Published online Oct 15, 2023. doi: 10.4239/wjd.v14.i10.1532
Peer-review started: July 6, 2023
First decision: July 27, 2023
Revised: August 2, 2023
Accepted: August 15, 2023
Article in press: August 15, 2023
Published online: October 15, 2023
Processing time: 95 Days and 2.4 Hours
Gestational diabetes mellitus (GDM) refers to hyperglycemia caused by insulin resistance or insufficient insulin secretion during pregnancy. Patients with GDM have a high risk of pregnancy complications, which can adversely affect both maternal and fetal health. Therefore, early diagnosis, treatment and monitoring of GDM are essential. In recent years, a new treatment scheme represented by insulin aspart combined with metformin has received increasing attention.
To explore the effects of insulin aspart combined with metformin on patients with GDM and inflammatory markers.
From April 2020 to September 2022, 124 patients with GDM in Sanya Women and Children’s Hospital Managed by Shanghai Children’s Medical Center were collected and analyzed retrospectively. The control group (CG) comprised 62 patients treated with insulin aspart alone, and 62 patients treated with insulin aspart and metformin formed the observation group (OG). Before and after treatment, improvement of blood-glucose-related indexes [fasting blood glucose (FBG), 2-h postprandial glucose (2h PG) and hemoglobin A1c (HbA1c)], serum related factor [serum homocysteine (Hcy)], serum inflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-6 and C-reactive protein (CRP)] were compared between the two groups. The clinical efficacy, adverse pregnancy outcomes and incidence of pregnancy complications were compared between the two groups.
After treatment, the levels of FBG, 2h PG, HbA1c, Hcy, TNF-α, IL-6 and CRP in both groups were significantly decreased (P < 0.05), and the levels of FBG, 2h PG, HbA1c, Hcy, TNF-α, IL-6 and CRP in the OG were lower than in the CG (P < 0.05). The total clinical effectiveness in the OG was higher than that in the CG (P < 0.05). The total incidence of adverse pregnancy outcomes and complications in the OG was significantly lower than in the CG (P < 0.05).
Insulin aspart combined with metformin are effective for treatment of GDM, which can reduce blood-glucose-related indexes, Hcy and serum inflammatory cytokines, and risk of adverse pregnancy outcomes and complications.
Core Tip: This study investigated the clinical effect of insulin aspart combined with metformin on gestational diabetes mellitus and serum homocysteine (Hcy), tumor necrosis factor-α, interleukin-6 and C-reactive protein, which represents a novel aspect in the field. The combination of drugs significantly improved the condition of patients, reduced adverse pregnancy outcomes and incidence of adverse reactions, decreased blood-glucose-related indicators, Hcy, and serum inflammatory cytokine levels, and exhibited a high level of safety. These findings provide an important basis for the clinical application and popularization of this combination therapy.
- Citation: Wang Y, Song M, Qi BR. Effects of insulin aspart and metformin on gestational diabetes mellitus and inflammatory markers. World J Diabetes 2023; 14(10): 1532-1540
- URL: https://www.wjgnet.com/1948-9358/full/v14/i10/1532.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i10.1532
Gestational diabetes mellitus (GDM) refers to different degrees of abnormal glucose metabolism that develops or is first found during pregnancy[1,2]. In recent years, with the change of life style and the fertility policy, the disease has shown a significant increasing trend[3]. It is reported that the incidence of GDM is 1%-14% globally and 1%-5% in China[4,5]. Although most parturients can return to normal after delivery, the risk of type II diabetes greatly increases[6]. If the patient is not treated in time, the disease can lead to various adverse pregnancy outcomes, such as fetal distress, fetal macrosomia, premature delivery, and abortion, and even endanger the life of the mother and baby. Therefore, the harm of GDM to the mother and newborn cannot be underestimated[7]. At present, the patients with GDM are treated with drugs, nutritional therapy, exercise therapy and blood sugar monitoring to keep their blood sugar in the normal range, thus reducing the complications to parturients and newborns, decreasing the perinatal mortality and improving adverse pregnancy outcome[8].
