Published online Sep 15, 2021. doi: 10.4239/wjd.v12.i9.1479
Peer-review started: May 17, 2021
First decision: June 5, 2021
Revised: June 18, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: September 15, 2021
Processing time: 112 Days and 23.6 Hours
Non-alcoholic fatty liver disease (NAFLD) has become one of the most common chronic liver diseases worldwide. A strong relationship exists between NAFLD and diabetes mellitus. There is growing evidence of a mechanistically complex and strong association between the two diseases. Current data also shows that one disease actually leads to worsening of the other and vice versa. Understanding of the various pathophysiological mechanisms involved, natural history and spectrum of these two diseases is essential not only for early diagnosis and management but also for prevention of severe disease forms. Despite the tremendous progress made in recent times in acquiring knowledge about these highly prevalent diseases, the guidelines and recommendations for screening and management of diabetics with NAFLD remain ambiguous. An interdisciplinary approach is required to not only raise awareness of the prevalence of NAFLD in diabetics but also for better patient management. This can help attenuate the development of significant complications, such as cirrhosis, decompensation and hepatocellular carcinoma in these patients, thereby halting NAFLD in its tracks. This review focuses on the pivotal role of primary care physicians and endocrinologists in identification of NAFLD in diabetics in early stages and the role of proactive screening for prompt referral to hepatologist.
Core Tip: With prevalence of non-alcoholic fatty liver disease (NAFLD) in diabetics being substantial, there is a need for its increased awareness and knowledge in the primary care physicians and endocrinologists. It is important to understand that these patients have the propensity to develop more severe forms of liver diseases, and their early identification and management can help in providing a stitch in time. We have reviewed in detail, the currently available societal guidance on screening of NAFLD in diabetics, especially with regards to high-risk patients that require hepatologist’s referral. We have even proposed a screening protocol for these patients based on available literature. This will not only help the treating physicians in identifying the disease in its incipient stages but also help in patient’s timely referral.
- Citation: Khandelwal R, Dassanayake AS, Conjeevaram HS, Singh SP. Non-alcoholic fatty liver disease in diabetes: When to refer to the hepatologist? World J Diabetes 2021; 12(9): 1479-1493
- URL: https://www.wjgnet.com/1948-9358/full/v12/i9/1479.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i9.1479
Non-alcoholic fatty liver disease (NAFLD) has assumed the status of major global health concern in recent times. It has become the most common chronic liver disease (CLD) worldwide, with prevalence among the general population being 25%-35%[1]. NAFLD is increasingly being recognized not only in adults but also in children and adolescents, adding further to the disease burden[2]. An individual is said to have NAFLD if the liver biopsy or imaging shows evidence of hepatic steatosis (≥ 5% liver fat) with background history of little or no alcohol consumption and in the absence of other liver diseases/conditions leading to hepatic steatosis[3]. The disease spectrum varies from simple steatosis (simple fatty liver) to the more severe and progressive non-alcoholic steatohepatitis (NASH) and cirrhosis[4,5]. Cirrhosis due to NASH is currently the second leading etiology for liver transplantation in the United States as well as in Europe and is projected to become the leading indication in the next decade[6,7]. The risk factors associated with NAFLD are also linked with other manifestations of metabolic syndrome viz diabetes, dyslipidemia, obesity and hypertension suggesting a relationship between these metabolic traits and the likelihood of developing NAFLD and also advanced fibrosis[8-10]. Studies have shown that 70%-80% of diabetics have NAFLD[11,12]. The presence of diabetes and obesity in patients with NAFLD has consistently been shown to be a key predictor of inflammatory disease progression leading to NASH and advanced fibrosis[13-15]. It is noteworthy that NAFLD is also frequent in subjects at increased risk for developing diabetes, including patients with the metabolic syndrome[13,16] and women with gestational diabetes or polycystic ovary syndrome[17], further underscoring the relationship between the two diseases. The strong association, as highlighted by various studies, is mechanistically complex, and NAFLD may precede or succeed diabetes onset[18,19]. Despite this, ambiguity remains in the guidelines and recommendations for screening of diabetic patients for NAFLD and vice versa, and their management. As most of the diabetic patients and patients with metabolic syndrome are under long-term treatment from an endocrinologist or a general physician, they are unlikely to visit a hepatologist for liver assessment and risk stratification, before they develop significant liver-related morbidity. This article represents an effort to understand and examine not only the link between the two diseases but to also review the available guidelines and screening strategies, and suggest future directives for these patients. Timely referral to a specialist can surely help nip ‘the epidemic of this liver disease’ in the bud.
NAFLD has often been referred to as the “hepatic manifestation” of metabolic syndrome[20]. It is known that lipid accumulation is the hallmark of NAFLD. In order to understand if NAFLD is the cause or consequence of diabetes or are they both just co-passengers, we need to delve deeper into the pathophysiology of the two entities.
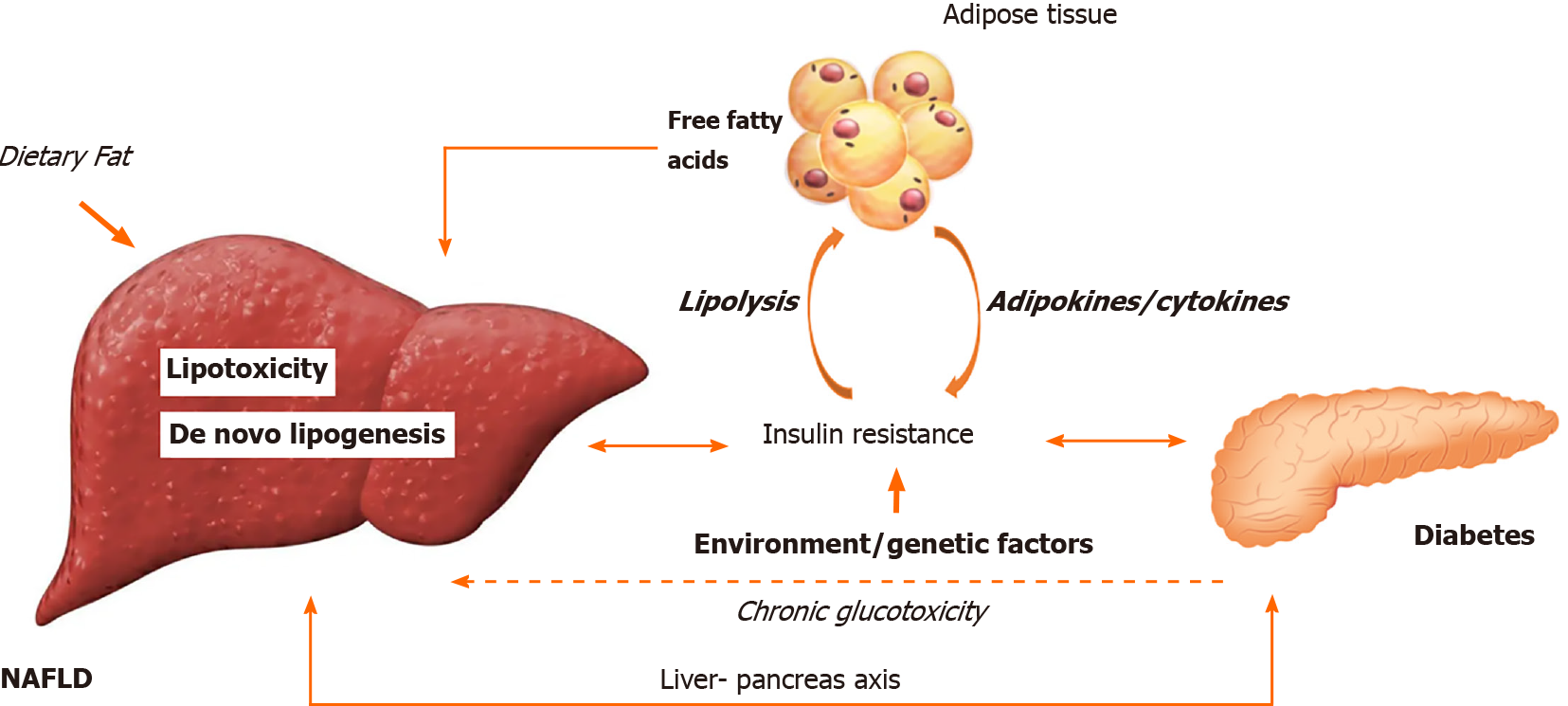
Adipose tissue present in the body not only acts as a storage depot but also prevents ectopic lipid deposition in muscle, liver, heart, and other tissues. Besides, it also acts as an endocrine gland, secreting many hormones, cytokines, and vasoactive substances[21]. It has been found that upon eating a meal, there is an increase in the rate of insulin secretion, that facilitates the entry of glucose into the adipocytes[22] enabling the generation of L-α-glycerophosphate needed for triglyceride formation[23]. Insulin also increases the lipoprotein lipase activity in adipose tissue, thus promoting the generation of free fatty acids (FFAs) from chylomicron-triglyceride which leads to increase in the rate of entry of FFAs into adipocytes[24]. Besides, insulin is known to inhibit the action of hormone-sensitive lipase, the enzyme that causes hydrolysis of the triglycerides already stored in the adipocytes, further reducing the levels of circulating FFAs and glycerol[25]. The various adipokines released from adipose tissue, including adiponectin and cytokines, regulate liver energy metabolism[26]. In addition, adiponectin also stimulates β oxidation in the liver and improves liver insulin sensitivity[27].
When carbohydrates are abundant, the liver not only utilizes glucose as the main metabolic fuel but also converts glucose into fatty acids[28]. Hepatocytes derive FFAs either from diet or from adipose tissue via lipolysis and/or from hepatic de novo lipogenesis (DNL)[28]. Once inside the hepatocytes, the FFAs are acted on by acyl-CoA synthases to form fatty acyl-CoAs, which may enter either esterification and/or β-oxidation pathways[29]. Studies have shown that 59% triacylglycerols (TAGs) that tend to accumulate in liver, come from circulating FFAs; DNL, which is the process in which carbohydrates are converted to lipids, contributes to 26% and the rest 14% is from the diet[30]. The TAGs and cholesterol esters are either stored in lipid droplets within hepatocytes or secreted into the circulation as very-low density lipoprotein (VLDL) particles. Insulin has an important action in liver as it potently suppresses gluconeogenesis in liver and stimulates lipogenesis[28]. TAG accumulation is not hepatotoxic per se and could represent a defensive mechanism to balance FFA excess, as demonstrated in mouse models[31]. Studies have shown that increased TAG concentration is an epiphenomenon which happens simultaneously with toxic metabolite generation, lipotoxicity and liver injury[32].
Normally, in the liver, there is a fine balance between lipid uptake (in the form of FFAs/DNL, and esterification) and lipid disposal (in the form of metabolism/β-oxidation and elimination as VLDLs)[33]. In patients with NAFLD, it has been demonstrated that VLDL removal, at times, is unable to keep pace with the increased rate of TAG uptake and intrahepatic production[33], leading to metabolic dis
The pathogenesis of NAFLD was earlier explained by the ‘two-hits hypothesis’, according to which the ‘first hit’ was hepatic accumulation of lipids, occurring secondary to sedentary lifestyle, high fat diet, obesity and insulin resistance (IR). This sensitized the liver to further insults, which acted as ‘second hit’, thereby leading to activation of inflammatory cascades and fibrogenesis[34]. The dictum that steatosis always precedes inflammation has now largely been discarded, as it was discovered that NASH can also present in liver ‘de novo’. Indeed, the timing and combination of genetic, external and intracellular events, rather than the simple sum of hepatic insults, result in different pathways leading to steatosis or NASH[35]. In order to overcome the shortcomings, the ‘multiple hit’ theory for pathogenesis of NAFLD has been proposed. Such hits include IR, hormones from the adipose tissue, nutritional factors, gut microbiota, and genetic and epigenetic factors[36].
IR has been highlighted as one of the key events occurring in NAFLD and diabetes, but difficulty lies in establishing if it is the cause or the consequence[37]. The link between diabetes and NAFLD can be described by a spectrum of metabolic changes represented by IR, defective hepatic lipid profile and TAG metabolism causing fat accumulation, immune responses, and/or subsequent hyperinsulinemia as determined by the β-cell dysfunction in diabetes[37].
Presence of IR in NAFLD has also been substantiated in one study which showed that lean non-diabetic men with increased liver fat (as quantified by MRS) had both hepatic and adipose tissue IR along with impaired insulin suppression of glucose production and serum FFAs, when compared with subjects matched for both body mass index (BMI) and intra-abdominal fat but having low levels of hepatic fat[38].
IR causes decrease in the rate of glycogen synthesis, along with increased rate of gluconeogenesis in liver[30]. The increase in intrahepatic glucose and resultant glycolysis provide substrates for DNL. There is increased production of acetylCoA which gets converted to malonylCoA that gets sequestered towards DNL as a substrate, thereby leading to hepatic steatosis[30]. Transcriptional regulation of DNL is primarily orchestrated by the sterol regulating element binding protein 1c (SREBP1c)[39]. Glucose and insulin promote lipogenesis through activation of the carbohydrate response element binding protein (ChREBP) and SREBP1c[39]. In states of IR, SREBP-1c is over-expressed and DNL is up-regulated[40]. SREBP1c can enhance the generation of harmful lipid molecules, such as diacylglycerol and ceramides, which further enhance IR. This results in a positive feedback loop in which hepatic DNL helps IR and IR stimulates hepatic DNL[41]. Also, β-oxidation of FFAs is inhibited in IR states, further promoting accumulation of hepatic lipids[42]. Excess of stored fat leads to abnormal lipid peroxidation and release of pro-inflammatory cytokines, high reactive oxygen species, and reactive nitrogen species causing liver disease[37]. The role of abnormal adipocyte and liver macrophage activity has also been highlighted in research models[37].
FFAs in the hepatocytes can induce defects in insulin signaling pathways through serine-kinase activation, thereby contributing to the IR[43]. Further, the increased FFAs released, due to excessive intra-hepatic TAGs, cause hepatic IR and inflammation[44], and localized intrahepatic inflammation can contribute to peripheral IR[45]. In addition to this, an increase in circulating FFAs impairs the ability of insulin to suppress endogenous glucose production and may directly enhance hepatic glucose production[25]. This explains why hepatic steatosis resolution can prevent diabetes onset[46].
IR by its action on adipose tissue, causes lipolysis, increasing the flux of FFAs to the liver. The adipocyte tissue becomes inflamed and dysfunctional and releases adipokines and inflammatory cytokines, such asinterleukin-6 and tumor necrosis factor (TNF)α-1, and there is decreased release of anti-inflammatory adiponectin[47]. The imbalance between pro-insulin (adiponectin, leptin) and anti-insulin (TNFα) cytokines further helps in the development of IR[48]. A novel adipokine, Gremlin 1, that antagonizes insulin signaling is positively correlated with the percentage of body fat and IR in diabetes and NAFLD/NASH subjects, and may become a potential biomarker or therapeutic target in the future[49].
Some researchers have proposed that chronic hyperglycemia (“glucose toxicity” or glucotoxicity), especially in patients with diabetes, may play an important role in development of NASH, further underscoring the interplay between NAFLD and diabetes[50]. Proposed mechanisms that need to be validated include hepatic inflammation and oxidative stress due to hyperglycemia, accelerated production of advanced glycosylation end-products and development of inflammation in Kupffer and hepatic stellate cells, alteration of the hepatocyte microenvironment by glucotoxicity, up-regulation by hyperglycemia of genes involved in key lipogenic and glycolytic pathways (such as the transcription factor ChREBP, stimulation of liver-pyruvate kinase, and many others), activation by high-fructose diets of DNL, along with up-regulation of inflammatory pathways[50]. Some theories also suggest that gut microbiome alteration and dietary habits are other mechanisms that induce and maintain diabetes and/or NAFLD[51].
As research to unravel the pathophysiological correlation between NAFLD and diabetes continues, the role of a new ‘liver-pancreas’ axis, existing between the liver and pancreatic α-cells, has emerged[52]. Pancreatic α-cells are known to secrete glucagon[53]. A study has shown that normal glucose-tolerant obese patients have fasting hyperglucagonemia, which is related to liver steatosis[53]. It has also been shown that besides hepatic IR, glucagon resistance can also putatively contribute to diabetes development in NAFLD patients[54].
Figure 1 schematically illustrates the pathophysiologic association between NAFLD and diabetes.
Many studies in NAFLD patients have also highlighted that both genetic and environmental factors interfere with the insulin signaling cascade and become cardinal in maintaining and worsening of IR[36]. Various molecular mechanisms that may be involved include serine phosphorylation of ‘insulin receptor substrate’ by inflammatory signal transducers, such as c-Jun N-terminal protein kinase 1 or inhibitor of nuclear factor-jB kinase-b, activation of nuclear factor kappa B and suppressors of cytokine signaling[36].
It is interesting to note that not all studies show the positive relationship of diabetes and NAFLD. Recently, few genotype/phenotype-related studies have shown a disproportional development of diabetes in patients of NAFLD having specific gene variants, such as the patatin-like phospholipase domain-containing 3 (PNPLA3 rs738409 GG) and transmembrane 6 superfamily member 2 protein (TM6SF2 rs58542926 C>T gene)[55]. Other NAFLD-related gene variants, such as LYPLAL1 and MBOAT7, have also shown no increase in the risk of diabetes in these patients[55].
While the pathophysiologic association of diabetes and NAFLD is partly because of the “common soil”, the clinical course of the two entities appears to be like an inextricably intertwined vine. NAFLD, as a disease, has been studied for many years and is broadly divided into two pathologically distinct conditions with different prognoses, namely: non-alcoholic fatty liver (NAFL) (i.e. pure steatosis or steatosis with mild lobular inflammation) and NASH[15]. The latter encompasses a wide spectrum of disease, including fibrosis and cirrhosis, and may be associated with hepatocellular carcinoma (HCC). NASH is further sub-classified as early NASH with no or mild (F0-F1) fibrosis, fibrotic NASH with significant (≥ F2) or advanced (≥ F3, bridging) fibrosis and NASH-cirrhosis with F4 fibrosis[15]. It is often difficult to differentiate NAFL from the progressive NASH, as patients are usually asymptomatic with normal liver enzyme levels, and imaging tests may at times fail to identify the steatosis and fibrosis[56,57]. Various studies have highlighted that about one-third patients with NAFL and NASH have progressive fibrosis and 20% may have some regression over an average follow-up between 2.2 and 13.8 years[58]. The rate of fibrosis progression has been found to be characteristically slow, with an average progression of one stage taking 7.7 years[58]. While the rate of progression in NASH subjects may be twice as high, there exists a sub-group of both NASH and NAFL patients who may progress rapidly from no fibrosis to advanced fibrosis over an average period of 6 years[58].
It has been observed that individuals with diagnosed NAFLD have a 2-fold increased risk of developing diabetes[59]. The prevalence of diabetes among diagnosed NAFLD and NASH patients is estimated to be 22.51% and 43.63%, respectively, which is much higher as compared to the prevalence of diabetes in the general population (8.5%)[60]. A study from India, conducted on 515 NAFLD patients, showed that the prevalence of diabetes and prediabetes in the cohort was about 24% and 23% respectively[61]. Diabetes seems to accelerate the course of NAFLD, and has also been found to be one of the strongest clinical predictors of progression of NAFLD to NASH and cirrhosis[62]. Furthermore, a strong pathophysiological link also exists between diabetes and HCC. The increased levels of inflammatory biomarkers and hyperinsulinemia, that are found in diabetics, may be responsible for the increased risk of HCC[63].
Increasing epidemiological evidence suggests that there is a bidirectional relationship between NAFLD and diabetes and that NAFLD may precede and/or promote the development of diabetes[64]. On evaluating and analyzing patients with diabetes, several studies have reported that the prevalence of NAFLD in these patients ranges broadly, between 34%-94%[65]. The prevalence of NAFLD is higher not just in diabetics but also in those at risk of developing diabetes[66]. These patients can be identified as having glycosylated hemoglobin A1c (HbA1c) values of 5.7%–6.4% (38.8–46.4 mmoL/moL), impaired fasting glucose (fasting glucose: 100–125 mg/dL [5.55–6.94 mmoL/L]) and/or impaired glucose tolerance (glucose: 140–199 mg/dL [7.77–11.04 mmoL/L]) at 2 h of the standardized 75 g oral glucose tolerance test (OGTT)[66]. Interestingly, insulin treatment, that increases body fat, does not appear to promote or worsen NAFLD in diabetics[67]. A study has shown the estimated prevalence of NASH and advanced fibrosis in individuals with coexisting NAFLD and diabetes to be 37.3% (95% confidence interval [CI]: 24.7–50.0) and 17.0% (95%CI: 7.2–34.8), respectively[68]. Further, the overall mortality ratio in 5–10 years was found to be of 585 per 100000, which was greater than mortality from other CLDs[68].
In patients with diabetes, NAFLD is believed to increase the risk of cardiovascular events by 1.87-fold after adjusting for confounders[69]. Co-existent NAFLD may also increase the risk of microvascular complications of diabetes, including chronic kidney disease and retinopathy[70]. Growing evidence shows that besides its effect on the liver, NAFLD in diabetics may also lead to development of sensory-motor and autonomic neuropathy[71,72].
Thus, a careful consideration and evaluation of diabetes in patients with NAFLD (NAFL/NASH) and vice versa not only helps in prognostication and therapy but also has a potential to prevent associated complications.
Before discussing the moot point of when to refer a diabetic with NAFLD to a hepatologist, it is essential to understand that NAFLD and diabetes are like a “two-way road”; detection of one entity in patients of the other can immensely help in reducing the disease burden of both. Patients with NAFLD have a significantly higher prevalence of abnormal glucose tolerance (prediabetes or diabetes) than those without NAFLD (20.6% vs 11%)[73]. It is therefore possible to decrease incident diabetes with improvement of NAFLD[46]. In view of this, European associations (EASL-EASD-EASO) in their guidelines have recommended mandatory screening for diabetes in all persons with NAFLD, by fasting or random blood glucose or HbA1c and if available, by the standardized 75-g OGTT in high-risk groups[15].
It was observed that diabetics with other components of metabolic syndrome were at a higher risk of having advanced CLD, thereby requiring further liver assessment[74]. It was found that development of NAFLD and, in particular, NASH-related fibrosis can have profound effects on morbidity and mortality in diabetics[60,75]. Studies have shown that steatosis in diabetics is associated with increased prevalence of altered albumin excretion rate, thus playing an important role in the development of diabetic nephropathy[76]. The earlier NICE NAFLD Guidelines of 2016 did not give any recommendations for screening of patients with diabetes for liver fibrosis, but with mounting evidence, this saw a gradual change.
Various non-invasive indicators were approved by the EASD for NAFLD diagnosis. These include NAFLD liver fat score, the fatty liver index (FLI)[77], fibrosis-4 index (FIB-4) and NAFLD fibrosis score (NFS)[78]. Besides, SteatoTest, NashTest, ActiTest and FibroTest too are handy tools in quantifying liver steatosis and fibrosis[79]. FibroScan®, a frequently used non-invasive test for liver stiffness measurement (LSM), can not only help to detect and stage fibrosis in NAFLD/NASH but can also predict macrovascular and microvascular complications of diabetes[80,81]. Experts have also proposed the use of intrahepatic TAG measurement with magnetic resonance imaging-derived proton density fat fraction as a standard test for detecting and grading hepatic steatosis[82,83]. Separation of diabetes patients with the relatively benign form of the disease (NAFL), from those who have NASH (with or without moderate-to severe fibrosis [F2]) requiring early intervention, has always been seen as a big challenge[84].
The gold standard for NAFLD diagnosis is liver biopsy. But this procedure, which requires specialist referral, being invasive and having several drawbacks, such as sampling error, high cost, inter- and intra- observer variability and risk of complications[85], cannot be applied to all the patients. In order to not miss at-risk patients while focusing on suitable and sustainable hepatologist referral, in 2016, the EASL-EASD-EASO guidelines were published[15]. It was recommended that in case of presence of obesity/diabetes or the incidental finding of raised liver enzymes with metabolic risk factors, patients should promptly be evaluated with non-invasive screening tests to identify steatosis, NASH, and fibrosis[15]. They suggested ultrasound evaluation along with application of steatosis biomarkers like FLI, SteatoTest, NAFLD Fat score to identify steatosis. Surrogate markers of fibrosis (NFS, FIB-4, ELF or FibroTest) are then to be calculated, in order to rule out significant fibrosis (≥ F2), which, if found, would mandate specialist referral for evaluation with or without liver biopsy[15].
In 2020, the American Diabetes Association also recommended checking for NASH and fibrosis in patients with elevated liver enzymes[86]. It was proposed that patients with type 2 diabetes or prediabetes and elevated liver enzymes (alanine aminotransferase [ALT]) or fatty liver on ultrasound should be evaluated for presence of NASH and liver fibrosis[86].
One study evaluated the performance of international (EASL-EASD-EASO) and national (DGVS) guidelines for NAFLD risk stratification in diabetic patients and compared it to their simplified referral strategy using Fibroscan-AST (FAST) score[87]. LSM values by FibroScan® in diabetics were defined as low (< 7.9/7.2kPa M/XL probe), intermediate (7.9–9.6/7.2–9.3kPaM/XL-probe) and high (> 9.6/9.3kPaM/XL probe)[87]. EASL-EASD-EASO recommended specialist referral for 60%–77% of the subjects depending on the fibrosis score, whereas the DGVS algorithm required LSM for 76%; 25% were referred for specialized care. The sensitivities of the diagnostic pathways were 47%–96%. The FAST score, when compared to these, revealed a sensitivity/specificity of 46V/88% for fibrosis risk and a specialist referral rate of 35%[87].
Thus, despite the societal guidance, debate continues as there is still no clarity as to which test is to be applied first and the suitable cut-offs that can be used so that the screening is simple, inexpensive with reasonable sensitivity and specificity.
With regards to the cut-off values used for plasma ALT concentration, studies have shown that although a threshold of 40 IU/L is frequently used in clinical practice and trials, lower cut-off points for normal (i.e. 30 IU/L for males and 19 IU/L for females) can improve the sensitivity of diagnosing prevalence of NAFLD[88]. Also, despite studies showing association of increased ALT values with increased risk of NASH, it has been found that, in some patients, especially those with diabetes and IR, NASH with/without advanced fibrosis may occur even when the ALT values are normal[89]. Thus, we cannot consider liver function test as a surrogate for disease severity in diabetics and relying solely on it may lead to missing of advanced liver disease cases.
As for abdominal ultrasonography, it is operator-dependent and is insensitive to mild steatosis[90]. When compared to this, Controlled Attenuation Parameter measurement by FibroScan can detect liver fat involving as little as 10% of the hepatocytes[91-93], thereby avoiding underreporting or missing cases. Various indices, such as FLI and hepatic steatosis index (HSI), have also been found useful in predicting hepatic steatosis. It has been shown that FLI < 30 can rule out hepatic steatosis with a sensitivity of 87% and a value of FLI ≥ 60 can help rule in hepatic steatosis with a specificity of 86%[77]. HSI, on the other hand, at values of < 30 or > 36.0, can rule out NAFLD with a sensitivity of 93.1% or detect NAFLD with a specificity of 92.4%, respectively[94].
Studies have also evaluated performance of other non-invasive scores such as aspartate aminotransferase (AST) to platelet ratio, FIB-4 and NFS that are used for evaluation of fibrosis while considering referral to hepatologist. Table 1 depicts a few of these non-invasive models that have been used for the evaluation of steatosis and fibrosis in patients of diabetes with NAFLD. In one study, FIB-4 and NFS models had a high negative predictive value (NPV) of 93.48% and 93.61%, respectively in patients with severe liver fibrosis (stages 3 and 4), thereby indicating that these models should actually only be used for excluding severe liver disease[95]. These scores also show lower specificity among older adults and lower accuracy in young adults[96]. It was found that the use of age-adjusted FIB-4 cut-offs can lead to appropriate referrals[97]. It was proposed that for those over the age of 65 years, a FIB-4 score of > 2.0 should be used as the cut-off for referral[96,98].
| Index | Components | Cut-offs | Sensitivity, % | Specificity, % |
| Steatosis | ||||
| FLI[77] | WC, BMI, TG and GGT | < 30 | 87.0 | 64.0 |
| ≥ 60 | 61.0 | 86.0 | ||
| HSI[94] | AST, ALT, BMI, diabetes, female sex | < 30 | 93.1 | 39.6 |
| > 36 | 45.1 | 92.4 | ||
| Fibrosis (stage 2, 3 or 4) | ||||
| APRI[95] | AST and platelet count | 0.518 | 50.00 | 89.19 |
| FIB-4[95] | Age, AST, ALT and platelet count | 1.743 | 63.33 | 94.59 |
| NFS[95] | Age, BMI, IFG and diabetes, AST-to-ALT ratio, platelet count and albumin | -0.054 | 50.00 | 86.21 |
The NASH Council, in their study, proposed that a patient who has a FIB-4 score of > 1.3 be referred to a specialist and for those that have a score ≤ 1.3 undergo lifestyle intervention with their primary provider[97,99]. Indian researchers have found that among patients of NAFLD, a FIB-4 cut-off of 1.0 instead of 1.3, showed 100% sensitivity and 94.3% specificity to rule out any fibrosis (F0 vs F1-F4) validated vs MRE in a cohort of 239 NAFLD patients, thus leading to inclusion of patients with F2 fibrosis in the primary care referral pathway[100]. However, validation of this is needed in diabetics and a larger patient cohort is needed to be further evaluated[100].
Another study that derived data from NASH Clinical Research Network studies and included patients with biopsy-proven NAFLD with diabetes, proposed a different model for NASH evaluation[101]. The parameters included were White race, BMI, waist circumference, ALT, AST, albumin, HbA1c, HOMA-IR and ferritin[101]. The specificity, sensitivity, NPV and pars plana vitrectomy (PPV) were 90.0%, 56.8%, 47.7%, and 93.2%, respectively, and the model correctly classified 67% of patients as having NASH[101]. The researchers also proposed a model for predicting advanced fibrosis using the parameters- age, Hispanic ethnicity, BMI, waist-to-hip ratio, hypertension, ALT/AST ratio, alkaline phosphatase, isolated abnormal alkaline phosphatase, bilirubin (total and direct), globulin, albumin, serum insulin, hematocrit, international normalized ratio, and platelet count. The specificity, sensitivity, NPV, and PPV were 90.0%, 57%, 75.1%, and 80.2%, respectively, and the model correctly classified 76.6% of patients as having advanced fibrosis[101]. It was concluded that proposed model performed better than the NAFLD fibrosis score in detecting advanced fibrosis[101].
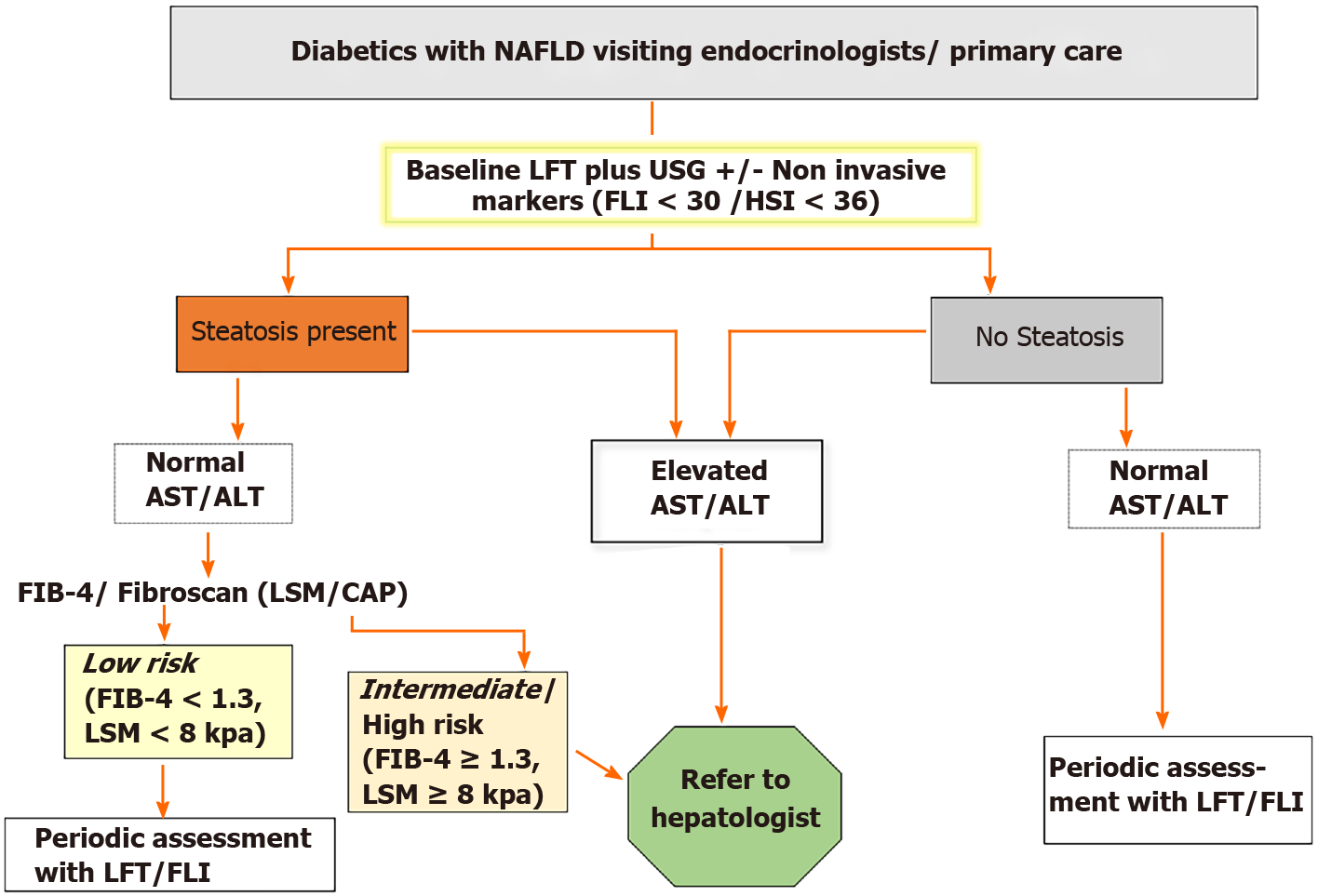
Based on the extensive review of the literature and pertaining to this ongoing debate of how best to manage diabetic patients with NAFLD, we propose a screening protocol that takes into account not only the current societal guidance but also the results of ongoing research. We believe that instead of only those diabetics who are at intermediate or high risk of having NAFLD, all diabetics must be evaluated using a baseline ultrasound abdomen and liver function test along with non-invasive markers as per the algorithm shown in Figure 2. However, more studies with larger patient cohorts are needed to further explore this simplified algorithm and for further re-strategizing the screening protocols.
Surveys in the Netherlands[102] and an urban western United States population[103] have shown that 84% of general practitioners and 83% of largely primary care providers respectively have endorsed the need for increased awareness and knowledge on NAFLD.
In diabetics with NAFLD, lifestyle interventions and weight loss have been found to be the most beneficial therapeutic strategies[104]. In a few randomized controlled trials, drugs such as pioglitazone, a potent and selective agonist for peroxisome proliferator-activated receptor-gamma, have also been consistently found to induce resolution of NASH and have shown modest effects on liver fibrosis[50,105]. Also, contrary to the popular belief that statins cannot be used in diabetics with NASH with elevated liver enzymes due to potential risk of hepatotoxicity, it has been shown that statin therapy is safe in these patients[106]. Trials are underway for evaluating efficacy of other treatment options.
As the prevalence of NAFLD in diabetics is substantial, and due to the influence of each disease on the other with regards to disease progression, the role of primary care physicians and diabetologists becomes pivotal. A knowledge of preventive measures and available treatment is needed in order to manage the milder disease forms at primary care level only. Diabetics must be monitored for NAFLD/NASH with a vigilant eye, similar to the way the other complications of diabetes, like retinopathy and nephropathy, are screened. This proactive approach of screening will surely help in not only prevention but also in early detection of the more sinister and progressive disease form, despite its benign phenotype. Where indicated, a prompt referral to hepatologists can make the patient turn the corner and can thereby attenuate the development of more severe forms of NASH, including cirrhosis and even HCC.
We sincerely thank Dr. Ankush R Pawar (Liverpool University Hospitals NHS Foundation Trust) for his helpful suggestions.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ji G, Wang CR S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li X
| 1. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3800] [Article Influence: 542.9] [Reference Citation Analysis (2)] |
| 2. | Wiegand S, Keller KM, Röbl M, L'Allemand D, Reinehr T, Widhalm K, Holl RW; APV-Study Group and the German Competence Network Adipositas. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes (Lond). 2010;34:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Puri P, Sanyal AJ. Nonalcoholic fatty liver disease: Definitions, risk factors, and workup. Clin Liver Dis (Hoboken). 2012;1:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1756] [Cited by in RCA: 1822] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 5. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, Rizzetto M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1018] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 6. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1384] [Article Influence: 138.4] [Reference Citation Analysis (1)] |
| 7. | Adam R, Karam V, Cailliez V, O Grady JG, Mirza D, Cherqui D, Klempnauer J, Salizzoni M, Pratschke J, Jamieson N, Hidalgo E, Paul A, Andujar RL, Lerut J, Fisher L, Boudjema K, Fondevila C, Soubrane O, Bachellier P, Pinna AD, Berlakovich G, Bennet W, Pinzani M, Schemmer P, Zieniewicz K, Romero CJ, De Simone P, Ericzon BG, Schneeberger S, Wigmore SJ, Prous JF, Colledan M, Porte RJ, Yilmaz S, Azoulay D, Pirenne J, Line PD, Trunecka P, Navarro F, Lopez AV, De Carlis L, Pena SR, Kochs E, Duvoux C; all the other 126 contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). 2018 Annual Report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl Int. 2018;31:1293-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 332] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 8. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1091] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 9. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 973] [Article Influence: 64.9] [Reference Citation Analysis (1)] |
| 10. | Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, Zein CO, Brunt EM, Kleiner DE, McCullough AJ, Sanyal AJ, Diehl AM, Lavine JE, Chalasani N, Kowdley KV; NASH Clinical Research Network. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913-924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 356] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 11. | Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 701] [Article Influence: 38.9] [Reference Citation Analysis (1)] |
| 12. | Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, Frier BM, Van Look LA, Johnston GI, Reynolds RM, Strachan MW; Edinburgh Type 2 Diabetes Study Investigators. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M, Kato T, Okuda J, Ida K. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 806] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 14. | Ong JP, Elariny H, Collantes R, Younoszai A, Chandhoke V, Reines HD, Goodman Z, Younossi ZM. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 506] [Article Influence: 56.2] [Reference Citation Analysis (2)] |
| 16. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1722] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 17. | Cerda C, Pérez-Ayuso RM, Riquelme A, Soza A, Villaseca P, Sir-Petermann T, Espinoza M, Pizarro M, Solis N, Miquel JF, Arrese M. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. J Hepatol. 2007;47:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 713] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 19. | Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 586] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 20. | Anstee QM, McPherson S, Day CP. How big a problem is non-alcoholic fatty liver disease? BMJ. 2011;343:d3897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Kahn BB. Adipose Tissue, Inter-Organ Communication, and the Path to Type 2 Diabetes: The 2016 Banting Medal for Scientific Achievement Lecture. Diabetes. 2019;68:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Unger RH, Eisentraut AM. Entero-insular axis. Arch Intern Med. 1969;123:261-266. [PubMed] |
| 23. | Renold AE. A brief and fragmentary introduction to some aspects of adipose tissue metabolism, with emphasis on glucose uptake. Ann N Y Acad Sci. 1965;131:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Garfinkel AG, Nilsson-ehle P, Schotz MC. Regulation of lipoprotein lipase.Induction by insulin. Biochim Biophys Acta. 1976;424:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Roden M, Stingl H, Chandramouli V, Schumann WC, Hofer A, Landau BR, Nowotny P, Waldhäusl W, Shulman GI. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes. 2000;49:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 202] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765-3773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 835] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 27. | Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3053] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 28. | Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1429] [Article Influence: 129.9] [Reference Citation Analysis (0)] |
| 29. | Ferramosca A, Zara V. Modulation of hepatic steatosis by dietary fatty acids. World J Gastroenterol. 2014;20:1746-1755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, Oyen T, Topal B, Fetter G, Nayler S, van der Merwe T, Windmolders P, Van Gaal L, Verrijken A, Hubens G, Gericke M, Cassiman D, Francque S, Nevens F, van der Merwe S. Association of Adipose Tissue Inflammation With Histologic Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:635-48.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 31. | Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, Naylor S, Rao M, Hubbard B, Farese RV Jr. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. 2010;120:756-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 843] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 33. | Mendez-Sanchez N, Cruz-Ramon VC, Ramirez-Perez OL, Hwang JP, Barranco-Fragoso B, Cordova-Gallardo J. New Aspects of Lipotoxicity in Nonalcoholic Steatohepatitis. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 34. | Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci. 2014;15:8591-8638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 35. | Yilmaz Y. Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (2)] |
| 36. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2121] [Article Influence: 235.7] [Reference Citation Analysis (1)] |
| 37. | Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. 2020;2020:3920196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 353] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 38. | Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, Halavaara J, Yki-Järvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023-3028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 644] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 39. | Liu W, Baker RD, Bhatia T, Zhu L, Baker SS. Pathogenesis of nonalcoholic steatohepatitis. Cell Mol Life Sci. 2016;73:1969-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 40. | Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 406] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 41. | Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, Sifuentes AJ, McDonald JG, Gordillo R, Scherer PE. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab. 2015;22:266-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 279] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 42. | Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 942] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 43. | Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54 Suppl 2:S73-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 44. | Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 833] [Cited by in RCA: 773] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 45. | Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1612] [Cited by in RCA: 1743] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 46. | Sung KC, Wild SH, Byrne CD. Resolution of fatty liver and risk of incident diabetes. J Clin Endocrinol Metab. 2013;98:3637-3643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 47. | Godoy-Matos AF, Silva Júnior WS, Valerio CM. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr. 2020;12:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 399] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 48. | Gastaldelli A, Gaggini M, DeFronzo RA. Role of Adipose Tissue Insulin Resistance in the Natural History of Type 2 Diabetes: Results From the San Antonio Metabolism Study. Diabetes. 2017;66:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 232] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 49. | Hedjazifar S, Khatib Shahidi R, Hammarstedt A, Bonnet L, Church C, Boucher J, Blüher M, Smith U. The Novel Adipokine Gremlin 1 Antagonizes Insulin Action and Is Increased in Type 2 Diabetes and NAFLD/NASH. Diabetes. 2020;69:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 50. | Cusi K. A diabetologist's perspective of non-alcoholic steatohepatitis (NASH): Knowledge gaps and future directions. Liver Int. 2020;40 Suppl 1:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 51. | Campo L, Eiseler S, Apfel T, Pyrsopoulos N. Fatty Liver Disease and Gut Microbiota: A Comprehensive Update. J Clin Transl Hepatol. 2019;7:56-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Knop FK. EJE PRIZE 2018: A gut feeling about glucagon. Eur J Endocrinol. 2018;178:R267-R280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 53. | Suppli MP, Bagger JI, Lund A, Demant M, van Hall G, Strandberg C, Kønig MJ, Rigbolt K, Langhoff JL, Wewer Albrechtsen NJ, Holst JJ, Vilsbøll T, Knop FK. Glucagon Resistance at the Level of Amino Acid Turnover in Obese Subjects With Hepatic Steatosis. Diabetes. 2020;69:1090-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 54. | Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, Gromada J, Vilstrup H, Knop FK, Holst JJ. The Liver-α-Cell Axis and Type 2 Diabetes. Endocr Rev. 2019;40:1353-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 55. | Xia MF, Bian H, Gao X. NAFLD and Diabetes: Two Sides of the Same Coin? Front Pharmacol. 2019;10:877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 56. | Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 653] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 57. | Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 417] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 58. | Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 457] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 59. | Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 541] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 60. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7542] [Article Influence: 838.0] [Reference Citation Analysis (0)] |
| 61. | Singh SP, Singh A, Pati GK, Misra B, Misra D, Kar SK, Panigrahi MK. A Study of Prevalence of Diabetes and Prediabetes in Patients of Non-Alcoholic Fatty Liver Disease and the Impact of Diabetes on Liver Histology in Coastal Eastern India. J Diab Mel. 2014;4:290-296. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2092] [Cited by in RCA: 2129] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 63. | Aleksandrova K, Boeing H, Nöthlings U, Jenab M, Fedirko V, Kaaks R, Lukanova A, Trichopoulou A, Trichopoulos D, Boffetta P, Trepo E, Westhpal S, Duarte-Salles T, Stepien M, Overvad K, Tjønneland A, Halkjaer J, Boutron-Ruault MC, Dossus L, Racine A, Lagiou P, Bamia C, Benetou V, Agnoli C, Palli D, Panico S, Tumino R, Vineis P, Bueno-de-Mesquita B, Peeters PH, Gram IT, Lund E, Weiderpass E, Quirós JR, Agudo A, Sánchez MJ, Gavrila D, Barricarte A, Dorronsoro M, Ohlsson B, Lindkvist B, Johansson A, Sund M, Khaw KT, Wareham N, Travis RC, Riboli E, Pischon T. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology. 2014;60:858-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 64. | Targher G, Marchesini G, Byrne CD. Risk of type 2 diabetes in patients with non-alcoholic fatty liver disease: Causal association or epiphenomenon? Diabetes Metab. 2016;42:142-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 65. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3720] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 66. | Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM; Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 368] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 67. | Juurinen L, Tiikkainen M, Häkkinen AM, Hakkarainen A, Yki-Järvinen H. Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E829-E835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 68. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1502] [Article Influence: 250.3] [Reference Citation Analysis (0)] |
| 69. | Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 408] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 70. | Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, Muggeo M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 71. | Williams KH, Burns K, Constantino M, Shackel NA, Prakoso E, Wong J, Wu T, George J, McCaughan GW, Twigg SM. An association of large-fibre peripheral nerve dysfunction with non-invasive measures of liver fibrosis secondary to non-alcoholic fatty liver disease in diabetes. J Diabetes Complications. 2015;29:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Ziegler D, Strom A, Kupriyanova Y, Bierwagen A, Bönhof GJ, Bódis K, Müssig K, Szendroedi J, Bobrov P, Markgraf DF, Hwang JH, Roden M; GDS Group. Association of Lower Cardiovagal Tone and Baroreflex Sensitivity With Higher Liver Fat Content Early in Type 2 Diabetes. J Clin Endocrinol Metab. 2018;103:1130-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Nobili V, Mantovani A, Cianfarani S, Alisi A, Mosca A, Sartorelli MR, Maffeis C, Loomba R, Byrne CD, Targher G. Prevalence of prediabetes and diabetes in children and adolescents with biopsy-proven non-alcoholic fatty liver disease. J Hepatol. 2019;71:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 74. | Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, Chan AW, Yeung MW, Chan JC, Kong AP, Wong VW. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 363] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 75. | Non-alcoholic Fatty Liver Disease Study Group; Lonardo A, Bellentani S, Argo CK, Ballestri S, Byrne CD, Caldwell SH, Cortez-Pinto H, Grieco A, Machado MV, Miele L, Targher G. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig Liver Dis. 2015;47:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 76. | Ciardullo S, Muraca E, Perra S, Bianconi E, Zerbini F, Oltolini A, Cannistraci R, Parmeggiani P, Manzoni G, Gastaldelli A, Lattuada G, Perseghin G. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 77. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1238] [Cited by in RCA: 2041] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 78. | Patel PJ, Cheng JC, Banh X, Gracen L, Radford-Smith D, Hossain F, Horsfall LU, Hayward KL, Williams S, Johnson T, Brown NN, Saad N, Stuart KA, Russell AW, Valery PC, Clouston AD, Irvine KM, Bernard A, Powell EE. Clinically Significant Fibrosis Is Associated With Longitudinal Increases in Fibrosis-4 and Nonalcoholic Fatty Liver Disease Fibrosis Scores. Clin Gastroenterol Hepatol. 2020;18:710-718.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 79. | Poynard T, Lassailly G, Diaz E, Clement K, Caïazzo R, Tordjman J, Munteanu M, Perazzo H, Demol B, Callafe R, Pattou F, Charlotte F, Bedossa P, Mathurin P, Ratziu V; FLIP consortium. Performance of biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in patients with severe obesity: meta analysis of individual patient data. PLoS One. 2012;7:e30325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 80. | Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 971] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 81. | Lombardi R, Airaghi L, Targher G, Serviddio G, Maffi G, Mantovani A, Maffeis C, Colecchia A, Villani R, Rinaldi L, Orsi E, Pisano G, Adinolfi LE, Fargion S, Fracanzani AL. Liver fibrosis by FibroScan® independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2020;40:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 82. | Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, Ajmera V, Bettencourt R, Collier S, Hooker J, Sy E, Rizo E, Richards L, Sirlin CB, Loomba R. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2018;67:1348-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 280] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 83. | Runge JH, Smits LP, Verheij J, Depla A, Kuiken SD, Baak BC, Nederveen AJ, Beuers U, Stoker J. MR Spectroscopy-derived Proton Density Fat Fraction Is Superior to Controlled Attenuation Parameter for Detecting and Grading Hepatic Steatosis. Radiology. 2018;286:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 84. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2613] [Article Influence: 201.0] [Reference Citation Analysis (1)] |
| 85. | Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 370] [Cited by in RCA: 469] [Article Influence: 42.6] [Reference Citation Analysis (2)] |
| 86. | American Diabetes Association. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S37-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 87. | Blank V, Petroff D, Beer S, Böhlig A, Heni M, Berg T, Bausback Y, Dietrich A, Tönjes A, Hollenbach M, Blüher M, Keim V, Wiegand J, Karlas T. Current NAFLD guidelines for risk stratification in diabetic patients have poor diagnostic discrimination. Sci Rep. 2020;10:18345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 1049] [Article Influence: 45.6] [Reference Citation Analysis (4)] |
| 89. | Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G, Fargion S. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 90. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1448] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 91. | Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 638] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 92. | Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, Poupon R, Cardoso AC, Marcellin P, Douvin C, de Ledinghen V, Trinchet JC, Beaugrand M. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012;19:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 93. | de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, Merrouche W, Foucher J, Brigitte le B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 94. | Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, Lee HS. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1070] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 95. | Nones RB, Ivantes CP, Pedroso MLA. Can FIB4 and NAFLD fibrosis scores help endocrinologists refer patients with non-alcoholic fat liver disease to a hepatologist? Arch Endocrinol Metab. 2017;61:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM, Tiniakos D, Burt A, Bugianesi E, Ratziu V, Day CP, Anstee QM. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 651] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 97. | Younossi ZM, Corey KE, Alkhouri N, Noureddin M, Jacobson I, Lam B, Clement S, Basu R, Gordon SC, Ravendhra N, Puri P, Rinella M, Scudera P, Singal AK, Henry L; US Members of the Global Nash Council. Clinical assessment for high-risk patients with non-alcoholic fatty liver disease in primary care and diabetology practices. Aliment Pharmacol Ther. 2020;52:513-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 98. | Tapper EB, Krajewski K, Lai M, Challies T, Kane R, Afdhal N, Lau D. Simple non-invasive biomarkers of advanced fibrosis in the evaluation of non-alcoholic fatty liver disease. Gastroenterol Rep (Oxf). 2014;2:276-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1610] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 100. | Shah S, Dhami-Shah H, Kamble S, Shukla A. FIB-4 cut-off of 1.3 may be inappropriate in a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2020;73:216-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 101. | Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, Doo E, Lavine J, Tonascia J, Loomba R. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015;38:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 102. | van Asten M, Verhaegh P, Koek G, Verbeek J. The increasing burden of NAFLD fibrosis in the general population: Time to bridge the gap between hepatologists and primary care. Hepatology. 2017;65:1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Wieland AC, Quallick M, Truesdale A, Mettler P, Bambha KM. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58:2809-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 104. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4954] [Article Influence: 707.7] [Reference Citation Analysis (9)] |
| 105. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 106. | Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Hecht J, Tio F, Cusi K. Liver Safety of Statins in Prediabetes or T2DM and Nonalcoholic Steatohepatitis: Post Hoc Analysis of a Randomized Trial. J Clin Endocrinol Metab. 2017;102:2950-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |