Published online Jan 15, 2022. doi: 10.4239/wjd.v13.i1.37
Peer-review started: February 18, 2021
First decision: April 20, 2021
Revised: April 26, 2021
Accepted: December 28, 2021
Article in press: December 28, 2021
Published online: January 15, 2022
Processing time: 326 Days and 8.5 Hours
When combined with vanadium salts, catecholamines strongly activate glucose uptake in rat and mouse adipocytes.
To test whether catecholamines activate glucose transport in human adipocytes.
The uptake of 2-deoxyglucose (2-DG) was measured in adipocytes isolated from pieces of abdominal subcutaneous tissue removed from women undergoing reconstructive surgery. Pharmacological approaches with amine oxidase inhibitors, adrenoreceptor agonists and antioxidants were performed to unravel the mechanisms of action of noradrenaline or adrenaline (also named epinephrine).
In human adipocytes, 45-min incubation with 100 µmol/L adrenaline or noradrenaline activated 2-DG uptake up to more than one-third of the maximal response to insulin. This stimulation was not reproduced with millimolar doses of dopamine or serotonin and was not enhanced by addition of vanadate to the incubation medium. Among various natural amines and adrenergic agonists tested, no other molecule was more efficient than adrenaline and noradrenaline in stimulating 2-DG uptake. The effect of the catecholamines was not impaired by pargyline and semicarbazide, contrarily to that of benzylamine or methylamine, which are recognized substrates of semicarbazide-sensitive amine oxidase. Hydrogen peroxide at 1 mmol/L activated hexose uptake but not pyrocatechol or benzoquinone, and only the former was potentiated by vanadate. Catalase and the phosphoinositide 3-kinase inhibitor wortmannin inhibited adrenaline-induced activation of 2-DG uptake.
High doses of catecholamines exert insulin-like actions on glucose transport in human adipocytes. At submillimolar doses, vanadium did not enhance this catecholamine activation of glucose transport. Consequently, this dismantles our previous suggestion to combine the metal ion with catecholamines to improve the benefit/risk ratio of vanadium-based antidiabetic approaches.
Core Tip: Our recent results indicated that the combination of catecholamines plus vanadium strongly stimulates glucose transport in rat adipocytes. We therefore proposed that catecholamine/vanadate salts could lead to the development of novel derivatives exhibiting potent insulin-like properties. Here, we found that adrenaline and noradrenaline stimulated glucose transport in human adipocytes but in a manner that was not dependent on and not enhanced by the presence of vanadate. Consequently, our previously proposed usefulness of the synergism of catecholamines/vanadium does not work in human fat cells. This might hamper the improvement of vanadium-based antidiabetic approaches, limited so far by toxicological issues.
- Citation: Carpéné C, Boulet N, Grolleau JL, Morin N. High doses of catecholamines activate glucose transport in human adipocytes independently from adrenoceptor stimulation or vanadium addition. World J Diabetes 2022; 13(1): 37-53
- URL: https://www.wjgnet.com/1948-9358/full/v13/i1/37.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i1.37
In a recent report, we demonstrated that catecholamines such as noradrenaline and adrenaline (also named norepinephrine and epinephrine) are capable of activating glucose transport in rodent adipocytes, essentially in the presence of vanadium[1]. These observations, providing the basis for novel research of vanadium/amine complexes exhibiting antidiabetic properties of the metal ions with less toxicological issues, needed further verification. Particularly, the demonstration of relevance to humans was lacking for this alleged insulin-like effect of high doses of catecholamines.
To extrapolate to humans our recent description of a stimulatory effect of catecholamines plus vanadium on glucose transport in rodent fat cells[1], we have reproduced our previous explorations in human adipocytes. Although cultured preadipocytes undergoing in vitro adipogenesis and immortalized cell lines have been successfully used to document the complex influence of pro- and antioxidants on insulin sensitivity[2,3], we have chosen to explore the effects of catecholamines in human mature adipocytes.
Since we have performed our previous observations on rodent white fat cells[1] and since white adipocytes store energy in adipose depots, it was of utmost importance to verify whether our findings are relevant for human mature adipocytes. Indeed, white adipocytes are not found in human adipose tissue because it is yellow mature fat cells that are present in fat depots, regardless of their anatomical location. The yellow coloration found in humans is attributed to the storage of natural lipophilic pigments such as carotenoids, which are slowly metabolized. The white fat cells are somewhat specific of rodents, and this mere difference in the color is not the sole difference between fat cells from animal models and humans[4]. The sensitivity to vanadium regarding glucose accumulation in adipocytes is also different between rodents and humans, as recently reviewed[5]. Thus, an interspecies extrapolation step was mandatory prior to further development of our proposed combination of catecholamines plus vanadium for potential blood glucose-lowering approaches[1]. In the case of vanadium, its insulin-like properties originally described several decades ago[6,7] still requires the setting of novel administration forms to increase the benefit/risk ratio in diabetic patients[8,9].
The advantage of white adipocytes freshly isolated from young laboratory rodents previously used for the demonstration of the potential insulin mimicry of amines plus vanadium[1,10] is that such fat cells are highly sensitive to insulin stimulation of glucose metabolism and to the catecholamine stimulation of lipolysis. Interspecies comparative functional explorations of triacylglycerol synthesis (lipogenesis), triacylglycerol breakdown (lipolysis) and metabolite or adipokine release have shown multiple differences between rodent and human adipocytes, with human adipocytes being less metabolically active[11]. For example, the adrenergic stimulation of lipolysis is predominantly mediated by β3-adrenergic receptor (β3-AR) activation in rat and mouse, while it depends only on β1-AR and β2-AR activation in human adipocytes[4,12]. Similarly, the activation of α2-ARs in human fat cells results in an antilipolytic response, which hardly occurs in rodent adipocytes since their equipment in α2-ARs is rather limited[4]. Finally, the atrial natriuretic peptides are more lipolytic in human adipocytes than in the rodent ones[13,14].
All these considerations prompted us to test whether adrenaline and noradrenaline were activating glucose uptake in human adipocytes freshly isolated from pieces of abdominal subcutaneous adipose tissue removed during reconstructive surgery interventions in premenopausal women, as in[15,16]. When deciphering the effects of catecholamines in rat and mouse adipocytes, it has been evidenced that β-AR activation is not involved since catecholamines were able to activate glucose transport in fat cells from “beta-less” mice with triple knock-out of the subtypes of β-ARs[1]. In view of the above mentioned interspecies differences regarding adrenoreceptor equipment, it was necessary to perform such verification in human fat cells, and this implied the use of specific adrenergic agonists as reported in the following results.
Moreover, among the amines already reported to activate hexose uptake in human fat cells in the absence of insulin, it is worth mentioning benzylamine[17] and methylamine[15]. They are substrates of a copper-containing amine oxidase, the AOC3 gene of which is highly expressed in human fat cells: the semicarbazide-sensitive amine oxidase (SSAO)[18], also known as primary amine oxidase[19], or vascular adhesion protein-1[20]. Benzylamine and methylamine have been included alongside insulin as positive controls of the hexose uptake activation in human adipocytes. As SSAO is not the sole amine oxidase present in adipocytes[21] [it coexists with monoamine oxidase (MAO-A, and to a lesser extend MAO-B)], their respective historical inhibitors semicarbazide and pargyline were also used. Also added in our control conditions was hydrogen peroxide, one of the reactive oxygen species (ROS) known to stimulate glucose uptake in fat cells[22] and is one of the historical insulin-mimetic compounds that act independently of insulin, while an excess of ROS hampers insulin action[3,23,24].
The following results, which can be considered as preclinical, will document that adrenaline and adrenaline stimulate hexose transport in human fat cells but in a manner that is not potentiated by sodium orthovanadate, not mimicked by β-AR or α2-AR agonists and not hampered by SSAO and MAO inhibitors.
(+/-)-Adrenaline (equivalent to epinephrine), (-)-noradrenaline (equivalent to norepinephrine), (-)-isoprenaline (equivalent to isoproterenol), dopamine, tyramine, benzylamine, sodium orthovanadate, collagenase A, human and bovine insulin and most of the other reagents were from Sigma-Aldrich-Merck (Saint Quentin Fallavier, France). [3H]-2-deoxyglucose (2-DG) was from Perkin Elmer (Boston, MA, United States). The adrenergic agonists CL 316243 and BRL 37344 were given by Dr. Lafontan M. (Toulouse, France), while UK 14304 and RX 821002 were a gift from the late Dr. Paris H. (Toulouse, France).
Samples of abdominal subcutaneous adipose tissue were obtained with informed consent from a total of 34 women undergoing reconstructive surgery at the Department of Plastic Surgery (Rangueil Hospital, Toulouse, France). Mean age was 37 years (range: 18-59), and mean body mass index was 25.04 ± 0.65 kg/m2 (range: 21-41). Adipose tissue samples were transferred to the laboratory in less than an hour after surgery and cut into small pieces then digested at 37 °C by collagenase under agitation. Preparations of buoyant adipocytes were obtained by filtration of the digested pieces through nylon stockings and two washes with Krebs–Ringer buffered at pH 7.5 with 15 mmol/L sodium bicarbonate, 10 mmol/L HEPES, supplemented with 3.5% of bovine serum albumin, as in[15]. No freezing/thawing sequences were inserted in the protocol for obtaining functional adipocytes, and 2-DG uptake assays were completed within 5 h after each surgical intervention. The study was in compliance with the INSERM guidelines and approved by the local ethics committee “Comité de Protection des Personnes Sud Ouest & Outre-Mer II” under the number DC-2014-2039.
Male Wistar rats from Charles River (L’Arbresle, France) and mice of both genders from a mixed genetic background (129 Sv/ev, 129 Sv/J, FVB/N, C57BL/6J, and DBA/2) were housed in separate rooms at constant temperature (20-22 °C) and with a 12-h light-dark cycle. All the rodents had free access to food and water and were treated in accordance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments)[25]. Only the mice that were considered as wildtype by Southern blot genotyping as described elsewhere[26] and were similar to those used as control for “β-less” mice in our previous study of catecholamine influence on adipocyte glucose transport[1] were euthanized after overnight fasting when 2- to 3-mo-old. Adipocyte preparations were obtained by collagenase digestion of perigonadic, retroperitoneal, perirenal and inguinal fat pads as previously described[1]. As for pieces of human adipose tissue, rodent fat pads were minced with scissors in Krebs-Ringer salt solution buffered at pH 7.5 and containing 3.5% fat-depleted bovine serum albumin.
The only source of glucose for the cell preparations during glucose uptake assays was the non-metabolizable analogue [3H]-2-DG, added at a final concentration of 0.1 mmol/L to fat cell suspensions as described previously[1]. Since the radioactive tracer (approximately 1300000 dpm/vial) was added for 10 min in the presence of fat cells after a 45 min preincubation period with the tested or reference agents, 2 mmol/L pyruvate was also present in the medium throughout the experiments for energy supply, as previously detailed[18]. Human or rodent fat cells were preincubated in 400 µL medium, then [3H]-2-DG was added as 100 µL portions, and hexose uptake assays were stopped 10 min later with 100 µL of 100 µmol/L cytochalasin B. Then, 200 µL of cell suspension were immediately transferred to plastic centrifugation microtubes prefilled with dinonyl-phthalate (density 0.98 g/mL) before a 40 s spin to separate the buoying adipocytes from the medium as described previously[1,18]. The upper part of the tubes, containing radiolabeled hexose internalized in intact fat cells above the silicon layer was then counted in scintillation vials. The extracellular [3H]-2-DG present in this upper part of the tubes, which was not internalized in cells, was determined with adipocytes whose transport activity was previously blocked by cytochalasin B at time 0. Though averaging 1%-5% of the radioactivity found in the upper phase, it was subtracted from all assays, as in[17]. Among the slight adaptations that differentiated assays with human fat cells from those with rat or mouse adipocytes was the use of human insulin instead of bovine insulin for rodent preparations and a higher richness in adipocytes: 20 mg lipids/400 µL instead of approximately 15 mg/400 µL.
Glucose was present at 5.5 mmol/L in the Krebs-Ringer-based medium used for lipolysis assays, for which 2-DG and pyruvate were omitted, as already reported[15]. As above, tested agents were added to 400 µL of fat cell suspension at the start of a 90-min incubation at 37 °C under gentle shaking. Incubations were stopped on ice. Lipolysis was determined by using glycerol release as an index as already documented, considering that free fatty acid release exhibits parallel variations in our experimental conditions[16].
Results are presented as means ± SE of the mean of (n) observations. All the statistical analyses for comparisons between parameters used analysis of variance followed by post-hoc Dunnett’s multiple comparisons test, analyzed with Prism 6 for Mac OS X (from GraphPad software, Inc). NS means non-significant difference.
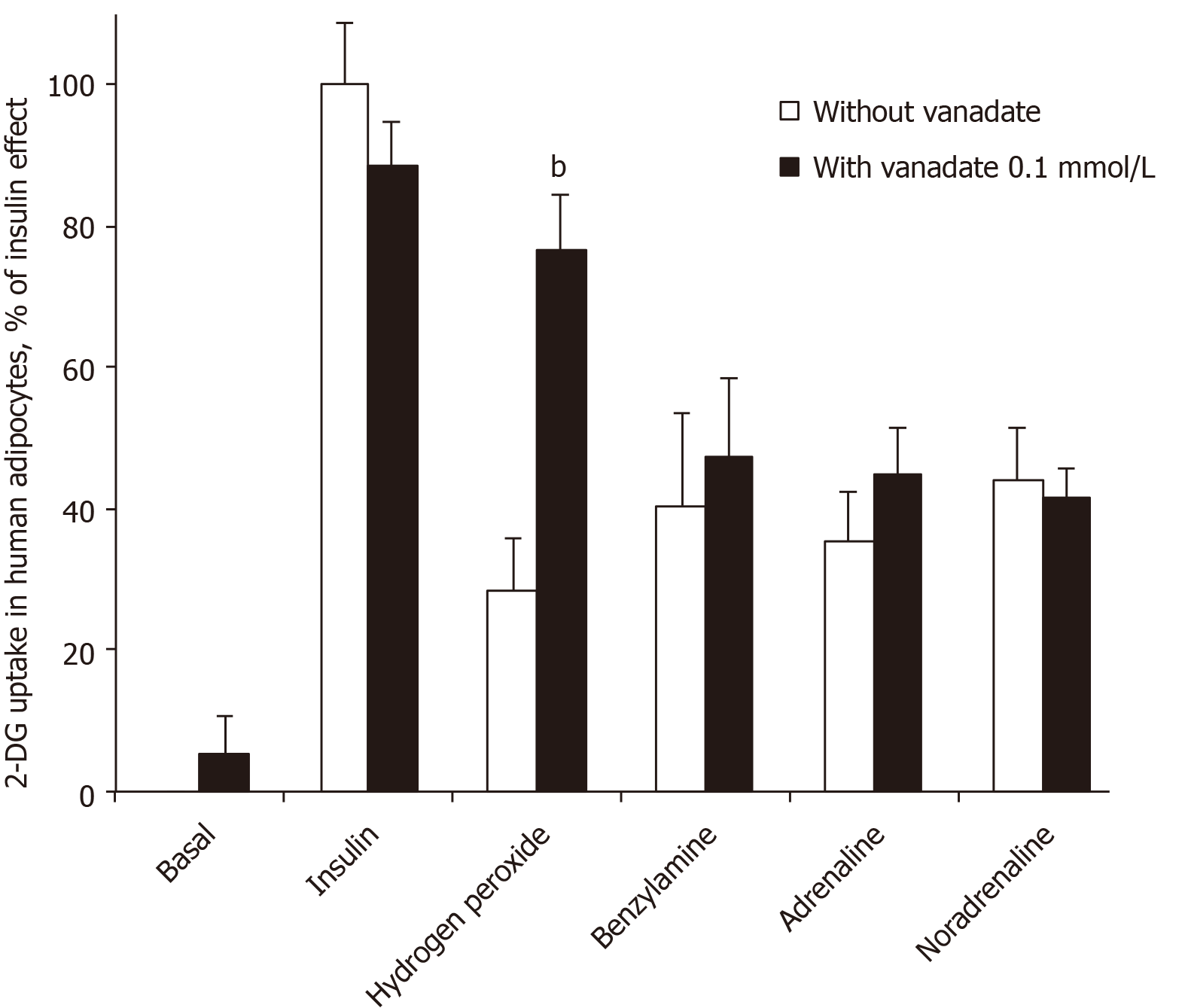
When incubation medium of human adipocytes was buffered at pH 7.5, no stimulation of basal hexose uptake was obtained with sodium orthovanadate at a final concentration of 100 µmol/L (Figure 1). Even the insulin-stimulated hexose transport, which was approximately three times higher than baseline, was not modified by 100 µmol/L vanadate. However, vanadium addition impressively potentiated the stimulatory effect of 1 mmol/L hydrogen peroxide on hexose uptake into human adipocytes (Figure 1), as already reported for rodent adipocytes[27]. Hydrogen peroxide was tested here as a reference because: (1) It stimulates hexose uptake in human adipocytes[17]; (2) Its action is potentiated by 100 µmol/L vanadate in rodent adipocytes[1]; and (3) It is one of the end-products of amine oxidase activity, regardless of the amine substrate or the type of amine oxidase activated[28].
All these control conditions confirmed our previous observations[15,17] and indicated that the human fat cell preparations were responsive to insulin regarding glucose transport activation. More importantly, the synergism between vanadium and hydrogen peroxide was in line with the characterization of the insulin-like properties of peroxovanadate, the compound generated by the combination of vanadate and hydrogen peroxide[5,29].
Figure 1 also shows that vanadate did not modify the effect of 100 µmol/L benzylamine, which elicited a stimulation that was equivalent to approximately one-third of the maximal insulin stimulation. Similarly, the stimulatory effect of adrenaline and noradrenaline on hexose uptake in human fat cells was not enhanced by vanadate (Figure 1). This was strikingly different from the synergism found between vanadate and amines regarding activation of glucose uptake in rodent adipocytes[1,27].
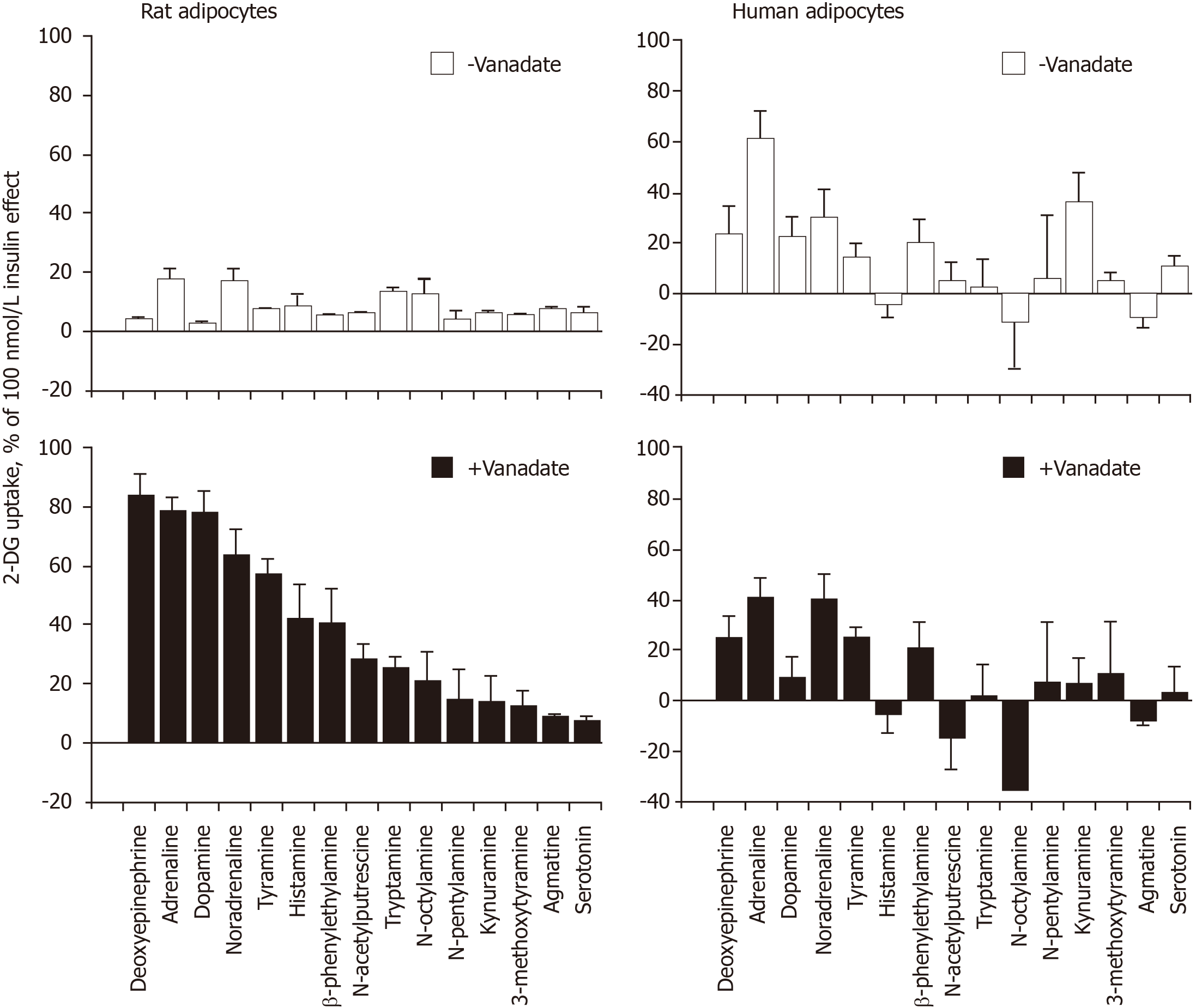
Together, these first observations indicated that high doses of adrenaline and noradrenaline can acutely activate glucose transport in human fat cells, at least when incubated with the cells at 100 µmol/L for 45 min. The unexpected difference when compared with animal models was that the ‘insulin-like’ effect of the amines was not enhanced by vanadium in human fat cells. In other words, catecholamines stimulated 2-DG uptake in human adipocytes without the need for vanadium. This capacity to enhance glucose transport deserved further study since it could constitute a novel rationale for increasing glucose consumption in peripheral tissues. We investigated whether other amines could activate 2-DG uptake in human adipocytes, either in a vanadium-dependent or independent manner. To this aim, we compared the responses of rat and human adipocytes.
Figure 2 shows that the behavior of human adipocytes was clearly different from that of rat adipocytes regarding the synergism between vanadium and amines. The clear potentiation occurring between vanadate and most of the tested amines, already evidenced in rat adipocytes[1], could not be merely extrapolated to human adipocytes. However, this interspecies comparative approach indicated that adrenaline and noradrenaline were the most powerful agents among the fifteen biogenic amines tested in human adipocytes and demonstrated that not any given amine was able to activate glucose uptake at 1 mmol/L. For unknown reasons, the relative rank order of potency for (either cyclic or aliphatic) amines activating hexose uptake was not the same in rat and human adipocytes. Another important finding drawn from this comparison is that the lack of potentiation between vanadium and amines was generalized to all the amines tested on glucose transport in human adipocytes, at least under our experimental conditions.
Methylamine, which does not contain any benzene or catechol ring in its chemical structure and is the simplest molecule well-recognized as an SSAO substrate, was then used for further comparison between rat and human adipocytes. The methylamine stimulation of 2-DG uptake, which occurred only in the presence of 100 µmol/L vanadate in rat adipocytes, was entirely abolished by 1 mmol/L semicarbazide, the reference inhibitor of SSAO, while it was partially resistant to the MAO inhibitor pargyline, even when present at 1 mmol/L (Table 1). In human adipocytes, methy
| Rat adipocytes with vanadate 0.1 mmol/L | Human adipocytes without vanadium | |
| Incubation condition | 2-DG uptake (nmol/100 mg lipids/10 min) | |
| Control | 1.30 ± 0.12 | 0.49 ± 0.05 |
| Insulin 100 nmol/L | 13.08 ± 0.31e | 1.49 ± 0.19e |
| Methylamine 1 mmol/L | 11.27 ± 1.17e | 0.86 ± 1.3b |
| Met + pargyline 1 mmol/L | 6.37 ± 0.88f | 0.84 ± 0.09 |
| Met + semicarbazide 1 mmol/L | 1.32 ± 0.19f | 0.60 ± 0.08c |
| Met + pargyline + semicarbazide | 1.18 ± 0.34f | 0.59 ± 0.07d |
Thus, in rats and humans, semicarbazide plus pargyline likely impaired the release of oxidation products during methylamine catabolism by SSAO and/or MAO, and this consequently prevented methylamine to activate 2-DG uptake. It can be postulated that activation of the amine oxidases expressed in adipocytes was supporting the 2-DG uptake activation in response to millimolar doses of methylamine. Hydrogen peroxide, one of the products generated during oxidative deamination was supposed to be involved in this hexose uptake stimulation, according to previous studies in adipocytes[1] or cardiomyocytes[30]. However, this paradigm does not address the different sensitivities of hydrogen peroxide and amines regarding potentiation by vanadium in human fat cells. Additional investigations were therefore required to depict the mechanisms underlying the stimulatory action of catecholamines on the glucose entry into human adipocytes. It was decided to search whether degradation products other than hydrogen peroxide could mediate the catecholamine effect on 2-DG uptake by further comparing rodent and human adipocytes.
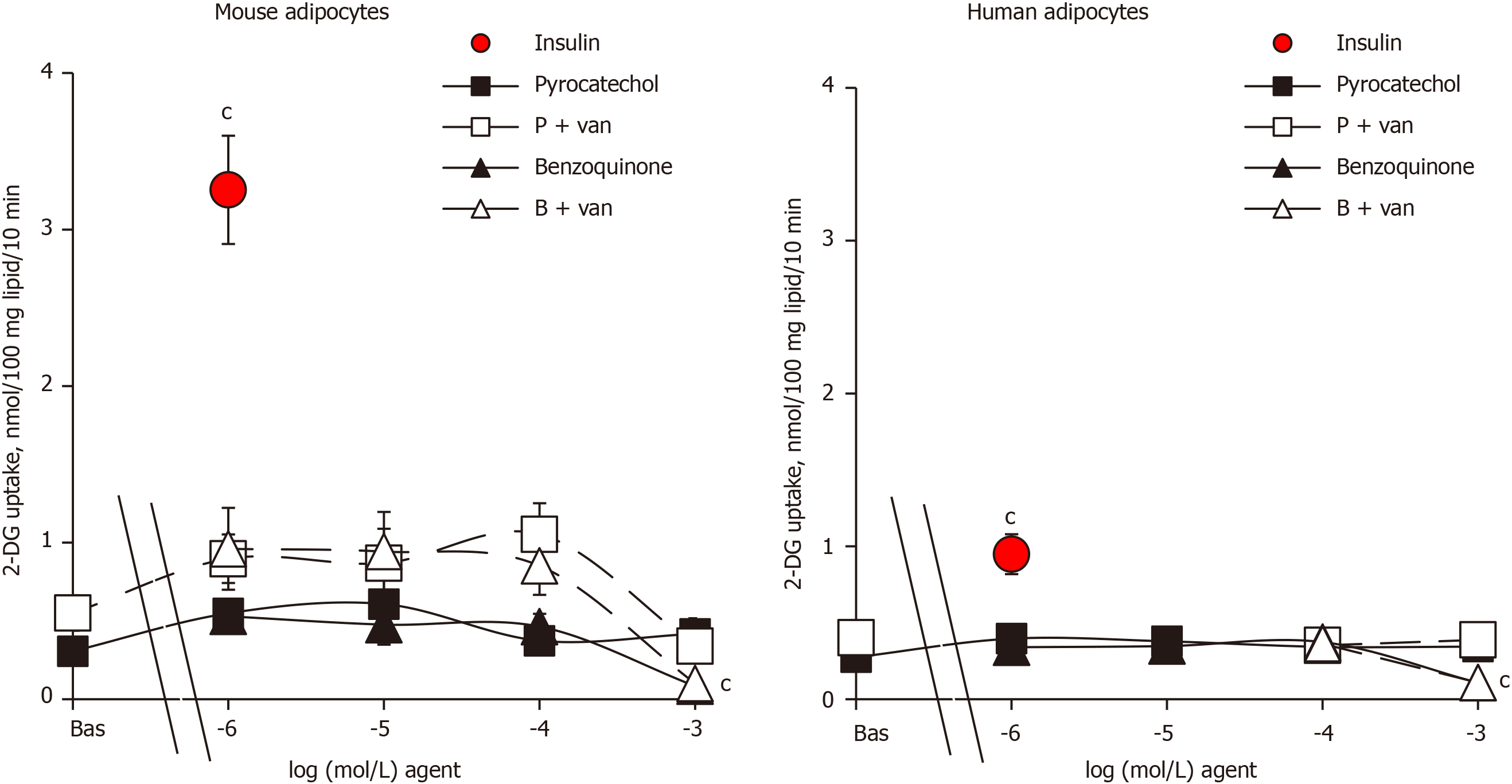
Since the reaction end-products of benzylamine oxidation by amine oxidases, benzaldehyde and ammonia, have been found to be inactive on glucose transport in human adipocytes[18], they were not further investigated here. By contrast, pyrocatechol and benzoquinone, which can be considered as final metabolites of catecholamine catabolism, have never been tested on glucose transport, at least to our knowledge. Thus, it was investigated how their putative effects could be improved by vanadate. The metabolite pyrocatechol, formed by a benzene core carrying two hydroxyl substituents, was inefficient on glucose transport in both mouse and human adipocytes when tested alone from 1 µmol/L up to 1 mmol/L (Figure 3). Pyrocatechol was also unable to activate hexose uptake in rat adipocytes (not shown). Even when tested with vanadate, pyrocatechol was inefficient in the three species, with only a tendency to generate higher uptake levels in mice, without reaching significance and with largely weaker magnitude than insulin stimulation (Figure 3).
In many biological materials, the oxidation of catechol gives reddish-brown melanoid pigments, derivatives of benzoquinone. We therefore tested benzoquinone on glucose transport. In mouse adipocytes that were highly responsive to insulin, benzoquinone did not notably activate 2-DG uptake, with or without vanadium (Figure 3). Benzoquinone even inhibited basal 2-DG uptake at 1 mmol/L, and a similar pattern was observed in human adipocytes (Figure 3). Apparently, these “waste” products of catecholamine catabolism were not responsible for the mild activation of hexose uptake by high doses of (nor)adrenaline either in rodent[1] or human adipocytes (see Figures 1 and 2). Moreover, benzoquinone was inhibitory at millimolar doses.
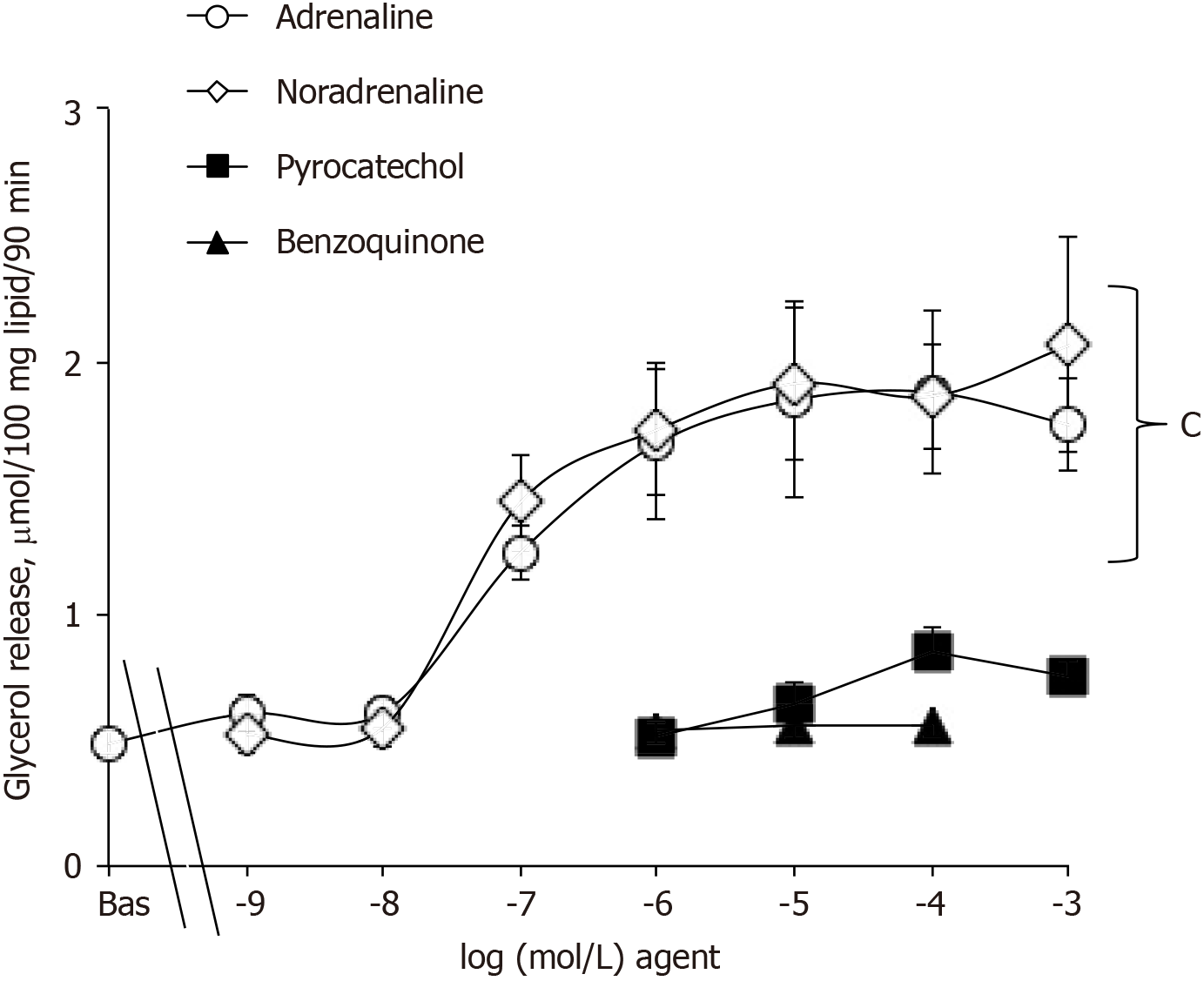
An additional investigation was performed with pyrocatechol and benzoquinone on mouse adipocytes and indicated that they were neither able to activate lipolysis as did adrenaline or adrenaline (Figure 4) nor able to impair the lipolytic effect of the catecholamines when tested at 1 µmol/L (not shown). Hence, these waste products cannot be suspected to impair or to support the effects of (nor)adrenaline on glucose entry in human fat cells.
During all these verifications, the sole 2-DG uptake activation demonstrated to depend on amine oxidase activity was that of methylamine. Thus, verifying whether the effects of (nor)adrenaline were sensitive to blockade by pargyline and semicarbazide in human adipocytes remained mandatory.
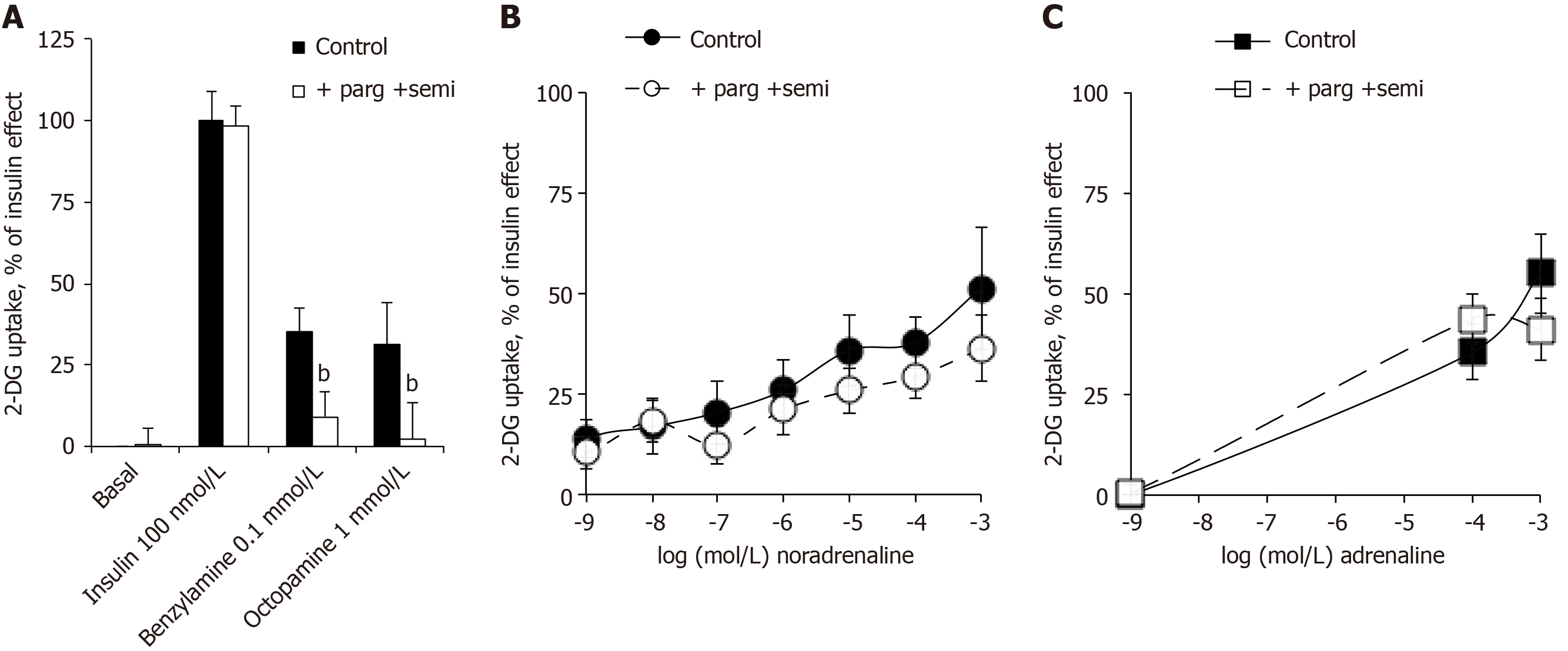
The glucose transport in human adipocytes incubated with 100 nmol/L insulin was defined as the maximal activation of 2-DG uptake and set at 100%. Basal and insulin-stimulated 2-DG uptake resisted the blockade by the combination of amine oxidase inhibitors: pargyline + semicarbazide (parg + semi) (Figure 5A). Nevertheless, as above with methylamine, the use of benzylamine and octopamine confirmed that ‘classical’ amine oxidase substrates were able to activate glucose entry in human fat cells in a manner that was abolished by parg + semi (Figure 5A). Surprisingly, this was not the case for the activation of 2-DG uptake by 0.1 and 1 mmol/L of noradrenaline and adrenaline, which was not impaired by parg + semi, ruling out the contribution of amine oxidase-dependent oxidation (Figure 5B and C). When tested separately, pargyline and semicarbazide were unable, even at 1 mmol/L to inhibit the adrenaline-induced hexose transport (respective 2-DG uptake levels were in nmol/100 mg lipid/10 min: adrenaline, 1.01 ± 0.12; adrenaline + semicarbazide, 1.01 ± 0.17; adrenaline + pargyline , 0.95 ± 0.10; n = 16; NS, not shown).
Being resistant to parg + semi, the stimulatory action of catecholamines on glucose entry in human adipocytes was not dependent on amine oxidase activity as observed in rodents. Albeit we recently ruled out the contribution of β-AR stimulation in the effects of catecholamines plus vanadium on glucose transport in rodent adipocytes[1], it became necessary to explore this putative mechanism in human adipocytes.
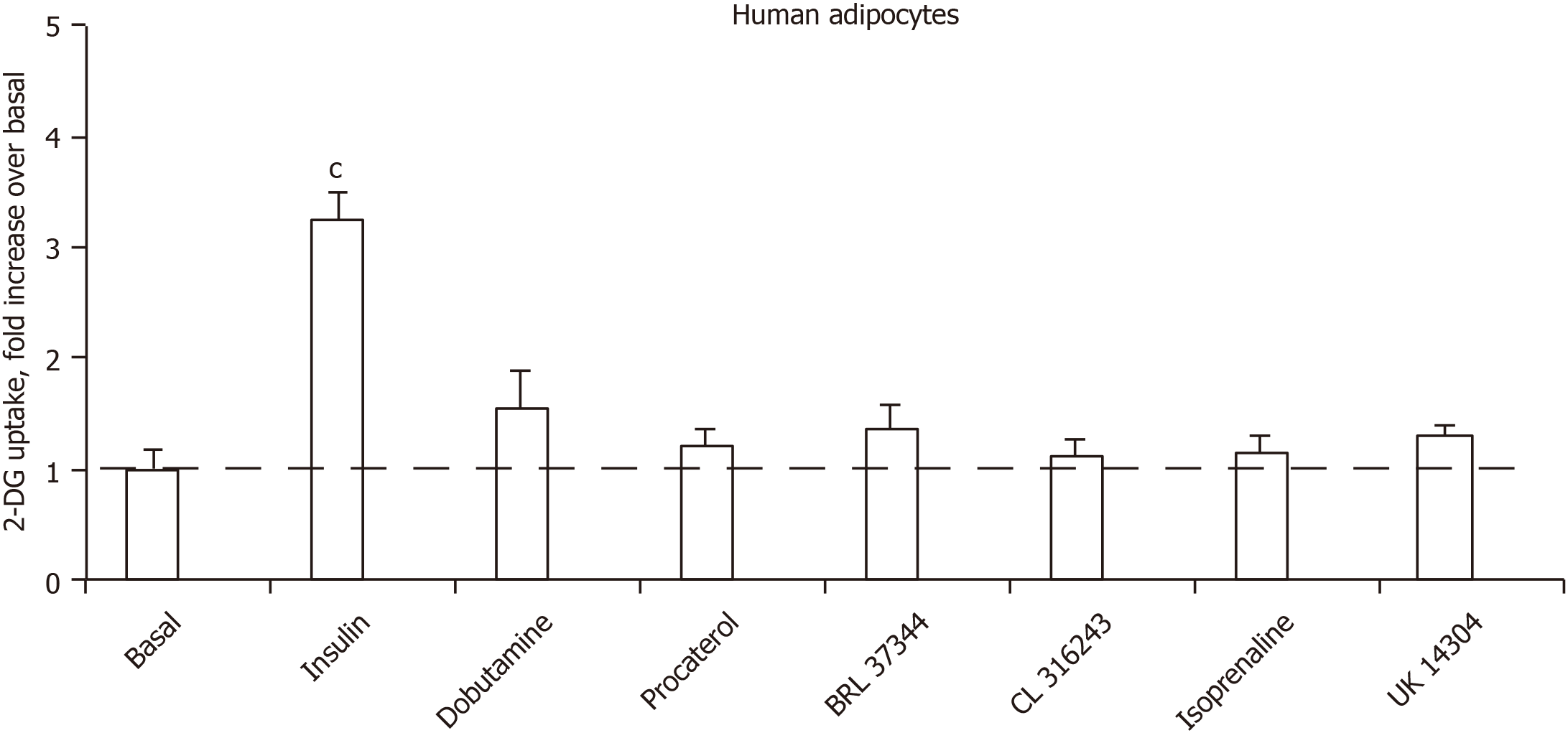
Figure 6 shows that the glucose transport of human adipocyte preparations that were responsive to human insulin could not be activated notably by any of the five β-adrenergic receptor agonists tested. The α2-AR agonist UK 14304 (also known as brimonidine) was also inefficient, arguing that α2-adrenergic activation does not enhance glucose uptake (Figure 6). Of note, the tested β1- and β2-adrenergic agonists were active at 1 µmol/L on lipolysis activation in human fat cells (or in provoking antilipolytic response in the case of UK 14304), as previously reported in independent studies[31,32].
In additional experiments performed to study the sensitivity to antagonists, RX 821002 was chosen for blocking α2-ARs, and the pan-antagonist bupranolol for blocking the β-ARs. Again, adrenaline at 100 µmol/L increased the basal 2-DG uptake (basal: 0.34 ± 0.02, adrenaline: 0.66 ± 0.04; n = 4; P < 0.01), and this was not impaired by 10 µmol/L of each of the antagonists (adrenaline + RX 821002: 0.61 ± 0.06; adrenaline + bupranolol: 0.58 ± 0.04 nmol 2-DG transported/100 mg lipids/10 min; n = 4; NS, not shown).
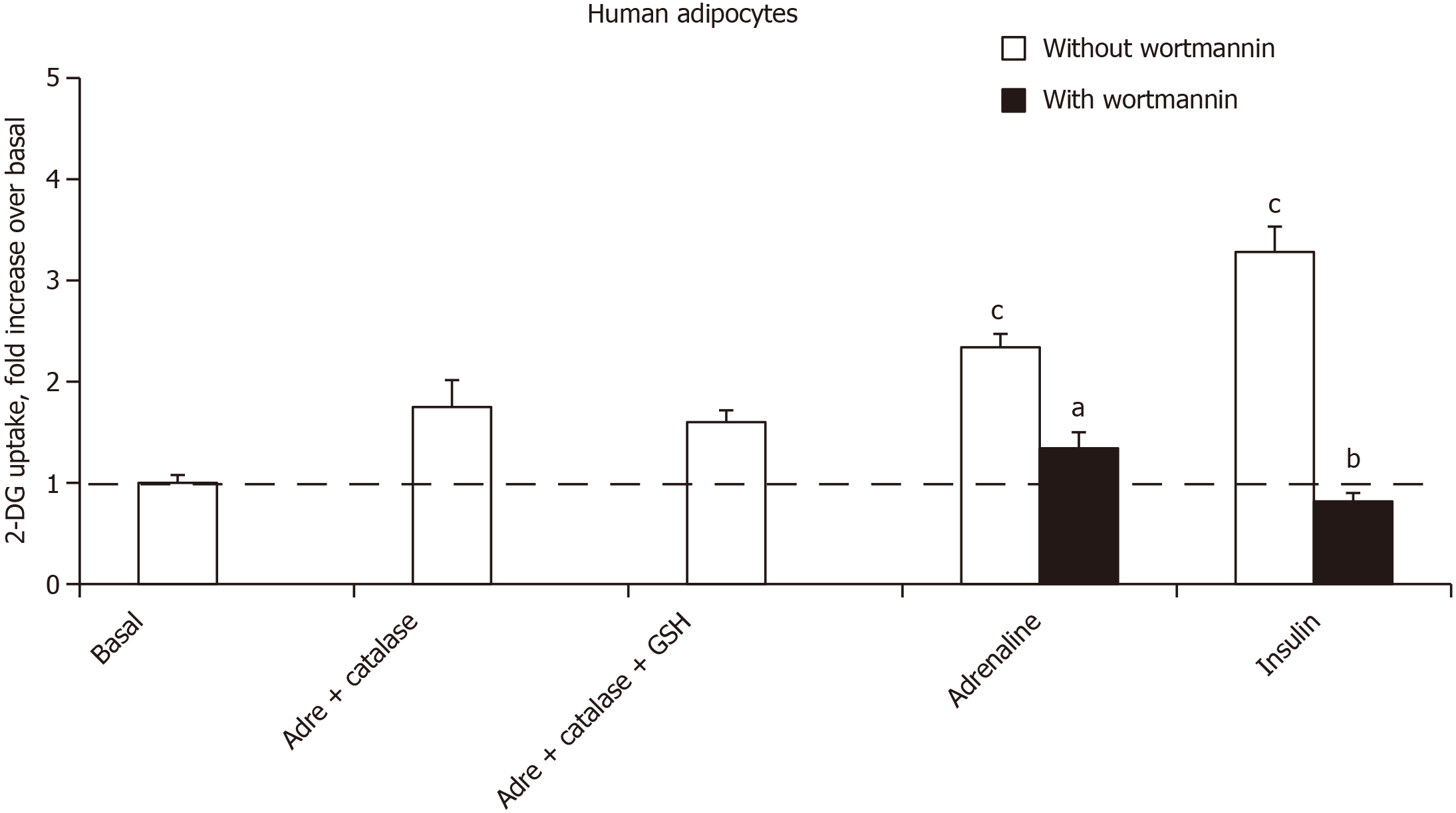
Thus, the use of adrenergic agents could not mimic or block the stimulatory effect of noradrenaline and adrenaline on hexose uptake. At this stage, the role of autoxidation products of the catecholamines was investigated. Alongside adrenochrome and noradrenochrome, the chemistry of catecholamine degradation encompasses numerous ROS, aldehydic molecules and oligomeres implied in neurotoxicity[33]. Rather than testing these highly reactive intermediates, which are rather unstable, it was investigated whether the relatively short-term effect of millimolar doses of adrenaline could be prevented by antioxidant pretreatment. As hydrogen peroxide is active on hexose uptake in adipocytes, it was verified whether its generation was prevented by catalase. Figure 7 shows that catalase impaired the adrenaline-induced stimulation of 2-DG uptake in human adipocytes. The addition of glutathione, expected to limit hydrogen peroxide dismutation by catalase, could not reach complete blockade of adrenaline effect. Lastly, the phosphoinositide 3-kinase inhibitor wortmannin was able at 1 µmol/L to inhibit the effect of adrenaline as well as that of insulin (Figure 7), suggesting that in both cases the activation of hexose uptake was due to a phosphoinositide 3-kinase/protein kinase B-induced glucose carrier recruitment to the cell surface.
In a recent study, in view of the powerful stimulating effect of the combination of catecholamines plus sodium orthovanadate on glucose transport in rodent adipocytes, we have proposed that the use of catecholamines might improve the antidiabetic effect of vanadium by reducing its efficient therapeutic doses and by lowering its toxicity[1]. In the present study, we aimed to extrapolate to human adipocytes the description of the insulin-like nature of the synergism between catecholamines and vanadium on glucose utilization. The results of our present human study clearly indicate that adrenaline and noradrenaline activate hexose uptake in human fat cells at doses comprised between 0.1 and 1 mmol/L. In fact, human fat cells respond to catecholamine exposure for 45 min by a stimulation of hexose uptake that represents one-third to one-half of the maximal response to insulin, depending on the individuals. To our knowledge, it is the first time that such a short-term, non-negligible, insulin-like effect of these two naturally occurring catecholamines is observed in human fat cells. However, this stimulation was not further enhanced by the presence of vanadium and never reached the 80%-90% of the maximal insulin-dependent stimulation of glucose uptake, as it was observed in rodents[1]. In other words, the synergism found between (nor)adrenaline and sodium orthovanadate in rat fat cells was not observed in human adipocytes. This interspecific difference, already observed for the sensitivity to decavanadate[5], abruptly ceased our proposal to use catecholamine derivatives in future strategies aimed at improving the benefit/risk ratio of vanadium-based antidiabetic treatments.
However, a potentiation of the mild activation effect of hydrogen peroxide with 0.1 mmol/L vanadate (a dose inefficient on its own to activate 2-DG uptake) occurred in both human and rodent adipocytes (Figure 1 and[1]). It is not the synergism between hydrogen peroxide and vanadium, which generates peroxovanadate, a powerful insulin-mimicking agent on glucose utilization, that was primarily involved in such unexpected interspecific differences. Curiously, in the same experimental conditions, catecholamines were not the sole amines that behaved differently between human and rodents fat cells. The widely recognized SSAO substrates, benzylamine and methy
Other unexpected differences between rat and human adipocytes did not facilitate a mere extrapolation of our previous findings regarding the synergism between catecholamines and vanadium. For instance, dopamine, which was as stimulatory as adrenaline and noradrenaline in rat adipocytes[1], was not active in human fat cells. Similarly, deoxyepinephrine was much more active on glucose uptake in rats than in humans. Unfortunately, we cannot provide at the moment any explanation for such differences, and this also applies for the rank order of potency for the various amines tested without and with vanadate on hexose uptake, since that found in the rat model is not at all predictive of that found in humans. The catabolism of the biogenic amines and the fates of the vanadate/vanadyl forms of the metal ion are probably different in the two species, as it is also the case for hydrogen peroxide generation/catabolism.
Nonetheless, several common features were observed in this comparative approach: first, no significant effect of serotonin was evidenced in both species; then, the same dose of vanadate that was inefficient on its own on basal or insulin-stimulated hexose uptake, i.e., 0.1 mmol/L, potentiated the hydrogen peroxide effect in both species. Other points of resemblance between rodent and human adipocytes are discussed below, but it must be kept in mind that the essential difference between the two models is that rat fat cells are definitely more metabolic active than the human ones, as attested by the absolute values of maximal hexose transport in response to insulin (see Figure 3 and Table 1).
Although impressed by the distinct intrinsic activity and vanadium sensitivity of the fifteen amines tested, we further attempted to decipher the mechanisms of action implied in the two most active on glucose uptake in human adipocytes: adrenaline and noradrenaline. As with rodent adipocytes, adrenaline and noradrenaline behaved differently from typical SSAO substrates (benzylamine, methylamine): only the latter were not able to activate glucose uptake in the presence of semicarbazide, alone or combined with the monoamine oxidase inhibitor pargyline. In this aspect, human adipocytes resemble the rat ones. Nevertheless, the lack of inhibition of (nor)
We used the combination of MAO and SSAO inhibitors in the present study since both MAO and SSAO substrates are able to mimic insulin-like effects in adipocyte models[27], and since methylamine is a product of adrenaline oxidation by MAO together with hydrogen peroxide. In turn, methylamine is a substrate for SSAO, also generating hydrogen peroxide[35]. This postulated two-step process could explain why adrenaline was the most powerful among the amines tested in stimulating glucose uptake in human adipocytes. But it cannot explain why the adrenaline effect was so weakly impaired by parg + semi combination, capable to block the methy
The absence of plateau in the dose-response curve to noradrenaline activation of 2-DG uptake in human fat cells was somewhat indicative that the mechanism involved is not mediated by a single receptor activation. Indeed, the linear increase of uptake in response to noradrenaline from 1 nmol/L to 1 mmol/L found in human adipocytes clearly contrasted with the typical sigmoid curve seen in mouse adipocytes when the adrenergic stimulation of glycerol release was determined. Only the latter response corresponds to a classical activation of the lipolytic cascade, implying an amplification system with successive activation of β-AR/Gs protein/adenylyl cyclase/protein kinase A/lipases (compare Figures 4 and 5). In keeping with this, none of the various adrenergic agonists tested was able to activate 2-DG uptake in human adipocytes, and the effect of adrenaline was insensitive to the α2- and β-AR antagonists used. These results were in perfect agreement with our recent report showing that β-AR or α2-adrenergic receptor stimulation was not involved in the stimulation by catecholamines plus vanadium of glucose transport in rodent adipocytes[1]. Our pharmacological approach still leaves open a putative mediation of the glucose transport stimulation by α1-AR activation, as proposed in a clinical study based on the effect of noradrenaline and the α1-AR agonist norfenefrine during microdialysis experiments in obese patients[37]. When keeping in mind that neither α1-AR agonist nor α1-AR antagonist modified 2-DG uptake in rat fat cells[1], such α1-AR contribution does not appear plausible and cannot be the sole mechanism supporting the glucose uptake stimulation by 100 µmol/L noradrenaline or adrenaline. Even the stimulation of glucose uptake in rat cardiomyocytes by the recognized α1-AR agonist phenylephrine has been reported to be biphasic: mediated partly by calcium release and by hydrogen peroxide[38].
It was then the products of catecholamine autoxidation that were suspected to produce activation of hexose uptake in view of: (1) The lack of classical sigmoid shape of the dose-response curve to adrenaline; (2) The resistance to amine oxidase inhibitors, although some hydrazine derivatives have been proven to limit the lipid oxidation by reactive carbonyl compounds[39]; and (3) The impairment caused by antioxidants on the activation by adrenaline + vanadate in rodent adipocytes[1].
The autoxidation of (nor)adrenaline, which generates (nor)adrenochrome and known to be increased by metal ions, can be delayed by EDTA or pH acidification. These two conditions have not been tested in the present study since they directly interfere with glucose transport activity. Although we did not assess whether the presence of vanadium was increasing adrenochrome generation in adipocyte preparations, we did not note any dark coloration in the incubation tubes under any condition. In addition, it must be repeated here that the addition of vanadium to adipocyte incubation medium did not increase the catecholamine-stimulated hexose uptake in human fat cells. However, we were aware that sodium vanadate can elicit pH alkalinization and thereby hexose uptake stimulation. For this reason, we prevented any pH elevation by 0.1 mmol/L vanadate owing to the strongly buffered incubation medium we used. Thus, the putative contribution of adrenochrome in the observed effects is not dealing with the lack of potentiation of adrenaline-induced uptake by vanadate since it has been reported that vanadate enhances the in vitro formation of adrenochrome from epinephrine, alongside a reduction of antioxidative defenses, a property that might be linked to vanadate toxic effects in various cell types[40,41]. The fact that the adrenaline stimulation of glucose transport was limited by catalase treatment is another element for discarding the involvement of adrenochrome. Nevertheless, its putative role remains to be definitely ruled out.
One of the limitations in our approach is that we cannot depict the signal transduction elicited by catecholamines when partially mimicking the insulin stimulation of glucose transport. Although we tested two among the numerous metabolites of catecholamines, we did not pay attention to the transient and highly reactive aldehydic molecules generated during either autoxidation or during catabolism by MAO and catechol-O-methyltransferase[33]. Moreover, we did not determine whether there was an appearance of the quinones that are produced during the degradation of (nor)adrenaline into (nor)adrenochrome[42]. However, in accordance with the cytotoxicity of these products, the millimolar dose of benzoquinone has been found to abolish transport activity in adipocytes. While various quinones probably occurred with dopamine also, they did not elicit a detectable effect on 2-DG uptake. Thus, the quinone-based toxic metabolites do not seem to support the catecholamine effect.
It cannot be excluded that others of the numerous metabolites of catecholamine degradation are involved in the in vitro effect we detected, but the participation of hydrogen peroxide, endowed with insulin-like effects, could not be clearly evidenced in our experiments, excepted by catalase treatment. Catalase and glutathione were used since they have been shown to protect neuroblastoma cells against the cytotoxicity of dopamine, due to oxidative stress by generating excessive ROS via MAO-catalyzed oxidative deamination and via autoxidation[43]. On the contrary, ascorbic acid has been reported to be unable to prevent the autoxidation of catecholamines that occurs readily in the oxygen-saturated incubation media of in vitro experiments[44,45]. At last, the inhibition by wortmannin allowed postulating that high doses of catecholamines were activating the recruitment of glucose transporters at the surface of human fat cells.
Finally, noradrenaline and adrenaline are vasoconstrictor agents that stimulate cardiac inotropism, strongly elevate blood pressure as well as increase blood glucose in order to better respond to stress conditions by a behavior well-known from invertebrates to vertebrates as the “fight or flight” response. It is not so astonishing to observe that at high doses these catecholamines are able to activate the glucose utilization in cells in order to facilitate energy consumption. Although the adipocytes are specialized for releasing their lipid stores when the organism requires energy supply, they have to increase glucose uptake/consumption at the same time to perform fatty acid re-esterification to avoid excessive lipolysis. The simultaneous activation of lipolysis and the enhancement of other metabolic pathways such as lipogenesis of fatty acid oxidation is therefore physiologically relevant under adrenergic activation and seems to occur in both animal and human adipocytes. It is the potentiation of the somewhat “insulin-mimicking” properties of catecholamines that does not occur with vanadium in human adipocytes. This does not preclude the interest of the current improvements of the antidiabetic therapeutic applications of vanadium[9,46,47] but seriously limits the relevance of the observations made on rat adipocytes[1,27,48] regarding the promising insulin mimicry of vanadium compounds.
This preclinical study describes in vitro the activation of hexose uptake in human adipocytes by high doses of catecholamines. It also demonstrates that this insulin mimicry has no interest for improving the benefit/risk ratio of vanadium-based antidiabetic complexes since there is no synergism between catecholamines and vanadate regarding glucose uptake in isolated human adipocytes. Moreover the puzzling effect of catecholamines is not entirely mediated by adrenoreceptor stimulation or by MAO- and SSAO-dependent amine oxidation. As lower doses of catecholamines are recognized to rise blood pressure and blood glucose in vivo, no therapeutic use of the present observations can be postulated at the present time.
We have recently reported a synergism between vanadium and catecholamines that generates a powerful activation of glucose transport in rodent adipose cells. Since the combination vanadium/adrenaline or vanadium/noradrenaline mimicked insulin activation of glucose handling in a manner depending on the production of reactive oxygen species, we proposed that further research on vanadate/catecholamine complexes could develop novel, less toxic antidiabetic therapeutic approaches for vanadium compounds.
To extrapolate to humans the potential antihyperglycemic properties of the vanadate/catecholamine combination found in animal models, we aimed to verify whether several amines, including adrenaline and noradrenaline, were able together with vanadate to reproduce the insulin-induced stimulation of glucose transport into human adipocytes.
To evaluate the impact of various biogenic amines, including the well-known catecholamines, adrenaline and noradrenaline, without and with vanadium, on glucose transport in human adipose cells.
Preparations of freshly isolated human adipocytes, obtained from patients undergoing plastic surgery, were subjected to a pharmacological exploration of glucose transport owing to short-term uptake assays performed with the non-metabolizable radiolabeled analogue 2-deoxyglucose. An interspecies approach compared the responses of rat, mouse and human adipocytes subjected to similar stimuli.
In human adipose cells, the stimulation of glucose transport by insulin increased by two-to three times the basal uptake. Neither basal nor insulin-stimulated glucose transport was altered by 100 µmol/L sodium orthovanadate, which clearly potentiated the mild stimulatory action of hydrogen peroxide. Among fifteen biogenic amines tested, adrenaline and noradrenaline were the most efficient in activating 2-deoxyglucose uptake. The stimulation occurred within 0.01-1 mmol/L dose range and was not enhanced with vanadium. Although known to be monoamine oxidase substrates, the stimulation induced by adrenaline and noradrenaline resisted the blockade by amine oxidase inhibitors, as previously found for rodent adipocytes. The tested α- and β-adrenergic agonists did not stimulate glucose uptake in human adipocytes, and the effects of catecholamines were not inhibited by adrenergic antagonists. Benzoquinone and pyrocatechol, two of the various metabolites of catecholamine catabolism were ineffective. Only catalase, together with the antioxidant glutathione, impaired the adrenaline stimulated glucose uptake.
The powerful synergism of vanadium/catecholamines previously reported on rodent adipocytes was not detectable in human fat cells. Nevertheless, adrenaline and noradrenaline were more stimulatory of hexose uptake than equivalent doses of vanadate, in a manner that was independent from adrenoceptor stimulation or amine oxidase activity.
If future studies demonstrate an improvement of the antidiabetic properties of vanadium complexes via their combination with catecholamines, such improvement will likely not be the result of a synergistic effect on the glucose handling by fat cells.
We thank the staff of Plastic Surgery Department of Rangueil Hospital (Toulouse, France) and the animal unit CREFRE, more especially its Rangueil satellite for housing rodents. The authors also thank Danielle Prévot for assistance, Xavier Testar (Univ Barcelona) for his grasp of knowledge about vanadium and Anne Bouloumié (Toulouse) for helpful discussions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozkok E, Wu QN S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Fontaine J, Tavernier G, Morin N, Carpéné C. Vanadium-dependent activation of glucose transport in adipocytes by catecholamines is not mediated via adrenoceptor stimulation or monoamine oxidase activity. World J Diabetes. 2020;11:622-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Chen CC, Hsu LW, Nakano T, Huang KT, Chen KD, Lai CY, Goto S, Chen CL. DHL-HisZn, a novel antioxidant, enhances adipogenic differentiation and antioxidative response in adipose-derived stem cells. Biomed Pharmacother. 2016;84:1601-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Lu B, Ennis D, Lai R, Bogdanovic E, Nikolov R, Salamon L, Fantus C, Le-Tien H, Fantus IG. Enhanced sensitivity of insulin-resistant adipocytes to vanadate is associated with oxidative stress and decreased reduction of vanadate (+5) to vanadyl (+4). J Biol Chem. 2001;276:35589-35598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Lafontan M. Historical perspectives in fat cell biology: the fat cell as a model for the investigation of hormonal and metabolic pathways. Am J Physiol Cell Physiol. 2012;302:C327-C359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Aureliano M. Recent perspectives into biochemistry of decavanadate. World J Biol Chem. 2011;2:215-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Shechter Y, Karlish SJ. Insulin-like stimulation of glucose oxidation in rat adipocytes by vanadyl (IV) ions. Nature. 1980;284:556-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 386] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Srivastava AK, Mehdi MZ. Insulino-mimetic and anti-diabetic effects of vanadium compounds. Diabet Med. 2005;22:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Aureliano M. Decavanadate Toxicology and Pharmacological Activities: V10 or V1, Both or None? Oxid Med Cell Longev. 2016;2016:6103457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Scibior A, Pietrzyk L, Plewa Z, Skiba A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J Trace Elem Med Biol. 2020;61:126508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 10. | Yu PH, Wang M, Fan H, Deng Y, Gubisne-Haberle D. Involvement of SSAO-mediated deamination in adipose glucose transport and weight gain in obese diabetic KKAy mice. Am J Physiol Endocrinol Metab. 2004;286:E634-E641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Langin D, Lucas S, Lafontan M. Millennium fat-cell lipolysis reveals unsuspected novel tracks. Horm Metab Res. 2000;32:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Arner P. Catecholamine-induced lipolysis in obesity. Int J Obes Relat Metab Disord. 1999;23 Suppl 1:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Sengenès C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Carpéné C, Mauriège P, Boulet N, Biron S, Grolleau JL, Garcia-Barrado MJ, Iglesias-Osma MC. Methylamine Activates Glucose Uptake in Human Adipocytes Without Overpassing Action of Insulin or Stimulating its Secretion in Pancreatic Islets. Medicines (Basel). 2019;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Carpéné C, Les F, Mercader J, Gomez-Zorita S, Grolleau JL, Boulet N, Fontaine J, Iglesias-Osma MC, Garcia-Barrado MJ. Opipramol Inhibits Lipolysis in Human Adipocytes without Altering Glucose Uptake and Differently from Antipsychotic and Antidepressant Drugs with Adverse Effects on Body Weight Control. Pharmaceuticals (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Morin N, Lizcano JM, Fontana E, Marti L, Smih F, Rouet P, Prévot D, Zorzano A, Unzeta M, Carpéné C. Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J Pharmacol Exp Ther. 2001;297:563-572. [PubMed] |
| 18. | Mercader J, Iffiú-Soltesz Z, Brenachot X, Földi A, Dunkel P, Balogh B, Attané C, Valet P, Mátyus P, Carpéné C. SSAO substrates exhibiting insulin-like effects in adipocytes as a promising treatment option for metabolic disorders. Future Med Chem. 2010;2:1735-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Olivieri A, Tipton KF, O'Sullivan J. Characterization of the in vitro binding and inhibition kinetics of primary amine oxidase/vascular adhesion protein-1 by glucosamine. Biochim Biophys Acta. 2012;1820:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Salmi M, Jalkanen S. Vascular Adhesion Protein-1: A Cell Surface Amine Oxidase in Translation. Antioxid Redox Signal. 2019;30:314-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 21. | Romauch M. Zinc-α2-glycoprotein as an inhibitor of amine oxidase copper-containing 3. Open Biol. 2020;10:190035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Czech MP. Differential effects of sulfhydryl reagents on activation and deactivation of the fat cell hexose transport system. J Biol Chem. 1976;251:1164-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 98] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Boden G, Homko C, Barrero CA, Stein TP, Chen X, Cheung P, Fecchio C, Koller S, Merali S. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci Transl Med. 2015;7:304re7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 24. | Fazakerley DJ, Minard AY, Krycer JR, Thomas KC, Stöckli J, Harney DJ, Burchfield JG, Maghzal GJ, Caldwell ST, Hartley RC, Stocker R, Murphy MP, James DE. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J Biol Chem. 2018;293:7315-7328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Kilpatrick IC, Traut M, Heal DJ. Monoamine oxidase inhibition is unlikely to be relevant to the risks associated with phentermine and fenfluramine: a comparison with their abilities to evoke monoamine release. Int J Obes Relat Metab Disord. 2001;25:1454-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3050] [Cited by in RCA: 3165] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 26. | Tavernier G, Jimenez M, Giacobino JP, Hulo N, Lafontan M, Muzzin P, Langin D. Norepinephrine induces lipolysis in beta1/beta2/beta3-adrenoceptor knockout mice. Mol Pharmacol. 2005;68:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Marti L, Morin N, Enrique-Tarancon G, Prevot D, Lafontan M, Testar X, Zorzano A, Carpéné C. Tyramine and vanadate synergistically stimulate glucose transport in rat adipocytes by amine oxidase-dependent generation of hydrogen peroxide. J Pharmacol Exp Ther. 1998;285:342-349. [PubMed] |
| 28. | O'Sullivan J, Davey G, O'Sullivan M, Tipton KF. Hydrogen peroxide derived from amine oxidation mediates the interaction between aminosugars and semicarbazide-sensitive amine oxidase. J Neural Transm (Vienna). 2007;114:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Lönnroth P, Eriksson JW, Posner BI, Smith U. Peroxovanadate but not vanadate exerts insulin-like effects in human adipocytes. Diabetologia. 1993;36:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Fischer Y, Rose H, Thomas J, Deuticke B, Kammermeier H. Phenylarsine oxide and hydrogen peroxide stimulate glucose transport via different pathways in isolated cardiac myocytes. Biochim Biophys Acta. 1993;1153:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Mauriège P, Marette A, Atgié C, Bouchard C, Thériault G, Bukowiecki LK, Marceau P, Biron S, Nadeau A, Després JP. Regional variation in adipose tissue metabolism of severely obese premenopausal women. J Lipid Res. 1995;36:672-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Laurencikiene J, Skurk T, Kulyté A, Hedén P, Aström G, Sjölin E, Rydén M, Hauner H, Arner P. Regulation of lipolysis in small and large fat cells of the same subject. J Clin Endocrinol Metab. 2011;96:E2045-E2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Goldstein DS, Kopin IJ, Sharabi Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol Ther. 2014;144:268-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Yraola F, García-Vicente S, Marti L, Albericio F, Zorzano A, Royo M. Understanding the mechanism of action of the novel SSAO substrate (C7NH10)6(V10O28).2H2O, a prodrug of peroxovanadate insulin mimetics. Chem Biol Drug Des. 2007;69:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | McDonald A, Tipton K, O'Sullivan J, Olivieri A, Davey G, Coonan AM, Fu W. Modelling the roles of MAO and SSAO in glucose transport. J Neural Transm (Vienna). 2007;114:783-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Schwelberger HG. Structural organization of mammalian copper-containing amine oxidase genes. Inflamm Res. 2010;59 Suppl 2:S223-S225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Flechtner-Mors M, Jenkinson CP, Alt A, Biesalski HK, Adler G, Ditschuneit HH. Sympathetic regulation of glucose uptake by the alpha1-adrenoceptor in human obesity. Obes Res. 2004;12:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Fischer Y, Thomas J, Holman GD, Rose H, Kammermeier H. Contraction-independent effects of catecholamines on glucose transport in isolated rat cardiomyocytes. Am J Physiol. 1996;270:C1204-C1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Galvani S, Coatrieux C, Elbaz M, Grazide MH, Thiers JC, Parini A, Uchida K, Kamar N, Rostaing L, Baltas M, Salvayre R, Nègre-Salvayre A. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic Biol Med. 2008;45:1457-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Donaldson J, LaBella F. Prooxidant properties of vanadate in vitro on catecholamines and on lipid peroxidation by mouse and rat tissues. J Toxicol Environ Health. 1983;12:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Chandra AK, Ghosh R, Chatterjee A, Sarkar M. Effects of vanadate on male rat reproductive tract histology, oxidative stress markers and androgenic enzyme activities. J Inorg Biochem. 2007;101:944-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633-643. [PubMed] |
| 43. | Lai CT, Yu PH. Dopamine- and L-beta-3,4-dihydroxyphenylalanine hydrochloride (L-Dopa)-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells. Effects of oxidative stress and antioxidative factors. Biochem Pharmacol. 1997;53:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Hugh D, Grennan A, Abugila MA, Weinkove C. Ascorbic acid as an antioxidant in measurements of catecholamines in plasma. Clin Chem. 1987;33:569-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Sutor B, ten Bruggencate G. Ascorbic acid: a useful reductant to avoid oxidation of catecholamines in electrophysiological experiments in vitro? Neurosci Lett. 1990;116:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Scior T, Guevara-Garcia JA, Do QT, Bernard P, Laufer S. Why Antidiabetic Vanadium Complexes are Not in the Pipeline of "Big Pharma" Drug Research? Curr Med Chem. 2016;23:2874-2891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Treviño S, Díaz A, Sánchez-Lara E, Sanchez-Gaytan BL, Perez-Aguilar JM, González-Vergara E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol Trace Elem Res. 2019;188:68-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 48. | Pereira MJ, Carvalho E, Eriksson JW, Crans DC, Aureliano M. Effects of decavanadate and insulin enhancing vanadium compounds on glucose uptake in isolated rat adipocytes. J Inorg Biochem. 2009;103:1687-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |