Published online Jun 15, 2022. doi: 10.4239/wjd.v13.i6.442
Peer-review started: March 1, 2022
First decision: April 17, 2022
Revised: April 25, 2022
Accepted: May 21, 2022
Article in press: May 21, 2022
Published online: June 15, 2022
Processing time: 98 Days and 16.9 Hours
At present, there is no ideal method to cure diabetes, and there are few reports on the treatment of diabetes with probiotics.
To propose a method for preparing a new type of chromium- and zinc-rich Acetobacter aceti (A. aceti) and explore its ability to enhance the hypoglycemic effects of probiotics in the treatment of diabetes.
A. aceti was cultured in a liquid medium that contained chromium trichloride and zinc chloride, both at a concentration of 64 mg/mL, with the initial concentration of the bacterial solution 1 × 104 CFU/mL. After the bacterial solution had been inducted for 48 h, the culture media was changed and the induction was repeated once. The levels of chromium and zinc in the bacteria were detected by inductively coupled plasma mass spectrometry, and the contents of NADH and glucose dehydrogenase were determined using an NAD/NADH kit and glucose dehydrogenase kit, respectively. Streptozotocin was used to establish a mouse model to evaluate the hypoglycemic effects of the proposed chromium- and zinc-rich A. aceti. Ten-times the therapeutic dose was administered to evaluate its biological safety. The effect on MIN6 islet cells was also assessed in vitro.
The levels of chromium metal, metallic zinc, NADH coenzyme, and glucose dehydrogenase in A. aceti prepared by this method were 28.58-34.34 mg/kg, 5.35-7.52 mg/kg, 5.13-7.26 μM, and 446.812-567.138 U/g, respectively. The use of these bacteria resulted in a better hypoglycemic effect than metformin, promoting the repair of tissues and cells of pancreatic islets in vivo and facilitating the growth of MIN6 pancreatic islet cells and increasing insulin secretion in vitro. Ten-times the therapeutic dose of treatment was non-toxic to mice.
Chromium trichloride and zinc chloride can be employed to induce the preparation of chromium- and zinc-rich A. aceti, which can then promote the hypoglycemic effect found in normal A. aceti. The bacteria biotransforms the chromium and zinc in a way that could increase their safety as a treatment for diabetes.
Core Tip: At present, there are no ideal drugs to treat diabetes. Acetobacter and other probiotics can be used in the treatment of diabetes, but their effect is not significant. The focus of this study is to determine if enriching chromium and zinc in Acetobacter aceti could enhance the hypoglycemic effect of this probiotic. In this study, metal compounds were used to induce A. aceti to enrich chromium and zinc concentrations, and the effects of these metal-enriched bacteria on the hypoglycemic effect were assessed. These chromium- and zinc-rich bacteria were able to increase the hypoglycemic effect and, due to low toxicity, have good prospects as a treatment for diabetes.
- Citation: Huang YY, Qin XK, Dai YY, Huang L, Huang GR, Qin YC, Wei X, Huang YQ. Preparation and hypoglycemic effects of chromium- and zinc-rich Acetobacter aceti. World J Diabetes 2022; 13(6): 442-453
- URL: https://www.wjgnet.com/1948-9358/full/v13/i6/442.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i6.442
Diabetes mellitus is a disease of chronic glucose metabolism disorders[1,2]. In 2020, the world’s population with diabetes was about 495 million, and this is expected to increase to 700 million by 2045[3,4]. The pain and burden of diabetes and its complications are major health and socio-economic concerns for people all over the world. Diabetic patients are often challenged by serious complications, such as diabetic nephropathy, diabetic retinopathy, cardiovascular system disease, etc, which pose a serious threat to human life and health[5-7]. Patients with type 2 diabetes mellitus (T2DM) account for 90% to 95% of all diabetic patients, making T2DM an important focus of much research into the epidemiology of diabetes. Scholars have heavily researched the causes of diabetes and methods for its prevention, for many years; however, there remains no effective cure, due to the disease’s unclear causes and complicated pathogenesis. Moveover, its underlying mechanism has yet to be fully elucidated[8,9]. The current comprehensive prevention and treatment measures for diabetes mainly include diet control, exercise, and drug therapy[10-12].
The drugs used for the treatment of diabetes are mainly chemical agents, with few biological drugs available. Health drinks, including kombucha (which contains yeast, lactic acid bacteria, and acetic acid bacteria and their metabolites) and black tea, can lower blood sugar, blood pressure, blood lipids, etc, and exert certain auxiliary effects on patients with hypertension, hyperlipidemia, and hyperglycemia; the main active ingredients in these drinks that lowers blood sugar are D-glucaric acid-1, 4-lactone and tea polyphenols, respectively[13]. However, the effect of bacterial metabolites from these drinks on reducing blood sugar is not fully elucidated. For example, Acetobacter aceti (A. aceti) contains dihydronicotinamide-adenine dinucleotide and glucuronic acid dehydrogenase, among other metabolites, which decompose glucose through glycolysis; however, kombucha fails to improve the effect of reducing blood sugar by fully utilizing the effects of these metabolic enzymes. Therefore, A. aceti can only be used as a healthy drink for adjuvant therapy. In order to capitalize on the ability of these bacteria to metabolize glucose, increase the contents of dihydronicotinamide-adenine dinucleotide glucuronic acid dehydrogenase, and other metabolic enzymes, and exert their hypoglycemic effects, their full effectiveness must be realized in vivo before these bacteria can be used as a hypoglycemic drug.
To improve the effect of A. aceti on lowering blood sugar, the present study provides a method for preparing and applying chromium- and zinc-rich A. aceti. This method demonstrated a significant increase in the amount of dihydronicotinamide-adenine dinucleotide, glucuronic acid dehydrogenase, chromium, and zinc inside the cells and other microelements in the specimens we prepared. These enriched bacteria had a notable repair effect on pancreatic islet cells, promoted insulin secretion, and demonstrated a good hypoglycemic effect. Therefore, chromium- and zinc-rich A. aceti can be used as a candidate drug for the treatment of T2DM.
Glucose (Guangdong Guanghua Technology Co., Ltd, batch number: 20200403); yeast extract (Beijing Aoboxing Bio-tech Co., Ltd, batch number: 20200422); calcium carbonate (Shanghai Titan Scientific Co., Ltd, batch number: P1260108); agar (Beijing Solarbio Technology Co., Ltd, batch number: 310C022); anhydrous alcohol (Shanghai MacLean Biochemical Technology Co., Ltd, batch number: C11974944); chromium chloride (Shanghai MacLean Biochemical Technology Co., Ltd, batch number: C10717130); zinc chloride (Shanghai MacLean Biochemical Technology Co., Ltd, batch number: C10730413); 50-mL centrifuge tubes and EP tubes (Jiangsu Lexinkang Medical Equipment Co., Ltd); streptozotocin (STZ) (Shanghai McLean Biochemical Technology Co., Ltd, batch number: C20PA038100B); citric acid (Shanghai McLean Biochemical Technology Co., Ltd, batch number: C10723907); sodium citrate (Shanghai McLean Biochemical Technology Co., Ltd, batch number: C10712912); universal pH indicator paper (Hangzhou Test Three Technology Co., Ltd); metformin hydrochloride tablets (Beijing Jingfeng Pharmaceutical Group Co., Ltd, batch number 2004032); glucose test strips (ACCU-CHEK; Roche Diabetes Care GmbH, batch number: 26020933, etc); specific pathogen-free (SPF) C57BL/6 mice, aged 6-8 wk (Changsha Tianqin Biological Co., Ltd); A. aceti, number: GIM1.67 (Guangdong Microorganism Conservation Centre); and MIN6 cells (China Centre for Type Culture Collection) were used. The Animal Experiment Ethics Approval Number was 20200620.
A. aceti were revived and cultured in a liquid enriched medium, with the concentration of the bacterial solution OD600 = 0.9 to 1.5, which is about 3 × 108 to 5 × 108 CFU/mL. To enrich the bacteria with chromium and zinc, A. aceti were cultured in a liquid medium (the concentrations of chromium trichloride and zinc chloride were both 64 mg/mL) with the initial concentration of the bacterial solution set to 1 × 104 CFU/mL and shaken for 48 h at 250 rpm; this cultivation was repeated once. Following the enrichment cultivations, the bacterial solution was collected, centrifuged to remove the supernatant, and washed by phosphate buffer saline (PBS); the precipitate was the chromium- and zinc-rich A. aceti.
Collection of A. aceti: Cell suspensions (100 mL) of A. aceti were removed and distributed across a sterile 96-microwell plate, with the culture solution used as a blank control. The absorption of the suspension and the A. aceti culture solution were measured at 600 nm using the microplate reader and calculated as OD1 and OD2, respectively, with the final OD value of the A. aceti suspension taken as the difference OD1-OD2. The A. aceti suspension was centrifuged at 8000 rpm for 10 min to remove the supernatant; the precipitate (the A. aceti) was then washed once with 1 mL sterile PBS and centrifuged at 13000 rpm for 1 min to remove the supernatant. Thereafter, the precipitate was weighed and resuspended with sterile water for a final concentration of A. aceti at 0.1 mg/mL.
Detection of chromium and zinc in A. aceti: Collected samples were sent to Shanghai WEIPU Chemical Technology Service Co., Ltd, and the contents of chromium and zinc were detected by inductively coupled plasma mass spectrometry.
Detection of NAD+/NADH in A. aceti: The concentrations of NAD+/NADH in the A. aceti was determined using the NAD+/NADH assay kit with WST-8 (Beyotime Biotechnology).
Detection of glucose dehydrogenase in A. aceti: Solarbio’s “glucose dehydrogenase microplate assay kit” was used to detect glucose dehydrogenase concentrations in A. aceti, according to the instruction manual.
Construction of a diabetes mouse model: For the preparation of citrate buffer, 2.10 g of citric acid was treated with 100 mL of double distilled water to make a citric acid mother liquor (solution A), after which 2.94 g of trisodium citrate was treated with 100 mL of double-distilled water to make a sodium citrate mother liquor (solution B). Solution A and solution B were mixed in a ratio of 1:1.32 (or 1:1), and the pH value was measured with a pH meter and adjusted from 4.2 to 4.5. This represented the 0.1 mol/L sodium citrate-hydrochloric acid buffer solution required to prepare streptozotocin (STZ).
Seventy SPF grade C57BL/6J female mice, aged 6 wk and weighing 20 ± 2 g, were allowed to eat and drink without restrictions during 5 d of adjustable feeding. Six mice were randomly selected as the normal control group, and the rest were used for model construction.
To prepare the STZ required to inject the mice to cause diabetes, 24 mL of 0.1 mol/L sodium citrate buffer was treated with 120 mg of STZ away from the light (equivalent to a concentration of 5 mg/mL) and placed in an ice environment. The mice were made to fast for 10 h and then treated with STZ at a dose of 0.15 mL/10 g of mouse weight (equivalent to 75 mg/kg). STZ was administered by intraperitoneal injection for 3 consecutive days. Before each intraperitoneal injection, the STZ liquid was carefully pipetted with a 1 mL syringe to mix the precipitate before it was extracted to maintain the STZ concentration. After each intraperitoneal injection, mice were deprived of food and water for 90 min. On the 7th d after the last administration (the mice were made to fast for 10 h before blood collection), blood was collected from their caudal veins, and the fasting blood glucose (FBG) levels of the mice were measured with a Roche glucometer. Pathological models of mice with diabetes were confirmed as having been successfully established when FBG ≥ 16.7 mmol/L.
The diabetic mice were designated from high to low, according to their blood glucose levels, and the model mice were divided into a model group (PBS), positive control group (metformin), metal chromium plus zinc group (concentrations of chromium and zinc calculated as 1 × 10-7 mg/mL and 2 × 10-8 mg/mL, respectively, according to the highest content of chromium- and zinc-rich A. aceti), A. aceti group (OD = 1), and chromium- and zinc-rich A. aceti group (OD = 1). There were six mice in each group, and each was given 0.5 mL of treatment by gavage.
Evaluation of hypoglycemic activity in vivo: After the diabetic models were successfully established, the mice were given intragastric administration of treatment on the second day, once a day, for 15 consecutive days. The normal control group and the model group were given the same amount of PBS-water. The positive drug control group was given diformin tablets (ground into powder, prepared into a suspension with reverse osmosis water, and administered intragastrically at 0.320 g/kg/d). The metal chromium plus zinc group was given chromium trichloride (1 × 10-7 mg/mL) and zinc chloride (2 × 10-8 mg/mL) intragastrically. Through general observation, the activity and spirit of the mice, their eating and drinking, urine and feces, and the dryness and wetness of the bedding were observed every day during the 15 d of administration. Starting at the beginning of treatment, fasting blood glucose was measured every 3 d. After 15 d of administration, the mice were fasted for 10 h and blood was collected from the eyeballs. Thereafter, the mice were sacrificed, with the tissue taken from their pancreas islets, fixed with formaldehyde, and processed for hematoxylin and eosin (HE) staining and immunohistochemistry with the apoptosis-related Bax proteins. Tissue from pancreatic islets was also prepared for electron microscopy to analyze the ultrastructural damage and possible repair on tissues and cells of pancreatic islets. The weights of mice were recorded every 3 d.
Evaluation of MIN6 cell growth: A. aceti was collected (OD = 10), sonicated, and stored at -20 °C for later use. MIN6 cells, a pancreatic islet cell line, were revived, the cell concentration adjusted to 1 × 105 CFU/mL, and then cultured in 5 mmol/L and 25 mmol/L high-glucose 1640 medium on a 96-well plate with 90 mL/well. After 12 h, the cells were diluted 10-fold and dosed with 10 mL of collected A. aceti (with or without metal enrichment; OD = 10), the dose of which was equivalent to 1 × 107 CFU/mL of bacteria. A positive drug control group (diformin tablets) and negative drug control group (PBS) were included. Approximately 24 h after dosing, cell growth was detected with the CCK-8 kit (Beyotime Biotechnology).
Determination of insulin secretion in MIN6 cells: A. aceti were collected (OD = 10), sonicated, and stored at -20 °C for later use. MIN6 pancreatic islet cells were revived, the cell concentration adjusted to 1 × 105 CFU/mL, and cultured in 5 mmol/L and 25 mmol/L high-glucose 1640 medium on a 6-well plate with 1.98 mL/well. After 12 h, the cells were dosed with 20 mL A. aceti (with or without metal enrichment; OD = 10), the dose of which was equivalent to 1 × 107 CFU/mL of bacteria. A positive drug control group (diformin tablets) and negative drug control group (PBS) were included. Some 24 h after dosing, the cell supernatant was collected, with the insulin content detected by a commercially-available enzyme-immunized mouse insulin ELISA kit.
Chromium- and zinc-rich A. aceti and control A. aceti were collected, with the initial concentration adjusted to 1 × 104 CFU/mL. The glucose concentration of the liquid medium was detected with a blood glucometer in A. aceti cultured at 30 °C for 12 h, 24 h, 36 h, and 48 h.
Chromium- and zinc-rich A. aceti were collected and adjusted to 10 times the therapeutic dose (OD = 10). Aliquots of 1-mL were administered, respectively, to the mice three times a day for 7 consecutive days, with the body weights and the pathological changes of the organs detected.
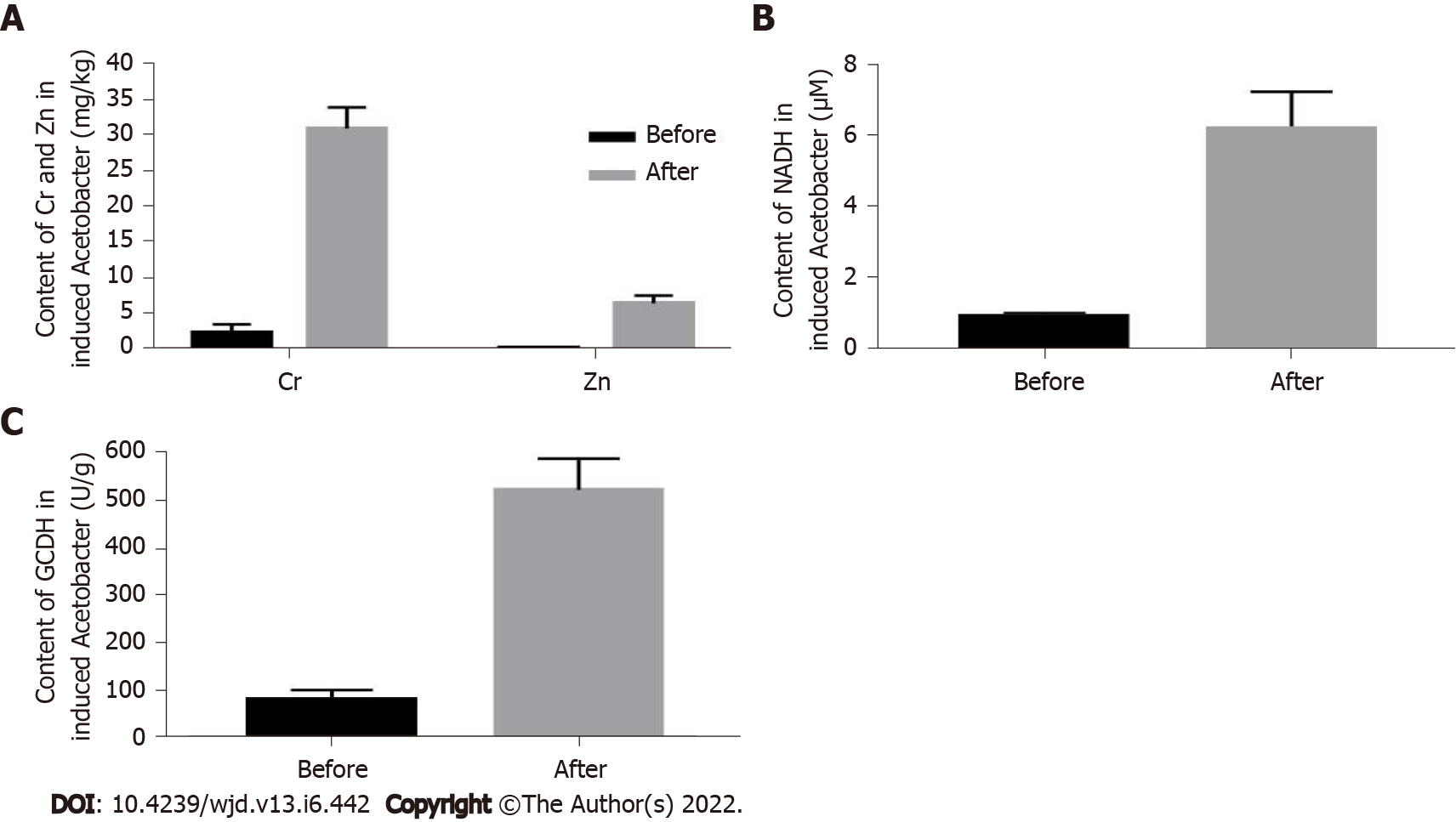
The concentration of chromium metal in the A. aceti prepared as described in the methods section above was 28.58-34.34 mg/kg, and the zinc metal concentration was 5.35-7.52 mg/kg, both of which were significantly higher than those in the untreated A. aceti (chromium: 1.05-2.29 mg/kg; zinc: 0.18-0.26 mg/kg) (Figure 1A). The concentration of NADH was 5.13 to 7.26 mM, which was significantly higher than that in the non-cultured A. aceti (0.86 to 1.02 mM) (Figure 1B). The concentration of glucose dehydrogenase was 446.812-567.138 U/g, which was significantly higher than that of non-cultivated A. aceti (54.126-93.651 U/g) (Figure 1C).
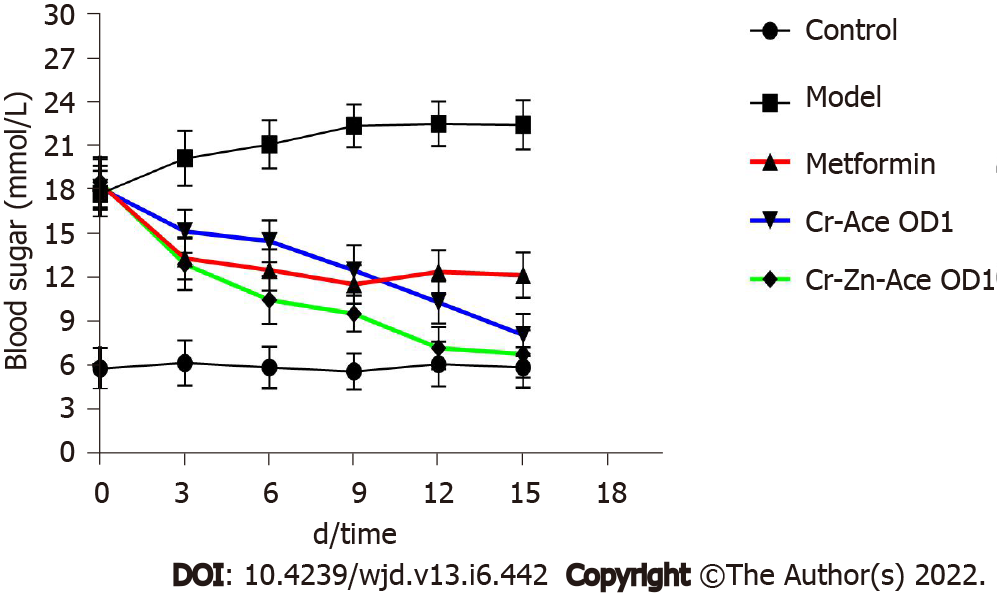
After 7 d of treatment, the diabetic mice (mice with FBG ≥ 16.7 mmol/L) treated with chromium- and zinc-rich A. aceti were found to be in a good mental state, with bright eyes and normal activity, normal intake of food and water, urinate, and normal defecation, with their beddings dry. The fasting blood glucose was detected every 3 d after the initial administration of treatment, and the diabetic mice treated with chromium- and zinc-rich A. aceti (OD = 1) had significantly lower blood glucose than mice in both the positive drug control group (diformin tablets) and the metal chromium plus zinc group (Figure 2).
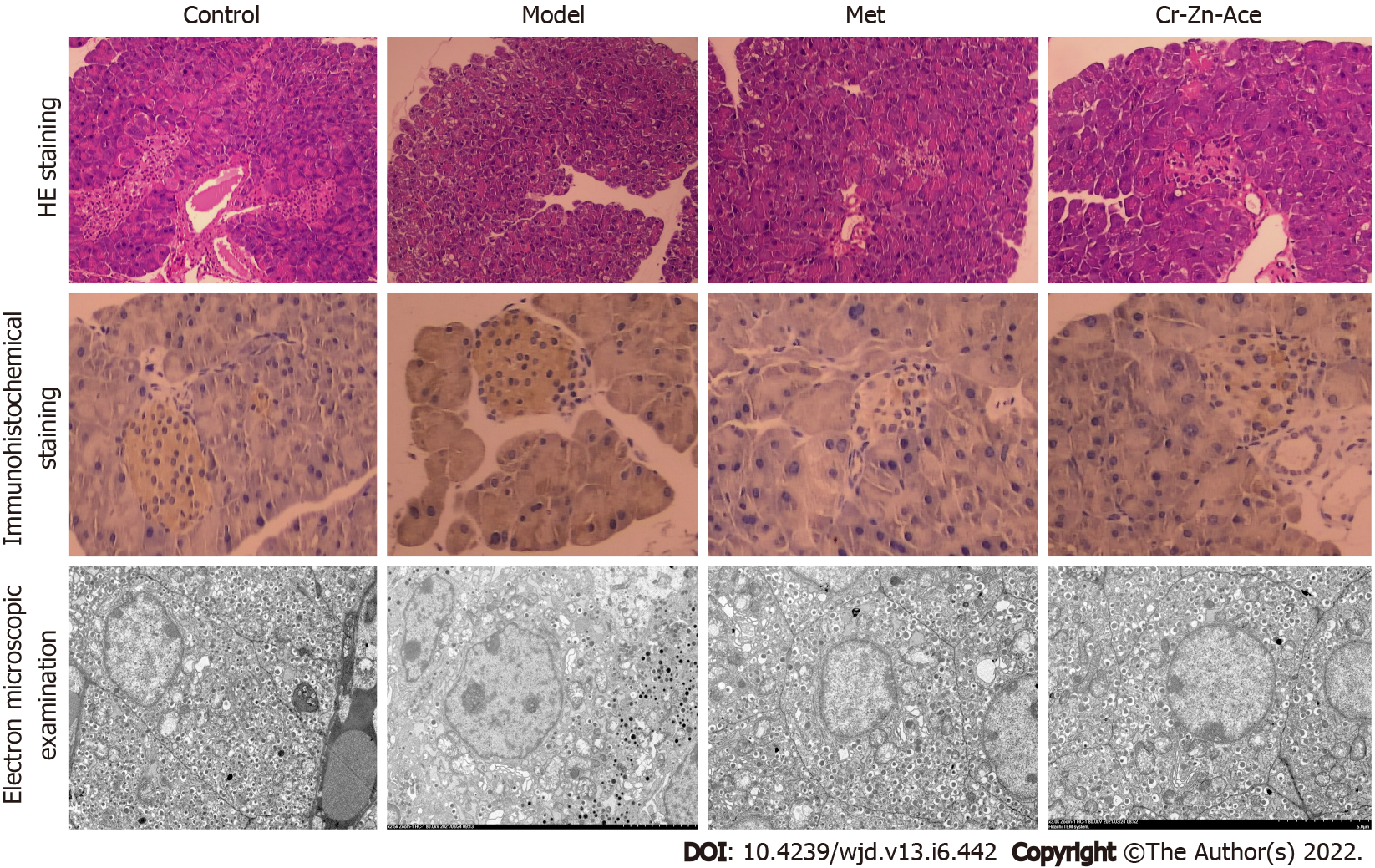
After 15 d of treatment, HE staining of the pancreas tissue indicated fewer cells undergoing apoptosis, less structural atrophy, and hardly any vacuoles detected in the chromium- and zinc-rich A. aceti group (OD = 1) (Figure 3). Immunohistochemistry analysis of apoptosis-related Bax proteins confirmed that the apoptosis of islet cells was significantly reduced (Figure 3). Electron microscopy of pancreas islet tissue confirmed that the ultrastructural damage was alleviated after treatment with the chromium- and zinc-rich A. aceti, with a small expansion range of endoplasmic reticulum, slightly swollen mitochondria, and the amount of autophagic vacuolization significantly reduced (Figure 3).
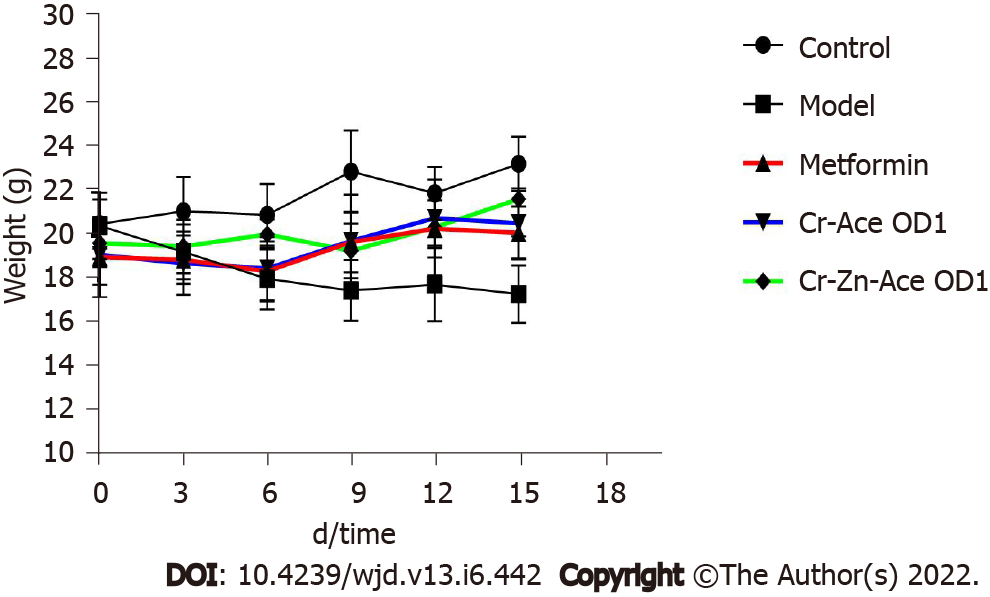
The body weights of the mice after treatment with chromium- and zinc-rich A. aceti were recorded every 3 d. The weights of diabetic mice in the chromium- and zinc-rich A. aceti (OD = 1) were found to recover well, with no significant difference from those of the positive drug control group (metformin), as displayed in Figure 4.
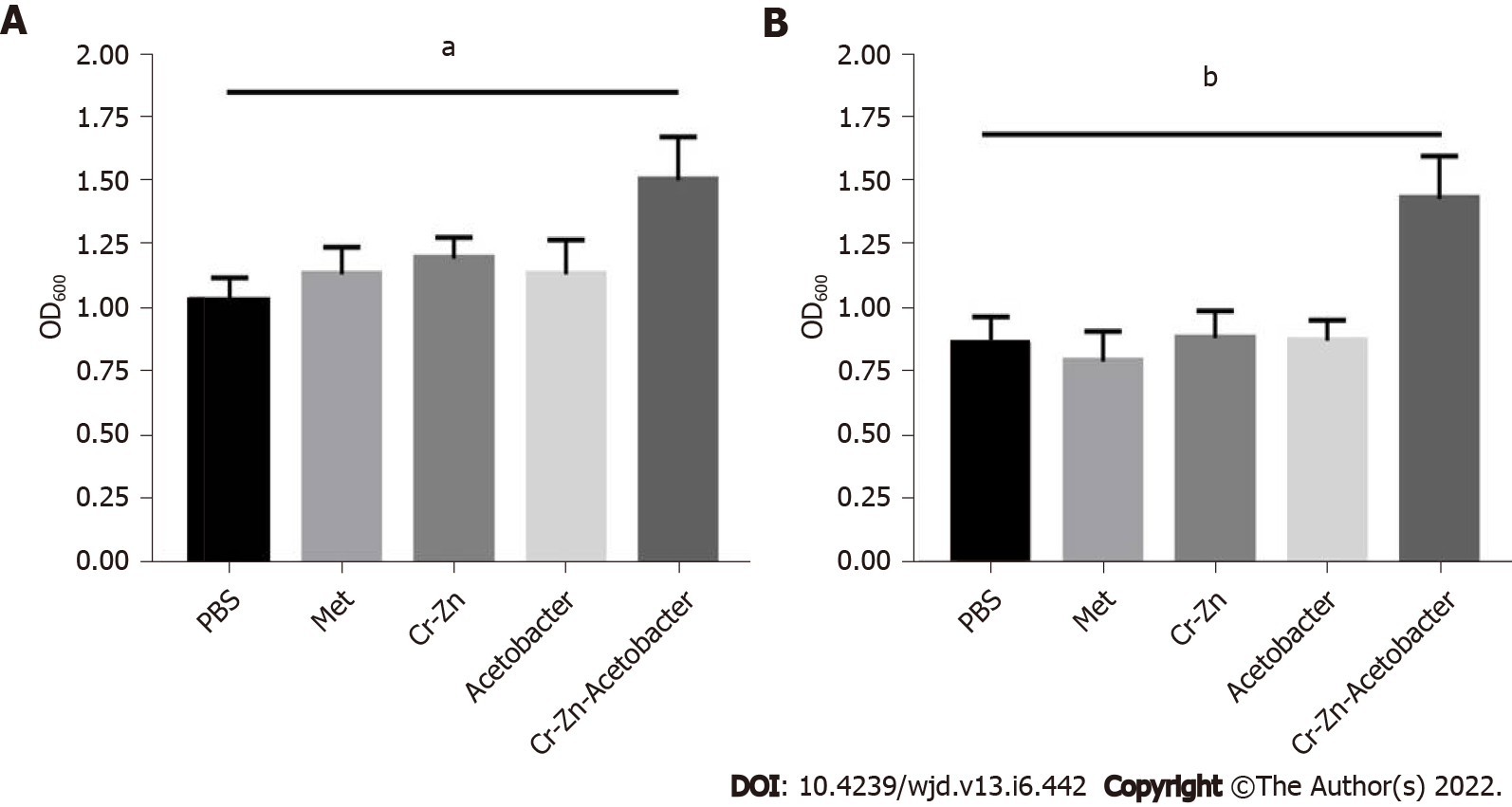
Chromium- and zinc-rich A. aceti promoted the growth of MIN6 cells significantly better than both the positive and negative control groups in the high glucose 1640 medium, as indicated by cytotoxicity measured with the CCK-8 kit (Figure 5). This result implied that chromium- and zinc-rich A. aceti could promote the growth of MIN6 pancreatic islet cells.
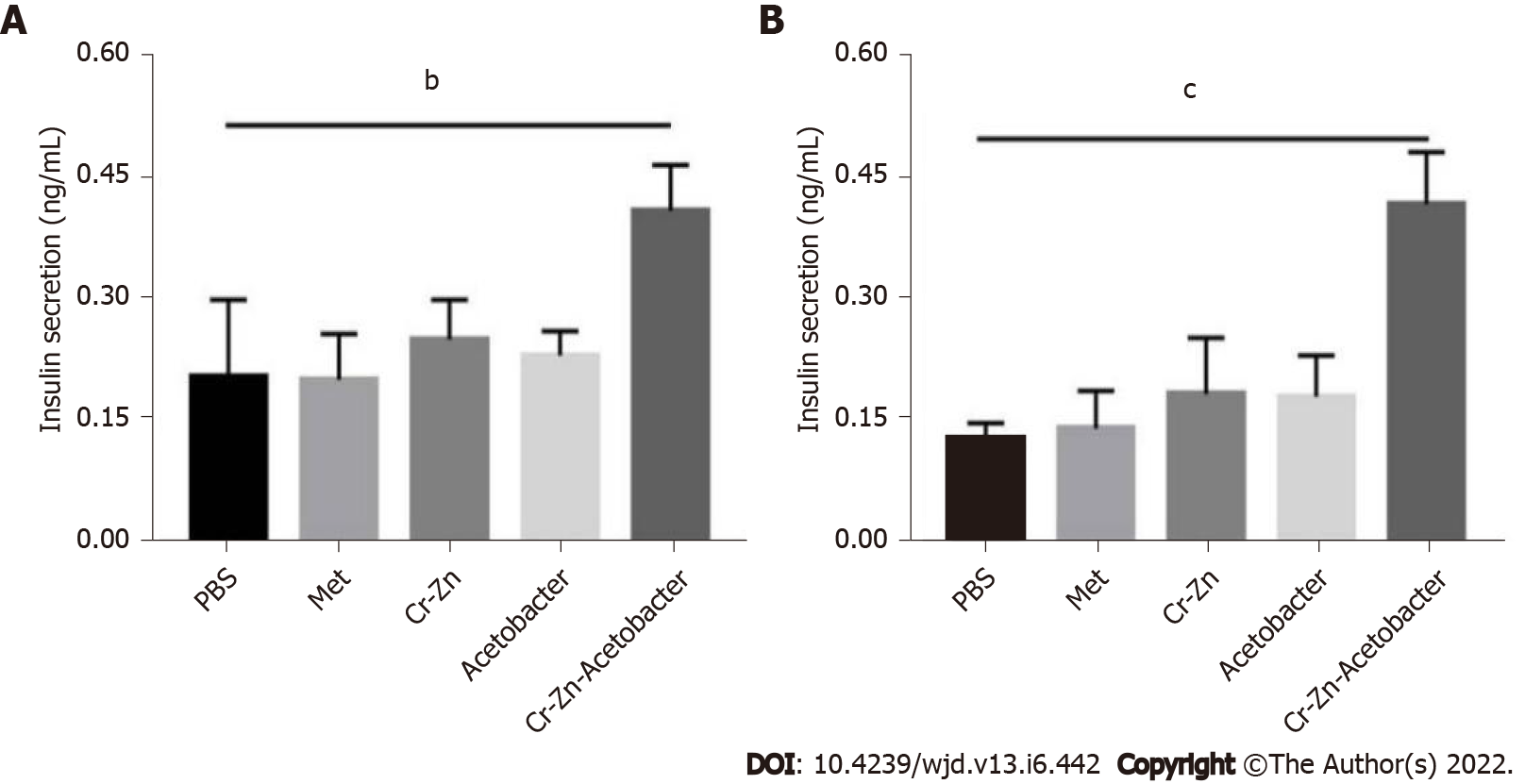
The insulin content of the supernatant in MIN6 cells incubated in the 25 mmol/L high-glucose 1640 medium treated with chromium- and zinc-rich A. aceti (OD = 10) group was significantly higher than that in the positive and negative control groups (Figure 6). This result implies that chromium- and zinc-rich A. aceti could promote insulin secretion of MIN6 islet cells.
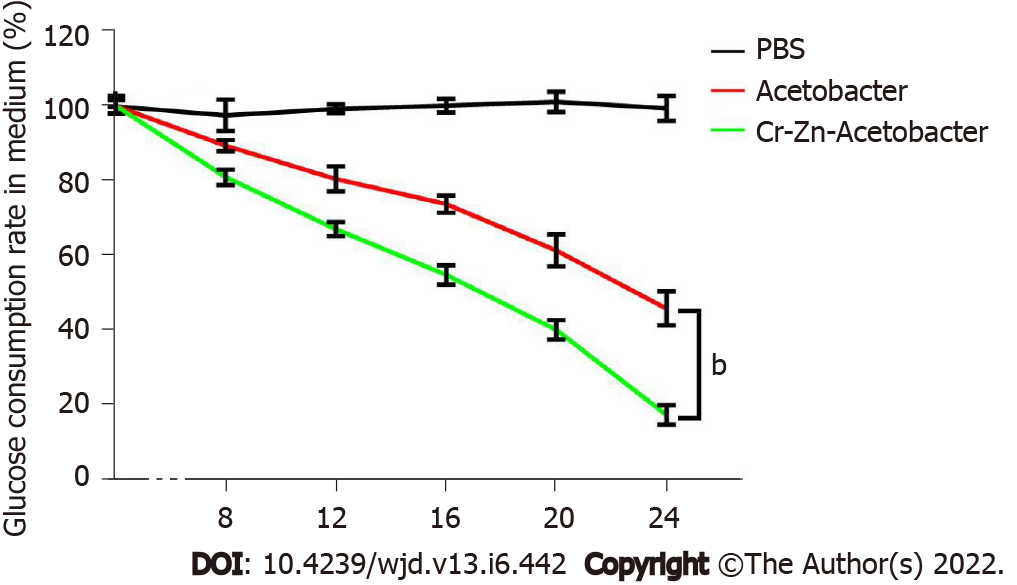
The glucose content in medium containing chromium- and zinc-rich A. aceti and control A. aceti both decreased significantly as incubation times increased; however, this decrease was significantly more pronounced for the chromium- and zinc-rich A. aceti compared to the A. aceti group (Figure 7). This result implies that chromium- and zinc-rich A. aceti are better at decomposing glucose than the untreated bacteria.
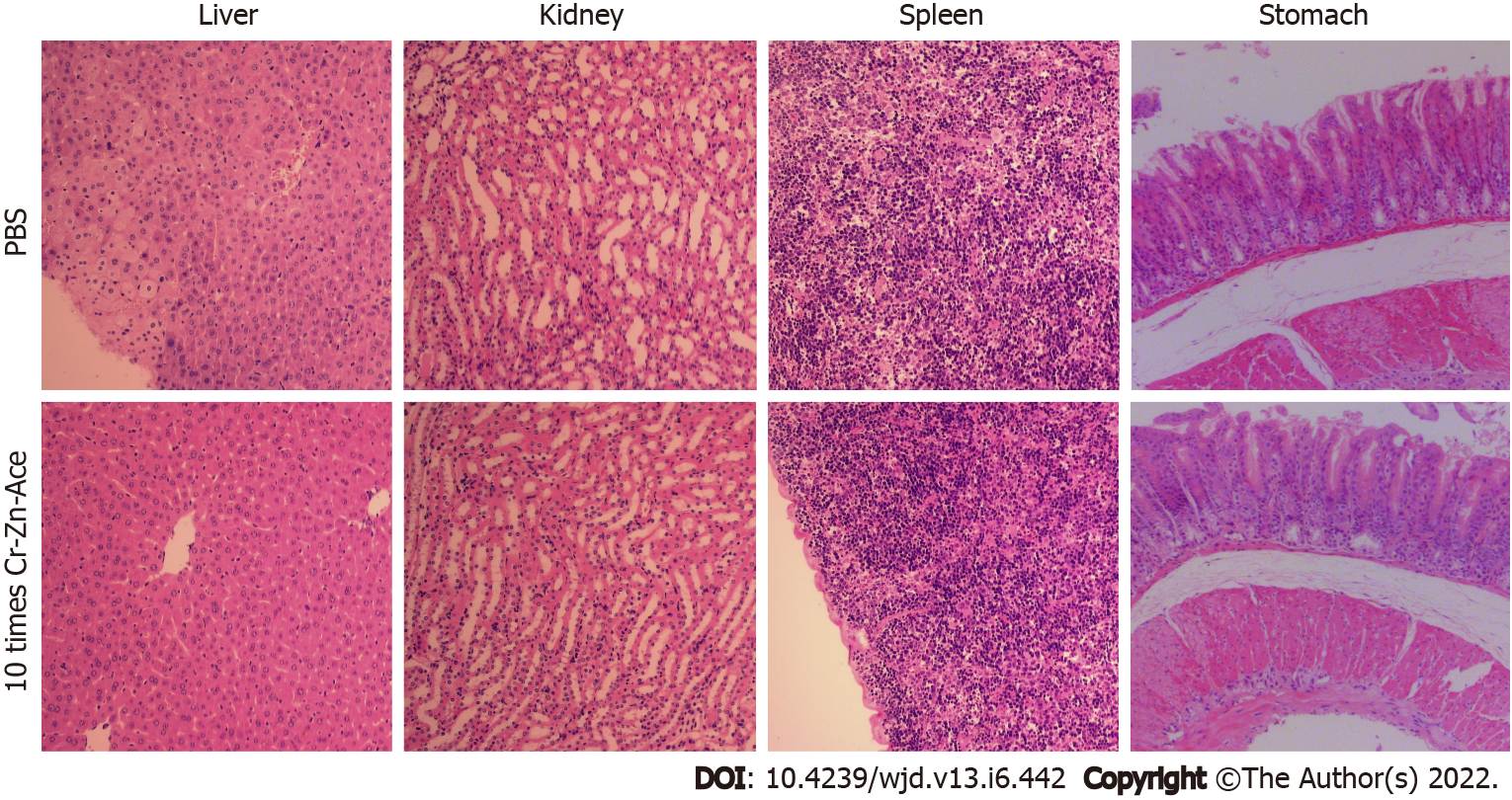
Ten times the dose of chromium- and zinc-rich A. aceti was administered to mice by gavage with no change detected in the weights and no pathological damage found in the liver, spleen, kidneys, and stomach (Figure 8). This result implies that A. aceti is biosafe.
At present, the incidence of diabetes is high, with no known cure. The conventional therapy is long-term use of hypoglycemic drugs and symptomatic treatment. However, in the long-term treatment process, drugs are prone to resistance among non-compliant patients who fail to adhere to treatment regimens. Therefore, in addition to helping patients control blood sugar long-term, it is vital that we clearly identify the risk factors for diabetes to prevent patients from developing this disease. An important area that may be significant in both the long-term treatment and prevention of diabetes is the use of supplemental micronutrients, as diabetic patients are often deficient in B vitamins and micronutrients, such as chromium, zinc, and selenium[14-16].
Chromium was designated as an essential microelement for the human body in 1989. The body absorbs Cr3+ mainly from food, including meat, whole grain, millet, pepper, etc. The daily intake for adults recommended by the United States Food and Drug Administration’s Center for Food Safety and Applied Nutrition Food Safety Recommendations Committee is 25-35 μg (as of 2001). Studies have found that plasma chromium levels are negatively correlated with the risk of type 2 diabetes and prediabetes. Chromium deficiency may lead to impaired glucose tolerance, insulin resistance, and elevated blood sugar. Chromium levels in the human body will decrease with age, with chromium deficiency becoming severe in old people[17-19]. According to evidence-based medicine meta-analysis studies, chromium increased insulin sensitivity mainly by activating insulin receptor kinase activity, inhibiting phosphatase activity, and increasing phosphorylation of insulin receptors[20,21]. Low molecular weight chromium binding substance was found to be combined with insulin receptors and activate tyrosine kinase activity on insulin P receptors, thus enhancing insulin signal transduction. However, at an insulin concentration of 100 nmol/L, the tyrosine kinase activity in insulin receptors was significantly enhanced if insulin was dosed with chromium[22,23]. In addition, it was found that chromium could also promote the translocation of GLUT4 to the cell membrane by activating protein kinase B and adenosine monophosphate to activate protein kinase signaling pathways and by activating the activity of p38 mitogen-activated protein kinase[24,25]. Despite much related research, the specific mechanism of lowering blood sugar through chromium supplementation remains unclear.
Zinc ions are an important part of the insulin molecule, maintaining the stability and biological effects of insulin[26-28]. Zinc plays a key role in the synthesis, storage, and secretion of insulin in pancreatic β cells and can increase the activity of the insulin signaling pathway. However, zinc deficiency can lead to a decrease in insulin secretion[29,30]. In addition, the formation of hexamers that contain zinc is required in the synthesis of insulin; therefore, zinc deficiency will lead to restricted insulin synthesis and reduced insulin sensitivity, thus increasing the risk of diabetes[31,32]. Studies have found that zinc lowers blood sugar mainly through antioxidant responses, inhibition of inflammatory factors, and anti-apoptosis effects[30,33]. Intracellular zinc has also been found to be regulated by zinc transporters, with the uptake, storage, and distribution regulated by metallothioneins[34,35]. Studies have found that zinc can inhibit the inflammatory response and has an anti-apoptosis effect at a concentration higher than 100 μmol/L[36]. Therefore, increasing the intake of zinc can increase the level of metallothionein, which helps mediate anti-apoptosis, etc[31,37]. Some scholars have suggested that the effects of a high-zinc intake on lowering blood sugar may be associated with its ability to reduce variation in zinc transporters[38]. Therefore, given the important role of zinc in glycemic control, a deficiency of zinc can lead to glucose metabolism disorders, while high intake may reduce the risk of glucose metabolism disorders and diabetes.
Above all, chromium and zinc supplementation can stabilize blood sugar in an indisputable manner. The key is how to supplement chromium and zinc through a proper, safe, and scientific approach. In the present study, chromium trichloride, zinc chloride, and A. aceti were co-cultured to induce the production of chromium- and zinc-rich A. aceti following simple protocols with high yield. Chromium- and zinc-rich A. aceti prepared using this method not only helped A. aceti exert the effect of decomposing glucose but also enhanced the hypoglycemic effect seen in untreated A. aceti. Because the chromium and zinc was transformed by the bacteria, biological safety was ensured, which means that it is possible that this can be used as a new hypoglycemic biological drug. The hypoglycemic mechanism of chromium- and zinc-rich A. aceti was preliminarily explored, with findings of these bacteria predominantly leading to increased dihydronicotinamide-adenine dinucleotide and glucuronide dehydrogenase levels in A. aceti, enhancing the ability to degrade glucose. In addition, its hypoglycemic mechanism was found to be not much different from those of chromium and zinc, which are metal microelements. However, because the source of nutrition for the growth of A. aceti is ethanol, these bacteria do not survive for long in the body, and, therefore, they cannot exert long-term hypoglycemic effects, which will be further addressed in future research.
Chromium trichloride and zinc chloride can be employed to induce the preparation of chromium- and zinc-rich A. aceti, which has a significantly enhanced hypoglycemic effect relative to normal A. aceti and can biotransform chromium and zinc in a way that improves the safety of administering these metals as a treatment for diabetes.
At present, there are no ideal drugs to treat diabetes. Acetobacter and other probiotics can play a role in the treatment of diabetes; however, their effect is not significant. In this study, metal compounds were used to enrich Acetobacter with chromium and zinc in an effort to enhance the hypoglycemic effect of these bacteria.
This research provides a theoretical basis for the application of new chromium- and zinc-rich Acetobacter aceti (A. aceti) to treat diabetes.
To prepare a new type of chromium- and zinc-rich A. aceti and explore its hypoglycemic effects on enhancing the application of probiotics in the treatment of diabetes.
A. aceti was cultured in a liquid medium that contained chromium trichloride and zinc chloride.
A new type of chromium and zinc rich A. aceti was successfully prepared.
Chromium- and zinc-rich A. aceti has a significantly enhanced hypoglycemic effect and can biotransform chromium and zinc to improve safety for administering these metals as a treatment.
The new method described has very good application prospects.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ajjan RA, United Kingdom; Lee CC, Taiwan; Yun JH, South Korea A-Editor: Liu X, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Kaneko K, Yatsuya H, Li Y, Uemura M, Chiang C, Hirakawa Y, Ota A, Tamakoshi K, Aoyama A. Risk and population attributable fraction of metabolic syndrome and impaired fasting glucose for the incidence of type 2 diabetes mellitus among middle-aged Japanese individuals: Aichi Worker's Cohort Study. J Diabetes Investig. 2020;11:1163-1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10:CD012661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 3. | National Centre for Statistics & Information of Oman Population Projections 2020-2040, 2017. [cited 1 January 2020]. Available from: https://ncsi.gov.om/Elibrary/LibraryContentDoc/ben_projection-Eng_87f44b27-0bf3-4a80-b2d2-1ead231a0165.pdf. |
| 4. | Awad SF, Al-Mawali A, Al-Lawati JA, Morsi M, Critchley JA, Abu-Raddad LJ. Forecasting the type 2 diabetes mellitus epidemic and the role of key risk factors in Oman up to 2050: Mathematical modeling analyses. J Diabetes Investig. 2021;12:1162-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Ryder JR, Northrop E, Rudser KD, Kelly AS, Gao Z, Khoury PR, Kimball TR, Dolan LM, Urbina EM. Accelerated Early Vascular Aging Among Adolescents With Obesity and/or Type 2 Diabetes Mellitus. J Am Heart Assoc. 2020;9:e014891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front Immunol. 2020;11:1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 7. | Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7:354-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 380] [Cited by in RCA: 372] [Article Influence: 41.3] [Reference Citation Analysis (13)] |
| 8. | Chawla R, Madhu SV, Makkar BM, Ghosh S, Saboo B, Kalra S; RSSDI-ESI Consensus Group. RSSDI-ESI Clinical Practice Recommendations for the Management of Type 2 Diabetes Mellitus 2020. Indian J Endocrinol Metab. 2020;24:1-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Hemmingsen B, Sonne DP, Metzendorf MI, Richter B. Dipeptidyl-peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;5:CD012204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Blahova J, Martiniakova M, Babikova M, Kovacova V, Mondockova V, Omelka R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 11. | Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335-1380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 12. | Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 253] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 13. | Zubaidah E, Afgani CA, Kalsum U, Srianta I, Blanc PJ. Comparison of in vivo antidiabetes activity of snake fruit Kombucha, black tea Kombucha and metformin. Biocatal Agric Biotechnol. 2018;17:465-469. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 14. | Cabral M, Kuxhaus O, Eichelmann F, Kopp JF, Alker W, Hackler J, Kipp AP, Schwerdtle T, Haase H, Schomburg L, Schulze MB. Trace element profile and incidence of type 2 diabetes, cardiovascular disease and colorectal cancer: results from the EPIC-Potsdam cohort study. Eur J Nutr. 2021;60:3267-3278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Bai J, Xun P, Morris S, Jacobs DR Jr, Liu K, He K. Chromium exposure and incidence of metabolic syndrome among American young adults over a 23-year follow-up: the CARDIA Trace Element Study. Sci Rep. 2015;5:15606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Nahas R, Moher M. Complementary and alternative medicine for the treatment of type 2 diabetes. Can Fam Physician. 2009;55:591-596. [PubMed] |
| 17. | Chen S, Jin X, Shan Z, Li S, Yin J, Sun T, Luo C, Yang W, Yao P, Yu K, Zhang Y, Cheng Q, Cheng J, Bao W, Liu L. Inverse Association of Plasma Chromium Levels with Newly Diagnosed Type 2 Diabetes: A Case-Control Study. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Ngala RA, Awe MA, Nsiah P. The effects of plasma chromium on lipid profile, glucose metabolism and cardiovascular risk in type 2 diabetes mellitus. A case - control study. PLoS One. 2018;13:e0197977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Ali A, Ma Y, Reynolds J, Wise JP Sr, Inzucchi SE, Katz DL. Chromium effects on glucose tolerance and insulin sensitivity in persons at risk for diabetes mellitus. Endocr Pract. 2011;17:16-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Feng W, Mao G, Li Q, Wang W, Chen Y, Zhao T, Li F, Zou Y, Wu H, Yang L, Wu X. Effects of chromium malate on glycometabolism, glycometabolism-related enzyme levels and lipid metabolism in type 2 diabetic rats: A dose-response and curative effects study. J Diabetes Investig. 2015;6:396-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Kolahian S, Sadri H, Shahbazfar AA, Amani M, Mazadeh A, Mirani M. The Effects of Leucine, Zinc, and Chromium Supplements on Inflammatory Events of the Respiratory System in Type 2 Diabetic Rats. PLoS One. 2015;10:e0133374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Zhang Q, Sun X, Xiao X, Zheng J, Li M, Yu M, Ping F, Wang Z, Qi C, Wang T, Wang X. Maternal Chromium Restriction Leads to Glucose Metabolism Imbalance in Mice Offspring through Insulin Signaling and Wnt Signaling Pathways. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Hua Y, Clark S, Ren J, Sreejayan N. Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem. 2012;23:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Ruth MR, Wang Y, Yu HM, Goruk S, Reaney MJ, Proctor SD, Vine DF, Field CJ. Vaccenic and elaidic acid modify plasma and splenocyte membrane phospholipids and mitogen-stimulated cytokine production in obese insulin resistant JCR: LA-cp rats. Nutrients. 2010;2:181-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Zhuo J, Zeng Q, Cai D, Zeng X, Chen Y, Gan H, Huang X, Yao N, Huang D, Zhang C. Evaluation of type 2 diabetic mellitus animal models via interactions between insulin and mitogenactivated protein kinase signaling pathways induced by a high fat and sugar diet and streptozotocin. Mol Med Rep. 2018;17:5132-5142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Hamedifard Z, Farrokhian A, Reiner Ž, Bahmani F, Asemi Z, Ghotbi M, Taghizadeh M. The effects of combined magnesium and zinc supplementation on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Lipids Health Dis. 2020;19:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Pompano LM, Boy E. Effects of Dose and Duration of Zinc Interventions on Risk Factors for Type 2 Diabetes and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Adv Nutr. 2021;12:141-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Cheng Y, Mao N, Fan J. Comment to Sylwia Olechnowicz-Tietz et al.: The risk of atherosclerosis in patients with chronic kidney disease. Int Urol Nephrol. 2013;45:1243-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Cooper-Capetini V, de Vasconcelos DAA, Martins AR, Hirabara SM, Donato J Jr, Carpinelli AR, Abdulkader F. Zinc Supplementation Improves Glucose Homeostasis in High Fat-Fed Mice by Enhancing Pancreatic β-Cell Function. Nutrients. 2017;9:1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Merriman C, Huang Q, Rutter GA, Fu D. Lipid-tuned Zinc Transport Activity of Human ZnT8 Protein Correlates with Risk for Type-2 Diabetes. J Biol Chem. 2016;291:26950-26957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Fukunaka A, Fujitani Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 32. | Liu Y, Batchuluun B, Ho L, Zhu D, Prentice KJ, Bhattacharjee A, Zhang M, Pourasgari F, Hardy AB, Taylor KM, Gaisano H, Dai FF, Wheeler MB. Characterization of Zinc Influx Transporters (ZIPs) in Pancreatic β Cells: roles in regulating cytosolic zinc homeostasis and insulin secretion. J Biol Chem. 2015;290:18757-18769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Choi S, Liu X, Pan Z. Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharmacol Sin. 2018;39:1120-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 253] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 34. | Aydemir TB, Troche C, Kim MH, Cousins RJ. Hepatic ZIP14-mediated Zinc Transport Contributes to Endosomal Insulin Receptor Trafficking and Glucose Metabolism. J Biol Chem. 2016;291:23939-23951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Giacconi R, Cai L, Costarelli L, Cardelli M, Malavolta M, Piacenza F, Provinciali M. Implications of impaired zinc homeostasis in diabetic cardiomyopathy and nephropathy. Biofactors. 2017;43:770-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Chang KL, Hung TC, Hsieh BS, Chen YH, Chen TF, Cheng HL. Zinc at pharmacologic concentrations affects cytokine expression and induces apoptosis of human peripheral blood mononuclear cells. Nutrition. 2006;22:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Sanna A, Firinu D, Zavattari P, Valera P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 38. | Fernández-Cao JC, Warthon-Medina M, H Moran V, Arija V, Doepking C, Serra-Majem L, Lowe NM. Zinc Intake and Status and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |