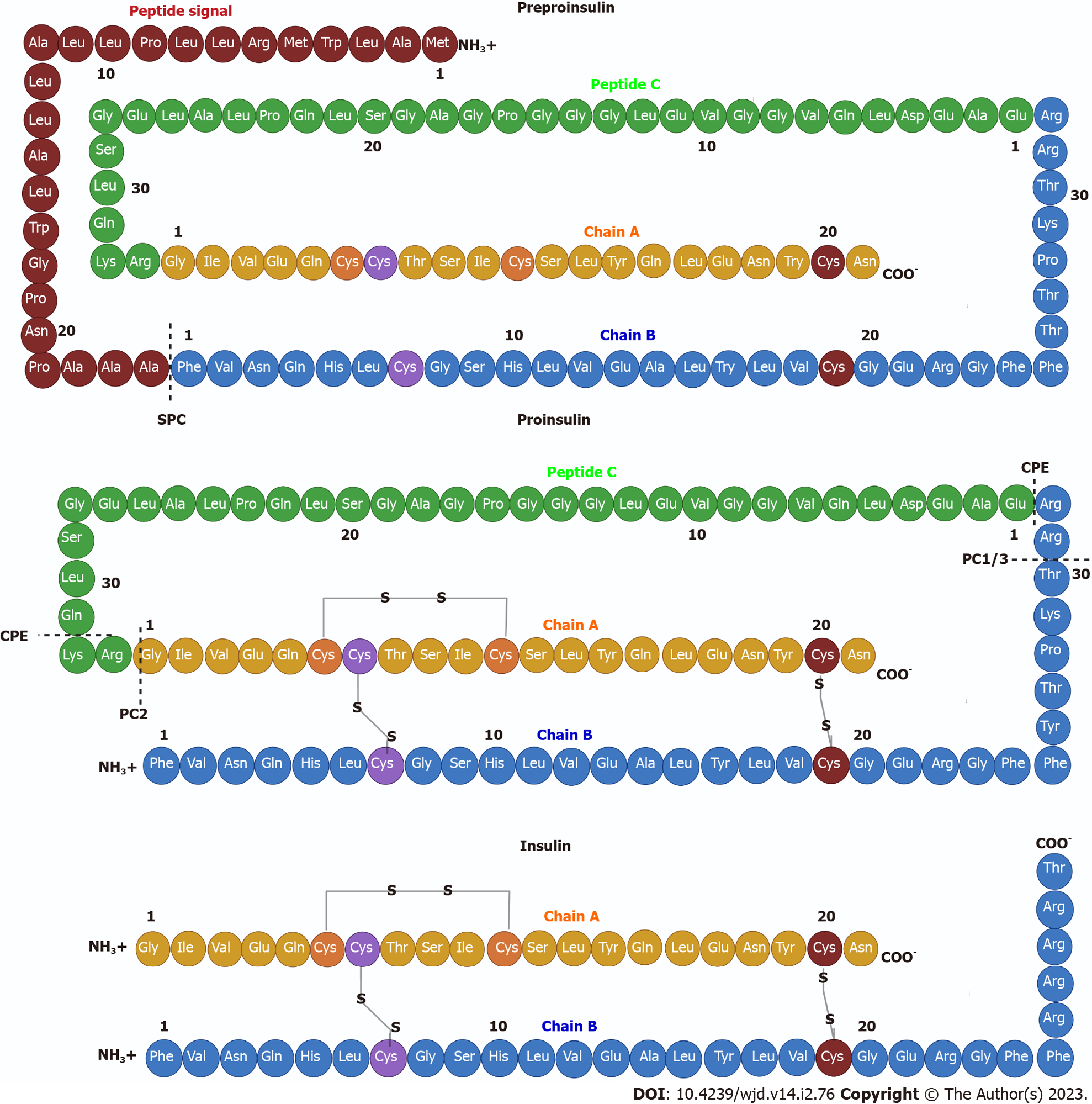
In β cells, insulin is translated as a 110 amino acid pre-proinsulin in the cytosol. Pre-proinsulin contains a 24 amino acid nuclear transport signal peptide (Ala-Ala-Ala-Ala-Pro-Asp-Pro-Gly-Trp-Leu-Ala-Leu-Leu-Leu-Ala-Leu-Leu-Leu-Pro-Leu-Leu-Leu-Arg-Met-Trp-Leu-Ala-Met)[30], which guides pre-proinsulin to the rough ER (RER) membrane for translocation to the RER cisternae via two mechanisms: (1) A signal recognition particle (SRP)-dependent cotranslational translocation mechanism where SRP recognizes and binds to the signal peptide of pre-proinsulin arising from ribosomes, forming a complex that interacts with the SRP receptor on the RER membrane, thereby directing nascent pre-proinsulin to the Sec61 translocon[31]; and (2) An SRP-independent post-translational translocation mechanism where in addition to the Sec61 translocon several RER and cytosolic molecular chaperones are involved, including heat shock protein 70, transmembrane recognition complex-4, calmodulin and protein complex Sec 62/63[31].
During translocation, the pre-proinsulin signal peptide must be correctly oriented within the Sec61 translocon so that the N-terminal end of the signal peptide faces the cytosolic side of the RER. This orientation allows the signal peptide cleavage site to be exposed to signal peptidase on the luminal side of the RER membrane[31], generating pro-insulin, a chain of 86 amino acids that folds and stabilizes in its three-dimensional configuration by linking peptide chains A and B through the formation of three disulfide bonds via chaperones such as thiol reductase. The first bond is between amino acids CysA6 and CysA11, the second is between amino acids CysA7 and CysB7, and the third bridge is between amino acids CysB19 and CysA20[23,32].
After acquiring three-dimensional folding, pro-insulin is transferred from the RER to the Golgi via vesicles where pro-insulin is converted to insulin as these immature vesicles acidify and mature[31] (Figure 1). In the secretory granules there are two endoproteases involved in the conversion of proinsulin to insulin called prohormone convertase 2 (PC2) and PC1/3. The former hydrolyzes between the basic amino acids Arg33-Gly1 at the C-peptide and A-chain junction, and the latter hydrolyzes between the dipeptide Thr30-Arg31 at the B-chain and C-peptide junction[33]. Subsequently, car-boxypeptidase E hydrolyzes between the Gln31-Lys32 amino acids as well as between Arg32 and Glu1 basic C-termini of the resulting peptide chains, producing a mature insulin protein of 51 amino acids[23] (Figure 2).
Insulin in its monomeric form tends to form dimers as insulin concentration increases. In the presence of zinc and pH optima (10 mmol/L Zn2+, pH 6.0), the hydrophobic amino acids in the dimeric structures interact and assemble into higher order conformations called hexamers, useful for insulin storage[2]. Once the hexamers are secreted into the circulation by exocytosis, they diffuse into the blood in favor of their concentration gradient. A combination of electrostatic repulsion and decrease in insulin concentration favors the dissociation of insulin into its monomeric form, releasing active insulin and an equimolar proportion of C-peptide[2,33].
This active structure is formed by two chains named “chain A” with 21 amino acid residues and “chain B” with 30 amino acid residues linked by three disulfide bonds between both chains (CysA7-CysB7, CysA20-CysB19 and CysA7-CysA11) (Figure 2). The secondary structure of the A chain contains two antiparallel α-helices connected near the two ends of the A chain. The secondary structure of the B chain contains α-helices and β-strands. This chain can generate two distinct conformations. In a taut state, there is a central α-helix from SerB9 to CysB19 as well as a β-twist from GlyB20-GlyB23 generating a “V” fold. This twist also allows the formation of a β-sheet with Phe24 and Tyr26 in contact with Leu11 and Leu15 of the α-helix of the B-chain. In a resting state, there is a continuous alpha helix from PheB1-CysB19. Disulfide bonds between residues CysA7-CysB7 and CysA20-CysB19 contribute to the stability of the native insulin structure[2,4]. The overall tertiary structure of the protein is highly organized and stabilized by specific interactions involving residues CysA6-CysA11 and LeuA11, PheB1 and LeuB15, IleA2, PheB24, ValA3, IleA13, ValB18 and ValB12 generating a hydrophobic core[2].
Following food intake, glucose is transported into pancreatic β cells via the glucose transporter (GLUT) 2 in humans and mice[30,33]. Once pancreatic β cells have internalized glucose and its degradation through glycolysis and the Krebs cycle is initiated, intracellular ATP levels increase, which generates the closure of K+ channels, causing a change in membrane permeability opening Ca2+ channels. This induces the remodeling of the cytoskeleton and the translocation of insulin granules to the plasma membrane to subsequently release the hormone, which through the bloodstream will reach all peripheral organs and the brain[5,30,33] (Figure 1).
Levels between approximately 2 mmol/L and 4 mmol/L glucose are required to promote insulin biosynthesis and levels greater than 5 mmol/L to promote insulin release[29]. Once insulin synthesis is stimulated in the β cells of the pancreas, it is exported through the portal vein to the liver. During this process, more than 50% of the insulin is eliminated by hepatocytes from the liver. The remaining insulin exits through the hepatic vein until it reaches the heart to be distributed through the arterial circulation to the rest of the body to fulfill its various functions. Finally, the remaining circulating insulin is degraded in the kidney[23].
In the peripheral organs that depend on insulin to bring glucose into the cells, the hormone will bind to its receptor and allow activation of the PI3K/AKT insulin signaling pathway. This will generate translocation of GLUT4 to the cell membrane thus allowing glucose to enter the cell. Therefore, insulin, through anabolic pathways, regulates blood glucose concentrations[6]. Whereas, the counter-regulatory hormone, glucagon, regulates glucose concentrations through catabolic pathways[1].
Within the positive regulators of insulin, in addition to glucose, are amino acids, glucagon, glucagon-like peptide 1 (GLP-1), growth hormone, secretin, gastrin, glucose-dependent insulinotropic peptide and cholecystokinin. Among the major negative regulators of insulin are adrenocorticosteroids, somatostatin, adrenaline, norepinephrine, neuropeptide Y and calcitonin gene-related peptide[33].
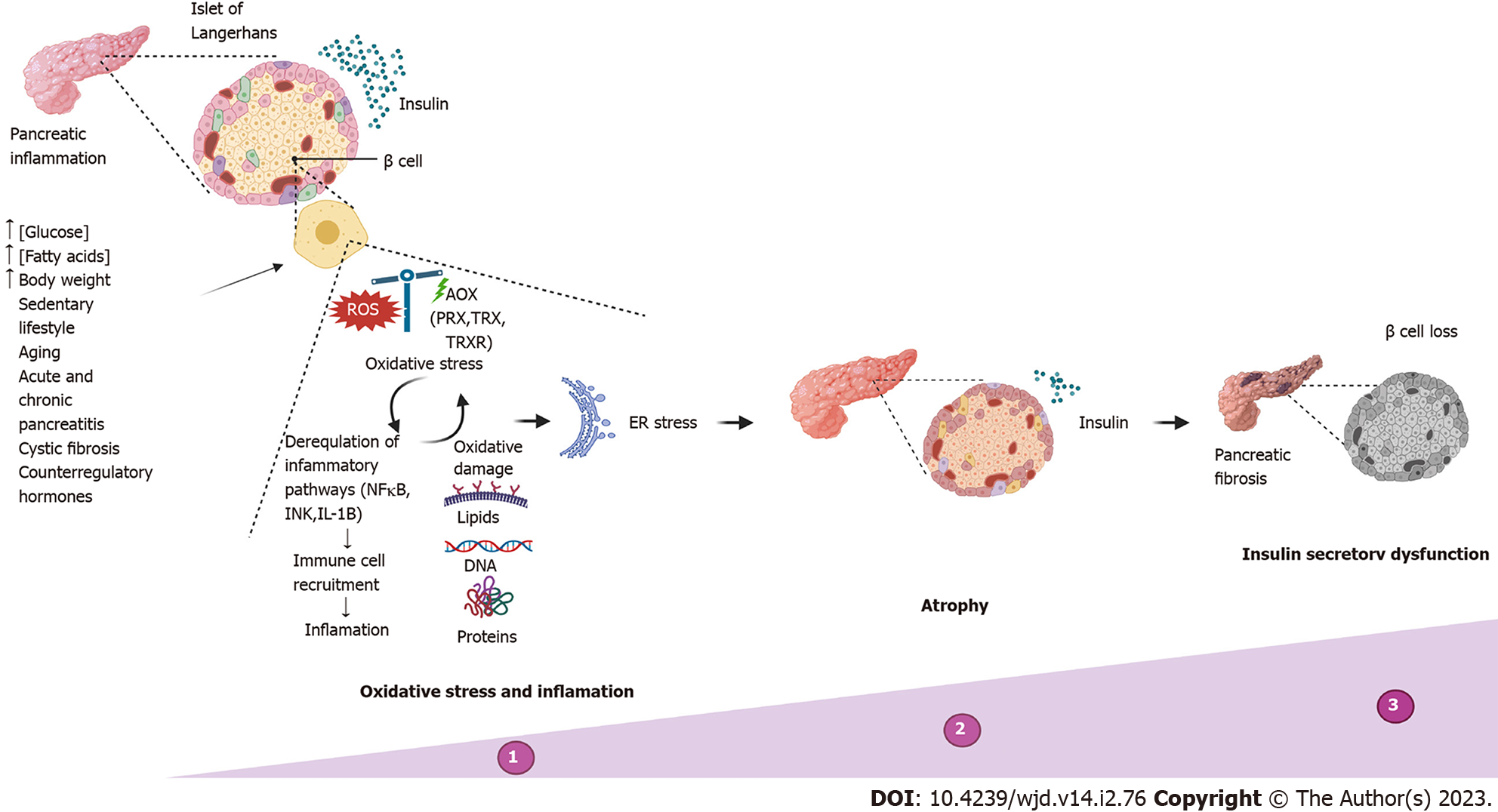
Insulin-secreting β cell dysfunction, defined as the loss of the ability of pancreatic β cells to produce and release insulin in concentrations sufficient to maintain euglycemia, occurs when high and prolonged insulin secretion in response to environmental insults leads to exhaustion of pancreatic β cells[34]. β cells can suffer from insulin secretory dysfunction due to multiple factors. The most common causes are overnutrition (excess nutrients such as glucose and fatty acids), increased body weight, a sedentary lifestyle and aging, which will lead to pathological conditions such as obesity and type 2 DM (T2DM)[34-36]. Other causes of β cell dysfunction, accounting for less than 5% of cases, include diseases that destroy the pancreas, such as acute pancreatitis, chronic pancreatitis and cystic fibrosis[37-39], that specifically inhibit insulin secretion (genetic β cell defects) or that alter counterregulatory hormones (Cushing’s syndrome, obesity)[34]. The clinical presentations in these cases depend on the exact nature of the process.
The most common causes of β cell dysfunction share the formation of ROS and cellular oxidative stress as the initiation mechanism[40-42]. Pancreatic β cells are especially vulnerable to stress and oxidative damage[38] due to the low expression of classical antioxidant enzymes such as catalases, glutathione peroxidases and superoxide dismutases compared to other cell types[43,44]. The main antioxidant system of β cells consists of peroxiredoxins, thioredoxins and thioredoxin reductase. This system has been shown to be sufficient to protect β cells against short-term oxidative stress and hypothetically provides a signaling role required for glucose-stimulated insulin secretion in both rodent and human cells[45]. However, long-term glycolipotoxic conditions compromise β cell metabolism and ATP production through glycolytic dysfunction and reduced activation of glyceraldehyde 3-phosphate dehydrogenase, which reduces the generation of pyruvate and promotes β-oxidation.
As a result of metabolic dysfunction, the generation of superoxide and hydrogen peroxide by the mitochondrial electron transport chain is increased[46], increasing cellular ROS concentrations. Excess ROS are capable of oxidizing DNA (mainly mitochondrial DNA), proteins and lipids and function as effector and signaling molecules in cell membranes that mediate signal transduction and inflammation pathways[46,47]. In addition, inflammation, which is also present in the aforementioned pathologies, aggravates the damage and functions as a feedback for stress and oxidative damage because poly-morphonuclear neutrophils at the site of inflammation release large amounts of ROS as an immune defense response, causing tissue damage and endothelial dysfunction[48]. Oxidative stress can induce and maintain a proinflammatory environment through the activation of proinflammatory pathways regulated by the transcription nuclear factor kB and c-Jun N-terminal kinase (JNK) and the production of inflammatory cytokines such as interleukin-1beta[34,38,40,49]. This improves polymorphonuclear neutrophil recruitment, which further stimulates the proinflammatory condition in the tissue, thus generating a feedback process oxidative stress-inflammation-oxidative stress[46].
Persistent inflammation of the pancreas causes ER stress, progressive atrophy and/or replacement with fibrotic tissue, pain, exocrine pancreatic insufficiency, trypsin activation leading to pancreatic autodigestion, loss of functional β cell mass and consequently the reduced ability of β cells to secrete insulin (Figure 3). This pathology is known as pancreatic endocrine dysfunction or DM[50,51].
DM is a complex and heterogeneous disorder defined by the presence of hyperglycemia[11] and can lead to life-threatening complications such as severe hypoglycemia or chronic micro- and macroangiopathic complications[52]. There are several types of diabetes, although type 1 DM (T1DM) and T2DM are the most common. The American Diabetes Association defines T1DM as the autoimmune destruction of β cells, usually leading to absolute insulin deficiency and T2DM as the progressive loss of insulin action in target tissues as well as a decrease in their secretion from β cells[53]. All cellular events are summarized in Figure 3.
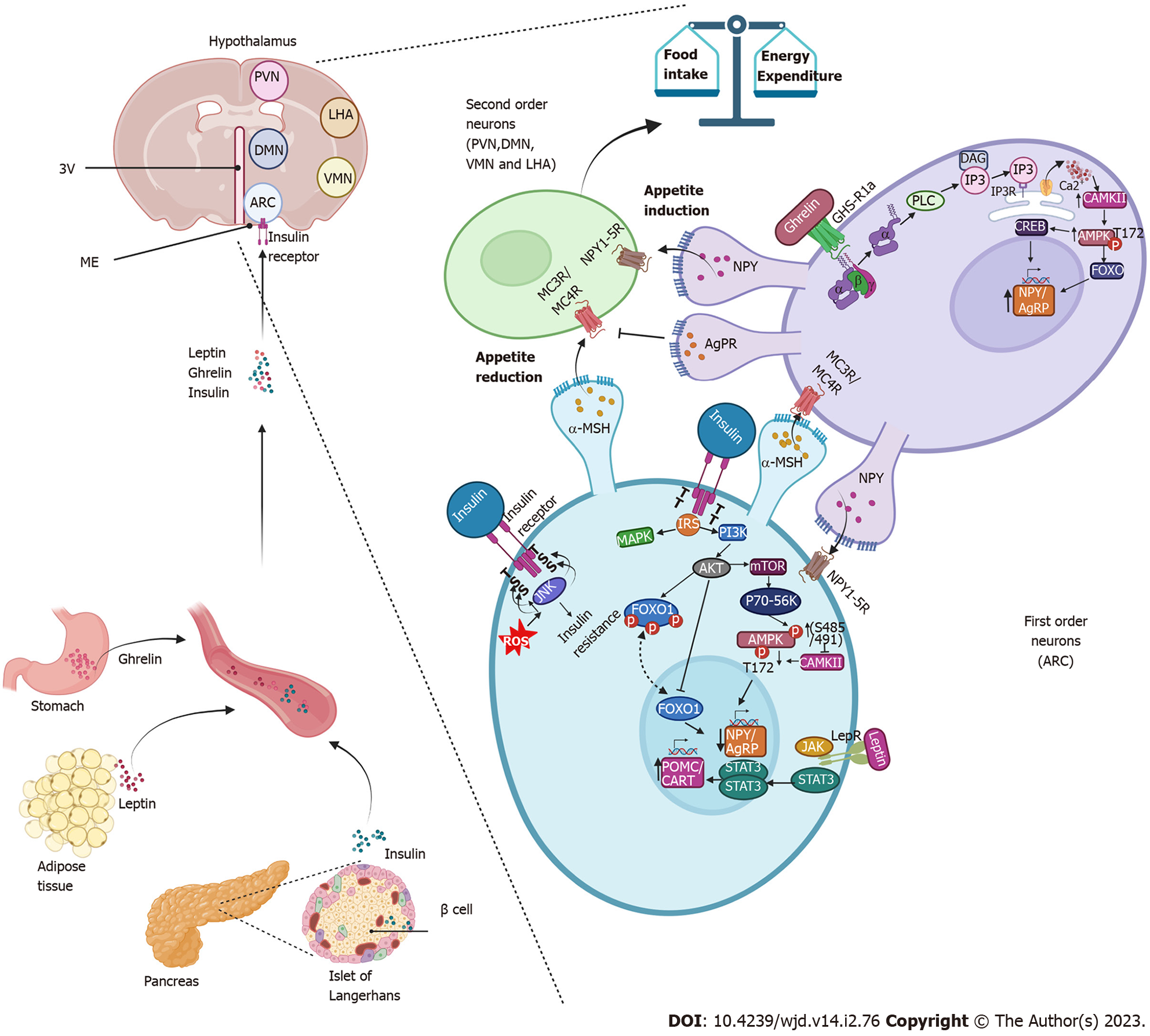
The hypothalamus is the specific area of the brain where eating behavior is regulated, which is directly related to glucose homeostasis[54]. The hypothalamus is located around the third ventricle, below the thalamus and above the median eminence, one of the circumventricular organs in which the blood brain barrier is slightly modified with semi-permeable capillaries that allow selective exchange between molecules of the blood and cerebrospinal flow with the neurons of the hypothalamus[55,56]. This region is divided into several nuclei, among which the arcuate nucleus (ARC), paraventricular nucleus, ventromedial nucleus, dorsomedial nucleus and lateral area nucleus stand out[57,58]. The ARC is located very close to the median eminence. It is made up of first-order neurons that first receive signals from peripheral organs such as the stomach, adipose tissue and the pancreas[56,59].
Insulin is the signal derived from the pancreas in response to the presence of nutrients (glucose) in the bloodstream[54]. After being secreted from pancreatic β cells, insulin via the bloodstream reaches the hypothalamus crossing the median eminence or crossing the vascular endothelium via transport proteins or via the insulin receptor itself, which is assumed to also act as its transporter (mechanism not fully defined)[60,61].
Insulin reaches the ARC and binds to its receptor in the first-order neurons. Once insulin binds to its receptor in the hypothalamus, it leads to rapid autophosphorylation of the insulin receptor, followed by tyrosine phosphorylation of insulin receptor substrates, which induces the activation of the PI3K/AKT and the mitogen-activated protein kinases (MAPK) cascades[61]. The PI3K/AKT pathway promotes the activation of the mammalian target of rapamycin complex 1/p70-S6 kinase[61,62], which is capable of phosphorylating AMP-activated protein kinase (AMPK) at serine 485/491 sites[63], reducing the ability of Ca2+/calmodulin-dependent kinase II to phosphorylate AMPK in the threonine 172 residue and resulting in the low expression of genes related to appetite induction (orexigenic), such as neuropeptide Y (NPY) and agouti-related protein (AgRP) in the ARC, the paraventricular nucleus and the lateral area nucleus, which decreases appetite[56,63,64].
Moreover, AKT induces the phosphorylation of the transcription factor forkhead box protein O1. When forkhead box protein O1 is phosphorylated it leaves the nucleus and therefore decreases the expression of genes that are activated by this factor, such as NPY and AgRP[56,64]. Therefore, insulin and the activation of it signaling pathway promotes an anorexigenic effect by inducing a decrease in the expression of the neuropeptides that induce appetite (NPY/AgRP).
Similar to insulin, another anorexigenic signaling pathway is activated by leptin[56,64]. Leptin is secreted from adipocytes in proportion to levels of body fat stores. Through the bloodstream it reaches first-order neurons, binds to its receptors and activates the Janus tyrosine kinase pathway and STAT3 pathway. STAT3 is a transcription factor that stimulates the expression of the precursor neuropeptide of α-melanocyte-stimulating hormone, named proopiomelanocortin (POMC) and the transcript regulated by cocaine and amphetamines (CART). These neuropeptides exert an anorexigenic effect[56,64]. Leptin and insulin signaling converge in the activation of PI3K/AKT, thus the anorexigenic effect is enhanced since the expression of NPY/AgRP is decreased by insulin and leptin, while POMC/CART expression is increased by leptin[56,64,65].
POMC/CART are the main anorexigenic neuropeptides expressed in neurons of the first-order (named neurons POMC/CART). These neurons release multiple cleavage products of POMC, including α-melanocyte-stimulating hormone, that bind in the second-order neurons located in the paraventricular nucleus, dorsomedial nucleus, ventromedial nucleus and lateral area nucleus to activate downstream melanocortin receptors (MC3R/MC4R) to promote satiety and control eating behavior, glucose homeostasis and body weight[54,58,64,66].
In periods of fasting, when glucose decreases, the release of insulin in the pancreas also decreases, and consequently the expression of POMC and CART decreases along with the satiety effect[56]. Meanwhile, the concentrations of ghrelin, a hormone secreted in the stomach during periods of starvation, increase[67]. This hormone reaches ARC through the bloodstream to activate the growth hormone receptor 1a, a G protein-coupled receptor, for the release of the α subunit from the βγ subunits of G protein. The α subunit activates phospholipase C. Phospholipase C induces the production of diacyl glycerol and phosphoinositol triphosphate. Phosphoinositol triphosphate is a second messenger that binds to its receptor in the ER and causes the release of Ca2+ into the cytosol[68]. Increasing Ca2+ activates the Ca2+/calmodulin-dependent kinase II, which phosphorylates AMPK in the threonine 172 residue. AMPK activates transcription factors such as the cAMP-response element binding protein and forkhead box protein O1, which act on the promoter region of the NPY and AgRP genes, promoting their expression and inducing appetite[14,56].
NPY exerts its orexigenic effect on second-order neurons through stimulation of the Gi-coupled NPY family of receptors[66,69], mediating the inhibition of adenylate cyclase, decreased levels of cAMP[57,70] and the activation of MAPK[61,70]. AgRP is a biased agonist of the melanocortin receptors (MC3R/MC4R) and prevents the binding of α-melanocyte-stimulating hormone to these receptors, blocking the induction of satiety and driving sustained increase in food intake[66]. This constitutes an orexigenic signal.
Therefore, under normal physiological conditions, the release of the specific signal (inducing or inhibiting appetite) in the peripheral organs will depend on the metabolic state of the organism and will induce a response in the form of orexigenic or anorexigenic neurotransmitters in the hypothalamus[9,56,64,66]. The strict regulation of these afferent and nutrient-related hormonal signals is necessary to avoid alterations in the regulation of appetite since an uncontrolled increase in POMC/CART would cause anorexia, but the uncontrolled increase in the expression of NPY/AgRP will generate hyperphagia, which due to excessive consumption of hypercaloric diets has been related to weight gain and obesity (characteristics that are linked to IR and T2DM)[9].
In studies in experimental models of hyperphagia and DM induced with streptozotocin, NPY/AgRP neurons are more active and the expression level of NPY and AgRP is increased, while POMC/CART neurons are less active and the expression level of POMC and CART is decreased. This change is explained in part to the inefficiency and/or deficiency of insulin[71,72] and leptin[73] and increased levels of circulating ghrelin[74,75].
During diabetic hyperphagia, high glucose intake will induce a proportional release of insulin from pancreatic β cells (hyperinsulinemia). The high concentration of insulin will induce the constant activation of the receptor at the cerebral and peripheral levels, which generates molecular and cellular regulation mechanisms such as: (1) Internalization of the receptor by clathrin-mediated endocytosis[76]; (2) Dephosphorylation in tyrosine residues of the insulin receptor by protein tyrosine phosphatase 1B, which is a nontransmembrane tyrosine phosphatase that acts as a potent negative modulator of insulin signaling by reversing insulin-induced phosphorylation in tyrosine residues and impairs insulin signal transduction; and (3) Phosphorylation on serine residues by serine-threonine kinases, such as JNK and the p38 MAPK[12,13]. This will generate a lack of response to the presence of the hormone (i.e. IR). At the level of the hypothalamus, this will decrease the activity of one of the pathways that induce satiety.
On the other hand, hyperphagia is often associated with the accumulation of visceral fat[77] and consequently elevated plasma leptin concentrations. This situation will induce the failure to respond to the hormone at central and peripheral levels, named leptin resistance[78,79]. In this way, there will be a decrease in the two central signals that induce satiety, favoring the persistence of hyperphagia and the onset of resistance to both hormones. This becomes a vicious circle: hyperphagia-hyperglycemia-hyperinsulinemia/hyperleptinemia-insulin/leptin resistance-hyperphagia.
In addition, excessive consumption of carbohydrates (glucose and/or fructose), coupled with a lack of physical activity, will generate an increase in glucose uptake in all cells but mainly in cells that have glucose transporters that act independently of the presence or absence of insulin[9,13,14] transporters, such as GLUT1 and GLUT3, mainly present in the brain[80]. With excessive intake of carbohydrates, glycolysis will increase, and therefore the release of ROS (species produced normally in glycolysis) will increase progressively until they overcome antioxidant barriers and oxidative stress develops[13,14,81].
It has been reported that during oxidative stress there is the activation of stress-sensitive kinases (JNK, p38 MAPK) that induce phosphorylation of serine residues in the insulin receptor and in the insulin receptor substrates, which blocks the pathway of insulin signaling aggravating the condition of IR[12,13]. In addition, studies carried out in rat models fed with fructose and subjected to an environmental stress protocol revealed that stress decreased body mass, adiposity and blood leptin level, decreased expression of the leptin receptor and POMC in the hypothalamus and led to a marked increase of AgRP, associated with AMPK phosphorylation and reduced Akt activity[14]. In parallel studies undertaken in normal rats, chronic blockade of hypothalamic insulin receptors caused hyperphagia and IR[82]. Furthermore, it has been reported that stimulation of hypothalamic insulin signaling would be sufficient to inhibit the glucose production in the liver through the intra-cerebroventricular administration of agonists and antagonists of insulin signaling[83], combined with evidence that mice with neuron-specific insulin receptor deletion are overweight, insulin-resistant and glucose-intolerant. These data demonstrate that neuronal insulin signaling is required for intact control of both body fat mass and glucose homeostasis[9]. Consequently, chronic stress can dysregulate the hypothalamus-adipose tissue[14,84] and hypothalamus-pancreas[64] axis over time, which affects glucose metabolism, promotes IR and influences multiple appetite-related hormones in the hypo-thalamus[64,84].
On the other hand, the effect of insulin has not only been studied in the hypothalamus at the level of glucose homeostasis. It has also been shown that the administration of insulin into the hippocampus of rats promotes Akt-dependent translocation of GLUT4[85]. Furthermore, hippocampal-specific suppression of insulin signaling reduces long-term potentiation in the hippocampus and significantly impairs memory and learning ability[86]. In hypothalamic neurons they have an important effect on body thermoregulation by signaling with brown adipose tissue[87]. Therefore, the effect of insulin at the brain level has been fully established. All cellular and molecular events are summarized in Figure 4.
Medical therapy is the first step to achieve adequate control of complications related to alterations in insulin secretion. Considering that DM is the main pathology related to this alteration, therapeutic treatments are focused on reducing hyperglycemia as well as stimulating the production and secretion of insulin in the cells of the pancreas and its signaling in the different tissues.
For T1DM, characterized by the destruction of the cells of the pancreas by autoantibodies as well as a decrease in the production and secretion of insulin, the first-line treatment is the administration of non-endogenous insulin[88]. Regarding T2DM, there are various therapeutic approaches, starting with improving eating habits[89] and increasing physical activity, which results in improving insulin sensitivity and helps control blood glucose[90]. When the above does not help control hyperglycemia, the therapeutic approach is based on the use of conventional drugs such as sulfonylureas (inducing insulin release from cells of the pancreas), biguanides (inducing glucose uptake by cells that are not insulin-dependent and reducing hepatic glucose production) and alpha-glucosidase inhibitors (blocking the absorption of glucose in the intestine)[91].
Currently, the use of incretin-based therapy has been implemented. Incretins are enteroendocrine hormones released after nutrient intake that stimulate glucose-dependent insulin secretion from β cells. To date, two incretins have been identified, glucose-dependent insulinotropic polypeptide (GIP) and GLP-1. In mice, deficiencies in GIP and GLP-1 secretion are associated with decreased insulin response and impaired glucose tolerance. In this context, the overexpression of GIP or GLP-1 improves β cell function and glucose tolerance, and enhances insulin sensitivity. However, GIP also has an obesogenic effect, at least in animal models. Therefore, investigations have focused on GLP-1, specifically on its receptor. Agonists for GLP-1 receptor activation have recently been used. These include liraglutide, albiglutide, dulaglutide and semaglutide, and the results have been favorable for the management of DM[92].
On the other hand, the importance of finding new therapies that help improve disease control and the use of nutraceuticals has been increasing in recent years[93]. A positive effect has been reported in compounds such as melatonin[94], aloe vera extract[95] and hibiscus sabdariffa leaf extract[96]. They have regenerated pancreatic β cells and enhanced insulin secretion in streptozotocin-induced diabetic animal models. In patients with metabolic syndrome, a nutraceutical diet composed of barberine, policosanol, red yeast rice or tocotrienols significantly reduced the Homeostatic Model Assessment for IR index, leading to the conclusion that they have beneficial effects on IR[97,98].
Resveratrol, a polyphenol, found in many types of red fruits, has beneficial effects both in vivo and in vitro, showing great antioxidant capacity while improving insulin sensitivity[99,100]. Resveratrol is capable of activating the AKT pathway to stimulate insulin action[15]. The activation of sirtuin-1/AMPK has also been reported[101], which has a positive impact on mitochondrial biogenesis, inhibition of lipogenesis and fatty acid oxidation[102] and improves insulin sensitivity in DM[103,104].
Another antioxidant compound that has been less studied than resveratrol but with positive effects in models of obesity[105] and diabetes[106] has been curcumin, a non-flavonoid polyphenol[107]. In diabetic animal models, curcumin improves insulin sensitivity and increases glucose uptake. This mechanism is mediated by the liver kinase B1-AMPK pathway. Adding curcumin induced an increase in fatty acid oxidation, an event that improves insulin sensitivity[108]. At the brain level, curcumin increases glucose metabolism and improves the insulin signaling pathway, improving learning and memory[16] both under non-pathological conditions and in Alzheimer’s disease[109]. Currently there are several studies on the use and beneficial effects of a wide variety of nutraceuticals, which are described in Table 1.
| Nutraceutical | Mechanism of action | Study model | Ref. |
| Resveratrol | Reduces blood glucose and serum insulin levels, improves insulin and glucose tolerance, increases Sirt1, p-AMPK, p-IRS1 and p-AKT levels in liver | Mice, KKAy | [110] |
| Enhances peripheral insulin signaling in diabetic mice in association with PTBP1 inhibition | Mice deficient in IRS2 (Irs2 -/-) and injected with STZ | [111] | |
| Reduces stress on the endoplasmic reticulum, thus improving insulin sensitivity and glucose levels | Mice C57BL/6J on a HFD | [112] | |
| Regulates protein expression of insulin receptor and GLUT4 | Rat, Goto-Kakizaki | [113] | |
| Counteracts insulin resistance caused by hyperinsulinemia by activating AMPK and regulating GLUT4 translocation in muscle cells | L6 cell line | [114] | |
| Reduces insulin levels and the HOMA-IR index | Patients with T2DM | [115] | |
| Curcumin | As a pretreatment, it protects pancreatic islets from cytokine-induced death | Mice, C57BL/6J | [116] |
| Protects pancreatic islets from glycolipotoxicity by inhibiting oxidative stress and NADPH oxidase activity | Rats, Sprague-Dawley | [117] | |
| Improves insulin sensitivity and energy metabolism through the FNDC5/p38 MAPK/ERK pathways | Mice, C57BL/6J | [118] | |
| HOMA-IR index decreases | Patients with T2DM | [119] | |
| Garlic | Decreases serum insulin level, HOMA-IR index and appetite | Patients with metabolic syndrome | [120] |
| Rhizoma polygonati odorati extract | Regulates serum insulin, adiponectin and leptin levels in mice on an HFD | Mice, C57BL/6 | [121] |
| Diospyros kaki (persimmon) extract | Increases the number of pancreatic islets, decreases the expression of TNFα and IL-6, which interferes with insulin action | Zebra fish | [122] |
| Morus alba leaves | Decreases in the fasting insulin level and the HOMA-IR index, resulting in decrease of insulin resistance | Mice, C57BL/6 with HFD and STZ | [123] |
| Hydrolyzed pea protein | Enhances insulin-stimulated phosphorylation of AKT and FOXO1, increases IRS1 expression | Cells AML-12 | [124] |
| Avocado oil | Improves insulin and glucose sensitivity | Mice, C57BL/6J | [125] |
| Eugenol | Improves glucose uptake in muscle, by insulin-independent pathway CaMKKβ/AMPK/GLUT4. | Mice, C57BL/6N with HFD and STZ | [126] |
| Okra leaf extract (Abelmoschus esculentus) | Regulates blood glucose level, food intake and changes in body weight | Wistar rats with STZ | [127] |
Insulin is a peptide hormone that plays an important role in various organs: in pancreas it participates in glucose homeostasis; in muscle it promotes glucose metabolism for energy generation and storage; in the vascular system it exerts an anti-atherogenic effect and participates in bone formation; in liver it decreases gluconeogenesis and favors glucose storage through glycogenesis; in adipose tissue it induces lipogenesis; and in brain it activates thermogenesis, regulates appetite, participates in glucose homeostasis and metabolism, reduces long-term potentiation and impairs memory and learning ability. Alterations in secretion or function of insulin considerably alter the cellular events regulated by the activation of its signaling pathway. Obesity and DM are pathologies associated with alterations in the function and secretion of insulin. In these pathologies, oxidative stress plays an important role since the uncontrolled increase in ROS derived from the increase in glycolysis due to the constant entry of glucose into the cells overcomes the antioxidant defenses. ROS induces alterations in insulin signaling and triggers a cascade of cellular alterations in various organs. Specifically in the hypothalamus, it can be the inducer of hyperphagia, which aggravates the diabetic condition and obesity. The use of antioxidants can be a complementary strategy to conventional treatment of DM.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Balbaa ME, Egypt; Cigrovski Berkovic M, Croatia S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Rahman MS, Hossain KS, Das S, Kundu S, Adegoke EO, Rahman MA, Hannan MA, Uddin MJ, Pang MG. Role of Insulin in Health and Disease: An Update. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (2)] |
| 2. | Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9:25-53. [PubMed] |
| 3. | Mziaut H, Trajkovski M, Kersting S, Ehninger A, Altkrüger A, Lemaitre RP, Schmidt D, Saeger HD, Lee MS, Drechsel DN, Müller S, Solimena M. Synergy of glucose and growth hormone signalling in islet cells through ICA512 and STAT5. Nat Cell Biol. 2006;8:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Baker EN, Blundell TL, Cutfield JF, Cutfield SM, Dodson EJ, Dodson GG, Hodgkin DM, Hubbard RE, Isaacs NW, Reynolds CD. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci. 1988;319:369-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 470] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Park SY, Gautier JF, Chon S. Assessment of Insulin Secretion and Insulin Resistance in Human. Diabetes Metab J. 2021;45:641-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 6. | Posner BI. Insulin Signalling: The Inside Story. Can J Diabetes. 2017;41:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Niswender KD. Basal insulin: physiology, pharmacology, and clinical implications. Postgrad Med. 2011;123:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 425] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 9. | Schwartz MW, Porte D Jr. Diabetes, obesity, and the brain. Science. 2005;307:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 585] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 10. | Scherer T, Sakamoto K, Buettner C. Brain insulin signalling in metabolic homeostasis and disease. Nat Rev Endocrinol. 2021;17:468-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 11. | Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 520] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 12. | Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 420] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol. 2019;234:8152-8161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 558] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 14. | Zidane Shirif A, Kovačević S, Bursać B, Lakić I, Veličković N, Jevdjovic T, Djordjevic A. Combination of chronic stress with fructose diet increases AMP-activated protein kinase phosphorylation and affects agouti-related protein and proopiomelanocortin expression in the hypothalamus of male Wistar rats. Acta Biochim Pol. 2022;69:647-655. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Sadi G, Pektaş MB, Koca HB, Tosun M, Koca T. Resveratrol improves hepatic insulin signaling and reduces the inflammatory response in streptozotocin-induced diabetes. Gene. 2015;570:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Wang P, Su C, Feng H, Chen X, Dong Y, Rao Y, Ren Y, Yang J, Shi J, Tian J, Jiang S. Curcumin regulates insulin pathways and glucose metabolism in the brains of APPswe/PS1dE9 mice. Int J Immunopathol Pharmacol. 2017;30:25-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Leung PS. Overview of the pancreas. Adv Exp Med Biol. 2010;690:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Dolenšek J, Rupnik MS, Stožer A. Structural similarities and differences between the human and the mouse pancreas. Islets. 2015;7:e1024405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 19. | Low MJ. Clinical endocrinology and metabolism. The somatostatin neuroendocrine system: physiology and clinical relevance in gastrointestinal and pancreatic disorders. Best Pract Res Clin Endocrinol Metab. 2004;18:607-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | A-Kader, HH, Ghishan, FK. The Pancreas. In: Elzouki, AY, Harfi, HA, Nazer, HM, Stapleton, FB, Oh, W, Whitley, RJ. Textbook of Clinical Pediatrics. Springer, Berlin, Heidelberg. 2012; 1925-1936. Available from: https://link.springer.com/referenceworkentry/10.1007/978-3-642-02202-9_198. |
| 21. | Bliss M, The Discovery of Insulin. Chicago: The University of Chicago Press. The American Historical Review 1983; 4: 964–965. [DOI] [Full Text] |
| 22. | Karamitsos DT. The story of insulin discovery. Diabetes Res Clin Pract. 2011;93 Suppl 1:S2-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Tokarz VL, MacDonald PE, Klip A. The cell biology of systemic insulin function. J Cell Biol. 2018;217:2273-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 281] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 24. | Soares MB, Schon E, Henderson A, Karathanasis SK, Cate R, Zeitlin S, Chirgwin J, Efstratiadis A. RNA-mediated gene duplication: the rat preproinsulin I gene is a functional retroposon. Mol Cell Biol. 1985;5:2090-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 84] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Irwin DM. Evolution of the Insulin Gene: Changes in Gene Number, Sequence, and Processing. Front Endocrinol (Lausanne). 2021;12:649255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Hay CW, Docherty K. Comparative analysis of insulin gene promoters: implications for diabetes research. Diabetes. 2006;55:3201-3213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 27. | Vanhoose AM, Samaras S, Artner I, Henderson E, Hang Y, Stein R. MafA and MafB regulate Pdx1 transcription through the Area II control region in pancreatic beta cells. J Biol Chem. 2008;283:22612-22619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Jeong DE, Heo S, Han JH, Lee EY, Kulkarni RN, Kim W. Glucose Controls the Expression of Polypyrimidine Tract-Binding Protein 1 via the Insulin Receptor Signaling Pathway in Pancreatic β Cells. Mol Cells. 2018;41:909-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Alarcón C, Lincoln B, Rhodes CJ. The biosynthesis of the subtilisin-related proprotein convertase PC3, but no that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic islets. J Biol Chem. 1993;268:4276-4280. [PubMed] |
| 30. | Thevis M, Thomas A, Schänzer W. Insulin. Handb Exp Pharmacol. 2010;209-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Liu M, Weiss MA, Arunagiri A, Yong J, Rege N, Sun J, Haataja L, Kaufman RJ, Arvan P. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes Metab. 2018;20 Suppl 2:28-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 32. | Hutton JC. Insulin secretory granule biogenesis and the proinsulin-processing endopeptidases. Diabetologia. 1994;37 Suppl 2:S48-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26:19-39. [PubMed] |
| 34. | Biondi G, Marrano N, Borrelli A, Rella M, Palma G, Calderoni I, Siciliano E, Lops P, Giorgino F, Natalicchio A. Adipose Tissue Secretion Pattern Influences β-Cell Wellness in the Transition from Obesity to Type 2 Diabetes. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (2)] |
| 35. | Čater M, Križančić Bombek L. Protective Role of Mitochondrial Uncoupling Proteins against Age-Related Oxidative Stress in Type 2 Diabetes Mellitus. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 36. | Murakami T, Inagaki N, Kondoh H. Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells. Front Endocrinol (Lausanne). 2022;13:869414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 37. |
Barrio-Castellano R.
Insuficiencia pancreática endocrina. |
| 38. | Yousef AI, Shawki HH, El-Shahawy AA, El-Twab SMA, Abdel-Moneim A, Oishi H. Polydatin mitigates pancreatic β-cell damage through its antioxidant activity. Biomed Pharmacother. 2021;133:111027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Nyirjesy SC, Sheikh S, Hadjiliadis D, De Leon DD, Peleckis AJ, Eiel JN, Kubrak C, Stefanovski D, Rubenstein RC, Rickels MR, Kelly A. β-Cell secretory defects are present in pancreatic insufficient cystic fibrosis with 1-hour oral glucose tolerance test glucose ≥155 mg/dL. Pediatr Diabetes. 2018;19:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Lin N, Chen H, Zhang H, Wan X, Su Q. Mitochondrial reactive oxygen species (ROS) inhibition ameliorates palmitate-induced INS-1 beta cell death. Endocrine. 2012;42:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Koshkin V, Wang X, Scherer PE, Chan CB, Wheeler MB. Mitochondrial functional state in clonal pancreatic beta-cells exposed to free fatty acids. J Biol Chem. 2003;278:19709-19715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Carlsson C, Borg LA, Welsh N. Sodium palmitate induces partial mitochondrial uncoupling and reactive oxygen species in rat pancreatic islets in vitro. Endocrinology. 1999;140:3422-3428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 842] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 44. | Miki A, Ricordi C, Sakuma Y, Yamamoto T, Misawa R, Mita A, Molano RD, Vaziri ND, Pileggi A, Ichii H. Divergent antioxidant capacity of human islet cell subsets: A potential cause of beta-cell vulnerability in diabetes and islet transplantation. PLoS One. 2018;13:e0196570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Stancill JS, Broniowska KA, Oleson BJ, Naatz A, Corbett JA. Pancreatic β-cells detoxify H(2)O(2) through the peroxiredoxin/thioredoxin antioxidant system. J Biol Chem. 2019;294:4843-4853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 46. | Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE, Newsholme P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer's Disease. Mediators Inflamm. 2015;2015:105828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 336] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 47. | Benáková Š, Holendová B, Plecitá-Hlavatá L. Redox Homeostasis in Pancreatic β-Cells: From Development to Failure. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 48. | Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr. 2019;13:1165-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 49. | Yang J, Tang X, Ke X, Dai Y, Shi J. Triptolide Suppresses NF-κB-Mediated Inflammatory Responses and Activates Expression of Nrf2-Mediated Antioxidant Genes to Alleviate Caerulein-Induced Acute Pancreatitis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, Mayerle J, Drewes AM, Rebours V, Akisik F, Muñoz JED, Neoptolemos JP. Chronic pancreatitis. Nat Rev Dis Primers. 2017;3:17060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 310] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 51. | Gukovskaya AS, Gukovsky I, Algül H, Habtezion A. Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology. 2017;153:1212-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 52. | Aslam M, Jagtap N, Karyampudi A, Talukdar R, Reddy DN. Risk factors for development of endocrine insufficiency in chronic pancreatitis. Pancreatology. 2021;21:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S13-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1621] [Cited by in RCA: 1967] [Article Influence: 327.8] [Reference Citation Analysis (0)] |
| 54. | Kim JD, Leyva S, Diano S. Hormonal regulation of the hypothalamic melanocortin system. Front Physiol. 2014;5:480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Harriette CJ. Behavioral Neuroscience for the Human Services: Foundations in Emotion, Mental Health, Addiction, and Alternative Therapies. 69 Part 2, Cap11. Brain Structure: Larger (visible to the human eye). Oxford University Press. 2014; 73-83. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 56. | Wang B, Cheng KK. Hypothalamic AMPK as a Mediator of Hormonal Regulation of Energy Balance. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 57. | Arora S, Anubhuti. Role of neuropeptides in appetite regulation and obesity--a review. Neuropeptides. 2006;40:375-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 296] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 58. | Parker JA, Bloom SR. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology. 2012;63:18-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 59. | Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 60. | Rhea EM, Banks WA. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front Neurosci. 2019;13:521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 61. | Zeng Y, Zhang L, Hu Z. Cerebral insulin, insulin signaling pathway, and brain angiogenesis. Neurol Sci. 2016;37:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Pena-Leon V, Perez-Lois R, Seoane LM. mTOR Pathway is Involved in Energy Homeostasis Regulation as a Part of the Gut-Brain Axis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake. Cell Metab. 2012;16:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 64. | Belgardt BF, Brüning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci. 2010;1212:97-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 65. | Lu SC, Akanji AO. Leptin, Obesity, and Hypertension: A Review of Pathogenetic Mechanisms. Metab Syndr Relat Disord. 2020;18:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Deem JD, Faber CL, Morton GJ. AgRP neurons: Regulators of feeding, energy expenditure, and behavior. FEBS J. 2022;289:2362-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 67. | Słotwińska SM. Ghrelin and oral diseases. Cent Eur J Immunol. 2020;45:433-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Dickson EJ, Falkenburger BH, Hille B. Quantitative properties and receptor reserve of the IP(3) and calcium branch of G(q)-coupled receptor signaling. J Gen Physiol. 2013;141:521-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 69. | Gericke MT, Schröder T, Kosacka J, Nowicki M, Klöting N, Spanel-Borowski K. Neuropeptide Y impairs insulin-stimulated translocation of glucose transporter 4 in 3T3-L1 adipocytes through the Y1 receptor. Mol Cell Endocrinol. 2012;348:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Gehlert DR. Introduction to the reviews on neuropeptide Y. Neuropeptides. 2004;38:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Havel PJ, Hahn TM, Sindelar DK, Baskin DG, Dallman MF, Weigle DS, Schwartz MW. Effects of streptozotocin-induced diabetes and insulin treatment on the hypothalamic melanocortin system and muscle uncoupling protein 3 expression in rats. Diabetes. 2000;49:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Sindelar DK, Mystkowski P, Marsh DJ, Palmiter RD, Schwartz MW. Attenuation of diabetic hyperphagia in neuropeptide Y--deficient mice. Diabetes. 2002;51:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 73. | German JP, Wisse BE, Thaler JP, Oh-I S, Sarruf DA, Ogimoto K, Kaiyala KJ, Fischer JD, Matsen ME, Taborsky GJ Jr, Schwartz MW, Morton GJ. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 74. | Dong J, Peeters TL, De Smet B, Moechars D, Delporte C, Vanden Berghe P, Coulie B, Tang M, Depoortere I. Role of endogenous ghrelin in the hyperphagia of mice with streptozotocin-induced diabetes. Endocrinology. 2006;147:2634-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Kamegai J, Tamura H, Shimizu T, Ishii S, Tatsuguchi A, Sugihara H, Oikawa S, Kineman RD. The role of pituitary ghrelin in growth hormone (GH) secretion: GH-releasing hormone-dependent regulation of pituitary ghrelin gene expression and peptide content. Endocrinology. 2004;145:3731-3738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Hall C, Yu H, Choi E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp Mol Med. 2020;52:911-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 77. | Durham HA, Truett GE. Development of insulin resistance and hyperphagia in Zucker fatty rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R652-R658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Grasso P. Harnessing the Power of Leptin: The Biochemical Link Connecting Obesity, Diabetes, and Cognitive Decline. Front Aging Neurosci. 2022;14:861350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 79. | Misch M, Puthanveetil P. The Head-to-Toe Hormone: Leptin as an Extensive Modulator of Physiologic Systems. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 80. | Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2-21. [PubMed] [DOI] [Full Text] |
| 81. | Samodien E, Johnson R, Pheiffer C, Mabasa L, Erasmus M, Louw J, Chellan N. Diet-induced hypothalamic dysfunction and metabolic disease, and the therapeutic potential of polyphenols. Mol Metab. 2019;27:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 82. | Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 644] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 83. | Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 484] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 84. | Deck CA, Honeycutt JL, Cheung E, Reynolds HM, Borski RJ. Assessing the Functional Role of Leptin in Energy Homeostasis and the Stress Response in Vertebrates. Front Endocrinol (Lausanne). 2017;8:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 85. | Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009;1296:35-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 86. | Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes. 2015;64:3927-3936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 87. | Sanchez-Alavez M, Tabarean IV, Osborn O, Mitsukawa K, Schaefer J, Dubins J, Holmberg KH, Klein I, Klaus J, Gomez LF, Kolb H, Secrest J, Jochems J, Myashiro K, Buckley P, Hadcock JR, Eberwine J, Conti B, Bartfai T. Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes. 2010;59:43-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Chiang JL, Kirkman MS, Laffel LM, Peters AL; Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37:2034-2054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 616] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 89. | Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552-1560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 90. | Amanat S, Ghahri S, Dianatinasab A, Fararouei M, Dianatinasab M. Exercise and Type 2 Diabetes. In: Xiao J, editor. Physical Exercise for Human Health. Singapore: Springer Singapore. 2020; 1228: 91-105. Available from: https://link.springer.com/chapter/10.1007/978-981-15-1792-1_6. |
| 91. | Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT, Kuriakose K, Sasapu A, Beebe A, Patil N, Musham CK, Lohani GP, Mirza W. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front Endocrinol (Lausanne). 2017;8:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 794] [Cited by in RCA: 736] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 92. | Chia CW, Egan JM. Incretins in obesity and diabetes. Ann N Y Acad Sci. 2020;1461:104-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 93. | Penlioglou T, Papanas N. Nutraceuticals for Insulin Resistance and Type 2 Diabetes Mellitus. In: Cicero AFG, Rizzo M, editors. Nutraceuticals and Cardiovascular Disease. Cham: Springer International Publishing. 2021; 107-115 Available from: https://link.springer.com/chapter/10.1007/978-3-030-62632-7_7. |
| 94. | Farid A, Moussa P, Youssef M, Haytham M, Shamy A, Safwat G. Melatonin relieves diabetic complications and regenerates pancreatic beta cells by the reduction in NF-kB expression in streptozotocin induced diabetic rats. Saudi J Biol Sci. 2022;29:103313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 95. | Noor A, Gunasekaran S, Vijayalakshmi MA. Improvement of Insulin Secretion and Pancreatic β-cell Function in Streptozotocin-induced Diabetic Rats Treated with Aloe vera Extract. Pharmacognosy Res. 2017;9:S99-S104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 96. | Peng CH, Chyau CC, Chan KC, Chan TH, Wang CJ, Huang CN. Hibiscus sabdariffa polyphenolic extract inhibits hyperglycemia, hyperlipidemia, and glycation-oxidative stress while improving insulin resistance. J Agric Food Chem. 2011;59:9901-9909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 97. | Affuso F, Mercurio V, Ruvolo A, Pirozzi C, Micillo F, Carlomagno G, Grieco F, Fazio S. A nutraceutical combination improves insulin sensitivity in patients with metabolic syndrome. World J Cardiol. 2012;4:77-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 98. | Cicero AF, Rosticci M, Parini A, Morbini M, Urso R, Grandi E, Borghi C. Short-term effects of a combined nutraceutical of insulin-sensitivity, lipid level and indexes of liver steatosis: a double-blind, randomized, cross-over clinical trial. Nutr J. 2015;14:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Gambini J, Inglés M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, Mas-Bargues C, Abdelaziz KM, Gomez-Cabrera MC, Vina J, Borras C. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid Med Cell Longev. 2015;2015:837042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 494] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 100. | Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, Mészáros LG, Sümegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 481] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 101. | Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1241] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 102. | Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 103. | Abbasi Oshaghi E, Goodarzi MT, Higgins V, Adeli K. Role of resveratrol in the management of insulin resistance and related conditions: Mechanism of action. Crit Rev Clin Lab Sci. 2017;54:267-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 104. | Zhu X, Wu C, Qiu S, Yuan X, Li L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: systematic review and meta-analysis. Nutr Metab (Lond). 2017;14:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 105. | Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549-3558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 333] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 106. | Marton LT, Pescinini-E-Salzedas LM, Camargo MEC, Barbalho SM, Haber JFDS, Sinatora RV, Detregiachi CRP, Girio RJS, Buchaim DV, Cincotto Dos Santos Bueno P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front Endocrinol (Lausanne). 2021;12:669448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 107. | Panahi Y, Khalili N, Sahebi E, Namazi S, Karimian MS, Majeed M, Sahebkar A. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacology. 2017;25:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 108. | Na LX, Zhang YL, Li Y, Liu LY, Li R, Kong T, Sun CH. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr Metab Cardiovasc Dis. 2011;21:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 109. | Das TK, Chakrabarti SK, Zulkipli IN, Abdul Hamid MRW. Curcumin Ameliorates the Impaired Insulin Signaling Involved in the Pathogenesis of Alzheimer's Disease in Rats. J Alzheimers Dis Rep. 2019;3:59-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Chen S, Li J, Zhang Z, Li W, Sun Y, Zhang Q, Feng X, Zhu W. Effects of resveratrol on the amelioration of insulin resistance in KKAy mice. Can J Physiol Pharmacol. 2012;90:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | González-Rodríguez Á, Santamaría B, Mas-Gutierrez JA, Rada P, Fernández-Millán E, Pardo V, Álvarez C, Cuadrado A, Ros M, Serrano M, Valverde ÁM. Resveratrol treatment restores peripheral insulin sensitivity in diabetic mice in a sirt1-independent manner. Mol Nutr Food Res. 2015;59:1431-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 112. | Zhao H, Zhang Y, Shu L, Song G, Ma H. Resveratrol reduces liver endoplasmic reticulum stress and improves insulin sensitivity in vivo and in vitro. Drug Des Devel Ther. 2019;13:1473-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 113. | Szkudelska K, Deniziak M, Sassek M, Szkudelski I, Noskowiak W, Szkudelski T. Resveratrol Affects Insulin Signaling in Type 2 Diabetic Goto-Kakizaki Rats. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 114. | Vlavcheski F, Den Hartogh DJ, Giacca A, Tsiani E. Amelioration of High-Insulin-Induced Skeletal Muscle Cell Insulin Resistance by Resveratrol Is Linked to Activation of AMPK and Restoration of GLUT4 Translocation. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 115. | Mahjabeen W, Khan DA, Mirza SA. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement Ther Med. 2022;66:102819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 116. | Kanitkar M, Gokhale K, Galande S, Bhonde RR. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br J Pharmacol. 2008;155:702-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 117. | Li J, Wu N, Chen X, Chen H, Yang X, Liu C. Curcumin protects islet cells from glucolipotoxicity by inhibiting oxidative stress and NADPH oxidase activity both in vitro and in vivo. Islets. 2019;11:152-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 118. | Zou T, Li S, Wang B, Wang Z, Liu Y, You J. Curcumin improves insulin sensitivity and increases energy expenditure in high-fat-diet-induced obese mice associated with activation of FNDC5/irisin. Nutrition. 2021;90:111263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 119. | Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem. 2014;25:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 120. | Sangouni AA, Alizadeh M, Jamalzehi A, Parastouei K. Effects of garlic powder supplementation on metabolic syndrome components, insulin resistance, fatty liver index, and appetite in subjects with metabolic syndrome: A randomized clinical trial. Phytother Res. 2021;35:4433-4441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 121. | Gu M, Zhang Y, Fan S, Ding X, Ji G, Huang C. Extracts of Rhizoma polygonati odorati prevent high-fat diet-induced metabolic disorders in C57BL/6 mice. PLoS One. 2013;8:e81724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 122. | Nuankaew W, Lee HK, Nam YH, Shim JH, Kim NW, Shin SW, Kim MC, Shin SY, Hong BN, Dej-Adisai S, Kwak JH, Kang TH. The Effects of Persimmon (Diospyros kaki L.f.) Oligosaccharides on Features of the Metabolic Syndrome in Zebrafish. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 123. | He L, Xing Y, Ren X, Zheng M, Yu S, Wang Y, Xiu Z, Dong Y. Mulberry Leaf Extract Improves Metabolic Syndrome by Alleviating Lipid Accumulation In Vitro and In Vivo. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 124. | Liao W, Cao X, Xia H, Wang S, Sun G. Pea Protein-Derived Peptides Inhibit Hepatic Glucose Production via the Gluconeogenic Signaling in the AML-12 Cells. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | de Oliveira Marques S, Muller AP, Luciano TF, Dos Santos Tramontin N, da Silva Caetano M, Luis da Silva Pieri B, Amorim TL, de Oliveira MAL, de Souza CT. Effects of Avocado Oil Supplementation on Insulin Sensitivity, Cognition, and Inflammatory and Oxidative Stress Markers in Different Tissues of Diet-Induced Obese Mice. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 126. | Jiang Y, Feng C, Shi Y, Kou X, Le G. Eugenol improves high-fat diet/streptomycin-induced type 2 diabetes mellitus (T2DM) mice muscle dysfunction by alleviating inflammation and increasing muscle glucose uptake. Front Nutr. 2022;9:1039753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 127. | Woumbo CY, Kuate D, Metue Tamo DG, Womeni HM. Antioxidant and antidiabetic activities of a polyphenol rich extract obtained from Abelmoschus esculentus (okra) seeds using optimized conditions in microwave-assisted extraction (MAE). Front Nutr. 2022;9:1030385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |