Published online Sep 15, 2021. doi: 10.4239/wjd.v12.i9.1576
Peer-review started: May 6, 2021
First decision: July 3, 2021
Revised: July 9, 2021
Accepted: August 12, 2021
Article in press: August 12, 2021
Published online: September 15, 2021
Processing time: 123 Days and 16.8 Hours
Nonalcoholic fatty liver disease (NAFLD) is a major chronic liver disorder worldwide, and there is no established treatment for this disease. We conducted a network meta-analysis (NMA) to compare existing treatments, which include four classes of antidiabetic drugs, and examined the optimum treatments for NAFLD.
To compare the effectiveness of different treatments for NAFLD.
An NMA was conducted using Stata 14.0 (Corporation LLC, College Station, United States) and R (X64 3.6.3 version) in this study. Eligible randomized controlled trials (RCTs) were searched in the PubMed, Cochrane Library, Embase, Medline and Web of Science databases from database inception to April 2021. Two researchers independently screened the available studies in strict accordance with inclusion and exclusion criteria. The Cochrane Risk of Bias tool was used to evaluate the risk of bias of the included studies. The variables with and without dimensional differences were calculated as the standardized mean difference and weighted mean difference, respectively. An inconsistency model and “node-splitting” technique were used to test for inconsistency. Funnel plots were used to evaluate publication bias.
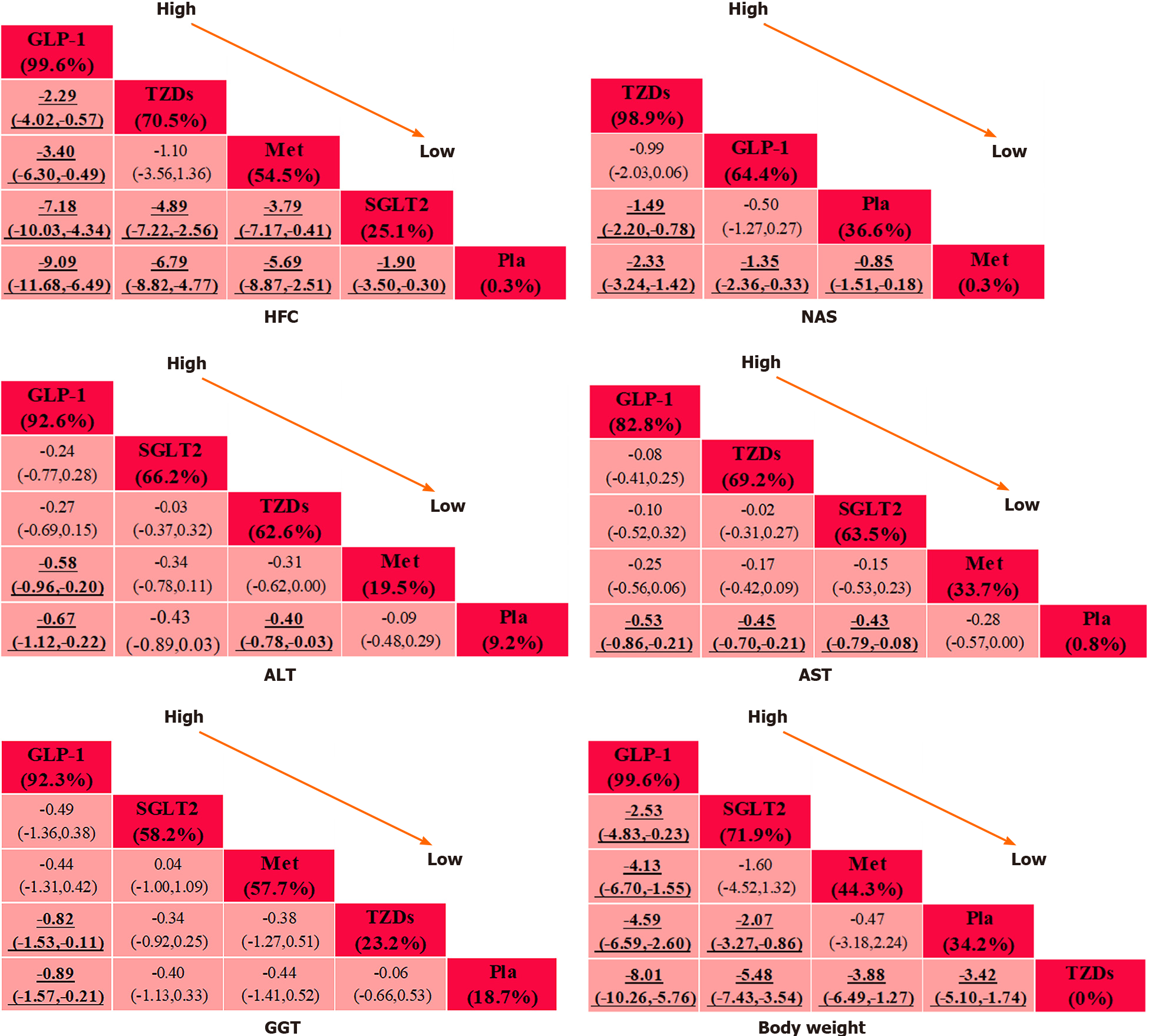
Twenty-two eligible RCTs involving 1377 participants were eventually included in our analysis. Data were pooled using a random-effects model. Our NMA results revealed that glucagon-like peptide-1 receptor agonists (GLP-1RAs) were the most effective treatment, yielding improvements in hepatic fat content (HFC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum γ-glutamyl transferase (GGT) and body weight [surface under the cumulative ranking curve (SUCRA) = 99.6%, 92.6%, 82.8%, 92.3% and 99.6%, respectively], while thiazolidinediones (TZDs) were the best intervention for reducing the NAFLD activity score (NAS; SUCRA = 98.9%). In addition, moderate performance was observed for the sodium glucose cotransporter-2 inhibitors groups (SUCRA = 25.1%, 66.2%, 63.5%, 58.2% and 71.9% for HFC, ALT, AST, GGT and body weight, respectively). However, metformin performed poorly according to most indicators (SUCRA = 54.5%, 0.3%, 19.5%, 33.7%, 57.7% and 44.3% for HFC, NAS, ALT, AST, GGT and body weight, respectively).
GLP-1RAs may be the optimum choice for most patients with NAFLD. However, TZDs are considered the most effective therapies in NAFLD patients with histological disease activity.
Core Tip: We performed a network meta-analysis and compared the effectiveness of different treatments for nonalcoholic fatty liver disease. In this study, glucagon-like peptide-1 receptor agonists and thiazolidinediones were revealed to be the best interventions for nonalcoholic fatty liver disease, and these findings could help clinicians make significant decisions in clinical practice. Furthermore, we address the possibility of using sodium glucose cotransporter-2 inhibitors in nonalcoholic fatty liver disease; however, trials with larger sample sizes are needed to obtain high-quality evidence.
- Citation: Huang YZ, Yang GY, Wang C, Chen XY, Zhang LL. Effectiveness of drug interventions in nonalcoholic fatty liver disease: A network meta-analysis. World J Diabetes 2021; 12(9): 1576-1586
- URL: https://www.wjgnet.com/1948-9358/full/v12/i9/1576.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i9.1576
Nonalcoholic fatty liver disease (NAFLD) has become one of the most common forms of chronic liver diseases worldwide and encompasses a spectrum of fatty liver diseases ranging from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis and eventually to cirrhosis and hepatocellular carcinoma[1,2]. The pathogenesis of NAFLD is not well understood; however, it has been indicated that the incidence of NAFLD often parallels the prevalence of obesity, and a large number of NAFLD patients experience metabolic disorder complications, including type 2 diabetes mellitus (T2DM), hyperlipidemia and metabolic syndrome[3,4]. These comorbidities increase the risk of adverse cardiovascular and cerebrovascular events. Therefore, it has been proposed that the term NAFLD be changed to metabolic-associated fatty liver disease for a better understanding of the disease[5]. In view of the above findings, changes to improve eating habits and lifestyle are recommended by clinicians, and this appears to be a basic strategy. To date, there have been no established pharmacotherapies for NAFLD; nonetheless, the application of antidiabetic drugs has emerged as a major therapeutic strategy.
Studies involving antidiabetic drugs in NAFLD patients have shown promising results. Thiazolidinediones (TZDs) and metformin have been confirmed to improve biochemical parameters and lipid metabolism[6,7]. Glucagon-like peptide-1 receptor agonists (GLP-1RAs), including liraglutide and exenatide, present good effects on decreasing hepatic fat content (HFC), body weight and liver enzymes. In addition, sodium glucose cotransporter-2 inhibitors (SGLT2), a new class of antidiabetic drugs, exert beneficial effects on body weight and abdominal fat area, which are accompanied by improvements in liver steatosis and fibrosis[8,9].
Although diverse interventions have been applied in an attempt to treat NAFLD, comprehensive comparisons among treatments are lacking. The aim of this network meta-analysis (NMA) research was to compare these interventions and assess drug options by analyzing the existing evidence. Based on the outcomes we defined, we identified those drugs that could improve the clinical outcomes of NAFLD. Additionally, outcomes with hierarchical ordering of interventions were determined to help clinicians make individualized treatment decisions.
The protocol of this review was registered on PROSPERO (ID: CRD42021250990). The search strategy was designed and performed separately by two researchers (Huang YZ and Zhang LL). A search for all NAFLD antidiabetic drug treatment randomized controlled trials (RCTs) was conducted in the PubMed, Cochrane Library, Embase, Medline and Web of Science databases from database inception to April 2021. Without language restriction, medical subject headings combined with free terms were conducted using “nonalcoholic fatty liver disease”, “nonalcoholic steatohepatitis”, “glucagon-like peptide-1 receptor agonists”, “metformin”, “thiazolidinediones”, “sodium glucose cotransporter-2 inhibitors”, “randomized controlled trials” and other relevant conceptual keywords.
The inclusion criteria were as follows: (1) Patients diagnosed with NAFLD; (2) Drug interventions including GLP-1RAs, metformin, TZDs or SGLT2; (3) Clearly reported outcome indicators; and (4) RCTs. The exclusion criteria were as follows: (1) Animal or cell models; (2) Duplicate articles; (3) Reviews, conference abstracts, retrospective studies or cross-sectional studies; and (4) Patients with fatty liver caused by alcohol or other known agents.
Three reviewers assessed the available studies independently (Chen XY, Wang C and Yang GY). The titles and abstracts of the obtained articles were screened, and articles that did not meet the inclusion criteria were excluded. A full-text read was implemented by the reviewers if an article met the inclusion criteria. Any discrepancies between researchers were resolved by discussion or arbitrated by an experienced investigator (Zhang LL). The predefined primary outcomes included (1) HFC; (2) NAFLD activity score (NAS); (3) Alanine aminotransferase (ALT); and (4) Aspartate aminotransferase (AST). Secondary outcomes were (1) serum γ-glutamyl transferase (GGT) and (2) body weight.
The Cochrane Risk of Bias tool was used to evaluate the risk of bias (ROB) of the included studies[10]. Seven domains of ROB were estimated to define the included studies as having a high, low, or unclear ROB, including “random sequence generation”, “allocation concealment”, “blinding of participants and personnel”, “blinding of outcome assessment”, “incomplete outcome data”, “selective reporting”, and “other bias”. The judgment of ROB was carried out by two authors separately in Review Manager (Version 5.4).
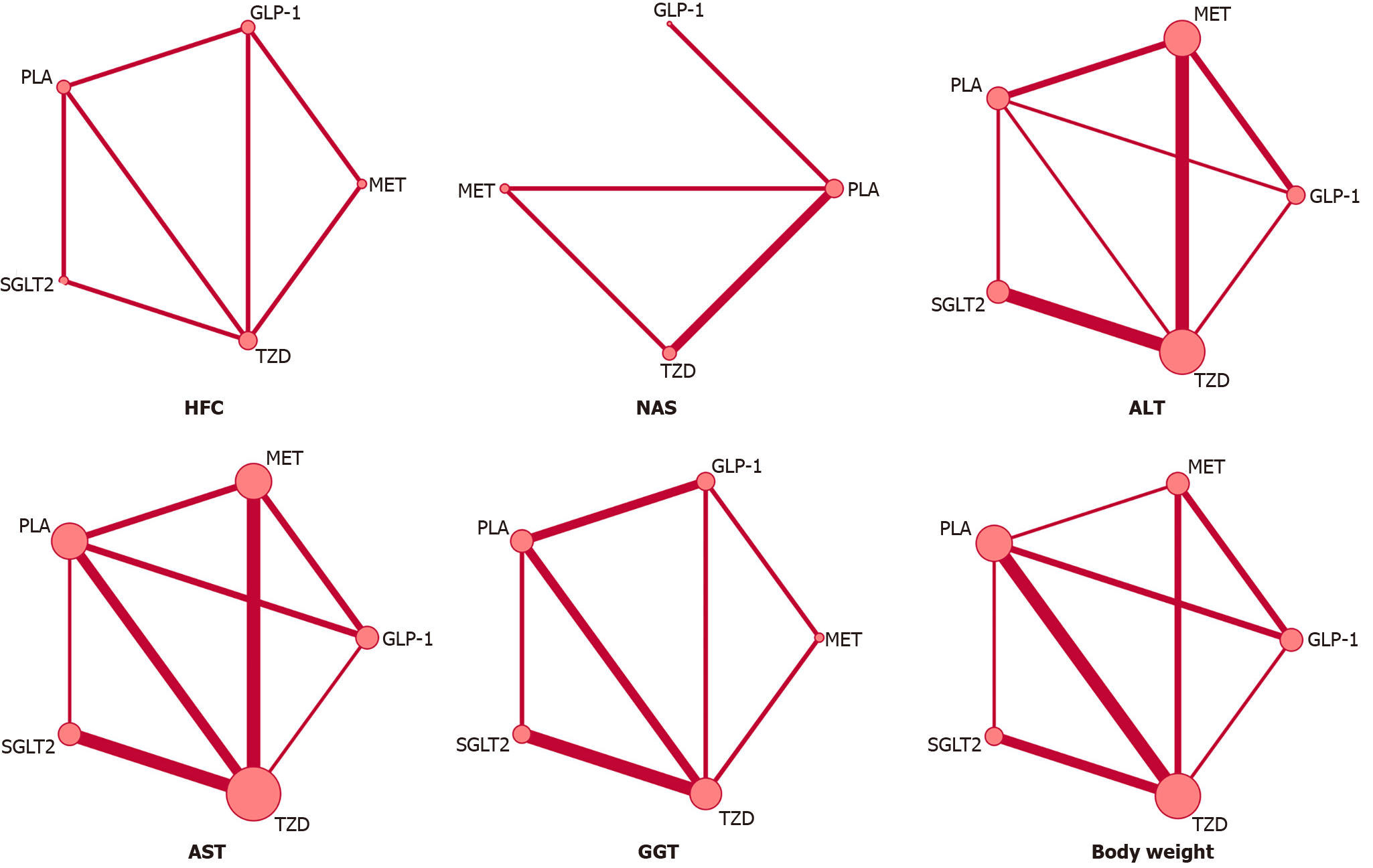
First, an inconsistency model was constructed for the measurement of global inconsistency generation, which outputs a P value. P < 0.05 was considered to indicate significant inconsistency. Then, we constructed network plots of outcome indicators to exhibit all the available evidence of each treatment (Figure 1). As the indicators were continuous variables, the variables with and without dimensional differences were calculated as the standardized mean difference (SMD) and weighted mean difference (WMD), respectively. To explore whether there was a potential source of local inconsistency in our network, the “node-splitting” technique was implemented by comparing the direct evidence to the indirect evidence from the entire network (with P value < 0.05 indicating local inconsistency). A comparison-adjusted funnel plot was constructed to evaluate publication bias. As an estimated probability used to rank the target interventions, the surface under the cumulative ranking curve (SUCRA) was displayed as a simple numerical statistical cumulative ranking probability plot for various interventions. The higher the SUCRA value, the greater the possibility of a given treatment being at the highest level or highly effective; a value of zero means that the treatment is the worst. All the analyses above were performed by Stata 14.0 (Corporation LLC, College Station, United States) and R (X64 3.6.3 version). Statistical review of this study was performed by a biomedical statistician.
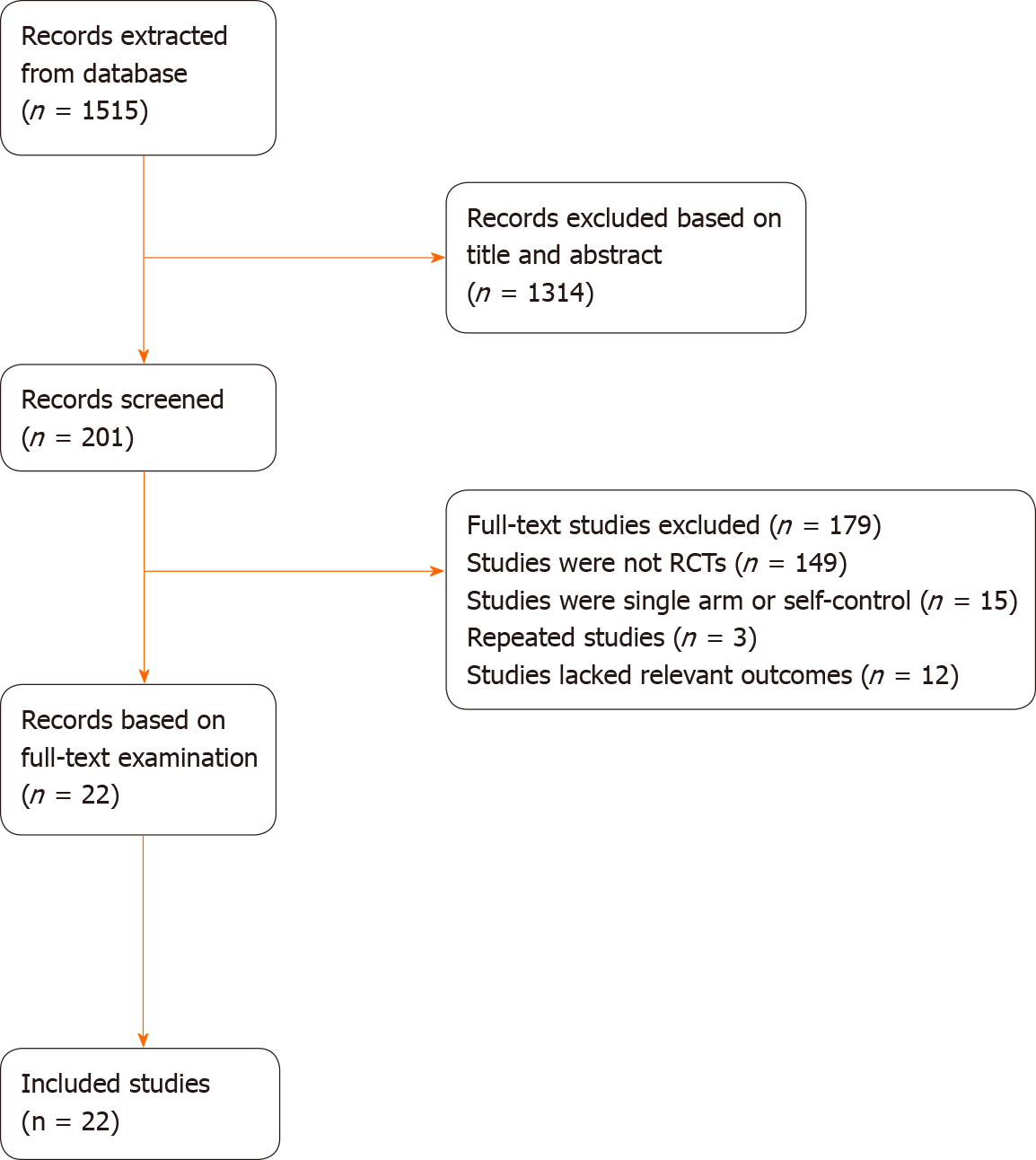
A total of 1515 records were initially screened from the database, and reading the title and abstract yielded 201 articles that were initially included. Subsequently, 179 articles were eliminated based on full-text examination: 149 articles describing studies that were not RCTs, 15 articles that involved single-arm research or self-controls with different doses in the control group, 3 articles that represented duplicate research, and 12 articles that lacked outcome indicators. Finally, only 22 studies including 1377 participants were considered eligible for this NMA. The literature selection process is shown in Figure 2.
Data were retrieved from studies published from November 2006 to February 2021. All the participants in the studies were diagnosed with NAFLD, and the duration of the trials varied from 2 mo to 24 mo (Table 1). Among the 22 included trials, all trials described in detail the generation of random sequences, 16 trials described the concealment approach, and 2 trials did not describe the blinding methods related to participants, implementers, or outcome measurers. Three trials did not have complete data, and only 1 trial exhibited selective outcome reporting. The quality assessment is shown in Supplementary Figure 1.
| Ref. | Treatment and sample size (n) | Baseline age (mean ± SD, median, range) | Treatment duration (mo) | Studying area | |
| Intervention group | Control group | ||||
| Zhang et al[12], 2020 | GLP-1RAs vs TZDs (30 vs 30) | 50.2 ± 11.5 | 51.5 ± 12.1 | 6 | China |
| Fan et al[33], 2013 | GLP-1RAs vs MET (49 vs 68) | 51.0 ± 10.1 | 54.7 ± 12.1 | 3 | China |
| Feng et al[28], 2017 | GLP-1RAs vs MET (29 vs 29) | 46.8 ± 9.7 | 46.3 ± 12.3 | 6 | China |
| Smits et al[34], 2016 | GLP-1RAs vs PLA (17 vs 17) | 60.8 ± 7.4 | 65.8 ± 5.8 | 3 | Netherlands |
| Armstrong et al[15], 2016 | GLP-1RAs vs PLA (26 vs 26) | 50.0 ± 11.0 | 52.0 ± 12.0 | 12 | United Kingdom |
| Hajiaghamohammadi et al[35], 2012 | MET vs TZDs (22 vs 22) | 32.6 ± 6.4 | 32.6 ± 6.4 | 2 | Iran |
| Razavizade et al[31], 2013 | MET vs TZDs (40 vs 40) | 36.4 ± 9.0 | 34.2 ± 6.8 | 4 | Iran |
| Shargorodsky et al[36], 2012 | MET vs PLA (32 vs 31) | 51.9 ± 10.9 | 55.2 ± 14.0 | 4 | Israel |
| Kazemi et al[37], 2011 | MET vs PLA (18 vs 15) | 41.5 (25-58) | 43.5 (26-62) | 6 | Iran |
| Haukeland et al[30], 2009 | MET vs PLA (20 vs 24) | 44.3 ± 9.0 | 49.9 ± 12.8 | 6 | Norway |
| Omer et al[29], 2010 | MET vs TZDs (22 vs 20) | 48.0 ± 9.8 | 49.3 ± 6.0 | 12 | Turkey |
| Anushiravani et al[38], 2019 | MET vs TZDs (30 vs 30) | NA | NA | 3 | Iran |
| Ito et al[9], 2017 | SGLT2 vs TZDs (32 vs 34) | 57.3 ± 12.1 | 59.1 ± 9.8 | 6 | Japan |
| Kinoshita et al[26], 2020 | SGLT2 vs TZDs (32 vs 33) | 58.7 ± 9.1 | 59.0 ± 10.9 | 7 | Japan |
| Eriksson et al[39], 2018 | SGLT2 vs PLA (21 vs 21) | 65.0 ± 6.5 | 65.6 ± 6.1 | 3 | Sweden |
| Chehrehgosha et al[8], 2021 | SGLT2 vs TZDs (35 vs 34) | 50.5 ± 8.4 | 52.5 ± 7.9 | 6 | Iran |
| Yoneda et al[27], 2021 | TZDs vs SGLT2 (19 vs 21) | 58.8 ± 8.1 | 58.4 ± 12.2 | 6 | Japan |
| Belfort et al[6], 2006 | TZDs vs PLA (26 vs 21) | 51.0 ± 7.0 | 51.0 ± 10.0 | 6 | United States |
| Ratziu et al[40], 2008 | TZDs vs PLA (32 vs 31) | 53.1 ± 11.5 | 54.1 ± 10.4 | 12 | France |
| Cusi et al[41], 2016 | TZDs vs PLA (50 vs 51) | 52.0 ± 10.0 | 49.0 ± 11.0 | 18 | United States |
| Sanyal et al[42], 2010 | TZDs vs PLA (80 vs 83) | 47.0 ± 12.6 | 45.4 ± 11.2 | 24 | United States |
| Aithal et al[43], 2008 | TZDs vs PLA (37 vs 37) | 55 (27-73) | 52 (28-71) | 12 | United Kingdom |
According the inconsistency model and “node-splitting” technique, the results regarding primary and secondary outcomes presented no statistical significance, which indicated the absence of inconsistency. Funnel plots were used to examine for publication bias, and the plots of the outcome indicators were symmetrical (Supplementary Figure 2). In addition, Begg’s test for asymmetry was applied to HFC, NAS, ALT, AST, GGT and body weight and yielded p values of 0.548, 0.669, 0.753, 0.675, 0.902 and 0.137, respectively, which confirmed the lack of publication bias.
The league plots of primary and secondary outcomes are displayed in Figure 3. Regarding the efficacy of the interventions, all the comparisons were statistically significant in the HFC set except for one comparison [TZDs vs metformin, mean difference (MD) = -1.10, confidence interval (CI) (-3.56, -1.36)]. Two comparisons had no statistical significance in the NAS set [GLP-1RAs vs placebo, MD = -0.50, CI (-1.27, 0.27); TZDs vs GLP-1RAs, MD =-0.99, CI (-2.03, 0.06)]. Three comparisons were observed to be significant in the ALT set [GLP-1RAs vs placebo, SMD = -0.67, CI (-1.12, -0.22); TZDs vs placebo, SMD = -0.40, CI (-0.78, -0.03); metformin vs GLP-1RAs, SMD = 0.58, (CI 0.20, 0.96)], and three comparisons were found to be significant in the AST set [GLP-1RAs vs placebo, SMD = -0.53, CI (-0.86, -0.22); SGLT2 vs placebo, SMD = -0.43, CI (-0.79, -0.08); TZDs vs placebo, SMD = -0.45, CI (-0.70, -0.21)]. A SUCRA line was generated to rank the hierarchy of each intervention and indicated that GLP-1RAs were the most effective treatment for the outcomes (SUCRA = 99.6%, 92.6% and 82.8% for HFC, ALT and AST, respectively). Nonetheless, TZDs were observed to satisfy rank probabilities for NAS and HFC (SUCRA = 98.9% and 70.5%, respectively) (Supplementary Figure 3).
We performed an NMA of secondary outcomes as well. Two comparisons were observed to be significant in the GGT set [GLP-1RAs vs placebo, SMD = -0.89, CI (-1.57, -0.21); TZDs vs GLP-1RAs, SMD = 0.82, CI (0.11, 1.53)]. Two comparisons had no significance for body weight [metformin vs placebo, MD = -0.47, CI (-3.18, 2.24); SGLT2 vs metformin, MD = -1.60, CI (-4.52, 1.32)]. According to the SUCRA lines, GLP-1RAs were the most effective treatment for secondary outcomes (SUCRA = 92.3% and 99.6% for GGT and body weight, respectively).
The present NMA provides important evidence supporting the use of GLP-1RAs in treating NAFLD, with effectiveness demonstrated for both primary and secondary outcomes except NAS. The probabilities of recommendation of GLP-1RAs reached a surprisingly high priority. Moreover, promising effectiveness of TZDs with regard to the NAS set was observed. These results provide useful evidence that can help clinicians prescribe individualized drugs for patients with different stages of NAFLD.
At present, NAFLD has been considered more of a hepatic manifestation of metabolic syndrome than a class of chronic liver disease due to its association with visceral obesity and insulin resistance[11,12]. In addition, NAFLD is reported to occur in 70%-90% of patients with T2DM. Therefore, antidiabetic drugs are utilized in an attempt to improve the situation for patients with NAFLD.
GLP-1RAs are a new class of glucose-lowering drugs approved for the treatment of T2DM and obesity[13,14]. The mechanism through which GLP-1RAs improve NAFLD is not only a decrease in weight but also a promotion of the ability of hepatocytes to resolve excessive lipid status through lipid transport, beta-oxidation, and de novo lipogenesis[15]. In addition, GLP-1RAs can improve NAFLD, especially HFC, through the augmentation of adiponectin levels, and a study has demonstrated that hypoadiponectinemia can induce fat deposition in the liver and the progression of fatty hepatitis[16]. As an anti-inflammatory factor, adiponectin has been proven to promote fatty acid oxidation in the liver by activating AMP-activated protein kinase[17]. However, according to the current literature, weight loss is the most important factor in NAFLD improvement[18,19]. A meta-analysis showed that weight loss ≥ 5% was associated with steatosis improvements, while weight loss ≥ 7% was correlated with improved histological disease activity[20]. In our research, GLP-1RAs presented an enormous advantage in weight loss (SUCRA = 99.6%) and achieved a significant improvement compared with other interventions, which may explain their priority being highest in other sets. Furthermore, liver cells express GLP-1R, and our previous animal study showed that liraglutide could protect against inflammatory stress by inhibiting the activation of JNK, indicating that the benefit of liraglutide treatment in NAFLD is not related solely to the net effect of weight loss[21]. However, GLP-1RAs did not appear to be the best option for NAS in this study (SUCRA = 64.4%). Among the included studies involving GLP-1RA intervention, only one study performed research on NAS[15]. Although liraglutide did not demonstrate significance for NAS, a greater proportion of patients had improvements in steatosis and hepatocyte ballooning in that trial[15]. Referencing the small population in their research, Armstrong et al[15] speculate that a significant change in NAS could be identified in a larger study. More studies on NAS are needed to verify the effectiveness of GLP-1RAs.
TZDs have been widely studied as a prospective treatment for NAFLD. The results of our NMA indicated that TZDs are beneficial for histological resolution (SUCRA = 98.9% and 70.5%, NAS and HFC, respectively), which is consistent with previous meta-analyses[22,23]. As insulin sensitizers, TZDs greatly reduce liver fat accumulation and inflammation by ameliorating insulin resistance[18,24]. Furthermore, the adhibition of TZDs increases serum adiponectin level and inhibits triglyceride synthesis in the liver. However, TZDs are not helpful for weight reduction. In fact, therapeutic use of TZDs has usually led to weight gain, which appears to conflict with the major goal of NAFLD treatment. The reason for this paradox may be the result of fat redistribution from visceral to subcutaneous adipose tissue[25]. In addition, research that directly compares GLP-1RAs and TZDs remains needed to assess their effectiveness regarding liver histology. Since the incidence of NAFLD in diabetic patients is high and the mechanisms of GLP-1RAs and TZDs are different, whether GLP-1RAs and TZDs in combination could have a synergistic effect on NAFLD warrants clinical study.
Moderate performance of SGLT2 regarding both primary and secondary outcomes was observed in this study. SGLT2 displayed great effects on weight loss and abdominal fat area; however, these effects were equivalent to those of TZDs in our included trials[8,9,26,27]. Moreover, there was no ranking of SGLT2 in the NAS set due to the lack of related research. Powerful evidence from high-quality, long-term and large-size studies is warranted to evaluate the effectiveness of SGLT2. Regarding metformin, it yielded poor results in our research. Although metformin has the ability to improve hepatic insulin sensitivity, it offers no advantage in improving HFC or liver histology compared with other interventions and placebo and no advantage in liver enzyme groups[28-31]. Nonetheless, new therapies, such as metformin combined with insulin, have presented promising effectiveness for HFC[32]. Further data from large multicenter RCTs are needed to assess its effectiveness.
Our NMA combined all the eligible direct and indirect evidence to simultaneously compare interventions in patients with NAFLD, which is the greatest advantage of our study. Furthermore, our study is significant because drug interventions for NAFLD are complex and multifaceted and no established treatments for this disease exist.
The limitations of our study need to be acknowledged. First, the duration of treatment varied from 2 mo to 24 mo, which may lead to false credibility in the endpoint assessment of patients. Second, the side effects of interventions, which may influence treatment options in clinical practice, were not analyzed in this study. Finally, potential factors that could introduce bias into our results exist.
In summary, our NMA indicated that GLP-1RAs are the optimum therapeutic approach to improve HFC, abnormally elevated liver enzymes and overweight, while TZDs are the most promising intervention to ameliorate liver inflammation. The evidence from our NMA can guide the development of clinical guidelines and thus help clinicians make individualized decisions in clinical practice. Large, multicenter prospective randomized trials with liver biopsy data regarding new classes of glucose-lowering drugs are needed to confirm our results.
Nonalcoholic fatty liver disease (NAFLD) is becoming a major chronic liver disorder worldwide. Patients with NAFLD usually experience metabolic disorder complications, including type 2 diabetes mellitus, hyperlipidemia and metabolic syndrome. However, there are no established pharmacotherapies for NAFLD.
The use of antidiabetic drugs, including thiazolidinediones (TZDs), metformin, glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium glucose cotransporter-2 inhibitors (SGLT2), has emerged as a major therapeutic strategy to treat patients with NAFLD. However, it is difficult for clinicians to decide which intervention is best for treating patients with NAFLD due to an absence of comprehensive comparisons among treatments.
In this study, we compared the effectiveness of different treatments for NAFLD. The results provide new evidence that can guide the development of clinical guidelines and thus help clinicians make individualized decisions in clinical practice.
The Cochrane Risk of Bias tool was used to assess the risk of bias of the included studies. Data analysis was performed by Stata 14.0 (Corporation LLC, College Station, United States) and R (X64 3.6.3 version) and included inconsistency modeling, the “node-splitting” technique, Begg’s test and the construction of plots of the surface under the cumulative ranking curve.
GLP-1RAs had a great advantage over other treatments in the improvement of liver enzymes and hepatic fat content (HFC), and promising effectiveness was observed with TZDs with regard to the NAFLD activity score (NAS) set. However, no ranking of SGLT2 was possible for the NAS set due to insufficient research. In addition, the side effects of these drugs were not analyzed in this study.
GLP-1RAs are the optimum therapeutic approach to improve HFC, abnormally elevated liver enzymes and overweight, while TZDs are the most promising intervention to ameliorate liver inflammation.
Large multicenter prospective randomized trials with liver biopsy data regarding new classes of glucose-lowering drugs are needed to obtain robust data and confirm our results.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdelbasset WK, Kvit K, Seetharaman RV S-Editor: Gao CC L-Editor: A P-Editor: Li X
| 1. | Petroni ML, Brodosi L, Bugianesi E, Marchesini G. Management of non-alcoholic fatty liver disease. BMJ. 2021;372:m4747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 2. | Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 999] [Article Influence: 199.8] [Reference Citation Analysis (0)] |
| 3. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3800] [Article Influence: 542.9] [Reference Citation Analysis (2)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7542] [Article Influence: 838.0] [Reference Citation Analysis (0)] |
| 5. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2212] [Article Influence: 442.4] [Reference Citation Analysis (1)] |
| 6. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 7. | Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin vs vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 487] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 8. | Chehrehgosha H, Sohrabi MR, Ismail-Beigi F, Malek M, Reza Babaei M, Zamani F, Ajdarkosh H, Khoonsari M, Fallah AE, Khamseh ME. Empagliflozin Improves Liver Steatosis and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Diabetes Ther. 2021;12:843-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 9. | Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, Akiyama Y, Morimoto Y, Noda M, Shimada A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care. 2017;40:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 10. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24860] [Article Influence: 1775.7] [Reference Citation Analysis (3)] |
| 11. | Luyckx FH, Lefebvre PJ, Scheen AJ. Non-alcoholic steatohepatitis: association with obesity and insulin resistance, and influence of weight loss. Diabetes Metab. 2000;26:98-106. [PubMed] |
| 12. | Zhang LY, Qu XN, Sun ZY, Zhang Y. Effect of liraglutide therapy on serum fetuin A in patients with type 2 diabetes and non-alcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. 2020;44:674-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 737] [Cited by in RCA: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 14. | Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, Maggioni AP, Öhman P, Poulter NR, Ramachandran A, Zinman B, Hernandez AF, Holman RR; EXSCEL Study Group. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 434] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 15. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1471] [Article Influence: 163.4] [Reference Citation Analysis (1)] |
| 16. | Arvaniti VA, Thomopoulos KC, Tsamandas A, Makri M, Psyrogiannis A, Vafiadis G, Assimakopoulos SF, Labropoulou-Karatza C. Serum adiponectin levels in different types of non alcoholic liver disease. Correlation with steatosis, necroinflammation and fibrosis. Acta Gastroenterol Belg. 2008;71:355-360. [PubMed] |
| 17. | Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3053] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 18. | Panunzi S, Maltese S, Verrastro O, Labbate L, De Gaetano A, Pompili M, Capristo E, Bornstein SR, Mingrone G. Pioglitazone and bariatric surgery are the most effective treatments for non-alcoholic steatohepatitis: A hierarchical network meta-analysis. Diabetes Obes Metab. 2021;23:980-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Byrne CD, Olufadi R, Bruce KD, Cagampang FR, Ahmed MH. Metabolic disturbances in non-alcoholic fatty liver disease. Clin Sci (Lond). 2009;116:539-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 21. | Zhang L, Yang M, Ren H, Hu H, Boden G, Li L, Yang G. GLP-1 analogue prevents NAFLD in ApoE KO mice with diet and Acrp30 knockdown by inhibiting c-JNK. Liver Int. 2013;33:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 23. | Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non-alcoholic steatohepatitis - a systematic review and meta analysis. J Hepatol. 2011;55:1383-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Miyazaki Y, Matsuda M, DeFronzo RA. Dose-response effect of pioglitazone on insulin sensitivity and insulin secretion in type 2 diabetes. Diabetes Care. 2002;25:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Shah PK, Mudaliar S, Chang AR, Aroda V, Andre M, Burke P, Henry RR. Effects of intensive insulin therapy alone and in combination with pioglitazone on body weight, composition, distribution and liver fat content in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:505-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Kinoshita T, Shimoda M, Nakashima K, Fushimi Y, Hirata Y, Tanabe A, Tatsumi F, Hirukawa H, Sanada J, Kohara K, Irie S, Kimura T, Nakamura Y, Nishioka M, Obata A, Nakanishi S, Mune T, Kaku K, Kaneto H. Comparison of the effects of three kinds of glucose-lowering drugs on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, open-label, three-arm, active control study. J Diabetes Investig. 2020;11:1612-1622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 27. | Yoneda M, Honda Y, Ogawa Y, Kessoku T, Kobayashi T, Imajo K, Ozaki A, Nogami A, Taguri M, Yamanaka T, Kirikoshi H, Iwasaki T, Kurihashi T, Saito S, Nakajima A. Comparing the effects of tofogliflozin and pioglitazone in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus (ToPiND study): a randomized prospective open-label controlled trial. BMJ Open Diabetes Res Care. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 28. | Feng W, Gao C, Bi Y, Wu M, Li P, Shen S, Chen W, Yin T, Zhu D. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes. 2017;9:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 29. | Omer Z, Cetinkalp S, Akyildiz M, Yilmaz F, Batur Y, Yilmaz C, Akarca U. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, Haaland T, Løberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 31. | Razavizade M, Jamali R, Arj A, Matini SM, Moraveji A, Taherkhani E. The effect of pioglitazone and metformin on liver function tests, insulin resistance, and liver fat content in nonalcoholic Fatty liver disease: a randomized double blinded clinical trial. Hepat Mon. 2013;13:e9270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Tang W, Xu Q, Hong T, Tong G, Feng W, Shen S, Bi Y, Zhu D. Comparative efficacy of anti-diabetic agents on nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized and non-randomized studies. Diabetes Metab Res Rev. 2016;32:200-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Fan H, Pan Q, Xu Y, Yang X. Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq Bras Endocrinol Metabol. 2013;57:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Pouwels PJ, Pieters-van den Bos IC, Hoekstra T, Diamant M, van Raalte DH, Cahen DL. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia. 2016;59:2588-2593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Hajiaghamohammadi AA, Ziaee A, Oveisi S, Masroor H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic Fatty liver disease: a randomized controlled pilot study. Hepat Mon. 2012;12:e6099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Shargorodsky M, Omelchenko E, Matas Z, Boaz M, Gavish D. Relation between augmentation index and adiponectin during one-year metformin treatment for nonalcoholic steatohepatosis: effects beyond glucose lowering? Cardiovasc Diabetol. 2012;11:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Kazemi R, Aduli M, Sotoudeh M, Malekzadeh R, Seddighi N, Sepanlou SG, Merat S. Metformin in nonalcoholic steatohepatitis: a randomized controlled trial. Middle East J Dig Dis. 2012;4:16-22. [PubMed] |
| 38. | Anushiravani A, Haddadi N, Pourfarmanbar M, Mohammadkarimi V. Treatment options for nonalcoholic fatty liver disease: a double-blinded randomized placebo-controlled trial. Eur J Gastroenterol Hepatol. 2019;31:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, Miliotis T, Forsberg GB, Risérus U, Lind L, Oscarsson J. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923-1934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 40. | Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E, Grimaldi A, Poynard T; LIDO Study Group. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 479] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 41. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 42. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2473] [Article Influence: 164.9] [Reference Citation Analysis (2)] |
| 43. | Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, Austin AS, Freeman JG, Morgan L, Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 556] [Article Influence: 32.7] [Reference Citation Analysis (0)] |