Insulin aspart is an analog of rapid-acting human insulin, and its activity is close to that of natural insulin. After subcutaneous injection, it can quickly help the body to ingest and utilize glucose in the blood, thus effectively maintaining the blood sugar level[9]. Insulin aspart takes effect faster and acts for a shorter time than soluble human insulin, so it should be injected immediately before meals. Studies have shown that insulin aspart is effective for treatment of GDM and pregestational diabetes, with stable control of blood sugar level and high safety[10]. Metformin is a biguanide hypoglycemic agent that mainly reduces blood sugar by inhibiting gluconeogenesis and glycogen decomposition and reducing the output of liver glucose[11]. In addition, metformin can improve the intake and utilization of glucose by muscle and adipose tissues, and can also play multiple roles in reducing body weight, improving insulin sensitivity and reducing insulin resistance[12]. Metformin can control the blood sugar level of parturients and newborns in the treatment of GDM, and reduce the risk of blood sugar, with a high level of safety[13]. Metformin has been widely used in clinical treatment, and has become the first choice to control blood sugar in overweight and obese patients with type II diabetes. Therefore, insulin aspart and metformin are potential effective drugs for the treatment of GDM.
However, the efficacy of the combination of the two drugs for treatment of GDM has not been systematically evaluated. Therefore, this study was designed to use insulin aspart and metformin for treatment of GDM, and explore the effect of the drug combination on patients and serum-related factors, to provide a reliable basis for clinical research.
From April 2020 to September 2022, 124 patients with GDM in Sanya Women and Children’s Hospital Managed by Shanghai Children’s Medical Center were collected and analyzed retrospectively. The control group (CG) comprised 62 patients treated with insulin aspart alone, and the other 62 patients treated with insulin aspart and metformin formed the observation group (OG). This study was approved by the Ethics Committee of Sanya Women and Children’s Hospital Managed by Shanghai Children’s Medical Center.
Inclusion criteria: (1) Patients met the diagnostic criteria for GDM; GDM was diagnosed if fasting blood glucose (FBG) during pregnancy was ≥ 5.1 mmol/L, blood glucose after taking glucose for 1 h was ≥ 10.0 mmol/L, or blood glucose after taking glucose for 2 h was ≥ 8.5mmol/L; (2) Blood sugar could not be controlled by diet or exercise, so drugs were needed for intervention; (3) Patients gave informed and signed consent for voluntary participation; and (4) Clinical data were complete.
Exclusion criteria: (1) Patients with multiple pregnancies; (2) Patients with diabetes before pregnancy or a family history of diabetes; (3) Comorbid hepatic and renal insufficiency; (4) Comorbid pregnancy complications; (5) Comorbid mental or cognitive dysfunction; and (6) Allergic to the drugs in this study.
In both groups, patients were given routine health education on GDM, diet control and exercise guidance. The CG was treated with subcutaneous injection of insulin aspartate before dinner at an initial dose of 0.2-0.3 IU/kg once daily. The dose of insulin aspart was adjusted according to the patient’s blood glucose regulation, and the maximum dose was not more than 30 IU/d. The OG was treated with insulin aspart at the same dose as in the CG, and the initial dose of metformin was 500 mg twice daily. The dose of metformin was adjusted according to the patient’s blood glucose, and the maximum dose was not more than 2000 mg. In both groups, the drug treatment lasted until delivery.
In the morning, 10 mL of median cubital vein blood was withdrawn from all patients after fasting for 8 h, and stored in three tubes. At 2 h after a meal, 4 mL of median elbow vein blood was withdrawn to detect the concentration of 2-h postprandial glucose (2h PG). An automatic biochemical analyzer was used to detect the blood-glucose-related indexes before and after treatment, including FBG, 2h PG and glycosylated hemoglobin (HbA1c). ELISA was used to detect serum-related factors [serum homocysteine (Hcy)] and serum inflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-6, and C-reactive protein (CRP)] before and after treatment.
Primary outcome measures: improvement of blood-glucose-related indexes before and after treatment was compared between the two groups, including FBG, 2h PG and HbA1c. Hcy levels were compared between the two groups before and after treatment. Serum inflammatory cytokines, including TNF-α, IL-6 and CRP, were compared between the two groups before and after treatment. The clinical therapeutic effect was compared between the two groups. The evaluation criteria for efficacy are shown in Table 1. Total effective rate = (markedly effective + effective) × 100%/total number of patients. Secondary outcome measures: Baseline data of the two groups were compared. Adverse pregnancy outcomes and pregnancy complications were compared between the two groups.
| Efficacy grade | Evaluative criteria |
| Markedly effective | After treatment, clinical symptoms disappeared and blood sugar decreased obviously |
| Effective | After treatment, clinical symptoms improved and blood sugar decreased |
| Ineffective | After treatment, clinical symptoms did not improve, and blood sugar did not decrease, or even worsened |
SPSS 20.0 (Chicago, IL, United States) was applied to analyze the collected data. GraphPad Prism 8 (La Jolla, CA, United States) was applied to visualize the data. The data were analyzed by t test. Classified variables were compared by c2 test. The difference was statistically significant with P < 0.05.
There was no significant difference between the two groups in age, gestational age, height, maternal category, or education level (P > 0.05) (Table 2).
| Factors | CG (n = 62) | OG (n = 62) | χ2 | P value |
| Age, yr | ||||
| ≤ 30 | 38 | 41 | 0.314 | 0.575 |
| > 30 | 24 | 21 | ||
| Gestational age, yr | ||||
| ≤ 30 | 34 | 30 | 0.517 | 0.472 |
| > 30 | 28 | 32 | ||
| Maternal category | ||||
| Primipara | 38 | 36 | 0.134 | 0.714 |
| Multipara | 24 | 26 | ||
| Educational level | ||||
| Below junior college | 27 | 25 | 0.133 | 0.716 |
| Junior college and above | 35 | 37 |
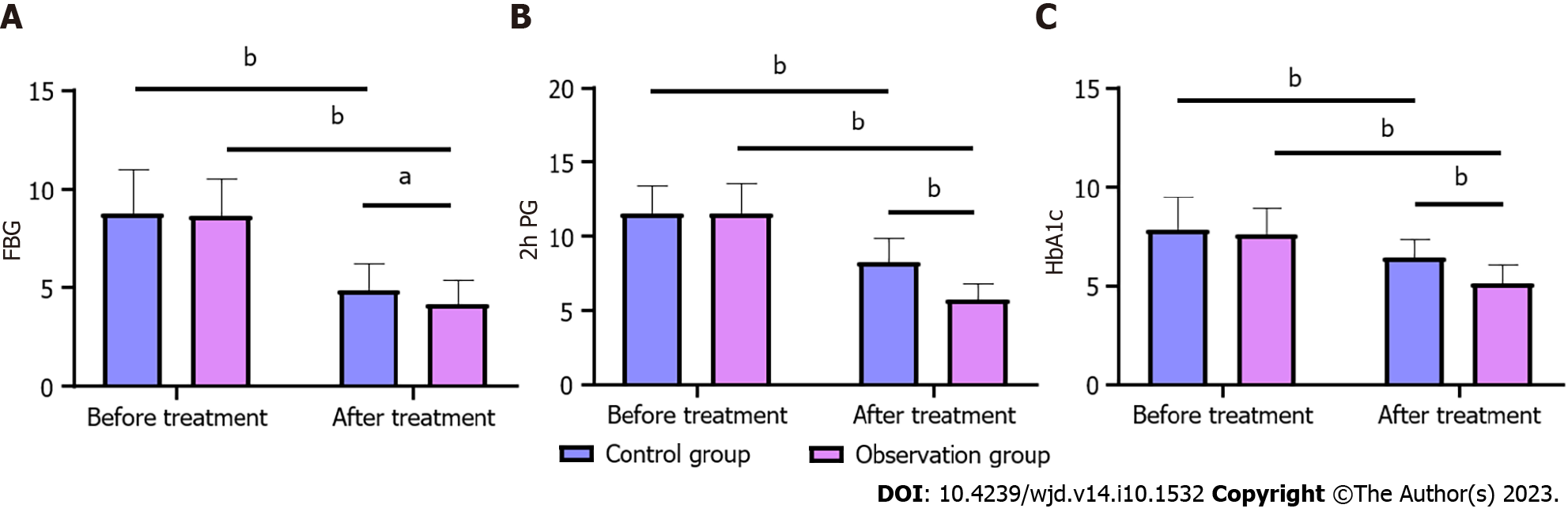
Compared with before treatment, FBG, 2h PG and HbA1c levels were significantly decreased in both groups after treatment (P < 0.05). In addition, the intergroup comparison showed that the levels of FBG, 2h PG and HbA1c in the two groups had no significant change before treatment (P > 0.05), but after treatment, the levels of FBG, 2h PG and HbA1c in the OG were significantly lower than in the CG (P < 0.05) (Figure 1).
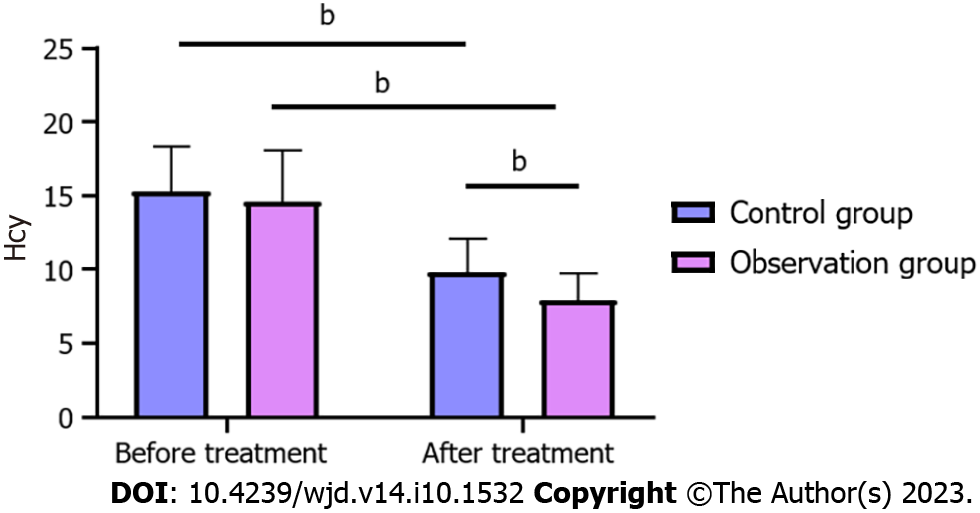
Compared with before treatment, Hcy level was significantly decreased in both groups after treatment (P < 0.05). In addition, the intergroup comparison showed that Hcy level in both groups had no significant change before treatment (P > 0.05), but after treatment, Hcy level in the OG was significantly lower than in the CG (P < 0.05) (Figure 2).
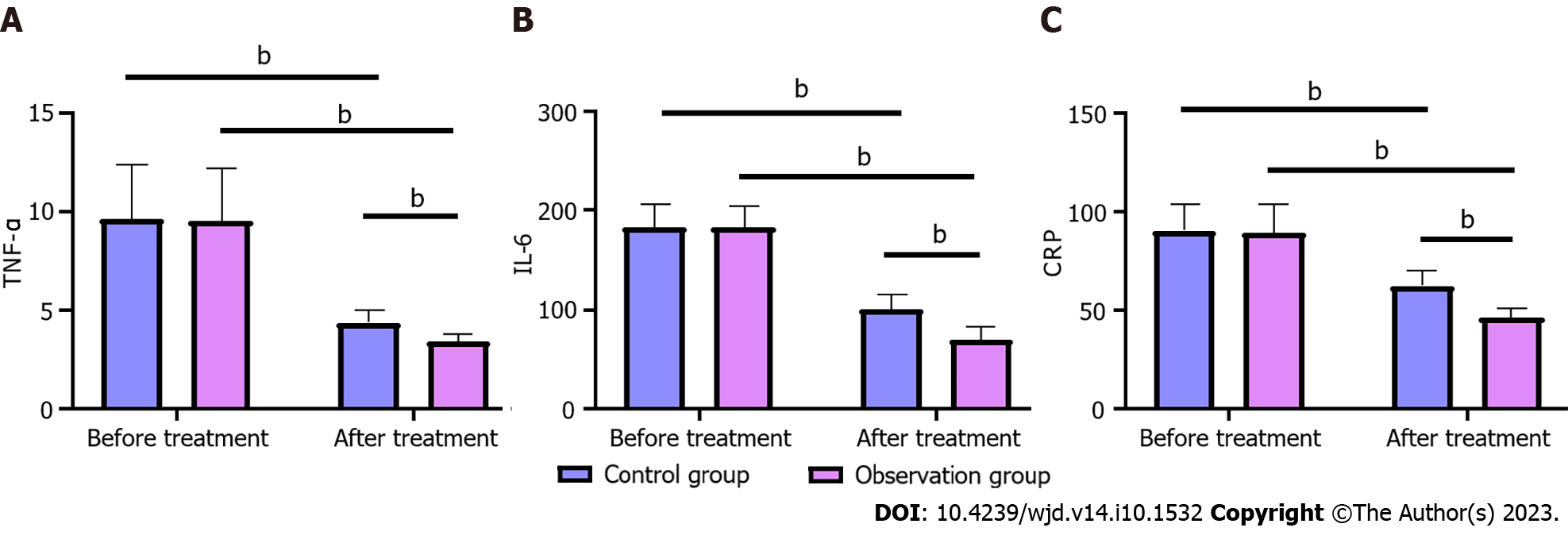
Compared with before treatment, TNF-α, IL-6 and CRP levels were significantly decreased in both groups after treatment (P < 0.05). In addition, the intergroup comparison showed that the levels of TNF-α, IL-6 and CRP in both groups had no significant change before treatment (P > 0.05), but after treatment, the levels of TNF-α, IL-6 and CRP in the OG were significantly lower than those in the CG (P < 0.05) (Figure 3).
The total clinical effectiveness rate in the OG was significantly higher than in the CG (P = 0.003) (Table 3).
| Groups | CG (n = 62) | OG (n = 62) | χ2 | P value |
| Markedly effective | 25 (40.32) | 32 (51.61) | ||
| Effective | 19 (30.65) | 25 (40.32) | ||
| Ineffective | 18 (29.03) | 5 (8.06) | ||
| Total effective rate | 44 (70.97) | 57 (91.94) | 9.021 | 0.003 |
The total incidence of adverse pregnancy outcomes and pregnancy complications in the OG was significantly lower than in the CG (P < 0.05) (Tables 4 and 5).
| Groups | Adverse pregnancy outcomes | |||
| Premature delivery | Induced labor | Cesarean section | Total incidence rate | |
| CG (n = 62) | 5 (8.06) | 3 (4.84) | 31 (50.0) | 39 (62.90) |
| OG (n = 62) | 2 (3.23) | 1 (1.61) | 8 (12.90) | 11 (18.33) |
| χ2 | 26.270 | |||
| P value | < 0.0001 | |||
| Groups | Pregnancy complications | ||||
| Pregnancy hypertension | Polyhydramnios | Hypoglycemia | Ketoacidosis | Total incidence rate | |
| CG (n = 62) | 5 (8.06) | 5 (8.06) | 4 (6.45) | 2 (3.23) | 16 (25.81) |
| OG (n = 62) | 1 (1.61) | 2 (3.23) | 1 (1.61) | 0 | 4 (6.45) |
| χ2 | 8.585 | ||||
| P value | 0.003 | ||||
Many hormones in pregnant women will change, and improper conditioning can lead to many complications. GDM is one of the more common complications[14,15]. Pregnant women in the second or third trimester produce a variety of insulin-resistant substances, such as placental lactogen, estrogen, progesterone, cortisol and placental insulinase, so that the sensitivity of pregnant women to insulin decreases with the increase of gestational age[16]. Pregnant women need more insulin to maintain blood glucose balance. For pregnant women with restricted insulin secretion, this physiological change cannot be compensated during pregnancy, which leads to an increase in blood sugar, thus aggravating the original diabetes or causing GDM[17]. GDM may be accompanied by three typical symptoms: Polydipsia, polyphagia and polyuria, and women may also experience blurred vision and abnormal touch, etc[18]. If GDM is not treated in time, it may have an impact on pregnant women and fetuses. GDM can lead to fetal macrosomia, fetal malformation, neonatal jaundice, neonatal respiratory distress syndrome, and increase fetal mortality[19,20]. Therefore, it is necessary to choose appropriate drugs and treat patients with GDM in time, so as to ensure the health and safety of parturients and fetuses.
At present, drugs are often used to control blood glucose. In the past, when patients were treated with biosynthetic human insulin, they were prone to hypoglycemia, so the therapeutic effect was not ideal. As a new type of rapid-acting insulin, insulin aspart has a short duration of action and peak time, takes effect rapidly, and reduces PG, which makes it a potential drug for treatment of GDM[21]. Studies have shown that insulin aspart combined with exercise diet can control the blood sugar level of patients with GDM, and ensure the health of parturients and fetuses[22]. However, long-term use of insulin can lead to resistance, thus reducing the control of blood sugar, so patients need to take corresponding hypoglycemic drugs to further control blood sugar. Metformin is a commonly used insulin-sensitizing agent that can improve insulin resistance and lower blood sugar[23]. Studies have shown that insulin aspart combined with metformin can control the blood sugar level of patients with GDM, reduce the adverse pregnancy outcomes of parturients and newborns, and play a positive role in clinical treatment[24]. In this study, insulin aspart plus metformin was used to treat patients with GDM. The total clinical effectiveness in the OG was higher than that in the CG, indicating that compared with single drug treatment, the two drugs complemented each other in combination, and significantly improved the therapeutic effect. The changes of blood-glucose-related indexes, Hcy and serum inflammatory cytokines were compared before and after treatment. Compared with before treatment, the levels of FBG, 2h PG, HbA1c, Hcy, TNF-α, IL-6 and CRP in both groups were significantly decreased, and the levels of FBG, 2h PG, HbA1c, Hcy, TNF-α, IL-6 and CRP in the OG were lower than those in the CG after treatment. These results indicated that the blood-glucose-related indexes, Hcy and serum inflammatory cytokines of patients receiving insulin aspart combined with metformin were significantly improved. There may be some limitations when insulin is used alone to treat GDM. Therefore, metformin combined with insulin aspart can play a significant role in lowering blood glucose and controlling blood glucose within a stable range.
At the end of the study, the adverse pregnancy outcomes and pregnancy complications were compared between the two groups. The total incidence of adverse pregnancy outcomes and pregnancy complications in the OG was lower than that in the CG, indicating that insulin aspart combined with metformin reduced the incidence of adverse pregnancy outcomes and complications in patients with GDM, with a high level of safety. In addition, some studies have shown that insulin aspart combined with metformin did not increase the risk of adverse reactions in patients GDM and fetal growth and development, and had good high safety, which is similar to our study[25].
In this study, insulin aspart combined with metformin controlled blood glucose level in patients with GDM. However, there were still some limitations to this study. First, this was a retrospective study with a small sample, so it was not as uniform as a randomized controlled trial. Second, the patients were not followed up in this study, so the long-term prognosis of the parturients and fetuses was not clear. Therefore, we hope to carry out follow-up studies in the future, so as to improve our conclusions.
Insulin aspart combined with metformin was effective in the treatment of GDM, which reduced blood-glucose-related indexes, Hcy and serum inflammatory cytokines, and reduced the risk of adverse pregnancy outcomes and complications during pregnancy. It is safe and worthy of clinical application and promotion.
Gestational diabetes mellitus (GDM) is a type of hyperglycemia during pregnancy, which requires timely diagnosis, treatment and monitoring to avoid negative effects on maternal and infant health. In order to improve the therapeutic effect of patients with GDM, a new combination drug regimen has received extensive attention in the past few years.
The study was designed to investigate the effect of insulin aspart combined with metformin on inflammatory cytokine levels and overall improvement of patients with GDM. By studying the therapeutic effect and pharmacological mechanism of this new combination drug regimen, it can provide a reference for the precise treatment of patients with GDM, and a scientific basis for the protection of high-risk groups during pregnancy.
This study aimed to explore the efficacy of insulin aspart combined with metformin in treating GDM, and evaluate the effect of combined medication on lowering blood glucose level, and improving inflammatory response and metabolic disorder. The study revealed the advantages and limitations of the combined therapy regimen in clinical application, thus providing a scientific reference for future improvement of treatment strategies for GDM.
This study was designed to retrospectively analyze 124 patients with GDM over a period of 2 years, divide them into two groups according to different treatment methods for analysis and comparison, and discuss the differences in blood-glucose-related indexes, serum-related factors, inflammatory cytokines, adverse pregnancy outcomes and pregnancy complications of patients with different treatment methods.
Insulin aspart combined with metformin can improve blood-glucose-related indexes (fasting blood glucose, 2-h postprandial glucose, glycosylated hemoglobin), serum-related factor (homocysteine) and serum inflammatory cytokines (tumor necrosis factor-α, interleukin-6, C-reactive protein) of patients, effectively reduce the incidence of adverse pregnancy outcomes and complications, and have a high level of safety.
We studied the effectiveness of combined therapy for treating GDM and made breakthroughs in the treatment plan for this disease. These research achievements will help to more accurately determine the treatment plan for patients and promote the further development of the prevention and treatment of GDM and its complications.
In follow-up studies, we hope to increase the sample size, extend the study duration, conduct follow-up, and explore the long-term prognosis of this combination therapy for both mothers and fetuses, thus improving our conclusions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rose AJ, Germany; Selvin E, United States S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Quintanilla Rodriguez BS, Mahdy H. Gestational Diabetes. 2022 Sep 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 2. | Kalra S, Gupta Y, Kumar A. Prevention of Gestational Diabetes Mellitus (GDM). J Pak Med Assoc. 2016;66:S107-S109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Li Y, Li D, Cheng X. The association between expression of lncRNAs in patients with GDM. Endocr Connect. 2021;10:1080-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Aktary WM, Pasichnyk D, Seida JC, Donovan L. Screening and diagnosing gestational diabetes mellitus. Evid Rep Technol Assess (Full Rep). 2012;1-327. [PubMed] |
| 5. | Jawad F, Ejaz K. Gestational diabetes mellitus in South Asia: Epidemiology. J Pak Med Assoc. 2016;66:S5-S7. [PubMed] |
| 6. | Szmuilowicz ED, Josefson JL, Metzger BE. Gestational Diabetes Mellitus. Endocrinol Metab Clin North Am. 2019;48:479-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 7. | Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The Pathophysiology of Gestational Diabetes Mellitus. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 817] [Cited by in RCA: 1021] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 8. | Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol Metab. 2018;29:743-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 519] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 9. | Rubin R, Khanna NR, McIver LA. Aspart Insulin. 2022 Nov 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 10. | Deepaklal MC, Joseph K, Rekha K, Nandita T. Insulin aspart in patients with gestational diabetes mellitus and pregestational diabetes mellitus. Indian J Endocrinol Metab. 2015;19:658-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Flory J, Lipska K. Metformin in 2019. JAMA. 2019;321:1926-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 323] [Article Influence: 53.8] [Reference Citation Analysis (1)] |
| 12. | Dodd JM, Grivell RM, Deussen AR, Hague WM. Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes. Cochrane Database Syst Rev. 2018;7:CD010564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Bashir M, Aboulfotouh M, Dabbous Z, Mokhtar M, Siddique M, Wahba R, Ibrahim A, Brich SA, Konje JC, Abou-Samra AB. Metformin-treated-GDM has lower risk of macrosomia compared to diet-treated GDM- a retrospective cohort study. J Matern Fetal Neonatal Med. 2020;33:2366-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Alfadhli EM. Gestational diabetes mellitus. Saudi Med J. 2015;36:399-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 15. | Chatzakis C, Cavoretto P, Sotiriadis A. Gestational Diabetes Mellitus Pharmacological Prevention and Treatment. Curr Pharm Des. 2021;27:3833-3840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Juan J, Yang H. Prevalence, Prevention, and Lifestyle Intervention of Gestational Diabetes Mellitus in China. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 17. | Homayouni A, Bagheri N, Mohammad-Alizadeh-Charandabi S, Kashani N, Mobaraki-Asl N, Mirghafurvand M, Asgharian H, Ansari F, Pourjafar H. Prevention of Gestational Diabetes Mellitus (GDM) and Probiotics: Mechanism of Action: A Review. Curr Diabetes Rev. 2020;16:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Sert UY, Ozgu-Erdinc AS. Gestational Diabetes Mellitus Screening and Diagnosis. Adv Exp Med Biol. 2021;1307:231-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377:e067946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 373] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 20. | Mistry SK, Das Gupta R, Alam S, Kaur K, Shamim AA, Puthussery S. Gestational diabetes mellitus (GDM) and adverse pregnancy outcome in South Asia: A systematic review. Endocrinol Diabetes Metab. 2021;4:e00285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 21. | Davis A, Kuriakose J, Clements JN. Faster Insulin Aspart: A New Bolus Option for Diabetes Mellitus. Clin Pharmacokinet. 2019;58:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Mu A, Chen Y, Lv Y, Wang W. Exercise-Diet Therapy Combined with Insulin Aspart Injection for the Treatment of Gestational Diabetes Mellitus: A Study on Clinical Effect and Its Impact. Comput Math Methods Med. 2022;2022:4882061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60:1586-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 364] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 24. | Wang W, Fan Y, Lin Q. Metformin combined with insulin aspart for ameliorating blood glucose levels and maternal and neonatal outcomes in women with gestational diabetes mellitus and chronic hypertension. Am J Transl Res. 2021;13:5596-5602. [PubMed] |
| 25. | Zhen XM, Li X, Chen C. Longer-term outcomes in offspring of GDM mothers treated with metformin versus insulin. Diabetes Res Clin Pract. 2018;144:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |