Published online Mar 15, 2022. doi: 10.4239/wjd.v13.i3.240
Peer-review started: August 12, 2021
First decision: October 3, 2021
Revised: October 13, 2021
Accepted: February 19, 2022
Article in press: February 19, 2022
Published online: March 15, 2022
Processing time: 215 Days and 5.5 Hours
Polycystic ovary syndrome (PCOS) is a common disorder in women of repro
To assess the hazard ratio (HR) of T2DM between women with/without PCOS.
This population-based, retrospective cohort study evaluated data retrieved from the National Health Insurance Research Database. The subjects were women with PCOS (n = 2545) identified on the basis of diagnosis, testing, or treatment codes, and women without PCOS as controls (n = 2545). The HR of T2DM between women with or without PCOS was the main outcome measure analyzed.
Our study found that, during a 10-year follow-up period, the overall incidence of T2DM was 6.25 per 1000 person-years in the PCOS group compared with 1.49 in the control group. After adjustment for potential confounding variables, the overall incidence of T2DM was higher in the PCOS group vs the control group (HR = 5.13, 95%CI: 3.51-7.48, P < 0.0001). The risk of developing T2DM subsequent to PCOS decreased with increasing diagnosis age: the adjusted HR was 10.4 in the 18–24-year age group, 5.28 in the 25-29-year age group, and 4.06 in the 29-34-year age group. However, no such significant association was noted in women older than 35 years.
These findings highlight the importance of prompting a more aggressive treatment to prevent diabetes in women diagnosed with PCOS at a young age, and, in contrast, the lessened importance of this type of intervention in women diagnosed with PCOS at a late reproductive age.
Core Tip: We aimed to evaluate the incidence of type 2 diabetes (T2DM) over time in women with polycystic ovary syndrome (PCOS) at different diagnosis ages, in comparison with non-PCOS controls. Our results showed that, among women diagnosed with PCOS at a young age, the incidence of T2DM was significantly higher than that of age-matched women in the general population. However, the risk disappeared among women diagnosed with PCOS after age 35. These findings highlight the importance of prompting a more aggressive treatment to prevent diabetes among women diagnosed with PCOS at a young age, and, in contrast, the lessened importance of this type of intervention in women diagnosed with PCOS at a late reproductive age.
- Citation: Liao WT, Huang JY, Lee MT, Yang YC, Wu CC. Higher risk of type 2 diabetes in young women with polycystic ovary syndrome: A 10-year retrospective cohort study. World J Diabetes 2022; 13(3): 240-250
- URL: https://www.wjgnet.com/1948-9358/full/v13/i3/240.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i3.240
Polycystic ovary syndrome (PCOS) is a common endocrine pathology that affects 5%-15% of women of reproductive age worldwide. The prevalence estimates vary according to the different diagnostic parameters applied[1]. It is also the most common cause of endocrine-related female infertility in the United States. This syndrome was first described by American gynecologists Irving F, Stein Sr. and Michael L Leventhal in 1935, when they reported a series of patients with enlarged polycystic ovaries, hirsutism, oligo/amenorrhea, and infertility. It has been demonstrated that PCOS includes a complex of systemic symptoms in addition to the reproductive apparatus. Over the last few decades, research studies have revealed that PCOS is strongly associated with metabolic disorders, including metabolic syndrome, obesity, insulin resistance, prediabetes, and type 2 diabetes (T2DM). The prevalence of metabolic syndrome in women with PCOS was approximately 6- to 7-fold higher than that detected in the controls[2,3]. According to a prospective case–control study, 64.4% of 271 patients with PCOS were noted to be insulin-resistant after adjusted for age, race, and body mass index (BMI)[4]. Based on clamp data, both obese and lean women with PCOS were more insulin-resistant compared with their weight-matched normal counterparts. In this study, insulin resistance (IR) was present in 75% of lean women with PCOS, 62% of overweight controls, and 95% of overweight women with PCOS[5]. Insulin resistance is defined as a reduced response of target tissues, such as the skeletal muscle and adipocytes. In women with PCOS, insulin-mediated glucose uptake is decreased by 35%-40% compared with age- and weight-comparable control women[6]. Because insulin resistance is the driving factor of hyperglycemia, women with POCS are particularly at risk of developing T2DM. The estimated prevalence of impaired glucose tolerance (IGT) and T2DM was 31%-37% and 7.5%-10.0%, respectively, in women with PCOS in the United States[7-9]. In two prospective trials of women with PCOS conducted in the United States and Turkey, after an average follow-up period of 2-3 years, both studies revealed a higher IGT and T2DM conversion rate compared with women without PCOS[9,10]. Abundant strong evidence supports the contention that diabetes is much more prevalent in women with PCOS than it is in the general population. We noticed that, even at a young age, women with PCOS also exhibit β-cell dysfunction, IGT, and T2DM[11,12]. Therefore, we aimed to evaluate the incidence of T2DM over time in women with PCOS at different diagnosis ages, in comparison with non-PCOS controls. We selected the National Health Insurance Research Database (NHIRD), which records age, gender, diagnosis codes, comorbidities, and the clinical prescriptions for each beneficiary, as the data source.
In this population-based retrospective cohort study, we used data from individuals in the Longitudinal Health Insurance Database 2000 (LHID2000), to evaluate the outcomes. LHID2000 is a subset of the NHIRD that contains the entire original claim data of 1000000 individuals randomly sampled from the 2000 registry for beneficiaries (ID) of the NHIRD, which maintains the registration data of everyone who was a beneficiary of the National Health Insurance (NHI) program during the period of 1996-2013. There are approximately 23.75 million individuals in this registry. The complete registration and claim data of these 1000000 individuals collected by the NHI program constitute the LHID2000. There was no significant difference in the gender distribution (χ2 = 1.74, df = 1, P value = 0.187) between the patients in the LHID2000 and those in the original NHIRD[13]. The data recorded in the LHID2000 include demographic information; prescription details; clinical events; diagnosis codes in accordance with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) system; and medical examinations and managements for all admitted patients and outpatients. In this study, we used LHID2000 from 1997 to 2010 as the research database, and followed to December 2013. This study was approved by the institutional review board of China Medical University in central Taiwan (CMUH104-REC2-115).
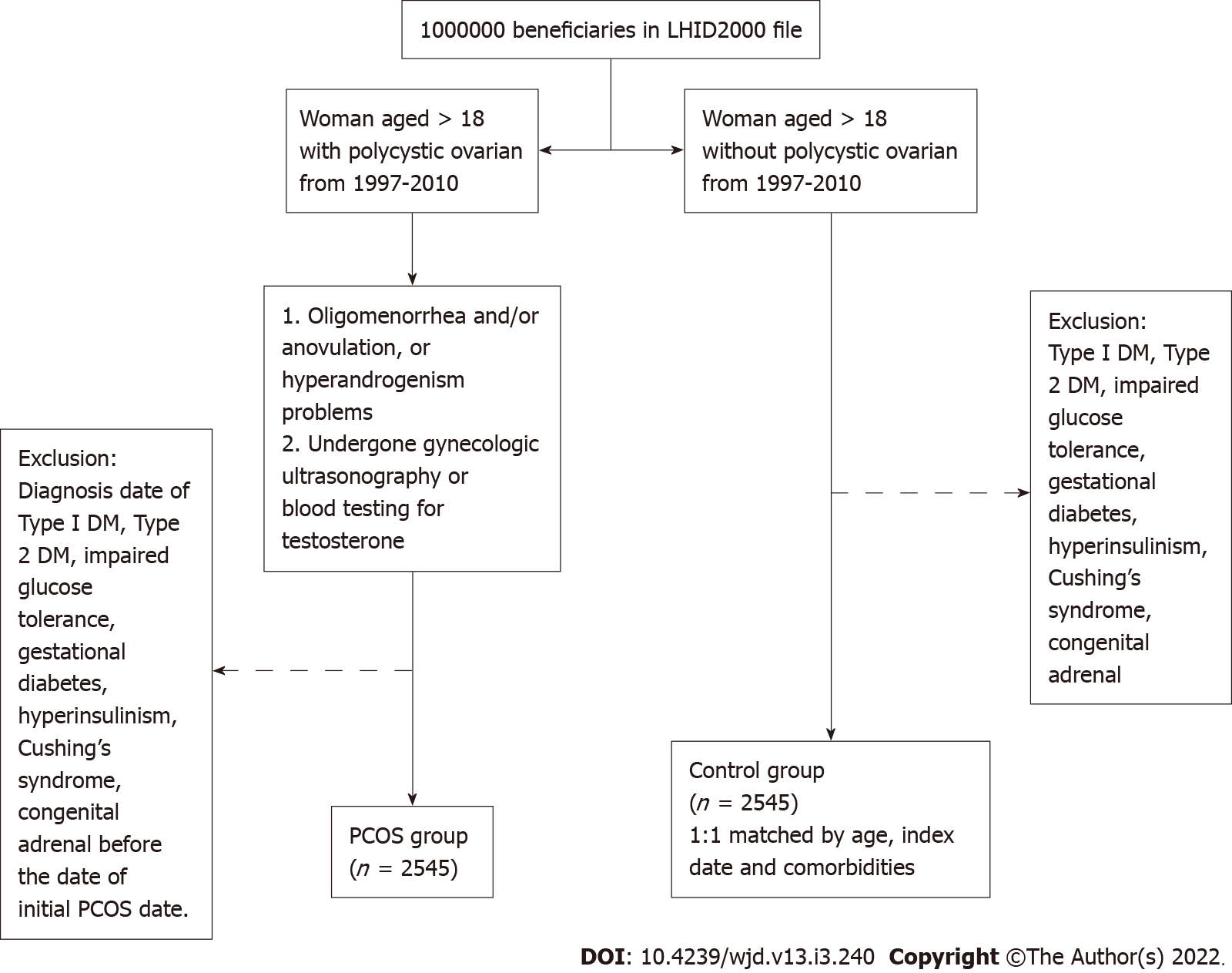
In this retrospective cohort study, we aimed to compare women with PCOS with those without PCOS. We selected the PCOS cohort as follows: (1) Women older than 18 years of age and diagnosed with polycystic ovarian morphology; (2) Clinical visit for oligomenorrhea and/or anovulation problems, or hyperandrogenism problems at least twice a year; and (3) Women who underwent gynecological ultrasonography or blood testing for testosterone or 17-hydroxyprogesterone levels. Women were eligible to participate when all three conditions were met. Patients with the diagnoses of type 1 diabetes, T2DM, IGT, gestational diabetes, hyperinsulinism, Cushing’s syndrome, and congenital adrenal hyperplasia before the date of initial PCOS diagnosis and those who were aged less than 18 years were excluded from the cohort. According to the inclusion and exclusion criteria, a total of 2545 people were defined as the PCOS group in this study (Figure 1). The factors associated with PCOS that are considered as potential confounding variables include lipid metabolism disorders, coronary artery disease, hypertension, chronic kidney disease, cerebrovascular accident, female infertility, obesity, chronic lymphocytic thyroiditis, major depression, and a history of anxiety before baseline. Prescriptions during follow-up for menstrual cycle regulation, ovulation induction, anti-androgen, and metformin were also considered potential confounding variables. The index date for the cohort group was assigned as the first time of recording of the ICD-9-CM code. The end point was set on the date of the new diagnosis of T2DM (more than three times at outpatient department or once in admission), the date of withdrawal from the NHI program, or the end of 2013. For the control group, women without PCOS were randomly selected and 1:1 frequency matched the cohort group by age, index date, and comorbidities. The comorbidities controlled in this study were lipid metabolism disorders, hypertension, coronary artery disease, chronic kidney disease, cerebrovascular accident, infertility, obesity, Hashimoto’s disease, major depression, and anxiety.
The baseline characteristics of women with PCOS and controls are described by numbers and percentages. An intergroup comparison was performed using the chi-squared and t-test for categorical variables and continuous variables, respectively. The incidence rates of T2DM were calculated in person-years. We used univariable and multivariable Cox proportional hazard regression models to estimate and adjust the crude hazard ratio (HR). After adjustment for key covariates (age, comorbidity), we calculated the adjusted HR together with 95%CIs with statistical significance set at P < 0.05. Survival curves were estimated for each group, considered separately using the Kaplan–Meier method and compared statistically using the log-rank test. The Kaplan–Meier curves of the cumulative incidence of T2DM between the PCOS group and the control group were performed to estimate the cumulative probability of T2DM between two groups. All analyses were performed using the SAS software (version 9.4 for windows; SAS Institute, Cary, NC, United States).
The study was reviewed by our expert biostatistician Dr. Jing-Yang Huang.
The first possible date for cohort entry (the study start date) was January 1, 1997, and patients could enter the cohort until December 31, 2010. The end of the follow-up time is December 2013. During the study period, we identified 2545 women with PCOS. These women were frequency matched at 1:1 to 2545 individuals in the non-POCS control group. The PCOS group and the control group were both followed for a mean period of 10 years, and the standard deviation was 3.14 vs 3.15 years (Table 1). In the baseline characteristic of the patients, all enrollees are stratified by age. The highest proportion of patients were into the 18-24-year age group (58.8%), followed by the 25-29-year age group (22.9%). The proportion of women over 30 years of age was only 18.3%. As expected, the age and comorbidity distributions of these two groups were similar because the groups were 1:1 propensity-score matched. Their mean age was 25 years, and there was no difference in the age stratification between the two groups. Women with PCOS were more likely to receive a prescription of metformin, oral contraceptive pills (OCPs), clomiphene citrate, and spironolactone.
| PCOS | |||||
| Yes (n = 2545) | No (n = 2545) | P value | |||
| n | % | n | % | ||
| Age, yr | > 0.99 | ||||
| 18-24 | 1497 | 58.8 | 1497 | 58.8 | |
| 25-29 | 583 | 22.9 | 583 | 22.9 | |
| 30-34 | 289 | 11.4 | 289 | 11.4 | |
| 35-39 | 117 | 4.60 | 117 | 4.60 | |
| 40-44 | 45 | 1.77 | 45 | 1.77 | |
| ≥ 45 | 14 | 0.55 | 14 | 0.55 | |
| mean ± SD | 25.1 ± 5.81 | 25.2 ± 5.91 | 0.63 | ||
| Comorbidity | |||||
| Disorders of lipid metabolism | 38 | 1.49 | 38 | 1.49 | > 0.99 |
| Cardiovascular disease | 3 | 0.12 | 8 | 0.31 | 0.13 |
| Hypertension | 27 | 1.06 | 27 | 1.06 | > 0.99 |
| Chronic kidney disease | 2 | 0.08 | 4 | 0.16 | 0.41 |
| Cerebrovascular accident | 7 | 0.28 | 8 | 0.31 | 0.80 |
| Infertility | 151 | 5.93 | 151 | 5.93 | > 0.99 |
| Obesity | 27 | 1.06 | 27 | 1.06 | > 0.99 |
| Hashimoto’s disease | 5 | 0.20 | 4 | 0.16 | 0.74 |
| Major depression | 18 | 0.71 | 27 | 1.06 | 0.18 |
| Anxiety | 169 | 6.64 | 169 | 6.64 | > 0.99 |
| Medication (during follow-up period) | |||||
| Metformin | 238 | 9.35 | 18 | 0.71 | < 0.0001 |
| OCPs | 443 | 17.4 | 72 | 2.83 | < 0.0001 |
| Clomiphene | 1384 | 54.4 | 302 | 11.9 | < 0.0001 |
| Spironolactone | 111 | 4.36 | 32 | 1.26 | < 0.0001 |
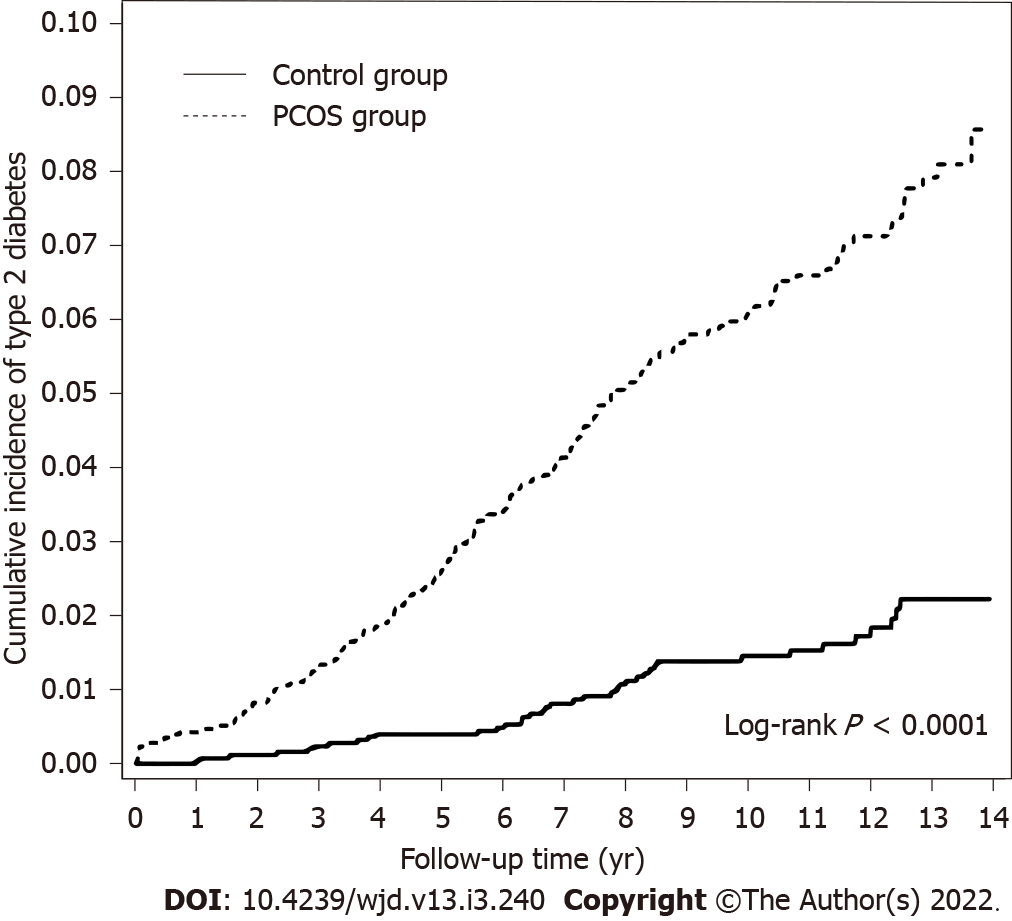
In the PCOS group, the overall incidence of T2DM was 6.25 per 1000 person-years compared with 1.49 in the control group (Table 2). After adjustment for potential confounding variables (age, all comorbidities, and the medications listed in Table 1), the incidence of T2DM was higher in the PCOS group compared with the control group (HR = 5.13, 95%CI: 3.51-7.48, P < 0.0001). Moreover, the PCOS group showed a higher incidence of T2DM in the 18-24-year age group (HR = 10.4, 95%CI: 5.04-21.4, P < 0.0001). The incidence of T2DM decreased with the increasing diagnosis age. However, no such significant association was noted in women older than 35 years. All participants were stratified according to the presence or absence of comorbidities or of medication. Among women without comorbidities and no medication, the PCOS group exhibited a higher incidence of T2DM compared with the control group (non-comorbidity stratifications: adjusted HR = 7.62, 95%CI: 4.68-12.4; non-metformin stratifications: adjusted HR = 5.41, 95%CI: 3.67-7.98; non-OCP stratifications: adjusted HR = 5.18, 95%CI: 3.54-7.58; non-clomiphene stratifications: adjusted HR = 5.93, 95%CI: 3.94-8.92; non-spironolactone stratifications: adjusted HR = 5.07, 95%CI: 3.47-7.41). The Kaplan–Meier curves present the differences in the cumulative incidence of T2DM between the PCOS group and the control group (Figure 2). The cumulative incidence of T2DM in the PCOS group (dashed line) was significantly higher than that observed in the control group (solid line) (log-rank test, P < 0.001).
| PCOS | ||||||||||
| No | Yes | Crude | Adjusted | |||||||
| Event | PY | IR | Event | PY | IR | HR (95%CI) | P value | HR (95%CI) | P value | |
| Overall | 38 | 25483 | 1.49 | 159 | 25460 | 6.25 | 4.19 (2.94, 5.97) | < 0.0001 | 5.13 (3.51, 7.48) | < 0.0001 |
| Age | ||||||||||
| 18-24 | 9 | 15453 | 0.58 | 74 | 15301 | 4.84 | 8.33 (4.17, 16.6) | < 0.0001 | 10.4 (5.04, 21.4) | < 0.0001 |
| 25-29 | 9 | 5630 | 1.60 | 40 | 5766 | 6.94 | 4.32 (2.10, 8.90) | < 0.0001 | 5.28 (2.42, 11.5) | < 0.0001 |
| 30-34 | 9 | 2753 | 3.27 | 27 | 2734 | 9.88 | 3.01 (1.42, 6.40) | 0.004 | 4.06 (1.73, 9.53) | 0.001 |
| 35-39 | 6 | 1117 | 5.37 | 11 | 1134 | 9.70 | 1.81 (0.67, 4.90) | 0.24 | 2.14 (0.72, 6.35) | 0.17 |
| 40-44 | 5 | 399 | 12.53 | 5 | 397 | 12.59 | 1.03 (0.30, 3.55) | 0.97 | 1.68 (0.38, 7.41) | 0.50 |
| ≥ 45 | 0 | 132 | 0.00 | 2 | 128 | 15.63 | ||||
| Comorbidity1 | ||||||||||
| Yes | 17 | 3864 | 4.40 | 32 | 3835 | 8.34 | 1.90 (1.06, 3.43) | 0.03 | 2.14 (1.14, 3.99) | 0.02 |
| No | 21 | 21620 | 0.97 | 127 | 21625 | 5.87 | 6.05 (3.81, 9.60) | < 0.0001 | 7.62 (4.68, 12.4) | < 0.0001 |
| Medication | ||||||||||
| Metformin | ||||||||||
| Yes | 2 | 191 | 10.47 | 21 | 2325 | 9.03 | 0.88 (0.21, 3.75) | 0.86 | 0.54 (0.1, 2.78) | 0.46 |
| No | 36 | 25292 | 1.42 | 138 | 23135 | 5.96 | 4.19 (2.91, 6.05) | < 0.0001 | 5.41 (3.67, 7.98) | < 0.0001 |
| OCPs | ||||||||||
| Yes | 0 | 789 | 0.00 | 17 | 4559 | 3.73 | ||||
| No | 38 | 24695 | 1.54 | 142 | 20901 | 6.79 | 4.42 (3.09, 6.32) | < 0.0001 | 5.18 (3.54, 7.58) | < 0.0001 |
| Clomiphene | ||||||||||
| Yes | 5 | 3349 | 1.49 | 72 | 14470 | 4.98 | 3.41 (1.38, 8.43) | 0.008 | 3.26 (1.3, 8.21) | 0.01 |
| No | 33 | 22135 | 1.49 | 87 | 10990 | 7.92 | 5.33 (3.57, 7.95) | < 0.0001 | 5.93 (3.94, 8.92) | < 0.0001 |
| Spironolactone | ||||||||||
| Yes | 0 | 349 | 0.00 | 4 | 1211 | 3.30 | ||||
| No | 38 | 25135 | 1.51 | 155 | 24249 | 6.39 | 4.23 (2.97, 6.04) | < 0.0001 | 5.07 (3.47, 7.41) | < 0.0001 |
To our knowledge, this was the first attempt to analyze large-scale data to evaluate the relationship between women with PCOS and the development of T2DM in an East-Asian cohort. Moreover, this was the only study that stratified the cohorts into subgroups based on the age at diagnosis.
Our study found that, during a 10-year follow-up period, women with PCOS were associated with 5-fold higher risk of developing T2DM compared with women without PCOS. In past studies, the incidence of T2DM in women with PCOS presented with substantial clinical heterogeneity (ranging from 2- to 7-fold). There may be several explanations for these marked differences. First, different ethnic backgrounds may be responsible for the higher prevalence of T2DM. A small-size prospective trial carried out in the eastern Mediterranean region showed that 11.5% of women with PCOS and normal glucose tolerance (NGT) at the baseline converted to IGT with an annualized incidence rate of 4.5%. Furthermore, the annualized incidence rate from IGT converted to T2DM was 10.4%. In comparison, another similar study conducted in the United States reported that, among women with PCOS, the annualized conversion risk was 16% from NGT to IGT, and 2% from IGT to T2DM[9,10]. A nationwide population-based retrospective cohort study performed in Denmark found that the HR for women with PCOS who developed T2DM was 3.5 (95%CI: 3.2-3.8) when gestational diabetes mellitus was excluded. The results of the Danish study were slightly lower than our current findings (HR = 5.13, 95%CI: 3.51-7.48)[13]. The different ethnic backgrounds may be responsible for the higher prevalence of T2DM detected in Taiwan. A meta-analysis of multiple quality studies calculated an increased prevalence of IGT and T2DM among women with PCOS and different ethnicities (OR for IGT, Asia = 5.22, Americas = 4.4, Europe = 2.59)[14]. Genome-wide association studies (GWASs) have become a feasible option for studying the genetic background of PCOS, thus providing the ability of surveying a large number of genomes at once[15]. Two GWASs targeting PCOS have been performed in China; they identified 11 variants associated with PCOS risk in Han Chinese women who were diagnosed with PCOS (i.e., who fulfilled all three Rotterdam criteria)[16,17]. However, not all loci for PCOS have been replicated in European women, which may speak to the variation in susceptible single-nucleotide polymorphisms (SNPs) among distinct racial and ethnic groups[18]. Some researchers believe that different combinations of SNPs may underlie the severity of the PCOS phenotypes, with Americans and Asians being more often characterized by the metabolic phenotype, and Europeans and Middle-Eastern women having a higher prevalence of hyperandrogenic phenotype[19]. Therefore, we assume that ethnicity may affect the transition from PCOS to diabetes[14].
Second, it may be related to the age at diagnosis of PCOS. This was also the most important finding of our study. There are indications that age may affect the incidence rate of conversion from PCOS to T2DM. According to a prospective study with a follow-up of 18 years performed in the United States, 53 women fulfilled the criteria for PCOS at ages 20-32 (average, 26 years). Compared with those without PCOS, women with persistent PCOS had a 7-fold odds of developing diabetes[20]. Another 10-year follow-up study performed in China among women with PCOS aged 30-39 years reported that the age-standardized incidence rate of T2DM was approximately 3-fold compared with women without PCOS[21]. It is well established that the PCOS phenotype changes with aging, the improvement of phenotype, and oligo-ovulation, as indicated by the decrease in serum androgen levels (e.g., testosterone, free androgen index, calculated free testosterone, androstenedione, and dehydroepiandrosterone sulfate) and increase in the number of regular menstrual cycles[22-25]. In healthy women, the positive correlation between age and worsening glucose tolerance is obvious after adjusting for BMI[26]. Interestingly, not only ovarian dysfunction and hyperandrogenism, but also insulin resistance, ameliorate during aging in women with PCOS[20]. According to a cross-sectional study, homeostasis model assessment (HOMA)-IR was negatively associated with age in women with PCOS as well as in different BMI subgroups, namely lean, normal-weight, and overweight subjects[27]. The observations that BMI and androgens are positively associated with HOMA-IR and that androgens decline with time suggest that these women achieved a better metabolic profile at their late reproductive ages. In a long-term prospective cohort study with a follow-up of more than 10 years, Kazemi Jaliseh et al[28] found that the adjusted HR for T2DM in women with PCOS aged ≤ 40 years was 4.9. In contrast, there was no difference between the two groups regarding the incidence rates of T2DM after the age of 40 years. The study included 178 women with PCOS and 1524 women without PCOS, and all PCOS cases were defined using the National Institutes of Health 1990 criteria, which carry the strongest clinical significance. The hazard differences between women with PCOS and those in the general population disappeared in their late reproductive years, which is in line with the results of the current study. Women who were diagnosed with PCOS before the age of 25 were 10 times more likely to develop T2DM compared with women without PCOS after adjusting for variance. The risk of developing T2DM subsequent to PCOS decreased with increasing diagnosis age: the adjusted HR was 10.4 in the 18-24-year age group, 5.28 in the 25-29-year age group, and 4.06 in the 29-34-year age group. Although the risk decreased with increasing age, it remained higher compared with that detected in women without PCOS. After age 35, the association between PCOS and T2DM was not statistically significant. Furthermore, among women without comorbidities and taking no medications, the incidence of T2DM was higher in the PCOS group than that in the control group. Several reasons for this result have been identified. First, women with PCOS who had no comorbidities showed a higher incidence of T2DM than the overall average, which means that the health problems caused by PCOS may be higher than previously recognized. Second, women without comorbidities and taking no medications may be relatively younger, which corroborates the previous assumption that women who are diagnosed with PCOS at a young age are more likely to develop T2DM. However, the sample size in the stratification of no medication is notably very small and may not provide reliable estimates and conclusive results.
The strength of our study consisted in the fact that NHIRD is one of the largest and most comprehensive nationwide population reimbursement databases in the world, as it covers almost 23 million residents in Taiwan with universal coverage. It provides a big sample size and complete records of medical visits and treatment, which are conducive to a longitudinal study design and age stratification. Furthermore, research conducted using NHIRD can avoid a selection bias and the possibility of recall bias in questionnaire assessments.
The limitation of this study was that certain prognostic factors that are associated with the incidence of T2DM are not available through the NHIRD; namely, BMI, waist-hip ratio, lifestyle, and the results of blood tests (androgen and plasma glucose levels). Thus, we were unable to rule out the possibility that the differences in HR detected between the two groups stemmed from these factors. Moreover, NHI covers 96%-99% of Taiwan’s population and 93% of hospitals and clinics are NHI-contracted. It subsidizes most medical treatments at a relatively low cost. However, there is still a possibility that patients reviewed in this study might have consulted other doctors before entering the NHI system. In addition, the sample size of the groups of women diagnosed with PCOS after the age of 35 years was relatively small, which may have led to imprecise estimates and statistical significance. Finally, the study population was homogeneous because all women were Asian. Therefore, additional research is required to substantiate this association among non-Asian women as well.
The data supplied here were from a relatively large population, spanning a long period. Our results showed that, among women diagnosed with PCOS at a young age, the incidence of T2DM was significantly higher than that of age-matched women in the general population. However, the risk disappeared among women diagnosed with PCOS after age 35. These findings highlight the importance of prompting a more aggressive treatment to prevent diabetes among women diagnosed with PCOS at a young age, and in contrast, the lessened importance of this type of intervention in women diagnosed with PCOS at a late reproductive age.
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women of reproductive age. Research over the last few decades has revealed that PCOS is strongly associated with metabolic disorders. Even at a young age, women with PCOS also exhibit β-cell dysfunction, impaired glucose tolerance, and type 2 diabetes (T2DM).
Although current evidence supports the contention that diabetes is much more prevalent in women with PCOS than it is in the general population. The majority of longitudinal studies regarding the incidence of T2DM in women with PCOS are from non-Asian countries.
We aimed to evaluate the incidence of T2DM over time in women with PCOS at different diagnosis ages, in comparison with non-PCOS controls.
The data retrieved from the Longitudinal Health Insurance Database 2000 (LHID2000). LHID2000 is a subset of the National Health Insurance Research Database (NHIRD) that contains the entire original claim data of 1000000 individuals randomly sampled from the 2000 registry for beneficiaries (ID) of the NHIRD, which maintains the registration data of everyone who was a beneficiary of the National Health Insurance program.
After adjustment for potential confounding variables (age, comorbidities and medications), the overall incidence of T2DM was higher in the PCOS group compared with the control group (HR = 5.13, 95%CI: 3.51-7.48, P < 0.0001). The risk of developing T2DM subsequent to PCOS decreased with increasing diagnosis age: the adjusted HR was 10.4 in the 18-24-year age group, 5.28 in the 25-29-year age group, and 4.06 in the 29-34-year age group. After age 35, the association between PCOS and T2DM was not statistically significant.
The risk of developing T2DM subsequent to PCOS decreased with increasing diagnosis age. No such significant association was noted in women older than 35 years.
These findings highlight the importance of prompting a more aggressive treatment to prevent diabetes among women diagnosed with PCOS at a young age, and, in contrast, the lessened importance of this type of intervention in women diagnosed with PCOS at a late reproductive age.
This study was based in part on data from the National Health Insurance Research Database, provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, Ministry of Health and Welfare, or National Health Research Institutes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nong X, Popova PV S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev. 2016;37:467-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 853] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 2. | Kazemi M, Pierson RA, Lujan ME, Chilibeck PD, McBreairty LE, Gordon JJ, Serrao SB, Zello GA, Chizen DR. Comprehensive Evaluation of Type 2 Diabetes and Cardiovascular Disease Risk Profiles in Reproductive-Age Women with Polycystic Ovary Syndrome: A Large Canadian Cohort. J Obstet Gynaecol Can. 2019;41:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Kyrkou G, Trakakis E, Attilakos A, Panagopoulos P, Chrelias C, Papadimitriou A, Vaggopoulos V, Alexiou E, Mastorakos G, Lykeridou A, Kassanos D, Papaevangelou V, Papantoniou N. Metabolic syndrome in Greek women with polycystic ovary syndrome: prevalence, characteristics and associations with body mass index. A prospective controlled study. Arch Gynecol Obstet. 2016;293:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril. 2005;83:1454-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 391] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 5. | Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, Teede HJ. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 6. | Dunaif A. Insulin action in the polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28:341-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 333] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 763] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 9. | Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236-3242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Celik C, Tasdemir N, Abali R, Bastu E, Yilmaz M. Progression to impaired glucose tolerance or type 2 diabetes mellitus in polycystic ovary syndrome: a controlled follow-up study. Fertil Steril. 2014;101:1123-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 180] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and Risk Factors of Type 2 Diabetes in a Nationwide Population of Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017;102:3848-3857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, Harrison CL, Ranasinha S, Teede HJ, Moran LJ. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24:455-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 15. | Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Li Z, You L, Zhao J, Liu J, Liang X, Zhao X, Sun Y, Zhang B, Jiang H, Zhao D, Bian Y, Gao X, Geng L, Li Y, Zhu D, Sun X, Xu JE, Hao C, Ren CE, Zhang Y, Chen S, Zhang W, Yang A, Yan J, Ma J, Zhao Y. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 17. | Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, Zhang B, Liang X, Li T, Chen J, Shen J, Zhao J, You L, Gao X, Zhu D, Zhao X, Yan Y, Qin Y, Li W, Yan J, Wang Q, Geng L, Ma J, Zhao Y, He G, Zhang A, Zou S, Yang A, Liu J, Li B, Wan C, Shi J, Yang J, Jiang H, Xu JE, Qi X, Sun Y, Zhang Y, Hao C, Ju X, Zhao D, Ren CE, Li X, Zhang W, Zhang J, Wu D, Zhang C, He L, Chen ZJ. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 429] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 18. | Welt CK, Duran JM. Genetics of polycystic ovary syndrome. Semin Reprod Med. 2014;32:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Casarini L, Brigante G. The polycystic ovary syndrome evolutionary paradox: a genome-wide association studies-based, in silico, evolutionary explanation. J Clin Endocrinol Metab. 2014;99:E2412-E2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, Sternfeld B, Wellons M, Schwartz SM, Lewis CE, Williams OD, Siscovick DS, Bibbins-Domingo K. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Ng NYH, Jiang G, Cheung LP, Zhang Y, Tam CHT, Luk AOY, Quan J, Lau ESH, Yau TTL, Chan MHM, Ho CS, Lim CKP, Ozaki R, Huang J, Liu KH, Tam WH, Sahota DS, Chu WCW, Goggins W, Woo J, Li TC, Chow CC, Chan JCN, Ma RCW. Progression of glucose intolerance and cardiometabolic risk factors over a decade in Chinese women with polycystic ovary syndrome: A case-control study. PLoS Med. 2019;16:e1002953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Brown ZA, Louwers YV, Fong SL, Valkenburg O, Birnie E, de Jong FH, Fauser BC, Laven JS. The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil Steril. 2011;96:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Pinola P, Piltonen TT, Puurunen J, Vanky E, Sundström-Poromaa I, Stener-Victorin E, Ruokonen A, Puukka K, Tapanainen JS, Morin-Papunen LC. Androgen Profile Through Life in Women With Polycystic Ovary Syndrome: A Nordic Multicenter Collaboration Study. J Clin Endocrinol Metab. 2015;100:3400-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Alsamarai S, Adams JM, Murphy MK, Post MD, Hayden DL, Hall JE, Welt CK. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Jacewicz-Święcka M, Wołczyński S, Kowalska I. The Effect of Ageing on Clinical, Hormonal and Sonographic Features Associated with PCOS-A Long-Term Follow-Up Study. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Chia CW, Egan JM, Ferrucci L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ Res. 2018;123:886-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 252] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 27. | Livadas S, Kollias A, Panidis D, Diamanti-Kandarakis E. Diverse impacts of aging on insulin resistance in lean and obese women with polycystic ovary syndrome: evidence from 1345 women with the syndrome. Eur J Endocrinol. 2014;171:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Kazemi Jaliseh H, Ramezani Tehrani F, Behboudi-Gandevani S, Hosseinpanah F, Khalili D, Cheraghi L, Azizi F. Polycystic ovary syndrome is a risk factor for diabetes and prediabetes in middle-aged but not elderly women: a long-term population-based follow-up study. Fertil Steril. 2017;108:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |