Published online Feb 15, 2023. doi: 10.4239/wjd.v14.i2.62
Peer-review started: August 28, 2022
First decision: October 21, 2022
Revised: November 4, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: February 15, 2023
Processing time: 169 Days and 17.7 Hours
Diabetes mellitus (DM) is one of the most common metabolic disorders characterized by elevated blood glucose levels. Prolonged uncontrolled hyperglycemia often leads to multi-organ damage including diabetic neuropathy, nephropathy, retinopathy, cardiovascular disorders, and diabetic foot ulcers. Excess production of free radicals causing oxidative stress in tissues is often considered to be the primary cause of onset and progression of DM and associated complications. Natural polyphenols can be used to induce or inhibit the expression of antioxidant enzymes such as glutathione peroxidase, heme oxygenase-1, superoxide dismutase, and catalase that are essential in maintaining redox balance, and ameliorate oxidative stress. Caffeic acid (CA) is a polyphenolderived from hydroxycinnamic acid and possesses numerous physiological properties includ-ing antioxidant, anti-inflammatory, anti-atherosclerotic, immune-stimulatory, cardioprotective, antiproliferative, and hepatoprotective activities. CA acts as a regulatory compound affecting numerous biochemical pathways and multiple targets. These include various transcription factors such as nuclear factor-B, tumor necrosis factor-α, interleukin-6, cyclooxygenase-2, and nuclear factor erythroid 2-related factor 2. Therefore, this review summarizes the pharmacological properties, molecular mechanisms, and pharmacokinetic profile of CA in mitigating the adverse effects of DM and associated complications. The bioavailability, drug delivery, and clinical trials of CA have also been discussed.
Core Tip: Diabetes mellitus has emerged as one of the most common metabolic disorders worldwide which can lead to other complications such as retinopathy, nephropathy, neuropathy, and foot ulcers. Free radical-induced oxidative stress is one of the primary causes of diabetes. Caffeic acid (CA) is a natural polyphenol obtained from various fruits and vegetables. CA and its derivatives act as an antioxidant and regulate the signaling pathways involved in lipid and carbohydrate metabolism. CA also exerts anti-diabetic effects by modulation of inflammatory cytokines and transcription factors.
- Citation: Ganguly R, Singh SV, Jaiswal K, Kumar R, Pandey AK. Modulatory effect of caffeic acid in alleviating diabetes and associated complications. World J Diabetes 2023; 14(2): 62-75
- URL: https://www.wjgnet.com/1948-9358/full/v14/i2/62.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i2.62
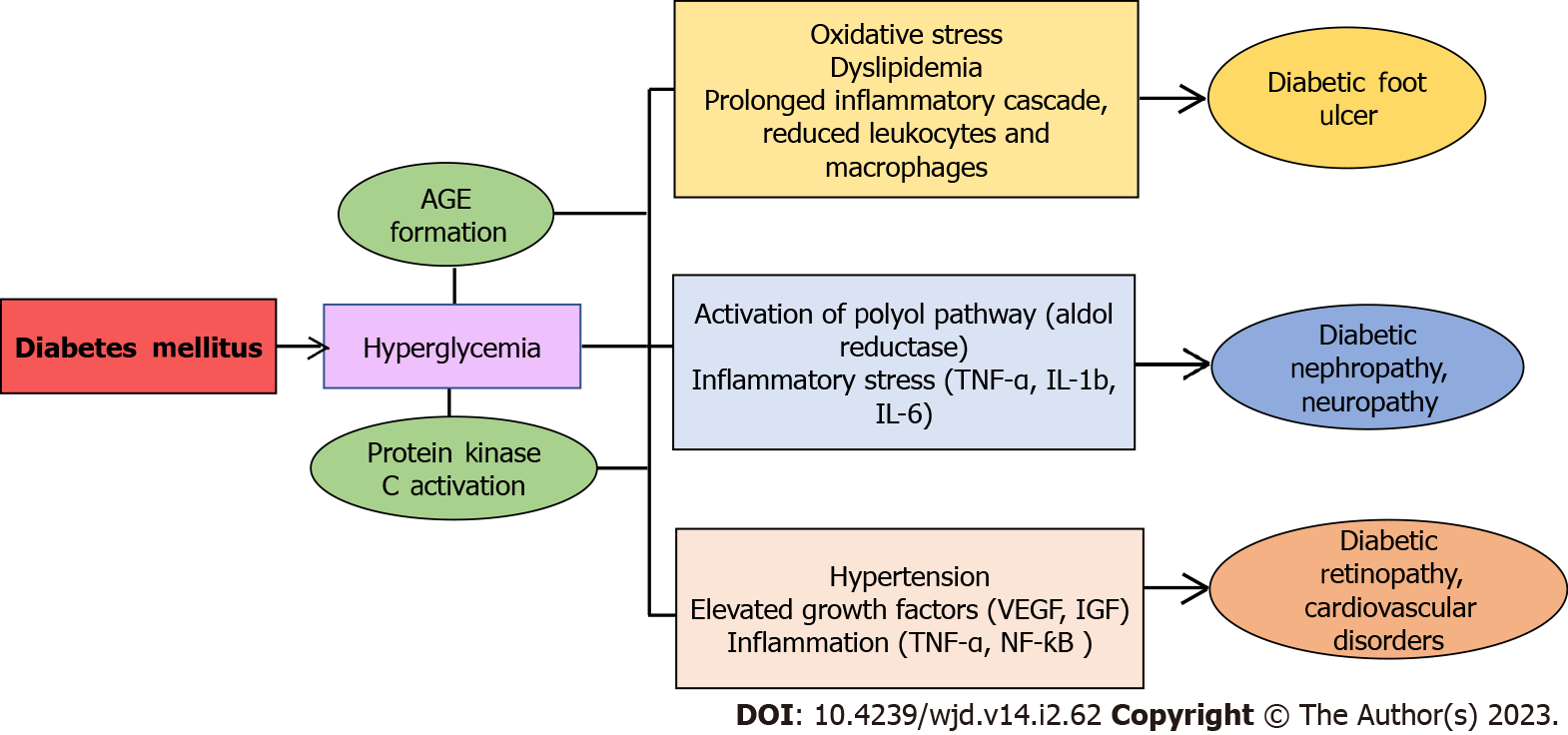
Diabetes mellitus (DM) is a metabolic disorder marked by elevated blood sugar levels that stems from the complete loss or dysfunction of insulin producing pancreatic β-cells and subsequently results in other complications in several organs of the body.DM is one of the most frequently occurring metabolic diseases worldwide and is the leading cause of death due to comorbidities[1,2]. The main subtypes of DM are type 1 diabetes (T1DM) and type 2 diabetes (T2DM). T1DM, also referred to as insulin dependent DM, is an autoimmune condition that is mediated by the dysfunction of pancreatic β-cells with complete loss of insulin production[3]. T2DM is the insulin resistance type that occurs when pancreatic β-cells are incapable of producing enough insulin. T2DM affects 90%–95% of diabetic individuals globally[4]. Several reports suggest that around 400 million people worldwide would be affected by DM by the year 2025[5]. Both types of DM are frequently linked to long-term consequences such as higher risk of cardiovascular diseases (CVD), retinopathy, neuropathy, nephropathy, foot ulcers, and other vascular anomalies. These complications consequently lead to blindness in diabetic patients, end-stage renal disease, atherosclerosis, and even mortality[6]. Compared to non-diabetic individuals, T2DM patients are at much higher risk of foot injuries and cardiovascular morbidity like atherosclerosis[7]. Studies have demonstrated that metabolic variables, oxidation/glucoxidation, and changes in vascular reactivity are some of the major factors that contribute to diabetic atherosclerosis[8]. Although the pathophysiological mechanism linking DM to its complications is yet to be extensively explored, oxidative stress appears to be a key factor[9-11]. Several reports have suggested that increased oxidative/nitrosative stress and cellular redox disturbances facilitate the etiology and development of both T1DM and T2DM. Uncontrolled hyperglycemia causes oxidative stress and further damages the cells primarily by targeting various metabolic pathways such as enhancement of polyol pathway, increased synthesis of advanced glycation end products (AGEs), activation of protein kinase C, and upregulated hexosamine pathway[9,10]. Therefore, hyperglycemia results in elevated levels of reactive oxygen species and reactive nitrogen species (RNS) in the majority of organs. Moreover, a decrease in cellular antioxidant defences is linked to an increase in oxidative stress in diabetic individuals[9,12,13]. The primary factor contributing to endothelial cell failure in diabetic complications may be due to increased lipid peroxidation caused by oxidative stress. Endothelial dysfunction in DM has been attributed to excessive generation and/or insufficient clearance of free radicals by the antioxidant defencesystem[14] (Figure 1). Since oxidative stress is involved in the development of T1DM, T2DM, and diabetes-associated complications, use of antioxidants as a counter measure could be beneficial. When cells are exposed to chemicals/oxidants, natural polyphenols can be used to induce or inhibit the expression of enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and heme oxygenase-1 (HO-1) that are essential in maintaining cellular homeostasis[15]. Natural polyphenols are secondary metabolites having lower risk of adverse effects when employed in conventional and alternative medicine[16].
Caffeic acid (CA) is a polyphenolic derivative of hydroxycinnamic acid, formed as a product of secondary metabolism in fruits and vegetables[17-19]. CA can be present in simple monomeric form as amides, glycosides, sugar, and organic acid esters, or in complex oligomeric forms as derivatives of flavonoids. CA can also be found attached to some cell wall proteins and polymers[19-20]. CA inhibits the growth of bacteria, fungi, and insects, protects plants from ultraviolet-Bradiations, and contributes to plants’ defensive mechanism against predators, pests, and illnesses[21]. Numerous biological effects of CA and its derivatives have been demonstrated through experimental studies, including antibacterial, antiviral, antioxidant, anti-inflammatory, anti-atherosclerotic, immune-stimulatory, cardioprotective, antiproliferative, and hepatoprotective activities[21-25]. Propolis, derived from honeybee, is rich in CA phenethyl ester (CAPE), a common naturally occurring derivative of CA having widespread applications in research and industry[26]. CAPE acts as a regulatory compound affecting numerous biochemical pathways and multiple intracellular targets including several transcription factors, namely, nuclear factor-kappa B (NF-κB), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), cyclooxygenase-2 (COX-2), nuclear factor erythroid 2-related factor 2, inducible nitric oxide synthase (iNOS), activated T-cell nuclear factor, and hypoxia-inducible factor-1[26-30]. Most of these pathways are usually involved in the regulation of inflammatory and oxidative stress markers. Numerous studies have reported the efficacy of CAPE in the treatment of stress-induced pathologies. Recent studies have shown the protective ability of CAPE against nephrotoxicity induced by a number of xenobiotics (methotrexate, doxorubicin, cisplatin, toluene, carbon tetrachloride, etc.) or by diverse toxic conditions[31]. Several reports suggest the application of CAPE in experimental and clinical studies for the treatment of several diseases such as cancer, thyroid, liver diseases, hepatic insulin resistance, non-alcoholic fatty liver disease, or hepatocellular carcinoma[32,33]. In vivo studies have reported that oral ingestion of CAPE stalled the progression of atherosclerosis in mice deficient in apolipoprotein E[32]. In addition, involvement of CAPE in molecular signaling pathways suggests that CAPE has therapeutic efficacy in diverse inflammatory diseases and cancer[31-32]. Similarly, CA treatment has also exhibited protective efficacy in various organs such as the brain, kidneys, lungs, ovaries, and heart from diabetes-induced damage[34-36]. Therefore, this review reports the structural and pharmacological properties of CA and its derivatives with special emphasis on the key mechanisms of action and pharmacokinetic properties of CA, especially in DM and associated complications.
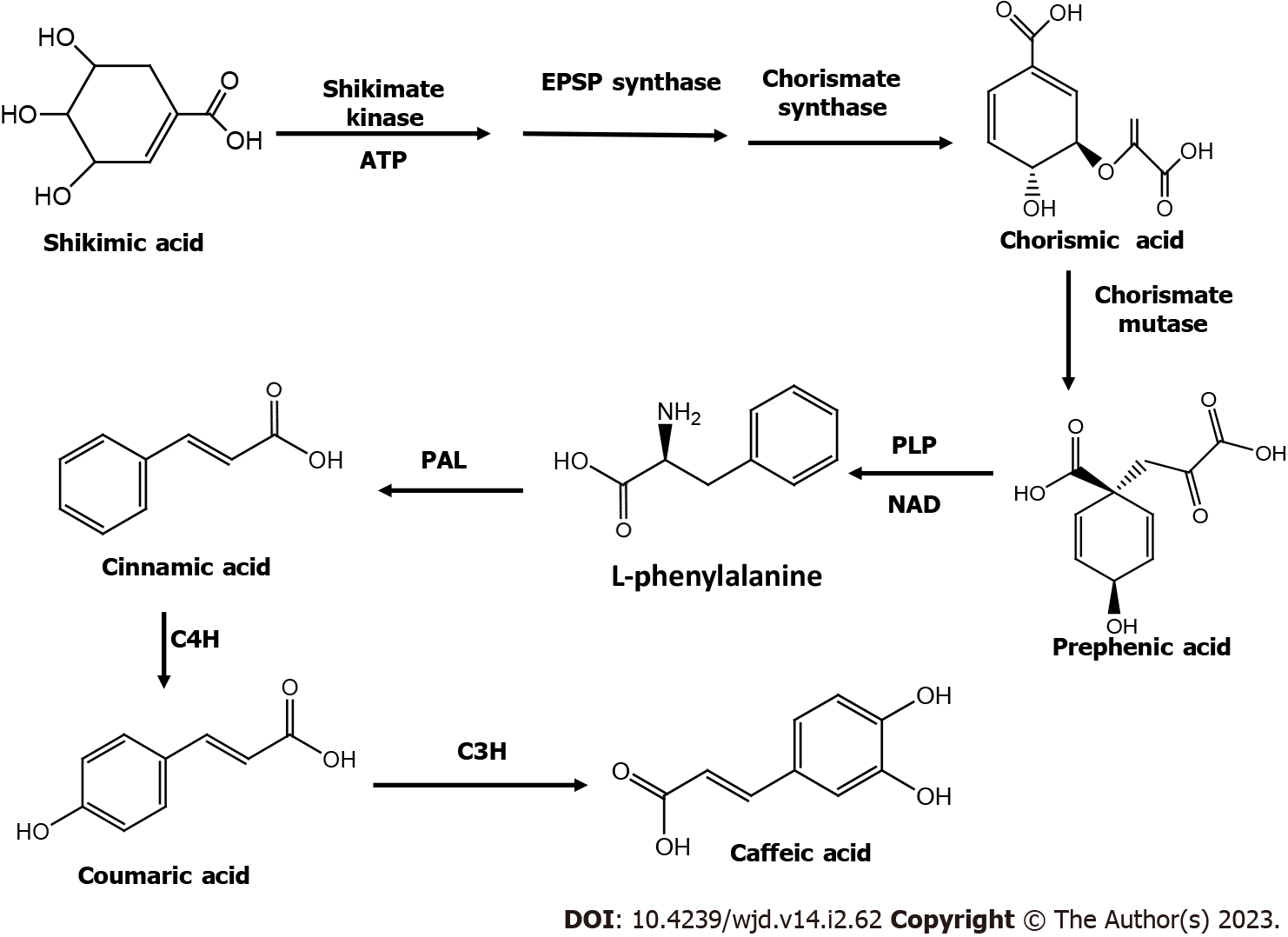
CA occurs naturally in several vegetables and fruits including kiwis, blueberries, plums, cherries, apples, cereals, carrots, and cabbage. CA can also be found in propolis, which is a resinous substance made by honeybees[37]. Different plant species have variable amounts of CA[38]. It is a very prevalent phenolic acid that accounts for 75 to 100 percent of the total hydroxycinnamic acid in fruits[39]. Structurally, CA is a phenylalanine-derived hydroxycinnamic acid with a 3,4-dihydroxyaromatic ring connected to carboxyl group through a trans-ethylene bond[37]. CA is synthesized naturally in plants via the endogenous shikimate pathway[23,37]. The biosynthesis of CA begins with precursor shikimic acid and involves three enzymatic reactions: (1) Phosphorylation by shikimate kinase; (2) The conjugation of phosphoenolpyruvate by 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase; and (3) Formation of intermediary metabolite chorismic acid by the enzyme chorismatesynthase[23,37]. Cinnamic acid is produced from the deamination of L-phenylalanine by enzyme phenylalanine ammonia lyase; it is converted into p-coumaric acid by the action of cinnamate-4-hydroxylase, which is subsequently converted into CA by enzyme 4-coumarate 3-hydroxylase[23] (Figure 2). CA is generally extracted from plant materials and by microbial synthesis using organisms like Escherichia coli. Two enzymes can be produced by genetic modifications in Escherichia coli strains: Tyrosine ammonia lyaseand 3-hydroxylase hydroxyphenylacetate which act on L-tyrosine to produce L-dopa and coumaric acid, respectively, leading to the synthesis of CA[36].
CA has a molecular weight of 180.16 g/mol and is typically found as a white, amorphous powder. The partition coefficient (logP) for CA ranges from 1 to 1.3[40,41]. In addition, propolis contains large amounts of naturally occurring derivative of CA, the CAPE that appears as a white crystalline solid and has a molecular weight of 284.31 g/mol. The intriguing aspect of CAPE is its ability to traverse the blood-brain barrier, which can be attributed to logP values of CAPE ranging between 3.2-13.8[41-43]. CA is essentially found in food in esterified form with chlorogenic acid, thus limiting its absorption in the body[44]. Human tissues such as the intestinal mucosa, stomach, and liver, and biological fluids such as plasma, duodenal fluid, and gastric juice lack the esterase enzymes that hydrolyze chlorogenic acid to release CA. Thus, it is hydrolyzed by intestinal microflora before its absorption[42,44]. As a result, the pharmacokinetic process starts when CA is consumed and enters the stomach in its esterified state, where a small amount of CA is absorbed[41-44]. Thereafter, the intestinal mucosa absorbs up to 95% of CA in its free form after the bacterial esterases in the colon break the ester part of CA[42-44]. Monocarboxylic acid transporters are involved in the active transport of CA across membranes into intestinal cells[36,42,44]. The peak plasma concentration of CA occurs after 1 h of meal digestion, and it takes repeated dosage every 2 h to sustain high levels of CA in plasma[36,42]. Under anaerobic conditions, gut bacteria having tyrosine decarboxylase can cause decarboxylation of CA, producing a compound known as 3-(3-hydroxyphenyl)-propionic acid that has stronger antioxidant activity than CA[45]. Sulfotransferases, uridine diphosphate-glucuronosyltransferases, and catechol-o-methyltransferases catalyze three main enzymatic conjugation processes of sulphation, glucuronidation, and methylation of CA, respectively, that occur immediately after absorption. This increases the hydrophilic properties of CA, thus reducing its toxicity and speeding up elimination. The liver and kidney are the major sites of CA metabolism. The primary elimination route of CA (5.9% to 27%) is via urine[43-45].
DM is characterized by hyperglycemia, altered lipid and carbohydrate metabolism, and oxidative stress[1,2,46]. The successful control of high blood sugar levels with natural polyphenols may be significant in minimizing diabetic complications, particularly micro- and macro-vascular disorders. Plant products used in traditional medicine constitute a potential alternative for effective control of diabetes, owing to their affordability, high efficacy, and minimal negative effects[47-49]. CA is a natural compound that is known to promote insulin secretion, inhibit α-amylase and β-glucosidase, prevent sodium-dependent glucose transporter-1 from absorbing glucose in the gut, and lower hepatic glucose output. Besides its anti-diabetic efficacy, CA also modifies the microbiome, facilitates insulin-dependent glucose uptake, activates adenosine monophosphate-activated protein kinase, and has immunomodulatory, antimicrobial, hypocholesteremic, and antioxidant properties[36,49]. Experimentally, in streptozotocin (STZ)-induced diabetic rats, and Balb/c and C57BL/KsJ-db/db mice, CA exhibited potential antihyperglycemic effects along with antioxidant and anti-inflammatory properties[50,51]. CA may exert its protective effects by activating and safeguarding intracellular antioxidant enzymes, and by transferring hydrogen atoms and single electrons, as well as by chelating metal ions[52]. In addition, CA helps to upregulate the transcription factor nuclear factor erythroid2-related factor 2 (NrF2) which controls the expression of over 200 genes involved in the cellular antioxidant and immune regulatory mechanism by binding with antioxidant response elements, which is also linked with the detoxification of xenobiotics. CA also regulates β-cell and adipocyte GLUT4 functions, increases activity of glucokinase in hepatocytes, inhibits glucose-6 phosphatase and phosphoenolpyruvate carboxykinase, and reduces glycosylated haemoglobin, thus resulting in controlled DM. CA aids in enhancing the utilization of glucose and glycogen synthases. This leads to reduced cholesterol biosynthesis and prevention of lipogenesis. CA suppresses iron-induced elevation of cholesterol and improves the levels of plasma insulin, C-peptide, and leptin[53]. In a study with STZ-induced diabetic rats, a significant decrease in malondialdehyde (MDA) level and SOD and CAT activities was observed in the liver, retina, and heart, post CA treatment. Insulin-like growth factors (IGFs) are known to be associated with the progression of DM where reduced serum IGF-I levels have been linked with poor glycemic control in DM, while elevated plasma IGF-II levels have been linked to the progression of DM[54,55]. In STZ-induced diabetic rats, the effects of CA administration led to amelioration of changes in gene expression as well as changes in the levels of IGF-I and IGF-II in the blood, liver, heart, and kidney[35,56].
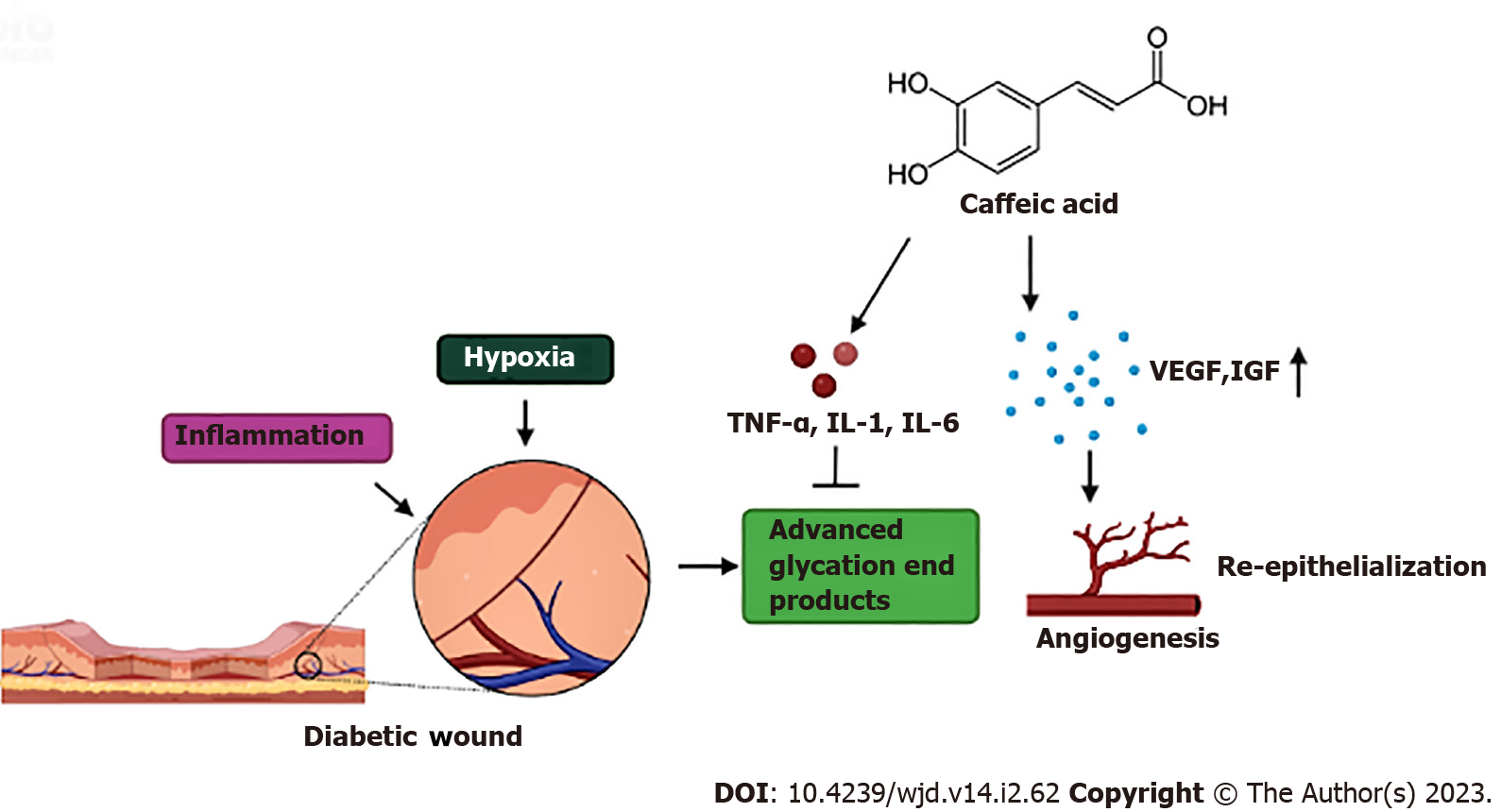
Chronic wounds below the ankle or foot lesions in diabetic patients that penetrate the dermis layer are known as diabetic foot ulcers (DFU)[57]. People with diabetes have a lifetime risk of developing foot ulcers in 25% cases, which may lead to 50%–70% of total non-traumatic amputations[58-61]. In recent years, amputation rates have increased significantly, which in turn has raised the rate of morbidity and death[62-65]. The wound healing cascade in diabetic patients is often hindered and delayed due to high blood sugar levels[66]. Hyperglycemia leads to a series of events such as formation of AGEs, non-enzymatic glycosylation, activation of the polyol pathway and the diacylglycerolprotein kinase C pathway, and hyperactivity of the hexosamine pathway[67,68]. These alterations are linked to a prolonged inflammatory phase causing stiffening of endothelial walls, which makes it challenging for blood to pass via tiny arteries near the surface of the incision[69]. As a result, there is also a lack of oxygen release and nutrition at the wound site, causing further elevation of blood sugar levels in the wound area. Therefore, the wound healing cascade is prolonged, leukocyte migration is reduced, and macrophage introduction is delayed[70]. Additionally, hyperglycemia also activates an inflammatory reaction by triggering NF-κB light-chain-enhancer of activated B cells[71,72]. Moreover, oxidative stress, dyslipidemia, and insulin resistance play a significant role in the development of DFU[73,74]. Thus, management of all these factors is crucial for the treatment of DFU.
Studies have shown that low glycemic index of CA and its derivatives is mainly responsible for their antidiabetic, antioxidant, and anti-inflammatory properties which aids in managing foot ulcers[75-78]. An early study on STZ-induced diabetic mice revealed that topical administration of propolis is well tolerated and aids in healing of human DFU[79]. CAPE increases wound contraction and re-epithelialization by reducing oxidative stress and accelerates cutaneous wound healing, which is mediated by its antioxidant action[80,81]. In another study on diabetic mice, topical application of propolis was found to stimulate the release of vascular endothelial growth factor (VEGF) in smooth muscle cells and facilitate the relaxation of arteries via the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) pathway which accelerated the healing of cutaneous diabetic wounds in mice[82,83] (Figure 3 and Table 1).
| Type of study/condition | Dose | Mode of action | Ref. |
| STZ-induced diabetic mice | Topical administration of propolis at 20 μL | Healing of human DFU | [79] |
| CAPE at 5 μmol/kg and 10 μmol/kg | Increased wound contraction and re-epithelialization by reducing oxidative stress | [80,81] | |
| Diabetic mice with DFU | Topical application of propolis | Stimulated VEGF and activated NO/cGMP pathway | [82,83] |
| Diabetic mice with renal damage | CA at 5% | Decreased AGEs, IL-1b, and IL-6, and reduced activity of renal AR and SDH. | [92] |
| STZ-induced diabetic mice, nephropathy | CA at 10-50 mg/kg | Modulation of autophagy pathway | [93] |
| CA at 40 mg/kg | Improved renal parameters, and downregulated the expression of miR-636 | [94] | |
| CAPE and CAPE-pNO2 at 20 μmol/kg/d | Inhibited inflammation through the Akt/NF-κB pathway and prevented renal fibrosis through the TGF-β/Smad pathway | [95] | |
| Diabetes induced in HUVECs | CAPE treatment at 3-10 μM | Reduced VEGF-induced angiogenesis | [96] |
| STZ-induced diabetic rats, retinopathy | CAF6 and CAF12 at 250 mM | Modulation of ERK1/2 and protein kinase-B/Akt signaling pathways | [98] |
| STZ-induced diabetic rats, neuropathy | CAPE at 10 μM/kg/d | Inhibition of iNOS enzyme | [102] |
| Alloxan-induced diabetic mice, CVD | CA at 50 mg/kg | Reduced atherogenic indices such as TG, LDL-c, VLDL-c, and TC | [53] |
| CA at 2% | Improved glycemic control and lipid metabolism, increased plasma antithrombin-III and protein C activities, and decreased MDA, IL-β, IL-6, and TNF-α levels | [34] | |
| STZ-induced T1D rat model, CVD | CAPA at 3 and 15 mg/kg | Reduced myocardial infarction and amelioration of cardiac dysfunction | [103] |
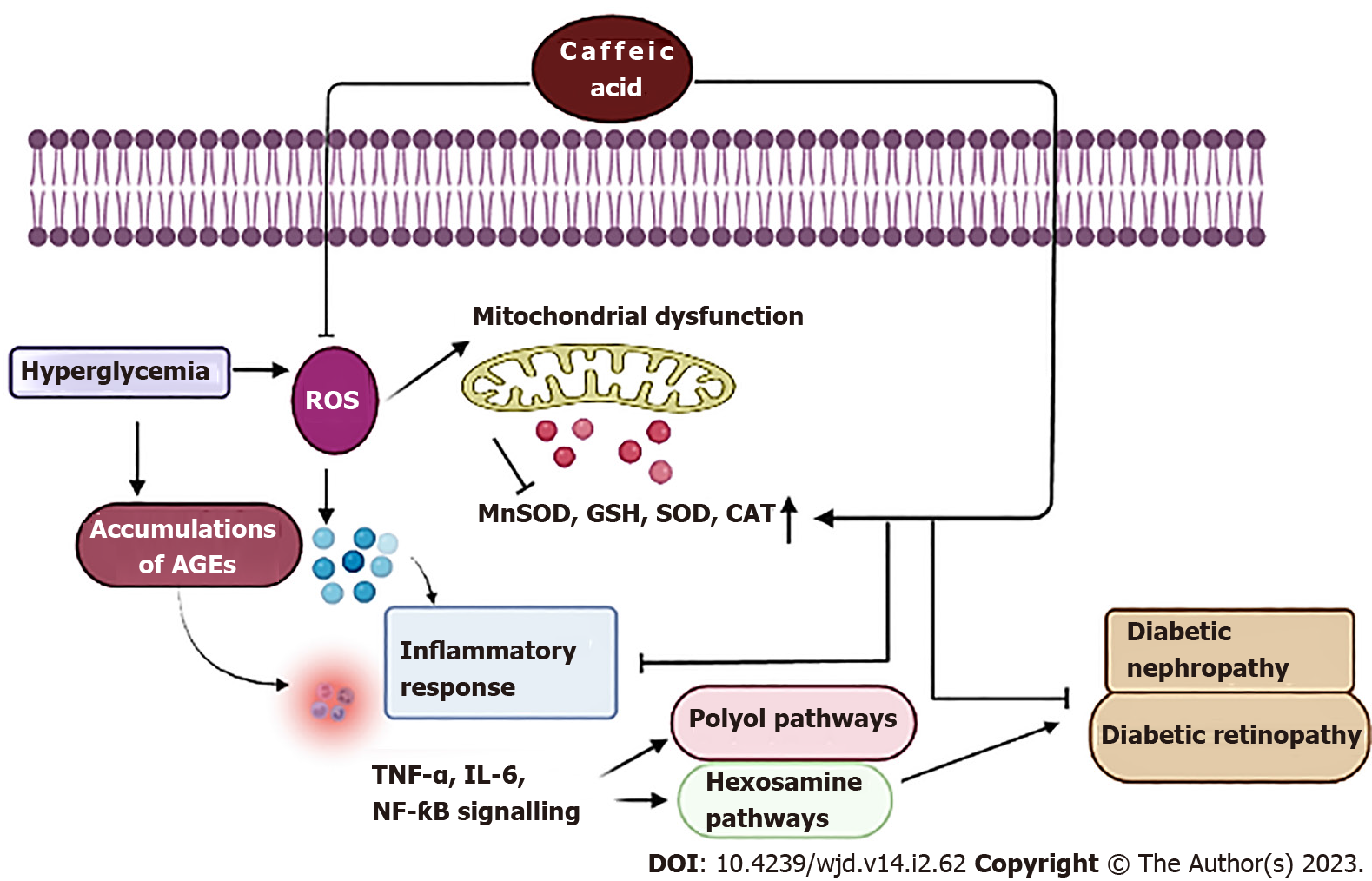
Diabetic nephropathy is a consequence of prolonged uncontrolled DM causing damage to the renal blood vessel clusters. The pathogenesis of diabetic nephropathy and other complications of diabetes have been linked to non-enzymatic glycation, with the formation of AGEs, also recognized as Maillard reaction products. These AGEs include glycated haemoglobin, glycated albumin, pentosidine, and carboxymethyllysine (CML)[84-86]. In addition, disruption in Th1-Th2 cytokine balance and over-production of pro-inflammatory cytokines result in increased inflammatory stress in diabetic patients, which further accelerates diabetic nephropathy[87,88]. Early investigations have suggested that CA lowers blood glucose by modulating the polyol pathway. Aldose reductase (AR) is the first and rate-limiting enzyme in the polyol pathway that reduces glucose to sorbitol, which could be further metabolised to fructose by the enzyme sorbitol dehydrogenase (SDH)[89,90]. The generation of AGEs was increased by the flux through SDH and an elevated fructose level, which enhanced diabetes-induced microvascular abnormalities[91] (Figure 4). In diabetic mice, CA significantly decreased the production of AGEs, inflammatory cytokines like IL-1b and IL-6, levels of plasma HbA1c, urinary glycated albumin, renal CML, pentosidine, sorbitol, and fructose, and considerably reduced the activity of renal AR and SDH along with suppression of renal AR mRNA expression[92]. In an in vivo study with STZ-induced diabetic rats, CA in a dose range of 10-50 mg/kg attenuated diabetic nephropathy via modulation of autophagy pathway by inhibiting autophagy-regulating miRNAs[93]. In another study on STZ-induced diabetic rats, oral treatment of CA at 40 mg/kg mitigated renal damage and significantly reduced fasting blood glucose, cholesterol, and triglyceride in diabetic rats. CA treatment also improved histological parameters in the diabetic kidney and downregulated the expression of miR-636[94]. In another study in STZ-induced diabetic mice, intraperitoneal treatment with CA derivatives CAPE and CA para-nitro phenethyl ester (CAPE-pNO2) at 20 μmol/kg/d resulted in improved renal biochemical parameters such as decreased serum creatinine, MDA, 24-h albumin excretion, blood urea nitrogen, myeloperoxidase levels, and SOD activity in diabetic mice. CAPE and CAPE-pNO2 also inhibited inflammation via the Akt/NF-κB pathway and prevented nephropathy through the transforming growth factor-β/Smad pathway[95] (Table 1).
Long-term DM results in diabetic retinopathy characterized by aberrant retinal blood vessel proliferation and microvascular retinal alterations, resulting in partial vision loss or even complete blindness. One of the major factors causing diabetic retinopathy is VEGF-driven angiogenesis. In a study using human umbilical vein endothelial cells (HUVECs), CAPE treatment in the dose range of 3-10 μM decreased VEGF-induced angiogenesis, indicating possible positive effects in the treatment of diabetic retinopathy[96] (Figure 4). In another study, HUVECs treated with CAPE at 5-20 μg/mL exhibited a reduction of VEGF-induced neovascularization and proliferation, tube formation, and migration. The protective efficacy of CAPE can be attributed to the inhibition of VEGF-induced VEGF receptor-2 activation and associated downstream pathways[97]. An in vivo study in a STZ-induced diabetic rat model demonstrated the protective efficacy of CA hexyl (CAF6) and dodecyl (CAF12) amide derivatives in diabetic retinopathy. Treatment with CAF6 and CAF12 at a dose of 250 mmol/L led to increased retinal SOD levels, and improved thickness of the whole retinal layer, outer nuclear layer, and ganglion cell count.The CA derivatives ameliorated diabetic retinopathy via modulation of the extracellular signal regulated kinase (ERK)1/2 and protein kinase-B/Akt signaling pathways[98] (Table 1).
The brain is another organ which is adversely affected by prolonged uncontrolled hyperglycemia, and cerebral dysfunction in diabetic patients is known to be a multifactorial process[99]. Free radical-mediated oxidative stress induced by hyperglycemia plays an important role in the pathogenesis of diabetic neuropathy[100]. It stimulates the production of the inflammatory cytokine TNF-α and promotes the expression of NF-κB[101]. The NO radical in the central nervous system acts as an important regulator leading to the generation of RNS via the enzyme iNOS and results in elevated oxidative stress in brain. In an in vivo study, STZ-induced diabetic rats post intraperitoneal treatment with CAPE at a dose of 10 μM/kg/day showed reduced NO radical and lipid peroxidation, and increased activities of antioxidant enzymes such as SOD, CAT, and GPx in the rat brain. In addition, CAPE was shown to inhibit the activity of iNOS enzyme, thus preventing excess production of RNS[102].
Hyperglycemia combined with dyslipidemia, oxidative stress, and inflammation cause CVD such as hypertension, cardiac myopathy, and atherosclerosis. DM-mediated CVD is characterized by elevated levels of triacylglycerol (TG), low density lipoprotein (LDL), very low-density lipoprotein (VLDL), and total cholesterol (TC). Atherogenic dyslipidemia in diabetic patients leads to increased risk of cardiac failure. Studies on alloxan-induced diabetic mice have revealed that CA at a dose of 50 mg/kg acts as a potent agent in controlling hyperglycemia and reducing atherogenic indices such as TG, LDL-c, VLDL-c, and TC. Thus, successful restoration of lipid and glucose metabolism parameters in mice by intraperitoneal CA administration led to improved cardiac function[53]. In another study, diabetic mice when orally fed2% CA, exhibited improved glycemic control and lipid metabolism. CA treatment led to a significant increase in plasma antithrombin-III and protein C activities, and decrease in MDA, IL-β, IL-6, and TNF-α levels[34]. Studies on a STZ-induced T1DM rat model demonstrated that intraperitoneal pre-treatment with CA phenethyl amide at doses 3 and 15 mg/kg led to reduced myocardial infarction and amelioration of cardiac dysfunction[103] (Table 1).
Plant-derived natural products including CA have several applications in the treatment of a wide range of diseases. However, there are many limitations that come in the way of using phytochemicals as alternative medicine. To ascertain the optimum utilization of plant-derived compounds in clinical investigations, it is important to design novel carriers for the delivery of natural products[104,105]. The use of CA as a pharmaceutical is constrained by a number of physicochemical and pharmacokinetic factors including poor water solubility and lack of specific tissue targeting[106]. Studies have also shown that CA has low oral bioavailability (14.7%) and low intestinal absorption (12.4%) in a rat model[107]. Therefore, numerous nanoparticles (NPs) have been created for the delivery of CA and related compounds in disease therapy with positive outcomes, including polymeric NPs, metal NPs, carbon nanomaterials, and lipid nanostructures[108,109]. The use of NPs for targeted delivery of CA is well reported. The combinations of gold and iron NPs (Au-Fe3O4) with CA, quercetin, and 5-fluorocytidine have been formulated for use in breast cancer treatment. Studies related with formulation and development of CA-NPs are mostly targeted for cancer therapy. The release of quercetin and CA from these nanostructures inhibits lactate secretion and prevents glycolytic reprogramming[106,107]. Additionally, NPs have also been designed for CAPE delivery for the treatment of cancer. In a recent study, methoxy poly (ethylene glycol)-b-poly(-caprolactone) was used to create polymeric nanostructures, which were subsequently loaded with CAPE[45,110,111]. There is still much work to be done in terms of NP formulation and design. Hence, further studies are required to examine the potential functions of NPs of CA for better delivery, in treatment of other diseases including DM.
Phytometabolites are pharmacologically active compounds and their clinical applications are constantly increasing[45,112,113]. Several reports have shown that approximately one fourth of all clinical compounds used as drugs are derived from natural products[114,115]. The pharmacological action of CA and its derivatives, particularly CAPE, as hepatoprotective, reno-protective, antioxidant, anti-diabetic, anti-inflammatory, and anticancer agents have been well documented. The activity of antioxidant enzymes such as SOD, CAT, and HO-1 is positively modulated by CA. CAPE treatment leads to protection against oxidative stress-mediated diabetic complications by regulating the transcription factors NF-κB, Nrf2, and COX-2 and associated molecular pathways. Moreover, CAPE shows notable efficacy in both in vitro and in vivo diabetic models with no substantial negative effects. CA exerts anti-diabetic efficacy in vitro and in vivo via reduced VEGF angiogenesis and decreased MDA, TNF-α, IL-β, IL-6, and other inflammatory and oxidative stress markers.
In order to examine the clinical trials’ data with respect to CA and related compounds in diabetic patients, we searched the largest clinical trial database at ‘https://clinicaltrials.gov’. No search results were obtained with the keywords ‘CA/CAPE and diabetes’. Since propolis found in beehive is a major source of CAPE, therefore we searched with keywords ‘propolis and diabetes’ on the database. Three studies were found in which propolis was administered orally or applied topically to diabetic patients. The results are summarized in Table 2.
| Number | Treatment | Condition | Outcome | ClinicalTrials.gov Identifier, phase, and status of trial |
| 1 | Propolis 300 mg twice a day for 12 wk | Type 2 DM | Propolis administration modified the glycemic control in patients with type 2 DM | ClinicalTrials.gov |
| Identifier: NCT03416127 | ||||
| Phase: 2 | ||||
| Status: Completed | ||||
| 2 | Propolis 400 mg for 6 mo, after performing scaling and root planing | Type 2 DM, periodontitis | Improvement in HbA1c, FPG, serum CML, and changes in periodontal parameters | ClinicalTrials.gov |
| Identifier: NCT02794506 | ||||
| Phase: 4 | ||||
| Status: Completed | ||||
| 3 | Propolis spray at the site of injury | Diabetic foot ulcer | Propolis possesses anti-inflammatory and antioxidant effects and its topical application is well tolerated, improving the healing of human diabetic foot ulcer | ClinicalTrials.gov |
| Identifier: NCT03649243 | ||||
| Phase: Not applicable | ||||
| Status: Completed |
CA has potential application in the treatment of several diseases including diabetes and associated complications. However, more in vivo research needs to be done for a better understanding of the mode of action of CA in DM and associated problems, particularly the role of cytoprotective enzymes like HO-1. Additionally, pharmacokinetic studies are required to entirely understand the metabolic pathway of CA post oral administration. Thus, further clinical investigations in humans are needed to determine the pharmacological potential of CA in major illnesses like diabetes.
DM has emerged as one of the most common metabolic disorders worldwide which can lead to other complications such as retinopathy, nephropathy, neuropathy, and foot ulcers. Free radical-induced oxidative stress is one of the primary factors causing DM. CA is a natural polyphenol obtained from various fruits and vegetables. CA and its derivatives act as an antioxidant to regulate the signaling pathways involved in lipid and carbohydrate metabolism. CA also exerts anti-diabetic effects by modulation of inflammatory cytokines and transcription factors. Furthermore, novel delivery strategies are being used for transport of CA to enhance its bioavailability, which has enabled the widespread use of CA in various disease therapies.
Risha Ganguly and Ramesh Kumar acknowledge financial support from University Grants Commission/Council of Scientific and Industrial Research, New Delhi, India in the form of UGC/CSIR-Senior Research Fellowships. Shiv Vardan Singh acknowledges UGC for Dr DS Kothari Fellowship. Kritika Jaiswal acknowledges financial support from University Grants Commission, New Delhi, India in the form of UGC-CRET Fellowship. All the authors also acknowledge DST-FIST and UGC-SAP facilities of the Department of Biochemistry, University of Allahabad, Prayagraj, India.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He Z, China; Karagun B, Turkey; Zeng Y, China S-Editor: Liu GL L-Editor: Wang TQ P-Editor: Liu GL
| 1. | Gupta A, Kumar R, Pandey AK. Antioxidant and antidiabetic activities of Terminalia bellirica fruit in alloxan induced diabetic rats. South Afr J Bot. 2020;130:308-315. [DOI] [Full Text] |
| 2. | Kumar R, Gupta A, Singh AK, Bishayee A, Pandey AK. The antioxidant and antihyperglycemic activities of Bottlebrush plant (Callistemon lanceolatus) stem extracts. Medicines (Basel). 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system? Nat Rev Endocrinol. 2021;17:150-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 351] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 4. | Laakso M. Biomarkers for type 2 diabetes. Mol Metab. 2019;27S:S139-S146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 5. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2531] [Article Influence: 281.2] [Reference Citation Analysis (0)] |
| 6. | International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 332] [Article Influence: 55.3] [Reference Citation Analysis (1)] |
| 7. | Pinto A, Tuttolomondo A, Di Raimondo D, Fernandez P, La Placa S, Di Gati M, Licata G. Cardiovascular risk profile and morbidity in subjects affected by type 2 diabetes mellitus with and without diabetic foot. Metabolism. 2008;57:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Harreiter J, Roden M. [Diabetes mellitus - Definition, classification, diagnosis, screening and prevention (Update 2019)]. Wien Klin Wochenschr. 2019;131:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 9. | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3956] [Cited by in RCA: 3623] [Article Influence: 241.5] [Reference Citation Analysis (0)] |
| 10. | Vikram A, Tripathi DN, Kumar A, Singh S. Oxidative stress and inflammation in diabetic complications. Int J Endocrinol. 2014;2014:679754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Ceriello A, Testa R, Genovese S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr Metab Cardiovasc Dis. 2016;26:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2020;2020:8609213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 376] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 13. | Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14:583-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (1)] |
| 14. | Esper RJ, Nordaby RA, Vilariño JO, Paragano A, Cacharrón JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 15. | Ganguly R, Kumar R, Pandey AK. Baicalin provides protection against fluoxetine-induced hepatotoxicity by modulation of oxidative stress and inflammation. World J Hepatol. 2022;14:729-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 16. | Sharma AK, Kumar S, Chashoo G, Saxena AK, Pandey AK. Cell cycle inhibitory activity of Piper longum against A549 cell line and its protective effect against metal-induced toxicity in rats. Indian J Biochem Biophys. 2014;51:358-364. [PubMed] |
| 17. | Verma RP, Hansch C. An Approach towards the quantitative structure-activity relationships of caffeic acid and its derivatives. Chembiochem. 2004;5:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Mishra AK, Mishra A, Bhargava A, Pandey AK. Antimicrobial activity of essential oils from the leaves of Cinnamomum spp. Natl Acad Sci Lett. 2008;31:341-345. |
| 19. | Huang Q, Lin Y, Yan Y. Caffeic acid production enhancement by engineering a phenylalanine over-producing Escherichia coli strain. Biotechnol Bioeng. 2013;110:3188-3196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Genaro-Mattos TC, Maurício ÂQ, Rettori D, Alonso A, Hermes-Lima M. Antioxidant activity of caffeic acid against iron-induced free radical generation-- a chemical approach. PLoS One. 2015;10:e0129963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Khan F, Bamunuarachchi NI, Tabassum N, Kim YM. Caffeic acid and its derivatives: antimicrobial drugs toward microbial pathogens. J Agric Food Chem. 2021;69:2979-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 22. | Lin Y, Yan Y. Biosynthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex. Microb Cell Fact. 2012;11:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Rodrigues JL, Araújo RG, Prather KL, Kluskens LD, Rodrigues LR. Heterologous production of caffeic acid from tyrosine in Escherichia coli. Enzyme Microb Technol. 2015;71:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Agunloye OM, Oboh G, Ademiluyi AO, Ademosun AO, Akindahunsi AA, Oyagbemi AA, Omobowale TO, Ajibade TO, Adedapo AA. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed Pharmacother. 2019;109:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 25. | Pandey AK, Kumar S. Perspectives of plant products as antimicrobial agents: A review. Pharmacologia. 2013;4:469-480. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Balaha M, De Filippis B, Cataldi A, di Giacomo V. CAPE and neuroprotection: A review. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Sidoryk K, Jaromin A, Filipczak N, Cmoch P, Cybulski M. Synthesis and antioxidant activity of caffeic acid derivatives. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Ayla Ş, Tunalı G, Bilgiç BE, Sofuoğlu K, Özdemir AA, Tanrıverdi G, Özdemir S, Soner BC, Öztürk B, Karahüseyinoğlu S, Aslan EG, Seçkin I. Antioxidant activity of CAPE (caffeic acid phenethyl ester) in vitro can protect human sperm deoxyribonucleic acid from oxidative damage. Acta Histochem. 2018;120:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Singh B, Singh JP, Kaur A, Singh N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res Int. 2020;132:109114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 289] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 30. | Zhang ZC, Xu M, Sun SF, Qiao X, Wang BR, Han J, Guo DA. Metabolic analysis of four phenolic acids in rat by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Yilmaz HR, Uz E, Yucel N, Altuntas I, Ozcelik N. Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver. J Biochem Mol Toxicol. 2004;18:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Okutan H, Ozcelik N, Yilmaz HR, Uz E. Effects of caffeic acid phenethyl ester on lipid peroxidation and antioxidant enzymes in diabetic rat heart. Clin Biochem. 2005;38:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Ganguly R, Gupta A, Pandey AK. Role of baicalin as a potential therapeutic agent in hepatobiliary and gastrointestinal disorders: A review. World J Gastroenterol. 2022;28:3047-3062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (2)] |
| 34. | Chao PC, Hsu CC, Yin MC. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab (Lond). 2009;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 35. | Pittalà V, Salerno L, Romeo G, Acquaviva R, Di Giacomo C, Sorrenti V. Therapeutic potential of caffeic acid phenethyl ester (CAPE) in diabetes. Curr Med Chem. 2018;25:4827-4836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Espíndola KMM, Ferreira RG, Narvaez LEM, Silva Rosario ACR, da Silva AHM, Silva AGB, Vieira APO, Monteiro MC. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front Oncol. 2019;9:541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 291] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 37. | Silva T, Oliveira C, Borges F. Caffeic acid derivatives, analogs and applications: a patent review (2009-2013). Expert Opin Ther Pat. 2014;24:1257-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | Khan FA, Maalik A, Murtaza G. Inhibitory mechanism against oxidative stress of caffeic acid. J Food Drug Anal. 2016;24:695-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 39. | Fraga CG, Croft KD, Kennedy DO, Tomás-Barberán FA. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10:514-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 632] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 40. | Reed AM, Gilding DK, Wilson J. Biodegradable elastomeric biomaterials--polyethylene oxide/polyethylene terephthalate copolymers. Trans Am Soc Artif Intern Organs. 1977;23:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Paracatu LC, Faria CM, Quinello C, Rennó C, Palmeira P, Zeraik ML, da Fonseca LM, Ximenes VF. Caffeic Acid phenethyl ester: consequences of its hydrophobicity in the oxidative functions and cytokine release by leukocytes. Evid Based Complement Alternat Med. 2014;2014:793629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Wei X, Zhao L, Ma Z, Holtzman DM, Yan C, Dodel RC, Hampel H, Oertel W, Farlow MR, Du Y. Caffeic acid phenethyl ester prevents neonatal hypoxic-ischaemic brain injury. Brain. 2004;127:2629-2635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Teke Z, Bostanci EB, Yenisey C, Sacar M, Simsek NG, Akoglu M. Caffeic acid phenethyl ester alleviates mesenteric ischemia/reperfusion injury. J Invest Surg. 2012;25:354-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Singh AK, Singla RK, Pandey AK. Chlorogenic acid: A dietary phenolic acid with promising pharmacotherapeutic potential. Curr Med Chem. 2022;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 45. | Mirzaei S, Gholami MH, Zabolian A, Saleki H, Farahani MV, Hamzehlou S, Far FB, Sharifzadeh SO, Samarghandian S, Khan H, Aref AR, Ashrafizadeh M, Zarrabi A, Sethi G. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol Res. 2021;171:105759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 46. | Toniolo A, Cassani G, Puggioni A, Rossi A, Colombo A, Onodera T, Ferrannini E. The diabetes pandemic and associated infections: suggestions for clinical microbiology. Rev Med Microbiol. 2019;30:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 47. | Joshi T, Singh AK, Haratipour P, Sah AN, Pandey AK, Naseri R, Juyal V, Farzaei MH. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J Cell Physiol. 2019;234:17212-17231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 48. | Singh AK, Rana HK, Singh V, Chand Yadav T, Varadwaj P, Pandey AK. Evaluation of antidiabetic activity of dietary phenolic compound chlorogenic acid in streptozotocin induced diabetic rats: Molecular docking, molecular dynamics, in silico toxicity, in vitro and in vivo studies. Comput Biol Med. 2021;134:104462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Gupta A, Kumar R, Ganguly R, Singh AK, Rana HK, Pandey AK. Antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicol Rep. 2021;8:44-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 50. | Jung UJ, Lee MK, Park YB, Jeon SM, Choi MS. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J Pharmacol Exp Ther. 2006;318:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Cheng JT, Liu IM, Tzeng TF, Chen WC, Hayakawa S, Yamamoto T. Release of beta-endorphin by caffeic acid to lower plasma glucose in streptozotocin-induced diabetic rats. Horm Metab Res. 2003;35:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Leopoldini M, Russo N, Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011;125:288-306. [RCA] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 746] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 53. | Oršolić N, Sirovina D, Odeh D, Gajski G, Balta V, Šver L, JazvinšćakJembrek M. Efficacy of caffeic acid on diabetes and its complications in the mouse. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 54. | Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 429] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 55. | Ajiboye TO, Ajala-Lawal RA, Adeyiga AB. Caffeic acid abrogates 1,3-dichloro-2-propanol-induced hepatotoxicity by upregulating nuclear erythroid-related factor 2 and downregulating nuclear factor-kappa B. Hum Exp Toxicol. 2019;38:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 56. | Tsai CF, Kuo YH, Yeh WL, Wu CY, Lin HY, Lai SW, Liu YS, Wu LH, Lu JK, Lu DY. Regulatory effects of caffeic acid phenethyl ester on neuroinflammation in microglial cells. Int J Mol Sci. 2015;16:5572-5589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 57. | Fernando ME, Seneviratne RM, Tan YM, Lazzarini PA, Sangla KS, Cunningham M, Buttner PG, Golledge J. Intensive vs conventional glycaemic control for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2016;CD010764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Bandyk DF. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin Vasc Surg. 2018;31:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 59. | Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1658] [Article Influence: 82.9] [Reference Citation Analysis (1)] |
| 60. | Monteiro-Soares M, Boyko EJ, Jeffcoate W, Mills JL, Russell D, Morbach S, Game F. Diabetic foot ulcer classifications: A critical review. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 61. | Jeffcoate WJ, Price P, Harding KG; International working group on wound healing and treatments for people with diabetic foot ulcers. Wound healing and treatments for people with diabetic foot ulcers. Diabetes Metab Res Rev. 2004;20 Suppl 1:S78-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 62. | Davis WA, Norman PE, Bruce DG, Davis TM. Predictors, consequences and costs of diabetes-related lower extremity amputation complicating type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2006;49:2634-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Ince P, Game FL, Jeffcoate WJ. Rate of healing of neuropathic ulcers of the foot in diabetes and its relationship to ulcer duration and ulcer area. Diabetes Care. 2007;30:660-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Ince P, Kendrick D, Game F, Jeffcoate W. The association between baseline characteristics and the outcome of foot lesions in a UK population with diabetes. Diabet Med. 2007;24:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 65. | Schofield CJ, Libby G, Brennan GM, MacAlpine RR, Morris AD, Leese GP; DARTS/MEMO Collaboration. mortality and hospitalization in patients after amputation: a comparison between patients with and without diabetes. Diabetes Care. 2006;29:2252-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Geethalakshmi R, Sakravarthi C, Kritika T, Arul Kirubakaran M, Sarada DV. Evaluation of antioxidant and wound healing potentials of SphaeranthusamaranthoidesBurm.f. Biomed Res Int. 2013;2013:607109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Luong KV, Nguyen LT. The impact of thiamine treatment in the diabetes mellitus. J Clin Med Res. 2012;4:153-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Gupta SK, Panda S, Singh SK. The etiopathogenesis of the diabetic foot: an unrelenting epidemic. Int J Low Extrem Wounds. 2010;9:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Tam JC, Lau KM, Liu CL, To MH, Kwok HF, Lai KK, Lau CP, Ko CH, Leung PC, Fung KP, Lau CB. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. J Ethnopharmacol. 2011;134:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Ekmektzoglou KA, Zografos GC. A concomitant review of the effects of diabetes mellitus and hypothyroidism in wound healing. World J Gastroenterol. 2006;12:2721-2729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | D'Souza DR, Salib MM, Bennett J, Mochin-Peters M, Asrani K, Goldblum SE, Renoud KJ, Shapiro P, Passaniti A. Hyperglycemia regulates RUNX2 activation and cellular wound healing through the aldose reductase polyol pathway. J Biol Chem. 2009;284:17947-17955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Abdullah FI, Chua LS, MohdBohari SP, Sari E. Rationale of Orthosiphon aristatus for healing diabetic foot ulcer. Nat Prod Comm. 2020;15:1934578X2095330. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Soleimani Z, Hashemdokht F, Bahmani F, Taghizadeh M, Memarzadeh MR, Asemi Z. Clinical and metabolic response to flaxseed oil omega-3 fatty acids supplementation in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J Diabetes Complications. 2017;31:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 74. | Ko CH, Yi S, Ozaki R, Cochrane H, Chung H, Lau W, Koon CM, Hoi SW, Lo W, Cheng KF, Lau CB, Chan WY, Leung PC, Chan JC. Healing effect of a two-herb recipe (NF3) on foot ulcers in Chinese patients with diabetes: a randomized double-blind placebo-controlled study. J Diabetes. 2014;6:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Mohamed EA, Siddiqui MJ, Ang LF, Sadikun A, Chan SH, Tan SC, Asmawi MZ, Yam MF. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complement Altern Med. 2012;12:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 76. | Yam MF, Lim V, Salman IM, Ameer OZ, Ang LF, Rosidah N, Abdulkarim MF, Abdullah GZ, Basir R, Sadikun A, Asmawi MZ. HPLC and anti-inflammatory studies of the flavonoid rich chloroform extract fraction of Orthosiphon stamineus leaves. Molecules. 2010;15:4452-4466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Gupta A, Pandey AK. Aceclofenac-induced hepatotoxicity: An ameliorative effect of Terminalia bellirica fruit and ellagic acid. World J Hepatol. 2020;12:949-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis C, Tousoulis D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 318] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 79. | McLennan SV, Bonner J, Milne S, Lo L, Charlton A, Kurup S, Jia J, Yue DK, Twigg SM. The anti-inflammatory agent Propolis improves wound healing in a rodent model of experimental diabetes. Wound Repair Regen. 2008;16:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Serarslan G, Altuğ E, Kontas T, Atik E, Avci G. Caffeic acid phenethyl ester accelerates cutaneous wound healing in a rat model and decreases oxidative stress. Clin Exp Dermatol. 2007;32:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Romana-Souza B, Dos Santos JS, Monte-Alto-Costa A. Caffeic acid phenethyl ester promotes wound healing of mice pressure ulcers affecting NF-κB, NOS2 and NRF2 expression. Life Sci. 2018;207:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 82. | Henshaw FR, Bolton T, Nube V, Hood A, Veldhoen D, Pfrunder L, McKew GL, Macleod C, McLennan SV, Twigg SM. Topical application of the bee hive protectant propolis is well tolerated and improves human diabetic foot ulcer healing in a prospective feasibility study. J Diabetes Complications. 2014;28:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 83. | Park SH, Song SY, Park EH, Kim E, Oh GC, Choo EH, Hwang BH, Chang K, Oak MH. Beneficial effects of caffeic acid phenethyl ester on wound healing in a diabetic mouse: role of VEGF and NO. Appl Sci. 2022;12:2320. [RCA] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 84. | Neelofar K, Ahmad J. An overview of in vitro and in vivo glycation of albumin: a potential disease marker in diabetes mellitus. Glycoconj J. 2017;34:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Grewal AS, Bhardwaj S, Pandita D, Lather V, Sekhon BS. Updates on aldose reductase inhibitors for management of diabetic complications and non-diabetic diseases. Mini Rev Med Chem. 2016;16:120-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 86. | Tang SCW, Leung JCK, Lai KN. Diabetic tubulopathy: an emerging entity. Contrib Nephrol. 2011;170:124-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 87. | Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 713] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 88. | Aso Y, Okumura K, Yoshida N, Tayama K, Kanda T, Kobayashi I, Takemura Y, Inukai T. Plasma interleukin-6 is associated with coagulation in poorly controlled patients with Type 2 diabetes. Diabet Med. 2003;20:930-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 90. | Maekawa K, Tanimoto T, Okada S, Suzuki T, Yabe-Nishimura C. Expression of aldose reductase and sorbitol dehydrogenase genes in Schwann cells isolated from rat: effects of high glucose and osmotic stress. Brain Res Mol Brain Res. 2001;87:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 91. | Garg SS, Gupta J. Polyol pathway and redox balance in diabetes. Pharmacol Res. 2022;182:106326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 92. | Chao CY, Mong MC, Chan KC, Yin MC. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol Nutr Food Res. 2010;54:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 93. | Matboli M, Eissa S, Ibrahim D, Hegazy MGA, Imam SS, Habib EK. Caffeic acid attenuates diabetic kidney disease via modulation of autophagy in a high-fat diet/streptozotocin- induced diabetic rat. Sci Rep. 2017;7:2263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 94. | Salem AM, Ragheb AS, Hegazy MGA, Matboli M, Eissa S. Caffeic Acid modulates miR-636 expression in diabetic nephropathy rats. Indian J Clin Biochem. 2019;34:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 95. | Momen H, Salles CA. Enzyme markers for Vibrio cholerae: identification of classical, El Tor and environmental strains. Trans R Soc Trop Med Hyg. 1985;79:773-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Izuta H, Shimazawa M, Tsuruma K, Araki Y, Mishima S, Hara H. Bee products prevent VEGF-induced angiogenesis in human umbilical vein endothelial cells. BMC Complement Altern Med. 2009;9:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 97. | Chung TW, Kim SJ, Choi HJ, Kwak CH, Song KH, Suh SJ, Kim KJ, Ha KT, Park YG, Chang YC, Chang HW, Lee YC, Kim CH. CAPE suppresses VEGFR-2 activation, and tumor neovascularization and growth. J Mol Med (Berl). 2013;91:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Fathalipour M, Eghtedari M, Borges F, Silva T, Moosavi F, Firuzi O, Mirkhani H. Caffeic acid alkyl amide derivatives ameliorate oxidative stress and modulate ERK1/2 and AKT signaling pathways in a rat model of diabetic retinopathy. Chem Biodivers. 2019;16:e1900405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, Tesfaye S. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7:938-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 100. | Uusitupa M, Khan TA, Viguiliouk E, Kahleova H, Rivellese AA, Hermansen K, Pfeiffer A, Thanopoulou A, Salas-Salvadó J, Schwab U, Sievenpiper JL. Prevention of type 2 diabetes by lifestyle changes: A systematic review and meta-analysis. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 101. | Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H, Shi Y. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 102. | Celik S, Erdogan S. Caffeic acid phenethyl ester (CAPE) protects brain against oxidative stress and inflammation induced by diabetes in rats. Mol Cell Biochem. 2008;312:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Ho YJ, Lee AS, Chen WP, Chang WL, Tsai YK, Chiu HL, Kuo YH, Su MJ. Caffeic acid phenethyl amide ameliorates ischemia/reperfusion injury and cardiac dysfunction in streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2014;13:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Kashyap D, Tuli HS, Yerer MB, Sharma A, Sak K, Srivastava S, Pandey A, Garg VK, Sethi G, Bishayee A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin Cancer Biol. 2021;69:5-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 105. | Ganguly R, Singh AK, Kumar R, Gupta A, Pandey AK. Nanoparticles as modulators of oxidative stress. In: Nanotehnology in modern animal biotechnology. 2019; Elsevier, 29-35.. |
| 106. | Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol Adv. 2020;38:107382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 107. | Wang SJ, Zeng J, Yang BK, Zhong YM. Bioavailability of caffeic acid in rats and its absorption properties in the Caco-2 cell model. Pharm Biol. 2014;52:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 108. | Fallahi F, Borran S, Ashrafizadeh M, Zarrabi A, Pourhanifeh MH, KhaksaryMahabady M, Sahebkar A, Mirzaei H. Curcumin and inflammatory bowel diseases: From in vitro studies to clinical trials. Mol Immunol. 2021;130:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 109. | Ashrafizadeh M, Ahmadi Z, Kotla NG, Afshar EG, Samarghandian S, Mandegary A, Pardakhty A, Mohammadinejad R, Sethi G. Nanoparticles targeting STATs in cancer therapy. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 110. | Klein S, Distel LVR, Neuhuber W, Kryschi C. Caffeic Acid, Quercetin and 5-Fluorocytidine-Functionalized Au-Fe3O4Nanoheterodimers for X-ray-triggered drug delivery in breast tumor spheroids. Nanomaterials (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Aguilar LE, Jang SR, Park CH, Lee KM. Supramolecular caffeic acid and bortezomib nanomedicine: prodrug inducing reactive oxygen species and inhibiting cancer cell survival. Pharmaceutics. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 112. | Kanwal N, Rasul A, Hussain G, Anwar H, Shah MA, Sarfraz I, Riaz A, Batool R, Shahbaz M, Hussain A, Selamoglu Z. Oleandrin: A bioactive phytochemical and potential cancer killer via multiple cellular signaling pathways. Food Chem Toxicol. 2020;143:111570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 113. | Atanasov AG, Zotchev SB, Dirsch VM; International Natural Product Sciences Taskforce, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3156] [Cited by in RCA: 2513] [Article Influence: 628.3] [Reference Citation Analysis (0)] |
| 114. | Rasul A, Millimouno FM, Ali Eltayb W, Ali M, Li J, Li X. Pinocembrin: a novel natural compound with versatile pharmacological and biological activities. Biomed Res Int. 2013;2013:379850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 115. | Dhupal M, Chowdhury D. Phytochemical-based nanomedicine for advanced cancer theranostics: Perspectives on clinical trials to clinical use. Int J Nanomedicine. 2020;15:9125-9157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |