Published online Dec 15, 2022. doi: 10.4239/wjd.v13.i12.1035
Peer-review started: August 28, 2022
First decision: September 26, 2022
Revised: October 27, 2022
Accepted: November 18, 2022
Article in press: November 18, 2022
Published online: December 15, 2022
Processing time: 109 Days and 9.4 Hours
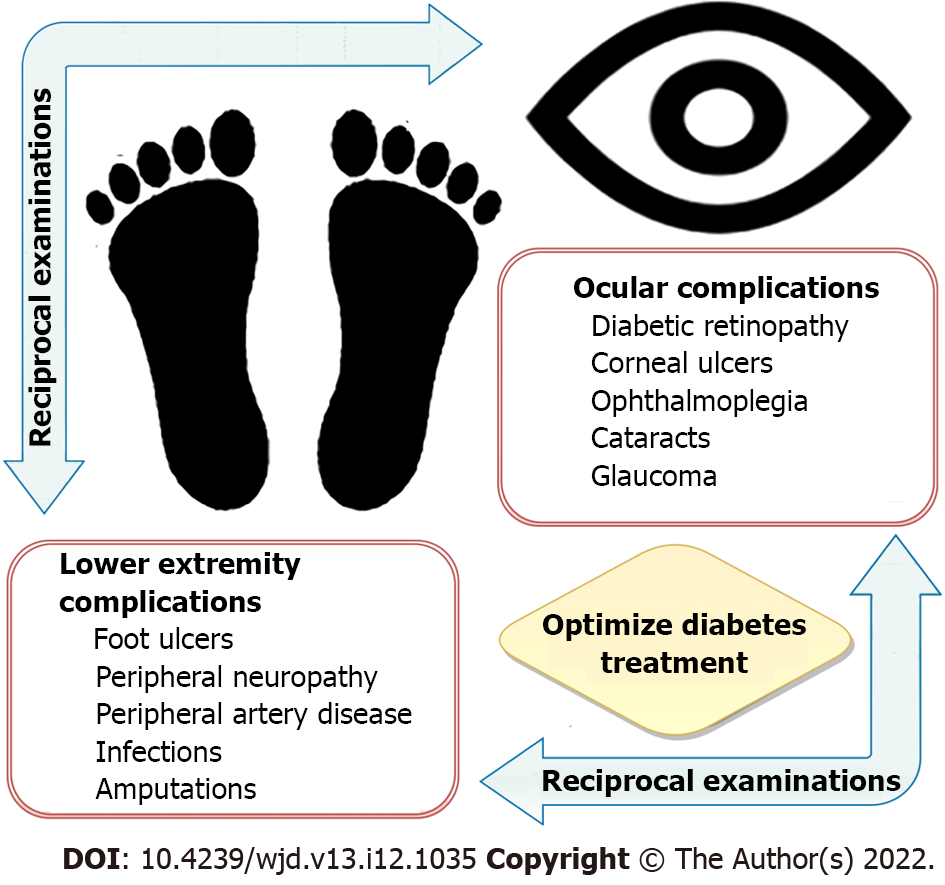
Diabetic eye disease is strongly associated with the development of diabetic foot ulcers (DFUs). DFUs are a common and significant complication of diabetes mellitus (DM) that arise from a combination of micro- and macrovascular compromise. Hyperglycemia and associated metabolic dysfunction in DM lead to impaired wound healing, immune dysregulation, peripheral vascular disease, and diabetic neuropathy that predisposes the lower extremities to repetitive injury and progressive tissue damage that may ultimately necessitate amputation. Diabetic retinopathy (DR) is caused by cumulative damage to the retinal mic-rovasculature from hyperglycemia and other diabetes-associated factors. The severity of DR is closely associated with the development of DFUs and the need for lower extremity revascularization procedures and/or amputation. Like the lower extremity, the eye may also suffer end-organ damage from macrovascular compromise in the form of cranial neuropathies that impair its motility, cause optic neuropathy, or result in partial or complete blindness. Additionally, poor perfusion of the eye can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy or neovascular glaucoma, both serious, vision-threatening conditions. Finally, diabetic corneal ulcers and DFUs share many aspects of impaired wound healing resulting from neurovascular, sensory, and immunologic compromise. Notably, alterations in serum biomarkers, such as hemoglobin A1c, ceruloplasmin, creatinine, low-density lipoprotein, and high-density lipoprotein, are associated with both DR and DFUs. Monitoring these parameters can aid in prognosticating long-term outcomes and shed light on shared pathogenic mechanisms that lead to end-organ damage. The frequent co-occurrence of diabetic eye and foot problems mandate that patients affected by either condition undergo reciprocal comprehensive eye and foot evaluations in addition to optimizing diabetes management.
Core Tip: This review explores the epidemiological and pathophysiological interconnections between diabetic foot and eye disease, especially the shared mechanisms that impact wound healing. Since diabetic foot and eye problems are often concurrent, it is imperative that patients affected by one or the other condition promptly undergo reciprocal examinations to reduce the risk of further complications. The best outcomes for patients with diabetic foot and eye disease are achieved by a team-based strategy that incorporates regular examinations, often performed by specialists, provides preventative health education, and delivers effective long-term management of the underlying diabetes and its associated metabolic consequences.
- Citation: Ramsey DJ, Kwan JT, Sharma A. Keeping an eye on the diabetic foot: The connection between diabetic eye disease and wound healing in the lower extremity. World J Diabetes 2022; 13(12): 1035-1048
- URL: https://www.wjgnet.com/1948-9358/full/v13/i12/1035.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i12.1035
An estimated 131 million people worldwide have lower extremity complications related to diabetes mellitus (DM), such as diabetic foot ulcers (DFUs), peripheral vascular disease (PVD), neuropathy, and amputations[1,2]. Similarly, an estimated 103 million people worldwide have diabetic eye disease, including nearly one million people aged 50 and older who are blind from diabetic retinopathy (DR)[3,4]. The frequent co-occurrence of diabetic eye and foot problems makes it imperative for patients affected by either condition to promptly undergo reciprocal examinations to reduce the risk of further complications (Figure 1). It is essential that individuals who have DM and the clinicians who care for them understand the likelihood of this association. With the worldwide prevalence of DM increasing because of changes in diet and lifestyle, aging of the population, and the ability of individuals to live longer with the disease, the need for well-informed clinicians has never been greater[3].
The connection between diabetic eye and foot problems is related, in part, to shared risk factors. In particular, the duration of DM and level of glycemic control as reflected by hemoglobin A1c (HbA1c) level strongly govern both the rate of onset and severity of diabetic foot disease[5,6] and DR[7]. Molecular biomarkers, particularly ceruloplasmin, have been demonstrated to be elevated in people with DM[8]. Other risk factors, such as age, male gender, race and ethnicity, smoking, insulin use, type of diabetes, and individual comorbid factors such as hypertension, elevated low-density lipoprotein (LDL), decreased high-density lipoprotein (HDL), coronary artery disease, cerebral vascular disease, PVD, neuropathy, and nephropathy, have been assessed, but not all studies agree on which of these risk factors affect the incidence or progression of these diabetic complications[5]. Some of this variation may possibly be ascribed to differences in DM care, improvements in treatment over time, and other less well-defined differences between individual populations studied. This paper reviews the shared pathogenic mechanisms underlying these conditions and the importance of comprehensive diabetes care to reduce morbidity and prevent disability.
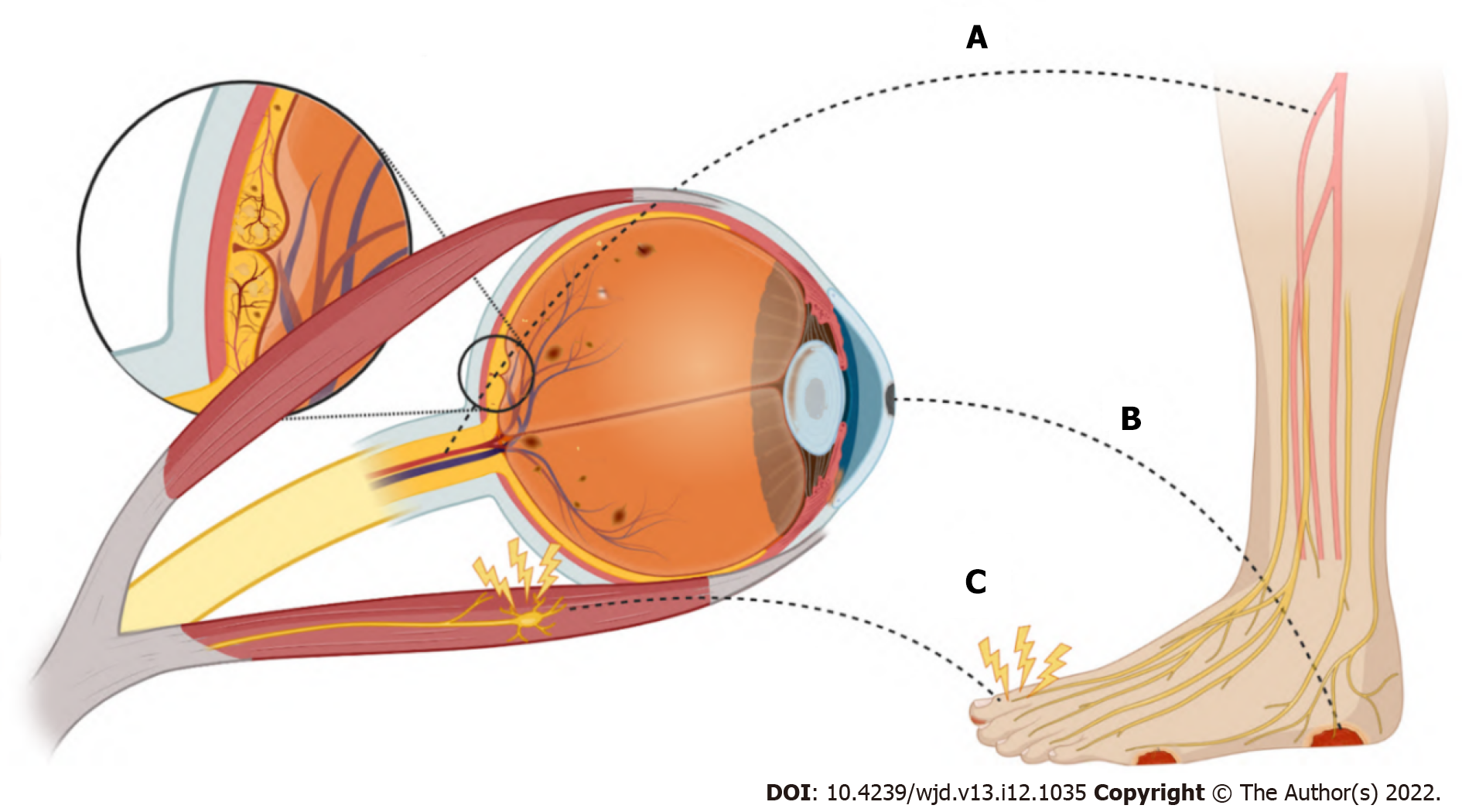
Individuals with DM are at a significantly increased risk of developing DFUs. DFUs are full-thickness wounds that penetrate the dermis (the deep vascular and collagenous inner layer of the skin) and are located below the ankle in patients with both type 1 and type 2 DM (Figure 2)[1,2]. DFUs arise from a combination of micro- and macrovascular compromise related to hyperglycemia and associated metabolic dysfunction that causes impaired growth and wound healing, immune dysregulation, and PVD[9]. In addition, the loss of protective sensation and proprioception caused by diabetic neuropathy and vision loss from diabetic eye disease predisposes patients to repetitive lower extremity trauma, with DFUs a common complication, especially among older adults[10,11]. Risk factors for DFUs include age, deformity or prior ulceration, repetitive trauma, sensory and autonomic neuropathy, peripheral arterial disease (PAD), and infection[12]. Up to one third of patients with DM will be affected by a DFU in their lifetime[13]. DFUs are associated with a 10- to 20-fold increased risk of amputation[1] and have a one-year mortality rate as high as 5%[14]. As many as 20% of DFUs remain unhealed after one year of treatment, with unhealed ulcers posing a risk for infections, gangrene, amputation, and even death[15,16].
There is a strong association between the development of DFUs and DR (Figure 2)[5]. Depending on the population studied, most individuals affected by DFUs also have DR[6,17-19], and those with DR are two to four times more likely to have DFUs or more serious forms of diabetic foot disease (Table 1)[20-22]. Even more concerning is the strong association between DFUs and proliferative diabetic retinopathy (PDR), with 31% to 55% of individuals having this more severe stage of DR (see below)[23]. Furthermore, patients with nonproliferative diabetic retinopathy (NPDR) who develop comorbid non-healing DFUs have a greater than 50% increased risk of progressing to PDR relative to those without this condition[24]. Finally, diabetic keratopathy is an important ocular parallel of DFUs. It is a disruption of normal corneal wound healing and loss of protective mechanisms of corneal sensation and aqueous tear production. These aberrations create an ideal environment for persistent corneal epithelial defects, microbial infection, and ulceration. Around half of patients with DM are affected by this condition[25]. The pathophysiology accounting for diabetic structural and functional alterations in the cornea is discussed in depth below.
| Ref. | Year | Type of study | Sample size (DFU; no DFU) | Source of population | Main findings |
| Jayaprakash et al[17] | 2009 | Prospective Case Study | 94 | India | 73.4% prevalence of DR in patients with DFUs |
| Hwang et al[22] | 2017 | Retrospective Cohort | 100 | South Korea | 90% prevalence of DR in patients with type 2 DM and DFUs; 55% had PDR |
| Karam et al[18] | 2018 | Cross-sectional | 182 | India | 67.6% prevalence of DR in patients diabetic foot disease (including neuropathy, deformation, DFUs, or amputation) |
| Zafar et al[19] | 2019 | Cross-sectional | 530 (225; 305) | Pakistan | 96% of patients with DFUs had DR |
| Sellman et al[23] | 2020 | Case Control | 270 (90; 180) | Sweden | 31% prevalence of PDR in patients with DFUs |
| Banik et al[21] | 2020 | Cross-sectional | 680 (8; 672) | Bangladesh | 65.9% prevalence of DR in patients with DFUs |
| Ye et al[20] | 2014 | Retrospective Cohort | 829 (61; 768) | China | OR 2.026 for DFUs in patients with DR |
| Al-Rubeaan et al[6] | 2015 | Retrospective Cohort | 62681 (2071; 60610) | Saudi Arabia | OR 4.45 for diabetic foot disease (including DFUs, gangrene, and amputation) in patients with DR |
| Harris Nwanyanwu et al[24] | 2013 | Retrospective Cohort | 4617 | United States | 1.54 HR for those with comorbid non-healing DFUs to progress from NPDR to PDR in three to five years |
Microvascular dysfunction in the lower extremity contributes to impaired function and impedes wound healing, which promotes the development of DFUs. Damage to endothelial cells from chronic hyperglycemia, oxidative stress-induced injury, generation of advanced glycation end-products, increased polyol flux regulated by aldose reductase, activation of protein kinase C (PKC), and other pro-inflammatory processes from immune dysregulation cumulatively disrupt normal blood flow and affect vascular permeability[26]. In its most severe form, this compromise of the microvasculature leads to ischemia and a relative hypoxic state in the involved tissue. As a result, there is increased expression of hypoxia-inducible factor-1 (HIF-1) leading to the production of vascular endothelial growth factor (VEGF), a protein principally responsible for restorative angiogenesis[27]. However, in DM there are disturbances in cytokine growth factor expression and locally decreased concentrations of VEGF, which render the lower extremity vulnerable to poor wound healing[9]. Failure of the microvasculature also contributes to peripheral neuropathy and local immune dysfunction, including impaired cellular response, cytokine expression, and vascular tone[28].
In the eye, these same pathways driven by hyperglycemia and other diabetes-associated factors lead to progressive damage to the retinal microvasculature and cause the development of DR[29]. DR develops in roughly one quarter of patients with DM, with the prevalence being highest in Africa (36%) and lowest in South and Central America (13%)[3,30]. Initially, the disease manifests as clinically detectable changes in the retinal vasculature, including the development of microaneurysms and loss of capillaries which are the hallmarks of early NPDR[31]. As the disease progresses, the production of VEGF and other diabetes-associated factors promotes further dysfunction, vascular leakage, and bleeding (dot-blot hemorrhages)[32]. At this stage, visual acuity is increasingly likely to be affected and is often further limited by swelling in the center of the retina, known as diabetic macular edema (DME)[33]. The development of neovascularization on the optic nerve or at locations in the peripheral retina signifies the progression to PDR. This is the most vision-threatening complications of the disease also primarily driven by the abnormal expression of VEGF[29,30,34]. These fragile new vessels, which grow into the vitreous cavity and along the inner retinal surface, often bleed, causing vitreous hemorrhages, traction, or retinal detachment and thereby impair vision[3,32]. Finally, excessive expression of VEGF may also affect the anterior segment of the eye by causing neovascularization on the iris and ciliary body. When the growth of this fibrovascular tissue extends to the anterior chamber angle it may block outflow of aqueous humor through the trabecular meshwork causing eye pressure to rise to levels capable of damaging the optic nerve in a disease process known as neovascular glaucoma[4,32]. When left unaddressed, irreversible blindness results.
Clinical examination supplemented by diagnostic color fundus photography and fluorescein angiography are the mainstays for staging DR; however, emerging modalities including ultrawide-angle imaging and optical coherence tomography and angiography increasingly allow clinicians to directly and noninvasively visualize the diseased retina and its microvasculature[31]. Advances in therapeutic modalities, such as intraocular injection of agents that target VEGF, steroids that target inflammation, and panretinal laser photocoagulation, have improved clinical outcomes for patients with DR[33-36]. However, effective long-term management is largely dependent upon regular follow-up care. Patients who fail to return for care are more likely to suffer vision loss[37,38].
The intraocular administration of agents that target VEGF are now the most common treatments for DR and DME[29,36]. Thankfully these agents are very unlikely to negatively impact wound healing in the lower extremity, especially at the doses employed to treat eye disease[39]. Similarly, intraocular corticosteroids, such as dexamethasone, and intravitreal steroid implants utilized to treat DME have been found to have no detectable influence on HbA1c or renal function[40,41]. However, when larger doses are administered as subconjunctival or peribulbar injections, some patients can experience elevations of blood glucose, similar to that observed with oral and intravenous administration of corticosteroids[42]. Finally, topical steroid drops have only very rarely been associated with endocrinological side effects in case reports[43].
Wound healing in the lower extremity requires coordinated cellular responses that cause an organized release of growth factors and cytokines. Under normal conditions, when an injury occurs, multiple cell types, including macrophages, fibroblasts, and epithelial cells, release VEGF and other cytokines in response to local ischemia caused by the wound[44]. However, in patients with DM, disturbances in cytokine and growth factor expression, including fibroblast growth factor, insulin-like growth factor, platelet derived growth factor, and VEGF, among others, lead to a condition that subsequently permits prolonged hypoxia[9,45]. Additionally, keratinocytes and fibroblasts in DFUs have demonstrated attenuated cellular migration, proliferation, and protein synthesis, resulting in impaired re-epithelialization which further exacerbates the oxygen-restricted wound[46]. Moreover, hyperglycemic states reduce the stability and function of HIF-1, which further impairs the wound healing response as a downstream consequence of sustained hypoxia[9]. Increased free radical damage is also a known causative factor in impaired wound healing in patients with DM. Inappropriately elevated concentrations of reactive oxygen species (ROS) and impaired antioxidant enzyme activity can cause nerve damage and directly contribute to the progression of peripheral neuropathy[47].
Individuals with DM also have abnormal wound healing pathways in the eye. Notably, corneal thinning is thought to be the earliest detectable pathological manifestation of DM in the eye[48]. Diabetic keratopathy leads to persistent corneal epithelial defects and neurotrophic corneal ulcers that respond poorly to treatments applied in the hyperglycemic environment[49]. Abnormalities in corneal cell morphology, varied number and disorganization of epithelial cell layers, impaired cellular migration, reduced endothelial cell number, and accumulation of acellular debris all contribute to poor wound healing[50]. Sectorial thinning, bullae, and persistent corneal epithelial defects from diabetic keratopathy often lead to corneal ulcers, scarring, and reactive neovascularization, which cause decreased visual acuity or permanent vision loss[50,51]. Although the cornea itself is avascular and ischemia does not play a significant role in diabetic keratopathy, wound healing in the cornea, like in the lower extremity, requires highly structured cellular processes which are impacted by hyp-erglycemia. These involve proliferation and migration of epithelial cells, fibroblasts, and the expression of numerous growth factors, including transforming growth factor beta, epidermal growth factor, insulin-like growth factor, and platelet derived growth factor[51]. Finally, diabetes-associated hyperglycemia may also impair vision by accelerating the progression of diabetic cataract and impact the health of the lens epithelium[52].
Most treatments for diabetic foot and eye problems are applied locally, but some treatments to aid the lower extremity may have theoretical consequences on the eye, and vice versa. Several adjuvant therapies have been found to reduce DFU healing times and amputation rates, including non-surgical debridement agents, topical dressings and agents, negative pressure wound therapy, oxygen therapies, acellular bioproducts, and human growth factors[53]. Oxygen is required for almost every step of the wound healing, affecting cell proliferation, collagen synthesis, and re-epithelialization, as well as immunologic defense against bacteria and other pathogens[54]. Oxygen may be delivered in the form of local, hyperbaric, or supplemental inspired oxygen therapy. Hyperbaric oxygen therapy has proven to be particularly useful in managing chronic, non-healing DFUs, especially in the relatively ischemic diabetic foot, albeit at a high financial cost[55]. Patients have been observed to have increased tissue concentrations of VEGF after completing hyperbaric therapy sessions; this has been attributed to the sharp decline of relative oxygen concentration once a session is completed[56]. As previously mentioned, the presence of VEGF is the primary driver of DR, so there is a theoretical risk that systemic or local oxygen therapy could exacerbate this condition. However, empiric evidence does not suggest that oxygen therapy is harmful to the diabetic eye[23]. It has even been reported that patients with concurrent DR have benefitted from the administration of hyperbaric oxygen therapy through supranormal levels of oxygen delivered to the retina[57]. Nevertheless, it remains essential that the status of DR is assessed and regularly monitored in any patient undergoing oxygen therapy for DFUs.
Many growth factors that have been identified as integral to wound healing are also potential therapeutic targets. In the diabetic foot, among the most promising are hydrogels which contain recombinant PDGF, approved by the United States Food and Drug Administration for topical administration having demonstrated improved rates of DFU healing in randomized clinical trials[58]. In the diabetic cornea, recombinant human nerve growth factor (NGF), epidermal growth factor, and metalloprotease inhibitors have demonstrated some success in trials for the treatment of diabetic keratopathy[59]. Of note, the opioid antagonist naltrexone has been demonstrated to improve wound healing, corneal surface sensitivity, and tear secretion in diabetic animal models[60,61]. The future will also likely include gene- and cell-based therapies to accelerate wound healing, including in DFUs and diabetic cornea[62,63].
Approximately half of adults with DM will be affected by peripheral neuropathy in their lifetime[64]. Peripheral neuropathy typically begins with diminution or loss of protective sensation. In addition, loss of proprioception contributes to injuries and falls[11]. Moreover, autonomic dysregulation in the foot may contribute to impaired cutaneous blood flow, sweating dysfunction, and loss of vascular tone that compromise integument integrity and wound healing[65]. Lower extremity deformities may also occur, such as hammer toes or claw toes, which are associated with loss of function[11]. Finally, delays in the identification of accidental and iatrogenic injuries because of reduced sensation may cause patients to fail to seek care in a timely fashion and increase the risk for infections[64].
As mentioned above, chronic hyperglycemia from DM causes microangiopathic changes. In the case of diabetic neuropathy, hyperglycemia may affect the endoneurial microvasculature by directly reducing perfusion and impairing nerve function[66]. Many of the same cellular and biochemical mechanisms linked to chronic hyperglycemia injure the peripheral nerves, including increased glycolytic processes producing oxidative stress, generation of advanced glycation end-products, increased polyol flux regulated by aldose reductase, PKC activation, and other pro-inflammatory processes from immune dysregulation[67]. Damage to mitochondria also plays an important role in the pathogenesis of diabetic neuropathy and contributes to nerve dysfunction, cell death, and loss of neurotrophic support provided by neurotrophin-3 and NGF[68]. Patients who develop PAD also have more severe diabetic neuropathy (see below)[64].
The cornea is the most densely innervated tissue of the human body and is 100 times more sensitive than skin[69], but this declines with age[70] and is further reduced by DM[71]. The loss of protective sensation of the diabetic cornea impacts various homeostatic functions, such as blinking, aqueous tear production, and the release of growth factors[51,52]. As a result, the incidence of dry eye disease and the need for artificial tears is increased among patients with DM, particularly among those with worse diabetes-related outcomes[72]. A recent meta-analysis estimated that DM conferred 30% increased odds for dry eye syndrome[58]. Dry eye disease and DR are also associated with each other[73]. These changes result in neurotrophic keratopathy marked by persistent epithelial defects and chronic erosions that may develop into corneal ulcers, corneal scarring, and neovascularization, all of which contribute to visual dysfunction[74] and predispose patients to infectious keratitis[75]. The recent application of in vivo confocal microscopy has allowed for visualization of diabetes-associated structural changes in the nerves of the corneal epithelium, including nerve thickening[76] and decreased nerve length and density[77]. Anterior segment optical coherence tomography is another emerging diagnostic modality used to evaluate and manage diabetic keratopathy by enabling the direct visualization of the cornea structure and nerves[50,71].
Diabetes-associated hyperglycemia has also been shown to cause direct injury to the neuronal retina, leading to thinning of the nerve fiber layer from the loss of ganglion cells and the death of other retinal neurons, including photoreceptors[78]. This may lead to decreased visual function, impaired contrast sensitivity, and diminished night vision[29,32]. Finally, the eye may also be suddenly and directly affected by diabetic cranial neuropathies, manifesting as double vision from ophthalmoplegia, which is the paralysis of the muscles that move the eye (see below).
Current recommendations for the management of painful diabetic neuropathy include gaba-pentinoids, serotonin and norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs)[79]. Gabapentin is a well-tolerated medication from an ophthalmic standpoint; its most common adverse effect is a reversible nystagmus[80]. SNRIs, such as venlafaxine, have been associated with acute angle closure glaucoma in some case reports[81], along with increased cataract development[82]. Finally, TCAs are associated with blurred vision in up to one third of patients, likely due to the anticholinergic action of these drugs[83]. These side effects further emphasize the importance of communication and collaboration with ophthalmologists when treating diabetes-associated complications of the lower extremity.
Common macrovascular complications of DM that affect the lower extremity include PAD and chronic venous insufficiency (CVI), which may lead to lower extremity amputation (Figure 1)[84]. DM induces and accelerates the development of atherosclerosis via multiple mechanisms that include metabolic derangements, smooth muscle dysfunction, oxidative stress, potentiated platelet function, increased coagulability, and chronic inflammation[85]. The availability of the potent vasodilator nitrous oxide, which is produced in the endothelium and is a primary mediator in local vascular endothelial tone, is reduced in hyperglycemic states; DM also promotes the production of endothelin-1, which indirectly increases vasoconstriction and vascular smooth muscle hypertrophy[86]. The result may be an overt occlusion, sometimes acutely when a thrombus forms, or when increasingly stenotic vessels result in reduced perfusion[86]. Patients with co-existing severe PAD are also more likely to have CVI[84], which contributes to poor wound healing by increasing hydrostatic pressure in the lower extremity, thereby promoting wound exudation[87].
While not directly a macrovascular complication, it is important to recognize that DR is strongly associated with lower-extremity PAD. Patients with DR have an approximately two-fold increase in the need for lower-limb revascularization and a five-fold increase in lower-limb amputation[88]. Patients with PAD benefit from additional medical management and risk factor modification for atherosclerotic disease. In addition to optimizing diabetes control, this includes counseling about smoking cessation, antiplatelet and statin therapies, as well as blood pressure control[89]. Exercise also plays a fundamental role in the treatment of PAD, leading to reductions in pain and improvement in functional capacity[89]. The clinical benefit of newer medications on amputation prevention remains uncertain.
In the eye, DM-associated macrovascular disease can manifest as an ocular ischemic syndrome (OIS), a rare, but vision-threatening condition associated with severe carotid artery occlusive disease that leads to ocular hypoperfusion[90]. Like PAD, atherosclerosis affecting the vessels supplying the eye is the main cause of the disease, and most patients with OIS have a diagnosis of DM[91]. Patients typically report dull eye or periorbital pain associated with gradual vision loss as the retina experiences progressive ischemia. Consequently, VEGF levels rise, which may cause neovascular glaucoma in the anterior segment and reduce the final visual potential; neovascularization can also develop in the retina, but it is less prominent than in DR because of reduced retinal perfusion[92]. OIS entails an overall poor visual prognosis, which means that the ophthalmologist’s diagnosis is crucial for the systemic health of those patients because OIS may be the presenting sign of impending serious cerebrovascular and ischemic heart disease. Finally, DM can sometimes cause an ischemic optic neuropathy, which is a direct infarct of the optic nerve[52].
Another condition involving a main function of the eye where macrovascular disease in DM manifests is ophthalmoplegia. Ophthalmoplegia is the paralysis of one or more of the extraocular muscles (EOM). It can arise from traumatic, autoimmune, infectious, and vascular etiologies. Usually involving the third (oculomotor), fourth (trochlear), or sixth (abducens) cranial nerves, double vision is the characteristic symptom of ophthalmoplegia[93]. The vascular supply for the EOMs comes from branches of the ophthalmic artery, which is itself a branch of the internal carotid artery. Additionally, the cranial nerves responsible for the EOMs themselves have a complex vascular supply. Focal cranial nerve ischemia due to atherosclerosis within the microvasculature is thought to contribute to the development of ophthalmoplegia in patients with DM[94].
Preventing diabetic foot and eye problems is best achieved through regular examinations, diabetes education, and optimal management of underlying DM and its associated metabolic consequences. Tight control of blood glucose, as reflected by HbA1c level, is the most important element for prevention of these two interrelated diabetes-associated complications, closely followed by optimization of blood pressure and lipid levels[1,7,37,38]. This is accomplished through a combination of regulation of diet, lifestyle modification, body mass reduction, and medications, such as insulin and/or oral antidiabetic therapies, as appropriate. Monitoring alterations in serum biomarkers, such as HbA1c, ceruloplasmin, creatinine, uric acid, LDL, and HDL, is also important because these biomarkers are associated with both the onset and severity of DR and DFUs[8,20].
The standard practices in DFU management include cleansing, surgical debridement, application of clean dressings to maintain a moist environment and control exudates, wound off-loading, vascular optimization (including revascularization procedures), treatment and prevention of infection, and glycemic control[88,95]. Proper instruction is also required to prevent accidental or iatrogenic injuries which can result from ordinary hygiene and grooming of the feet and lower extremity[29]. Infection prevention is best achieved through protective footwear, proper hygiene, and offloading interventions[11]. Similarly, preventing complications from diabetic keratopathy focuses on limiting repetitive trauma, neurosensory deformities, exposure, and infections. Injuries may be caused by eye droppers, abnormal eye lashes, cosmetic applicators, fingers, facial towels, and bedding[96]. Infection can occur from overgrowth of the ocular flora or opportunistic infection enhanced by hyperglycemia, or it can take the form of chronic and recurrent herpes simplex and zoster[97].
Many parallels exist between management of ulcers in the cornea and those in the lower extremity. Both are treated with clean dressings, antimicrobial ointments, and salves. Wound infections may be polymicrobial, but the bacterial species most associated with DFUs include gram-positive species, e.g., Staphylococcus aureus and Streptococcus species, but gram-negative infections with Pseudomonas aeruginosa and Enterobacteriaceae species also occur and are notably more common in ischemic or deep wounds[98]. Infection of corneal ulcers involve many of these same organisms, including Staphylococcus, Pseudomonas aeruginosa, and Streptococcus pneumoniae[70,99]. Special dressing and vacuum-assisted wound closure have been used with good result in the management of DFUs[100]. Non-healing diabetic corneal ulcers are often treated in conjunction with bandage contact lenses, which can lengthen the time antibiotic treatments are in contact with the ocular surface and serve as a reservoir for pharmacologically active compounds to aid wound healing[69]. In contrast to DFU management, patching should generally be avoided in patients with DM and corneal disease because of an increased risk of infection[101]. Amniotic membrane grafts have been studied for their potential of facilitating epithelial migration and healing of corneal ulcers and in very severe cases, corneal transplantation may be necessary[102].
Emerging research has placed an emphasis on developing therapeutic options that offer additional ways of preventing diabetic complications, treating them at earlier stages, or in more effective ways. As discussed earlier, inflammation has been implicated in the pathogenesis of diabetes-associated complications. Cytokines, such as interferon-γ, are being investigated as potential therapeutic targets in attenuating inflammatory cascades given that many of these cytokines contribute to altered vascular permeability and angiogenesis[103]. Given the high metabolic rate of the retina in conjunction with the metabolic stress induced by chronic hyperglycemia, reducing free radical stress may be an effective strategy[104]. Polyphenols, such as epigallocatechin-3-gallate found in green tea, are known for their antioxidant and anti-inflammatory properties and in diabetic animal models, have been shown to attenuate ROS concentrations in the retina[105]. Other polyphenol compounds, carotenoids, thiols, and vitamin supplementation are being investigated to address the several pathways involved in ROS generation and inflammation[104].
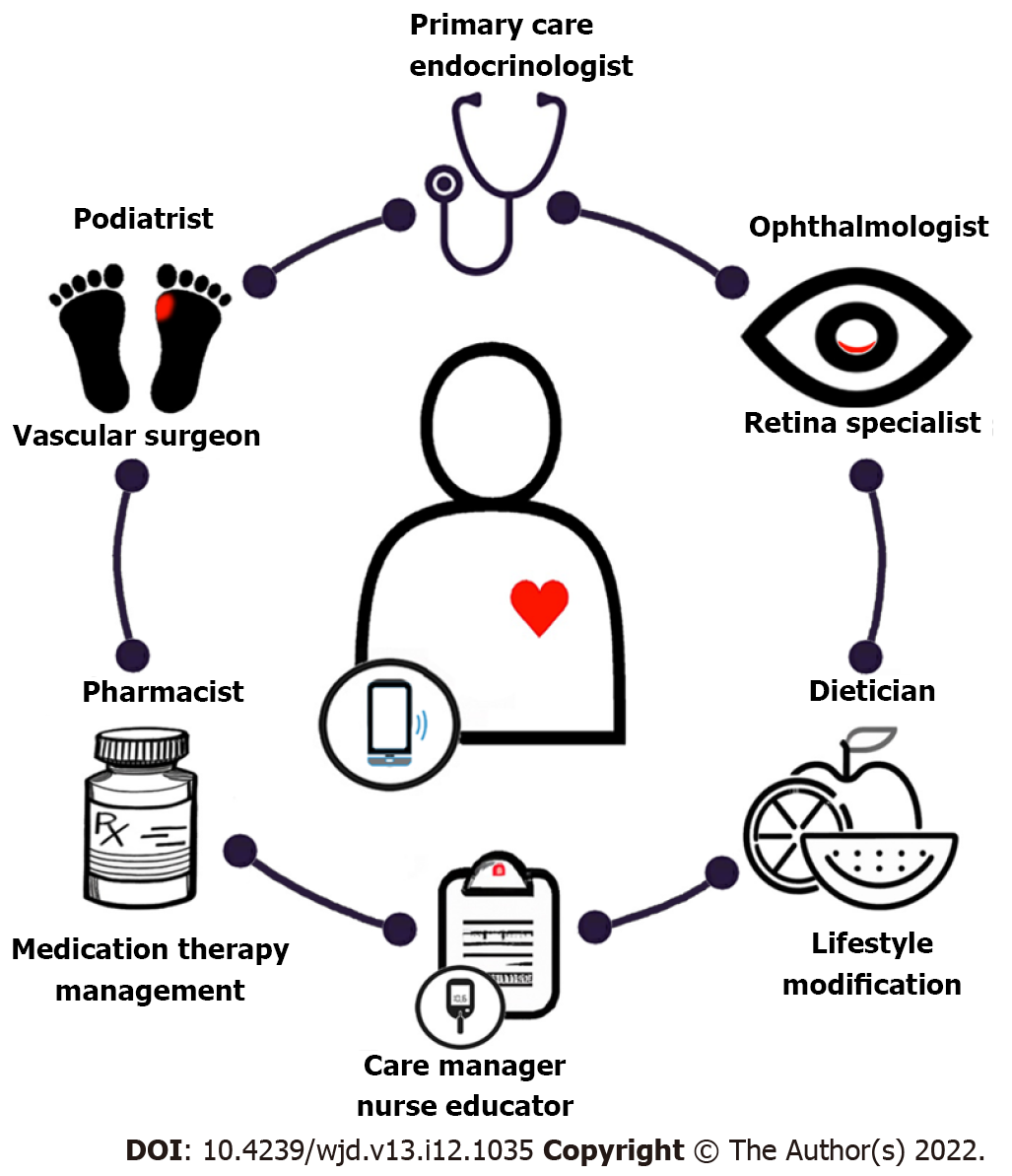
Finally, a multidisciplinary care team is essential to care optimally for the diabetic foot and eye, preserving function and quality of life for those with DM (Figure 3). Primary care providers and endocrinologists play a crucial role in coordinating care, including providing a formal assessment of the degree of diabetic control, screening for symptoms related to diabetic complications affecting other organ systems such as diabetic nephropathy, prescribing DM treatment, and involving specialists who manage diabetic complications such as foot or eye problems[1]. A diabetes care team should also include pharmacists who provide medication therapy management, dieticians, psychologists, diabetes care managers, and nurse educators. By working together, a coordinated care team can effectively reduce the healthcare burden associated with DM and its complications through prevention, screening, and management. In the future, the integration of smartphone technology and telehealth may not only streamline care coordination, but also allow for remote diagnosis and long-term monitoring of disease[106].
The identification of any ophthalmic or lower extremity complication in a patient with type 1 or type 2 DM should immediately prompt a review of DM management and coordination of diabetes care, including referral for reciprocal comprehensive foot or eye evaluations in patients with either complication[1,37,38]. Although diabetic foot disease is slightly more common among patients with type 1 DM and those who use insulin[6], optimizing diabetes management remains the most important step in preventing diabetes-associated complications no matter what the type of DM[37,38]. While many patients may report symptoms related to diabetic foot disease or observe vision loss in the setting of diabetic eye problems, many others may be asymptomatic or have such mild signs and symptoms that they are easily overlooked, dismissed, or fail to receive clinical attention unless specifically assessed[64,107]. Primary care providers and endocrinologists should perform regular diabetic foot examinations because they provide insight into the presence and degree of PVD, neuropathy, skin breakdown, and other pre-ulcerative changes. Providers must also screen for signs and symptoms of eye disease, in part because their identification may help triage the urgency of any necessary referrals[37,38]. Because diabetic eye and foot diseases so commonly occur in conjunction, it is essential that clinicians take the necessary steps to reduce the impact of these diseases through regular screening, prompt referral to specialists, and providing a coordinated, team-based approach to management.
The authors thank Dr. Andrew Popelka Jr., Dr. Shiyoung Roh, Dr. Sarkis Soukiasian, Dr. Adam Romeiser III, Dr. Angela Jellison, Rebecca Rick Longo, as well as Carol Spencer, Lahey Hospital Librarian, for research support. D.J. Ramsey is supported by the Harry N. Lee Family Chair in Innovation at the Lahey Hospital and Medical Center, Beth Israel Lahey Health. Study was performed as part of regular employment duties at the Lahey Hospital and Medical Center, Beth Israel Lahey Health. No additional funding was provided.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morya AK, India; Wu QN, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | International Diabetes Federation. Clinical practice recommendations on the diabetic foot, 2017. Brussels, Belgium, 2022. [cited 24 August 2022]. Available from: https://www.idf.org/e-library/guidelines/119-idf-clinical-practice-recommendations-on-diabetic-foot-2017.html. |
| 2. | Zhang Y, Cramb S, McPhail SM, Pacella R, van Netten JJ, Cheng Q, Derhy PH, Kinnear EM, Lazzarini PA; Diabetic Foot Working Group, Queensland Statewide Diabetes Clinical Network, Australia. Factors Associated With Healing of Diabetes-Related Foot Ulcers: Observations From a Large Prospective Real-World Cohort. Diabetes Care. 2021;44:e143-e145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, Wong IY, Ting DSW, Tan GSW, Jonas JB, Sabanayagam C, Wong TY, Cheng CY. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 1025] [Article Influence: 256.3] [Reference Citation Analysis (1)] |
| 4. | GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144-e160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1680] [Cited by in RCA: 1517] [Article Influence: 379.3] [Reference Citation Analysis (0)] |
| 5. | Huang ZH, Li SQ, Kou Y, Huang L, Yu T, Hu A. Risk factors for the recurrence of diabetic foot ulcers among diabetic patients: a meta-analysis. Int Wound J. 2019;16:1373-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Al-Rubeaan K, Al Derwish M, Ouizi S, Youssef AM, Subhani SN, Ibrahim HM, Alamri BN. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 7. | Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 382] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 8. | Memişoğullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipid peroxidation in the serum of patients with Type 2 diabetes mellitus. J Diabetes Complications. 2004;18:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev Endocr Metab Disord. 2019;20:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 10. | Gohdes DM, Balamurugan A, Larsen BA, Maylahn C. Age-related eye diseases: an emerging challenge for public health professionals. Prev Chronic Dis. 2005;2:A17. [PubMed] |
| 11. | van Deursen R. Footwear for the neuropathic patient: offloading and stability. Diabetes Metab Res Rev. 2008;24 Suppl 1:S96-S100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017;110:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 13. | Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2336] [Article Influence: 292.0] [Reference Citation Analysis (2)] |
| 14. | Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 332] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 15. | Senneville É, Lipsky BA, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M, van Asten SA, Urbančič-Rovan V, Peters EJG. Diagnosis of infection in the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 501] [Article Influence: 100.2] [Reference Citation Analysis (2)] |
| 17. | Jayaprakash P, Bhansali S, Bhansali A, Dutta P, Anantharaman R. Magnitude of foot problems in diabetes in the developing world: a study of 1044 patients. Diabet Med. 2009;26:939-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Karam T, Kamath YS, Rao LG, Rao KA, Shenoy SB, Bhandary SV. Diabetic retinopathy in patients with diabetic foot syndrome in South India. Indian J Ophthalmol. 2018;66:547-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Zafar S, Rahim K, Khan IU, Yasin M, Dawood M, Saleha S. Prevalence and association of diabetic retinopathy with diabetic foot ulcer: a cross-sectional observational study. In: Ziaei A, editor, Frontiers in Ophthalmology and Ocular Imaging. London, UK: IntechOpen Limited, 2019. [cited 24 August 2022]. Available from: https://www.intechopen.com/chapters/65026. |
| 20. | Ye X, Cao Y, Gao F, Yang Q, Zhang Q, Fu X, Li J, Xue Y. Elevated serum uric acid levels are independent risk factors for diabetic foot ulcer in female Chinese patients with type 2 diabetes. J Diabetes. 2014;6:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 21. | Banik PC, Barua L, Moniruzzaman M, Mondal R, Zaman F, Ali L. Risk of diabetic foot ulcer and its associated factors among Bangladeshi subjects: a multicentric cross-sectional study. BMJ Open. 2020;10:e034058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Hwang DJ, Lee KM, Park MS, Choi SH, Park JI, Cho JH, Park KH, Woo SJ. Association between diabetic foot ulcer and diabetic retinopathy. PLoS One. 2017;12:e0175270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Sellman A, Katzman P, Andreasson S, Lõndahl M. Long-term effects of hyperbaric oxygen therapy on visual acuity and retinopathy. Undersea Hyperb Med. 2020;47:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Harris Nwanyanwu K, Talwar N, Gardner TW, Wrobel JS, Herman WH, Stein JD. Predicting development of proliferative diabetic retinopathy. Diabetes Care. 2013;36:1562-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180-199. [PubMed] |
| 26. | Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6145] [Cited by in RCA: 6204] [Article Influence: 258.5] [Reference Citation Analysis (0)] |
| 27. | Crafts TD, Jensen AR, Blocher-Smith EC, Markel TA. Vascular endothelial growth factor: therapeutic possibilities and challenges for the treatment of ischemia. Cytokine. 2015;71:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Ahmed AS, Antonsen EL. Immune and vascular dysfunction in diabetic wound healing. J Wound Care. 2016;25:S35-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Ramsey DJ, Arden GB. Hypoxia and Dark Adaptation in Diabetic Retinopathy: Interactions, Consequences, and Therapy. Curr Diab Rep. 2015;15:118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 695] [Cited by in RCA: 915] [Article Influence: 183.0] [Reference Citation Analysis (1)] |
| 31. | Elnahry AG, Ramsey DJ. Automated Image Alignment for Comparing Microvascular Changes Detected by Fluorescein Angiography and Optical Coherence Tomography Angiography in Diabetic Retinopathy. Semin Ophthalmol. 2021;36:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Arden GB, Ramsey DJ. Diabetic retinopathy and a novel treatment based on the biophysics of rod photoreceptors and dark adaptation. 2015 Jul 14. In: Webvision: The Organization of the Retina and Visual System [Internet]. Salt Lake City (UT): University of Utah Health Sciences Center; 1995–. [PubMed] |
| 33. | Kim EJ, Lin WV, Rodriguez SM, Chen A, Loya A, Weng CY. Treatment of Diabetic Macular Edema. Curr Diab Rep. 2019;19:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 34. | Bressler SB, Beaulieu WT, Glassman AR, Gross JG, Jampol LM, Melia M, Peters MA, Rauser ME; Diabetic Retinopathy Clinical Research Network. Factors Associated with Worsening Proliferative Diabetic Retinopathy in Eyes Treated with Panretinal Photocoagulation or Ranibizumab. Ophthalmology. 2017;124:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Techniques for scatter and local photocoagulation treatment of diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report no. 3. The Early Treatment Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin. 1987;27:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1061] [Cited by in RCA: 1029] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 37. | Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:412-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 445] [Cited by in RCA: 600] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 38. | Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology. 2020;127:P66-P145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 411] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 39. | Ramsey DJ, Haddock LJ, Young LH, Eliott D. Complications of subspecialty ophthalmic care: systemic complications from the intravitreal administration of agents that target the vascular endothelial growth factor pathway. Semin Ophthalmol. 2014;29:263-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Valverde-Megías A, Cifuentes-Canorea P, Ruiz-Medrano J, Peña-García P, Megías-Fresno A, Donate-López J, García-Feijoo J. Systemic Effects of Repeated Intraocular Dexamethasone Intravitreal Implant in Diabetic Patients: A Retrospective Study. Diabetes Ther. 2017;8:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Ehlers JP, Yeh S, Maguire MG, Smith JR, Mruthyunjaya P, Jain N, Kim LA, Weng CY, Flaxel CJ, Schoenberger SD, Kim SJ. Intravitreal Pharmacotherapies for Diabetic Macular Edema: A Report by the American Academy of Ophthalmology. Ophthalmology. 2022;129:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 42. | Feldman-Billard S, Du Pasquier-Fediaevsky L, Héron E. Hyperglycemia after repeated periocular dexamethasone injections in patients with diabetes. Ophthalmology. 2006;113:1720-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Roters S, Aspacher F, Diestelhorst M. The influence of dexamethasone 0.1% eye drops on plasma cortisol and ACTH concentrations after cataract surgery. Ophthalmologica. 1996;210:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6:37-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 291] [Cited by in RCA: 326] [Article Influence: 32.6] [Reference Citation Analysis (11)] |
| 45. | Chen Z, Fu S, Wu Z, Chen J, Huang Y, Wang Y, Fu M. Relationship between plasma angiogenic growth factors and diabetic foot ulcers. Clin Chim Acta. 2018;482:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1170] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 47. | Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic Wound-Healing Science. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 338] [Article Influence: 84.5] [Reference Citation Analysis (1)] |
| 48. | Busted N, Olsen T, Schmitz O. Clinical observations on the corneal thickness and the corneal endothelium in diabetes mellitus. Br J Ophthalmol. 1981;65:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S. Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol. 1977;95:2193-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Ljubimov AV. Diabetic complications in the cornea. Vision Res. 2017;139:138-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 51. | Zhu L, Titone R, Robertson DM. The impact of hyperglycemia on the corneal epithelium: Molecular mechanisms and insight. Ocul Surf. 2019;17:644-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Skarbez K, Priestley Y, Hoepf M, Koevary SB. Comprehensive Review of the Effects of Diabetes on Ocular Health. Expert Rev Ophthalmol. 2010;5:557-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 53. | Kavitha KV, Tiwari S, Purandare VB, Khedkar S, Bhosale SS, Unnikrishnan AG. Choice of wound care in diabetic foot ulcer: A practical approach. World J Diabetes. 2014;5:546-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (3)] |
| 54. | de Smet GHJ, Kroese LF, Menon AG, Jeekel J, van Pelt AWJ, Kleinrensink GJ, Lange JF. Oxygen therapies and their effects on wound healing. Wound Repair Regen. 2017;25:591-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 55. | Lipsky BA, Berendt AR. Hyperbaric oxygen therapy for diabetic foot wounds: has hope hurdled hype? Diabetes Care. 2010;33:1143-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Sheikh AY, Gibson JJ, Rollins MD, Hopf HW, Hussain Z, Hunt TK. Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Arch Surg. 2000;135:1293-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 194] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Kaldırım H, Yazgan S, Ceylan B, Atalay K. The effect of hyperbaric oxygen therapy on retinal thickness and progression of retinopathy in patients with Type 2 diabetes: a prospective cohort study. Cutan Ocul Toxicol. 2019;38:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Yoo TK, Oh E. Diabetes mellitus is associated with dry eye syndrome: a meta-analysis. Int Ophthalmol. 2019;39:2611-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 59. | Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2237] [Cited by in RCA: 2541] [Article Influence: 149.5] [Reference Citation Analysis (2)] |
| 60. | Lou-Bonafonte JM, Bonafonte-Marquez E, Bonafonte-Royo S, Martínez-Carpio PA. Posology, efficacy, and safety of epidermal growth factor eye drops in 305 patients: logistic regression and group-wise odds of published data. J Ocul Pharmacol Ther. 2012;28:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. 2014;8:571-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 62. | Winkler MA, Dib C, Ljubimov AV, Saghizadeh M. Targeting miR-146a to treat delayed wound healing in human diabetic organ-cultured corneas. PLoS One. 2014;9:e114692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 63. | Eleftheriadou I, Samakidou G, Tentolouris A, Papanas N, Tentolouris N. Nonpharmacological Management of Diabetic Foot Ulcers: An Update. Int J Low Extrem Wounds. 2021;20:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Hicks CW, Selvin E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr Diab Rep. 2019;19:86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 419] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 65. | Sun PC, Chen CS, Kuo CD, Lin HD, Chan RC, Kao MJ, Wei SH. Impaired microvascular flow motion in subclinical diabetic feet with sudomotor dysfunction. Microvasc Res. 2012;83:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Malik RA, Veves A, Masson EA, Sharma AK, Ah-See AK, Schady W, Lye RH, Boulton AJ. Endoneurial capillary abnormalities in mild human diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1992;55:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: Where are we now and where to go? J Diabetes Investig. 2011;2:18-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 320] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 68. | Zherebitskaya E, Akude E, Smith DR, Fernyhough P. Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes. 2009;58:1356-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 69. | Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 70. | Millodot M. The influence of age on the sensitivity of the cornea. Invest Ophthalmol Vis Sci. 1977;16:240-242. [PubMed] |
| 71. | Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Retin Eye Res. 2019;73:100762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 72. | Kaiserman I, Kaiserman N, Nakar S, Vinker S. Dry eye in diabetic patients. Am J Ophthalmol. 2005;139:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 73. | Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008;8:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 74. | Priyadarsini S, Whelchel A, Nicholas S, Sharif R, Riaz K, Karamichos D. Diabetic keratopathy: Insights and challenges. Surv Ophthalmol. 2020;65:513-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 75. | Badawi AE, Moemen D, El-Tantawy NL. Epidemiological, clinical and laboratory findings of infectious keratitis at Mansoura Ophthalmic Center, Egypt. Int J Ophthalmol. 2017;10:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Zhao H, He Y, Ren YR, Chen BH. Corneal alteration and pathogenesis in diabetes mellitus. Int J Ophthalmol. 2019;12:1939-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 77. | Han SB, Yang HK, Hyon JY. Influence of diabetes mellitus on anterior segment of the eye. Clin Interv Aging. 2019;14:53-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 78. | Chhablani J, Sharma A, Goud A, Peguda HK, Rao HL, Begum VU, Barteselli G. Neurodegeneration in Type 2 Diabetes: Evidence From Spectral-Domain Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2015;56:6333-6338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 79. | Price R, Smith D, Franklin G, Gronseth G, Pignone M, David WS, Armon C, Perkins BA, Bril V, Rae-Grant A, Halperin J, Licking N, O'Brien MD, Wessels SR, MacGregor LC, Fink K, Harkless LB, Colbert L, Callaghan BC. Oral and Topical Treatment of Painful Diabetic Polyneuropathy: Practice Guideline Update Summary: Report of the AAN Guideline Subcommittee. Neurology. 2022;98:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 80. | Jensen TS. Anticonvulsants in neuropathic pain: rationale and clinical evidence. Eur J Pain. 2002;6 Suppl A:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 81. | Wang HY, Tseng PT, Stubbs B, Carvalho AF, Li DJ, Chen TY, Lin PY, Hsueh YT, Chen YZ, Chen YW, Chu CS. The risk of glaucoma and serotonergic antidepressants: A systematic review and meta-analysis. J Affect Disord. 2018;241:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Fu Y, Dai Q, Zhu L, Wu S. Antidepressants use and risk of cataract development: a systematic review and meta-analysis. BMC Ophthalmol. 2018;18:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24:501-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 84. | Ammermann F, Meinel FG, Beller E, Busse A, Streckenbach F, Teichert C, Weinrich M, Neumann A, Weber MA, Heller T. Concomitant chronic venous insufficiency in patients with peripheral artery disease: insights from MR angiography. Eur Radiol. 2020;30:3908-3914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 638] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 86. | Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1841] [Cited by in RCA: 1826] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 87. | Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 511] [Article Influence: 73.0] [Reference Citation Analysis (1)] |
| 88. | Foussard N, Saulnier PJ, Potier L, Ragot S, Schneider F, Gand E, Monlun M, Baillet-Blanco L, Velho G, Marre M, Roussel R, Rigalleau V, Mohammedi K, Hadjadj S; SURDIAGENE Study Group. Relationship Between Diabetic Retinopathy Stages and Risk of Major Lower-Extremity Arterial Disease in Patients With Type 2 Diabetes. Diabetes Care. 2020;43:2751-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Ratchford EV. Medical management of claudication. J Vasc Surg. 2017;66:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 90. | Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 131] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 91. | Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol. 1988;11:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 92. | Sivalingam A, Brown GC, Magargal LE. The ocular ischemic syndrome. III. Visual prognosis and the effect of treatment. Int Ophthalmol. 1991;15:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 93. | Al Kahtani ES, Khandekar R, Al-Rubeaan K, Youssef AM, Ibrahim HM, Al-Sharqawi AH. Assessment of the prevalence and risk factors of ophthalmoplegia among diabetic patients in a large national diabetes registry cohort. BMC Ophthalmol. 2016;16:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Smith BE, Dyck PJ. Subclinical histopathological changes in the oculomotor nerve in diabetes mellitus. Ann Neurol. 1992;32:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | International Working Group on the Diabetic Foot (IWGDF). Practical guidelines on the prevention and management of diabetic foot disease. IWGDF, 2019. [cited 24 August 2022]. Available from: https://iwgdfguidelines.org/wp-content/uploads/2019/05/IWGDF-Guidelines-2019.pdf. |
| 96. | Margolis TP. Neurotrophic Keratopathy: Ophthalmology's Diabetic Foot Problem. Eye Contact Lens. 2021;47:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Liesegang TJ. Corneal complications from herpes zoster ophthalmicus. Ophthalmology. 1985;92:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Dörr S, Freier F, Schlecht M, Lobmann R. Bacterial diversity and inflammatory response at first-time visit in younger and older individuals with diabetic foot infection (DFI). Acta Diabetol. 2021;58:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Sand D, She R, Shulman IA, Chen DS, Schur M, Hsu HY. Microbial keratitis in Los Angeles: the Doheny Eye Institute and the Los Angeles County Hospital experience. Ophthalmology. 2015;122:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 100. | Lone AM, Zaroo MI, Laway BA, Pala NA, Bashir SA, Rasool A. Vacuum-assisted closure versus conventional dressings in the management of diabetic foot ulcers: a prospective case-control study. Diabet Foot Ankle. 2014;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 101. | Malafa MM, Coleman JE, Bowman RW, Rohrich RJ. Perioperative Corneal Abrasion: Updated Guidelines for Prevention and Management. Plast Reconstr Surg. 2016;137:790e-798e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 507] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 103. | Li BY, Tan W, Zou JL, He Y, Yoshida S, Jiang B, Zhou YD. Role of interferons in diabetic retinopathy. World J Diabetes. 2021;12:939-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 104. | Kang Q, Yang C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 573] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 105. | Silva KC, Rosales MA, Hamassaki DE, Saito KC, Faria AM, Ribeiro PA, Faria JB, Faria JM. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:1325-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 106. | Varney JE, Weiland TJ, Inder WJ, Jelinek GA. Effect of hospital-based telephone coaching on glycaemic control and adherence to management guidelines in type 2 diabetes, a randomised controlled trial. Intern Med J. 2014;44:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 107. | Konstantinidis L, Carron T, de Ancos E, Chinet L, Hagon-Traub I, Zuercher E, Peytremann-Bridevaux I. Awareness and practices regarding eye diseases among patients with diabetes: a cross sectional analysis of the CoDiab-VD cohort. BMC Endocr Disord. 2017;17:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |