Published online Jul 15, 2022. doi: 10.4239/wjd.v13.i7.532
Peer-review started: February 17, 2022
First decision: April 17, 2022
Revised: April 26, 2022
Accepted: June 16, 2022
Article in press: June 16, 2022
Published online: July 15, 2022
Processing time: 143 Days and 20.3 Hours
Diabetes is a serious public health concern in China, with 30% of patients developing retinopathy, and diabetic macular edema (DME) having the biggest impact on vision. High blood glucose level can cause retinal cell hypoxia, thus promoting vascular endothelial growth factor (VEGF) formation and increasing vascular permeability, which induces DME. Moreover, cell hypoxia can accelerate the rate of apoptosis, which leads to the aging of patients. In severe cases, optic cell apoptosis or retinal fibrosis and permanent blindness may occur.
To investigate and compare the efficacy, mechanism, and differences between two anti-VEGF drugs (Compaq and ranibizumab) in DME patients.
Ninety-six patients with DME who attended our hospital from April 2018 to February 2020 were included and randomly divided into two groups (Compaq group and ranibizumab group). The groups received vitreal cavity injections of 0.5 mg Compaq and 0.5 mg ranibizumab, respectively, once a month. The best corrected visual acuity (BCVA), intraocular pressure (IOP), macular retinal thickness (CMT), macular choroidal thickness (SFCT), foveal no perfusion area (FAZ), superficial capillary density, deep capillary density, treatment effect, and adverse reactions were compared before and after treatment and between the two groups.
Before treatment and 1-mo post-treatment, there was no statistically significant difference in the estimated BCVA in both groups (P > 0.05). BCVA decreased in the Compaq group 3 mo after treatment, and the difference was statistically significant (P < 0.05). Before treatment, and 1 mo and 3 mo post-treatment, there was no statistically significant difference in the estimated IOP in either group (P > 0.05). Before treatment and 1-mo post-treatment, there was no statistically significant difference in the estimated CMT, SFCT, or FAZ in either group (P > 0.05). CMT and SFCT values decreased in the Compaq group 3 mo post-treatment, and the difference was statistically significant (P < 0.05). Before treatment, and 1 mo and 3 mo post-treatment, there were no statistically significant differences in vascular density in the shallow or deep capillary plexi of the fovea, parafovea, or overall macular area between the two groups (P > 0.05). Marked efficient, effective, and invalid rates were 70.83% and 52.08%, 27.08% and 39.58%, and 2.08% and 8.33% in the Compaq and ranibizumab groups, respectively. The differences between the two groups were statistically significant (P < 0.05).
Anti-VEGF drugs can effectively improve CMT and SFCT, without affecting microcirculation, thus providing an effective and safe treatment for patients with DME.
Core Tip: The main pathological feature of diabetic macular edema (DME) is abnormal neovascularization throughout the retinal pigment epithelium. New vessels develop rapidly and are fragile, thus resulting to rupture and retinal detachment, macular edema, impaired, vision and blind spots. Without effective treatment, vision declines rapidly, causing irreversible impairment. Compaq has a strong affinity with vascular endothelial growth factor (VEGF) receptors, and as a novel VEGF biological agent, it has a relatively strong inhibition of vascular growth in ocular lesions. Our study investigated the effect and mechanism of anti-VEGF drugs in DME patients to improve clinical DME treatment.
- Citation: Li YF, Ren Q, Sun CH, Li L, Lian HD, Sun RX, Su X, Yu H. Efficacy and mechanism of anti-vascular endothelial growth factor drugs for diabetic macular edema patients. World J Diabetes 2022; 13(7): 532-542
- URL: https://www.wjgnet.com/1948-9358/full/v13/i7/532.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i7.532
Diabetic macular edema (DME), which manifests clinically as visual impairment, is a common complication of diabetes[1-3]. Long-term high blood glucose is the basis of DME, as it causes increased endothelial cell permeability in the tight junctions of retinal capillaries, allowing protein and fluid to accumulate in the area of the macula, resulting in macular edema. Previous studies have also found that along with leaking capillary endothelial cells, an increase in glycosylated hemoglobin due to high blood sugar is also a risk factor for cystoid macular edema. Strict blood glucose control within a reasonable range can slow down the development of DME.
Vascular endothelial growth factor (VEGF) play an important role in the pathophysiology of DME[4]. In a high blood glucose environment, glycosylation products increase, and active oxygen is at a relatively high level, leading to further diglyceride production. This activates protein kinase C, which mediates the generation of VEGF[5]. VEGF is the primary regulatory factor for neovascularization and vascular permeability, which characterizes DME. The interaction of vascular receptors on the endothelial cell surface can be inactivated through VEGF inhibition to prevent vascular endothelial hyperplasia, thereby reducing retinal neovascularization and blood vessel leakage in the macular area. Currently, according to evidence from multiple regions, the pharmaceutical drug Compaq (Chengdu Kanghong Biotechnology Co., LTD., Chengdu, China; National Drug approval S20130012)[6] acts directly on new vessels in retinal lesion tissue, thus reducing the incidence of blood vessel hyperplasia caused by photocoagulation. Moreover, inreverse rate of retinal tissue can be reduced by laser photocoagulation reduction. For these reasons, anti-VEGF drugs are recommended as the first-line treatment for DME to improve clinical manifestations. Examples of anti-VEGF drugs used for macular edema in China include bevacizumab, Compaq, and ranibizumab[7]. Vitreal cavity injection is the preferred route of administration for anti-VEGF drugs.
Compaq is a novel anti-VEGF drug developed independently in China, which can specifically bind to VEGF-A, VEGF-B, and placenta growth factors to inhibit the activation of VEGF families, thus preventing neovascularization and reducing blood retinal barrier (BRB) damage and minor microvasculature leakage. Studies have shown[8,9] Compaq as an efficacious DME treatment, but whether it can inhibit neovascularization, reduce the perfusion area in the macular area, and improve microcirculation remains unclear.
There are a few studies comparing the effects of Compaq with that of ranibizumab for DME administered via vitreal cavity injection. For this study, we used optical coherence tomography angiography (OCTA) to measure the treatment effect and mechanism of action in these two anti-VEGF drugs. Based on the segment frequency amplitude of B-scan correlation calculation and the real-time flow of red blood cells in retinal vascular correlation calculation, OCTA can remove the correlation of fixed organization, highlight regular blood flow images of the organization, and recombine all images to obtain structures, such as the retina and choroid blood vessels, to facilitate blood flow evaluation[10].
A total of 96 patients with DME who visited our hospital from April 2018 to February 2020 were included in the study. Patients were randomly divided into two groups: Compaq (n = 48) and ranibizumab (n = 48) by assigning even and odd-numbered sequences. The inclusion criteria were as follows: (1) Age 54–79 years; (2) Diagnosed according to the criteria and treatment guidelines for diabetic retinopathy (DR)[11] (with DME within the proliferative phase of the DR lesion stage); (3) Hospital admission and receipt of fundoscopic angiography, with diagnosis by OCTA examination; (4) Retinal fovea thickness in the macular area > 250 μm; and (5) Ocular IOP range: 10-21 mmHg. The exclusion criteria were as follows: (1) Presence of eye tumors; (2) History of ocular trauma; (3) Retinal macular spots and tissue hyperplasia; (4) Reticular vein obstruction and senile macular lesions; (5) Hypertensive retinopathy; (6) Combined cataract and glaucoma; and (7) Other systematic major disease.
The study was approved by the Medical Ethics Committee of our hospital, and informed consent from the patients' families was obtained for the treatment plan of this study.
Patients were placed in the supine position, and the cornea and conjunctival sac were cleaned. Routine pre-anesthesia operations were performed, followed by surface anesthesia. Under the microscope (Nikon, Tokyo, Japan), the doctor opened the patient’s eyelid. Compaq (0.10 mg/mL, 0.2 mL/injection) was injected into the vitreous chamber of patients in both groups. After injection, the needle was withdrawn slowly, and 0.5 mg/eye/time was infused in the vitreous cavity once per month for the first 3 mo (0.05 mL), followed by intravitreal administration once every 3 mo.
Basic treatment
All patients underwent lacrimal tract irrigation 3 d before surgery and were treated with levofloxacin eye drops four times a day for 5 d (Shentian Pharmaceutical Co., LTD. Noto Factor, Japan; National medicine approval number J20150106; 10 mL/branch/box) after surgery.
The best corrected visual acuity (BCVA), intraocular pressure (IOP), macular retinal thickness (CMT), macular choroidal thickness (SFCT), foveal no perfusion area (FAZ), superficial capillary density, deep capillary density, treatment effect, and adverse reactions were compared between the two groups before and after treatment.
BCVA was measured using a TDRS visual acuity chart at a distance of 4 m, and the maximum number of letters obtained was recorded at four timepoints: pre-treatment, and at 1, 3, and 6 mo post-treatment.
For IOP measurement, a non-contact ophthalmometer (model AD-1900; Neusoft Xikang Co., LTD., Shenyang, China) was used, and the average value of three inspections were taken.
Astigmatism was examined using the slit slice method, and an OCT instrument (American BD company production, model AU-300; GE Company, Chicago, Illinois) was used to scan the macular area within a range of 6 mm × 6 mm. Consequently, the retinal thickness of the macular fovea was measured.
Treatments were evaluated with reference to the diagnosis and treatment of diabetic retinopathy[12]. Treatments were considered markedly effective if edema had disappeared, retinal hemorrhage had been completely absorbed, and there was no obvious neovascularization, leakage, or perfusion in the macular area 3 mo after treatment. Treatments were considered effective if retinal hemorrhage had partially absorbed, vascular leakage was reduced, the perfusion density (PD) in the non-perfusion area was < 5, and no new vessels were assumed to be functioning. Lastly, treatments were considered invalid if patients did not meet the above criteria.
Ophthalmic testing included the following: (1) Routine examination: BCVA was measured using an international standard visual acuity chart; IOP was measured using a non-contact tonometer; and (2) OCT examination: A Zeiss Cirrus 5000 OCT instrument (Carl Zeiss Meditec, Jena, Germany) was used. CMT was measured with 2 PD on the optic disc temporal side and 1.5 PD below the macular fovea. Three measurements were obtained, and the average value was considered. SFCT was measured using the caliper function of the instrument. The retinal blood flow imaging mode was selected, and the scanning range of the macular area was 3 mm × 3 mm, scanning signal intensity index was > 45, and transverse and longitudinal scanning required 3 s. The patients were instructed not to move their eyes during the scan. Supporting analysis software was used to measure the vessel density of the shallow capillary plexus (SCP) and deep capillary plexus (DCP) in the FAZ within the scanning range of 3 mm × 3 mm in the macular area at the SCP level. All examinations were performed by the same physician and reviewed independently by two surgeons clinically experienced fundus imaging analysis.
A normal distribution test showed that the BCVA values of the patients in this study were consistent with an approximate normal distribution or normal distribution, and was expressed as mean ± SD. Test was used for comparisons between groups. The counting data were expressed as percentages, and comparisons were based on χ2 test or the Mann–Whitney U test. Professional SPSS 21.0 software (IBM Corp., Armonk, NY) was used for data processing, and the significance level was set at α = 0.05.
The age, sex, body mass index, and BCVA before treatment and the distribution of the affected side were compared between the two groups, and no statistically significant difference was found (P > 0.05), as shown in Table 1.
| Baseline data | Compaq group (n = 48) | Ranibizumab group (n = 48) | t/χ2 value | P value |
| Age (yr) | 64.8 ± 7.2 | 66.3 ± 6.9 | -1.042 | 0.300 |
| BMI (kg/m2) | 23.5 ± 2.3 | 23.2 ± 2.8 | 0.574 | 0.568 |
| Before treatment: BCVA (LogMAR) | 0.78 ± 0.12 | 0.80 ± 0.11 | -0.851 | 0.397 |
| Gender, n (%) | 2.043 | 0.153 | ||
| Male | 27 (56.25) | 20 (41.67) | ||
| Female | 21 (43.75) | 28 (58.33) | ||
| Distribution of affected side, n (%) | 0.667 | 0.414 | ||
| Left | 22 (45.83) | 26 (54.17) | ||
| Right | 26 (54.17) | 22 (45.83) |
Before and 1-mo post-treatment, there was no statistically significant difference between the estimated value of BCVA in either group (P > 0.05). After 3 mo, a decrease was observed in the Compaq group, and the difference was statistically significant (P < 0.05). However, there was no statistically significant difference in the estimated value of IOP before, 1 mo, or 3 mo after the treatment in either group (P > 0.05), as shown in Table 2.
| Groups | BCVA (LogMAR) | IOP (mmHg) | ||||
| Before treatment | 1 mo after treatment | 3 mo after treatment | Before treatment | 1 mo after treatment | 3 mo after treatment | |
| Compaq group (n = 48) | 0.78 ± 0.12 | 0.72 ± 0.13 | 0.51 ± 0.10 | 16.84 ± 2.77 | 16.40 ± 2.81 | 16.39 ± 2.64 |
| Ranibizumab group (n = 48) | 0.80 ± 0.11 | 0.75 ± 0.14 | 0.57 ± 0.13 | 16.50 ± 2.80 | 16.72 ± 2.76 | 16.81 ± 2.82 |
| t value | -0.851 | -1.088 | -2.535 | 0.598 | -0.563 | -0.753 |
| P value | 0.397 | 0.279 | 0.013 | 0.551 | 0.575 | 0.453 |
There were no statistically significant differences in the estimated values of CMT, SFCT, or FAZ before or 1-mo post-treatment in either group (P > 0.05). Three months post-treatment, the estimated values of CMT and SFCT in the Compaq group were lower than those in the ranibizumab group, and the difference was statistically significant (P < 0.05) (Table 3).
| Groups | Before treatment | 1 mo after treatment | 3 mo after treatment |
| CMT (μm) | |||
| Compaq group (n = 48) | 445.8 ± 89.6 | 372.1 ± 76.0 | 210.6 ± 66.4 |
| Ranibizumab group (n = 48) | 452.7 ± 93.2 | 384.0 ± 80.6 | 243.1 ± 73.5 |
| t value | -0.370 | -0.744 | -2.273 |
| P value | 0.712 | 0.459 | 0.025 |
| SFCT (μm) | |||
| Compaq group (n = 48) | 335.1 ± 55.9 | 323.4 ± 59.5 | 281.6 ± 54.0 |
| Ranibizumab group (n = 48) | 340.5 ± 58.3 | 330.5 ± 63.0 | 306.2 ± 57.3 |
| t value | -0.463 | -0.568 | -2.165 |
| P value | 0.644 | 0.572 | 0.033 |
| FAZ (mm2) | |||
| Compaq group (n = 48) | 0.74 ± 0.10 | 0.72 ± 0.12 | 0.73 ± 0.11 |
| Ranibizumab group (n = 48) | 0.75 ± 0.12 | 0.74 ± 0.14 | 0.74 ± 0.11 |
| t value | -0.444 | -0.751 | -0.445 |
| P value | 0.658 | 0.454 | 0.657 |
Before, 1 mo, and 3 mo post-treatment, there were no statistically significant differences in the vascular density of the SCP and DCP of the fovea, parafovea, or overall macular area between the Compaq and ranibizumab groups (P > 0.05), as shown in Table 4 and Table 5.
| Groups | Before treatment | 1 mo after treatment | 3 mo after treatment |
| Fovea | |||
| Compaq group (n = 48) | 20.64 ± 4.40 | 20.30 ± 3.95 | 20.28 ± 3.77 |
| Ranibizumab group (n = 48) | 20.90 ± 4.83 | 20.48 ± 4.20 | 20.37 ± 4.14 |
| t value | -0.276 | -0.216 | -0.111 |
| P value | 0.783 | 0.829 | 0.912 |
| Parafovea | |||
| Compaq group (n = 48) | 38.56 ± 4.82 | 38.10 ± 4.50 | 37.73 ± 4.72 |
| Ranibizumab group (n = 48) | 39.10 ± 5.57 | 38.67 ± 5.53 | 38.38 ± 5.28 |
| t value | -0.508 | -0.554 | -0.636 |
| P value | 0.613 | 0.581 | 0.526 |
| Overall macular area | |||
| Compaq group (n = 48) | 35.74 ± 5.10 | 35.43 ± 4.85 | 34.92 ± 5.51 |
| Ranibizumab group (n = 48) | 36.30 ± 5.34 | 35.67 ± 5.11 | 34.58 ± 5.18 |
| t value | -0.525 | -0.236 | 0.311 |
| P value | 0.601 | 0.814 | 0.756 |
| Groups | Before treatment | 1 mo after treatment | 3 mo after treatment |
| Fovea | |||
| Compaq group (n = 48) | 18.58 ± 3.80 | 18.23 ± 3.75 | 17.86 ± 4.12 |
| Ranibizumab group (n = 48) | 19.14 ± 4.00 | 18.78 ± 4.24 | 18.47 ± 3.96 |
| t value | -0.703 | -0.673 | -0.740 |
| P value | 0.484 | 0.502 | 0.461 |
| Parafovea | |||
| Compaq group (n = 48) | 40.92 ± 5.73 | 40.51 ± 4.85 | 40.38 ± 5.22 |
| Ranibizumab group (n = 48) | 40.40 ± 5.51 | 40.10 ± 5.28 | 39.56 ± 4.87 |
| t value | 0.453 | 0.396 | 0.796 |
| P value | 0.651 | 0.693 | 0.428 |
| Overall macular area | |||
| Compaq group (n = 48) | 39.64 ± 4.85 | 39.40 ± 4.77 | 38.78 ± 4.62 |
| Ranibizumab group (n = 48) | 40.43 ± 5.18 | 39.93 ± 5.03 | 39.52 ± 4.85 |
| t value | -0.771 | -0.530 | -0.765 |
| P value | 0.442 | 0.598 | 0.446 |
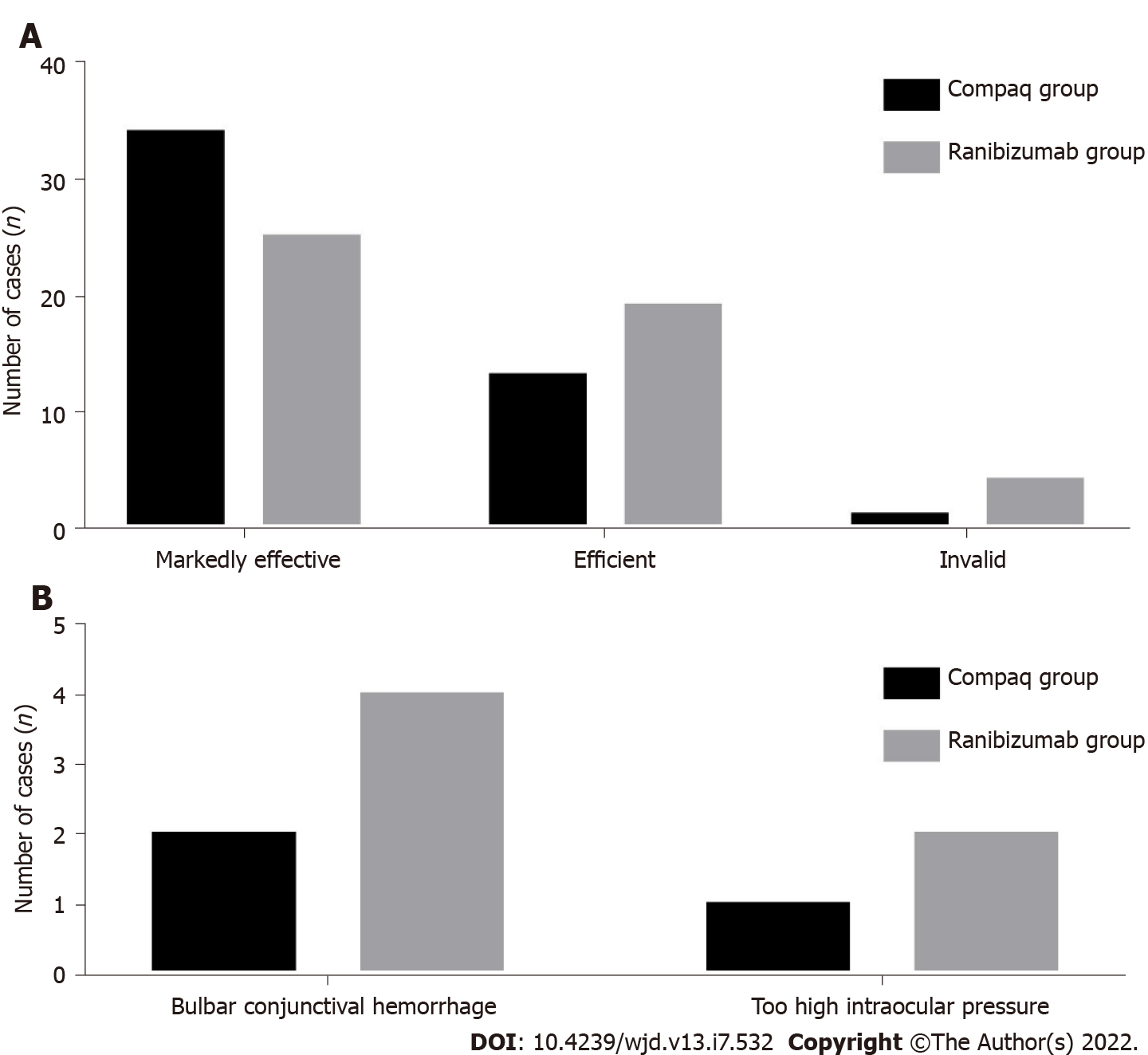
Three months post-treatment, the rates of marked efficiency, effective, and invalid in the Compaq and ranibizumab groups were 70.83% and 52.08%, 27.08% and 39.58%, and 2.08% and 8.33%, respectively, and the difference between the two groups was statistically significant (P < 0.05), as shown in Table 6 and Figure 1A.
| Groups | Markedly efficiency | Efficient | Invalid |
| Compaq group (n = 48) | 34 (70.83) | 13 (27.08) | 1 (2.08) |
| Ranibizumab group (n = 48) | 25 (52.08) | 19 (39.58) | 4 (8.33) |
| Z value | -1.993 | ||
| P value | 0.046 | ||
The rates of adverse reactions in the Compaq and ranibizumab groups were 6.25% and 12.50%, respectively, and there was no statistically significant difference between the two groups (P > 0.05), as shown in Table 7 and Figure 1B.
| Groups | Bulbar conjunctival hemorrhage | Too high intraocular pressure | Adverse reaction |
| Compaq group (n = 48) | 2 | 1 | 3 (6.25) |
| Ranibizumab group (n = 48) | 4 | 2 | 6 (12.50) |
| χ2 value | 1.333 | ||
| P value | 0.248 |
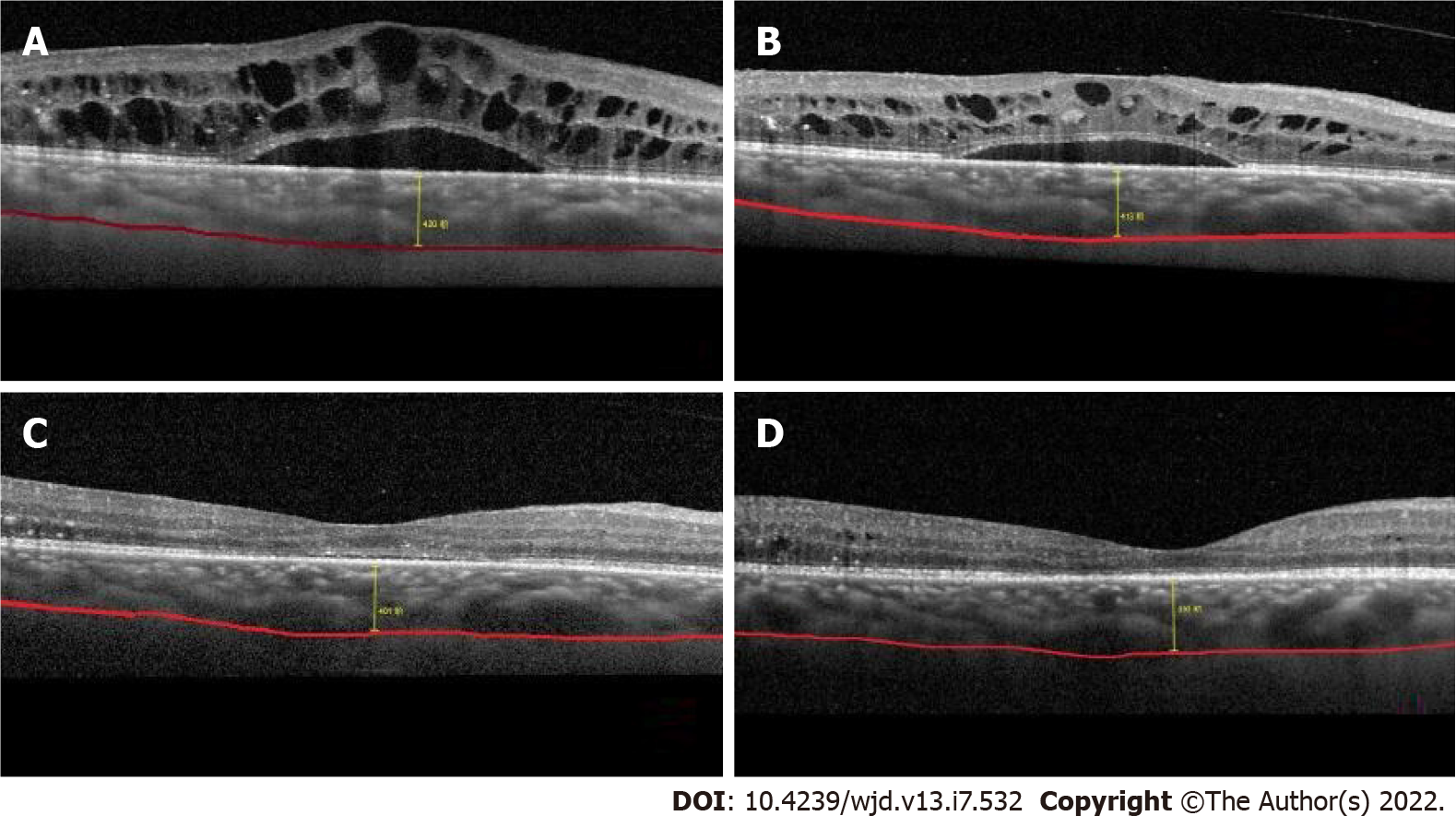
A 71-year-old male patient, with a history of diabetes over 12 years, reported a significant decrease in visual acuity in the prior 6 mo. After admission, he was diagnosed with diabetic macular edema by fundus angiography and optical coherence tomography. Before treatment, the foveal thickness in the macular area was > 477.2 μm, and the BCVA value was 0.83 LogMAR. The patient was treated with intravitreal injection of Conbercept 0.5 mg once a month. The OCT examination results before and after treatment are shown in Figure 2. The patient’s visual acuity BCVA recovered to 0.55 LogMAR 3 mo after treatment.
In diabetes, the retina is prone to injury due to oxidative stress. A high blood glucose environment can increase active oxygen levels as well as oxygen production in the mitochondria, thus impeding balance between the deoxidation and neutralization of mitochondrial reactive oxygen species in the body. This increased oxidative stress level results in macular edema[13,14]. Research has verified that the excessive production of mitochondrial reactive oxygen species and the decrease of antioxidant enzymes promote the progression of diabetes.
As a novel anti-VEGF, developed independently in China, Compaq can be used to inhibit the increase of vascular wall permeability and neovascularization. The VEGF concentrations in the vitreous chamber and perivascular vessels on the retinal surface are abnormally elevated in DME patients, resulting in retinal neovascularization and increased vascular wall permeability, finally leading to edema. Our study showed that before treatment and 1-mo post-treatment, there was no statistical difference in BCVA between the two groups. However, 3 mo post-treatment, BCVA was significantly lower in the Compaq group than in the ranibizumab group.
Various kinds of anti-VEGF drugs are commonly used in DME treatment, including imported drugs, such as ranibizumab. These drugs bind to and inhibit VEGF receptors to prevent the formation of specific receptors of neovascularization, and therefore, blood glucose and its effects such as retinal capillary permeability can be reduced to improve vision[15]. Compaq eye injections have a significant effect on neovascularization inhibition to reduce VEGF concentration and vascular wall permeability in the eyes and reduce the infiltration of blood vessels; therefore, retinal edema can be absorbed and the degree of macular edema can be relieved to significantly improve visual performance. At present, Compaq is used globally in the field of ophthalmology to reduce macular central retina thickness and choroid thickness of the macular fovea to improve vision in DME patients. Compaq treatment has been proven to be effective and safe[16].
DME treatment of the retina and choroid can sometimes lead to retinal capillary cell loss and degeneration and vascular endothelial cell hyperplasia in diabetes patients, thereby destroying the blood-retinal barrier[17]. Meanwhile, VEGF secretion can be increased, leading to retinal tight junction dysfunction and pericyte loss, as well as an impaired BRB; thus, vascular wall permeability can be increased, causing fluid to leak into retinal tissue and accumulate, resulting in macular edema. Severe cases may develop macular edema, thickening, vision loss, or even blindness. Some studies have found that DR patients may have a relatively thin choroid compared to DME patients. Upon OCTA examination, the thicknesses and changes in each retinal layer can be clearly observed, and the structural image and thickness of the choroid can be distinguished[18]. However, according to our study results, before treatment and 1-mo post-treatment, there was no statistical difference in CMT, SFCT, or FAZ level between the two groups. Three months post-treatment, the estimated values of CMT and SFCT in the Compaq group were significantly lower than those in the ranibizumab group.
Moreover, previous studies have shown that increased total cholesterol, triglycerides, and low-density lipoprotein is related to DME in diabetic patients. Anti-VEGF administered through vitreal cavity injection can improve glucose and lipid levels and decrease levels of oxidative stress throughout the body, which significantly reduces VEGF production, thus reducing retinal vein and artery diameter. This also reduces the permeability of retinal capillaries, inhibiting neovascularization and reducing the extent of damage in the BRB. The thicknesses of the macular central retina and choroid can be reduced once macular edema is relieved. After anti-VEGF treatment in DME, the detailed reasons for decreased macular central choroid thickness remain unclear, which may be because anti-VEGF drugs such as Compaq can inhibit signaling, and the activity of VEGF can be inhibited by binding to VEGF to reduce respiration and edema caused by retinal vascular leakage, resulting in decreased macular central retina and choroid thickness for improved vision.
DME is a complex pathologic progress caused by multiple factors. Currently, it is believed that microvascular lesion in diabetes can cause blood flow changes in retinal microvasculature, and histanoxia can lead to aggravated inflammation, resulting in the release of various inflammatory factors, such as prostaglandins, leukocyte trienes, intercellular adhesion molecule-1, integrin, and tumor necrosis factor α. These factors cause vascular endothelial injury and increased VEGF secretion, thus promoting macular edema[19]. Conversely, macular edema can aggravate histanoxia, and VEGF can be further increased to stimulate the growth of new blood vessels, as well as the formation of a microaneurysm, which becomes a negative feedback loop. Our study showed that, before and after treatment, there were no statistical differences in vascular density in the SCP and DCP in the overall macular area between the two groups. There was also no significant difference in treatment efficacy, indicating that the two drugs selected in our study had no influence on microcirculation, and were safe. Compaq can directly act on new blood vessels in retinal lesions and reduce the damage caused by laser photocoagulation on the retina, thereby resulting in a decreased inflammatory response rate in retinal tissue.
Some studies have shown that both ranibizumab and Compaq can reduce the macular FAZ area and increase vascular density in the SCP to improve microcirculation[20]. With the progression of diabetes, VEGF secretion can be abnormally increased and BRB irreversibility can be aggravated, resulting in the interrupted integrity of the macular arch, and forming of the microaneurysm. Normal vascular tissue is destroyed, and the overall vascular density of the macular area is also reduced. Reports on the quantitative observation of the FAZ area and vascular density using OCTA in the treatment of DME remain unclear.
Previous studies[21] used Conbercept for treatment and found that it can exert strong affinity and multi-target characteristics in the treatment, and can reach the target concentration in a short time. Anti-VEGF therapy decreased SFCT, which has become a relevant parameter for drug selection and follow-up evaluation. DME has a very large impact on the choroid and has a very large impact on the patient's visual acuity. Anti-VEGF drugs can inhibit the biological activity of abnormal VEGF in new blood vessels in the body. From the results of this study, it can be concluded that anti-VEGF drugs can effectively improve CMT and SFCT in patients with DME, restore good visual effects, and represent a safe and efficient treatment regimen for DME. At present, there is no accurate software for measuring choroidal thickness, and the measurement of SFCT is performed by highly qualified physicians. Inevitably, there will be some errors. Automatic analysis software may reduce these errors and reduce the number of times choroidal thickness is measured.
Previous studies on the effect of anti-VEGF drugs on DME have mostly analyzed the changes in visual acuity, central macular retinal thickness, and choroid thickness, while other studies have focused on the changes and correlation in eye axis before and after treatment[22]. This study is unique in that it observed and analyzed the effects of anti-VEGF drugs on SFCT, FAZ, and microcirculation. Concurrently, it also explored the improvements in conventional indicators, such as vision and intraocular pressure, which are of high clinical value.
This study was a clinical controlled study on the treatment of DME patients with vitreous injection of ranibizumab and Compaq. During the follow-up process, we discovered that both drugs could reduce CMT and SFCT to a certain extent and improve the visual acuity of patients. However, this study was a preliminary clinical application study with a small sample size and a short follow-up period; hence, further studies are warranted to confirm our findings.
In summary, anti-VEGF drugs can effectively improve CMT and SFCT, without affecting microcirculation, thus resulting in positive treatment outcomes.
Diabetes is a serious public health concern in China, with 30% of patients developing retinopathy, and diabetic macular edema (DME) having the biggest impact on vision.
Compaq as an efficacious DME treatment, but whether it can inhibit neovascularization, reduce the perfusion area in the macular area, and improve microcirculation remains unclear.
This study aimed to investigate and compare the efficacy, mechanism, and differences between two anti-vascular endothelial growth factor (VEGF) drugs (Compaq and ranibizumab) in DME patients.
Total 96 patients with DME were divided into two groups with different treatment modalities.
Marked efficient, effective, and invalid rates were 70.83% and 52.08%, 27.08% and 39.58%, and 2.08% and 8.33% in the Compaq and ranibizumab groups, respectively.
Anti-VEGF drugs can effectively improve macular retinal thickness and macular choroidal thickness, without affecting microcirculation.
This study was a preliminary clinical application study with a small sample size and a short follow-up period; hence, further studies are warranted to confirm our findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hilliard ME, United States; Kilanowska A, Poland; Ortega AL, Spain S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Alnagdy AA, Abouelkheir HY, El-Khouly SE, Tarshouby SM. Impact of topical nonsteroidal anti-inflammatory drugs in prevention of macular edema following cataract surgery in diabetic patients. Int J Ophthalmol. 2018;11:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Rinaldi M, dell'Omo R, Morescalchi F, Semeraro F, Gambicorti E, Cacciatore F, Chiosi F, Costagliola C. ILM peeling in nontractional diabetic macular edema: review and metanalysis. Int Ophthalmol. 2018;38:2709-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Chaudhary S, Zaveri J, Becker N. Proliferative diabetic retinopathy (PDR). Dis Mon. 2021;67:101140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Kang YK, Park HS, Park DH, Shin JP. Incidence and treatment outcomes of secondary epiretinal membrane following intravitreal injection for diabetic macular edema. Sci Rep. 2020;10:528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Akkaya S, Açıkalın B, Doğan YE, Çoban F. Subthreshold micropulse laser versus intravitreal anti-VEGF for diabetic macular edema patients with relatively better visual acuity. Int J Ophthalmol. 2020;13:1606-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Mathis T, Dot C, Mauget-Faÿsse M, Kodjikian L. Variation of choroidal thickness in diabetic macular edema: friend or foe? Acta Ophthalmol. 2021;99:e282-e283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Busch C, Fraser-Bell S, Zur D, Rodríguez-Valdés PJ, Cebeci Z, Lupidi M, Fung AT, Gabrielle PH, Giancipoli E, Chaikitmongkol V, Okada M, Laíns I, Santos AR, Kunavisarut P, Sala-Puigdollers A, Chhablani J, Ozimek M, Hilely A, Unterlauft JD, Loewenstein A, Iglicki M, Rehak M; International Retina Group. Real-world outcomes of observation and treatment in diabetic macular edema with very good visual acuity: the OBTAIN study. Acta Diabetol. 2019;56:777-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Maloney MH, Schilz SR, Herrin J, Sangaralingham LR, Shah ND, Barkmeier AJ. Risk of Systemic Adverse Events Associated with Intravitreal Anti-VEGF Therapy for Diabetic Macular Edema in Routine Clinical Practice. Ophthalmology. 2019;126:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Mastropasqua L, Di Staso S, D'Aloisio R, Mastropasqua A, Di Antonio L, Senatore A, Ciancaglini M, Di Nicola M, Di Martino G, Tognetto D, Toto L. Anatomical and functional changes after dexamethasone implant and ranibizumab in diabetic macular edema: a retrospective cohort study. Int J Ophthalmol. 2019;12:1589-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O'Neal DN, Januszewski AS. Biomarkers in Diabetic Retinopathy. Rev Diabet Stud. 2015;12:159-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 11. | Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 2296] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 12. | Seery CW, Betesh S, Guo S, Zarbin MA, Bhagat N, Wagner RS. Update on the Use of Anti-VEGF Drugs in the Treatment of Retinopathy of Prematurity. J Pediatr Ophthalmol Strabismus. 2020;57:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Osathanugrah P, Sanjiv N, Siegel NH, Ness S, Chen X, Subramanian ML. The Impact of Race on Short-term Treatment Response to Bevacizumab in Diabetic Macular Edema. Am J Ophthalmol. 2021;222:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Euswas N, Phonnopparat N, Morasert K, Thakhampaeng P, Kaewsanit A, Mungthin M, Rangsin R, Sakboonyarat B. National trends in the prevalence of diabetic retinopathy among Thai patients with type 2 diabetes and its associated factors from 2014 to 2018. PLoS One. 2021;16:e0245801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Ruiz-Moreno JM, de Andrés-Nogales F, Oyagüez I. Cost-consequence analysis of extended loading dose of anti-VEGF treatment in diabetic macular edema patients. BMC Ophthalmol. 2020;20:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Starr MR, Mahr MA, Smith WM, Iezzi R, Barkmeier AJ, Bakri SJ. Outcomes of Patients With Active Diabetic Macular Edema at the Time of Cataract Surgery Managed With Intravitreal Anti-Vascular Endothelial Growth Factor Injections. Am J Ophthalmol. 2021;229:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Yoshitake T, Murakami T, Yoshitake S, Suzuma K, Dodo Y, Fujimoto M, Tsujikawa A. Anti-Fumarase Antibody as a Predictor of Functional Efficacy of Anti-VEGF Therapy for Diabetic Macular Edema. Invest Ophthalmol Vis Sci. 2019;60:787-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Imazeki M, Noma H, Yasuda K, Motohashi R, Goto H, Shimura M. Anti-VEGF Therapy Reduces Inflammation in Diabetic Macular Edema. Ophthalmic Res. 2021;64:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Jagadish P, Dalziel D. Discharge outcomes of patients referred to specialist eye clinic from diabetic retinopathy screening in Northland (2014-15). N Z Med J. 2017;130:89-93. [PubMed] |
| 20. | Rajeshuni N, Zubair T, Ludwig CA, Moshfeghi DM, Mruthyunjaya P. Evaluation of Racial, Ethnic, and Socioeconomic Associations With Treatment and Survival in Uveal Melanoma, 2004-2014. JAMA Ophthalmol. 2020;138:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Ming S, Xie K, Yang M, He H, Li Y, Lei B. Comparison of intravitreal dexamethasone implant and anti-VEGF drugs in the treatment of retinal vein occlusion-induced oedema: a meta-analysis and systematic review. BMJ Open. 2020;10:e032128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Kelkar A, Webers C, Shetty R, Kelkar J, Labhsetwar N, Pandit A, Malode M, Tidke S. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in Indian patients with retinal vein occlusion, age-related macular degeneration, and diabetic macular edema. Indian J Ophthalmol. 2020;68:2143-2147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |