Published online Feb 15, 2022. doi: 10.4239/wjd.v13.i2.70
Peer-review started: February 26, 2021
First decision: April 20, 2021
Revised: April 27, 2021
Accepted: January 20, 2022
Article in press: January 20, 2022
Published online: February 15, 2022
Processing time: 347 Days and 17.1 Hours
Metabolically healthy obese (MHO) individuals are reported to have a lower risk of developing cardiovascular diseases in comparison with individuals with metabolic syndrome. However, the association between MHO and type 2 diabetes (T2DM) is still controversial. Some studies indicated that MHO is a favorable phenotype for T2DM, but more studies showed that MHO individuals have an increased risk of developing T2DM compared with metabolically healthy normal-weight individuals, especially among those who would acquire metabolically unhealthy obesity. This has been supported by finding insulin resistance and low-grade inflammatory responses in MHO individuals with a tendency for impaired beta-cell dysfunction. Studies also showed that liver fat accumulation increased the risk of incidence of T2DM in MHO. Here, we reviewed current literature on the relationship between MHO and T2DM, discussed the determinants for the development of diabetes in MHO, and summarized the measures for the prevention of T2DM in MHO.
Core Tip: Metabolically healthy obese individuals have already developed impaired insulin sensitivity with dysfunction of insulin action on subcutaneous tissue, as well as a tendency for beta-cell dysfunction and a chronic low-grade inflammatory status compared with metabolically healthy normal-weight individuals. Thus, it is an unfavorable phenotype for type 2 diabetes, with metabolic changes preceding the incidence of diabetes. Liver fat content might be an important contributor to the development of diabetes in metabolically healthy obesity among all risk factors. More attention should be paid to the weight management and metabolic status of these individuals.
- Citation: Wu Q, Xia MF, Gao X. Metabolically healthy obesity: Is it really healthy for type 2 diabetes mellitus? World J Diabetes 2022; 13(2): 70-84
- URL: https://www.wjgnet.com/1948-9358/full/v13/i2/70.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i2.70
Obesity and diabetes have been growing public health problems for decades. The prevalence of obesity had doubled worldwide in 2015 compared with that in 1980[1]. Individuals with obesity are generally likely to develop type 2 diabetes mellitus (T2DM), since obesity is linked to increased risk of insulin resistance, beta-cell dysfunction, and imbalanced fat tissue metabolism[2]. However, there is a subset of obese individuals who are at low risk of cardiovascular disease with a relatively normal metabolic profile compared with metabolic unhealthy obesity (MUO) individuals, a condition known as metabolically healthy obesity (MHO)[3]. Some studies showed that MHO individuals were not at increased risk for diabetes compared with those who are classified as metabolically healthy normal weight (MHNW)[4,5], but others indicated that MHO was associated with an increased risk of developing T2DM over a lifetime than MHNW[6,7]. Whether MHO is a real health status, or more specifically, whether it predisposes individuals to T2DM, is still controversial.
In this review, we address the above questions by discussing controversies related to metabolically healthy obesity, including the causal relationship between MHO and T2DM and its related diseases as well as the underlying mechanisms.
MHO was described by Sims in 2001 as obesity with the absence of metabolic syndrome and metabolic complications[8]. Most definitions of MHO are based on the criteria for metabolic syndrome based on the definition provided by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP-III)[9], which include: (1) The presence of central obesity, waist circumference ≥ 102 cm ( 90 cm for Asians) in men and ≥ 88 cm ( 80 cm for Asians) in women; (2) Systolic blood pressure ≥ 17.3 kPa (130 mmHg) and/or diastolic blood pressure ≥ 11.3 kPa (85 mmHg); (3) Triglycerides ≥ 1.7 mmol/L (150 mg/dL); (4) Fasting blood glucose ≥ 5.6 mmol/L (100 mg/dL); and (5) High-density lipoprotein cholesterol (HDL-C) less than 1.03 mmol/L (40 mg/dL) in men or less than 1.30 mmol/L (50 mg/dL) in women. Most definitions of MHO require fewer than two or the absence of any metabolic abnormalities except for waist circumference[7,10-13]. However, the details of the MHO definitions are slightly different. One study defined MHO as individuals who possess no more than two of four metabolic abnormalities except waist circumference[14]. Some researchers believe that those who use anti-hypertension drugs, lipid-lowering agents, or glucose-lowering medicines are also metabolically abnormal even though their metabolic levels are good[15,16]. The level of low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC) were also included in the definition of MHO by Karelis et al[17]. Insulin resistance evaluated by the homeostasis model assessment for insulin resistance (HOMA-IR) and inflammatory status expressed by C-reactive protein (CRP) has been added to the criteria for MHO by Wildman et al[18]. Lwow et al[19] proposed using a combined lipid accumulation product with the criteria mentioned above as new criteria for MHO. Smith et al[20] decreased the cut point of triglyceride to a level of 95 mg/dL and includes the criteria for the evaluation of intrahepatic lipid content. MHO was also defined as the absence of metabolic diseases such as hypertension, T2DM, and dyslipidemia[15]. The detailed information of common definitions of MHO was showed in Table 1.
| Ref. | BP, kPa (mmHg) | Plasma glucose, mmol/L | TG, mmol/L | HDL-C, mmol/L | LDL-C, mmol/L | TC, mmol/L | WC, cm | Insulin sensitivity | CRP, mg/L | Intrahepatic lipid content | Others | Metabolic health | |
| NECP ATP III[9] | SBP ≥ 17.3 (130) and/or DBP ≥ 11.3 (85) and/or treatment | FPG ≥ 5.60 | ≥ 1.70 | < 1.29 in women, < 1.03 in men | - | - | > 88 in women, >102 in men | - | - | - | - | < 3 of above | |
| Karelis et al[17] | - | - | ≤ 1.70 | ≥ 1.30 and no treatment | ≤ 2.60 and no treatment | ≤ 5.20 | - | HOMA-IR ≤ 1.95 | - | - | - | > 3 of above | |
| Meigs et al[4] | SBP ≥ 17.3 (130) or DBP ≥ 11.3(85) or treatment | 5.6 < FPG ≤ 6.9 | ≥ 1.70 | < 1.30 in women, < 1.00 in men | - | - | > 88 in women, > 102 in men | - | - | - | - | < 2 of above | |
| Meigs et al[4] | - | - | - | - | - | - | - | HOMA-IR ≥ 75th percentile | - | - | - | None of above | |
| Aguilar-Salinas et al[89] | SBP > 18.6 (140) and/or DBP > 12.0 (90) and/or treatment | FPG ≥ 7.0, or 2-h OGTT ≥ 11.1, or RBG ≥ 11.11 or treatment | - | < 1.04 | - | - | - | - | - | - | - | None of above | |
| Wildman et al[18] | SBP ≥ 17.3 (130) or DBP ≥ 11.3 (85) or treatment | FPG ≥ 5.56 or treatment | ≥ 1.70 | < 1.30 in women, < 1.04 in men or treatment | - | - | - | HOMA-IR > 90th percentile | > 90th percentile | - | - | < 2 of above | |
| van Vliet-Ostaptchouk et al[90] | SBP ≥ 17.3 (130) or DBP ≥ 11.3 (85) or treatment | FPG ≥ 6.10 or treatment or history/diagnosis of type 2 diabetes | ≥ 1.70 or treatment | < 1.03 in men or < 1.30 in women or treatment | - | - | - | - | - | - | - | <2 of above | |
| Jana V van Vliet-Ostaptchouk et al[90] | SBP ≥ 18.6 (140) or DBP ≥ 12.0(90) or treatment | FPG ≥ 7.0 or treatment or history/diagnosis of type 2 diabetes | ≥ 1.70 or treatment | < 1.03 in men or < 1.30 in women or treatment | - | - | - | - | - | - | - | < 2 of above | |
| Smith et al[20] | SBP < 17.3 (130) and/or DBP < 11.3 (85) | FPG < 5.60, or 2-h OGTT glucose < 7.80 | < 1.07 | ≥ 1.29 in women, ≥ 1.04 in men | - | - | - | GIR > 8 mg/kg FFM/min during an HECP (insulin infusion rate: 40 mU/m2/min) | - | < 5% of liver volume by imaging or < 5% of hepatocytes with intracellular TG by histology | Basic criteria: Absence of diagnosis or therapy of cardiometabolic diseases | all of above | |
The prevalence of MHO differs from 2.2% to 11.9% in the general population according to the different definitions of MHO[21]. The prevalence of MHO in Americans from the National Health and Nutrition Examination Survey was 19.9% when metabolic health was defined as the absence of components of NCEP ATP-III; the prevalence decreased to 16.0% when the threshold of glucose was reduced to 100 mg/dL, and it decreased to 14.8% when HbA1c was included in the definition of MHO. The prevalence further decreased to 12.2% when the cut-off point of blood pressure was reduced from 17.3/11.3 kPa (130/85 mmHg) to 16.0/10.6 kPa (120/80 mmHg)[22]. Using the criteria of less than three components of NCEP ATP-III, the prevalence of MHO was 8.6% in Spanish[23] and 10.3% in China[24]. There was an age-related reduction in the proportion of MHO regardless of different definitions[24]. Besides, obese patients with higher body mass index (BMI) levels had a lower proportion of MHO, which accounted for 53.7% of participants with BMI at 30-34.9 kg/m2 and 4.9% of participants with BMI at 35-39.9 kg/m2[25]. When a more stringent criterion of having no components of NECP ATP-III was applied to the definition of MHO, there was no metabolic healthy individual with BMI ≥ 35 kg/m2[23]. It means that there might be a cut-off point in individuals with MHO, beyond which their metabolic status would no longer be healthy.
Metabolically healthy individuals will develop metabolic disorders over time. Feng et al[7] discovered that only 42.84% of individuals in a group of MHO remained metabolic healthy after a 4-year follow-up. Gilardini et al[26] reported that 44% of MHO became metabolically unhealthy after 6-year follow-up, and the proportion increased to 62% after 12-year follow-up. The proportion of transition from MHO to MUO might differ because of different definitions of MHO and various lengths of follow-up[27]. Generally speaking, MHO is not a health status according to the current definitions of having one or two abnormal conditions but rather a transient state that can transition to an unhealthy state over time. Thus, it is fundamentally inaccurate to define those groups of people as “healthy” and worthwhile to investigate the relationship between MHO and T2DM.
The association between T2DM and MHO has been studied with diverse results, as shown in Table 2. Although MHO is believed to be a healthier phenotype for T2DM when compared with metabolically unhealthy normal weight and MUO individuals, most of the current studies supported that MHO phenotype relates to an increased incidence of T2DM in cohort studies compared to MHNW individuals, independent of the length of follow-up[7,16,28-31]. Wei et al[30] examined 17801 individuals in the Dongfeng-Tongji cohort study and showed that the hazard ratio [95% confidence interval (CI)] of diabetes for MHO was 1.74 (1.16-2.59). The multivariate-adjusted hazard ratio (95%CI) of diabetes for MHO without non-alcoholic fatty liver diseases (NAFLD) was 1.57 (1.14-2.16) after an average 4.1-year follow-up in The Kangbuk Samsung Health Study[16]. However, studies have also found that different sub
| Ref. | Definition of “metabolic health” | MHO, n | Main findings |
| Wei et al[30], 2020 | Having < 2 of the following criteria: (1) TG ≥ 1.7 mmol/L or lipid-lowering drugs; (2) SBP ≥ 17.3 kPa (130 mmHg) or DBP ≥ 11.3 kPa (85 mmHg) or anti-hypertensive drugs; (3) FPG ≥ 5.6 mmol/L; and (4) HDL-C < 1.04 mmol/L for men and < 1.29 mmol/L for women. | 693 | MHO was associated with an increased incidence of diabetes, and the association did not differ by the presence or absence of NAFLD. |
| Feng et al[7], 2020 | Having < 2 of the following criteria: (1) Hyperglycemia, defined as FPG ≥ 5.6 mmol/L (100 mg/dL); (2) Elevated blood pressure, defined as SBP ≥ 17.3 kPa (130 mmHg) and/or DBP ≥11.3 kPa (85 mmHg) or antihypertensive drug treatment; (3) Hypertriglyceridemia, defined as TG ≥ 1.7 mmol/L (150 mg/dL); and (4) Reduced HDL-C levels, defined as drug treatment to increase HDL-C levels. | 3728 | The MHO phenotype was associated with an increased incidence of diabetes in older adults. The presence of metabolic disorders in the group with MHO was associated with increased diabetes risk and was predicted by the waist circumference at baseline. |
| Kim et al[32], 2019 | Having two or fewer metabolic abnormalities as follows: (1) WC ≥ 90 cm in men and ≥ 85 cm in women; (2) SBP ≥ 17.3 kPa (130 mmHg) or DBP ≥ 11.3 kPa (85 mmHg) or medication use; (3) FPG ≥ 5.6 mmol/L (100 mg/dL) or claim for T2DM or on anti-diabetic medications; (4) Hypertriglyceridemia ≥ 1.7 mmol/L (150 mg/dL) or on lipid medications; and (5) HDL-C < 1.04 mmol/L (40 mg/dL) in men and < 1.29 mmol/L (50 mg/dL) in women, or medication use. | 796371 | MHO and MHNW phenotypes were transient phenotypes, and their change into metabolic unhealthy status was an important risk factor for the development of T2DM both in obese and normal-weight subjects. Transition into a metabolically unhealthy phenotype was a more significant risk factor of developing T2DM than obesity itself. |
| Wang et al[33], 2018 | Having < 2 of the following criteria: (1) SBP ≥ 17.3 kPa (130 mmHg) or DBP ≥ 11.3 kPa (85 mmHg) or current treatment for hypertension; (2) Fasting TG level ≥ 1.7 mmol/L; (3) HDL-C level < 1.03 mmol/l for males or < 1.29 mmol/L for females; and (4) FPG ≥ 5.60 mmol/L. | 2153 | Stable metabolically healthy overweight/obesity Individuals and those who transitioned to the metabolically healthy status from MUNW did not have an increased risk of incident T2DM. Participants who transitioned from the metabolically healthy overweight/obesity to metabolically unhealthy overweight/obesity phenotype and stable MUNW phenotype showed an increased risk of incident T2DM. |
| Fingeret et al[31], 2018 | Having two or fewer metabolic abnormalities as follows: (1) FPG ≥ 5.6 mmol/L or drug treatment; (2) Fasting TG ≥ 1.7 mmol/L or drug treatment; (3) Fasting HDL-C < 1.30 mmol/L in women and < 1.00 mmol/L in men or drug treatment; (4) SBP ≥ 17.3 kPa (130 mmHg), DBP ≥ 11.3 kPa (85 mmHg), or drug treatment; and (5) WC ≥ 102 cm for men and ≥ 88 cm for women. | 170 | MHO leads to a higher risk of developing cardiovascular risk factors such as hypertension, diabetes, dyslipidemia as compared with MHNW. MHO is transient and should be regarded by clinicians as a warning sign. |
| Liu et al[91], 2018 | Having < 2 of metabolic abnormalities as follows: (1) TG ≥ 1.7 mmol/L; (2) HDL-C < 1.0 mmol/L; (3) SBP ≥ 17.3 kPa (130 mmHg) and/or DBP ≥ 11.3 kPa (85 mmHg); and (4) FPG ≥ 5.6 mmol/ L (≥ 100 mg/dL). | 1184 | MHO and MUNW phenotypes had an increased risk for diabetes. Both baseline metabolic status and follow-up changes played more important roles than obesity for diabetes incidence after adjusted for potential confounding factors. MHO is a transient condition. |
| Janghorbani et al[29], 2017 | Having none of metabolic abnormalities as follows: (1) TG ≥ 1.7 mmol/L (150 mg/dL); (2) HDL < 1.04 mmol/L(40 mg/dL) in men and < 1.29 mmol/L(50 mg/dL) in women; (3)BP ≥ 17.3/11.3 kPa (130/85 mmHg) or on antihypertensive medication; and (4) FPG ≥ 5.6 mmol/L (100 mg/dL). | 75 | Metabolic abnormalities increased risk for incident T2D at any BMI status. Also, obesity is a risk factor for the incidence of T2DM, even in the absence of any metabolic abnormalities. |
| Latifi et al[25], 2017 | Having none of metabolic abnormalities as follows: (1) WC ≥ 102 cm in men and ≥ 88 cm in women; (2) TG ≥ 1.7 mmol/L (150 mg/dL) or drug use; (3) HDL < 1.04 mmol/L (40 mg/dL) in men and 1.29 mmol/L (50 mg/dL) in women or drug consumption for hyperlipidemia; (4) BP ≥ 17.3/10.6 kPa (130/80 mmHg) or a history of anti-hypertensive drug consumption; and (5) FPG ≥ 5.6 mmol/L (100 mg/dL), or a history of diabetes mellitus or consumption of anti-diabetes drugs. | NA | There was a specific higher risk of developing metabolic syndrome and diabetes in MHO. |
| Navarro-Gonzalez et al[14], 2016 | Having < 3 of the following criteria: (1) TG ≥ 1.7 mmol/L (150 mg/dL); (2) HDL-C > 1.04 mmol/L (40 mg/dL) for men and > 1.29 mmol/L (50 mg/dL) for women; (3) BP ≥ 17.3/11.3 kPa (130/85 mmHg); or (4) FPG ≥ 5.6 mmol/L (100 mg/dL). All individuals currently taking a pharmacological treatment for hypertension were assumed to have raised BP. | 389 | MHO individuals had an increased risk of incident type 2 diabetes but mainly among those who progressed MUO. MHO individuals who remained with one or no metabolic health risk factors or lost weight overtime did not have a significant risk of diabetes. Metabolically unhealthy individuals had a greater risk of diabetes compared with subjects with MHO. |
| Guo et al[3], 2016 | Having all three components as follows: (1) Untreated SBP < 17.3 kPa (130 mmHg) and DBP < 11.3 kPa (85 mmHg); (2) Untreated FPG < 5.6 mmol/L (100 mg/dl) or HbA1c < 5.7%; and (3) Untreated TC < 6.2 mmol/L (240 mg/dL) and HDL ≥ 1.04 mmol/L (40 mg/dL) in men and ≥ 1.29 mmol/L (50 mg/dL) in women. | 260 | People with healthy obesity have lower risks for diabetes, coronary heart disease, stroke, and mortality compared with unhealthy subjects regardless of their BMI status. Obesity did not affect the risks of coronary heart disease, stroke, and mortality, but did increase diabetes risk. |
| Jung et al[40], 2016 | Having < 2 of the following criteria: (1) SBP ≥ 17.3 kPa (130 mmHg) and/or a DBP ≥ 11.3 kPa (85 mmHg), or on antihypertensive treatment; (2) TG ≥ 1.7 mmol/L; (3) FPG ≥ 5.6 mmol/L (impaired fasting glucose, IFG); (4) HDL-C < 1.0 mmol/L in men and < 1.3 mmol/L in women; (5) HOMA-IR ≥ 90th percentile (≥ 2.91); and (6) Hs-CRP ≥ 90th percentile (≥ 2.0 mg/L). | 4635 | MHO subjects have a substantially increased risk of incident type 2 diabetes compared with MHNO subjects in an Asian population. The presence of FLD assessed by FLI partially explains this increased risk. |
| Chang et al[16], 2016 | Having none of the following criteria: (1) BP ≥ 17.3/11.3 kPa (130/85 mmHg) or current use of blood pressure-lowering agents; (2) FPG ≥ 5.6 mmol/L (100 mg/dL) or current use of blood glucose-lowering agents; (3) TG ≥ 1.7 mmol/L (150 mg/dL) or current use of lipid-lowering agents (15); (4) HDL-C < 1.04 mmol/L (40 mg/dL) in men or < 1.29 mmol/L (50 mg/dL) in women; or (5) Insulin resistance, defined as HOMA-IR score ≥ 2.5. | 8140 | Metabolically healthy overweight and obese individuals were both associated with an increased incidence of diabetes, even in the absence of NAFLD. Obese phenotype itself can drive the development of diabetes, even in the absence of metabolic abnormalities and NAFLD. |
| Ryoo et al[34], 2015 | Having < 2 of the following criteria: (1) SBP ≥ 17.3 kPa (130 mmHg) and/or DBP ≥ 11.3 kPa (85 mmHg); (2) TG ≥ 1.7 mmol/L; (3) FPG ≥ 5.6 mmol/L; (4) HDL-C < 1.0 mmol/L; and (5) HOMA-IR ≥ 90th percentile. | 240 | The risk for diabetes was in proportion to both metabolic health status and degree of obesity in Korean men. Additionally, metabolically healthy status was a more significant determinant for the development of diabetes than obesity itself. |
Several factors might contribute to the development of T2DM in the MHO participants. Baseline body weight is an important factor associated with the high risk of incidence of diabetes. It is universally known that obesity can increase the risk of T2DM. One study found that obese individuals (BMI ≥ 30 kg/m2) with a healthy metabolic status were at greater risk of developing diabetes than either overweight or normal-weight subjects, and the risk was in proportion to the degree of obesity[14,34]. The previous study has also shown that all metabolically unhealthy individuals, regardless of their body weight, have a higher risk of diabetes[14]. Unstable MHO individuals who progress into unhealthy metabolic statuses also have an elevated risk of developing diabetes. Weight gain was a risk factor for the progression from a healthy condition to an unhealthy one, which further develops into T2DM. In one study, MHO individuals who developed cardiometabolic risk complications gained 6% ± 14% of their body weight (4.9 ± 11.8 kg) compared to 5% ± 14% (3.9 ± 11.3 kg) for those that retained a healthy status[35]. Besides, MHO participants with larger waist circumference at baseline are more likely to transition into an unhealthy phenotype[7]. This has been supported by studies showing that visceral abdominal fat accumulation and fatty liver in MHO contribute to this transition[12,36,37]. Thus, MHO individuals with high liver fat content or large waist circumference are possibly associated with a high risk of diabetes as they have a trend to transferring into MUO phenotype. Our previous study found that visceral adipose area measured by visceral adiposity index in Chinese adults has a more favorable function to predict the development of diabetes than BMI and waist circumference in MHO individuals[38]. Some researchers found that MHO individuals with a high fatty liver index[39] have an increased risk of incident T2DM[40].
NAFLD is believed to be significantly associated with the long-term risk of T2DM, and increased liver fat can predict the incidence of T2DM independent of obesity[41,42]. Bian et al[43] found that elevated liver fat content (LFC) showed a positive association with insulin resistance and a higher level of nocturnal mean blood concentration before the onset of diabetes. The presence of NAFLD will promote the transition from MHO to a metabolic unhealthy state, and further increases the long-term risk of incidence of T2DM and even aggravates the deterioration of liver diseases in MHO. Hwang et al[12] found that the presence of NAFLD in MHO could predict the conversion from a metabolic health status into a metabolic unhealthy status independent of age, sex, BMI, lifestyle factors, components of metabolic syndrome, and insulin resistance evaluated by HOMA-IR. This result was supported by Hashimoto et al[37] with findings that fatty liver index was a predictor for the transition from MHO to MUO phenotype even adjusted for body weight change. However, Hwang et al[12] also found that the association between the NAFLD and future transition of MHO into MUO weakened as BMI increased, and the relationship was more prominent in lower BMI individuals. Studies also found that the risk of NAFLD, non-alcoholic steatohepatitis, and liver fibrosis increased as BMI elevated in MHO[16,44]. The unstable MHO status predicted by NAFLD would increase the risk for the development of T2DM, as mentioned above, and therefore the presence of NAFLD in MHO might increase the risk of incident T2DM. Chang et al[16] supported this with the result that the risk of incidence of T2DM in MHO subjects with NAFLD increased compared to those free of NAFLD. Ampuero et al[45] also found that MHO individuals with biopsy-proven NAFLD or with an intermediate-to-high risk of significant fibrosis evaluated by Hepanet Fibrosis Score (> 0.12) were at risk of developing T2DM.
However, despite the presence of elevated LFC in MHO increasing the risk for the transition of MHO and the incidence of T2DM, few studies regarded intrahepatic lipids content as one of the criteria for the definition of metabolic health. Our previous study found that LFC was positively associated with metabolic disorders independent of related anthropometric and metabolic parameters, and the risk for metabolic diseases increased in an LFC-dependent manner when LFC ≥ 5%[46]. Besides, part of normal individuals without metabolic disorders had a higher LFC[46]. Hence, we agree with Smith et al[20] that the evaluation of LFC should be regarded as another crucial criterion for defining “metabolic health”.
Studies have found that subjects with MHO have a lower risk of cardiovascular disease (CVD) than MUO individuals over their lifetimes but still have a higher risk than MHNW subjects[47-50]. The transition to an unhealthier metabolic status and the longer duration of unhealthy metabolic conditions contribute to the increased risk of developing CVD among MHO subjects[50-52]. Furthermore, the risk of developing CVD for MHO subjects who initially develop diabetes, hypertension, or hypercholesterolemia tends to be higher than in MHNW subjects[53]. Obesity might increase the risk of CVD independently. A meta-analysis concluded that CVD risk is increased in metabolically healthy overweight or obese participants than in MHNW individuals even when there are no metabolic risk factors[54]. Similarly, obese individuals have been reported to be at higher risk of coronary heart disease irrespective of metabolic health, which challenges the concept of “metabolically healthy obesity”[55].
Previous studies have shown an increased risk of developing chronic kidney disease (CKD), defined as an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2 in metabolically healthy overweight/obese subjects compared to MHNW individuals at follow-up, with metabolic health judged as having less than two metabolic abnormalities[56]. Another study showed a similar result in which MHO individuals with no metabolic abnormalities had a higher risk of developing CKD, and this risk was greater in those 40 years or older than in the young[57]. Systemic inflammation measured by high sensitivity-CRP (hs-CRP) might partially contribute to the association between MHO and CKD[11]. Furthermore, individuals who progress to MUO at follow-up show a higher risk of CKD compared with remaining MHO subjects[58,59]. However, Chen et al[60] found that the risk difference was not significant in MHO subjects compared to MHNW individuals in the early stage of CKD. This discrepancy might come from the different definitions of CKD, as Chen et al[60] combined proteinuria and structural changes in the kidney as indicators.
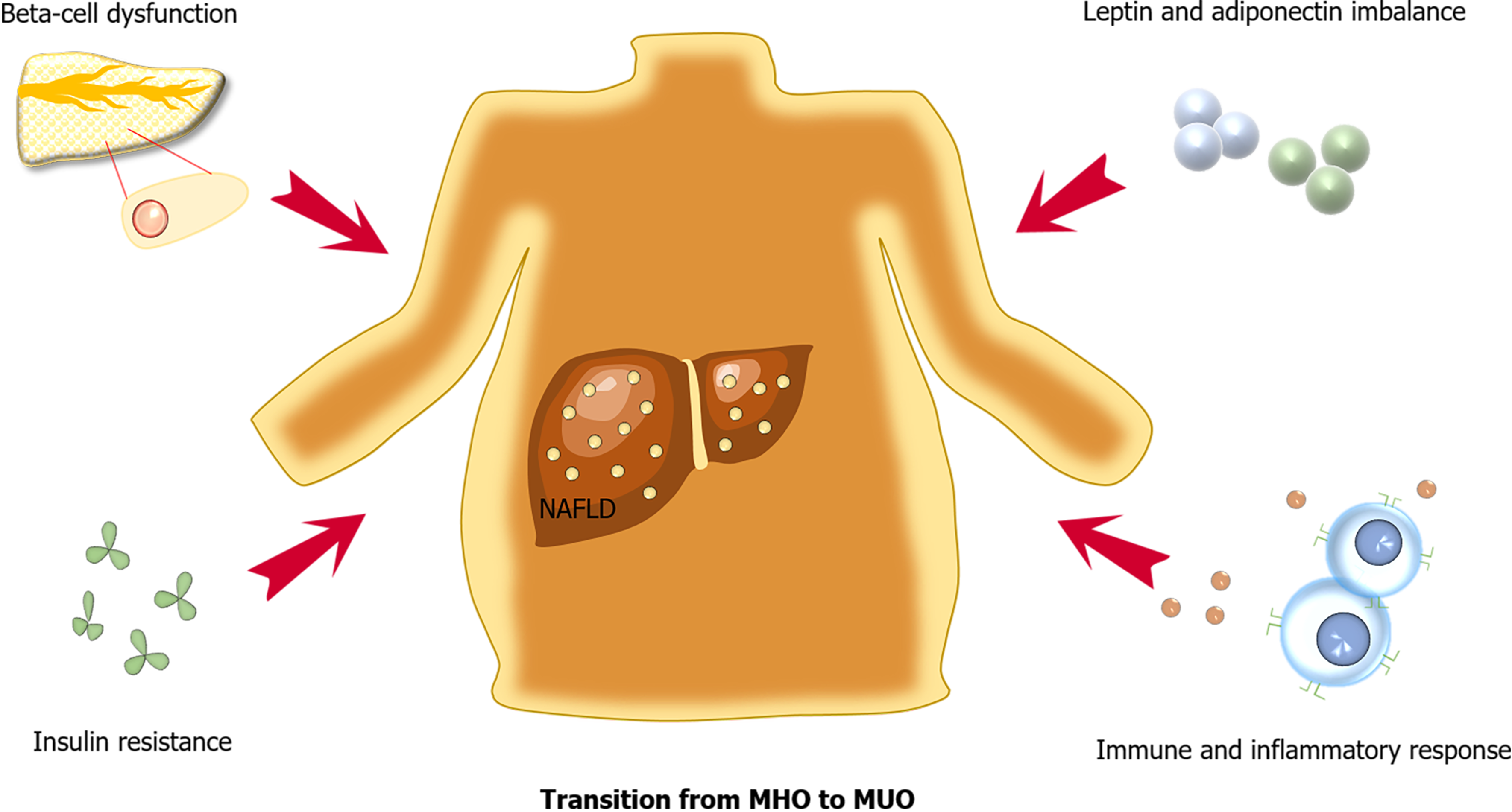
The possible mechanisms underlying the pathophysiology of incident T2DM in MHO include beta-cell dysfunction, insulin resistance, leptin and adiponectin imbalance, as well as a chronic low-grade inflammatory status (Figure 1). The presence of NAFLD in MHO is also an important factor for the development of T2DM.
Mature insulin and C-peptide are produced from the precursor proinsulin, and increased proinsulin is observed in insulin-resistant and/or glucose-intolerant individuals[61]. Significantly increased levels of plasma proinsulin, split proinsulin, and C-peptide are observed in MHO subjects compared to MHNW subjects[62]. A similar result has been found in a Chinese population, in which the serum insulin of MHO subjects is significantly elevated[63]. Studies have confirmed the above results showing that HOMA-IR evaluations are significantly different between MHO and MHNW subjects, with a higher value of HOMA-IR in MHO individuals[62,63].
The action of insulin on subcutaneous adipocytes is impaired as well. Rydén et al[10] compared the inhibitory action on lipolysis and the stimulatory effect on lipogenesis of insulin in metabolically healthy subjects who were lean, overweight, or obese and found that insulin resistance was already observed in metabolically healthy overweight and obese subjects. In the classical agonist-receptor interaction model, the half-maximum effects for insulin to inhibit adipocyte lipolysis and lipogenesis in overweight/obese people were 10 times and 100 times higher than that in lean people, respectively. The above model suggested that alterations in intracellular events downstream of the insulin receptor and their initial signaling steps have already happened in those individuals. The decreased expression of the insulin signaling mediator AKT2 might partially explain the increased maximum concentration of insulin hormones needed for an antilipolytic effect and lipogenesis, as AKT2 is an early signaling factor common to the two pathways[64]. Furthermore, the impaired lipogenic function might in part result from a decrease in SLC2A4 (glucose transporter type 4) mRNA expression, which is essential for insulin-induced glucose uptake by fat cells and stimulating lipogenesis[64]. When testing the maximum insulin action on subcutaneous adipocytes, Rydén et al[10] found that the lipogenic effect of insulin hormone was reduced by more than 50% in healthy overweight/obese subjects comparing to lean individuals, and the effect was further impaired in the unhealthy obese groups. Thus, there are reasons to believe that insulin resistance is already present in MHO individuals.
There is no apparent evidence that beta cells in MHO subjects are severely impaired, but they may be partially impaired according to previous studies. Hjelmgren et al[62] found that MHO individuals are at increased risk for having β-cell dysfunction, as evaluated by proinsulin levels > 11 pmol/L compared to MHNW subjects, with a relative risk of 18.2 (95%CI: 2.1-159.3). However, Zhao et al[63] failed to find a significant difference in HOMA-β between MHNW and obese subjects, though the value of HOMA-β in MHO tended to be higher than that in MHNW individuals among middle-aged subjects. The discrepancy between the two studies might come from their different definitions of metabolic health, differences in race and age, and the relatively small sample size in Zhao’s study for evaluating statistical differences. Overall, studies on beta-cell dysfunction are too few to confirm their impaired function in MHO individuals.
Obesity has always been believed to be a chronic low-grade inflammatory status[65], referred to as meta-inflammation. This chronic low-grade inflammation is believed to be a central link between obesity and T2DM[66,67]. A previous study showed that meta-inflammation is presented in MHO subjects as well[68].
Macrophage infiltration in adipose tissue causes increased proinflammatory cytokines and contributes to the development of insulin resistance and T2DM[69]. Christou et al[70] found that circulating inflammatory intermediate monocytes [Mon2 (CD14++CD16+)] are upregulated in MHO individuals, and nonclassical monocytes [Mon3 (CD14+CD16++)] tended to be higher in comparison to metabolically healthy lean individuals when metabolic health was defined as fewer than two metabolic disabilities. The absolute counts of nonclassical Mon3 showed a positive association with HOMA-IR in that study. However, that result differed from previous studies, as the participants they recruited were taking antidiabetic medications, which might have disturbed the relationship between Mon3 and the level of insulin resistance[71,72].
A previous study found that an imbalance of T cell subsets is responsible for the pathogenesis of obesity and T2DM[68]. Th22 subsets might play a role in obesity and T2DM progression, with MHO and T2DM individuals having significantly elevated peripheral blood Th22 frequencies[73]. This might partially result from the signifi
Adipose tissue is not only an energy storage depot, it also has endocrine functions and produces some cytokines that influence metabolism throughout the human body. White fat tissue can participate in regulating insulin sensitivity, lipid metabolism, and low-grade inflammation[74,75]. Leptin and adiponectin are important factors in these conditions. Leptin is responsible for food intake and metabolism regulation, while adiponectin release contributes to energy metabolism, insulin action, lipid metabolism regulation, and oxidative stress. Increased adiponectin is associated with better insulin sensitivity in the human body[76]. A previous study found that adiponectin is significantly decreased in MHO Han Chinese adolescents compared with a normal-weight control group, and a similar result was also found in middle-aged Norwegians[77,78]. Thus, insulin sensitivity might be disturbed in MHO individuals with elevated adiponectin. However, Carvalho et al[79] found an inconsistent result that the serum adiponectin concentration in MHO subjects had no significant difference with MHNW individuals. The small sample size of the latter study might have contributed to the inability to find statistically significant differences in adiponectin. Taken together, most studies indicated an increased leptin/adiponectin ratio in MHO compared to MHNW individuals[77-79], which was already regarded as a sensitive indicator of metabolic syndrome and insulin sensitivity[80]. Thus, no matter whether adiponectin is decreased in MHO individuals, it can be deduced that insulin sensitivity has been already impaired in MHO subjects with an elevated leptin/adiponectin ratio.
There are few clinical procedures for MHO individuals to prevent the high risk of incidence of T2DM, but studies have shown evidence of benefits of weight loss for MHO with the improvement of metabolic parameters and inflammatory biomarkers.
A cohort study has found that bariatric surgery could significantly achieve a great deal of total weight loss in MHO patients at follow-up[81]. Some studies have shown that MHO could achieve more weight loss than that in MUO participants after bariatric surgery[82-84], suggesting that the MHO phenotype is an independent predictor for greater body weight loss and more effective bariatric surgery in obese individuals before metabolic abnormalities appear[83]. Furthermore, cardiovascular risk factors such as blood pressure, lipid levels, and plasma glucose are improved after bariatric surgery, even when some of these levels are ”normal” preoperatively[81]. Otherwise, these indexes show more improvement in metabolically unhealthy individuals[81,84]. However, Pelascini et al[82] failed to find significant improvements in HDL-C and plasma glucose in MHO participants, which might have resulted from their relatively small sample size and strict definition of “metabolic health” plus HOMA-IR and hs-CRP. In summary, the benefits of bariatric surgery for the MHO phenotype are considerable, potentially comparable in benefit to the unhealthier phenotype with much better weight loss[84]. However, this has only been tested and observed in MHO subjects whose BMI was ≥ 40 kg/m2. For the majority of MHO individuals, the application of bariatric surgery is not recommended in the current clinical environment with no more solid testimonies.
For the majority of those individuals with MHO, cultivating a favorable lifestyle might be a more feasible method to achieve weight loss. Studies have demonstrated that a healthier diet with a higher proportion of fruit, vegetables, and fish and longer mealtimes (more than 10 min) in women and higher degrees of physical activity is associated with the MHO phenotype compared with the MUO phenotype[85,86]. Gomez-Huelgas et al[87] found that intensive lifestyle modification could induce clinically significant weight loss in MHO phenotype women, leading to the reduction of serum adipokines and inflammatory biomarkers such as hs-CRP, interleukin-6, and tumor necrosis factor-α, which play important roles in the pathological mechanism of obesity and insulin resistance.
In a prospective cohort study of the MHO population, it was found that air pollution had a significantly positive correlation with adiponectin and hs-CRP, which suggests that air pollution plays an important role in the occurrence and development of diabetes in MHO individuals[88]. It will be interesting to compare the risk of the incident in MHO with and without exposure to polluted air.
Current MHO diagnostic criteria are insufficient to exclude all obese people with the potential to develop future metabolic disorders. How to define MHO is an issue worth discussing. MHO is not absolutely “metabolically healthy” compared to MHNW with potential risks for T2DM and its related metabolic disorders. This might be explained by mechanisms such as the expansion and hypoxia of adipose tissue, increased inflammation, and decreased adiponectin concentrations in the MHO population. Liver fat accumulation is also a crucial risk factor for the incidence of T2DM in MHO. Thus, we recommend adding the intrahepatic fat content into the criteria for “metabolic health”. Weight control might effectively protect the MHO individuals from the development of diabetes and its related metabolic diseases. In addition, MHO is a transitional phenotype between MHNW and MUO. It will be worthwhile to investigate the crucial factors that are responsible for the transition from MHO to MUO. The advance of multi-omics technology might help us to identify better MHO with a higher risk of developing diabetes and multiple metabolic disorders.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Belete R S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5077] [Article Influence: 634.6] [Reference Citation Analysis (2)] |
| 2. | Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3786] [Cited by in RCA: 3676] [Article Influence: 167.1] [Reference Citation Analysis (0)] |
| 3. | Guo F, Garvey WT. Cardiometabolic disease risk in metabolically healthy and unhealthy obesity: Stability of metabolic health status in adults. Obesity (Silver Spring). 2016;24:516-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 727] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 5. | Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, Adams RJ; North West Adelaide Health Study Team. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36:2388-2394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 6. | Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev. 2014;15:504-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 327] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 7. | Feng S, Gong X, Liu H, Lu R, Duan T, Wang M, Wang J, Wang H, Chen J, Liu Y, Li C, Ma J, Wu L, Lin Y, Hou F, Zhang Y, Lu C, Yu P, Cui Z. The Diabetes Risk and Determinants of Transition from Metabolically Healthy to Unhealthy Phenotypes in 49,702 Older Adults: 4-Year Cohort Study. Obesity (Silver Spring). 2020;28:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 332] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7515] [Cited by in RCA: 8298] [Article Influence: 414.9] [Reference Citation Analysis (0)] |
| 10. | Rydén M, Petrus P, Andersson DP, Medina-Gómez G, Escasany E, Corrales Cordón P, Dahlman I, Kulyté A, Arner P. Insulin action is severely impaired in adipocytes of apparently healthy overweight and obese subjects. J Intern Med. 2019;285:578-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Lin L, Peng K, Du R, Huang X, Lu J, Xu Y, Xu M, Chen Y, Bi Y, Wang W. Metabolically healthy obesity and incident chronic kidney disease: The role of systemic inflammation in a prospective study. Obesity (Silver Spring). 2017;25:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Hwang YC, Ahn HY, Park CY. Association Between Nonalcoholic Fatty Liver Disease and Future Deterioration of Metabolic Health: A Cohort Study. Obesity (Silver Spring). 2019;27:1360-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Chang Y, Jung HS, Cho J, Zhang Y, Yun KE, Lazo M, Pastor-Barriuso R, Ahn J, Kim CW, Rampal S, Cainzos-Achirica M, Zhao D, Chung EC, Shin H, Guallar E, Ryu S. Metabolically Healthy Obesity and the Development of Nonalcoholic Fatty Liver Disease. Am J Gastroenterol. 2016;111:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 14. | Navarro-González D, Sánchez-Íñigo L, Fernández-Montero A, Pastrana-Delgado J, Alfredo Martínez J. Are all metabolically healthy individuals with obesity at the same risk of diabetes onset? Obesity (Silver Spring). 2016;24:2615-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Ahl S, Guenther M, Zhao S, James R, Marks J, Szabo A, Kidambi S. Adiponectin Levels Differentiate Metabolically Healthy vs Unhealthy Among Obese and Nonobese White Individuals. J Clin Endocrinol Metab. 2015;100:4172-4180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Chang Y, Jung HS, Yun KE, Cho J, Ahn J, Chung EC, Shin H, Ryu S. Metabolically healthy obesity is associated with an increased risk of diabetes independently of nonalcoholic fatty liver disease. Obesity (Silver Spring). 2016;24:1996-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004;30:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med. 2008;168:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1129] [Cited by in RCA: 1178] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 19. | Lwow F, Jedrzejuk D, Milewicz A, Szmigiero L. Lipid accumulation product (LAP) as a criterion for the identification of the healthy obesity phenotype in postmenopausal women. Exp Gerontol. 2016;82:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129:3978-3989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 405] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 21. | Phillips CM. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 273] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 22. | Araújo J, Cai J, Stevens J. Prevalence of Optimal Metabolic Health in American Adults: National Health and Nutrition Examination Survey 2009-2016. Metab Syndr Relat Disord. 2019;17:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 23. | Goday A, Calvo E, Vázquez LA, Caveda E, Margallo T, Catalina-Romero C, Reviriego J. Prevalence and clinical characteristics of metabolically healthy obese individuals and other obese/non-obese metabolic phenotypes in a working population: results from the Icaria study. BMC Public Health. 2016;16:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Liu C, Wang C, Guan S, Liu H, Wu X, Zhang Z, Gu X, Zhang Y, Zhao Y, Tse LA, Fang X. The Prevalence of Metabolically Healthy and Unhealthy Obesity according to Different Criteria. Obes Facts. 2019;12:78-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Latifi SM, Karandish M, Shahbazian H, Taha JM, Cheraghian B, Moradi M. Prevalence of Metabolically Healthy Obesity (MHO) and its relation with incidence of metabolic syndrome, hypertension and type 2 Diabetes amongst individuals aged over 20 years in Ahvaz: A 5 Year cohort Study (2009-2014). Diabetes Metab Syndr. 2017;11 Suppl 2:S1037-S1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Gilardini L, Zambon A, Soranna D, Croci M, Invitti C. Predictors of the transition from metabolically healthy obesity to unhealthy obesity. Eat Weight Disord. 2018;23:739-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Mongraw-Chaffin M, Foster MC, Kalyani RR, Vaidya D, Burke GL, Woodward M, Anderson CA. Obesity Severity and Duration Are Associated With Incident Metabolic Syndrome: Evidence Against Metabolically Healthy Obesity From the Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2016;101:4117-4124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Hashimoto Y, Hamaguchi M, Tanaka M, Obora A, Kojima T, Fukui M. Metabolically healthy obesity without fatty liver and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obes Res Clin Pract. 2018;12:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Janghorbani M, Salamat MR, Amini M, Aminorroaya A. Risk of diabetes according to the metabolic health status and degree of obesity. Diabetes Metab Syndr. 2017;11 Suppl 1:S439-S444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Wei Y, Wang J, Han X, Yu C, Wang F, Yuan J, Miao X, Yao P, Wei S, Wang Y, Liang Y, Zhang X, Guo H, Zheng D, Tang Y, Yang H, He M. Metabolically healthy obesity increased diabetes incidence in a middle-aged and elderly Chinese population. Diabetes Metab Res Rev. 2020;36:e3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Fingeret M, Marques-Vidal P, Vollenweider P. Incidence of type 2 diabetes, hypertension, and dyslipidemia in metabolically healthy obese and non-obese. Nutr Metab Cardiovasc Dis. 2018;28:1036-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Kim JA, Kim DH, Kim SM, Park YG, Kim NH, Baik SH, Choi KM, Han K, Yoo HJ. Impact of the Dynamic Change of Metabolic Health Status on the Incident Type 2 Diabetes: A Nationwide Population-Based Cohort Study. Endocrinol Metab (Seoul). 2019;34:406-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Wang B, Zhang M, Wang S, Wang C, Wang J, Li L, Zhang L, Ren Y, Han C, Zhao Y, Zhou J, Wang G, Shen Y, Wu D, Pang C, Yin L, Feng T, Zhao J, Luo X, Hu D. Dynamic status of metabolically healthy overweight/obesity and metabolically unhealthy and normal weight and the risk of type 2 diabetes mellitus: A cohort study of a rural adult Chinese population. Obes Res Clin Pract. 2018;12:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Ryoo JH, Park SK, Ye S, Choi JM, Oh CM, Kim SY, Shin JY, Park JH, Hong HP, Ko TS. Estimation of risk for diabetes according to the metabolically healthy status stratified by degree of obesity in Korean men. Endocrine. 2015;50:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Espinosa De Ycaza AE, Donegan D, Jensen MD. Long-term metabolic risk for the metabolically healthy overweight/obese phenotype. Int J Obes (Lond). 2018;42:302-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, Boyko EJ. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int J Obes (Lond). 2015;39:1365-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 37. | Hashimoto Y, Hamaguchi M, Fukuda T, Ohbora A, Kojima T, Fukui M. Fatty liver as a risk factor for progression from metabolically healthy to metabolically abnormal in non-overweight individuals. Endocrine. 2017;57:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Xia MF, Chen Y, Lin HD, Ma H, Li XM, Aleteng Q, Li Q, Wang D, Hu Y, Pan BS, Li XJ, Li XY, Gao X. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6:38214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 39. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1238] [Cited by in RCA: 2041] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 40. | Jung CH, Kang YM, Jang JE, Hwang JY, Kim EH, Park JY, Kim HK, Lee WJ. Fatty liver index is a risk determinant of incident type 2 diabetes in a metabolically healthy population with obesity. Obesity (Silver Spring). 2016;24:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2018;41:372-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 430] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 42. | Sung KC, Wild SH, Kwag HJ, Byrne CD. Fatty liver, insulin resistance, and features of metabolic syndrome: relationships with coronary artery calcium in 10,153 people. Diabetes Care. 2012;35:2359-2364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 43. | Bian H, Yan H, Zeng M, Rao S, Yao X, Zhou J, Jia W, Gao X. Increased liver fat content and unfavorable glucose profiles in subjects without diabetes. Diabetes Technol Ther. 2011;13:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Kim Y, Chang Y, Cho YK, Ahn J, Shin H, Ryu S. Metabolically healthy versus unhealthy obesity and risk of fibrosis progression in non-alcoholic fatty liver disease. Liver Int. 2019;39:1884-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Ampuero J, Aller R, Gallego-Durán R, Crespo J, Calleja JL, García-Monzón C, Gómez-Camarero J, Caballería J, Lo Iacono O, Ibañez L, García-Samaniego J, Albillos A, Francés R, Fernández-Rodríguez C, Diago M, Soriano G, Andrade RJ, Latorre R, Jorquera F, Morillas RM, Escudero D, Estévez P, Guerra MH, Augustín S, Banales JM, Aspichueta P, Benlloch S, Rosales JM, Salmerón J, Turnes J, Romero Gómez M; HEPAmet Registry. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J Hepatol. 2020;73:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 46. | Xia MF, Bian H, Yan HM, Lin HD, Chang XX, Li XM, Ma H, He WY, Zhao NQ, Xia P, Gao X. Assessment of liver fat content using quantitative ultrasonography to evaluate risks for metabolic diseases. Obesity (Silver Spring). 2015;23:1929-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Nirantharakumar K. Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J Am Coll Cardiol. 2017;70:1429-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 378] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 48. | Yeh TL, Chen HH, Tsai SY, Lin CY, Liu SJ, Chien KL. The Relationship between Metabolically Healthy Obesity and the Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 49. | Gao M, Lv J, Yu C, Guo Y, Bian Z, Yang R, Du H, Yang L, Chen Y, Li Z, Zhang X, Chen J, Qi L, Chen Z, Huang T, Li L; China Kadoorie Biobank (CKB) Collaborative Group. Metabolically healthy obesity, transition to unhealthy metabolic status, and vascular disease in Chinese adults: A cohort study. PLoS Med. 2020;17:e1003351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 50. | Kouvari M, Panagiotakos DB, Yannakoulia M, Georgousopoulou E, Critselis E, Chrysohoou C, Tousoulis D, Pitsavos C; ATTICA Study Investigators. Transition from metabolically benign to metabolically unhealthy obesity and 10-year cardiovascular disease incidence: The ATTICA cohort study. Metabolism. 2019;93:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 51. | Mongraw-Chaffin M, Foster MC, Anderson CAM, Burke GL, Haq N, Kalyani RR, Ouyang P, Sibley CT, Tracy R, Woodward M, Vaidya D. Metabolically Healthy Obesity, Transition to Metabolic Syndrome, and Cardiovascular Risk. J Am Coll Cardiol. 2018;71:1857-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 287] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 52. | Guo X, Li Z, Zhou Y, Yu S, Yang H, Sun G, Zheng L, Afzal J, Liu Y, Sun Y. The effects of transitions in metabolic health and obesity status on incident cardiovascular disease: Insights from a general Chinese population. Eur J Prev Cardiol. 2020;2047487320935550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses' Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6:714-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 54. | Opio J, Croker E, Odongo GS, Attia J, Wynne K, McEvoy M. Metabolically healthy overweight/obesity are associated with increased risk of cardiovascular disease in adults, even in the absence of metabolic risk factors: A systematic review and meta-analysis of prospective cohort studies. Obes Rev. 2020;21:e13127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 55. | Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, Huerta JM, Agnoli C, Freisling H, Weiderpass E, Wennberg P, van der A DL, Arriola L, Benetou V, Boeing H, Bonnet F, Colorado-Yohar SM, Engström G, Eriksen AK, Ferrari P, Grioni S, Johansson M, Kaaks R, Katsoulis M, Katzke V, Key TJ, Matullo G, Melander O, Molina-Portillo E, Moreno-Iribas C, Norberg M, Overvad K, Panico S, Quirós JR, Saieva C, Skeie G, Steffen A, Stepien M, Tjønneland A, Trichopoulou A, Tumino R, van der Schouw YT, Verschuren WMM, Langenberg C, Di Angelantonio E, Riboli E, Wareham NJ, Danesh J, Butterworth AS. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. Eur Heart J. 2018;39:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 56. | Wang Y, Sun B, Sheng LT, Pan XF, Zhou Y, Zhu J, Li X, Yang K, Guo K, Zhang X, He M, Yang H, Wu T, Pan A. Association between weight status, metabolic syndrome, and chronic kidney disease among middle-aged and elderly Chinese. Nutr Metab Cardiovasc Dis. 2020;30:2017-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Chang Y, Ryu S, Choi Y, Zhang Y, Cho J, Kwon MJ, Hyun YY, Lee KB, Kim H, Jung HS, Yun KE, Ahn J, Rampal S, Zhao D, Suh BS, Chung EC, Shin H, Pastor-Barriuso R, Guallar E. Metabolically Healthy Obesity and Development of Chronic Kidney Disease: A Cohort Study. Ann Intern Med. 2016;164:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 58. | Nam KH, Yun HR, Joo YS, Kim J, Lee S, Lee C, Park KS, Park JT, Chang TI, Kang EW, Yoo TH, Kang SW, Han SH. Changes in obese metabolic phenotypes over time and risk of incident chronic kidney disease. Diabetes Obes Metab. 2018;20:2778-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Cho YK, Lee J, Kim HS, Park JY, Lee WJ, Kim YJ, Jung CH. Impact of Transition in Metabolic Health and Obesity on the Incident Chronic Kidney Disease: A Nationwide Cohort Study. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 60. | Chen HY, Lu FH, Chang CJ, Wang RS, Yang YC, Chang YF, Wu JS. Metabolic abnormalities, but not obesity per se, associated with chronic kidney disease in a Taiwanese population. Nutr Metab Cardiovasc Dis. 2020;30:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Vangipurapu J, Stančáková A, Kuulasmaa T, Kuusisto J, Laakso M. Both fasting and glucose-stimulated proinsulin levels predict hyperglycemia and incident type 2 diabetes: a population-based study of 9,396 Finnish men. PLoS One. 2015;10:e0124028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Hjelmgren O, Gummesson A, Bergström G, Schmidt C. Beta-Cell Function, Self-rated Health, and Lifestyle Habits in 64-Year-Old Swedish Women with Metabolically Healthy Obesity Phenotype. J Obes Metab Syndr. 2020;29:39-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Zhao RX, He Q, Sha S, Song J, Qin J, Liu P, Sun YJ, Sun L, Hou XG, Chen L. Increased AHR Transcripts Correlate With Pro-inflammatory T-Helper Lymphocytes Polarization in Both Metabolically Healthy Obesity and Type 2 Diabetic Patients. Front Immunol. 2020;11:1644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Hellmér J, Arner P, Lundin A. Automatic luminometric kinetic assay of glycerol for lipolysis studies. Anal Biochem. 1989;177:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Prospective Studies Collaboration. , Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3582] [Cited by in RCA: 3265] [Article Influence: 204.1] [Reference Citation Analysis (0)] |
| 66. | Shu CJ, Benoist C, Mathis D. The immune system's involvement in obesity-driven type 2 diabetes. Semin Immunol. 2012;24:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 67. | Ndisang JF, Rastogi S, Vannacci A. Immune and inflammatory processes in obesity, insulin resistance, diabetes, and related cardiometabolic complications. J Immunol Res. 2014;2014:579560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Zhao R, Tang D, Yi S, Li W, Wu C, Lu Y, Hou X, Song J, Lin P, Chen L, Sun L. Elevated peripheral frequencies of Th22 cells: a novel potent participant in obesity and type 2 diabetes. PLoS One. 2014;9:e85770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 69. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6517] [Cited by in RCA: 6528] [Article Influence: 296.7] [Reference Citation Analysis (0)] |
| 70. | Christou KA, Christou GA, Karamoutsios A, Vartholomatos G, Gartzonika K, Tsatsoulis A, Tigas S. Metabolically Healthy Obesity Is Characterized by a Proinflammatory Phenotype of Circulating Monocyte Subsets. Metab Syndr Relat Disord. 2019;17:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Patiño R, Ibarra J, Rodriguez A, Yagüe MR, Pintor E, Fernandez-Cruz A, Figueredo A. Circulating monocytes in patients with diabetes mellitus, arterial disease, and increased CD14 expression. Am J Cardiol. 2000;85:1288-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Terasawa T, Aso Y, Omori K, Fukushima M, Momobayashi A, Inukai T. Bezafibrate, a peroxisome proliferator-activated receptor α agonist, decreases circulating CD14(+)CD16(+) monocytes in patients with type 2 diabetes. Transl Res. 2015;165:336-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Dalmas E, Venteclef N, Caer C, Poitou C, Cremer I, Aron-Wisnewsky J, Lacroix-Desmazes S, Bayry J, Kaveri SV, Clément K, André S, Guerre-Millo M. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014;63:1966-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 74. | Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1073] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 75. | Jaganathan R, Ravindran R, Dhanasekaran S. Emerging Role of Adipocytokines in Type 2 Diabetes as Mediators of Insulin Resistance and Cardiovascular Disease. Can J Diabetes. 2018;42:446-456.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 76. | Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, Staiger H, Maerker E, Häring H, Stumvoll M. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003;52:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 426] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 77. | Fu J, Li Y, Esangbedo IC, Li G, Feng D, Li L, Xu L, Han L, Li M, Li C, Gao S, Willi SM. Circulating Osteonectin and Adipokine Profiles in Relation to Metabolically Healthy Obesity in Chinese Children: Findings From BCAMS. J Am Heart Assoc. 2018;7:e009169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Larsen MA, Isaksen VT, Moen OS, Wilsgaard L, Remijn M, Paulssen EJ, Florholmen J, Goll R. Leptin to adiponectin ratio - A surrogate biomarker for early detection of metabolic disturbances in obesity. Nutr Metab Cardiovasc Dis. 2018;28:1114-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Carvalho LP, Basso-Vanelli RP, Di Thommazo-Luporini L, Mendes RG, Oliveira-Junior MC, Vieira RP, Bonjorno-Junior JC, Oliveira CR, Luporini R, Borghi-Silva A. Myostatin and adipokines: The role of the metabolically unhealthy obese phenotype in muscle function and aerobic capacity in young adults. Cytokine. 2018;107:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Zhuo Q, Wang Z, Fu P, Piao J, Tian Y, Xu J, Yang X. Comparison of adiponectin, leptin and leptin to adiponectin ratio as diagnostic marker for metabolic syndrome in older adults of Chinese major cities. Diabetes Res Clin Pract. 2009;84:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 81. | Goday A, Benaiges D, Parri A, Ramón JM, Flores-Le Roux JA, Pedro Botet J; Obemar Group. Can bariatric surgery improve cardiovascular risk factors in the metabolically healthy but morbidly obese patient? Surg Obes Relat Dis. 2014;10:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Pelascini E, Disse E, Pasquer A, Poncet G, Gouillat C, Robert M. Should we wait for metabolic complications before operating on obese patients? Surg Obes Relat Dis. 2016;12:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 83. | Genua I, Tuneu L, Ramos A, Stantonyonge N, Caimari F, Balagué C, Fernández-Ananin S, Sánchez-Quesada JL, Pérez A, Miñambres I. Effectiveness of Bariatric Surgery in Patients with the Metabolically Healthy Obese Phenotype. Obes Surg. 2021;31:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Barzin M, Aryannezhad S, Khalaj A, Mahdavi M, Valizadeh M, Ghareh S, Azizi F, Hosseinpanah F. Effects of bariatric surgery in different obesity phenotypes: Tehran Obesity Treatment Study (TOTS). Obes Surg. 2020;30:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Slagter SN, Corpeleijn E, van der Klauw MM, Sijtsma A, Swart-Busscher LG, Perenboom CWM, de Vries JHM, Feskens EJM, Wolffenbuttel BHR, Kromhout D, van Vliet-Ostaptchouk JV. Dietary patterns and physical activity in the metabolically (un)healthy obese: the Dutch Lifelines cohort study. Nutr J. 2018;17:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 86. | de Winter M, Rioux BV, Boudreau JG, Bouchard DR, Sénéchal M. Physical Activity and Sedentary Patterns among Metabolically Healthy Individuals Living with Obesity. J Diabetes Res. 2018;2018:7496768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Gomez-Huelgas R, Ruiz-Nava J, Santamaria-Fernandez S, Vargas-Candela A, Alarcon-Martin AV, Tinahones FJ, Bernal-Lopez MR. Impact of Intensive Lifestyle Modification on Levels of Adipokines and Inflammatory Biomarkers in Metabolically Healthy Obese Women. Mediators Inflamm. 2019;2019:4165260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Lucht S, Hennig F, Moebus S, Führer-Sakel D, Herder C, Jöckel KH, Hoffmann B; Heinz Nixdorf Recall Study Investigative Group. Air pollution and diabetes-related biomarkers in non-diabetic adults: A pathway to impaired glucose metabolism? Environ Int. 2019;124:370-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Aguilar-Salinas CA, García EG, Robles L, Riaño D, Ruiz-Gomez DG, García-Ulloa AC, Melgarejo MA, Zamora M, Guillen-Pineda LE, Mehta R, Canizales-Quinteros S, Tusie Luna MT, Gómez-Pérez FJ. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93:4075-4079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 90. | van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, Gaye A, Gögele M, Heier M, Hiekkalinna T, Joensuu A, Newby C, Pang C, Partinen E, Reischl E, Schwienbacher C, Tammesoo ML, Swertz MA, Burton P, Ferretti V, Fortier I, Giepmans L, Harris JR, Hillege HL, Holmen J, Jula A, Kootstra-Ros JE, Kvaløy K, Holmen TL, Männistö S, Metspalu A, Midthjell K, Murtagh MJ, Peters A, Pramstaller PP, Saaristo T, Salomaa V, Stolk RP, Uusitupa M, van der Harst P, van der Klauw MM, Waldenberger M, Perola M, Wolffenbuttel BH. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 91. | Liu M, Tang R, Wang J, He Y. Distribution of metabolic/obese phenotypes and association with diabetes: 5 years' cohort based on 22,276 elderly. Endocrine. 2018;62:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |