Published online Oct 15, 2023. doi: 10.4239/wjd.v14.i10.1478
Peer-review started: July 6, 2023
First decision: August 4, 2023
Revised: August 16, 2023
Accepted: September 14, 2023
Article in press: September 14, 2023
Published online: October 15, 2023
Processing time: 94 Days and 20.9 Hours
Diabetes mellitus is a kind of typical metabolic disorder characterized by elevated blood sugar levels. Atherosclerosis (AS) is one of the most common complications of diabetes. Modern lifestyles and trends that promote overconsumption and unhealthy practices have contributed to an increase in the annual incidence of diabetic AS worldwide, which has created a heavy burden on society. Several studies have shown the significant effects of glycolysis-related changes on the occurrence and development of diabetic AS, which may serve as novel thera-peutic targets for diabetic AS in the future. Glycolysis is an important metabolic pathway that generates energy in various cells of the blood vessel wall. In particular, it plays a vital role in the physiological and pathological activities of the three important cells, Endothelial cells, macrophages and vascular smooth muscle cells. There are lots of similar mechanisms underlying diabetic and common AS, the former is more complex. In this article, we describe the role and mechanism underlying glycolysis in diabetic AS, as well as the therapeutic targets, such as trained immunity, microRNAs, gut microbiota, and associated drugs, with the aim to provide some new perspectives and potentially feasible programs for the treatment of diabetic AS in the foreseeable future.
Core Tip: Diabetic atherosclerosis (AS) is becoming increasingly common today. Glycolysis, as a metabolic process that plays a significant role in its occurrence and development, has great potential to become an important therapeutic target in the future. We herein discuss the specific mechanisms of glycolysis in the development of diabetic AS and possible directions of therapeutic targets.
- Citation: Liu QJ, Yuan W, Yang P, Shao C. Role of glycolysis in diabetic atherosclerosis. World J Diabetes 2023; 14(10): 1478-1492
- URL: https://www.wjgnet.com/1948-9358/full/v14/i10/1478.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i10.1478
The global incidence and prevalence of diabetes are rapidly increasing. Notably, China, the most populous country in the world, bears the heaviest burden of diabetes[1]. A recent study revealed that in 2021, the global population of individuals living with diabetes was projected to reach 529 million, with an age-standardized prevalence of 6.1%. It is estimated that by 2050, 1.31 billion people will be diagnosed with diabetes worldwide[2]. Atherosclerosis (AS) is among the most common complications of diabetes, a well-established independent risk factor for AS[3]. AS is classified as a chronic inflammatory disease associated with complex etiopathogenesis. The disease originates from intimal lesions and is characterized by local lipid accumulation, fibrous tissue hyperplasia, and calcareous deposition with formation of plaques that reduce vessel elasticity and cause hardening of the vessel walls[4]. The disorder is referred to as AS owing to the yellowish appearance of lipids that accumulate in the arterial lining. AS may result in stroke, heart failure, coronary heart disease, and other serious cardiovascular complications[5].
AS develops earlier and progresses more rapidly in patients with diabetes than in the general population[6]. Compared with individuals without diabetes, those with diabetes show coronary plaques with typically larger necrotic cores and more pronounced inflammation, characterized by abundant macrophages[7]. The plaque load measured using the mean area and maximum wall thickness, is significantly higher in patients with diabetes than in those without diabetes, with a well-documented higher incidence of vascular calcification[8]. Diabetes-associated hyperglycemia (HG) disturbs vascular endothelial function, triggers inflammation, and promotes the formation of advanced glycosylation end-products (AGE) and a series of adverse effects[9,10]. Diabetes may also be associated with defective autophagy and destroys the internal homeostasis of smooth muscle cells (SMCs), leading to plaque expansion, core necrosis, and fibrous cap thinning, all of which favor plaque instability and increase the risk of plaque rupture[11]. Diabetic AS causes greater blood vessel damage, thereby emerging as the leading cause of disability and mortality in patients with diabetes globally[12]. Consequently, it gives rise to a substantial socioeconomic burden on society. Further research is warranted to gain deeper understanding of diabetic AS plaques, with the objective of exploring novel therapeutic approaches.
Endothelial cells (ECs), vascular SMCs (VSMCs), and macrophages play key roles in AS plaque formation, in which glycolysis is also an important contributor[13]. Abnormalities at any stage of the glycolytic pathway may promote AS. Numerous studies have investigated the mechanism underlying glycolysis in AS and plaque formation and described the effects of trained immunity, microRNAs, gut microbiota (GM), and other factors on glycolysis. Nevertheless, the comprehensive and precise mechanism of glycolysis in diabetic AS remains incompletely understood. The current findings provide novel concepts and potential strategies for targeted therapy of AS in the future. In this review, we summarize and discuss the role of glycolysis in the promotion, inhibition, and treatment of diabetic AS.
Glucose is the primary energy source for most body cells, serving as an essential substrate for various physiological and pathological activities. Glycolysis includes a series of biochemical reactions involved in the degradation of glucose by glycolytic enzymes, with the ultimate goal to produce pyruvate and adenosine triphosphate (ATP)[14]. Glycolysis is a common pathway in glucose metabolism that, under anaerobic conditions, culminates in the production of lactic acid, which subsequently enters the tricarboxylic acid cycle for oxidative phosphorylation when oxygen is available. However, these processes may differ under specific conditions. In the 1920s, the German scientist, Warburg, discovered significantly higher glycolytic activity in cancer cells than in normal cells[15]. Compared with the large amount of energy produced by mitochondrial oxidative phosphorylation, glycolysis generates limited amounts of energy. However, in contrast to normal cells, cancer cells rely on glycolysis for energy even under aerobic conditions, a phenomenon referred to as the Warburg effect[16]. Currently, a growing body of evidence suggests that aerobic glycolysis is not unique to tumor cells, and this phenomenon also significantly affects cells associated with AS[17,18].
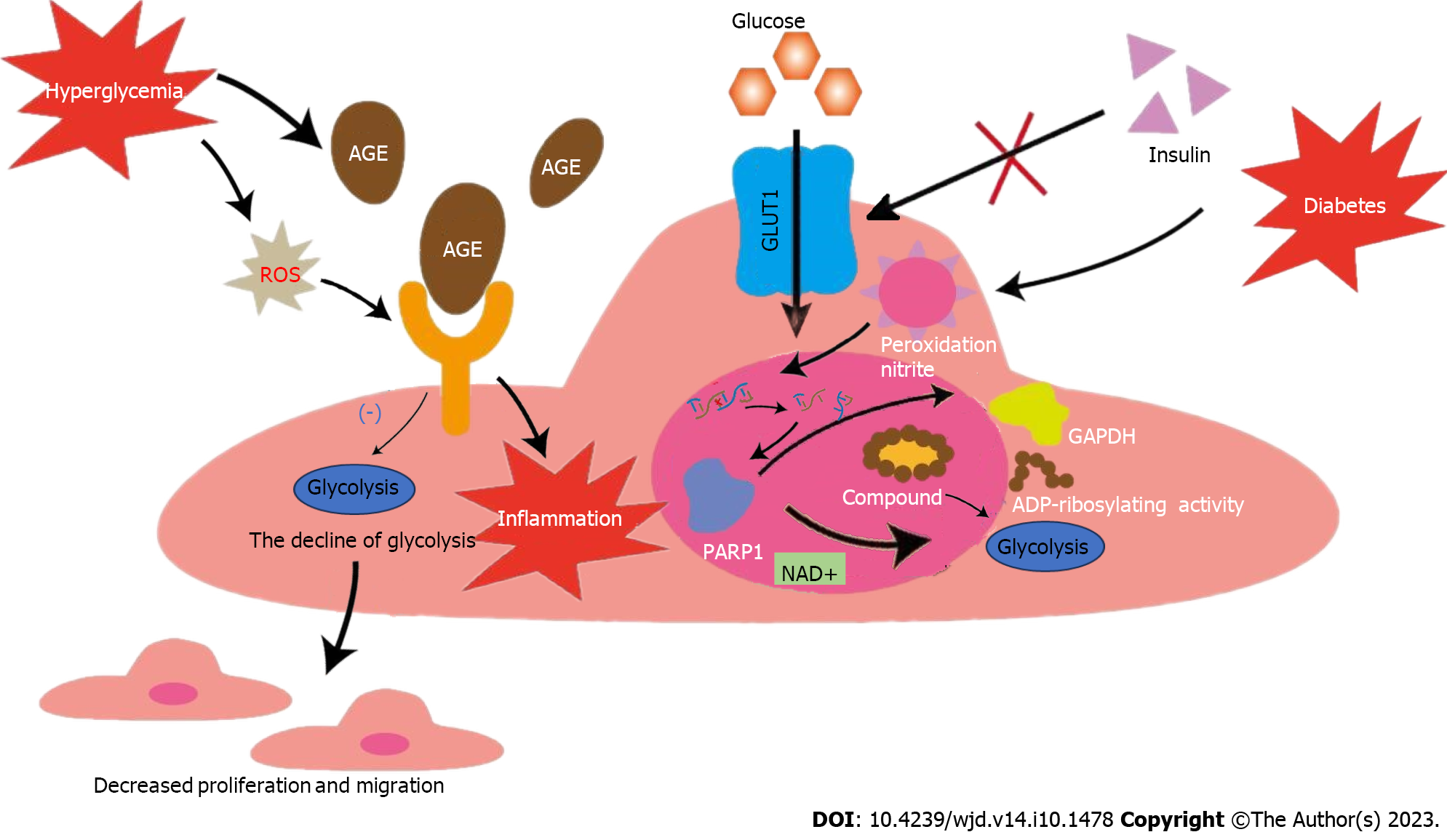
The process of AS plaque formation commences with EC injury. Although ECs are in close proximity to oxygenated blood and receive abundant oxygen compared with other cells, they primarily rely on glycolysis for energy[19]. Under physiological conditions, glycolysis provides 85% of the ATP required by the entire EC unit[20]. ECs depend on glycolysis for energy production based on the following features: (1) The mitochondrial content in ECs is too low to provide sufficient ATP through oxidative phosphorylation[21]; and (2) glycolysis is the source of energy for survival and maintenance of the cell itself because the ATP production rate during glycolysis is much higher than that during oxidative phosphorylation[22]. HG is an important sign of diabetes[23]. Glucose is transported into ECs via the glucose transporter 1 (GLUT-1), a receptor whose activity is regulated by extracellular glucose concentration independent of insulin[24,25]. ECs in patients with diabetes are therefore more vulnerable. An intact vascular barrier composed of quiescent ECs is essential to maintain vascular homeostasis[26], which is a favorable factor against AS. However, in the context of diabetes, HG can trigger overproduction of reactive oxygen species (ROS) in ECs, which disrupts the normal physiological state[27]. An increasing body of evidence shows that cardiovascular complications in diabetes mellitus occur secondary to an increase in nitrosative stress. Oxidative and nitrosative stress can lead to DNA injury, subsequently triggering the activation of the ribozyme, and poly polymerase 1 (PARP-1), and thus mediate the onset and progression of diabetic cardiovascular complications[28]. This ribozyme is a key enzyme involved in glycolysis in the nuclei of DNA-injured ECs[29]. PARP-1 not only slows glycolytic efficiency by facilitating NAD+ consumption but also promotes adenosine diphosphate (ADP) ribosylation of proteins. However, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity decreases after ADP ribosylation. Therefore, GAPDH entry into the nucleus to form a complex with nuclear proteins (ADP ribosylation) inhibits glycolysis. Glycolytic intermediates are also transferred to other pathways (including the hexosamine and polyol pathways). These changes lead to endothelial dysfunction in individuals with diabetes and worsens AS[30-32]. Additionally, non-enzymatic glycosylation of proteins or lipids in patients with diabetes leads to the production of AGE, which bind to the receptor for AGE (RAGE) on ECs and produce inflammation and dysfunction of ECs[33-35]. Human umbilical vein ECs (HUVECs) treated with AGE reportedly showed a decrease in glycolysis, which leads to the conclusion that AGE inhibits the migration and proliferation of ECs[36]. Moreover, HG-induced ROS was shown to increase the expression of RAGE and its pro-inflammatory endogenous ligands[37]. Additionally, enhanced endothelial superoxide production in patients with diabetes results in increased AGE accumulation[38]. These processes collectively lead to a vicious cycle of endothelial dysfunction. In summary, the hyperglycemic environment itself in people with diabetes, as well as the accompanying oxidative stress, AGEs and other adverse factors interfere with glycolysis, leading to ECs dysfunction. These changes eventually disrupt vascular homeostasis, which leads to AS in people with diabetes. Therefore, a series of internal environmental changes caused by diabetes-related HG may play an important role in diabetic AS by influencing glycolysis, which deserves further study.
However, every situation has dual implications. Inhibit glycolysis disrupt the normal physiological state of ECs, thus destroying vascular homeostasis. Nonetheless, in patients with diabetic AS, glycolysis inhibition could potentially reduce angiogenesis within atherosclerotic plaques and stabilize them to a certain extent. Energy metabolism of ECs, which is intricately associated with their germination, migration, and proliferation, is an important prerequisite for angiogenesis[39]. In patients with diabetes, HG can significantly alter EC metabolism, resulting in higher risk of pathological neovascularization in these cells than that in healthy cells under normal conditions[40]. Plaque rupture is a primary contributor to acute cardiovascular events. Many studies have shown that plaque angiogenesis promotes AS progression, particularly plaque instability[41]. Restricted oxygen diffusion coupled with activation of inflammatory mediators leads to plaque hypoxia, which eventually accelerates neovascularization[42]. Newly formed vessels are fragile and highly vulnerable to bleeding or leakage, resulting in increased plaque instability and rupture[43]. Excessive or abnormal neovascularization in plaques significantly increases capillary permeability and tissue edema. This, in turns results in a likelihood of bleeding or rupture of diabetic AS plaques[44]. In glycolysis, 6-phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFKFB3) is a key activator that provides active ATP and essential biosynthetic products for angiogenesis[45]. Based on these findings, previous studies have shown that the knockdown of PFKFB3-related genes in ECs can lead to defective angiogenesis, and the use of 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO, a PFKFB3 inhibitor) can reduce vascular germination via inhibiting EC proliferation and migration[46]. Notably, 3PO does not affect the glycolysis necessary for normal EC to maintain homeostasis but only reduces excess glycolysis required for EC germination[47]. Nonetheless, research has shown that 3PO can reduce T cell activation in vitro, which may adversely affect the body's immune suppressive function[48]. A recent study reported that partial inhibition of glycolysis prevented plaque angiogenesis without a particularly significant effect on the size, composition, and vulnerability of pre-existing plaques, although it reduced the frequency of plaque formation. The study also reported that 3PO-induced metabolic stress could stimulate autophagosome formation and promote autophagy in ECs[49]. Aging of ECs can lead to loss of function and transition to a pro-inflammatory state, which stimulates transformation of mononuclear macrophages into a pro-inflammatory phenotype[50]. The simultaneous increase in the expression of adhesion molecules in aging ECs accelerates macrophage migration and activation in plaques[51]. The aforementioned factors act synergistically to promote AS plaque progression. The previously described autophagic response can effectively inhibit EC senescence and apoptosis, thereby limiting plaque formation and development. Additionally, another study has shown that PFKFB-3 not only promotes glycolysis, but also directly participates in glycolytic-dependent DNA repair of diabetic ECs under oxidative stress damage, which may play a certain role in vascular protection of diabetes[52]. Therefore, the use of the glycolytic inhibitor 3PO may disrupt this protective effect, making the application of glycolytic inhibitors uncertain. In conclusion, although a substantial body of evidence suggests that targeting glycolysis inhibition can effectively improve plaque stability in patients with diabetic AS and prevent plaque rupture and bleeding by limiting EC metabolism and preventing plaque neovascularization, the safety and efficacy of this approach require further elucidation and warrant further research (Figure 1).
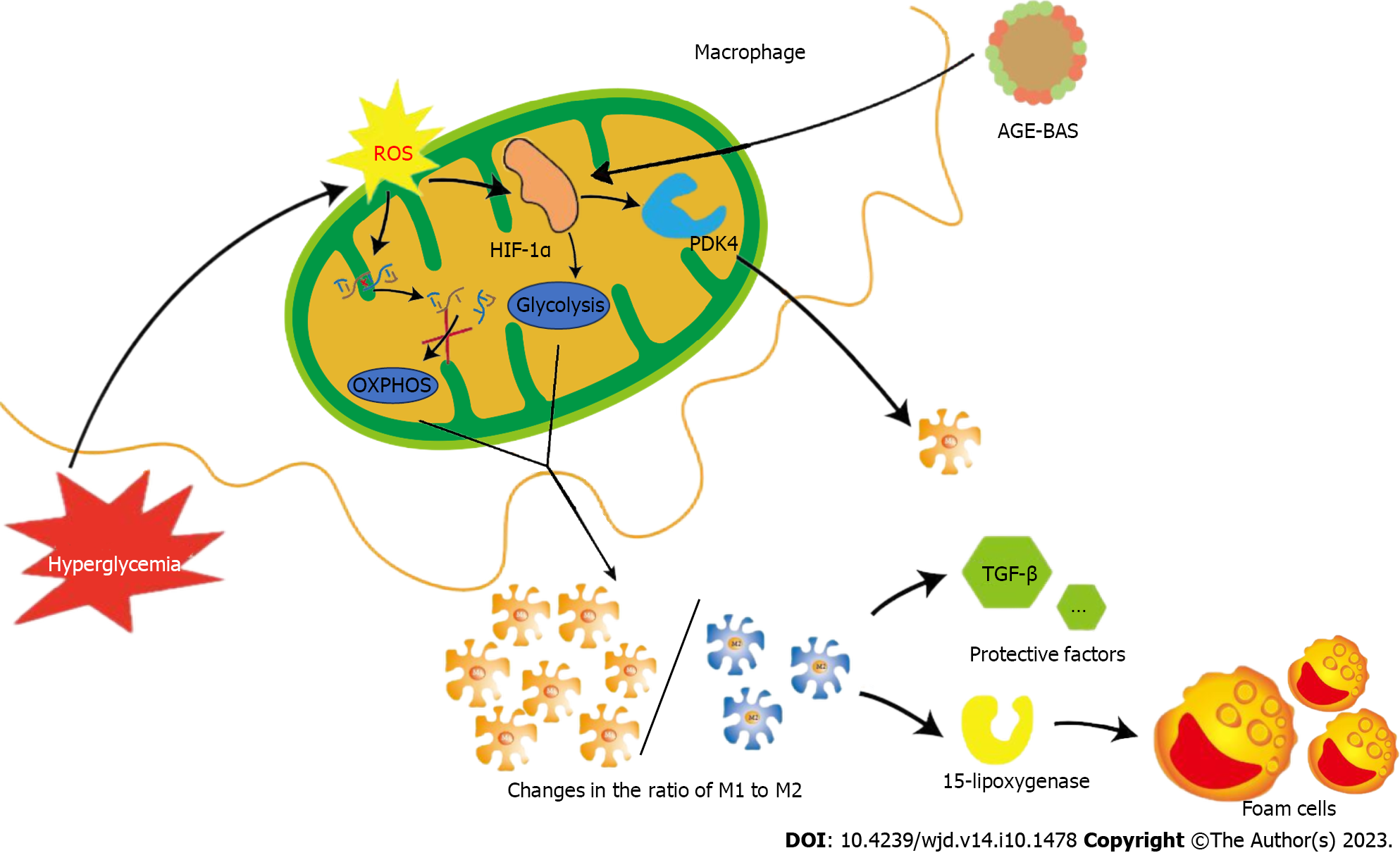
Macrophage polarization is a typical phenomenon associated with AS[53]. M1 and M2 constitute the common macrophage phenotypes. M1 macrophages show a pro-inflammatory phenotype and play a major role in unstable plaques[54], they are more commonly observed in the shoulder area of unstable and vulnerable-to-rupture plaques[55]. M2 macrophages show an anti-inflammatory phenotype and are more commonly expressed in stable plaques[56]. In contrast, M2 cells play a key role in the occurrence and progression of AS and maintain the stability of AS plaques. Notably, 15-lipoxygenase expressed by M2 macrophages is closely associated with the formation of foam cells and actively promotes AS plaque formation and progression. Simultaneously, M2 macrophages secrete various anti-inflammatory factors, such as transforming growth factor-β, which protects against AS[57]. Usually, the ratio of M1 to M2 cells plays an important role in AS plaque progression[58]. Diabetes-induced HG can enhance glucose metabolism and inflammatory response in macrophages[59]. ROS produced by macrophages under oxidative stress conditions can injure mitochondrial DNA, leading to mitochondrial dysfunction and inhibiting oxidative phosphorylation. Simultaneously, ROS induces sustained expression of hypoxia-inducible factor 1α (HIF-1α), consequently promoting glycolysis[60]. The combination of the aforementioned factors eventually stimulates M1 and suppresses M2 activation[61], which favors plaque instability. Moreover, local intraplaque hypoxia can trigger a similar response via the HIF-1α pathway[62]. These changes in macrophage energy metabolism interfere with stable lipid metabolism and significantly increase the intracellular lipid content[63], which promotes transformation of macrophages into foam cells that participate in AS plaque formation. The production of large amounts of AGE is a typical feature of patients with diabetes. HIF-1α is up-regulated in AGE-bovine serum albumin (BAS)-induced M1 polarization, and HIF-1α knockdown reduces AGE-BAS-induced M1 polarization via regulation of pyruvate dehydrogenase kinase 4[64]. This study further highlighted the association between glucose energy metabolism in macrophages and diabetes-related inflammation. A recent study comparing macrophages in diabetic and non-diabetic mice found that glucose uptake and glycolysis were not increased but rather decreased in the former group[65]. Although it is premature to conclude whether these results were coincidental or influenced by some interfering factors, this finding challenges the notion that diabetes-induced HG directly promotes glucose metabolism in macrophages. Recently, other researchers have suggested that HG is not the only, or even the most important, factor in diabetes-related cardiovascular disease[66]. Diabetes is an extremely complex background, so the conditions to prove this point are very demanding. However, we still believe that this finding is worthy of further verification, which has important significance for us to understand the specific mechanism of glycolysis in diabetic AS and explore related treatment methods. Nevertheless, changes in glycolysis significantly affect macrophage energy metabolism and polarization in diabetic AS and therefore plays a crucial role in the development of diabetic AS and plaque stabilization (Figure 2).
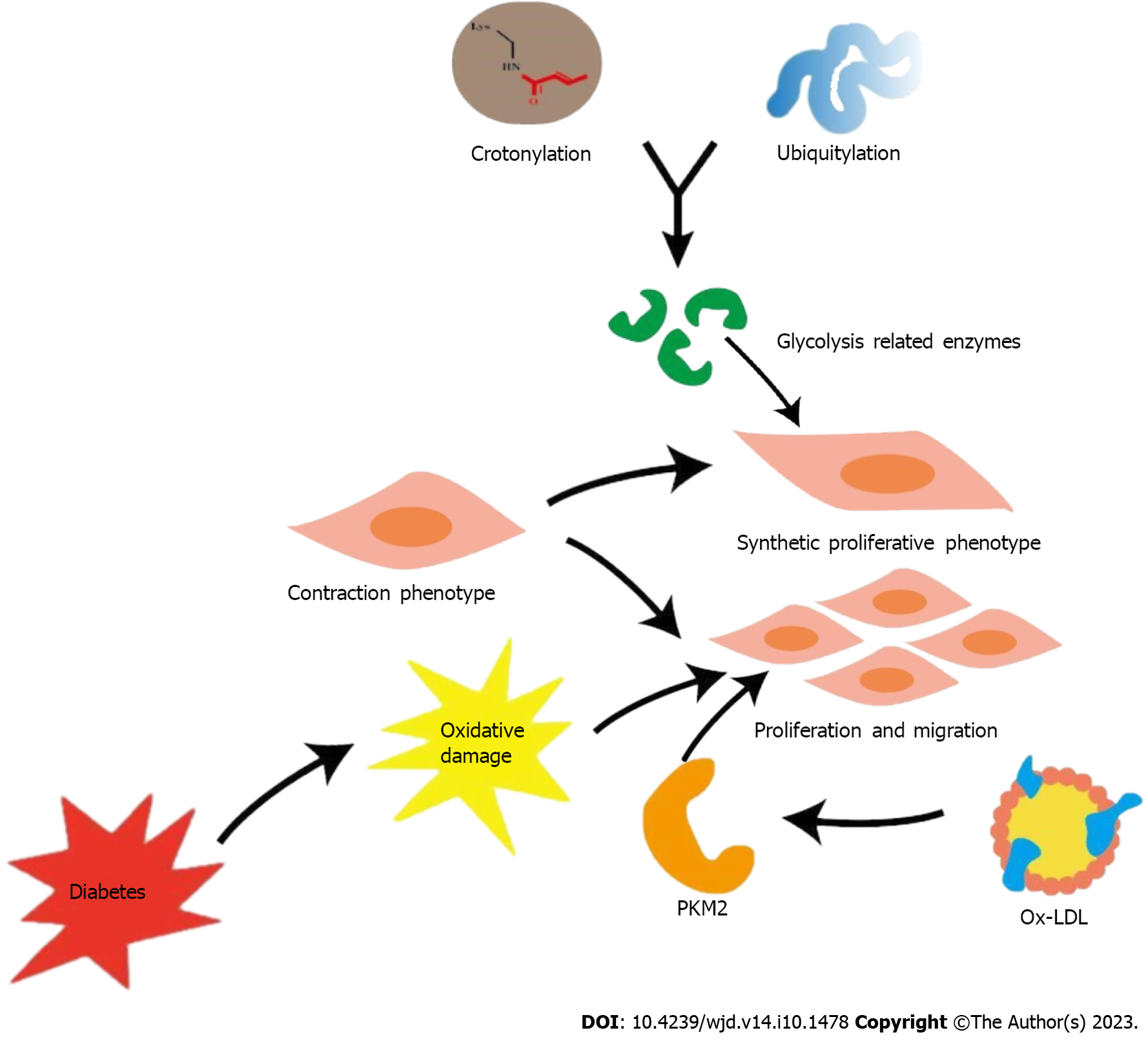
Proliferation and subintimal migration of VSMCs is a key event in AS formation[67]. VSMCs, which are viewed as plaque stabilizers, proliferate and migrate to plaque fibrous caps and produce collagen and extracellular matrix, which contribute to plaque stability[68,69]. Enhanced glycolysis in VSMCs is important for platelet-derived growth factor-induced VSMC proliferation and migration, which is an important contributor to AS[70,71]. In the context of diabetes, VSMC proliferation secondary to oxidative injury is a primary process that accelerates AS progression[72]. Intracellular transportation of low-density lipoprotein (LDL), followed by subintimal oxidation, results in the formation of oxidized LDL (ox-LDL)[73]. Ox-LDL significantly influences VSMC proliferation and migration and, through this effect, contributes to plaque vulnerability and AS progression[74,75]. Pyruvate kinase subtype M2 (PKM2) is a key rate-limiting enzyme involved in glycolysis. Ox-LDL up-regulates PKM2-dependent glycolysis to trigger VSMC proliferation and migration and eventually promotes AS[76]. Additionally, AS vascular remodeling is accompanied by a shift in the VSMC phenotype from a contractile phenotype under normal physiological conditions to a synthetic proliferative phenotype under pathological conditions[77]. Furthermore, inflammatory stimulation in a diabetic environment with high glucose levels may also promote such phenotypic transformation of VSMCs[78]. A significant percentage of enzymes involved in glycolysis are crotonylated and ubiquitinated. Some researchers have suggested that the crosstalk between crotonylation and ubiquitination in glycolysis may be a potential mechanism underlying the phenotypic remodeling of VSMCs[79]. The results of a study on SMC-specific PKM2-deficient mouse model support the hypothesis that SMC-derived PKM2 promotes injury-induced neointimal hyperplasia via enhanced phenotypic conversion, proliferation, and migration from a genetic perspective[80]. However, the specific role of glycolysis-induced metabolic mechanisms in phenotypic switching of VSMCs remains unclear and requires further investigation. Nonetheless, glycolysis significantly affects VSMC participation in diabetic AS development (Figure 3).
Earlier researchers held the belief that only adaptive immunity can establish immune memory. However, following extensive experimental studies have contradicted this perspective[81]. Innate immune cells produce immune memory following antigenic stimulation and tend to respond more strongly to reinfection in a nonspecific manner, a phenomenon termed as trained immunity[82]. Although the long-term activation of trained immune system strengthens the body’s ability to fight infection, it also negatively affects chronic inflammation[83].
AS is a typical chronic low-grade vascular inflammatory disease; therefore, trained immunity has a major role in this disorder. β-glucan–induced trained immunity is a typical model[84]. In vitro studies using monocytes trained with dextran have shown that AKt-mTOR-HIF-1α signaling pathway is the metabolic basis underlying trained immunity[85]. Another study has also shown that the transition from oxidative phosphorylation to a significant increase in aerobic glycolysis was the driving force for the development of trained immunophenotype[86]. These changes in cellular metabolism observed during the induction of trained immunity constitute metabolic reprogramming, which can induce epigenetic remodeling and chromatin structure changes (such as methylation or an increase in mRNA of the key enzymes involved in glycolysis)[81]. Ultimately, these changes increase transcription of inflammatory genes, triggering the immune response (these genes encode not only cytokines and chemokines associated with AS but also proteins associated with foam cells and plaque vulnerability)[87]. Another study observed that shear stress at AS sites throughout the vasculature shows similar effects of glycolytic up-regulation of histone modifications and signaling pathways[88], which reiterates the tight association between trained immunity and AS. Moreover, the HG environment in patients with diabetes induces long-term epigenetic regulation of inflammatory genes[89]. Corresponding research suggests that hypoglycemic therapy for patients with diabetes is likely to be affected by these findings, potentially reducing its efficacy and subsequently, increasing the risk of AS[90]. Glycolysis provides the energy for trained immunity, serving as its dynamic foundation. Thus, changes in glycolysis profoundly influence the role of trained immunity on diabetic AS.
Glycolysis forms the metabolic basis of both ECs and trained immunity, thereby implying a plausible correlation between the two. Atherogenic factors induce trained immunity in ECs via oxidative phosphorylation, glycolytic metabolic transformation, epigenetic modification of pro-inflammatory genes, and activation of the Akt-mTOR-HIF-1a signaling pathway[91]. Ox-LDL, a lipid that promotes AS, drives the production of innate immune cells and trained immune phenotypes in ECs[92]. Reportedly, training monocytes with this substance can also induce trained immunity, accompanied by a significant increase in glycolysis[93]. Additionally, trained immune phenotype is reversed after treatment with mammalian target of rapamycin (mTOR) inhibitors, glycolytic inhibitor 3, or the HIF-1a inhibitor following Ox-LDL exposure[85]. Therefore, it is conceivable to speculate that the reversal of trained immunity using glycolysis inhibitors or genes involved in glycolysis knockout may be a potential therapeutic option for AS.
To summarize, the relationship between trained immunity and glycolysis provides a new perspective on the treatment of diabetic AS. However, most current studies on trained immunity have been performed in vitro or in animal experimental models. Available and clinical data are insufficient to establish definitive conclusions[84]. Therefore, the clinical efficacy of these methods remains a potential research topic requiring further investigation.
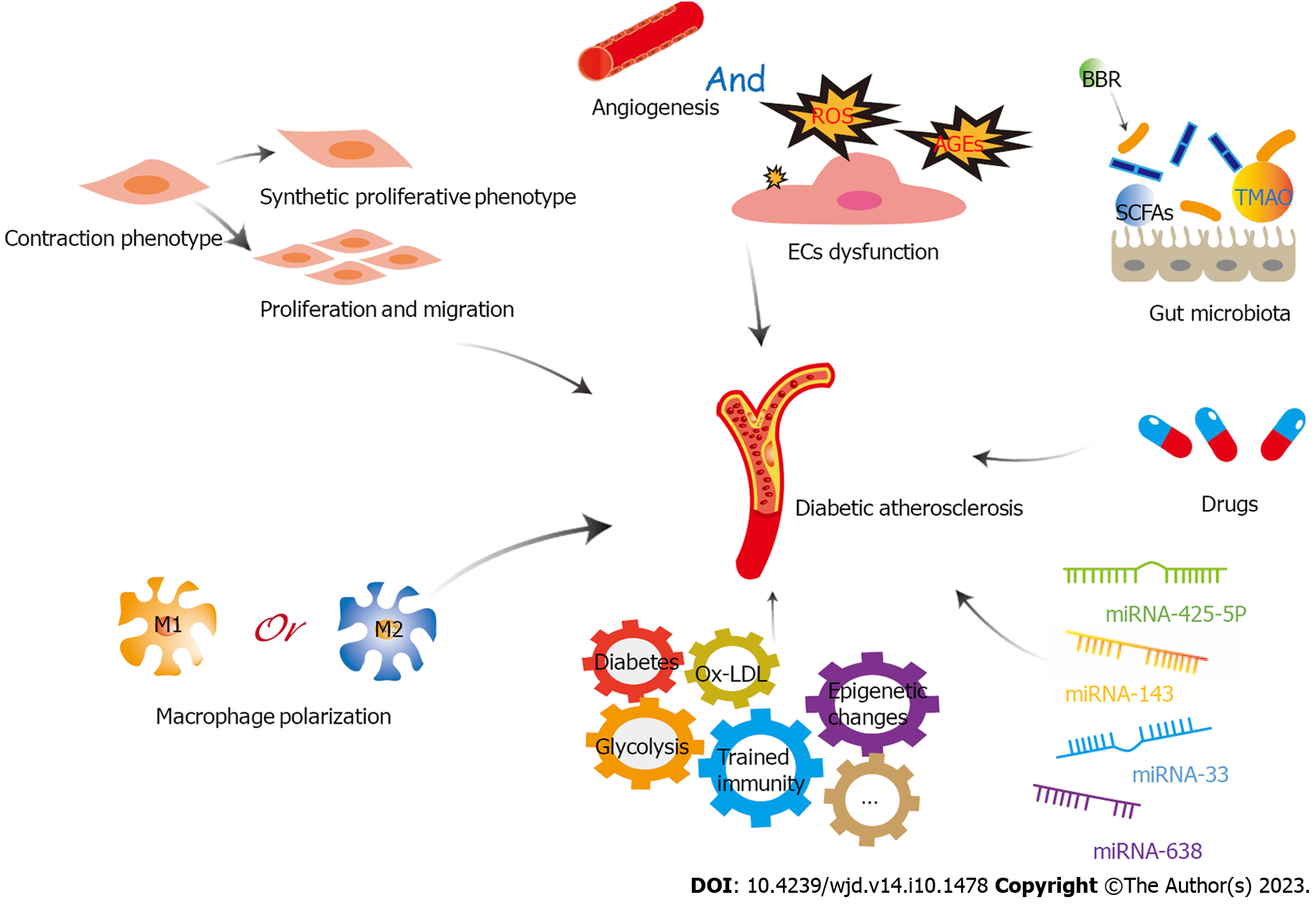
MicroRNAs (miRNAs) are a class of non-coding single-stranded RNA encoded by endogenous genes, approximately 22 nucleotides in length[94]. Their role as regulators of gene expression has long been of interest to researchers. An increasing number of recent studies have highlighted the role of miRNAs in AS and its progression. The NF-KB/miRNA-425-5P/MCT4 signaling axis can down-regulate the expression of monocarboxylate transporters (MCT4) within HUVECs derived from patients with diabetes, as well as HUVECs subjected to high glucose levels and interleukin (IL)-1β. This process leads to impaired lactate transport, thereby inducing EC dysfunction and even apoptosis[95]. The study discussed the mechanism underlying endothelial vascular injury in diabetes mellitus from a new perspective of glycolysis and lactate transport disorders. Therefore, inhibition of miRNA-425-5P expression and improvement in ECs may be a potential strategy for diabetic AS treatment. Another study on HUVECs showed that miR-143 inhibited glycolysis by directly targeting hexokinase 2 (HK2), leading to endothelial dysfunction and an increased risk of AS[96]. Therefore, down-regulation of the miR-143 Level to restore HK2 expression and restoration of the balance of glycolysis in EC are necessary for the prevention and treatment of AS plaques. miR-638 can inhibit proliferation, migration, and glycolysis of VSMCs by targeting lactate dehydrogenase A (LDHA)[97]. It is plausible to speculate that miR-638 can effectively inhibit the development and progression of AS plaques by targeting LDHA and inhibiting glycolysis in VSMCs. M1-type macrophages use aerobic glycolysis to rapidly generate energy[98]. miR-33 regulates the inflammatory phenotypic polarization program of macrophages via alteration of the balance between cellular fatty acid oxidation and glycolysis, which affects AS plaque progression. Anti-miR-33 was also found to exert a protective effect against AS[99], suggesting the potential value of therapeutically silencing miR-33 in the context of AS. Through the regulation of gene expression, such as down-regulating the amount of certain glycolytic enzymes or inhibiting their activity, miRNA significantly affects glycolysis, playing a non-negligible role in the occurrence and development of diabetic AS. Thus, targeting miRNA to regulate glycolysis, may potentially be an important therapeutic approach for treating diabetic AS.
GM refers to the diverse microorganisms, including bacteria and fungi that colonize the gastrointestinal tract of humans and other animals including insects. Microbiota participate in the synthesis of various bioactive substances, which play key roles in human health and diseases[100,101]. GM-derived metabolites transmit signals for effective communication between the host and microbiota and are indispensable mediators in several important reactions[102]. Several metabolic diseases including diabetes are attributable to a dysfunctional gut microbiome[103]. Bacterial DNA is shared between the gut and AS plaques[104]. With regard to the microbial composition of unstable and stable plaques, the feces of patients with unstable plaques show a decrease in the Roseburiam species[105]. AS plaque and its stability may be closely associated with the human GM. Additionally, transplantation of GM affects the host’s susceptibility to AS[106]. In conclusion, various phenomena suggest that GM and its metabolites are intricately and closely associated with diabetic AS.
Trimethylamine-N-oxide (TMAO) is mainly derived from the metabolism of methylamine-rich nutrients by the GM. TMA, produced and processed in the liver in the presence of flavin monooxygenase, results in the generation of TMAO[107]. Experimental and clinical studies have reported the role of TMAO in the etiology of diabetes[108], and TMAO has also gained increasing attention as a contributor to AS. High blood TMAO concentrations can promote cholesterol transport in macrophages for the formation of foam cells, eventually causing AS[109]. The amount of Bacteroides in the human intestine was positively correlated with plasma TMA concentrations[110].
Metagenomic analysis of the GM has revealed that the percentage of Bacteroidetes was significantly higher in patients with symptomatic AS than in controls[111]. Notably, plaques in patients with high levels of TMAO tend to show thinner fibrous caps and a greater number of microvessels[112]. The association between elevated TMAO plasma levels and unstable AS plaques is readily evident. This notion has been supported by an animal study[113], and further research underscores that high TMAO plasma levels can increase pro-inflammatory gene expression, consequently leading to a marked increase in inflammatory cytokines levels, adhesion molecules, and chemokines[114]. In summary, TMAO produces adverse effects in AS. The NLP3 is a multiprotein complex formed by pattern recognition receptor activation[115]. Studies performed using carotid ECs in mice with partially ligated carotid arteries have reported TMAO-induced NLP3 inflammasome activation, which increased IL-1B levels, caspase-1 activity, and cell permeability, subsequently leading to EC injury in AS[116]. Among these, caspase-1 activation was shown to play a vital role in mitochondrial injury and proteolytic cleavage of glycolytic enzymes[117]. Some studies have also shown that glycolytic changes are closely associated with NLRP3 activation[118]. Therefore, we hypothesize that intervening in glycolysis may, to some extent, counteract the adverse effects of TMAO in diabetic AS.
Butyrate, a short-chain fatty acid (SCFA), is another product of the GM[119] that plays a key role in the regulation of cellular energy metabolism, particularly glycolysis. For example, butyrate can cross-link with T cell receptors to switch cells from mitochondrial respiration to glycolysis during T cell activation[120]; butyrate can inhibit glucose transport and glycolysis by reducing GLUT1 and cytoplasmic glucose-6-phosphate dehydrogenase abundance regulated by GPR109A-AKT signaling in colorectal cancer cells[121]. Patients with diabetes have lower levels of butyrate-producing bacteria than those without diabetes[122]. Some researchers argue that the effects of butyrate-producing bacteria on AS are attributed to the metabolic effect of butyrate in the intestines. They showed that gut-associated butyrate-producing bacteria interact with dietary plant polysaccharides and affect gene expression of distal intestinal cells with a switch from glycolytic metabolism toward fatty acid utilization, which consequently reduces systemic inflammation and improves AS[123]. In addition, studies have shown that the use of live B. vulgatus and B. dorei can also help suppress the pro-inflammatory immune response to prevent coronary AS. However, the specific mechanism and whether glycolysis is involved have not been clarified, which also deserves further study[124]. As a result, the concept of supplementing anti-atherosclerotic bacteria for patients with diabetes hold promise as a topic worthy of study. This approach may contribute to the treatment of diabetic AS through glycolysis regulation.
Many studies have reported good efficacy of berberine (BBR), an isoquinoline alkaloid extracted from herbs, including Coptis coptidis, in the treatment of metabolic and cardiovascular diseases[125,126]. BBR can simultaneously regulate insulin signaling, inhibit A-glucosidase, induce glycolysis, and inhibit gluconeogenesis; therefore, it plays a significant role in reducing blood glucose levels in diabetes[127,128]. Following the upsurge in GM research, the effect of BBR on GM has attracted widespread attention. BBR can cause changes in more than 20 genera in DB/DB mice (C57BLKS/JNju, an animal model of type 2 diabetes). Notably, significant alterations were observed in the expression of seven operational taxonomic units, including increased prevalence of a series of SCFA-producing bacteria[129]. For example, Butyricimonas promotes glycolysis of branched-chain amino acids to produce SCFA as a source of energy[130]. Effects of BBR on AS are also closely associated with GM activity. A comparison between BBR-treated and untreated mice fed the same high-fat diet showed significant differences in the abundance of Firmicutes and Verrucobacteria; the BBR-treated mice showed reduced expression of inflammatory factors and a lower incidence of AS[131]. Other studies also support the role of BBR in inhibition or destruction of some harmful intestinal bacteria and in increasing the numbers of bacteria that reduce TMAO concentration and AS plaque size[132]. In conclusion, BBR not only has a significant effect on the treatment of diabetes through inducing glycolysis but also the capacity for the prevention and treatment of AS by affecting the GM.
Hence, it is reasonable to conclude that glycolysis metabolism-related therapies, such as regulating GM through probiotic supplements or fecal transplantation[133], have a huge potential for future treatments of diabetes AS.
Drugs constitute an important component of the therapeutic arsenal against diabetic AS. Many researchers have emphasized the rapid development of drugs from the perspective of glycolysis. Ethyl pyruvate is a stable pyruvate derivative[134], that has been shown to provide energy for the differentiation of regulatory T cells (Tregs) and enhance their proliferation via the up-regulation of glycolysis, which increases the number and function of Tregs and improves the clinical symptoms of type 1 diabetes in mice[135]. Furthermore, that aspalathin and its associated compounds can specifically inhibit sirtuin 6 at a certain concentration, improve insulin-mediated activation of AKT, enhance glycolysis, and inhibit gluconeogenesis through the activation of non-repressor protein 5 and peroxisome proliferator-activated receptor-γ coactivator. Maintaining glucose homeostasis is important in patients with type 2 diabetes mellitus[136]. Diabetes is the root cause of a more severe and complex symptoms of diabetic AS; therefore, improving diabetes is fundamental to the treatment of diabetic AS.
Rapamycin, a classic glycolysis inhibitor, has pharmacological effects that can inhibit the mTOR pathway and suppress cell glycolysis in addition to its anti-inflammatory and antiproliferative actions; it can also effectively inhibit AS progression[137]. Polylactic acid glycolic acid copolymers coated with rapamycin can target AS plaques in mice and may locally deliver glycolysis inhibitors. This method has been shown to be safe in vitro[138]. Therefore, it can be concluded that nanoparticles coated with glycolytic inhibitors on macrophage membrane coating may be useful for precise treatment through targeted inhibition of AS. Several studies have shown that glutamine antagonists down-regulate mTORC1 activity and attenuate the up-regulation of glycolysis in response to growth factor stimulation and effectively control vascular restenosis caused by excessive VSCM proliferation[139,140]. Therefore, glutamine antagonists may be useful for the treatment of AS and AS-induced vascular occlusive disease. Recent studies have demonstrated a positive correlation between PFKFB3 expression and unstable plaque phenotypes in human coronary and carotid arteries. Mice in which the glycolysis inhibitor, PFK158, repressed PFKFB3 showed improved AS plaque stability and fibrous cap thickening[141]. AS plaque progression is closely associated with the influx of specific immune cells[142]; therefore, inhibiting PFKFB3 also reduces the glycolysis flux of immune cells, effectively inhibits their metabolism and further minimizes AS progression[141]. Therefore, PFK158 may be important for AS plaque stabilization and reducing the risk of AS development.
In conclusion, targeting both diabetes and AS can effectively reduce the risk of acute cardiovascular events secondary to diabetic AS. Furthermore, this approach can also counteract the role of a major risk factor for diabetic AS in the long term, which can facilitate better treatment of diabetic AS (Figure 4).
With the global incidence of diabetes increasing year by year, diabetic AS is a growing threat to society. The mechanisms of diabetic AS are highly intricate, and this paper aims to stimulate interest in the emerging field hat delves into the roles of glycolysis in the occurrence and development of diabetic AS. Glycolysis is the metabolic basis of main cells involved in the development of diabetic AS, including ECs, macrophages, and VSMCs. Glycolysis plays an important role in diabetic AS by influencing the functional status of ECs, polarization of macrophages, and proliferation, migration and phenotypic transformation of VSMCs. Therefore, glycolysis should be considered as a potential target for treating diabetic AS. However, it must be acknowledged that, in comparison with the study of glycolysis in cancer treatment, the study concerning the role of glycolysis in diabetic AS does not go that far. Nonetheless, as the significant metabolic mechanism shared by cancer and AS cells, the role of glycolysis in cancer cells also has a considerable reference significance AS cells. As mentioned above, butyrate produced by intestinal flora can inhibit glycolysis in colon cancer cells, providing an important foundation for research on the role of butyrate-producing bacteria in AS. Therefore, in-depth exploration of GM, trained immunity, miRNA, and drugs, which play important roles in the regulation of tumor glycolysis, is also expected to significantly improve our research on diabetic AS glycolysis. HG is the main reason for the abnormal function of vascular ECs and the polarization of macrophages due to the change of energy metabolism, causing AS. However, many scholars argue that HG is not a single factor that affects glycolysis in diabetic AS. We believe that diabetes products such as AGEs and the inflammatory environment caused by diabetes may be involved in this effect. However, the specific influencing mechanism requires further research. In addition, most studies on the role of glycolysis in diabetic AS remain in animal or cellular models at present. Thus, there is a need to bridge the gap between results of research and their clinical applications. Nevertheless, we firmly believe that the that regulation of glycolysis is a potentially promising therapeutic strategy for diabetic AS in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emran TB, Bangladesh; Nawab M, India; Horowitz M, Australia S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317:2515-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1356] [Article Influence: 169.5] [Reference Citation Analysis (0)] |
| 2. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1683] [Cited by in RCA: 1744] [Article Influence: 872.0] [Reference Citation Analysis (18)] |
| 3. | Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, Cameron AJ, Dwyer T, Taylor HR, Tonkin AM, Wong TY, McNeil J, Shaw JE. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 518] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 4. | Kobiyama K, Ley K. Atherosclerosis. Circ Res. 2018;123:1118-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 391] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 5. | Huang D, Refaat M, Mohammedi K, Jayyousi A, Al Suwaidi J, Abi Khalil C. Macrovascular Complications in Patients with Diabetes and Prediabetes. Biomed Res Int. 2017;2017:7839101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 6. | Gärtner V, Eigentler TK. Pathogenesis of diabetic macro- and microangiopathy. Clin Nephrol. 2008;70:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, Virmani R. Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 2017;37:191-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 377] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 8. | Gao X, Song J, Watase H, Hippe DS, Zhao X, Canton G, Tian F, Du R, Ji S, Yuan C; CARE-II Investigators. Differences in Carotid Plaques Between Symptomatic Patients With and Without Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 2019;39:1234-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Domingueti CP, Dusse LM, Carvalho Md, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 455] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 10. | Ping S, Liu S, Zhou Y, Li Z, Li Y, Liu K, Bardeesi AS, Wang L, Chen J, Deng L, Wang J, Wang H, Chen D, Zhang Z, Sheng P, Li C. Protein disulfide isomerase-mediated apoptosis and proliferation of vascular smooth muscle cells induced by mechanical stress and advanced glycosylation end products result in diabetic mouse vein graft atherosclerosis. Cell Death Dis. 2017;8:e2818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Masuyama A, Mita T, Azuma K, Osonoi Y, Nakajima K, Goto H, Nishida Y, Miyatsuka T, Mitsumata M, Watada H. Defective autophagy in vascular smooth muscle cells enhances atherosclerotic plaque instability. Biochem Biophys Res Commun. 2018;505:1141-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and Risk of Amputation in Patients With Diabetes Mellitus and Peripheral Artery Disease. Arterioscler Thromb Vasc Biol. 2020;40:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 303] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 13. | Matsuura Y, Yamashita A, Zhao Y, Iwakiri T, Yamasaki K, Sugita C, Koshimoto C, Kitamura K, Kawai K, Tamaki N, Zhao S, Kuge Y, Asada Y. Altered glucose metabolism and hypoxic response in alloxan-induced diabetic atherosclerosis in rabbits. PLoS One. 2017;12:e0175976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Falkenberg KD, Rohlenova K, Luo Y, Carmeliet P. The metabolic engine of endothelial cells. Nat Metab. 2019;1:937-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Urbano AM. Otto Warburg: The journey towards the seminal discovery of tumor cell bioenergetic reprogramming. Biochim Biophys Acta Mol Basis Dis. 2021;1867:165965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11782] [Article Influence: 736.4] [Reference Citation Analysis (0)] |
| 17. | Stienstra R, Netea-Maier RT, Riksen NP, Joosten LAB, Netea MG. Specific and Complex Reprogramming of Cellular Metabolism in Myeloid Cells during Innate Immune Responses. Cell Metab. 2017;26:142-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 18. | Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19:526-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 395] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 19. | Goveia J, Stapor P, Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol Med. 2014;6:1105-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 20. | De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1137] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 21. | Du W, Ren L, Hamblin MH, Fan Y. Endothelial Cell Glucose Metabolism and Angiogenesis. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Wang R, Wang M, Ye J, Sun G, Sun X. Mechanism overview and target mining of atherosclerosis: Endothelial cell injury in atherosclerosis is regulated by glycolysis (Review). Int J Mol Med. 2021;47:65-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Knapp M, Tu X, Wu R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol Sin. 2019;40:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 24. | Mandarino LJ, Finlayson J, Hassell JR. High glucose downregulates glucose transport activity in retinal capillary pericytes but not endothelial cells. Invest Ophthalmol Vis Sci. 1994;35:964-972. [PubMed] |
| 25. | Kaiser N, Sasson S, Feener EP, Boukobza-Vardi N, Higashi S, Moller DE, Davidheiser S, Przybylski RJ, King GL. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993;42:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 196] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Gimbrone MA Jr, García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res. 2016;118:620-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1448] [Cited by in RCA: 2346] [Article Influence: 260.7] [Reference Citation Analysis (0)] |
| 27. | Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ Res. 2018;122:877-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1347] [Article Influence: 192.4] [Reference Citation Analysis (0)] |
| 28. | Pacher P, Obrosova IG, Mabley JG, Szabó C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Garcia Soriano F, Virág L, Jagtap P, Szabó E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabó C. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 453] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 30. | Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 504] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 31. | Hopp AK, Grüter P, Hottiger MO. Regulation of Glucose Metabolism by NAD(+) and ADP-Ribosylation. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Pacher P, Szabó C. Role of poly(ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid Redox Signal. 2005;7:1568-1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Stitt AW, Li YM, Gardiner TA, Bucala R, Archer DB, Vlassara H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am J Pathol. 1997;150:523-531. [PubMed] |
| 34. | Chavakis T, Bierhaus A, Nawroth PP. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 2004;6:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 35. | Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507-1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 472] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 36. | Li Y, Chang Y, Ye N, Chen Y, Zhang N, Sun Y. Advanced glycation end productsinduced mitochondrial energy metabolism dysfunction alters proliferation of human umbilical vein endothelial cells. Mol Med Rep. 2017;15:2673-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 38. | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3956] [Cited by in RCA: 3617] [Article Influence: 241.1] [Reference Citation Analysis (0)] |
| 39. | Perrotta P, de Vries MR, Peeters B, Guns PJ, De Meyer GRY, Quax PHA, Martinet W. PFKFB3 gene deletion in endothelial cells inhibits intraplaque angiogenesis and lesion formation in a murine model of venous bypass grafting. Angiogenesis. 2022;25:129-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Wang L, Liu WX, Huang XG. MicroRNA-199a-3p inhibits angiogenesis by targeting the VEGF/PI3K/AKT signalling pathway in an in vitro model of diabetic retinopathy. Exp Mol Pathol. 2020;116:104488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | Perrotta P, Emini Veseli B, Van der Veken B, Roth L, Martinet W, De Meyer GRY. Pharmacological strategies to inhibit intra-plaque angiogenesis in atherosclerosis. Vascul Pharmacol. 2019;112:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218:7-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 267] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 43. | Owusu J, Barrett E. Early Microvascular Dysfunction: Is the Vasa Vasorum a "Missing Link" in Insulin Resistance and Atherosclerosis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Madonna R, Pieragostino D, Balistreri CR, Rossi C, Geng YJ, Del Boccio P, De Caterina R. Diabetic macroangiopathy: Pathogenetic insights and novel therapeutic approaches with focus on high glucose-mediated vascular damage. Vascul Pharmacol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Li X, Kumar A, Carmeliet P. Metabolic Pathways Fueling the Endothelial Cell Drive. Annu Rev Physiol. 2019;81:483-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 46. | Xu Y, An X, Guo X, Habtetsion TG, Wang Y, Xu X, Kandala S, Li Q, Li H, Zhang C, Caldwell RB, Fulton DJ, Su Y, Hoda MN, Zhou G, Wu C, Huo Y. Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler Thromb Vasc Biol. 2014;34:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 47. | Bousseau S, Vergori L, Soleti R, Lenaers G, Martinez MC, Andriantsitohaina R. Glycosylation as new pharmacological strategies for diseases associated with excessive angiogenesis. Pharmacol Ther. 2018;191:92-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Telang S, Clem BF, Klarer AC, Clem AL, Trent JO, Bucala R, Chesney J. Small molecule inhibition of 6-phosphofructo-2-kinase suppresses t cell activation. J Transl Med. 2012;10:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Perrotta P, Van der Veken B, Van Der Veken P, Pintelon I, Roosens L, Adriaenssens E, Timmerman V, Guns PJ, De Meyer GRY, Martinet W. Partial Inhibition of Glycolysis Reduces Atherogenesis Independent of Intraplaque Neovascularization in Mice. Arterioscler Thromb Vasc Biol. 2020;40:1168-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114:622-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 51. | Uryga AK, Bennett MR. Ageing induced vascular smooth muscle cell senescence in atherosclerosis. J Physiol. 2016;594:2115-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 52. | Sun D, Chen S, Li S, Wang N, Zhang S, Xu L, Zhu S, Li H, Gu Q, Xu X, Wei F. Enhancement of glycolysis-dependent DNA repair regulated by FOXO1 knockdown via PFKFB3 attenuates hyperglycemia-induced endothelial oxidative stress injury. Redox Biol. 2023;59:102589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 53. | Yang S, Yuan HQ, Hao YM, Ren Z, Qu SL, Liu LS, Wei DH, Tang ZH, Zhang JF, Jiang ZS. Macrophage polarization in atherosclerosis. Clin Chim Acta. 2020;501:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 54. | Weisser SB, McLarren KW, Kuroda E, Sly LM. Generation and characterization of murine alternatively activated macrophages. Methods Mol Biol. 2013;946:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Stöger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, Daemen MJ, Lutgens E, de Winther MP. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 56. | Gong M, Zhuo X, Ma A. STAT6 Upregulation Promotes M2 Macrophage Polarization to Suppress Atherosclerosis. Med Sci Monit Basic Res. 2017;23:240-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 57. | Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 809] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 58. | Jin X, Yao T, Zhou Z, Zhu J, Zhang S, Hu W, Shen C. Advanced Glycation End Products Enhance Macrophages Polarization into M1 Phenotype through Activating RAGE/NF-κB Pathway. Biomed Res Int. 2015;2015:732450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 59. | Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 469] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 60. | Wang Y, Wang GZ, Rabinovitch PS, Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-κB-mediated inflammation in macrophages. Circ Res. 2014;114:421-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 61. | Thapa B, Lee K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019;52:360-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 62. | Sergin I, Evans TD, Bhattacharya S, Razani B. Hypoxia in plaque macrophages: a new danger signal for interleukin-1β activation? Circ Res. 2014;115:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Parathath S, Yang Y, Mick S, Fisher EA. Hypoxia in murine atherosclerotic plaques and its adverse effects on macrophages. Trends Cardiovasc Med. 2013;23:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Han X, Ma W, Zhu Y, Sun X, Liu N. Advanced glycation end products enhance macrophage polarization to the M1 phenotype via the HIF-1α/PDK4 pathway. Mol Cell Endocrinol. 2020;514:110878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Eckel RH, Bornfeldt KE, Goldberg IJ. Cardiovascular disease in diabetes, beyond glucose. Cell Metab. 2021;33:1519-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 66. | Matsuura Y, Shimizu-Albergine M, Barnhart S, Kramer F, Hsu CC, Kothari V, Tang J, Gharib SA, Kanter JE, Abel ED, Tian R, Shao B, Bornfeldt KE. Diabetes Suppresses Glucose Uptake and Glycolysis in Macrophages. Circ Res. 2022;130:779-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1041] [Cited by in RCA: 1590] [Article Influence: 176.7] [Reference Citation Analysis (0)] |
| 68. | Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 2018;114:540-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 362] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 69. | Docherty CK, Strembitska A, Baker CP, Schmidt FF, Reay K, Mercer JR. Inducing Energetic Switching Using Klotho Improves Vascular Smooth Muscle Cell Phenotype. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Heiss EH, Schachner D, Donati M, Grojer CS, Dirsch VM. Increased aerobic glycolysis is important for the motility of activated VSMC and inhibited by indirubin-3'-monoxime. Vascul Pharmacol. 2016;83:47-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Grootaert MOJ, Bennett MR. Vascular smooth muscle cells in atherosclerosis: time for a re-assessment. Cardiovasc Res. 2021;117:2326-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 262] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 72. | Chen GP, Yang J, Qian GF, Xu WW, Zhang XQ. Geranylgeranyl Transferase-I Knockout Inhibits Oxidative Injury of Vascular Smooth Muscle Cells and Attenuates Diabetes-Accelerated Atherosclerosis. J Diabetes Res. 2020;2020:7574245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Li L, Li Y, Dai Z, Liu M, Wang B, Liu S, Wang L, Chen L, Tan Y, Wu G. Lipid Metabolism in Vascular Smooth Muscle Cells Infuenced by HCMV Infection. Cell Physiol Biochem. 2016;39:1804-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 561] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 75. | Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 520] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 76. | Zhao X, Tan F, Cao X, Cao Z, Li B, Shen Z, Tian Y. PKM2-dependent glycolysis promotes the proliferation and migration of vascular smooth muscle cells during atherosclerosis. Acta Biochim Biophys Sin (Shanghai). 2020;52:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 77. | Oh S, Son M, Park CH, Jang JT, Son KH, Byun K. Pyrogallol-Phloroglucinol-6,6-Bieckolon Attenuates Vascular Smooth Muscle Cell Proliferation and Phenotype Switching in Hyperlipidemia through Modulation of Chemokine Receptor 5. Mar Drugs. 2020;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Carrillo-Sepulveda MA, Matsumoto T. Phenotypic modulation of mesenteric vascular smooth muscle cells from type 2 diabetic rats is associated with decreased caveolin-1 expression. Cell Physiol Biochem. 2014;34:1497-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Cao SH, Chen ZH, Ma RY, Yue L, Jiang HM, Dong LH. Dynamics and Functional Interplay of Nonhistone Lysine Crotonylome and Ubiquitylome in Vascular Smooth Muscle Cell Phenotypic Remodeling. Front Cardiovasc Med. 2022;9:783739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 80. | Jain M, Dhanesha N, Doddapattar P, Nayak MK, Guo L, Cornelissen A, Lentz SR, Finn AV, Chauhan AK. Smooth Muscle Cell-Specific PKM2 (Pyruvate Kinase Muscle 2) Promotes Smooth Muscle Cell Phenotypic Switching and Neointimal Hyperplasia. Arterioscler Thromb Vasc Biol. 2021;41:1724-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 81. | van der Heijden CDCC, Noz MP, Joosten LAB, Netea MG, Riksen NP, Keating ST. Epigenetics and Trained Immunity. Antioxid Redox Signal. 2018;29:1023-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 82. | Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O'Neill LA, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1767] [Article Influence: 196.3] [Reference Citation Analysis (0)] |
| 83. | Keating ST, Plutzky J, El-Osta A. Epigenetic Changes in Diabetes and Cardiovascular Risk. Circ Res. 2016;118:1706-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 84. | Zhong C, Yang X, Feng Y, Yu J. Trained Immunity: An Underlying Driver of Inflammatory Atherosclerosis. Front Immunol. 2020;11:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 85. | Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O'Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1104] [Cited by in RCA: 1565] [Article Influence: 142.3] [Reference Citation Analysis (0)] |
| 86. | Sohrabi Y, Godfrey R, Findeisen HM. Altered Cellular Metabolism Drives Trained Immunity. Trends Endocrinol Metab. 2018;29:602-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 87. | Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1679] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 88. | Li J, Fang Y, Wu D. Mechanical forces and metabolic changes cooperate to drive cellular memory and endothelial phenotypes. Curr Top Membr. 2021;87:199-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Lisco G, Giagulli VA, De Pergola G, Guastamacchia E, Jirillo E, Triggiani V. Hyperglycemia-Induced Immune System Disorders in Diabetes Mellitus and the Concept of Hyperglycemic Memory of Innate Immune Cells: A Perspective. Endocr Metab Immune Disord Drug Targets. 2022;22:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | van Diepen JA, Thiem K, Stienstra R, Riksen NP, Tack CJ, Netea MG. Diabetes propels the risk for cardiovascular disease: sweet monocytes becoming aggressive? Cell Mol Life Sci. 2016;73:4675-4684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 91. | Drummer C, Saaoud F, Shao Y, Sun Y, Xu K, Lu Y, Ni D, Atar D, Jiang X, Wang H, Yang X. Trained Immunity and Reactivity of Macrophages and Endothelial Cells. Arterioscler Thromb Vasc Biol. 2021;41:1032-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 92. | Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, Riksen NP. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol. 2014;34:1731-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 495] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 93. | Groh LA, Riksen NP. Macrophage mitochondrial superoxides as a target for atherosclerotic disease treatment. Int J Biochem Cell Biol. 2020;129:105883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 94. | Tiwari A, Mukherjee B, Dixit M. MicroRNA Key to Angiogenesis Regulation: MiRNA Biology and Therapy. Curr Cancer Drug Targets. 2018;18:266-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 95. | Luo E, Wang D, Yan G, Qiao Y, Zhu B, Liu B, Hou J, Tang C. The NF-κB/miR-425-5p/MCT4 axis: A novel insight into diabetes-induced endothelial dysfunction. Mol Cell Endocrinol. 2020;500:110641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 96. | Xu RH, Liu B, Wu JD, Yan YY, Wang JN. miR-143 is involved in endothelial cell dysfunction through suppression of glycolysis and correlated with atherosclerotic plaques formation. Eur Rev Med Pharmacol Sci. 2016;20:4063-4071. [PubMed] |
| 97. | Chen S, Chen H, Yu C, Lu R, Song T, Wang X, Tang W, Gao Y. MiR-638 Repressed Vascular Smooth Muscle Cell Glycolysis by Targeting LDHA. Open Med (Wars). 2019;14:663-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Bobryshev YV, Nikiforov NG, Elizova NV, Orekhov AN. Macrophages and Their Contribution to the Development of Atherosclerosis. Results Probl Cell Differ. 2017;62:273-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, Rinehold K, van Solingen C, Fullerton MD, Cecchini K, Rayner KJ, Steinberg GR, Zamore PD, Fisher EA, Loke P, Moore KJ. MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest. 2015;125:4334-4348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 316] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 100. | Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 425] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 101. | Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9:416-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 308] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 102. | Zhao J, Zhang X, Liu H, Brown MA, Qiao S. Dietary Protein and Gut Microbiota Composition and Function. Curr Protein Pept Sci. 2019;20:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 103. | Ren X, Wang L, Chen Z, Hou D, Xue Y, Diao X, Shen Q. Foxtail Millet Improves Blood Glucose Metabolism in Diabetic Rats through PI3K/AKT and NF-κB Signaling Pathways Mediated by Gut Microbiota. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 104. | Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4592-4598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 855] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 105. | Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Bäckhed F, Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 711] [Cited by in RCA: 932] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 106. | Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647-5660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 107. | Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. 2019;20:461-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 737] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 108. | Nowiński A, Ufnal M. Trimethylamine N-oxide: A harmful, protective or diagnostic marker in lifestyle diseases? Nutrition. 2018;46:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 109. | Wilson A, McLean C, Kim RB. Trimethylamine-N-oxide: a link between the gut microbiome, bile acid metabolism, and atherosclerosis. Curr Opin Lipidol. 2016;27:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 110. | Wang Q, Guo M, Liu Y, Xu M, Shi L, Li X, Zhao J, Zhang H, Wang G, Chen W. Bifidobacterium breve and Bifidobacterium longum Attenuate Choline-Induced Plasma Trimethylamine N-Oxide Production by Modulating Gut Microbiota in Mice. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 111. | Liu Z, Li J, Liu H, Tang Y, Zhan Q, Lai W, Ao L, Meng X, Ren H, Xu D, Zeng Q. The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis. 2019;284:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 112. | Liu X, Xie Z, Sun M, Wang X, Li J, Cui J, Zhang F, Yin L, Huang D, Hou J, Tian J, Yu B. Plasma trimethylamine N-oxide is associated with vulnerable plaque characteristics in CAD patients as assessed by optical coherence tomography. Int J Cardiol. 2018;265:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 113. | Koay YC, Chen YC, Wali JA, Luk AWS, Li M, Doma H, Reimark R, Zaldivia MTK, Habtom HT, Franks AE, Fusco-Allison G, Yang J, Holmes A, Simpson SJ, Peter K, O'Sullivan JF. Plasma levels of trimethylamine-N-oxide can be increased with 'healthy' and 'unhealthy' diets and do not correlate with the extent of atherosclerosis but with plaque instability. Cardiovasc Res. 2021;117:435-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 114. | Hoseini-Tavassol Z, Hasani-Ranjbar S. Targeting TMAO and its metabolic pathway for cardiovascular diseases treatment. J Diabetes Metab Disord. 2021;20:1095-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 115. | Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 2358] [Article Influence: 393.0] [Reference Citation Analysis (0)] |
| 116. | Boini KM, Hussain T, Li PL, Koka S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell Physiol Biochem. 2017;44:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 117. | Hughes MM, O'Neill LAJ. Metabolic regulation of NLRP3. Immunol Rev. 2018;281:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 118. | Próchnicki T, Latz E. Inflammasomes on the Crossroads of Innate Immune Recognition and Metabolic Control. Cell Metab. 2017;26:71-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 119. | Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 1709] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 120. | Häselbarth L, Ouwens DM, Teichweyde N, Hochrath K, Merches K, Esser C. The small chain fatty acid butyrate antagonizes the TCR-stimulation-induced metabolic shift in murine epidermal gamma delta T cells. EXCLI J. 2020;19:334-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 121. | Geng HW, Yin FY, Zhang ZF, Gong X, Yang Y. Butyrate Suppresses Glucose Metabolism of Colorectal Cancer Cells via GPR109a-AKT Signaling Pathway and Enhances Chemotherapy. Front Mol Biosci. 2021;8:634874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 122. | Gouaref I, Detaille D, Wiernsperger N, Khan NA, Leverve X, Koceir EA. The desert gerbil Psammomys obesus as a model for metformin-sensitive nutritional type 2 diabetes to protect hepatocellular metabolic damage: Impact of mitochondrial redox state. PLoS One. 2017;12:e0172053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Kasahara K, Krautkramer KA, Org E, Romano KA, Kerby RL, Vivas EI, Mehrabian M, Denu JM, Bäckhed F, Lusis AJ, Rey FE. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3:1461-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 358] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 124. | Yoshida N, Emoto T, Yamashita T, Watanabe H, Hayashi T, Tabata T, Hoshi N, Hatano N, Ozawa G, Sasaki N, Mizoguchi T, Amin HZ, Hirota Y, Ogawa W, Yamada T, Hirata KI. Bacteroides vulgatus and Bacteroides dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation. 2018;138:2486-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 125. | Han Y, Xiang Y, Shi Y, Tang X, Pan L, Gao J, Bi R, Lai X. Pharmacokinetics and Pharmacological Activities of Berberine in Diabetes Mellitus Treatment. Evid Based Complement Alternat Med. 2021;2021:9987097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 126. | Cai Y, Xin Q, Lu J, Miao Y, Lin Q, Cong W, Chen K. A New Therapeutic Candidate for Cardiovascular Diseases: Berberine. Front Pharmacol. 2021;12:631100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 127. | Liu LZ, Cheung SC, Lan LL, Ho SK, Xu HX, Chan JC, Tong PC. Berberine modulates insulin signaling transduction in insulin-resistant cells. Mol Cell Endocrinol. 2010;317:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 128. | Jiang SJ, Dong H, Li JB, Xu LJ, Zou X, Wang KF, Lu FE, Yi P. Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J Gastroenterol. 2015;21:7777-7785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (2)] |
| 129. | Yue SJ, Liu J, Wang AT, Meng XT, Yang ZR, Peng C, Guan HS, Wang CY, Yan D. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am J Physiol Endocrinol Metab. 2019;316:E73-E85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 130. | Zhang X, Wang H, Xie C, Hu Z, Zhang Y, Peng S, He Y, Kang J, Gao H, Yuan H, Liu Y, Fan G. Shenqi compound ameliorates type-2 diabetes mellitus by modulating the gut microbiota and metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. 2022;1194:123189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 131. | Shi Y, Hu J, Geng J, Hu T, Wang B, Yan W, Jiang Y, Li J, Liu S. Berberine treatment reduces atherosclerosis by mediating gut microbiota in apoE-/- mice. Biomed Pharmacother. 2018;107:1556-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 132. | Li X, Su C, Jiang Z, Yang Y, Zhang Y, Yang M, Zhang X, Du Y, Zhang J, Wang L, Jiang J, Hong B. Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbiomes. 2021;7:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 133. | Iatcu CO, Steen A, Covasa M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 205] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 134. | Koprivica I, Vujičić M, Gajić D, Saksida T, Stojanović I. Ethyl Pyruvate Stimulates Regulatory T Cells and Ameliorates Type 1 Diabetes Development in Mice. Front Immunol. 2018;9:3130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 135. | Koprivica I, Gajić D, Pejnović N, Paunović V, Saksida T, Stojanović I. Ethyl Pyruvate Promotes Proliferation of Regulatory T Cells by Increasing Glycolysis. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 136. | Muller CJF, Joubert E, Chellan N, Miura Y, Yagasaki K. New Insights into the Efficacy of Aspalathin and Other Related Phytochemicals in Type 2 Diabetes-A Review. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 137. | Kennedy BK, Lamming DW. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016;23:990-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 394] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 138. | Wang Y, Zhang K, Li T, Maruf A, Qin X, Luo L, Zhong Y, Qiu J, McGinty S, Pontrelli G, Liao X, Wu W, Wang G. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics. 2021;11:164-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 230] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 139. | Park HY, Kim MJ, Kim YJ, Lee S, Jin J, Choi YK, Park KG. V-9302 inhibits proliferation and migration of VSMCs, and reduces neointima formation in mice after carotid artery ligation. Biochem Biophys Res Commun. 2021;560:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 140. | Park HY, Kim MJ, Lee S, Jin J, Kim JG, Choi YK, Park KG. Inhibitory Effect of a Glutamine Antagonist on Proliferation and Migration of VSMCs via Simultaneous Attenuation of Glycolysis and Oxidative Phosphorylation. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 141. | Poels K, Schnitzler JG, Waissi F, Levels JHM, Stroes ESG, Daemen MJAP, Lutgens E, Pennekamp AM, De Kleijn DPV, Seijkens TTP, Kroon J. Inhibition of PFKFB3 Hampers the Progression of Atherosclerosis and Promotes Plaque Stability. Front Cell Dev Biol. 2020;8:581641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 142. | Sotiriou SN, Orlova VV, Al-Fakhri N, Ihanus E, Economopoulou M, Isermann B, Bdeir K, Nawroth PP, Preissner KT, Gahmberg CG, Koschinsky ML, Chavakis T. Lipoprotein(a) in atherosclerotic plaques recruits inflammatory cells through interaction with Mac-1 integrin. FASEB J. 2006;20:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |