Published online Feb 15, 2023. doi: 10.4239/wjd.v14.i2.120
Peer-review started: November 26, 2022
First decision: December 12, 2022
Revised: December 20, 2022
Accepted: January 19, 2023
Article in press: January 19, 2023
Published online: February 15, 2023
Processing time: 80 Days and 0.4 Hours
Exposure to proton pump inhibitors (PPIs) has been reported to have a potential role in the development of diabetes.
To determine the association between PPIs and diabetes.
This meta-analysis is registered on PROSPERO (CRD42022352704). In August 2022, eligible studies were identified through a comprehensive literature search. In this study, odds ratios were combined with 95% confidence intervals using a random-effects model. The source of heterogeneity was assessed using sensitivity analysis and subgroup analysis. The publication bias was evaluated using Egger’s test and Begg’s test.
The meta-analysis included 9 studies with a total of 867185 participants. Results showed that the use of PPIs increased the risk of diabetes (odds ratio = 1.23, 95% confidence interval: 1.05-1.43, n = 9, I2 = 96.3%). Subgroup analysis showed that geographic location and study type had significant effects on the overall results. Both Egger’s and Begg’s tests showed no publication bias (P > 0.05). Sensitivity analysis also confirmed the stability of the results.
The results of this study indicated that the use of PPIs was related to an increased risk of diabetes. However, more well-designed studies are needed to verify these results in the future.
Core Tip: Exposure to proton pump inhibitors has been reported to have a potential role in the development of diabetes. There are no consistent results for the association between proton pump inhibitors use and diabetes risk. This meta-analysis aimed to provide a more reliable assessment.
- Citation: Guo YR, Liu XM, Wang GX. Exposure to proton pump inhibitors and risk of diabetes: A systematic review and meta-analysis. World J Diabetes 2023; 14(2): 120-129
- URL: https://www.wjgnet.com/1948-9358/full/v14/i2/120.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i2.120
Diabetes is one of the fastest-growing chronic diseases in the 21st century and is characterized by inadequate insulin production or insulin resistance. Based on the 2021 International Diabetes Federation’s Diabetes Atlas, approximately 537 million adults aged 20-79 have diabetes, which is expected to reach 783 million by 2045[1]. Drug-induced diabetes is widely reported clinically and is a global problem[2].
Proton pump inhibitors (PPIs) that act on H+/K+-ATPase and inhibit gastric acid secretion are often used in the treatment of gastroesophageal reflux disease, peptic ulcer disease, and bleeding caused by nonsteroidal anti-inflammatory drugs[3]. As one of the most widely used drugs in the world, the overuse of PPIs is increasingly prominent and may cause a variety of adverse effects, including fractures, chronic kidney disease, cancer, etc[3-5]. Several recent studies have shown that there is a relationship between PPIs use and diabetes risk, with the potential mechanisms including changes in gut microbiota, PPI-induced hypomagnesemia, reduction of insulin-like growth factor-1, activation of pregnane X receptor, and effects of gastrin[6].
Some studies support a link between PPI use and diabetes risk, but there have been reports of conflicting conclusions. For example, two recent case-control studies and four cohort studies confirmed that PPIs were related to an increased risk of diabetes[6-10]. By contrast, PPIs were related to a reduced risk of diabetes in another cohort study[11]. However, there was no relationship between PPI use and diabetes risk in one randomized controlled trial and one cohort study[10,12]. Moreover, a recent meta-analysis involving eight studies from six articles showed that the use of PPIs was not related to the risk of diabetes[13]. Given the high prevalence of diabetes, the widespread use of PPIs, and conflicting findings about the association between PPI use and diabetes risk, this meta-analysis aimed to provide more reliable evidence on the relationship between PPI use and diabetes risk.
This meta-analysis was conducted according to the standard Preferred Reporting Items for Systematic Review and Meta-Analysis[14]. The study protocol has been registered on the PROSPERO International Prospective Register for Systematic Review (CRD42022352704), which provides more details.
A comprehensive search was performed in Web of Science, PubMed, Cochrane, and Embase to collect all eligible studies published before August 2022 (Supplementary Tables 1-4). The following retrieval strategy was used: Diabetes Mellitus, DM, T2DM, Diabetes, Type 1 Diabetes or Type 2 Diabetes, and Proton Pump Inhibitor, Proton Pump Inhibitors, PPI, PPIs, Esomeprazole, Rabeprazole, Pantoprazole, and Lansoprazole or Omeprazole.
The following studies were considered to be eligible for inclusion: (1) Randomized controlled trial, cohort study, or case-control study; (2) PPI use as an exposure of interest (no limitation on the type of PPIs); (3) Studies showing the association between PPI use and diabetes risk; and (4) Studies with relative risks, odds ratios (ORs), or hazard ratios with corresponding 95% confidence intervals (CIs). The exclusion criteria included: (1) The subject of the study was not human; (2) Reviews, systematic reviews, meta-analyses, comments, reports, letters, guides, conference abstracts, books, etc; (3) Results of interest not provided; and (4) Data could not be extracted or calculated.
Two authors separately and independently extracted data using a predesigned Excel spreadsheet. Any disagreements were resolved through discussion with the remaining authors. From the included articles, the following data were obtained: Authors’ names, year of publication, country, study type, comparison, total population, age, sex, adjustment factors, and effect sizes [effect quantities (ESs), including hazard ratios or ORs] with corresponding 95%CIs.
The randomized controlled trial was assessed using the Jadad scale based on randomization and blinding and whether to describe the details of participants’ exits or withdrawals from the study. The study quality was graded as follows: Low quality = 1-3 and high quality = 4-7. The Newcastle-Ottawa Scale was used to assess the quality of observational studies, including three aspects: selection, comparability, and outcome/exposure. The study quality was graded as follows: low quality = 0-3, medium quality = 4-6, and high quality = 7-9.
The data were analyzed using Stata version 15.0. (Stata Corporation, College Station, TX, United States). Due to the potential clinical heterogeneity of the studies included, multivariate-adjusted ORs were combined with 95%CIs using a random-effects model. The I2 statistics were used to evaluate the heterogeneity between studies, and significant heterogeneity was expressed as I2 > 50%, P < 0.1. The subgroup analysis was conducted to further address heterogeneity. The following three aspects were considered: (1) Sex; (2) Geographic location; and (3) Study type. The sensitivity analysis was carried out by eliminating one article at a time and recalculating aggregated effect values. Egger’s and Begg’s tests were used to assess the publication bias, and statistical significance was expressed as the two-sided P value < 0.05.
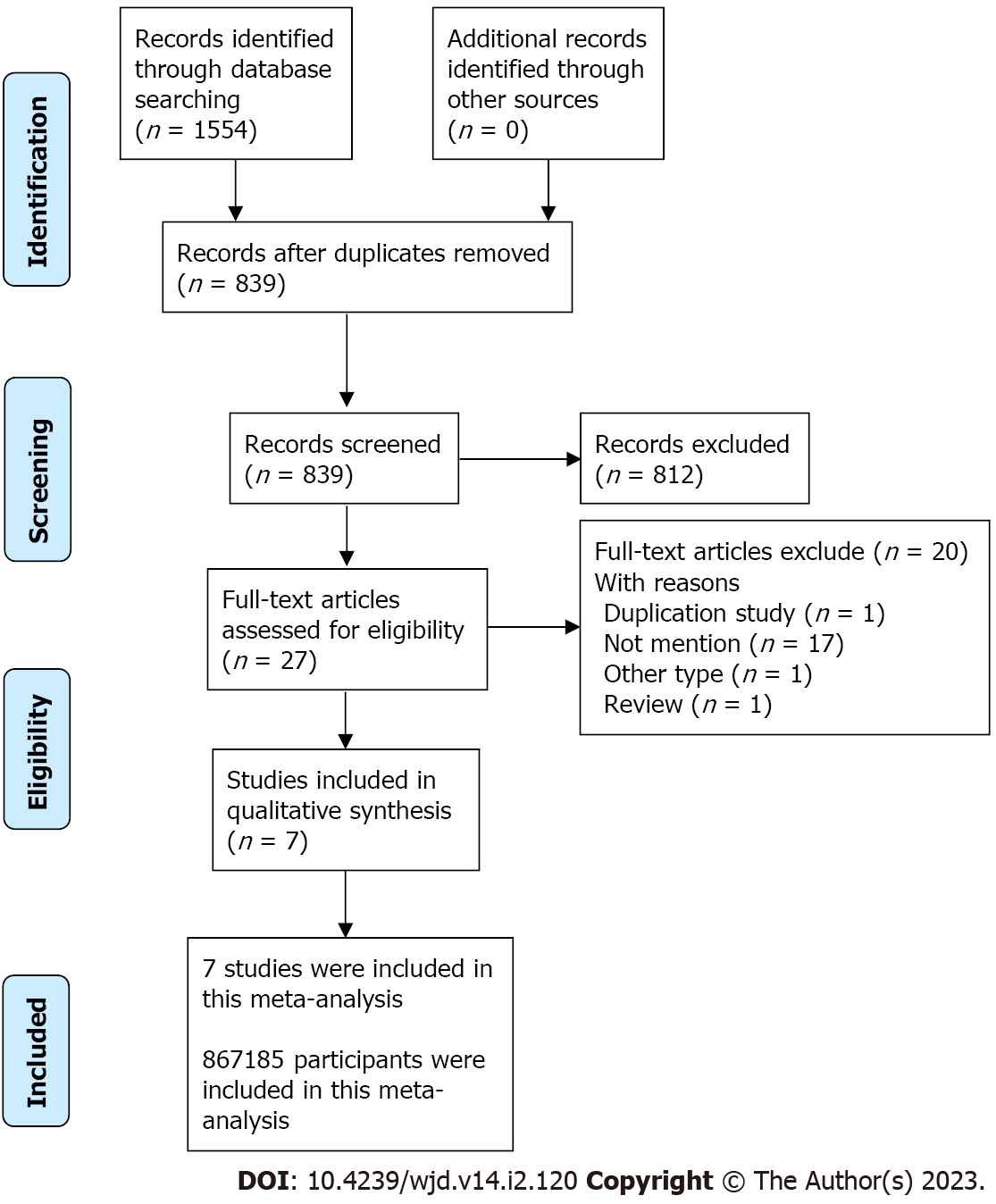
A total of 1554 articles were included after a comprehensive search of the four databases. After removing duplication and filtering by title and abstract, 27 articles required full-text evaluation. As shown in Figure 1, this meta-analysis contained nine studies from seven articles published from 2016 to 2022, including one randomized controlled trial, two case-control studies, and six cohort studies, which involved 867185 participants. Table 1 shows the baseline characteristics and quality assessments of the studies included.
| Ref. | Country | Study design | Quality | Population | Age in yr | Sex | Comparison | Adjustment | ESs (95%CI) |
| Ciardullo et al[7], 2022 | Italian | Case-control | High | 101070 | ≥ 40 | M/F | PPIs vs non-PPIs | Adjusted1 | 1.56 (1.49, 1.64) |
| Kuo et al[8], 2022 | China | Case-control | High | 41880 | 55.85 ± 13.48 | M/F | PPIs vs non-PPIs | Adjusted2 | 1.34 (1.23, 1.46) |
| Czarniak et al[6], 2022 | Netherlands | Cohort | High | 9531 | ≥ 45 | M/F | PPIs vs non-PPIs | Adjusted3 | 1.49 (1.14, 1.95) |
| He et al[9], 2021 | United Kingdom | Cohort | Moderate | 470265 | 56.34 ± 8.11 | M/F | PPIs vs non-PPIs | Adjusted4 | 1.56 (1.46, 1.66) |
| Yuan et al[10], 2021 | United States | Cohort | High | 80500 | 30-55 | F | PPIs vs non-PPIs | Adjusted5 | 1.22 (1.12, 1.33) |
| Yuan et al[10], 2021 | United States | Cohort | High | 95550 | 25-42 | F | PPIs vs non-PPIs | Adjusted5 | 1.27 (1.17, 1.38) |
| Yuan et al[10], 2021 | United States | Cohort | High | 28639 | 40-75 | M | PPIs vs non-PPIs | Adjusted5 | 1.12 (0.91, 1.38) |
| Moayyedi et al[12], 2019 | Mixed | Randomized controlled trial | High | 17598 | 67.7 ± 8.1 | M/F | PPIs vs placebo | Unadjusted | 0.96 (0.85, 1.09) |
| Lin et al[11], 2016 | China | Cohort | High | 22152 | 55.38 ± 16.95 | M/F | PPIs vs non-PPIs | Adjusted6 | 0.80 (0.73, 0.88) |
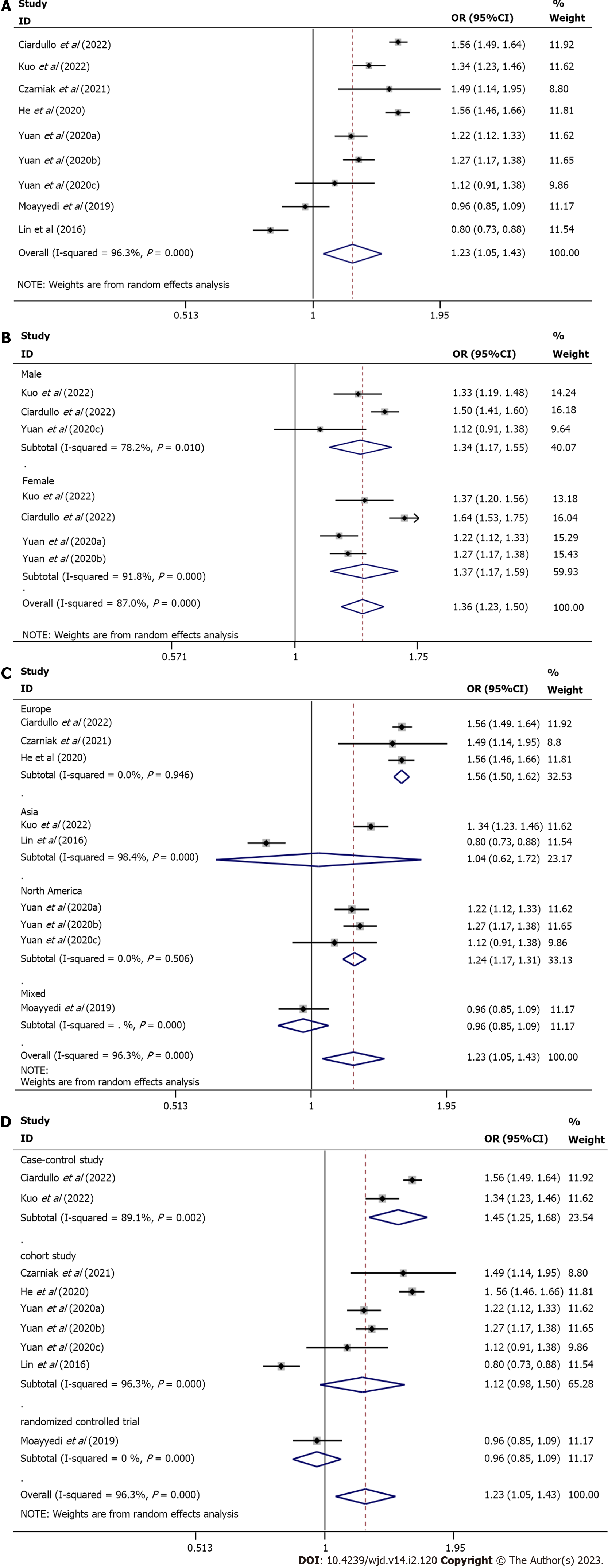
Meta-analysis using a random-effects model indicated a statistically significant association between PPI use and diabetes risk compared to placebo or no PPI use (OR = 1.23, 95%CI: 1.05-1.43, n = 9, I2 = 96.3 %) (Figure 2A).
The subgroup analysis was conducted based on sex, geographic location, and study type since the I2 analysis revealed significant heterogeneity: (1) Sex. The use of PPIs was related to an increased risk of diabetes in both females (pooled ES = 1.37, 95%CI: 1.17-1.59, n = 4, I2 = 91.8%) and males (pooled ES = 1.34, 95%CI: 1.17-1.55, n = 3, I2 = 78.2%) (Figure 2B); (2) Geographic location. A statistical association was found in Europe (pooled ES = 1.56, 95%CI: 1.50-1.62, n = 3, I2 = 0%) and North America (pooled ES = 1.24, 95%CI: 1.17-1.31, n = 3, I2 = 0%) but not in Asia (pooled ES = 1.04, 95%CI: 0.62-1.72, n = 2, I2 = 98.4%) (Figure 2C); and (3) Study type. A statistical association was detected in case-control studies (pooled ES = 1.45, 95%CI: 1.25-1.68, n = 2, I2 = 89.1%) but not in cohort studies (pooled ES = 1.21, 95%CI: 0.98-1.50, n = 6, I2 = 96.3%) (Figure 2D).
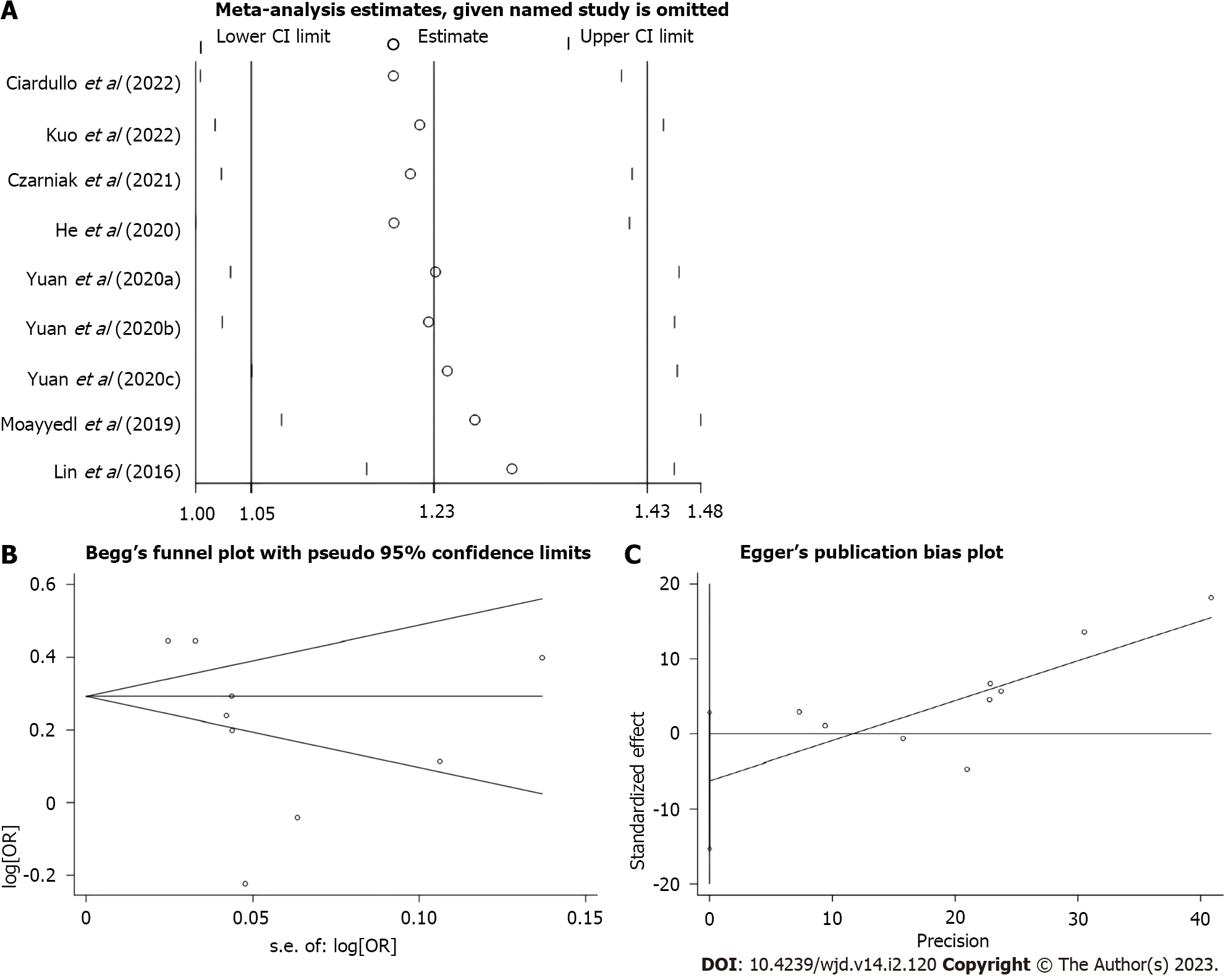
The sensitivity analysis was performed by excluding one study at a time and recalculating pooled risk estimates. The results showed no significant change in risk estimates after combination (Figure 3A). This analysis verified the robustness of the results of this study.
The meta-analysis, including nine studies with a total of 867185 participants, showed that the use of PPIs increased the risk of diabetes, which is consistent with some previous studies. A case-control study of 41880 participants in China and a cohort study of 9531 participants in Europe, after adjusting for known risk factors, showed that the use of PPIs was related to an increased risk of type 2 diabetes, and efficacy was dose-dependent[6,8]. However, the former showed an increased risk of type 2 diabetes in patients treated with pantoprazole, lansoprazole, and omeprazole, while no increased risk was found in patients treated with esomeprazole or rabeprazole[8]. Another case-control study from Europe involving 101070 participants showed that the long-term use of PPIs was related to an increased risk of diabetes and increased risk over time with treatment[7]. Similarly, there was a positive relationship between PPI use and diabetes risk in two cohort studies in North America and one in Europe but not in another study in North America[9,10].
In contrast, a randomized controlled trial of 17598 participants from mixed regions found no statistical difference between pantoprazole and diabetes risk, without adjusting for confounding factors[12]. Moreover, a cohort study from China, including 22152 participants, showed that the use of PPIs was related to a reduced risk of diabetes[11]. Recently, a systematic review and meta-analysis summarized the evidence on this topic, including eight studies of 850019 participants, showing that the use of PPIs was not related to an increased or decreased risk of diabetes. However, PPIs were only used as controls in two unadjusted included studies, with glucocorticoids and antipsychotics that have been reported to have a risk of diabetes in experimental groups[15-18]. The criteria for the included studies were standardized, and three recent high-quality studies were supplemented in the meta-analysis[6-8].
The mechanism between PPI use and diabetes risk is still unclear, and several hypotheses exist. First, the use of PPIs can alter the gut microbiome[19,20]. Changes in the gut microbiome environment play an important role in metabolism that are related to obesity, metabolic syndrome, insulin resistance, and the development of diabetic microvascular and macrovascular complications[21,22].
Second, studies have shown that the use of PPIs can lead to hypomagnesemia[23,24]. Magnesium, an essential mineral in the human body and the second richest cationic ion in cells, activates enzymes and plays an important role as a cofactor in various biochemical reactions[25]. It has been also reported that lower serum magnesium concentrations are associated with higher insulin resistance and diabetes risk, with a nonlinear dose-response relationship[26,27].
Third, studies have shown a negative correlation between PPIs and insulin-like growth factor-1 (IGF-1) levels[28]. IGF-1 is a polypeptide protein substance that is similar to insulin in molecular structure. It is able to enhance the absorption of glucose and amino acids, promote glycogen synthesis and lactate secretion, inhibit glycogenolysis, and increase insulin sensitivity. Studies have found that low IGF-1 levels are associated with diabetes risk[29,30].
The fourth mechanism may be associated with the use of PPIs to activate progesterone X receptor (PXR)[6]. PXR is a ligand-dependent member of the nuclear receptor family that regulates target genes and senses the chemical environment, which is activated by many clinically used drugs and environmental pollutants. When activated, it can regulate the expression of multiple drug metabolizing enzymes and transporters[31]. The true mechanism by which PXR impairs glucose metabolism is not fully understood, but its role in inducing hyperglycemia/diabetes by impairing glucose metabolism in the liver has been demonstrated[32].
The fifth mechanism is considered to be associated with gastrin. PPIs have been shown to increase endogenous gastrin levels in both animals and humans by inhibiting gastric acid secretion, which is associated with islet growth/regeneration[33]. Interestingly, there is also conflicting evidence regarding the role of PPIs in glycemic control for patients with diabetes[34,35]. Perhaps gastrin may be depleted over time, increasing the risk of diabetes[7].
It is well known that heterogeneity and study quality may influence the relationship resulting from the final analysis. Due to the high degree of heterogeneity in this study, a subgroup analysis was performed to understand the origin of heterogeneity. In the geographic location subgroup, a positive association between PPI use and diabetes risk was observed in Europe and North America but not in Asia or other mixed regions. In addition, an association between PPI use and diabetes risk was also found in the case-control study but not in the cohort study. This suggests that more high-quality studies from diverse geographic locations may be required in the future. Adjusting for confounding factors has an important influence on the reliability of meta-analysis results, and only one randomized controlled trial of the included studies was not adjusted. Sensitivity analysis showed robust results in the present meta-analysis, and there was no published bias in this study. Taken together, the results of this meta-analysis are robust.
In summary, PPI use is associated with diabetes risk. It is expected that the inclusion of more high-quality studies with detailed data on the use of PPIs can be required in the future, such as different types of PPIs, frequency of use, duration of use, and indications. H2 receptor antagonists could also be included in the analysis to see if there is an association between antacids and diabetes.
Based on the available evidence, it can be concluded that the use of PPIs is related to an increased risk of diabetes. In addition, this connection between the use of PPIs and the risk of diabetes is also found in both females and males. However, accounting for the limitations and the presence of bias in the primary studies, future research should still focus on the use of different types of PPIs and the risk of diabetes, especially in people with different backgrounds.
There is a still controversial connection between the widespread use of proton pump inhibitors (PPIs) and the risk of diabetes.
In a previous meta-analysis, the use of PPIs was shown to not be associated with the risk of diabetes. However, three recent high-quality studies found that the use of PPIs was associated with an increased risk of diabetes. Therefore, a meta-analysis was carried out to determine the association between PPIs and diabetes.
To provide more reliable evidence on the relationship between PPI use and diabetes risk.
Meta-analysis was used to realize the objectives. Statistical analyses were performed by using Stata version 15.0.
Results showed that the use of PPIs increased the risk of diabetes (odds ratio = 1.23, 95% confidence interval: 1.05-1.43, n = 9, I2 = 96.3%). In the subgroup analysis, geographic location and study type had significant effects on the overall results. No publication bias (P > 0.05) was found in Egger’s or Begg’s tests. Also, sensitivity analysis confirmed the stability of the results.
The results of this study indicated that the use of PPIs was associated with an increased risk of diabetes.
It is expected that more research from diverse geographic locations with detailed data on the use of PPIs is required in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Suravajhala PN, India; Thongon N, Thailand S-Editor: Chen YL L-Editor: Filipodia A P-Editor: Chen YL
| 1. | IDF Diabetes Atlas. Brussels International Diabetes Federation 2021, 10th ed. [cited 3 December 2022]. Available from: https://diabetesatlas.org/. |
| 2. | Liu MZ, He HY, Luo JQ, He FZ, Chen ZR, Liu YP, Xiang DX, Zhou HH, Zhang W. Drug-induced hyperglycaemia and diabetes: pharmacogenomics perspectives. Arch Pharm Res. 2018;41:725-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 567] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 4. | Turshudzhyan A, Samuel S, Tawfik A, Tadros M. Rebuilding trust in proton pump inhibitor therapy. World J Gastroenterol. 2022;28:2667-2679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Bridoux M, Simon N, Turpin A. Proton Pump Inhibitors and Cancer: Current State of Play. Front Pharmacol. 2022;13:798272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Czarniak P, Ahmadizar F, Hughes J, Parsons R, Kavousi M, Ikram M, Stricker BH. Proton pump inhibitors are associated with incident type 2 diabetes mellitus in a prospective population-based cohort study. Br J Clin Pharmacol. 2022;88:2718-2726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Ciardullo S, Rea F, Savaré L, Morabito G, Perseghin G, Corrao G. Prolonged Use of Proton Pump Inhibitors and Risk of Type 2 Diabetes: Results From a Large Population-Based Nested Case-Control Study. J Clin Endocrinol Metab. 2022;107:e2671-e2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Kuo HY, Liang CS, Tsai SJ, Chen TJ, Chu CS, Chen MH. Dose-Dependent Proton Pump Inhibitor Exposure and Risk of Type 2 Diabetes: A Nationwide Nested Case-Control Study. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 9. | He Q, Yang M, Qin X, Fan D, Yuan J, Pan Y. Risk stratification for proton pump inhibitor-associated type 2 diabetes: a population-based cohort study. Gut. 2021;70:2212-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Yuan J, He Q, Nguyen LH, Wong MCS, Huang J, Yu Y, Xia B, Tang Y, He Y, Zhang C. Regular use of proton pump inhibitors and risk of type 2 diabetes: results from three prospective cohort studies. Gut. 2021;70:1070-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Lin HC, Hsiao YT, Lin HL, Uang YS, Cheng HW, Wang Y, Wang LH. The use of proton pump inhibitors decreases the risk of diabetes mellitus in patients with upper gastrointestinal disease: A population-based retrospective cohort study. Medicine (Baltimore). 2016;95:e4195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Moayyedi P, Eikelboom JW, Bosch J, Connolly SJ, Dyal L, Shestakovska O, Leong D, Anand SS, Störk S, Branch KRH, Bhatt DL, Verhamme PB, O’Donnell M, Maggioni AP, Lonn EM, Piegas LS, Ertl G, Keltai M, Bruns NC, Muehlhofer E, Dagenais GR, Kim JH, Hori M, Steg PG, Hart RG, Diaz R, Alings M, Widimsky P, Avezum A, Probstfield J, Zhu J, Liang Y, Lopez-Jaramillo P, Kakkar AK, Parkhomenko AN, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Vinereanu D, Tonkin AM, Lewis BS, Felix C, Yusoff K, Metsarinne KP, Fox KAA, Yusuf S; COMPASS Investigators. Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology. 2019;157:682-691.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 340] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 13. | Chen Y, Hu L, Sun C, Bao J, Liu J, Bhan C, Kim KY, Manem R, Thapa P, Ma S, Liu M, Cheng X, Cheng C, Zhou Q. Will Proton Pump Inhibitors Increase the Risk of Diabetes Mellitus? Turk J Gastroenterol. 2022;33:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 4683] [Article Influence: 1170.8] [Reference Citation Analysis (0)] |
| 15. | Blackburn D, Hux J, Mamdani M. Quantification of the Risk of Corticosteroid-induced Diabetes Mellitus Among the Elderly. J Gen Intern Med. 2002;17:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Barnett M, Argo T, Alexander B, Perry P. A regional comparison of developing diabetes among VA patients exposed to typical and atypical antipsychotics relative to corticosteroids and proton pump inhibitors. Ann Clin Psychiatry. 2006;18:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Li JX, Cummins CL. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat Rev Endocrinol. 2022;18:540-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 124] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 18. | Ouyang F, He J, Cheng X, Zhou W, Xiao S, Fang J. Antipsychotic-Related Risks of Type 2 Diabetes Mellitus in Enrollees With Schizophrenia in the National Basic Public Health Service Program in Hunan Province, China. Front Psychiatry. 2022;13:754775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 19. | Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 937] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 20. | Hamilton MK, Wall ES, Robinson CD, Guillemin K, Eisen JS. Enteric nervous system modulation of luminal pH modifies the microbial environment to promote intestinal health. PLoS Pathog. 2022;18:e1009989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Martínez-López YE, Esquivel-Hernández DA, Sánchez-Castañeda JP, Neri-Rosario D, Guardado-Mendoza R, Resendis-Antonio O. Type 2 diabetes, gut microbiome, and systems biology: A novel perspective for a new era. Gut Microbes. 2022;14:2111952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Iatcu CO, Steen A, Covasa M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 23. | Novotny M, Klimova B, Valis M. PPI Long Term Use: Risk of Neurological Adverse Events? Front Neurol. 2018;9:1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Srinutta T, Chewcharat A, Takkavatakarn K, Praditpornsilpa K, Eiam-Ong S, Jaber BL, Susantitaphong P. Proton pump inhibitors and hypomagnesemia: A meta-analysis of observational studies. Medicine (Baltimore). 2019;98:e17788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 25. | Patoa S, Das D. Serum Magnesium Levels and its Association with Glycated Hemoglobin in Type 2 Diabetes Mellitus Patients. J Assoc Physicians India. 2022;70:11-12. [PubMed] |
| 26. | Li W, Jiao Y, Wang L, Wang S, Hao L, Wang Z, Wang H, Zhang B, Ding G, Jiang H. Association of Serum Magnesium with Insulin Resistance and Type 2 Diabetes among Adults in China. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Park JW, Kim EK, Lee J, Chung SH, Boo G, Do SH. Effect of Intraoperative Magnesium Sulfate Administration on Blood Glucose Control following Total Joint Arthroplasty in Patients with Diabetes. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 28. | Maggio M, Lauretani F, De Vita F, Buttò V, Cattabiani C, Masoni S, Sutti E, Bondi G, Dall’aglio E, Bandinelli S, Corsonello A, Abbatecola AM, Lattanzio F, Ferrucci L, Ceda GP. Relationship between use of proton pump inhibitors and IGF system in older subjects. J Nutr Health Aging. 2014;18:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Teppala S, Shankar A. Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care. 2010;33:2257-2259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Meyer NMT, Kabisch S, Dambeck U, Honsek C, Kemper M, Gerbracht C, Arafat AM, Birkenfeld AL, Schwarz PEH, Machann J, Osterhoff MA, Weickert MO, Pfeiffer AFH. Low IGF1 and high IGFBP1 predict diabetes onset in prediabetic patients. Eur J Endocrinol. 2022;187:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Hassani-Nezhad-Gashti F, Rysä J, Kummu O, Näpänkangas J, Buler M, Karpale M, Hukkanen J, Hakkola J. Activation of nuclear receptor PXR impairs glucose tolerance and dysregulates GLUT2 expression and subcellular localization in liver. Biochem Pharmacol. 2018;148:253-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Liu P, Jiang L, Kong W, Xie Q, Li P, Liu X, Zhang J, Liu M, Wang Z, Zhu L, Yang H, Zhou Y, Zou J, Liu L. PXR activation impairs hepatic glucose metabolism partly via inhibiting the HNF4α-GLUT2 pathway. Acta Pharm Sin B. 2022;12:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 33. | Bödvarsdóttir TB, Hove KD, Gotfredsen CF, Pridal L, Vaag A, Karlsen AE, Petersen JS. Treatment with a proton pump inhibitor improves glycaemic control in Psammomys obesus, a model of type 2 diabetes. Diabetologia. 2010;53:2220-2223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Peng CC, Tu YK, Lee GY, Chang RH, Huang Y, Bukhari K, Tsai YC, Fu Y, Huang HK, Munir KM. Effects of Proton Pump Inhibitors on Glycemic Control and Incident Diabetes: A Systematic Review and Meta-analysis. J Clin Endocrinol Metab. 2021;106:3354-3366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Sánchez-García A, Simental-Mendía M, Simental-Mendía LE. Effect of Proton-Pump Inhibitors on Glucose and Insulin Metabolism on Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr Pharm Des. 2020;26:4007-4013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |