Published online Sep 15, 2021. doi: 10.4239/wjd.v12.i9.1507
Peer-review started: January 22, 2021
First decision: May 3, 2021
Revised: May 11, 2021
Accepted: July 15, 2021
Article in press: July 15, 2021
Published online: September 15, 2021
Processing time: 227 Days and 14.2 Hours
The escalating global burden of type 2 diabetes mellitus necessitates the implementation of strategies that are both more reliable and faster in order to improve the early identification of insulin resistance (IR) in high-risk groups, including overweight and obese individuals. The use of salivary biomarkers offers a promising alternative to serum collection because it is safer, more comfortable, and less painful to obtain saliva samples. As obesity is the foremost contributory factor in IR development, the adipocytokines such as leptin, adiponectin, resistin, and visfatin secreted from the adipose tissue have been studied as potential reliable biomarkers for IR. Measurement of salivary adipokines as predictors for IR has attracted widespread attention because of the strong correlation between their blood and salivary concentrations. One of the adipokines that is closely related to IR is resistin. However, there are conflicting findings on resistin’s potential role as an etiological link between obesity and IR and the reliability of measuring salivary resistin as a biomarker for IR. Hence this study reviewed the available evidence on the potential use of salivary resistin as a biomarker for IR in order to attempt to gain a better understanding of the role of resistin in the development of IR in obese individuals.
Core Tip: The worldwide increased prevalence of obesity-induced insulin resistance (IR) highlights the limitations of the long-term, invasive methods currently being used in detecting and monitoring IR. Measurement of salivary concentrations of adipokines such as resistin offers a good alternative to serum collection for early detection and monitoring of glycaemic control among obese individuals. However, there are conflicting findings on the association between resistin and IR. Hence this review of the available evidence aims to provide a better understanding of the role of resistin in the development of IR and the potential use of salivary resistin as a biomarker for IR.
- Citation: Abdalla MMI. Salivary resistin level and its association with insulin resistance in obese individuals. World J Diabetes 2021; 12(9): 1507-1517
- URL: https://www.wjgnet.com/1948-9358/full/v12/i9/1507.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i9.1507
The World Health Organization reported a marked increase in the number of diabetic patients from 108 million in 1980 to 422 million in 2014[1]. There was also a 5% increase in premature mortality from diabetes between 2000 and 2016, and the World Health Organization estimated that diabetes was the seventh leading cause of death in 2016[1]. More recently, in 2020, an epidemiological study estimated that 462 million individuals or 6.28% of the global population are affected by type 2 diabetes mellitus (T2DM), a condition that seems to be more prevalent in developed countries despite the promotion and implementation of a range of public health measures[2].
T2DM is a non-insulin-dependent type of diabetes that was previously largely considered to be a disease of middle and old age. However, during recent decades, there has been a global rise in the prevalence of T2DM among children and young adults[3,4]. This rise has coincided with an increased prevalence of obesity, the foremost contributory factor to insulin resistance (IR) and T2DM[5,6]. The earlier the onset of the disease, the longer its duration and the higher the incidence of complications, which subsequently leads to higher mortality among the younger generation[7].
T2DM is characterized by increased insulin secretion, IR, and impaired glucose tolerance[8]. Early detection of impaired glycaemic control among prediabetics as well as maintenance of good control of the blood glucose level is, therefore, crucial in reducing mortality and delaying the onset of complications[9]. The glucose tolerance test and measurement of glycated haemoglobin are the methods most commonly used for early detection of IR in high-risk individuals[10]. However, the global burdens of T2DM and obesity highlight the limitations of the long-term, invasive screening methods currently employed in the early identification of individuals at high-risk of these two conditions.
The chronic inflammatory state in obesity is known to be a significant pathogenic mechanism for obesity-associated complications[11]. Several polypeptides known as adipokines or pro-inflammatory cytokines that are secreted from adipocytes and adipose tissue macrophages have been found to play a role in inflammatory response as well as in the regulation of energy balance, food intake, and insulin sensitization[12]. Among the known adipokines, adiponectin, leptin, resistin, interleukin-6, tumour necrosis factor-alpha, plasminogen activator inhibitor-1, and monocyte chemo attractant protein-1 have been found to be directly related to the pathogenesis of IR in obesity[13].
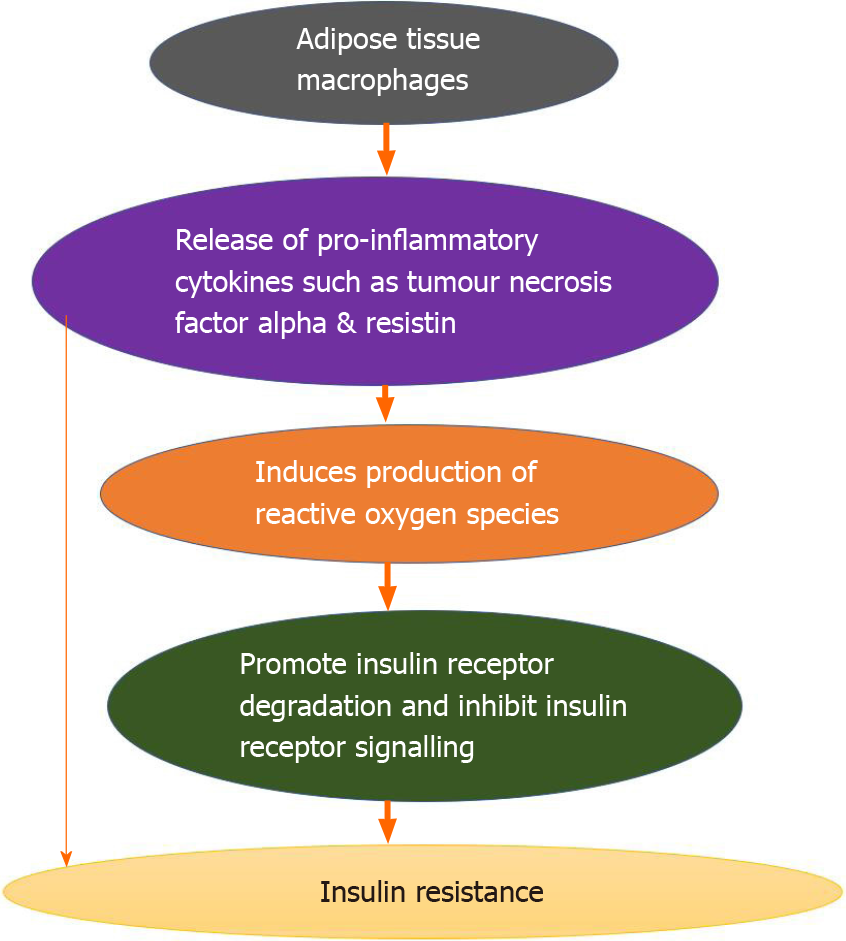
Moreover, the chronic inflammatory state in obesity is found to induce a state of oxidative stress that is caused by enhanced production of reactive oxygen species, which is induced by pro-inflammatory cytokines such as tumour necrosis factor-alpha and resistin. It has therefore been suggested that a combination of inflammation and oxidative stress is involved in the process of the pathogenesis of IR[14], as illustrated in Figure 1.
The chronic inflammation, macrophage infiltration, increased leptin level, decreased adiponectin, mitochondrial dysfunction, endoplasmic reticulum stress, and adipocyte apoptosis could be attributed to the adipose tissue hypoxia response and adipocyte dysfunction[15], which are found to be associated with the downregulation of insulin receptors resulting in systemic IR[16]. In addition, the inflammation and oxidative stress associated with obesity may be attributed to the aging of the adipose tissue. This process includes molecular changes in the cells, such as deactivation of P53 tumour suppressor and inflammation[17,18]. Oxidative stress is also associated with endoplasmic reticulum stress, which occurs due to excess nutrient intake in obesity[19].
Even with little macrophage infiltrates, lipotoxicity caused due to excess and ectopic fat accumulation in adipocytes, liver, and muscle is found to damage the pancreatic beta cells, leading to T2DM[20]. In addition, lipid overload leads to cellular dysfunction, endoplasmic reticulum stress, activation of pro-inflammatory stress pathways, and occurrence of IR, which may be attributed to the increased production of biologically active lipid intermediates such as ceramides and diacylglycerol[21-23].
Insulin, a pleiotropic peptide secreted by beta cells in the pancreatic islets, regulates the blood glucose level by increasing glucose uptake and utilization in muscles and adipose tissues through stimulating the translocation of glucose transporter 4 to the plasma membrane, inhibiting glucose production in the liver through inhibiting the expression of key gluconeogenic enzymes and promoting lipolysis[24]. The effects of insulin are mediated by the binding of insulin-to-insulin receptors and insulin-like growth factor-1 receptors, which results in phosphorylation of the receptor substrate, followed by activation of the intracellular signalling pathways phosphoinositide 3-kinase/protein kinase B pathway and the mitogen-activated protein kinase pathways[25].
IR is a disease condition in which insulin-dependent cells, such as those found in skeletal muscle, the liver, and adipocytes, are unable to respond properly to the normal circulatory levels of insulin[14]. This inability to respond results in hyperglycaemia, which is caused by decreased removal of glucose from the blood and by increased production of glucose in the liver, the latter of which is associated with decreased fatty acid release from adipose tissues[26].
Studies have proved the usefulness of assessing the serum levels of many of the adipokines, including resistin and adiponectin, as biomarkers for IR[9,27]. The positive correlation between serum and salivary proteome levels[28,29] has attracted attention because of the implication that salivary biomarkers could be used in preference to serum due to the potential benefits of the former in reducing the suffering, pain, and stress associated with serum sampling[30]. The use of salivary biomarkers in diabetes is further supported by the fact that the increased permeability of the basement membrane in diabetes is associated with increased leakage of proteins from serum into saliva[30,31].
Resistin is a cysteine-rich polypeptide that was discovered by Steppan et al[32]. It has been proposed that resistin is a potential link between obesity and T2DM because it is upregulated in rodent models of obesity and IR and downregulated by insulin sensitizers. Resistin is also known as an adipose-tissue-specific secretory factor, which in humans is encoded by the RETN gene located on chromosome 19[33].
The normal serum level of human resistin ranges from 7 to 22 ng/mL[34]. There are two circulating forms of resistin: high molecular weight resistin, which is the predominant form, and low molecular weight resistin, which is the bioactive form in which bioactivity is initiated by disulphide cleavage in its hexametric structure[35].
Human resistin is quite different from rodent resistin in terms of both its structure and distribution. Murine resistin is a 114 amino acid polypeptide that is produced primarily in white adipose tissue[36], whereas human resistin is a 108 amino acid polypeptide expressed by adipose tissue, particularly visceral fat, pre-adipocytes, adipocytes[37,38], peripheral blood mononuclear cells[39], skeletal muscle[40], the pancreas[41], hypothalamus, adrenal gland, spleen, bone marrow, gastrointestinal tract, lungs[42], pituitary gland[43], and placenta[44]. Here it is worth mentioning that several studies have proved the role of peripheral blood mononuclear cells – the primary producers of human resistin[39,45] – in the inflammatory process involved in the pathogenesis of obesity-induced IR[46,47]. The dissimilar genetic organization of murine and human resistin[48] may be the reason for the conflicting findings on the potential role of resistin as an aetiological link between obesity and diabetes reported in studies on murine vs human resistin.
Since the discovery of resistin two decades ago, many research studies have been conducted on humans and rodents in order to investigate its potential role as a link between obesity and IR. Higher circulating levels of resistin have been reported in murine and rodent models of obesity compared to lean[32,49], and higher circulating levels of resistin have also been reported in obese individuals compared with lean[50,51] and positively correlated with body mass index (BMI) and visceral fat[51,52]. The increase in resistin in obese rodents may represent a negative feedback mechanism that acts to control adipocyte differentiation[49]. It has been suggested that an increase in the accumulation of adipose tissue, which reflects an increase in adipocyte differentiation and the pool of adipocytes, results in an increase in the secretion of resistin from the adipocytes, where the secreted resistin acts as a paracrine polypeptide that autoregulates its secretion by inhibiting adipocyte differentiation[53]. It has also been reported that resistin increases in parallel with increases in insulin and glucose and decreases in parallel with their decrease, hence the serum level of resistin seems to be regulated by the levels of insulin and glucose[53]. Other regulators of resistin include age, gender, thyroid hormones, and gonadal hormones[54].
Human studies have revealed contradictory findings on the correlation between circulating resistin and IR in T2DM and obesity. Some studies have reported a positive correlation[55-60], whereas others have found a negative[61] or lack of correlation[62,63].
A recent systematic review and meta-analysis conducted in 2019 on the correlation between serum resistin and IR in T2DM and obesity concluded that, overall, the results were in favour of there being a positive correlation between circulating resistin and IR in T2DM and obese individuals with hyperresistinaemia but not in those with normal circulating levels of resistin[64], which implies that resistin needs to reach a certain critical level to cause IR[60]. The meta-analysis was performed on 15 studies that were undertaken during the period 2005–2017 and involved a total of 1227 patients of diverse age, gender, and ethnicity. In the meta-analysis, these patients were classified into 20 clinical groups: 10 with simple T2DM, 7 with simple obesity, 2 with T2DM and obesity, and 1 group of T2DM patients with or without obesity[64]. The difference in resistin concentration among these different groups, which led to conflicting results on the association between resistin and IR, may be explained by the several single nucleotide polymorphisms of the resistin RETN gene in the different ethnic groups studied[65], differences in the levels of insulin and leptin, which have been found to stimulate resistin expression[66], and differences in the methods used to assess resistin levels, specifically, commercially available enzyme-linked immuno
Overexpression of resistin in transgenic mice has been found to result in the im
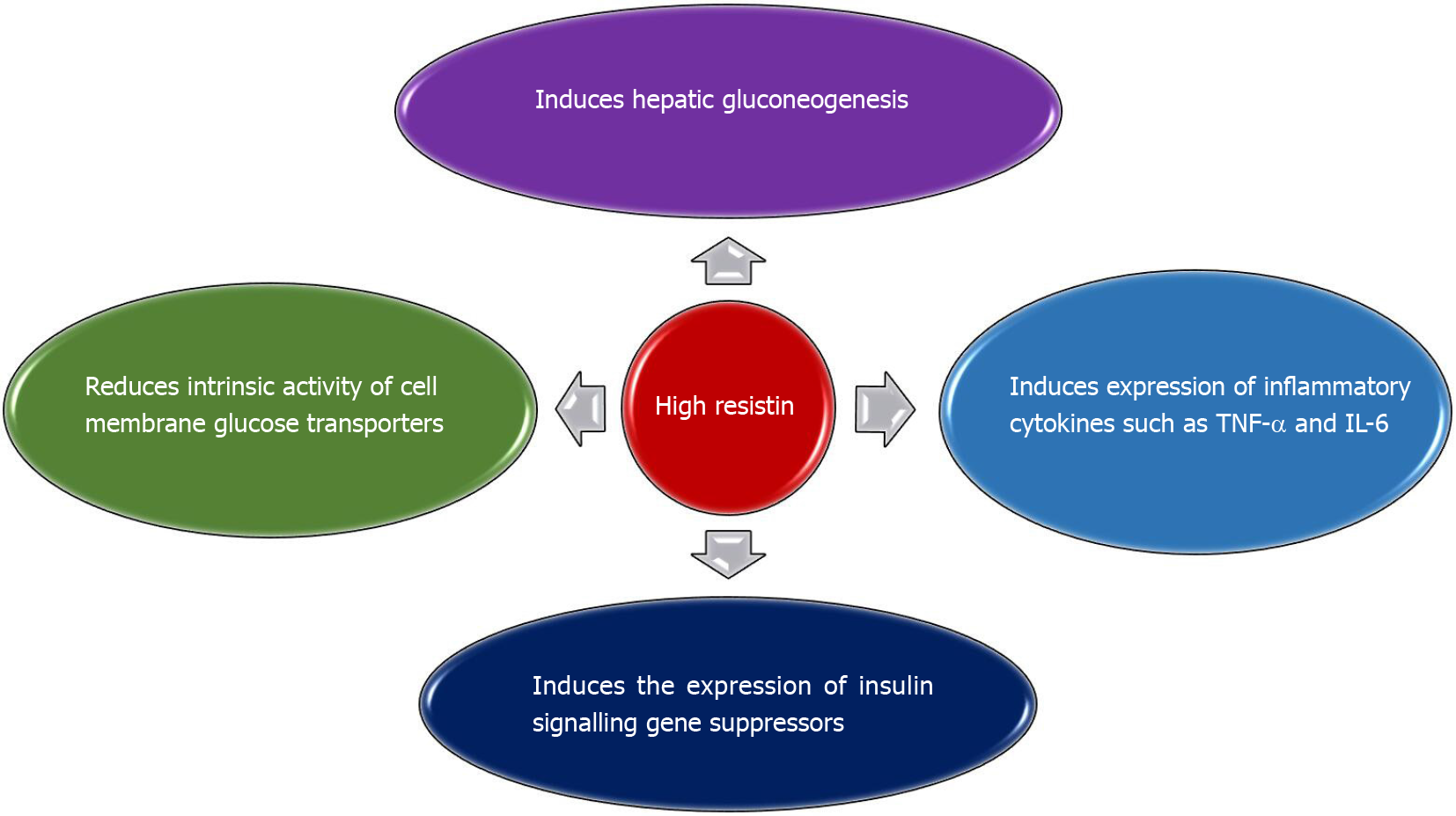
High levels of resistin stimulate the expression of tumour necrosis factor-alpha and interleukin-6 in both human and murine macrophages via the NF-kB-dependent pathway, which results in IR[73]. In addition, an increased resistin level leads to leptin resistance[74], which contributes to the development of IR[75], as illustrated in Figure 2.
Due to the increased global and economic burden of T2DM, IR, and obesity across age groups, including children and young adults, the need for effective, easy, and non-invasive methods for early detection and monitoring of IR has increased. The use of saliva as a diagnostic tool is evolving not only due to the rapid advances that are being made in the fields of nanotechnology and molecular diagnostics, but also because saliva contains biomarkers that are ideal for early detection and monitoring of oral as well as systemic diseases[30,76].
To review the available evidence on the association between salivary resistin and IR in obese individuals, the scientific literature published up to 31 December 2020 was searched in the following databases: PubMed, ProQuest, Scopus, Ovid, Science Direct, Springer Link, and Trip. The search was limited to papers published in the English language. A search of the references in the identified papers was also done to identify any additional relevant papers. At the end of the search process, a total of six papers were identified for review. These papers, which were published between 2011 and 2020, are discussed in ascending chronological order.
The first selected study, conducted by Mamali et al[77] in Patras, Greece and published in 2011, involved the measurement of resistin in saliva and an assessment of the association between its salivary and serum levels. Salivary and serum resistin was measured using a commercial enzyme immunoassay method. Samples were measured in duplicate. Serum samples were diluted five-fold while saliva samples were diluted three-fold. The study reported a strong positive correlation between the serum and salivary levels of resistin (r = 0.441, P = 0.003) with no significant correlation between their levels with age, body fat percentage, or BMI. The ratio of the serum level of resistin to its salivary level was 0.2[77]. The positive correlation between the salivary and serum levels of resistin that was reported indicated that resistin was transported from the blood to saliva, which supports the potential use of the salivary levels of resistin rather than its serum levels for early detection of IR[78]. The absence of a correlation between the salivary as well as the serum levels of resistin with age, BMI, and body fat percentage could be attributed to the characteristics of the participants, who were healthy with almost normal BMI and body fat. It could also be attributed to the measurement method that was used.
A positive correlation between saliva and serum levels of resistin was further evidenced in a 2012 study conducted in China by Yin et al[29] who investigated for the first time the differences in the serum and salivary levels of resistin in a sample of 38 patients who were newly diagnosed with T2DM (18 males/20 females) compared with a control group of 35 non-diabetic individuals (18 males/17 females). The study revealed a significantly higher level of serum resistin as compared with salivary resistin in both diabetic and control groups. It also found significantly higher levels of both serum and salivary resistin in T2DM patients as compared with the control group. Furthermore, the study revealed significant correlations between salivary resistin and BMI (r = 0.39), glycated haemoglobin (r = 0.31) and the homeostatic model assessment of IR (r = 0.20)[29]. Collectively, these findings along with the presence of a consistent fluctuating trend of salivary and serum resistin levels during the oral glucose tolerance test provide evidence that indicates that the source of resistin in the saliva of newly diagnosed T2DM is mainly derived from the blood[29] rather than from local production by the salivary glands[79].
The above findings are further supported by a study conducted by Sarhat et al[80] in Iraq in which a significantly higher concentration of resistin was found in the saliva of patients with T2DM as compared with a healthy control group.
In 2017, Al-Rawi and Al-Marzooq[81] undertook a study in the United Arab Emirates to assess concentrations of resistin in the saliva of 26 obese diabetics, 26 obese non-diabetics, and 26 non-obese non-diabetics. The study found no difference in resistin concentration between obese diabetics (14.7 ± 2.8 ng/mL) and obese non-diabetics (14.4 ± 3.6 ng/mL), but the concentration level in these two groups was significantly higher than in non-obese non-diabetics (10.8 ± 6.1 ng/mL, P = 0.01). The study also reported a significant correlation between salivary resistin and BMI. However, there was no correlation between salivary resistin and glucose level[81].
A study by Srinivasan et al[82] in 2018 in the United States assessed not only the level of resistin, but also the levels of adiponectin, visfatin, and ghrelin in unstimulated whole saliva as biomarkers for T2DM. The study involved two groups: 20 periodontally healthy patients with self-reported T2DM and a control group of 20 individuals with no known oral or systemic diseases. Salivary resistin was measured using an enzyme-linked immunosorbent assay kit. The study found that the glycated haemo
More recently, in 2020, Gürlek and Çolak[83] investigated the effectiveness of evaluating salivary resistin concentrations as a screening marker for gestational diabetes mellitus (GDM). Gestational diabetes mellitus is a type of diabetes that occurs during pregnancy with an incidence that varies from 1% to 25%[84,85]. Studies have revealed that GDM is associated with decreased insulin sensitivity and release of pro-inflammatory cytokines such as resistin[86]. Also, a recent meta-analysis supported the use of serum resistin concentrations to screen for GDM[87]. Studies have also shown that the risk of GDM is far higher among overweight and obese pregnant women, especially those with central obesity[88-90]. Gürlek and Çolak[83] included 81 pregnant women in their study: 41 with newly diagnosed GDM and 40 with normal pregnancy without GDM. Fasting blood and unstimulated saliva samples were collected, and resistin was estimated using an enzyme-linked immunosorbent assay. The study revealed that pregnant women with GDM had significantly higher pre-gestational BMI, BMI at the time of sampling, and salivary resistin concentrations as compared with the pregnant women without GDM. Hence their study provided evidence for the first time that resistin concentrations in saliva might be a useful screening marker for GDM[83].
A summary of the findings related to the concentration of resistin in saliva and its correlation with BMI, homeostatic model assessment of IR, and blood glucose level are presented in Table 1.
| Study group | BMI | Salivary resistin (ng/mL) | Correlation with BMI | Correlation with blood glucose | Correlation with HOMA-IR | Correlation test used | Ref. |
| Healthy | 22.39 ± 3.65 | 1.69 | NS | - | - | Partial correlation | Mamali et al[77] |
| T2DM | 25.5 ± 4.9 | 3.4± 0.41 | -0.391 | -0.14 | -0.201 | Pearson’s test | Yin et al[29] |
| Control | 23.9 ± 3.3 | 1.5 ± 0.3 | -0.17 | -0.281 | -0.19 | ||
| T2DM | - | 4 ± 0.451 | - | - | - | - | Sarhat et al[80] |
| Control | - | 1.73 ± 0.34 | - | - | - | - | |
| Obese diabetic | 34.3 ± 3.9 | 14.7 ± 2.8 | Significant | NS | Al-Rawi and Al-Marzooq[81] | ||
| Obese non-diabetic | 34.2 ± 2.9 | 14.4 ± 3.6 | Significant | ||||
| Control “Non-obese-non -diabetic | 7.1 ± 2.1 | 10.8 ± 6.1 | Significant | - | |||
| T2DM | - | 9.2 ± 2.31 | - | - | - | Srinivasan et al[82] | |
| Control | - | 5.7 ± 1.3 | - | - | - | ||
| GDM | 32 (20.4–43.7) | 13.11 | - | - | - | Gürlek and Çolak[83] | |
| Control | 27.2 (19.4–42) | 5.9 | - | - | - |
The available evidence indicates that resistin plays a role in the development of IR. Prior studies also support the use of serum resistin as a reliable marker for IR. The evidence also supports the potential use of salivary resistin as a reliable biomarker for IR. However, the number of studies that assessed the correlation of salivary resistin with IR among obese, newly diagnosed T2DM patients is still limited.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Geng TY S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Liu JH
| 1. | World Health Organization. Global report on diabetes. 2016. Available from: https://apps.who.int/iris/handle/10665/204871. |
| 2. | Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 1637] [Article Influence: 409.3] [Reference Citation Analysis (2)] |
| 3. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3375] [Article Influence: 482.1] [Reference Citation Analysis (0)] |
| 4. | Molnár D. The prevalence of the metabolic syndrome and type 2 diabetes mellitus in children and adolescents. Int J Obes Relat Metab Disord. 2004;28 Suppl 3:S70-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Caceres M, Teran CG, Rodriguez S, Medina M. Prevalence of insulin resistance and its association with metabolic syndrome criteria among Bolivian children and adolescents with obesity. BMC Pediatr. 2008;8:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Pulungan AB, Puspitadewi A, Sekartini R. Prevalence of insulin resistance in obese adolescents. Paediatr Indones. 2013;53:167-172. |
| 7. | Huo L, Magliano DJ, Rancière F, Harding JL, Nanayakkara N, Shaw JE, Carstensen B. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997-2011. Diabetologia. 2018;61:1055-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Meigs JB. Multiple biomarker prediction of type 2 diabetes. Diabetes Care. 2009;32:1346-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Faerch K, Borch-Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009;52:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 1443] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 12. | Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 532] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 13. | Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol. 2019;234:8152-8161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 554] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 15. | Wondmkun YT. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab Syndr Obes. 2020;13:3611-3616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 368] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 16. | Arcidiacono B, Chiefari E, Foryst-Ludwig A, Currò G, Navarra G, Brunetti FS, Mirabelli M, Corigliano DM, Kintscher U, Britti D, Mollace V, Foti DP, Goldfine ID, Brunetti A. Obesity-related hypoxia via miR-128 decreases insulin-receptor expression in human and mouse adipose tissue promoting systemic insulin resistance. EBioMedicine. 2020;59:102912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Ahima RS. Connecting obesity, aging and diabetes. Nat Med. 2009;15:996-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Liu Z, Wu KKL, Jiang X, Xu A, Cheng KKY. The role of adipose tissue senescence in obesity- and ageing-related metabolic disorders. Clin Sci (Lond). 2020;134:315-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 19. | Burgos-Morón E, Abad-Jiménez Z, Marañón AM, Iannantuoni F, Escribano-López I, López-Domènech S, Salom C, Jover A, Mora V, Roldan I, Solá E, Rocha M, Víctor VM. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 354] [Article Influence: 59.0] [Reference Citation Analysis (1)] |
| 20. | Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci USA. 1994;91:10878-10882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 622] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 21. | Chaurasia B, Summers SA. Ceramides - Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol Metab. 2015;26:538-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 456] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 22. | Han J, Kaufman RJ. The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res. 2016;57:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 461] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 23. | Hauck AK, Bernlohr DA. Oxidative stress and lipotoxicity. J Lipid Res. 2016;57:1976-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 24. | Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. 2011;93 Suppl 1:S52-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 385] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 25. | Ieronymaki E, Daskalaki MG, Lyroni K, Tsatsanis C. Insulin Signaling and Insulin Resistance Facilitate Trained Immunity in Macrophages Through Metabolic and Epigenetic Changes. Front Immunol. 2019;10:1330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med. 2016;280:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 368] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 27. | Lau CH, Muniandy S. Novel adiponectin-resistin (AR) and insulin resistance (IRAR) indexes are useful integrated diagnostic biomarkers for insulin resistance, type 2 diabetes and metabolic syndrome: a case control study. Cardiovasc Diabetol. 2011;10:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Loo JA, Yan W, Ramachandran P, Wong DT. Comparative human salivary and plasma proteomes. J Dent Res. 2010;89:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 266] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 29. | Yin J, Gao H, Yang J, Xu L, Li M. Measurement of salivary resistin level in patients with type 2 diabetes. Int J Endocrinol. 2012;2012:359724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Desai GS, Mathews ST. Saliva as a non-invasive diagnostic tool for inflammation and insulin-resistance. World J Diabetes. 2014;5:730-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 31. | Williamson S, Munro C, Pickler R, Grap MJ, Elswick RK Jr. Comparison of biomarkers in blood and saliva in healthy adults. Nurs Res Pract. 2012;2012:246178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 32. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3205] [Cited by in RCA: 3208] [Article Influence: 133.7] [Reference Citation Analysis (1)] |
| 33. | Steppan CM, Lazar MA. The current biology of resistin. J Intern Med. 2004;255:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 34. | Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304:1154-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 36. | Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, Scherer PE, Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Fain JN, Cheema PS, Bahouth SW, Lloyd Hiler M. Resistin release by human adipose tissue explants in primary culture. Biochem Biophys Res Commun. 2003;300:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | McTernan PG, McTernan CL, Chetty R, Jenner K, Fisher FM, Lauer MN, Crocker J, Barnett AH, Kumar S. Increased resistin gene and protein expression in human abdominal adipose tissue. J Clin Endocrinol Metab. 2002;87:2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 676] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 40. | Dietze D, Koenen M, Röhrig K, Horikoshi H, Hauner H, Eckel J. Impairment of insulin signaling in human skeletal muscle cells by co-culture with human adipocytes. Diabetes. 2002;51:2369-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Minn AH, Patterson NB, Pack S, Hoffmann SC, Gavrilova O, Vinson C, Harlan DM, Shalev A. Resistin is expressed in pancreatic islets. Biochem Biophys Res Commun. 2003;310:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Nohira T, Nagao K, Kameyama K, Nakai H, Fukumine N, Okabe K, Kitano S, Hisatomi H. Identification of an alternative splicing transcript for the resistin gene and distribution of its mRNA in human tissue. Eur J Endocrinol. 2004;151:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Morash BA, Ur E, Wiesner G, Roy J, Wilkinson M. Pituitary resistin gene expression: effects of age, gender and obesity. Neuroendocrinology. 2004;79:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Yura S, Sagawa N, Itoh H, Kakui K, Nuamah MA, Korita D, Takemura M, Fujii S. Resistin is expressed in the human placenta. J Clin Endocrinol Metab. 2003;88:1394-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 329] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 46. | Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789-5795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 723] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 47. | Kapłon-Cieślicka A, Postuła M, Rosiak M, Peller M, Kondracka A, Serafin A, Trzepla E, Opolski G, Filipiak KJ. Association of adipokines and inflammatory markers with lipid control in type 2 diabetes. Pol Arch Med Wewn. 2015;125:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Ghosh S, Singh AK, Aruna B, Mukhopadhyay S, Ehtesham NZ. The genomic organization of mouse resistin reveals major differences from the human resistin: functional implications. Gene. 2003;305:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Asensio C, Cettour-Rose P, Theander-Carrillo C, Rohner-Jeanrenaud F, Muzzin P. Changes in glycemia by leptin administration or high- fat feeding in rodent models of obesity/type 2 diabetes suggest a link between resistin expression and control of glucose homeostasis. Endocrinology. 2004;145:2206-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Vendrell J, Broch M, Vilarrasa N, Molina A, Gómez JM, Gutiérrez C, Simón I, Soler J, Richart C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 380] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 51. | Degawa-Yamauchi M, Bovenkerk JE, Juliar BE, Watson W, Kerr K, Jones R, Zhu Q, Considine RV. Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab. 2003;88:5452-5455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 330] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 52. | Yannakoulia M, Yiannakouris N, Blüher S, Matalas AL, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab. 2003;88:1730-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 291] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 53. | Rajala MW, Qi Y, Patel HR, Takahashi N, Banerjee R, Pajvani UB, Sinha MK, Gingerich RL, Scherer PE, Ahima RS. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53:1671-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 54. | Nogueiras R, Gualillo O, Caminos JE, Casanueva FF, Diéguez C. Regulation of resistin by gonadal, thyroid hormone, and nutritional status. Obes Res. 2003;11:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Al-Harithy RN, Al-Ghamdi S. Serum resistin, adiposity and insulin resistance in Saudi women with type 2 diabetes mellitus. Ann Saudi Med. 2005;25:283-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Jung HS, Youn BS, Cho YM, Yu KY, Park HJ, Shin CS, Kim SY, Lee HK, Park KS. The effects of rosiglitazone and metformin on the plasma concentrations of resistin in patients with type 2 diabetes mellitus. Metabolism. 2005;54:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Mojiminiyi OA, Abdella NA. Associations of resistin with inflammation and insulin resistance in patients with type 2 diabetes mellitus. Scand J Clin Lab Invest. 2007;67:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Tokuyama Y, Osawa H, Ishizuka T, Onuma H, Matsui K, Egashira T, Makino H, Kanatsuka A. Serum resistin level is associated with insulin sensitivity in Japanese patients with type 2 diabetes mellitus. Metabolism. 2007;56:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Mabrouk R, Ghareeb H, Shehab A, Omar K, El-Kabarity RH, Soliman DA, Mohamed NA. Serum visfatin, resistin and IL-18 in A group of Egyptian obese diabetic and non diabetic individuals. Egypt J Immunol. 2013;20:1-11. [PubMed] |
| 60. | Zaidi SI, Shirwany TA. Relationship of serum resistin with insulin resistance and obesity. J Ayub Med Coll Abbottabad. 2015;27:552-555. [PubMed] |
| 61. | Bu J, Feng Q, Ran J, Li Q, Mei G, Zhang Y. Visceral fat mass is always, but adipokines (adiponectin and resistin) are diversely associated with insulin resistance in Chinese type 2 diabetic and normoglycemic subjects. Diabetes Res Clin Pract. 2012;96:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Owecki M, Miczke A, Nikisch E, Pupek-Musialik D, Sowiński J. Serum resistin concentrations are higher in human obesity but independent from insulin resistance. Exp Clin Endocrinol Diabetes. 2011;119:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Park H, Hasegawa G, Obayashi H, Fujinami A, Ohta M, Hara H, Adachi T, Tamaki S, Nakajima Y, Kimura F, Ogata M, Fukui M, Yoshikawa T, Nakamura N. Relationship between insulin resistance and inflammatory markers and anti-inflammatory effect of losartan in patients with type 2 diabetes and hypertension. Clin Chim Acta. 2006;374:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Su KZ, Li YR, Zhang D, Yuan JH, Zhang CS, Liu Y, Song LM, Lin Q, Li MW, Dong J. Relation of Circulating Resistin to Insulin Resistance in Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. Front Physiol. 2019;10:1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 65. | Hivert MF, Manning AK, McAteer JB, Dupuis J, Fox CS, Cupples LA, Meigs JB, Florez JC. Association of variants in RETN with plasma resistin levels and diabetes-related traits in the Framingham Offspring Study. Diabetes. 2009;58:750-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 66. | Tsiotra PC, Boutati E, Dimitriadis G, Raptis SA. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. Biomed Res Int. 2013;2013:487081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 67. | Youn BS, Yu KY, Park HJ, Lee NS, Min SS, Youn MY, Cho YM, Park YJ, Kim SY, Lee HK, Park KS. Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 68. | Fujinami A, Obayashi H, Ohta K, Ichimura T, Nishimura M, Matsui H, Kawahara Y, Yamazaki M, Ogata M, Hasegawa G, Nakamura N, Yoshikawa T, Nakano K, Ohta M. Enzyme-linked immunosorbent assay for circulating human resistin: resistin concentrations in normal subjects and patients with type 2 diabetes. Clin Chim Acta. 2004;339:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 69. | Moon B, Kwan JJ, Duddy N, Sweeney G, Begum N. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. Am J Physiol Endocrinol Metab. 2003;285:E106-E115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol. 2005;25:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 71. | Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, Steppan CM, Ahima RS, Obici S, Rossetti L, Lazar MA. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 517] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 72. | Rangwala SM, Rich AS, Rhoades B, Shapiro JS, Obici S, Rossetti L, Lazar MA. Abnormal glucose homeostasis due to chronic hyperresistinemia. Diabetes. 2004;53:1937-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 73. | Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 454] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 74. | Asterholm IW, Rutkowski JM, Fujikawa T, Cho YR, Fukuda M, Tao C, Wang ZV, Gupta RK, Elmquist JK, Scherer PE. Elevated resistin levels induce central leptin resistance and increased atherosclerotic progression in mice. Diabetologia. 2014;57:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Zhang J, Pronyuk K, Kuliesh O, Chenghe S. Adiponectin, resistin and leptin: Possible markers of metabolic syndrome. Endocrinol Metab Syndr. 2015;4:2161-1017.1000212. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 77. | Mamali I, Roupas ND, Armeni AK, Theodoropoulou A, Markou KB, Georgopoulos NA. Measurement of salivary resistin, visfatin and adiponectin levels. Peptides. 2012;33:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Gröschl M. [Current status of salivary hormone analysis]. Ann Biol Clin (Paris). 2009;67:493-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 79. | Boström EA, d'Elia HF, Dahlgren U, Simark-Mattsson C, Hasséus B, Carlsten H, Tarkowski A, Bokarewa M. Salivary resistin reflects local inflammation in Sjögren's syndrome. J Rheumatol. 2008;35:2005-2011. [PubMed] |
| 80. | Sarhat ER, Najim RS, Abdulla EH. Estimation of salivary resistin, malondialdehyde and lipid profile levels in patients with diabetes mellitus. Tikrit J Pure Sci. 2018;20:167-170. |
| 81. | Al-Rawi N, Al-Marzooq F. The Relation between Periodontopathogenic Bacterial Levels and Resistin in the Saliva of Obese Type 2 Diabetic Patients. J Diabetes Res. 2017;2017:2643079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 82. | Srinivasan M, Meadows ML, Maxwell L. Assessment of Salivary Adipokines Resistin, Visfatin, and Ghrelin as Type 2 Diabetes Mellitus Biomarkers. Biochem Res Int. 2018;2018:7463796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Gürlek B, Çolak S. Saliva resistin as a screening marker of gestational diabetes mellitus. Gynecol Endocrinol. 2021;37:324-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Barbour LA. New concepts in insulin resistance of pregnancy and gestational diabetes: long-term implications for mother and offspring. J Obstet Gynaecol. 2003;23:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 85. | Moyer VA; U. S. Preventive Services Task Force. Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 86. | Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol. 2005;186:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 87. | Hu SM, Chen MS, Tan HZ. Maternal serum level of resistin is associated with risk for gestational diabetes mellitus: A meta-analysis. World J Clin Cases. 2019;7:585-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, Dietz PM. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 692] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 89. | Sathyapalan T, Mellor D, Atkin SL. Obesity and gestational diabetes. Semin Fetal Neonatal Med. 2010;15:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Yao D, Chang Q, Wu QJ, Gao SY, Zhao H, Liu YS, Jiang YT, Zhao YH. Relationship between Maternal Central Obesity and the Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Cohort Studies. J Diabetes Res. 2020;2020:6303820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |