Published online Mar 15, 2022. doi: 10.4239/wjd.v13.i3.224
Peer-review started: October 30, 2021
First decision: December 27, 2021
Revised: January 29, 2022
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: March 15, 2022
Only 50% of patients with type 2 diabetes mellitus (T2DM) can control their blood glucose levels. Dapagliflozin is a selective inhibitor of sodium-glucose co-transporter 2 (SGLT-2) that improves the insulin sensitivity of the liver and peripheral tissues. Many studies confirmed that SGLT2 inhibitors reduce blood glucose and have multiple beneficial effects such as weight loss, lipid regulation, and kidney protection. Nevertheless, the mechanisms of the renal and cardiovascular protective effects of dapagliflozin from the perspective of differentially expressed proteins in the serum of T2DM patients have not been intensively explored so far.
To identify differentially expressed proteins associated with dapagliflozin treatment in patients with T2DM.
Twenty T2DM patients [hemoglobin A1c (HbA1c) 7.0%-10.0%] were enrolled at The Affiliated Hospital of Inner Mongolia Medical University between January 1, 2017 and December 1, 2018. They received dapagliflozin (10 mg/d) for 3 mo, and the HbA1c < 7.0% target was achieved. The changes in clinical indexes were compared before and after treatments. Label-free quantitative proteomics was used to identify differentially expressed proteins using the serum samples of five patients. The identified differentially expressed proteins were analyzed using various bioinformatics tools.
Dapagliflozin significantly improved the clinical manifestation of the patients. There were 18 downregulated proteins and one upregulated protein in the serum samples of patients after dapagliflozin administration. Bioinformatics analyses, including subcellular localization, EuKaryotic Orthologous Groups, Gene Ontology, and Kyoto Encyclopedia of Genes and Genomes annotations, were used to profile the biological characteristics of the 19 differentially expressed proteins. Based on the literature and function enrichment analysis, two downregulated proteins, myeloperoxidase (MPO) and alpha II B integrin (ITGA2B), and one upregulated protein, podocalyxin (PCX), were selected for enzyme linked immunosorbent assay validation. These validated differentially expressed proteins had multiple correlations with clinical indexes, including HbAc1 and fasting C-peptide.
Dapagliflozin has hypoglycemic effects and regulates the serum expressions of MPO, ITGA2B, and PCX, possibly contributing to the effects of dapagliflozin on oxidative stress, insulin resistance, and lipid metabolism.
Core Tip: This study aimed to identify differentially expressed proteins associated with dapagliflozin treatment in patients with type 2 diabetes mellitus. Changes in blood indexes were examined in 20 patients treated with dapagliflozin for 3 mo. Quantitative proteomics was used to identify differentially expressed proteins using the serum samples of five patients. Dapagliflozin has hypoglycemic effects and regulates the serum expressions of myeloperoxidase, alpha II B integrin, and podocalyxin, possibly contributing to the effects of dapagliflozin on oxidative stress, insulin resistance, and lipid metabolism.
- Citation: Zhao YX, Borjigin S, Yan ZL. Functional annotation and enrichment analysis of differentially expressed serum proteins in patients with type 2 diabetes after dapagliflozin. World J Diabetes 2022; 13(3): 224-239
- URL: https://www.wjgnet.com/1948-9358/full/v13/i3/224.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i3.224
With the aging global population and the increase in the prevalence of obesity, it is expected that type 2 diabetes mellitus (T2DM) will affect more than 381.8 million people worldwide in 2035[1]. In the United States alone, T2DM is projected to affect nearly one in three people by 2050[2]. T2DM manifests through the development of fasting and postprandial hyperglycemia, which is the primary contributor to numerous life-threatening complications and co-morbidities[3,4]. These alarming projections suggest an urgent need for the development and implementation of novel preventative and treatment strategies to fight the rise in T2DM prevalence worldwide[4]. Unfortunately, despite the best care, only 50% of patients with T2DM can control their blood glucose levels[5-7].
In recent years, the participation of the kidney in glucose metabolism and homeostasis attracted much attention, and this participation has begun to be explored in clinical studies[8]. The mechanisms mainly include the renal tubular reabsorption of glucose, largely dependent on the expression of sodium-glucose co-transporter 2 (SGLT-2) localized at the proximal small tubules S1 and S2[9]. Dapagliflozin is a selective inhibitor of SGLT-2, reducing the reabsorption of SGLT-2 receptor glucose in renal tubular epithelial cells and allowing excess glucose to be excreted in the urine[10]. Thus, the insulin sensitivity of the liver and peripheral tissues can be improved, and the hepatic glucose output can return to the normal range[10,11]. Furthermore, many investigators proposed that SGLT-2 inhibitors have renal and cardiovascular protective roles in addition to their glucose-lowering effects[12-14]. Thereby, dapagliflozin is recommended for T2DM patients[15].
Several studies confirmed that SGLT2 inhibitors reduce blood glucose and have multiple beneficial effects such as weight loss, lipid regulation, and kidney protection[13-15]. Powell et al[16] suggested that SGLT2 inhibitor alone could reduce hemoglobin A1c (HbA1c) by 0.37%-1.16%. Several randomized, double-blind, controlled trials have confirmed that dapagliflozin can significantly reduce HbA1c (by up to 1.16%) and blood glucose and that the efficacy of dapagliflozin (10 mg) in reducing HbA1c is comparable to that of metformin sustained-release tablets (2000 mg)[17,18]. Ji et al[19] proposed that SGLT2 inhibitors can reduce blood glucose and hyperglycemic toxicity by reducing the stress reaction in the endoplasmic reticulum and reducing the beta-cell apoptosis caused by glycolipid toxicity, thereby improving insulin secretion. They also proposed that the damaged function of the beta-cells will also be improved[19]. Nevertheless, the mechanisms of the renal and cardiovascular protective effects of dapagliflozin from the perspective of differentially abundant proteins in serum of T2DM patients have not been intensively explored so far.
Proteomics techniques have attracted more and more attention since these techniques can be used to identify the expression of differential proteins in cells and tissues of patients with T2DM[20]. Label-free quantitative proteomics can replace or supplement the traditional two-way gel electrophoresis approach, and it has become an important mass spectrometry method in recent years because of its powerful protein identification ability[21]. The changes in protein abundances of different samples can be analyzed by comparing mass spectrometry frequency or mass spectrometry peak intensity[22]. Without expensive isotope labeling and with liquid chromatography-mass spectrometry analysis of peptides obtained from protein enzymatic digestion, the relative abundance of the corresponding proteins can be quantified according to the signal strength of the peptide segments[22]. In this study, using this technique, we explored the differentially abundant proteins in the serum samples of T2DM patients before and after dapagliflozin treatments and conducted functional annotation analysis of the differential proteins. In addition, the levels of some differential proteins in serum samples were validated, and the correlations between their levels and clinical indexes were analyzed.
Forty-six patients with T2DM were enrolled at the Department of Endocrinology, Affiliated Hospital of Inner Mongolia Medical University, between January 1, 2017 and December 1, 2018. There were 26 participants in the dapagliflozin group, and 20 participants who controlled their blood glucose levels through diet and exercise alone were enrolled in the control group during the same period (these patients did not receive drugs as per their own choice but were still followed in case diet and exercise became insufficient to control their T2DM). All participants met the diagnostic criteria for T2DM according to the World Health Organization diagnostic criteria for type 2 diabetes in 2017[23] The course of T2DM was < 5 years. All participants were 25-55 years of age, and they did not have a blood relationship. All participants had complete physical examination data and other disease information. The exclusion criteria were: (1) Acute or chronic complications of T2DM; (2) Cardiovascular and cerebrovascular diseases such as hypertension or coronary heart disease; (3) Other antidiabetic, antihypertensive, or lipid-regulating drugs; (4) Ketoacidosis, genital fungal infection, or urinary tract infection; (5) Recent history of surgery and trauma; or (6) Infectious diseases, tumors, hematological diseases, severe impairment of heart, liver and kidney functions, autoimmune diseases and other endocrine and metabolic diseases.
This study was approved by the Ethics Committee of Affiliated Hospital of Inner Mongolia Medical University. All participants signed the informed consent form before the start of the study.
For the participants in the dapagliflozin group, their HbA1c levels ranged from 7.0% to 10.0%. If the participants were taking other hypoglycemic drugs, the participants were admitted to the group after a wash-out period of 1-2 wk. The participants were instructed to adhere strictly to diet and exercise during the wash-out period. After wash-out, the participants were treated with dapagliflozin alone after clinical evaluation. The participants without the need for wash-out were treated with dapagliflozin directly after clinical evaluation. In order to avoid complications such as urinary tract infection and ketoacidosis, the participants were advised to drink more water during the study period, which was also conducive to the excretion of urinary glucose. No other drugs, such as lipid-lowering drugs, were allowed during the study. After 3 mo of treatment, the participants with HbA1c < 7.0% were considered as reaching the HbA1c target level. Six patients in the dapagliflozin group dropped out due to self-discontinuation, failure to meet the HbA1c target, or refusal to be reviewed after 3 mo. The average age of the 20 eligible patients in this group was 39.8 ± 5.1 years.
For the participants in the control group, their HbA1c levels reached the target level (HbA1c < 7.0%). These participants did not take any hypoglycemic drugs or other drugs within 3 mo before sampling, and they controlled their blood glucose levels only through diet and exercise. There were no dropouts. Their average age was 39.8 ± 6.0 years.
All participants received diet and exercise guidance. The dietary guidance referred to the balanced dietary plan recommended by the Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2013 edition) and suggested 1/3 structure of the energy intake ratio of three meals or 2:2:1 distribution. According to the “Guidelines for Exercise in Type 2 Diabetes Mellitus”, the exercise program was mainly composed of low-intensity aerobic exercise such as walking, swimming, cycling, etc. After a meal, the participants were required to exercise for about 30 min and 3-5 times per week. During the study, no adverse reactions such as urinary tract infection, ketoacidosis, or other adverse reactions occurred.
Fasting venous blood was drawn from the participants in the morning. Blood samples were subjected to centrifugation. The supernatant was stored at -80 °C until analysis. Blood samples were also sent to the laboratory professionals of Affiliated Hospital of Inner Mongolia Medical University for quantification of indicators, including retinol-binding protein 4 (RBP4), fasting blood glucose (FBG), fasting C-peptide (FCP), fasting plasma insulin level (FINS), HbA1c, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol(LDL-C), triglycerides(TG), total cholesterol (TC), apolipoprotein A1 (ApoA1), ApoB100, homocysteine (HCY), non-esterified fatty acids (NEFA), C-reactive protein. These indicators were determined using Roche Cobas 8000 and Aikelai H-8160 automatic biochemical analyzers in the Laboratory Department of Affiliated Hospital of Inner Mongolia Medical University. Quality control was carried out by the professionals in the Laboratory Department. Insulin resistance indexes, including updated homeostasis model assessment-insulin resistance (HOMA2-IR), updated homeostasis model assessment-beta cell (HOMA2-B), and updated homeostasis model assessment-insulin sensitivity (HOMA2-S%), were obtained using the HOMA Calculator v2.2.3 software based on FPG, C-peptide, and islet function. The non-HDL-C value was calculated by subtracting the HDL-C value from the TC value.
Serum samples from five patients in the dapagliflozin group before and after dapagliflozin treatments for 3 mo were selected for the exploratory proteomics study. Individual differences such as diabetes duration, blood glucose levels, and body mass index (BMI) were minimized to ensure the reliability of the results. High-abundance proteins were removed from the samples using Pierce™ Top 12 Abundant Protein Depletion Spin Columns Kit following the manufacturer’s instruction, and total protein concentration was determined by a BCA kit (Pierce). The proteins were resolved by 10% SDS-PAGE and visualized with Coomassie Blue staining. Each lane was excised and cut into bands containing proteins with different molecular weights. Each gel fraction was subjected to in-gel tryptic digestion. Peptide segments were classified by high-pH reverse HPLC with Agilent 300 Extend C18 (5 µm diameter, 4.6 mm inner diameter, 250 mm long).
Digested peptides were analyzed by liquid chromatography-mass spectrometry (LC-MS)/MS using a ThermoScientific Easy nLC-1000 in tandem with a Q-Exactive Orbitrap mass spectrometer. Each sample (5 µL) was resolved using a 60 min gradient (Buffer A: 0.1 formic acid in 2% acetonitrile; Buffer B: 0.1% formic acid in 90% acetonitrile) on a 2 cm Acclaim 100 PepMap Nanoviper C18 trapping column in tandem with a Thermo EASY-Spray column (PepMap® RSLC, C18, 3 µm, 100 A, 75 µm × 150 mm).
The database was SwissProt Human (20387 sequences), which included the counter-library in calculating the false positive rate (FDR) caused by randomly matching. The common contamination library was considered to eliminate contaminations. The enzyme cutting method was set to trypsin/P; the number of leakage sites was set to 2. The first-stage mother ion mass error tolerance to the first search and main search was set to 20 ppm and 5 ppm. The error tolerance of the secondary fragment ions mass was set as 0.02 Da. The alkylation of cysteine was set as fixed modification, while the oxidation of methionine and acetylation of protein N-terminus were set as alternative modifications. The FDR of protein identification and PSM identification was set as 1%.
For data-dependent analysis, full scans were acquired at a 35000 resolution range of 400-200 m/z, while a 17500 resolution was used for MS/MS scans. Only the top 15 ions with +2 and +3 charges were selected for MS/MS with 10-s dynamic exclusion to prevent continuous reanalysis of abundant peptides. Following data acquisition, raw data files were compiled for each gel lane and searched with Proteome Discoverer 1.4’s SEQUEST search algorithm using the reviewed, non-redundant homo sapiens complete proteome retrieved from UniProtKB.
For protein functional enrichment evaluation, Gene Ontology (GO) and pathway enrichment analyses were carried out. GO annotations (including biological process (BP), molecular function (MF), and cellular component) were performed using the InterProScan database (v.5.14–53.0 http://www.ebi.ac.uk/interpro/) and according to an existing report[16]. The Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used for pathway enrichment analysis[16]. KAAS (v.2.0 http://www.genome.jp/kaas-bin/kaas_main) and KEGG mapper (v.2.5 http://www.kegg.jp/kegg/mapper.html) are the main tools used with the KEGG database. Prediction of subcellular localization was carried out using the wolfpsort software (v.0.2 http://www.genscript.com/psort/wolf_psort.html). Cluster memberships were visualized using the heat map drawn by the function heatmap.2 in the R package gplots. For each annotation, Fisher’s exact test was applied to compare the enrichment of the differentially abundant protein against all identified proteins, and a P value < 0.05 was considered significant.
Enzyme linked immunosorbent assay (ELISA) was performed for the quantification of alpha II B integrin, myeloperoxidase (MPO), and podocalyxin (PCX) using the kits from Bio-Rad Laboratories, United States (CSB-EL0118644HU for integrin, CSB-E08721h for MPO, and CSB-E09891h for PCX). All reagents were equilibrated to room temperature (18-25 °C) for at least 30 min and prepared according to the instructions of the relevant kits. The optical density of each well was measured sequentially at 450 nm with an enzyme-labeled instrument within 5 min after the termination of the reaction.
The statistical methods of this study were reviewed by Xue-Mei Wang. The quantitative data were described using means ± SD. The paired t-test was used to compare the indexes obeying the normal distribution before and after treatment. Variance analysis was used to compare the indexes obeying normal distribution, and a nonparametric rank-sum test was used to compare the indexes not obeying normal distribution. Pearson correlation analysis was used for the two variables obeying normal distribution, and Spearman grade correlation analysis was used for the variables not obeying normal distribution. The chi-square test was used to analyze the categorical data. For the enrichment test, Fisher’s exact test was used to show the functional classification and pathway of significant enrichment of differentially expressed proteins (P < 0.05) using bubble diagrams. Two-sided P < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS 22.0.
Twenty participants were treated from January 1, 2017 to December 1, 2018. Table 1 presents the characteristics of the participants in the dapagliflozin (after treatment) and control groups. Compared with the controls, the patients under dapagliflozin treatment had slightly higher FBP, lower FINS, and lower HOMA2-B, but no significant differences in HOMA2-S% and HOMA2-IR. Compared with the controls, patients with dapagliflozin had higher apoA1 Levels and lower NEFA levels. Table 2 presents the comparison before/after dapagliflozin. Compared with baseline, the participants after dapagliflozin treatment had significantly decreased BMI, waist circumference, and waist-hip ratio (P < 0.05) (Table 2). In addition, their blood glucose-related indexes, including HbA1c, FCP, FINS, FBP, and HOMA2-IR, also demonstrated significantly decreased levels (P < 0.05), while HOMA2-S% and HOMA2-B significantly increased (P < 0.05) (Table 2). Regarding the lipid metabolism-related indexes, non-HDL-C decreased (P < 0.05), while ApoA1 increased (P < 0.05) (Table 2). Moreover, RBP, HCY, and NEFA were significantly decreased levels after treatment (P < 0.05) (Table 2). Taken together, dapagliflozin substantially improved the clinical manifestation of patients with T2DM.
| Parameter | Dapagliflozin | Control | Wilcoxon W | P value |
| Age | 39.80 ± 5.136 | 39.75 ± 5.990 | 397.000 | 0.724 |
| Smoking history | 16.55 ± 9.720 | 10.25 ±11.639 | 353.000 | 0.111 |
| Height (m) | 1.7670 ± 0.07255 | 1.7505 ± 0.090 | 389.000 | 0.569 |
| Weight (kg) | 74.950 ± 8.9176 | 72.525 ± 6.6718 | 381.500 | 0.440 |
| BMI (kg/m2) | 24.3695 ± 1.82674 | 24.014 ± 1.6715 | 385.00 | 0.499 |
| Waist circumference (cm) | 96.350 ± 2.3402 | 93.525 ± 5.7502 | 325.000 | 0.021 |
| Hip circumference (cm) | 96.975 ± 2.4413 | 99.225 ± 6.4062 | 398.500 | 0.755 |
| Waist-hip ratio | 0.9937 ± 0.0132391 | 0.9494 ± 0.04815 | 308.000 | 0.006 |
| SBP (mmHg) | 124.80 ± 5.297 | 120.40 ± 5.423 | 323.500 | 0.018 |
| DBP (mmHg) | 78.85 ± 5.724 | 77.95 ± 5.969 | 394.500 | 0.671 |
| Diabetes course (yr) | 2.575 ± 1.2169 | 2.825 ± 1.8229 | 396.500 | 0.713 |
| TG | 1.884 ± 1.2172 | 0.979 ± 0.2376 | 1.2172203 | 0.002 |
| LDL | 2.8585 ± 1.0440 | 1.5975 ± 0.5079 | 1.0439867 | < 0.001 |
| HDL | 1.0855 ± 0.2457 | 1.023 ± 0.1246 | 0.2457100 | 0.560 |
| APOA1 | 1.3630 ± 0.2022 | 1.2625 ± 0.1882 | 0.2022271 | 0.010 |
| APOB100 | 0.9425 ± 0.2651 | 1.0345 ± 0.1521 | 0.2651092 | 0.424 |
| CRP | 1.311 ± 0.9305 | 1.1635 ± 0.5462 | 0.9305511 | 0.967 |
| HbA1c | 6.7500 ± 0.6863 | 6.3700 ± 0.3827 | 0.6863327 | 0.032 |
| THCY | 12.030 ± 2.1451 | 12.4215 ± 1.2702 | 2.1450899 | 0.213 |
| RBP4 | 37.150 ± 5.6033 | 35.800 ± 4.3842 | 5.6033 | 0.446 |
| NEFA | 0.8245 ± 0.5849 | 1.026 ± 0.1099 | 0.5849019 | < 0.001 |
| FCP | 2.2770 ± 0.7903 | 2.231 ± 0.4151 | 0.7903104 | 0.525 |
| TC | 4.1950 ± 0.8378 | 3.7875 ± 0.4683 | 344.500 | 0.076 |
| IR | 2.7120 ± 1.7245 | 2.8440 ± .8424 | 358.500 | 0.163 |
| FINS | 8.4905 ± 4.4824 | 10.313 ± 3.2099 | 328.500 | 0.027 |
| FBP | 6.80 ± 1.105 | 6.15 ± 0.671 | 335.500 | 0.034 |
| Non-HDL | 3.05 ± 0.826 | 2.80 ± 0.410 | 364.000 | 0.142 |
| HOMA2- insulin | 75.6150 ± 20.339 | 89.460 ± 18.246 | 324.000 | 0.020 |
| HOMA2-S% | 61.6750 ± 21.1603 | 60.440 ± 14.789 | 400.000 | 0.787 |
| HOMA2-IR | 1.8185 ± 0.7285 | 1.7210 ± 0.3760 | 399.000 | 0.766 |
| Parameter | Before dapagliflozin | After dapagliflozin | Wilcoxon W | P value |
| Weight (kg) | 80.600 ± 10.4549 | 74.950 ± 8.9176 | 351.000 | 0.110 |
| BMI | 26.1875 ± 2.1889 | 24.3695 ± 1.8267 | 313.000 | 0.009 |
| Waist circumference (cm) | 98.300 ± 2.4570 | 96.350 ± 2.3402 | 310.50 | 0.007 |
| Hip circumference (cm) | 97.425 ± 3.1131 | 96.975 ± 2.4413 | 395.000 | 0.684 |
| Waist-hip ratio | 1.009350 ± 0.0172 | 0.9937 ± 0.0132 | 311.500 | 0.007 |
| TG | 2.3020 ± 1.7483 | 1.884 ± 1.2172 | 387.000 | 0.534 |
| LDL | 2.9310 ± 0.9873 | 2.8585 ± 1.0440 | 405.000 | 0.892 |
| HDL | 1.0525 ± 0.2722 | 1.0855 ± 0.2457 | 381.000 | 0.432 |
| APOA1 | 1.2450 ± 0.2176 | 1.3630 ± 0.2022 | 334.500 | 0.041 |
| APOB100 | 1.0345 ± 0.3400 | 0.9425 ± 0.2651 | 392.000 | 0.626 |
| CRP | 1.7090 ± 1.1454 | 1.311 ± 0.9305 | 349.000 | 0.098 |
| HbA1c | 8.0550 ± 1.0842 | 6.7500 ± 0.6863 | 277.000 | < 0.001 |
| THCY | 14.6850 ± 3.0387 | 12.030 ± 2.1451 | 311.500 | 0.008 |
| RBP4 | 43.310 ± 8.8547 | 37.150 ± 5.6033 | 315.500 | 0.010 |
| NEFA | 0.4525 ± 0.3246 | 0.8245 ± 0.5849 | 321.500 | 0.017 |
| FCP | 2.7980 ± 0.9510 | 2.2770 ± 0.7903 | 337.000 | 0.048 |
| TC | 4.6830 ± 1.1643 | 4.1950 ± 0.8378 | 347.500 | 0.091 |
| IR | 5.7385 ± 3.2238 | 2.7120 ± 1.7245 | 266.000 | < 0.001 |
| FINS | 13.3500 ± 5.7057 | 8.4905 ± 4.4824 | 289.000 | 0.001 |
| FBP | 9.60 ± 2.437 | 6.80 ± 1.105 | 245.000 | < 0.001 |
| Non-HDL | 3.70 ± 1.129 | 3.05 ± 0.826 | 337.000 | 0.037 |
| HOMA2-insulin | 53.4450 ± 21.1716 | 75.6150 ± 20.339 | 305.000 | 0.005 |
| HOMA2-S% | 44.7950 ± 16.2213 | 61.6750 ± 21.1603 | 310.500 | 0.007 |
| HOMA2-IR | 2.5185 ± 0.9686 | 1.8185 ± 0.7285 | 313.000 | 0.009 |
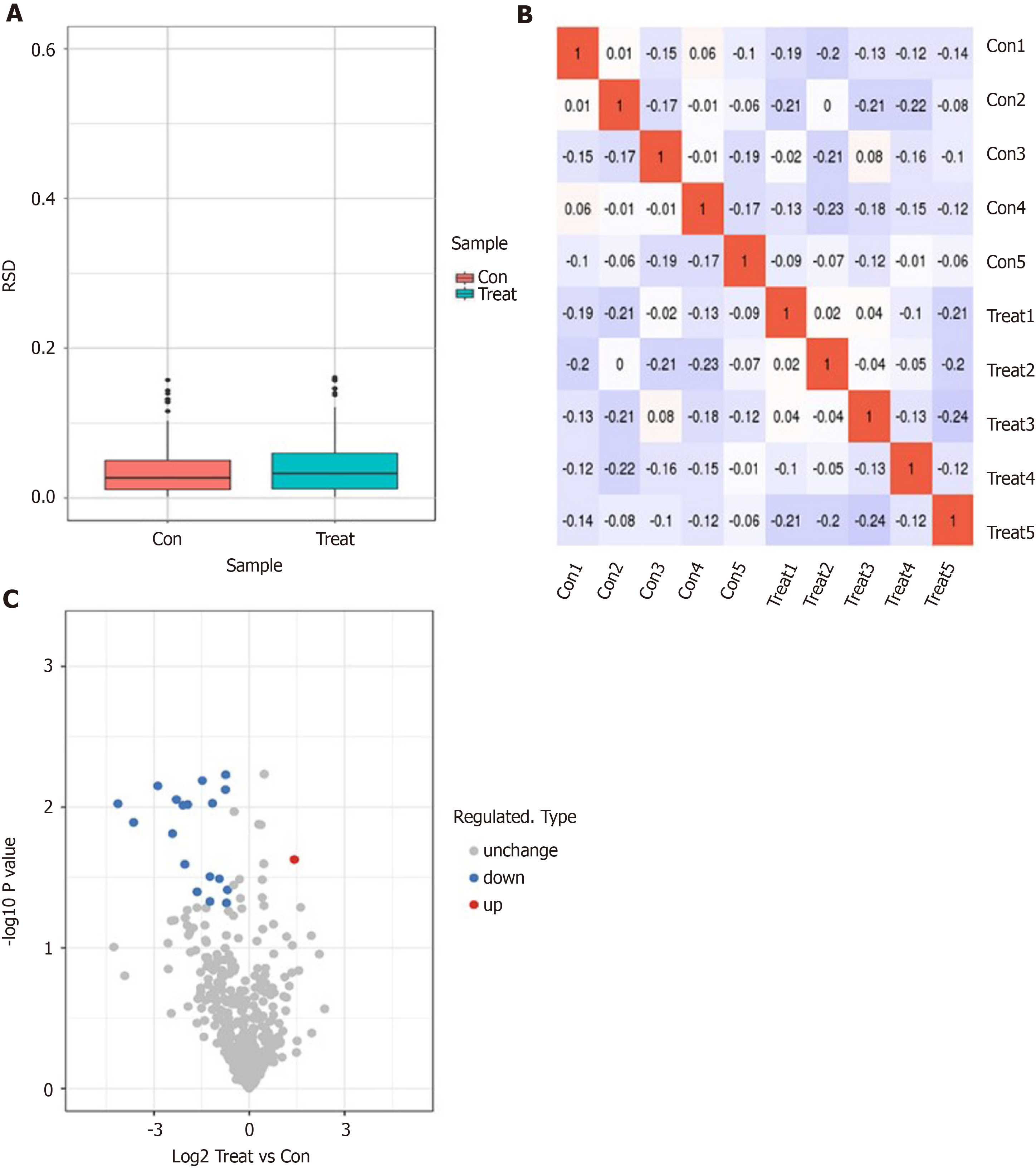
Next, we performed a label-free proteomics analysis of serum samples from five patients before and after dapagliflozin treatments. A total of 665007 sary spectra were obtained through mass spectrometry. A total of 4732 peptides were identified through spectral analysis, of which 3389 were specific peptides. We identified 534 proteins, all of which could be quantified (quantitative protein means that at least one comparison group has quantitative information). The evaluation of quantitative proteome reproducibility was performed by relative standard deviation (RSD) analysis, which showed that the biological replicates were statistically consistent (Figure 1A). The heatmap of the Pearson correlation coefficients from all quantified proteins between each pair of samples demonstrated that the linear correlation degree of the two metrics was not high (Figure 1B). Notably, 19 proteins exhibited significant differences before and after dapagliflozin treatments (P < 0.05; FC > 1.5), of which 18 were downregulated, and one was upregulated (Figure 1C).
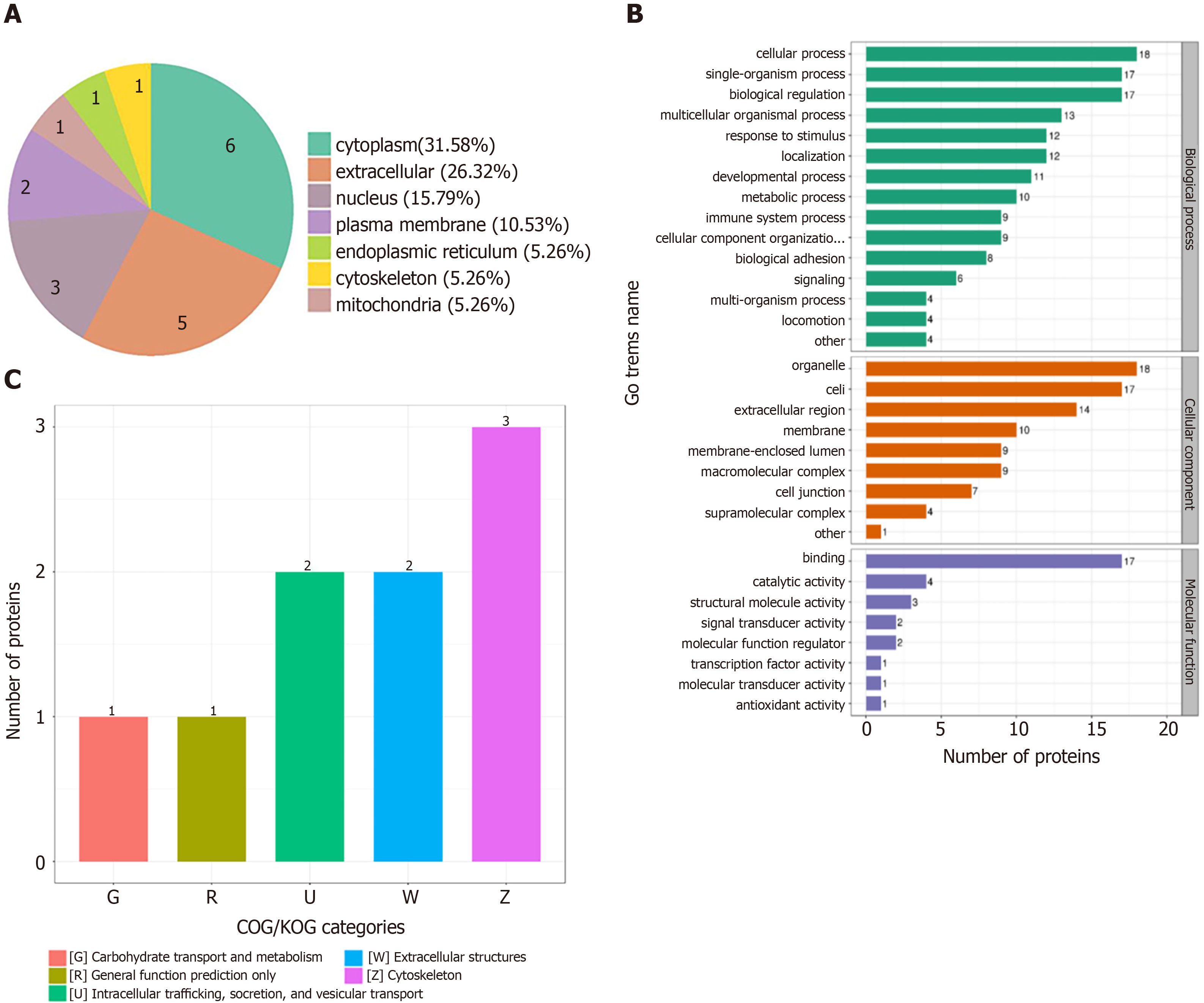
In order to determine the characteristics of the differentially expressed proteins, we annotated the subcellular localization, Clusters of Orthologous Groups of proteins (KOG), Gene Ontology (GO), and KEGG pathway of the 19 differentially expressed proteins. The annotation of the subcellular localization showed that 31.6% of all differentially expressed proteins were localized to the cytoplasm, 26.3% to the extracellular space, 15.8% to the nucleus, 10.5% to the plasma membrane, 5.3% to the mitochondria, 5.3% to the endoplasmic reticulum, and 3.8% to the cytoskeleton (Figure 2A). In the GO analysis, the GO annotations are divided into three categories (biological process, cellular component, and molecular function), explaining the biological role of proteins from different perspectives. In the biological process classification of GO, the cellular process had the largest proportion, followed by the single-organism process and biological regulation. In the cellular composition classification, differentially expressed proteins were mostly expressed in organelles, followed by cells and extracellular regions. In the molecular function classification, binding molecules accounted for the largest proportion (Figure 2B). In terms of subcellular structure location, these differentially expressed proteins were mainly located in the cytoplasm, extracellular, nucleus, and plasma membrane. COG/KOG functional classification revealed that most of these differentially expressed proteins played a role in the cytoskeleton, extracellular structures, intracellular trafficking, secretion, vesicular transport, carbohydrate transport, and metabolism, as general function predictions (Figure 2C).
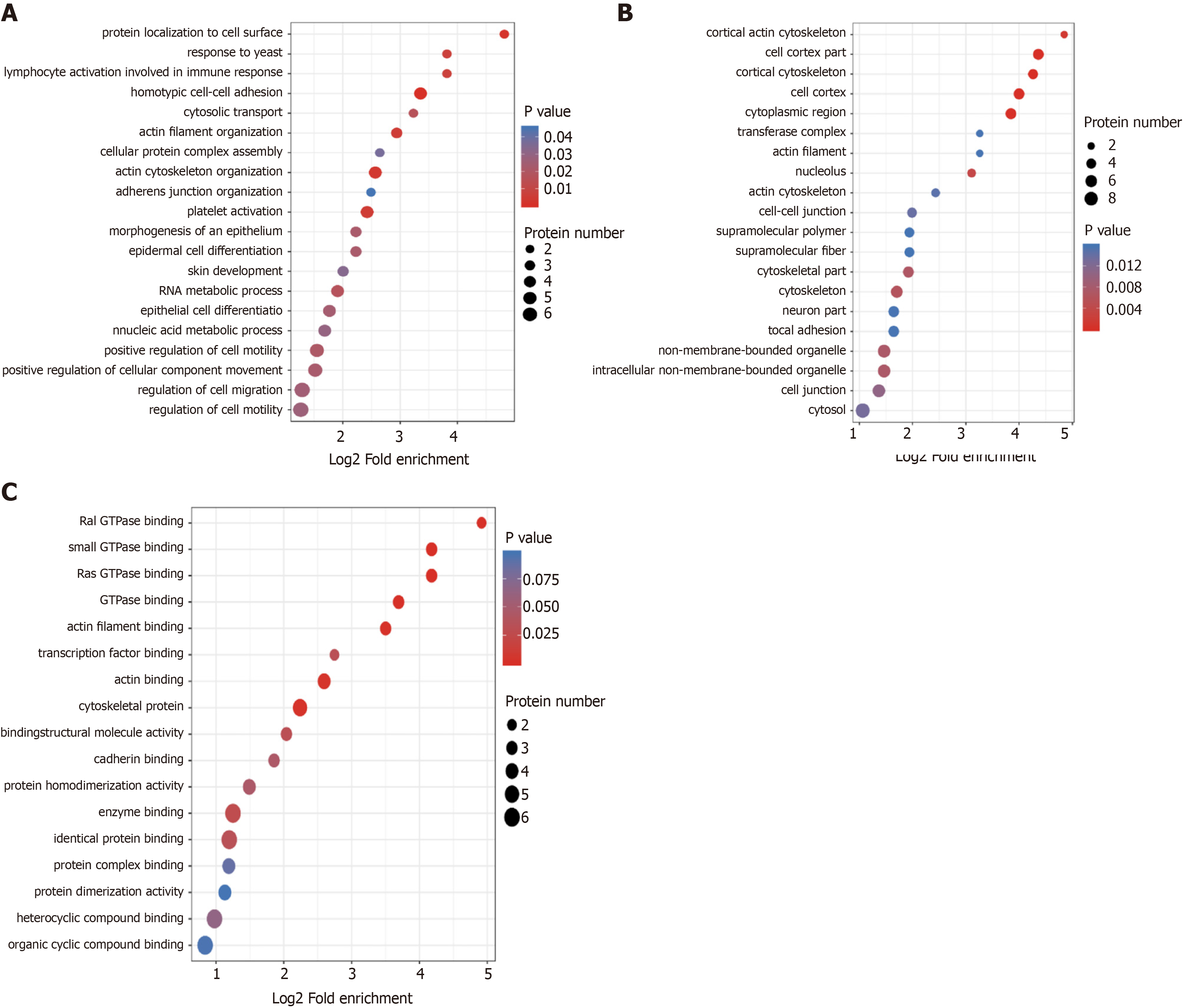
For the annotation of all identified proteins and the screening of differentially expressed proteins, the differentially expressed proteins in our comparison groups were enriched at three levels: GO classification, KEGG pathway, and protein domains. The purpose was to determine whether the differentially expressed proteins had significant enrichment trends in some functional types. GO functional classification found that the homotypic intercellular adhesion pathway, lymphocyte activation pathway, and actin cytoskeleton regulation pathway were highly enriched in the classification of cell composition (Figure 3A). The pathways such as Cortex and Cytoskeleton were enriched significantly in the KEGG pathway (Figure 3B). In the function of molecular biology, the small GTP enzyme binding pathway and the Ras GTPase binding pathway were enriched significantly (Figure 3C).
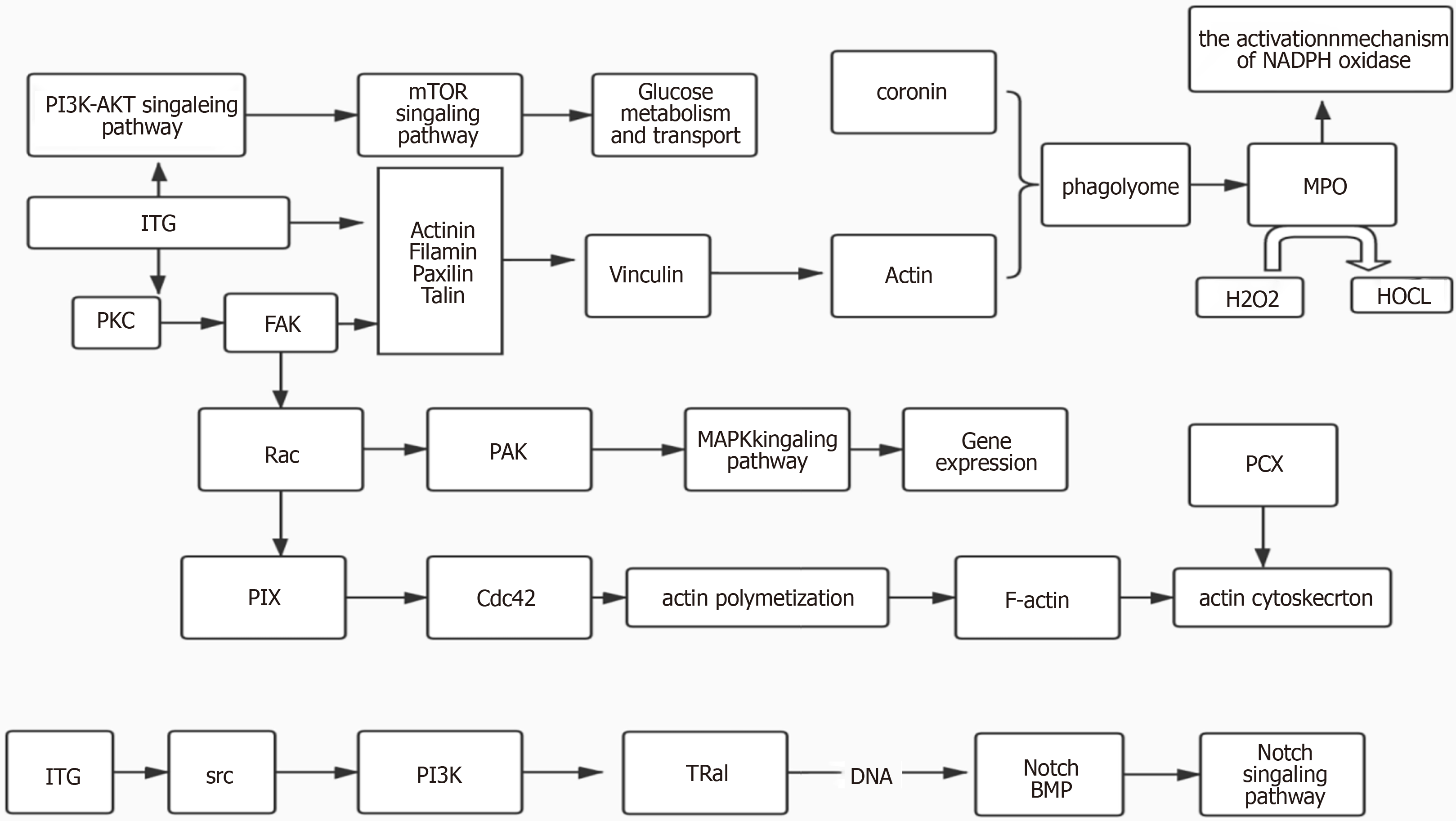
We found that these proteins, such as actin, alpha II beta integrin, MPO, and PCX, were closely related by the KEGG pathway. Therefore, we performed a protein-protein interaction analysis and identified a network among the integrin protein, MPO, and PCX (Figure 4). The integrin protein was used to modulate the production of cytoplasmic actin by participating in the PKC and the FAK pathway to function on actin filament and vinculin. On the other hand, by participating in the PKC and FAK pathway to function on Rac family proteins, the integrin protein modulates the MAPK signal through the PAK pathway to eventually manipulate gene expression. Besides, the integrin protein can also act on the PI3K pathway through the SRC family, affecting Notch signaling pathway. The cytoplasmic actin is involved in the behavior of phagolysosome with coronin, and the MPO production is increased during the formation of autophagolysosome. PCX protein and actin are involved in regulating the actin skeleton, and the Notch signaling pathway also participates in this process (Figure 4).
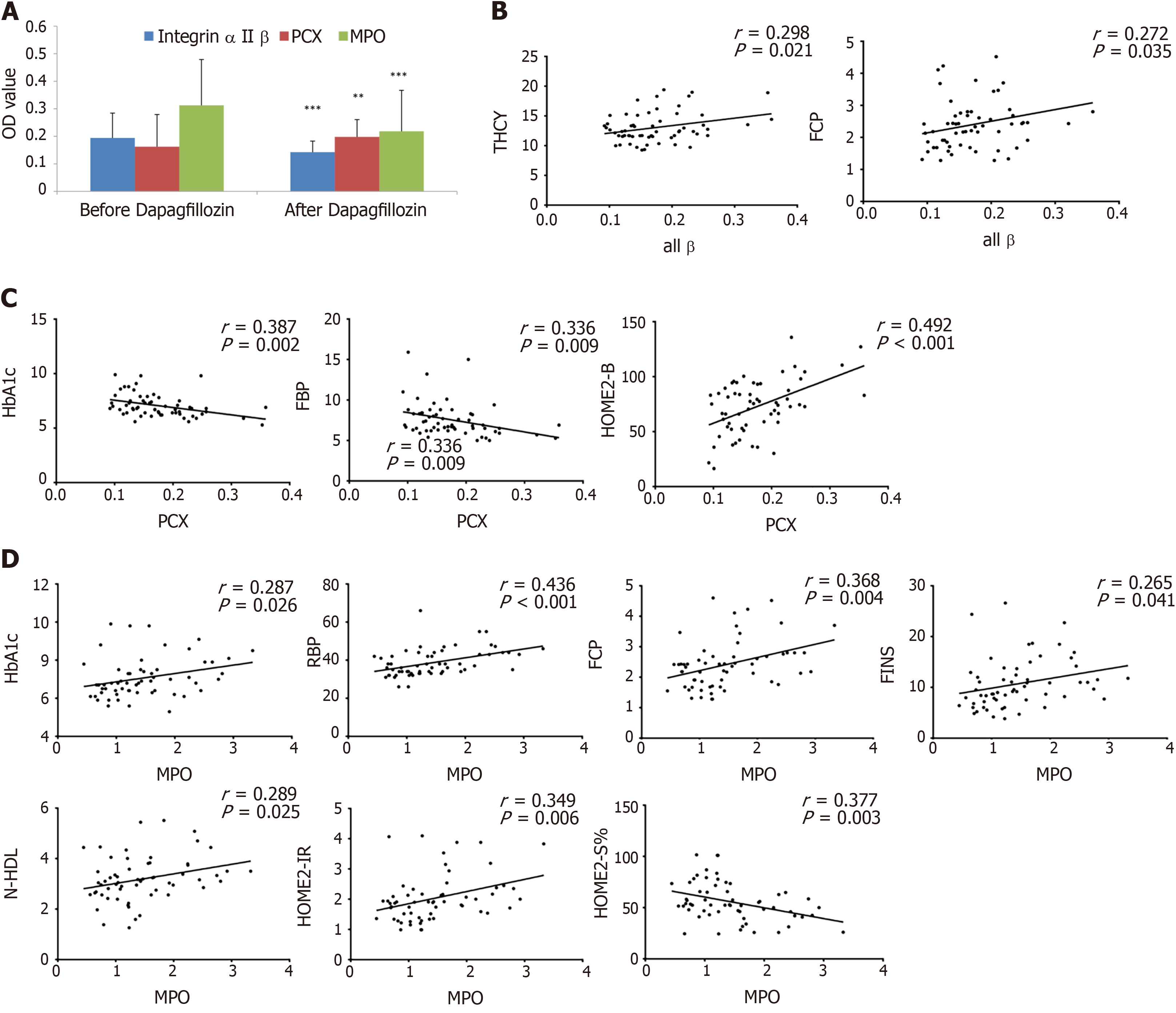
Based on the literature and our analysis of the biological function of candidate proteins that might be closely related to diabetes mellitus, three differentially expressed proteins (including MPO, alpha II B integrin, and PCX) were validated by ELISA. The results showed that the expression of MPO and alpha II B integrin protein in serum samples was significantly downregulated (P < 0.05), and the PCX protein levels were significantly upregulated (P < 0.05) after dapagliflozin treatment (Figure 5A). In addition, compared with the control group, the dapagliflozin group also had a significantly downregulated expression of MPO after treatment (P < 0.05) (Supplementary Figure 1). We also conducted a correlation analysis between the expression levels of these three differentially expressed proteins and clinical indexes (Supplementary Table 1). We found that the serum alpha II B integrin protein levels were positively correlated with THCY (homocysteine) and FCP (P < 0.05, Figure 5B), while that of PCX was positively correlated with HOMA2-B and negatively correlated with HbAc1 and FBP (P < 0.05, Figure 5C). In addition, the serum protein levels of MCP were positively correlated with multiple parameters, including HbAc1, RBP, FCP, FINS, N-HDL, and HOMA2-IR, and negatively correlated with HOMA2-S% (P < 0.05, Figure 5D).
The SGLT-2 inhibitor dapagliflozin, as a new hypoglycemic drug, plays a role in lowering blood glucose by reducing the reabsorption of SGLT2 receptor glucose in renal tubular epithelial cells in patients with T2DM. Here, we performed label-free quantitative proteomics analysis of serum samples in patients before and after dapagliflozin treatments and identified differentially expressed proteins associated with dapagliflozin treatment. Notably, our function annotation and enrichment analysis suggested that three differential proteins (including αIIβ integrin, MPO, and PCX) potentially contribute to the renal and cardiovascular protective roles of dapagliflozin through participating in the regulation of multiple pathways. Furthermore, the serum differential expressions of these proteins were validated by ELISA, and their levels were correlated with some clinical indexes in patients with T2DM.
This study used dapagliflozin as the representative drug for SGLT-2 inhibitors. We found that the related indexes of islet function, such as FBG, HbA1c, FCP, FINS, and HOMA2-IR, decreased after dapagliflozin treatment. The lipid metabolism of T2DM patients was significantly improved after dapagliflozin treatment, as evidenced particularly by decreased non-HDL-C and increased ApoA1 Levels. Compared with baseline, the participants after dapagliflozin administration had significantly decreased BMI and hip circumference. We also found that RBP4, NEFA, and HCY were decreased after treatment with dapagliflozin. The controls were patients with mild T2DM in whom diet and exercise were sufficient to control their condition. Of note, dapagliflozin decreased FBG from 9.60 ± 2.44 to 6.80 ± 1.11 mmol/L, close to the value in controls (6.15 ± 0.67 mmol/L). Dapagliflozin also decreased FINS to lower levels than in controls, but HOMA2-B was lower than in controls, while there were no significant differences in HOMA2-S% and HOMA2-IR between the two groups. Compared with the controls, patients with dapagliflozin had higher apoA1 Levels and lower NEFA levels, also supporting the benefits of dapagliflozin. These results support the known effects of dapagliflozin in patients with T2DM[15]. RBP4, as a fat-derived factor, is closely related to obesity, insulin resistance, and other diseases[24]. Ost et al[25] found that RBP4 can prevent insulin-stimulated serine phosphorylation at position 307 of IRS1 by interfering with RBP4 and its antibodies in primitive adipocytes and correspondingly increasing the effective concentration of IRS1 tyrosine phosphorylation by half, as well as preventing the phosphorylation of ERK1/2. Therefore, it can be concluded that RBP4 might interfere with the Ras/MAPK signal of the insulin receptor by interfering with the phosphorylation of ERK1/2, thus participating in insulin resistance. In this study, after label-free quantitative proteomics, we also found that the differentially expressed proteins were significantly enriched in the Ras GTPase pathway. Since these two metabolic pathways share a common pathway, whether dapagliflozin could play a role through this pathway and the mechanism of RBP4 in dapagliflozin effectiveness need to be further studied.
Our comparative analysis with non-standard quantification demonstrated that 18 proteins were downregulated, and one was upregulated in serum samples of patients after dapagliflozin treatment. After consulting the literature, three candidate differential proteins closely related to T2DM were selected for ELISA validation. The differential expressions of these proteins were validated using serum samples from all the patients before and after dapagliflozin treatments. Notably, the correlation analysis suggested that the serum MPO protein levels were positively correlated with multiple clinical indexes, and the alpha II B protein levels were positively correlated with THCY and FCP levels. In addition, the serum PCX levels were negatively correlated with HOMA2-B and positively correlated with HbA1c and FBP. Based on these results and the literature, these three differential proteins might contribute to the beneficial roles of dapagliflozin in treating T2DM patients. Of note, PCX is the main surface antigen of podocytes and is normally expressed in renal podocytes, endothelial cells, and vascular endothelial cells and participates in maintaining the vascular endothelial cell barrier and reducing vascular inflammation. High glucose levels downregulate the expression of PCX in cultured podocytes via ERK1/2 MAPKs and inhibit the expression of PCX protein and mRNA by WT1 tumor protein and advanced glycation end-products[26], possibly resulting in the reduction of PCX in the blood. Second, we agree that integrins are cellular proteins that would not be expected to be found in circulation. Still, this study was not designed to determine the source of these proteins in the plasma. On the other hand, numerous studies report serum/plasma levels of various integrins as markers of diseases[27-29]. It could be hypothesized that the systemic inflammatory condition observed in T2DM increases cell death, releasing those proteins in circulation, but the present study cannot provide an answer regarding that point. Future studies will have to examine that specifically.
MPO is a heme enzyme and is the major protein in neutrophils and, to a lesser extent, in monocytes. MPO uses H2O2 to generate HOCl, a potent bactericidal agent, generating ROS[30]. MPO plays an essential part in the innate immune system by catalyzing the production of HOCl[31], but MPO has also been implicated as a very harmful agent in an increasing number of inflammatory-mediated disorders[32]. It has been reported that MPO is related to insulin resistance and inflammation parameters in overweight subjects with first-degree relatives of T2DM[31]. In addition, plasma MPO levels were positively correlated with the degree of coronary artery stenosis in T2DM patients, and increasing blood glucose might amplify the association between MPO and coronary artery disease[33]. Patients with uremic diabetic nephropathy with a low MPO level might be at a lower risk for any cardiac event than uremic patients with high MPO levels, suggesting that MPO might be a biomarker to predict coronary events in diabetic patients end-stage renal disease[34]. In this study, MPO levels in the dapagliflozin group decreased after treatment, related to the improvement of blood glucose control and inflammatory oxidation. Since our correlation analysis suggested that MPO was positively correlated with indexes including RBP, FCP, FINS, N-HDL, and HOMA2-IR, MPO very likely participated in oxidative stress regulation and acute, chronic inflammation of T2DM through a certain metabolic pathway. However, the specific mechanisms of MPO in contributing to the beneficial roles of dapagliflozin are still under investigation.
Integrin is a transmembrane protein that exists on the cell membrane to mediate cell-to-cell interaction. It can interact with various growth factors at the receptor level and regulate cell adhesion, survival, growth, differentiation, proliferation, and migration[35]. There are 24 kinds of integrins in humans, formed by heterodimerization of 18 alpha subunits and eight beta subunits. Alpha II beta integrin is one of the important components of the integrin family[35]. Studies have confirmed that platelet apoptosis in patients with T2DM is higher than in normal patients, increasing alpha II beta integrin levels[36]. The mechanism might be that integrins participate in RAS/MAPK signal transduction through interaction with growth factors and synergism between integrins, related to insulin resistance and secretion deficiency[37,38]. It has not been reported through which pathway dapagliflozin can induce the downregulation of alpha II beta integrin. Our analysis indicated that the Ras GTP pathway enrichment might be related to the downregulation of alpha II beta integrin in the differential protein function enrichment pathway in this study, which needs further confirmation by additional experiments.
The normal expression of PCX can prevent negatively charged proteins from leaking into human urine, resist adhesion between adjacent foot processes, and prevent adhesion between parietal epithelial cells and capillary loops[39]. Through animal experiments, Qi et al[40] demonstrated that high glucose could activate the ERK1/2 MAPK pathway of podocytes and decrease PCX expression. Although hyperglycemia inhibits PCX expression during glomerular injury, it can aggravate podocyte injury, increasing PCX with urine excretion[40]. These results suggest that urinary PCX is increased in urinary nephropathy, but PCX expression decreases with high glucose levels. We found that PCX was negatively correlated with HbA1c and FBP. The lipid metabolism disorders in diabetic nephropathy were higher than in diabetic patients without nephropathy, and there were obvious disorders in the early stage of diabetic nephropathy (Figure 5B). This study suggests that dapagliflozin might participate in the protective effect of PCX protein in kidney through lipid metabolism-related mechanisms, and there are many pieces of evidence. Dapagliflozin has a protective effect on kidney by affecting glomerular feedback, restoring renal blood flow and glomerular filtration rate, thus preventing the progression of diabetic nephropathy in the initial stage, and posing potential renal protective effect on patients with mild to moderate renal insufficiency[13-15]. SGLT2 inhibitors can also limit the glycotoxicity of the kidney itself and reduce renal hypertrophy[13-15]. Vallon et al[41] suggested that SGLT2 inhibitors could inhibit the expression of inflammatory markers and fibrotic markers, and whether PCX was involved in these processes needs further study.
This study has several limitations. Because it was an exploratory study, no power analysis was initially performed, and the participants were enrolled using convenience sampling. Five serum samples with relatively small heterogeneity were directly selected from the dapagliflozin group for LC-MS. The abundance of proteins in the different samples was analyzed by comparing the frequency of mass spectrometry analysis or the peak intensity of mass spectrometry. Because a single sample can only be analyzed separately, the analytical flux was relatively low, and repeated experiments are needed to improve the accuracy of the analytical flux. Moreover, the sample size of this study is insufficient. Due to limited time and funding, the mechanisms of the differentially expressed proteins associated with dapagliflozin treatments need to be further studied. Many proteins’ bioinformatics database data collection might be needed for analyzing the interaction between identified differentially expressed proteins and predicting the correlation between these differentially expressed proteins and clinical indicators. In addition, the relationship between dapagliflozin treatments and the changes of isoproteins still needs to be further validated.
Dapagliflozin has obvious hypoglycemic effects, and it can also improve weight loss, lipid metabolism, and islet function of patients with T2DM. After dapagliflozin treatment, 18 proteins (including MPO and alpha II beta integrin) were downregulated, and PCX protein was upregulated in the serum of T2DM patients. Subsequent function annotation and enrichment analysis, as well as ELISA validation and correlation analysis with the clinical indexes, suggested that MPO, alpha II beta integrin, and PCX might contribute to the beneficial roles of dapagliflozin through their regulations on oxidative stress, insulin resistance, and lipid metabolism.
Only 50% of patients with type 2 diabetes mellitus (T2DM) can control their blood glucose levels. Dapagliflozin is a selective inhibitor of sodium-glucose co-transporter 2 (SGLT-2) that improves the insulin sensitivity of the liver and peripheral tissues. Many studies confirmed that SGLT2 inhibitors reduce blood glucose and have multiple beneficial effects such as weight loss, lipid regulation, and kidney protection.
The mechanisms of the renal and cardiovascular protective effects of dapagliflozin from the perspective of differentially expressed proteins in the serum of T2DM patients have not been intensively explored so far.
This study aimed to identify differentially expressed proteins associated with dapagliflozin treatment in patients with T2DM. The results could help understand the mechanisms of dapagliflozin in patients with T2DM.
Twenty T2DM patients [hemoglobin A1c (HbA1c) 7.0%-10.0%] were enrolled at The Affiliated Hospital of Inner Mongolia Medical University between January 1, 2017 and December 1, 2018. They received dapagliflozin (10 mg/d) for 3 mo, and the HbA1c < 7.0% target was achieved. The changes in clinical indexes were compared before and after treatments. Label-free quantitative proteomics was used to identify differentially expressed proteins using the serum samples of five patients. The identified differentially expressed proteins were analyzed using various bioinformatics tools.
Dapagliflozin significantly improved the clinical manifestation of the patients. There were 18 downregulated proteins and one upregulated protein in the serum samples of patients after dapagliflozin administration. Bioinformatics analyses, including subcellular localization, EuKaryotic Orthologous Groups, Gene Ontology, and Kyoto Encyclopedia of Genes and Genomes annotations, were used to profile the biological characteristics of the 19 differentially expressed proteins. Based on the literature and function enrichment analysis, two downregulated proteins, myeloperoxidase (MPO) and alpha II B integrin, and one upregulated protein, podocalyxin (PCX), were selected for enzyme linked immunosorbent assay (ELISA) validation. These validated differentially expressed proteins had multiple correlations with clinical indexes, including HbAc1 and fasting C-peptide.
Dapagliflozin has obvious hypoglycemic effects, and it can also improve weight loss, lipid metabolism, and islet function of patients with T2DM. After dapagliflozin treatment, 18 proteins (including MPO and alpha II beta integrin) were downregulated, and PCX protein was upregulated in the serum of T2DM patients.
Subsequent function annotation and enrichment analysis, as well as ELISA validation and correlation analysis with the clinical indexes, suggested that MPO, alpha II beta integrin, and PCX might contribute to the beneficial roles of dapagliflozin through their regulations on oxidative stress, insulin resistance, and lipid metabolism.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Belosludtseva NV, Cure E S-Editor: Zhang H L-Editor: A P-Editor: Wu RR
| 1. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2860] [Cited by in F6Publishing: 2812] [Article Influence: 281.2] [Reference Citation Analysis (1)] |
| 2. | Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 868] [Cited by in F6Publishing: 870] [Article Influence: 62.1] [Reference Citation Analysis (1)] |
| 3. | Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5819] [Cited by in F6Publishing: 5689] [Article Influence: 237.0] [Reference Citation Analysis (0)] |
| 4. | Javeed N, Matveyenko AV. Circadian Etiology of Type 2 Diabetes Mellitus. Physiology (Bethesda). 2018;33:138-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 5. | United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310:83-88. [PubMed] [Cited in This Article: ] |
| 6. | Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016;37:278-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 840] [Cited by in F6Publishing: 988] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 7. | Brunton S. Pathophysiology of Type 2 Diabetes: The Evolution of Our Understanding. J Fam Pract. 2016;65. [PubMed] [Cited in This Article: ] |
| 8. | Wilding JP. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism. 2014;63:1228-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci (Lond). 2018;132:1329-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | Aso Y, Kato K, Sakurai S, Kishi H, Shimizu M, Jojima T, Iijima T, Maejima Y, Shimomura K, Usui I. Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase-4 in patients with type 2 diabetes and non-alcoholic fatty liver disease. Int J Clin Pract. 2019;73:e13335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 587] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 12. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2953] [Cited by in F6Publishing: 3502] [Article Influence: 700.4] [Reference Citation Analysis (0)] |
| 13. | Saleem F. Dapagliflozin: Cardiovascular Safety and Benefits in Type 2 Diabetes Mellitus. Cureus. 2017;9:e1751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int J Environ Res Public Health. 2019;16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 15. | Dhillon S. Dapagliflozin: A Review in Type 2 Diabetes. Drugs. 2019;79:1135-1146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 16. | Powell J, Miller SA, Taylor JR. Sodium-glucose cotransporter 2 inhibitors: the new option for diabetes mellitus management. South Med J. 2015;108:82-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with Type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med. 2015;32:531-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Ghosh RK, Bandyopadhyay D, Hajra A, Biswas M, Gupta A. Cardiovascular outcomes of sodium-glucose cotransporter 2 inhibitors: A comprehensive review of clinical and preclinical studies. Int J Cardiol. 2016;212:29-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Ji L, Li H, Guo X, Li Y, Hu R, Zhu Z. Impact of baseline BMI on glycemic control and weight change with metformin monotherapy in Chinese type 2 diabetes patients: phase IV open-label trial. PLoS One. 2013;8:e57222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | López-Villar E, Martos-Moreno GÁ, Chowen JA, Okada S, Kopchick JJ, Argente J. A proteomic approach to obesity and type 2 diabetes. J Cell Mol Med. 2015;19:1455-1470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Fu J, Luo Y, Mou M, Zhang H, Tang J, Wang Y, Zhu F. Advances in Current Diabetes Proteomics: From the Perspectives of Label- free Quantification and Biomarker Selection. Curr Drug Targets. 2020;21:34-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Välikangas T, Suomi T, Elo LL. A systematic evaluation of normalization methods in quantitative label-free proteomics. Brief Bioinform. 2018;19:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 23. |
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.
Report of the expert committee on the diagnosis and classification of diabetes mellitu |
| 24. | Rychter AM, Skrzypczak-Zielińska M, Zielińska A, Eder P, Souto EB, Zawada A, Ratajczak AE, Dobrowolska A, Krela-Kaźmierczak I. Is the Retinol-Binding Protein 4 a Possible Risk Factor for Cardiovascular Diseases in Obesity? Int J Mol Sci 2020; 21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Ost A, Danielsson A, Lidén M, Eriksson U, Nystrom FH, Strålfors P. Retinol-binding protein-4 attenuates insulin-induced phosphorylation of IRS1 and ERK1/2 in primary human adipocytes. FASEB J. 2007;21:3696-3704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Drossopoulou GI, Tsotakos NE, Tsilibary EC. Impaired transcription factor interplay in addition to advanced glycation end products suppress podocalyxin expression in high glucose-treated human podocytes. Am J Physiol Renal Physiol. 2009;297:F594-F603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Yamauchi M, Mizuhara Y, Maezawa Y, Toda G. Serum levels of integrins in chronic liver diseases. Pathol Res Pract. 1994;190:984-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Radwan AF, Ismael OE, Fawzy A, El-Mesallamy HO. Evaluation of Serum Integrin αvβ3 & Vitronectin in the Early Diagnosis of Breast Cancer. Clin Lab. 2019;65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Lenggenhager D, Bengs S, Fritsch R, Hussung S, Busenhart P, Endhardt K, Töpfer A, The FO, Bütikofer S, Gubler C, Scharl M, Morell B. β6-Integrin Serves as a Potential Serum Marker for Diagnosis and Prognosis of Pancreatic Adenocarcinoma. Clin Transl Gastroenterol. 2021;12:e00395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899-2937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 31. | Gómez García A, Rivera Rodríguez M, Gómez Alonso C, Rodríguez Ochoa DY, Alvarez Aguilar C. Myeloperoxidase is associated with insulin resistance and inflammation in overweight subjects with first-degree relatives with type 2 diabetes mellitus. Diabetes Metab J. 2015;39:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Nauseef WM. Contributions of myeloperoxidase to proinflammatory events: more than an antimicrobial system. Int J Hematol. 2001;74:125-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Song P, Xu J, Song Y, Jiang S, Yuan H, Zhang X. Association of Plasma Myeloperoxidase Level with Risk of Coronary Artery Disease in Patients with Type 2 Diabetes. Dis Markers. 2015;2015:761939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Kusuma K, Vasudha K, Vanitha Gowda M. A comparative study of serum myeloperoxidase activity in type 2 diabetes and diabetic nephropathy. 2009. [Cited in This Article: ] |
| 35. | Green HJ, Brown NH. Integrin intracellular machinery in action. Exp Cell Res. 2019;378:226-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Springer DL, Miller JH, Spinelli SL, Pasa-Tolic L, Purvine SO, Daly DS, Zangar RC, Jin S, Blumberg N, Francis CW, Taubman MB, Casey AE, Wittlin SD, Phipps RP. Platelet proteome changes associated with diabetes and during platelet storage for transfusion. J Proteome Res. 2009;8:2261-2272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Williams AS, Kang L, Wasserman DH. The extracellular matrix and insulin resistance. Trends Endocrinol Metab. 2015;26:357-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 38. | Tschoepe D, Menart B, Ferber P, Altmann C, Haude M, Haastert B, Roesen P. Genetic variation of the platelet- surface integrin GPIIb-IIIa (PIA1/A2-SNP) shows a high association with Type 2 diabetes mellitus. Diabetologia. 2003;46:984-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Jitrapakdee S, St Maurice M, Rayment I, Cleland WW, Wallace JC, Attwood PV. Structure, mechanism and regulation of pyruvate carboxylase. Biochem J. 2008;413:369-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 289] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 40. | Qi J, Xiao YF, Zhang DJ, Yang GR, Huang HC. [High glucose downregulates the expression of podocalyxin protein in glomerular podocytes of mice]. Beijing Da Xue Xue Bao Yi Xue Ban. 2007;39:167-170. [PubMed] [Cited in This Article: ] |
| 41. | Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2014;306:F194-F204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |