Published online Aug 15, 2021. doi: 10.4239/wjd.v12.i8.1325
Peer-review started: January 28, 2021
First decision: March 30, 2021
Revised: April 12, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: August 15, 2021
Processing time: 193 Days and 5.8 Hours
The prevalence of diabetes as a catastrophic disease in childhood is growing in the world. The search for novel biomarkers of β-cell failure has been an elusive task because it requires several clinical and biochemical measurements in order to integrate the risk of metabolic syndrome.
To determine which biomarkers are currently used to identify β-cell failure among children and adolescents with high risk factors for diabetes mellitus.
This systematic review was carried out using a modified version of the PICO protocol (Participants/Intervention/Comparison/Outcome). Once our research question was established, terms were individually researched on three different databases (PubMed, BIREME and Web of Science). The total articles obtained underwent a selection process from which the 78 most relevant articles were retrieved to undergo further analysis. They were assessed individually according to quality criteria.
First, we made the classification of the β-cell-failure biomarkers by the target tissue and the evolution of the disease, separating the biomarkers in relation to the types of diabetes. Second, we demonstrated that most biomarkers currently used as early signs of β-cell failure are those that concern local or systemic inflammation processes and oxidative stress as well as those related to endothelial dysfunction processes. Third, we explored the novelties of diabetes as a protein conformational disease and the novel biomarker called real human islet amyloid polypeptide amyloid oligomers. Finally, we ended with a discussion about the best practice of validation and individual control of using different types of biomarkers in type 1 and type 2 diabetes in order to assess the role they play in the progress of diabetes in childhood.
This review makes widely evident that most biomarkers currently used as early signs of β-cell failure are those that concern local or systemic inflammation processes and oxidative stress as well as those related to endothelial dysfunction processes. Landing in the clinical practice we propose that real human islet amyloid polypeptide amyloid oligomers is good for identifying patients with β-cell damage and potentially could substitute many biomarkers.
Core Tip: β-cell failure biomarkers have been an elusive task, and the searching of a molecule that will work as a biomarker and therapeutic target is a challenge. The data obtained in this study demonstrated that real human islet amyloid polypeptide amyloid oligomers could be used as a sensitive and specific marker for diabetes as a protein conformational disease.
- Citation: Calderón-Hernández MF, Altamirano-Bustamante NF, Revilla-Monsalve C, Mosquera-Andrade MB, Altamirano-Bustamante MM. What can we learn from β-cell failure biomarker application in diabetes in childhood? A systematic review. World J Diabetes 2021; 12(8): 1325-1362
- URL: https://www.wjgnet.com/1948-9358/full/v12/i8/1325.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i8.1325
The β-cell of the endocrine pancreas is responsible for the synthesis, storage and release of insulin into the bloodstream in response to metabolic demand. Human β-cells correspond to 50%-80% of pancreatic islets mass. However, this amount could vary depending on the species analyzed. The distribution of β-cells in humans is scattered, though predominantly in the tail of the pancreas. Furthermore, they present a very characteristic appearance with secretory granules on microscopic observation. Upon food intake, insulin is co-expressed and co-secreted with the human islet amyloid polypeptide (hIAPP), an intrinsically disordered protein of 37 residues in the β-cell to stimulate the internalization of glucose and amino acids, allowing the storage of glycogen, protein and lipid deposits, which are used to maintain energy demands during prolonged fasting. The hIAPP aggregation-oligomerization process causes apoptosis, disrupts the production of insulin[1-12] and forms amyloid oligomers and fibers in the pancreas of postmortem patients of diabetes mellitus (DM)[13-15].
DM represents a worldwide public health problem. In 2014, the World Health Organization estimated that 422 million people worldwide had diabetes, most of them presenting with type 2 DM (T2DM)[16].
Type 1 DM (T1DM) is a heterogeneous autoimmune disorder, which results from the gradual destruction of beta cell mass that ultimately results in absolute deficiency of insulin[17]. T1DM has shown to increase the risk two to four times of developing cardiovascular complications in comparison with a non-diabetic population. This condition causes early endothelial dysfunction as well as early atherosclerosis. Hence, these conditions may be used as early indicators for future cardiovascular events[18].
According to the International Diabetes Federation the incidence rate in T1DM is increasing steadily, particularly in the pediatric population. In 2019, 1106200 children and adolescents under 20-years-old were affected by T1DM worldwide thus worsening their life quality and presenting early in life with complications that may even result into life-threatening conditions[19]. The pathophysiology underlying the development of T1DM has been widely studied and has not yet been completely elucidated. T1DM is a T cell-driven autoimmune disease caused by a lack of tolerance towards β-cells in the pancreas. Different studies have shown the role of several chemokines, and interestingly β-cells are able to secrete specific CXC chemokines in response to inflammation states and thereby induce a vicious pathogenic loop[20].
Nonetheless, other studies have shown that dysfunction and/or death of pancreatic β-cells underlies the pathophysiology of all type of diabetes. β-cell failure in T2DM is thought to result as a compensatory mechanism of chronic peripheral insulin resistance. Furthermore, this is thought to cause loss of mature β-cell phenotype without necessarily causing cell death[21]. Different hypotheses underlying T2DM pathogenesis are still under debate: whether it comes as a result of reversible β-cell dysfunction or a decrease in β-cell mass[22].
Insulin resistance, which is worsened with obesity (which precedes chronic hyperglycemia), β-cell dysfunction and apoptosis (usually triggered by chronic inflammation and oxidative stress) are factors involved in the pathogenesis of T2DM[16]. Further study is required to understand the pathophysiology underlying the disease and to identify possible specific biomarkers of β-cell failure.
As DM represents a condition that increases the risk of cardiovascular complications, among a wide spread of other multisystem complications, the identification of early risk biomarkers, either of developing DM upon high-risk subjects or as prognostic factors of presenting complications associated with either T1DM and T2DM subjects, represents an important global challenge upon the scientific and medical community.
Despite the great effort and substantial progress that has been made in the understanding of the pathophysiology underlying the micro- and macrovascular complications of DM, further investigation is still required. Advanced glycation end products and their receptors, adhesive molecules, pro- and anti-inflammatory cytokines, enzymes such as N-acetyl-β-D-glucosaminidase and growth factors represent the main subject of ongoing studies[19].
The global escalation of childhood obesity is a major concern as it is believed to be the root of major cardiovascular complications and related mortality[23]. Pediatric obesity currently affects around 13%-20% of children and youth in western countries[24], and its prevalence continues to rise internationally[25]. It is associated with many deleterious health outcomes such as decreased neurocognitive function in childhood and increased risk of conformational diseases such as Alzheimer´s disease and other types of dementia. Notwithstanding that this chronic systemic inflammation state is closely linked to glycemic dysregulation and thus the initial steps on the patho
Several studies have shown the relationship between obesity and endothelial dysfunction, whereas insulin resistance has been proposed as a mediator of adiposity on vascular function. In physiologic conditions, insulin has vasodilator effects via nitric oxide (NO) production. Yet under insulin resistance conditions, NO production is impaired, and production of vasoconstrictors such as endothelin 1 are increased[26].
Obesity has been associated with a low-inflammation chronic state. Thus, inflammatory pathways could be critical for the understanding of the mechanisms underlying obesity and its complications. This inflammatory process originates mainly in the adipose tissue, and it secretes cytokines such as leptin and adiponectin[23]. Both of these cytokines have been widely studied as possible early biomarkers associated to β-cell failure.
Moreover, the worldwide prevalence of metabolic syndrome (MS) has a marked increase related to the severity of obesity, and it reaches 50% in young people presenting with severe obesity. Some studies have shown each unit of body mass index (BMI) increased is associated to the increased risk of presenting MS[23].
In this review, we aimed to identify the main biomarkers in the pediatric population as early signs of pancreatic β-cell failure in patients presenting with risk factors such as being overweight, obesity, diabetes and MS.
First, we made the classification of the β-cell-failure biomarkers by the target tissue and the evolution of the disease, separating the biomarkers according to the types of diabetes. Second, we demonstrated that most biomarkers currently used as early signs of β-cell failure are those that concern local or systemic inflammation processes and oxidative stress as well as those related to endothelial dysfunction processes. Third, we explored the novelties of diabetes as a protein conformational disease and the novel biomarker called real hIAPP amyloid oligomers (RIAO). Finally, we ended with a discussion about the best practice of validation, and individual control of using different types of biomarkers in type 1 and type 2 diabetes in order to assess the role they play in the progress of diabetes in childhood.
This systematic review was carried out using a modified version of the PICO (Participants/Intervention/Comparison/Outcome) protocol. On the first step we established our research question, which was: “What are the biomarkers of an early β pancreatic cell failure?”
No ethical approval or letter of informed consent were required to carry out this research, and all the files from the articles taken into account may be obtained from the different databases used.
The electronic databases used were PubMed, BIREME and Web of Science. The research started in February 2020. Following the PICO protocol the following terms were used for the research. Each term was individually searched for on each database. It should be clarified that for this review the C (Comparison) terms were excluded due to the fact that the goal of this research was not to compare biomarkers but rather look for new information regarding our research question.
P (Participants): Obesity, prediabetes, diabetes mellitus type 1, diabetes mellitus type 2, diabetes mellitus and metabolic syndrome.
I (Intervention): Metabolic biomarkers, biomarkers, cytotoxic oligomers, amyloid oligomers, protein aggregation, amyloid protein, coaggregation.
O (Outcome): β cell failure, Beta cell failure, Beta cell, B-cell, B-cell failure, glucotoxicity, lipotoxicity, endothelial dysfunction, fibrosis, apoptosis, inflammation, hyperfunction, dedifferentiation, comorbidities, retinopathy, microangiopathy, Macroangio
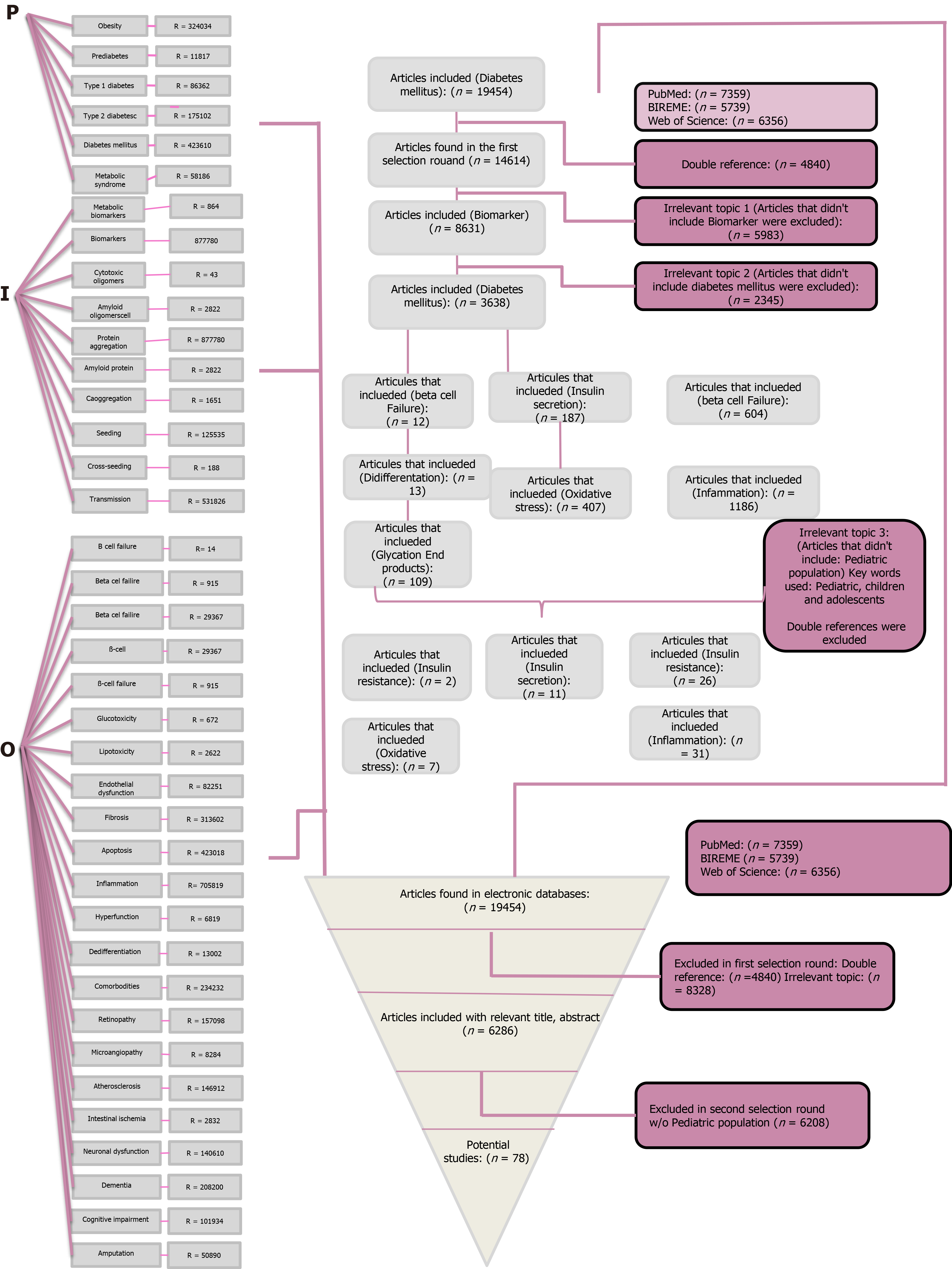
Each of the terms were individually researched on each database and saved as BibTex or RIS format for future reference. The terms belonging to the same group were researched with an algorithm using the Boolean operator “OR.” This algorithm of research was saved, and the references were saved as BibTex or RIS format as well and downloaded onto the Mendeley platform. Then within the groups of the PIO research the Boolean operator “AND” was used as well. The research for PIO was filtered by year of publication on a 10 year lapse (2010-2020) and by language (English and Spanish). The whole algorithm was saved, and the references were downloaded onto the Mendeley platform. A flow-chart was constructed in order to show the history of the research and the concepts used. This diagram describes in detail the research strategy, the key words used and moreover the number of articles resulting for each step of the process.
The total articles retrieved from the three electronic databases used were 19454. In order to obtain the most relevant articles each one of them underwent a selection process based on our initial research question. This process consisted of a three selection round process in which articles were excluded. For the first selection round, the double references were excluded. For the second selection round, all articles that had an irrelevant topic were excluded. Articles with the following terms were excluded following the next order: first the ones that didn´t include “Biomarker” and then “Diabetes Mellitus” in order to make the research more specific and reduce the number of articles. At this point, there were 3638 articles. More irrelevant topics were added to the exclusion, thus articles that did not include the following terms were excluded as well: β cell failure, insulin secretion, insulin resistance, dedifferentiation, oxidative stress, inflammation and glycation end products. Finally, articles that did not refer to the pediatric population were discarded as well, ending up with a 78 potential studies (Figure 1 and Table 1).
| Type 1 diabetes | |||||
| Ref. | Setting | Target population and sample size | Biomarker | Type of study | Quality (%) |
| Abdel-Moneim, et al[17], 2020 | Egypt | Sample size: 100 subjects; 70 children diagnosed with T1DM and 30 healthy control subjects | Inflammation biomarker (MDA); Oxidative stress biomarker (NO) | Transversal | 100 |
| Babar et al[18], 2011 | United States | Sample size: 36 children 21 with T1DM (aged 8.3 ± 0.3 yr with a diabetes duration of 4.3 ± 0.4 yr) and 15 group-matched healthy siblings (aged 7.6 ± 0.3 yr) | Vascular inflammatory biomarkers (hs-CRP, homocysteine C-reactive protein) | Transversal | 87.5 |
| Cabrera et al[39], 2018 | United States | Sample size: 42 pediatric subjects | Innate immunity | Cohort | 75.0 |
| Cazeau et al[29], 2016 | United States | Sample size: 9 children and adolescents aged 8-15 yr (mean age 12.9 ± 0.9 SD) with a mean T1DM duration of 5.0 ± 2.5 yr were included | Oxidative stress and endothelial dysfunction | Clinical trial/cohort | 100 |
| Cree-Green et al[41], 2018 | United States | Sample size: 35 youth subjects with T1DM [median age 16-yr-old) and 22 nondiabetic youth subjects of similar age | Insulin resistance | Observational | 100 |
| Cummings et al[51], 2018 | Canada | Participants (n = 199) were included in the observational arm of the Canadian AdDIT cohort | Urinary inflammatory biomarkers | Retrospective | 62.5 |
| Elbarbary et al[33], 2018 | Egypt | Sample size: 60 children and adolescents with T1DM (aged ≤ 18 yr with at least 5 yr disease duration). Patients were compared with 30 age- and sex-matched healthy controls | Neopterin | Cross-sectional | 75.0 |
| Heier et al[52], 2015 | Norway | Sample size: 299 patients with T1DM and 112 healthy controls at baseline and 241 patients and 128 controls at follow-up | AGEs methylglyoxal-derived hydroimidazolone-1 and carboxymethyllysine | Cohort | 62.5 |
| Ho et al[53], 2016 | Canada | Sample size: Children aged 8 to 17 yr with T1DM for at least 1 yr | Serum C-peptide, HbA1c, serum inflammatory markers (IL-6, IFN-gamma, TNF-alpha, and IL-10), GLP-1 and GLP-2, intestinal permeability | Single-center, randomized, double-blind, placebo-controlled trial | 62.5 |
| Hoffman et al[54], 2016 | United States | Sample size: 17 adolescents (8 F/9 M; age, 13.1 ± 1.6 yr (mean ± SD); duration, 4.8 ± 3.8 yr, BMI, 20.3 ± 3.1 kg/m2; HbA1c, 8.3% ± 1.2% | Inflammatory (C-reactive protein), oxidative (total anti-oxidative capacity) and endothelial injury (soluble intracellular adhesion molecule 1) markers | Cohort | 50.0 |
| Kingery et al[55], 2012 | United States | Sample size: 34 patients of European ancestry with new-onset T1DM, aged between 3 and 17 yr (10.70 ± 3.45), at Nationwide Children’s Hospital in Columbus, Ohio | Complement C4A and C4B | Longitudinal | 62.5 |
| Lakhter et al[35], 2018 | United States | Human experiment. Sample size: 16 healthy non-diabetic children (as a control group) and 19 children diagnosed with T1DM. Animal experiment: Serum was collected weekly from 8-wk-old female NOD mice until diabetes onset | miR-21-5pin Beta cell extracellular vesicle | Human experiment: cross-sectional; Animal experiment: longitudinal | 75.0 |
| Marchand et al[56], 2016 | France | Sample size: 22 children at onset of T1DM within 2 d of clinical diagnosis (immediate insulin-requiring diabetes with at least one positive autoantibody) and before subcutaneous insulin treatment and sera from 10 control subjects (no obese and nondiabetic child) | miRNA-375 | Transversal | 62.5 |
| Marek-Trzonkowska et al[57], 2016 | Poland | Sample size: 12 children with recently diagnosed T1DM as well as that of untreated subjects | C peptide | Cohort | 50.0 |
| Redondo et al[58], 2014 | United States | Sample size: 32 lean and 18 obese children (age range: 2-18 yr) with new-onset autoimmune T1DM and followed them for up to 2 yr | Leptin, total and high molecular weight adiponectin, omentin, resistin, chemerin, visfatin, cytokines [interferon-gamma, interleukin (IL)-10, IL-12, IL-6, IL-8, and tumor necrosis factor-alpha] and C-reactive protein | Cohort | 62.5 |
| Ryba-Stanisławowska et al[59], 2014 | Poland | Sample size: 47 young patients (mean age; 14.25 ± 3.50 yr) diagnosed with T1DM. Control group consisted of 28 age- and sex- matched individuals | IL-12, IL-18. Blood glucose levels, lipid status, C-reactive protein, HbA1c | Transversal | 62.5 |
| Singh et al[60], 2017 | United States | Sample size: 220 adolescents and children, half of which had lived with T1DM for an average of 7 years and half of which were healthy siblings | Leucine-rich alpha-2-glycoprotein, CD14, AZGP1, NAGA, CTSD, GM2A, GNS, CTSS | Cases and controls prospective | 50.0 |
| Şiraz et al[61], 2017 | Turkey | Sample size: 80 patients who had T1DM for at least 5 yr and who had no chronic complications or an autoimmune disorder | Fetuin-A, Hb1Ac | Transversal | 62.5 |
| Thorsen et al[20], 2014 | England | Sample size: 482 cases and 479 sibling frequencies matched on age and sample year distribution were included | Five different inflammatory chemokines (CCL2, CCL3, CCL4, CCL5 and CXCL8) | Cases and controls | 100 |
| Vorobjova et al[32], 2019 | Estonia | Sample size: 72 patients (median age 10.1 yr) who had undergone small bowel biopsy were studied | Inflammation markers. Association with Enterovirus infection. Immunity | Longitudinal | 87.5 |
| Wołoszyn-Durkiewicz et al[19], 2019 | Poland | NA | Advanced glycation end-products and their receptors, adhesive molecules, pro- and anti-inflammatory cytokines, growth factors or enzymes, such as N-acetyl-β-D-glucosaminidase | Review | 25.0 |
| Zhang et al[62], 2014 | United States | NA | Autoantibodies [pancreatic islet antigens (insulin, glutamic acid decarboxylase and/or tyrosine phosphatase islet antigen 2)] | Review | 37.5 |
| Type 1 and 2 diabetes | |||||
| Aburawi et al[30], 2016 | United Arab Emirates | Sample size: 181 subjects; 79 patients with T1DMs, 55 patients with T2DM and 47 control subjects | Endothelial dysfunction biomarkers (sICAM-1, and sVCAM-1). Inflammation biomarkers Interleukin-6, tumor necrosis factor-α, high-sensitivity C reactive protein | Transversal | 100 |
| Krapfenbauer[63], 2017 | Austria | NA | Novel protein chip technology | Review | 50.0 |
| Roberts et al[64], 2012 | Australia | NA | Glucose in respiratory fluids | Review | 12.5 |
| Type 2 diabetes | |||||
| Arenaza et al[36], 2017 | Spain | Sample size: 84 children (age 8-12-yr-old) with high risk of T2DM. Control (n = 42) or intervention (exercise) (n = 42) group | microRNA expression in circulating exosomes and in peripheral blood mononuclear cells | Randomized control trial | 87.5 |
| Arslanian et al[65], 2019 | United States | Sample size: 662 patients between 10-yr-old to 18-yr-old with DM for 2 yr (median duration 8 mo) with overweight or obesity | OGTT glucose response curve | Cohort | 62.5 |
| Bacha et al[40], 2014 | United States | Sample size: 90 obese adolescents (37 normal glucose tolerant, 27 with prediabetes and 26 T2DM); Mean age for adolescents with T2DM was 15.1 ± 0.4 yr and duration of diabetes was 10.8 ± 2.5 mo | Inflammatory markers (Leptin, Interleukin-6, VCAM-1, ICAM-1, E-selectin) | Transversal | 87.5 |
| Barbosa-Cortés et al[31], 2017 | Mexico | Sample size: 52 children (leukemia n = 26, lymphoma n = 26), who were within the first 5 yr after cessation of therapy. Median age of the subjects was 12.1 yr | Leptin. Adiponectin. Vascular cell adhesion molecule, intercellular cell adhesion molecule | Cohort | 100 |
| Berezin[66] 2016 | Ukraine | NA | Growth differentiation factor-15 | Review | 25.0 |
| Blum et al[21], 2014 | United States | Animal experiment: Sample: Mouse strains: C57BL/6, Lep ob/ob, Lepr db/db, Ins2akita, Insulin2-Cre transgenic mice, Ucn3-GFP transgenic mice | Urocorti3 | Cohort | 50.0 |
| Burke et al[67], 2017 | United States | Animal experiment: Sample: 9-wk-old C57BL/6j and 5-wk-old male db/ and db/db mice | B-cell dedifferentiation biomarkers | Cohort | 37.5 |
| Can et al[68], 2016 | Turkey | Sample size: 43 (18 males, 25 females) MetS adolescents between the ages of 13 and 17 yr (14.70 ± 1.15) and 43 lean controls were matched for age and sex | High-sensitive C-reactive protein, haptoglobin, α2-macroglobulin, platelet factor-4, fetuin-A, serum amyloid P and α1-acid glycoprotein | Transversal | 62.5 |
| Carlbom et al[34], 2017 | Sweden | Sample size: 39 participants. 31 individuals with T2DM divided in 4 groups based on their current DM treatment. Control group with 8 participants | Functional Beta cell mass | Clinical trial | 75.0 |
| Cui et al[69], 2018 | China | Human experiment: Sample size: 183 children and adults with obesity; Animal experiment: Obese ob/ob mice (3-wk-old to 4-wk-old), diabetic db/db mice (8-wk-old) and age-matched male C57BL/6J wild-type mice | Circulating microRNAs (miR-486, miR-146b and miR-15b), | Observational | 62.5 |
| DeBoer[70] 2013 | United States | NA | Fasting insulin, adiponectin, retinol-binding protein 4 | Review | - |
| Dentelli et al[27], 2013 | Germany | Sample size: 25 patients with T2DM and 15 non-diabetic participants who underwent abdominal surgery (gallbladder removal, in situ colorectal cancer) were included. Non-diabetic participants were used as controls | Adipose-derived stem cell pluripoten | Transversal | 75.0 |
| Fanjul et al[71], 2010 | France | Sample size: Human pancreases (n56) were harvested from brain- dead adult donors between 24 and 64-yr-old | Epithelial-mesenchymal transition biomarkers | Retrospective | 50.0 |
| Garanty-Bogacka et al[44], 2011 | Poland | Sample size: 50 obese children and adolescents (boys and girls), aged 8 yr to 18 yr | High-sensitive C-reactive protein, interleukin-6, fibrinogen, white blood count, glucose, insulin, insulin resistance index, glycosylated hemoglobin, lipids as well as systolic and diastolic blood pressure | Cohort | 50.0 |
| Garnett et al[72], 2010 | Australia | Sample size: 108 (54 each treatment arm) 10-yr-old to 17-yr-old with clinical features of insulin resistance and/or prediabetes | Insulin sensitivity | Randomized controlled clinical trial | 37.5 |
| Hansen et al[73], 2014 | Denmark | NA | Iron | Review | 25.0 |
| Ishida et al[74], 2017 | United States | Animal experiment: B6.BKS(D)-Leprdb/J heterozygous mice (db/+) were purchased from The Jackson Laboratory (Sacramento, CA) and bred to homozygosity to obtain wild-type, db/+, and db/db mice | Blood glucose and plasma insulin | Cohort | 50.0 |
| Jaberi-Douraki et al[75], 2014 | Canada | NA | Autoantibodies (Auto-antigen-2) | Review | 25.0 |
| Janem et al[43], 2017 | United States | Sample size: 3 pediatric cohorts ages 10-19 years were studied: lean (normal weight-C), obese (Ob) and obese with T2DM | Salivary glucose. Nitric oxide, CRP and IL-1B | Cohort | 62.5 |
| Kaya et al[76], 2017 | Turkey | Sample size: 50 obese adolescents with IR were included in the study | Serum lipids, adiponectin, and interleukin-6 levels | Transversal | 75.0 |
| Kim-Muller et al[38], 2016 | United States | Animal experiment. Groups: Mice were maintained in a mixed 120J-C57BL/6 background. Sample size calculations were based on the variance observed in prior experiments. No randomization or blinding was used | B cell dedifferentiation markers; Impaired mitochondrial function | Observational | 87.5 |
| Kim et al[77], 2016 | United States | Sample size: 277 obese adolescents without diabetes completed a 2-h OGTT and were categorized to either a monophasic or a biphasic group | In vivo insulin sensitivity, insulin secretion, and β-cell function relative to insulin sensitivity | Observational | 62.5 |
| Kuo et al[78], 2016 | United States | Animal experiment: Mice lacking the three FoxO isoforms in pancreas (TKO) using Tg (Ipf1-cre)1Tuv transgenics to excise the floxed Foxo1, Foxo3a, and Foxo4 genes | FoxO1, FoxO3a, and FoxO4 | Longitudinal | 62.5 |
| Mastrangelo et al[79], 2016 | Spain | Sample size: 60 prepubertal obese children (30 girls/30 boys, 50% IR and 50% non-IR in each group, but with similar BMIs) | 47 metabolites (Taurodeoxycholate, LysoPC, LysoPE, LysoPS, acetylcarnitine, biliverdin, pyruvate, lactate, alanine, proline, valine, isoleucine, tryptophan, tyrosine, arginine, aspartate, glutamate) | Transversal | 62.5 |
| Neelankal et al[80], 2017 | United States | Animal experiment: Pancreatic B cell line mouse insulinoma 6 | Insulin. GLUT-2, Pdx1 | Transversal | 62.5 |
| Park et al[81], 2016 | Korea | Sample size: 214 children 7-9 yr of age | Persistent organic pollutants | Cohort | 62.5 |
| Rachdi et al[82], 2014 | France | Animal experiment: mBACTgDyrk1A mice | DYRK1A | Cohort | - |
| Santoro et al[83], 2014 | United States | Sample size: 80 adolescents (age 13.30 ± 3.31 yr; BMI 33.0 ± 6.79 kg/m2) | Cytokeratin 18 | Transversal | 62.5 |
| Serbis et al[84], 2018 | United States | Sample size: 88 children with obesity divided into two groups according to 1h-GL during an OGTT: group 1 (n = 57) consisted of those with 1h-GL < 8.6 mmol/L and group 2 (n = 31) of those with 1h-GL ≥ 8.6 mmol/L | HOMA-IR, Matsuda index and Cederholm insulin sensitivity index. Adiponectin, leptin, visfatin and interleukin-6 together with lipid levels | Transversal | 62.5 |
| Sheng et al[37], 2016 | China | Animal experiment C57BLKS/J-Leprdb/Leprdb (db/db) and C57BLKS/J-Leprdb/m (db/m) male mice from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences (SLAC, CAS) | B-cell dedifferentiation | Longitudinal | 87.5 |
| Solimena et al[42], 2018 | Germany | Sample size: 103 organ donors, including 84 non-diabetic and 19 type 2 diabetic individuals, including 32 non-diabetic, 36 with type 2 diabetes, 15 with impaired glucose tolerance and 20 with recent-onset diabetes (< 1 yr) | Genes including TMEM37, which inhibited Ca2+ influx and insulin secretion in beta cells, and ARG2 and PPP1R1A | Retrospective | 62.5 |
| Spagnuolo et al[23], 2010 | Italy | Sample size: 34 children (25 males; median age 10.8 ± 3.4 yr) with severe obesity; Groups: Normal glucose tolerance (n = 10), Impaired glucose tolerance (n = 24) and T2DM (n = 7) | Systemic and bowel inflammation markers | Transversal | 75.0 |
| Stansfield et al[28], 2016 | United States | Sample size: 575 adolescents aged 14-18 yr (52% female, 46% black population) | Leptin. Adiponectin. C-reactive protein | Transversal | 87.5 |
| Tenenbaum and Fisman[85], 2012 | Israel | NA | PPAR isoforms | Review | NA |
| Walker et al[25], 2014 | Italy | Sample size: 33 children (mean BMI 28.1 ± 5.1 kg/m2 and mean age 11.6 ± 2.2 yr) with confirmed NAFLD. Bio | WAT inflammation biomarkers; Liver fibrosis biomarkers | Transversal | 100 |
| Walsh et al[24], 2018 | Canada | Sample size: 66 boys and 136 girls aged 14-18 yr (15.4 ± 1.4 yr), who volunteered for the HEARTY trial | Glucose, HOMA-B, HOMA-IS, HbA1c, insulin | Randomized controlled trial | 50.0 |
| White et al[22], 2013 | United States | Sample size: 3 individuals with diabetes and 5 nondiabetic control subjects | Insulin, vimentin, glucagon | Transversal | 50.0 |
| Xu et al[16], 2017 | China | Animal experiment: Male Sprague-Dawley rats. 5 experimental groups: Normal control group. Model control group. Ginseng oligopeptides: Low-dose group, Medium-dose group, High-dose group | Antioxidant biomarkers; NF-KB, Bax, cleaved Caspase-3 and Bcl2 | Clinical trial | 100 |
The potential studies from the first selection (n = 78) were evaluated individually according on whether they met the following criteria: (1) Clear objectives specified; (2) Research question; (3) Definition of the concepts that were researched and measured; (4) Extensive and detailed description of the methodology used; (5) Description of the measuring instruments (reliable and valid); (6) Sample size and characteristics of the target population and the subjects participating; (7) Analysis of missing values; and (8) Adequate statistical analysis.
For each met criteria 12.5 points were given, thus each article was graded out of 100 points. This data was organized in a table with just the most relevant information from each article, author(s), year of publication, setting, target population and sample size, biomarker, method of quantification and the quality score given (%).
In order to make the research more valuable, only articles with a score of 75 points or more were taken into consideration for a deeper analysis, which was synthesized in another table which included: author, year of publication and setting, biomarker analyzed, target molecule(s), antibodies/kits used, pretreatment before determination of biomarker, methods of quantifying, target population and sample size, exclusion criteria, if informed consent was signed, clinical data, relevant results, correlation with other biomarkers and sensibility/specificity (Figure 1).
This review process was initiated by retrieving articles from three different databases, PubMed (n = 7359), BIREME (n = 5739) and Web of Science (n = 6356) for a total of 19454 articles related to our initial research question “Which are the biomarkers of an early β pancreatic cell failure?” Using a modified version of the PICO protocol, the articles were put through a selection process in which they were discarded, first by double reference, then by multiple irrelevant topics and finally by excluding all articles that did not refer to the pediatric population. The 78 potential studies underwent a more exhaustive selection process in which (Figure 1) they were qualified over certain criteria, and only the articles (n = 20) with a score over 75 points were considered for a deep further analysis (Table 2). The methodology is described in detail in the corresponding section.
| Ref. | Setting | Biomarker | Target molecule | Antibodies/ kits | Pretreatment before determi | Methods of quantifying biomarkers | Target population/ sample size | Exclusion criteria | Written informed consent | Clinical data/Lab/Rx | Relevant results | Correlation with other biomarkers | Chronic complica |
| Type 1 diabetes | |||||||||||||
| Abdel-Moneim, et al[17], 2020 | Egypt | Inflammation biomarker (MDA); Oxidative stress biomarker (NO) | MDANO | NO and MDA → Colorimetric kits (BioVision, Milpitas, California, United States) | No pretreatment was specified | Colorimetry | Sample size: 100 subjects; 70 children diagnosed with T1DM and 30 healthy control subjects. Healthy subjects (control group) (n = 30, 2-16-yr-old), children newly diagnosed with T1DM (up to 1 yr) (n = 21, 3-14-yr-old) children with established T1DM (within 1.1 to 10.0 yr) (n = 49, 3-14-yr-old) | Infectious diseases, autoimmune disorders, allergies, respiratory disorders, thyroid dysfunction, hematologic disorders, malignancies and chronic cardiac, kidney and liver diseases | Yes | Body mass index; Sex and age; Time of DM1 diagnose; Fasting blood glucose; Glycated hemoglobin; Microalbu | This study demonstrates that the hematologic profile in pediatric patients with T1DM showed significant abnormalities such as decreased red blood cells, and platelet count, hemoglobin and hematocrit elevation as well as elevation of inflammation and oxidative stress markers, which can be used in early detection of children with T1DM at risk of complications | Erythrocyte count; Platelet count; Leucocyte count; Neutrophile count; Hemoglobin; Hematocrit; Red blood cell distribution width; Micro albuminuria | Nephropathy |
| Babar et al[18], 2011 | United States | Vascular inflammatory biomarkers (hs-CRP, Homocysteine C-reactive protein) | hs-CRP, Homocysteine C-reactive protein | hs-CRP → solid- phase ELISA from MP Biomedicals (West Chester, PA); Homocysteine→ Siemens Immulite platforms and kit (Diagnostic Products, Flanders, NJ) | No pretreatment was specified | Solid-phase ELISAs; Chemilumine | Sample size: 36 children, 21 with T1DM (aged 8.3 ± 0.3 yr with a diabetes duration of 4.3 ± 0.4 yr) and 15 group-matched healthy siblings (aged 7.6 ± 0.3 yr) | Known dyslipidemia, hypertension, microvascular complications, anemia (hemoglobin, 11.0 g/dL), congenital heart disease, allergy to ultrasound gel, or family history of hypercholesterolemia or premature cardiovascular disease | Yes | Age, sex, BMI, blood pressure, blood cell count, plasma glucose, HbA1c, lipids, hs-CRP, fibrinogen, chemistry panel, homocysteine, and erythrocyte folate, lipid profile. Diabetes duration in study group. Insulin regimen and dose | T1DM preadolescent children showed increased vascular inflammation and presented with higher fasting plasma glucose, HbA1c and hs-CRP; the flow-mediated dilatation was attenuated; this suggests endothelial dysfunction, harbingers of cardiovascular risk | Vascular inflammatory biomarkers. Endothelial dysfunction marker; Fasting plasma glucose, oral glucose tolerance curve, lipid profile, HbA1c, hs-CRP, fibrinogen, homocysteine, and erythrocyte (red blood cell) | Macroangio |
| Cazeau et al[29], 2016 | United States | Oxidative stress and endothelial dysfunction | Endothelial function/ dysfunction: (ECFCs: CD34+, CD133+, CD45-). Oxidative stress: TAOC, hs-CRP, endothelial progenitor cells | Antibodies (2.5 μmol/L of each), PE-AC133, FITC-CD34, and PECy5-CD45. Oxidative stress → TAOC (Biovision Research Products, Mountain View, CA) | A 50 μmol/L volume anticoagulated peripheral blood was incubated with 50 μmol/L 3% BSA in PBS (without Ca++ and Mg++) at room temperature for 30 min. Fluorescent antibodies were added and incubated for 30 min. FACS lysis buffer was added and incubated for another 30 min | ECFCs → polychromatic flow cytometry method | Sample size: 9 children and adolescents aged 8-15 yr (mean age 12.9 ± 0.9 SD) with a mean T1DM duration of 5.0 ± 2.5 yr were included | BMI ≥ 95%, BP > 95%, Tanner 1 or 5 pubertal status, pregnancy, smoking, history of oral hypoglycemic use, acanthosis nigricans, fasting c-peptide ≥ 0.4 ng/mL, abnormal thyroid function tests, random urine albumin to creati | Yes | Age, mean duration of diabetes, diabetes treatment (insulin), BMI, HbA1c, pubertal stage of Tanner stages 2-4, normal thyroid function tests, random urine albumin/creatinine ratio < 0.02, creatinine < 1 | This study demonstrated that there is no significant benefits for antioxidative therapy; there were no findings of decreased oxidative damage, improved endothelial function or increased vascular repair capacity | Endothelial function/dysfun | Prevention of Macroangio |
| Cree-Green et al[41], 2018 | United States | Insulin resistance | 2H5 glycerol and 6,6-2H2 glucose; hs-CRP; Adiponectin, leptin, and C-peptide | hs-CRP → immune-turbidimetric assay (Beckman Coulter, Brea, CA) | No pretreatment was specified | 2H5 glycerol and 6,6-2H2 glucose → gas chromatog | Sample size: 35 youth subjects with T1DM (median age 16-yr-old) and 22 non-diabetic youth subjects of similar age | ALT 80 IU/mL; blood pressure 140/90 mm Hg; hemoglobin, 9 mg/dL; serum creatinine.1.5 mg/dL; smoking; medications affecting IR, blood pressure, or lipids; and, in youths with T1DM, HbA1c, 12% | Yes | Age. Female/male, n/n (% female) Height, cm. Weight, kg. BMI, kg/m2. BMI percentile. Ethnicity. White Hispanic Black Asian. Tanner stage. Waist circumference, cm Waist-to-hip ratio. Lean mass %. Total body fat %. Liver fat. Visceral fat %. Daily METs from 3DPAR Accelerometer data. Sedentary Lifestyle, light, moderate, vigorous, very vigorous activity | Insulin resistance (adipose, hepatic and peripheral) was present in adolescents despite obesity or metabolic syndrome, an elevated lipolysis rate and endogenous glucose production and a lower peripheral glucose uptake supported these results. | Insulin sensitivity (adipose, hepatic and peripheral). Inflammation biomarkers | β-cell function |
| Elbarbary et al[33], 2018 | Egypt | Neopterin | Neopterin | Neopterin → (ELISA) using kit supplied by IBL International GmbH, Hamburg, Germany | Serum obtained from clotted samples by centrifugation for 15 min at 1000g was used for chemical analysis and stored at -20 °C until subsequent usein ELISA | ELISA | Sample size: 60 children and adolescents with T1DM (aged ≤ 18 yr with at least 5 yr disease duration). Patients were compared with 30 age- and sex-matched healthy controls | Chronic infection, neurological disease, history of drugs that may affect neural function, nutrition deficiency, liver or renal dysfunction, connective tissue disease or other autoimmune disorders and any other conditions that could influence hs-CRP. Patients with hs- CRP > 10 mg/L, other microvascular nephropathy and retinopathy and macrovascular diabetic complications | Yes | Age of onset of diabetes, disease duration, insulin therapy and chronic diabetic complications (nephropathy, neuropathy, retinopathy or cardiovascular ischemic events). Anthropo | Neopterin, a marker of inflammation and cellular response, was significantly higher in pediatric patients that presented with diabetic peripheral neuropathy, which demonstrates it may be used as an early biomarker for DPN. | Inflammation biomarkers; Cellular immune response; Neurological neuropathy | Diabetic; Neuropathy |
| Lakhter et al[35], 2018 | United States | miR-21-5p in Beta cell extracellular vesicle | miR-21-5pin Beta cell extracellular vesicle | EV isolation→ culture media using ExoQuick Tc reagent (System Biosciences, Palo Alto, CA, United States); miRNeasy and miScipt II RT kits (Qiagen, Valencia, CA, United States); Agilent Small RNA kit Bioanalyzer instrument (Agilent Technologies, Santa Clara, CA, United States) | To model the inflammatory milieu of T1DM, samples were exposed to cytokine mix consisting of 5 ng/ml IL-1β, 100 ng/ml IFN-γ and 10 ng/ml TNF-α (R&D Systems, Minneapolis, MN, United States); To inhibit cytokine- induced apoptosis, 25 μmoL/L of the pancaspase inhibitor Z-VAD-FMK (R&D Systems) | RNA isolation and reverse transcription; Quantitative real-time PCR. | Human experiment; Sample size: 16 healthy non-diabetic children (as a control group) and 19 children diagnosed with T1DM. Animal experiment; Serum was collected weekly from 8-wk-old female NOD mice until diabetes onset | Diabetic ketoacidosis requiring an intensive care unit stay, diabetes other than type 1 diabetes, history of prior chronic illness known to affect glucose metabolism or use of medications known to affect glucose metabolism | Yes | Age, sex, BMI percentile, HbA1c mmol/mol | This study demonstrated cytokine treatment in beta cell lines resulted in a 1.5-3.0 increase in miR-21-5p, however nanoparticle tracking showed that cytokines have no effect on the number of circulating miR-21-5p in beta cell extracellular vesicle cargo | miR-21-5p | Pancreatic reserve |
| Vorobjova et al[32], 2019 | Estonia | Inflammation markers; Association with Enterovirus infection; Immunity | 33 inflammation cytokinesanti-EV IgA and IgG; Density of Tregs and dendritic cells in small intestine mucosa | Cytokines → kits of Milliplex® MAP Magnetic BeadAnti | Patients’ sera were collected and stored at -80 °C prior to analyses. Microtiter plates (Nunc Immunoplate, Nunc, Glostrup, Denmark) were coated with the synthetic enterovirus peptide (KEVPALTA- VETGATC, Storkbio Ltd., Estonia) derived from an immunodomi | Immunohistochemistry; ELISAs | Sample size: 72 patients (median age 10.1 yr) who had undergone small bowel biopsy were studied. The study group consisted of 24 patients with CD (median age 6.5 years), 9 patients with CD + T1D (median age 7.0 years), two patients with T1DM (median age 8.5 years), and 37 patients (median age 14.0 years) with functional gastrointesti | No exclusion criteria were specified | Yes | Age, sex, status of small bowel mucosa | This study demonstrated a positive correlation between elevated cytokines (specially 12 of the 33 cytokines studied) in pediatric patients presenting T1DM and celiac disease | No other biomarkers were associated | Co-morbidities: celiac disease |
| Type 1 and 2 diabetes | |||||||||||||
| Aburawi et al[30], 2016 | United Arab Emirates | Endothelial dysfunction biomarkers (sICAM-1, and sVCAM-1). Inflammation biomarkers Interleukin-6, tumor necrosis factor-α high-sensitivity C-reactive protein | sICAM-1, sVCAM-1) | sICAM-1 and sVCAM-1→ ELISAs from R&D Systems (Human TNFα Quantikine, DTA00C) | No pretreatment was specified | ELISAs | Sample size: 181 subjects; 79 patients with T1DM, 55 patients with T2DM and 47 control subjects. Mean age was 20 ± 6 (age range 12-31-yr-old) | Active infection, chronic illness (e.g., rheumatoid arthritis, hyperthyroi | Yes | Age (12-31-yr-old). Anthropo | T1DM patients usually present subclinical inflammation and endothelial dysfunction (with higher levels of sICAM-1 and sVCAM-1). Poor control associates with higher levels of adiponectin and haptoglobin, while obesity correlated with lower levels of it but higher inflammatory and endothelial biomarkers | Glucose, HbA1c, low- density lipoprotein, high-density lipoprotein, triglycerides, total cholesterol. Adiponectin | Macroangio |
| Type 2 diabetes | |||||||||||||
| Arenaza et al[36], 2017 | Spain | microRNA expression in circulating exosomes and in peripheral blood mononuclear cells | miRNA in circulating exosomes | Exosomes → Total Exosome Kit (Thermo Fisher Scientific). Exosomal fractions → Qiagen miRNeasy kit. miRNA → RNA-seq methodology on Illumina’s MiSeq Next Generation Sequencing system | Exosomes were obtained from 1 mL of plasma usingthe Total Exosome Isolation Kit (Thermo Fisher Scientific) following manufac | FASTQ files generationand sequence alignment were performed using specific software packages (MiSeq Reporter, Bowtie, SAMtools, DESeq2 and miRDeep) | Sample size: 84 children (age 8-12-yr-old) with high risk of T2DM; Control (n = 42) or intervention (exercise) (n = 42) groups | Children with any medical condition that could affect the results of the study or that limits physical activity were excluded | Yes | Anthropome | The PREDIKID project helped to identify miRNAs potentially associated with early onset of IRS in overweight/ obese children overweight/ obesity | Fasting insulin, glucose and hemoglobin A1c; ectopic fat. Carotid intima-media thickness. Inflammation and biochemical cardiovascular disease risk factors | T2DM prediction: Macroangio |
| Bacha et al[40], 2014 | United States | Inflammatory markers (Leptin, Interleukin-6, VCAM-1, ICAM-1, E-selectin) | Leptin; Interleukin-6; VCAM-1 ICAM-1; E-selectin PAI-1 | Leptin → Radio- immunoassay (Linco Research Inc., St. Charles, MO) Il-6, VCAM-1, ICAM-1, and E-selectin → Double-sandwich ELISAs (R&D Systems, Minneapolis, MN); PAI-1 and TNF-a Cytometer– based platform (Luminex MAP 200; Milli- pore, St. Charles, MO) | No pretreatment was specified | Double-sandwich ELISAs | Sample size: 90 obese adolescents (37 normal glucose tolerant, 27 with prediabetes and 26 T2DM) Mean age for adolescents with T2DM was 15.1 ± 0.4 yr and duration of diabetes was 10.8 ± 2.5 mo | No exclusion criteria were specified | Yes | Male/female. AA/white. Smoking history (no/yes/not available) Age. BMI. Fat mass. Body fat (%). Total abdominal fat (cm2) SAT (cm2) VAT (cm2). Blood pressure, lipid profile, glucose levels, glycated hemoglobin, adiponectin, leptin, smoking history | Coronary artery calcifications were unexpected in pediatric population; in this study CAC+ group had higher BMI, waist circumference and fat mass, evidence of CAC highlight the increased cardiovascular risk in obese pediatric population | Subclinical atherosclerosis (vascular markers): CACs. Aortic pulse wave velocity. Carotid intima-media thickness | Macroangio |
| Barbosa-Cortés et al[31], 2017 | Mexico | Leptin; Adiponectin; VCAM; ICAM | Leptin; Adiponectin; VCAM; ICAM | Leptin and adiponectin levels, VCAM and ICAM → ELISA (Human Adiponectin, Leptin, VCAM and ICAM ELISA Kits, Millipore, St. Charles, MO, Unites States), | Serum aliquots were separated and frozen at -80 °C until biochemical determination | ELISAs | Sample size: 52 children (leukemia n = 26, lymphoma n = 26), who were within the first 5 yr after cessation of therapy. Median age of the subjects was 12.1 yr | Subjects with a relapse, second neoplasm or bone marrow transplants | Yes | Anthropome | This study revealed that there were diverse biomarkers, such as HOMA-IR, leptin and leptin/adipo | Insulin, HOMA-IR, Adiponectin, Leptin, Leptin to Adiponectin ratio Body fat. Glucose (mg/dL); Lipid profile | T2DM prediction |
| Cabrera et al[39], 2018 | United States | Innate immunity | CD4 lymphocytes; T-cells (CD4+/CD45RA−/FOXP3high) | PBMCs were stained with fixable LIVE/DEAD Violet dye followed by blocking Fc receptors and staining for anti-CD4, anti-CD25, anti-CD45RO, anti-CD45RA and anti-CD127 | Cryopreserved PBMCs were obtained from each of the subjects. PBMCs were pre-treated in medium with CTLA4-Ig (Bristol-Myers Squibb, New York, NY, Unites States) at 0 μg/mL, 25 μg/mL and 82 μg/mL for 45 min prior to the addition of plasma from a recently diagnosed local individual | LSR II flow cytometer (BD Bioscience) | Sample size: 42 pediatric subjects | No exclusion criteria were specified | Yes | Clinical data was not specified | The results of this study showed the existence of multiple T1DM subtypes, which differentiate by varying levels of innate inflammation and its association with C-peptide. It also demonstrated the different responsiveness to therapeutic intervention with CTLA4-Ig (abatacept) | C-peptide; Innate inflammatory activity | |
| Carlbom et al[34], 2017 | Sweden | Functional Beta cell mass | Functional Beta cell mass (HOMA2-B and HOMA2-IR) | Function of B cell mass → Intravenous arginine test and a glucose-potentiated arginine test | No pretreatment was specified | Arginine test and glucose-potentiated arginine test | Sample size: 39 participants. 31 individuals with T2DM divided in 4 groups based on their current DM treatment. Control group with 8 participants | No exclusion criteria were specified | Yes | Sex, age, diabetes duration (years), anti-diabetes treatment, BMI, plasma glucose, plasma c-peptide, HbA1c, HOMA, plasma cholesterol, plasma triglycerides, HDL cholesterol, LDL cholesterol | [11C]5-HTP uptake and retention in pancreas was a surrogate marker for the endogenous islet mass. Nonetheless, the major cause of B-cell failure in T2DM was attributed to cell dedifferentiation and not cell loss, which is why this is not mirrored by a decrease of [11C]5-HTP uptake in the pancreas | HbA1c levels, plasma glucose and plasma C-peptide levels | Function β-cell |
| Dentelli et al[27], 2013 | Germany | Adipose-derived stem cell pluripoten | Octamer-binding transcription factor and NANOG; NOX-generated reactive oxygen species | ASCs → FACS analysis (FACS-Calibur flow cytometer; BD Biosciences, San Jose, CA, United States) | Visceral N-ASCs were maintained in normal glucose DMEM(5 mmol/L D-glucose), high glucose DMEM (25 mmol/l D-glucose)or high mannitol DMEM (19 mmol/l D-mannitol, asosmotic control) | ASC proliferation → direct cell count (FACS); Apoptosis → ELISAs; mRNA isolation and quantitative real-time PCR;Western blot | Sample size: 25 patients with T2DM and 15 non-diabetic participants who underwent abdominal surgery (gallbladder removal, in situ colorectal cancer) were included. Non-diabetic participants were used as controls | No exclusion criteria were specified | Yes | Gender, age, BMI, HbA1c, triglycerides, total cholesterol, LDL and HDL cholesterol, creatinine, retinopathy, blood pressure, diabetes duration | This study demonstrated that adipose-derived stem cell were higher in diabetic patients than in control group as well as production of OCT4 and NANOG, which were upregulated, supporting the use of ASCs in regenerative medicine | Adipose-derived stem cells (ASCs); NOX-generated reactive oxygen species | |
| Kim-Muller et al[38], 2016 | United States | B cell dedifferentiation markers; Impaired mitochondrial function | Aldehyde dehydro | No antibodies or kits were specified | Cells were incubated with aldefluor and selected for RFP (red) and aldefluor (green) fluorescence, yielding ALDH andALDH+ cells | Immunohistochemistry | Animal experiment. Groups: Mice were maintained in a mixed 120J-C57BL/6 background. Sample size calculations were based on the variance observed in prior experiments. No randomization or blinding was used. | No exclusion criteria were specified | No | Not applied | This study demonstrated that in adolescents with obesity the risk of presenting T2DM was higher if they present a monophasic oral glucose tolerance test, they present higher glucose, insulin and C-peptide and lower in vivo hepatic and peripheral insulin sensitivity, thus this can be used as a biomarker for T2DM | Aldehyde dehydro | T2DM prediction |
| Sheng et al[37], 2016 | China | B-cell dedifferen | Glut2, Pdx1, Nkx6.1; MafA; Foxo1; GLP-1; PKC | Antibodies: polyclonal rabbit anti-Pdx1 Ab), polyclonal rabbit anti-MafA Ab), polyclonal rabbit anti-Nkx6.1 Ab, polyclonal rabbit anti-Glut2Ab, monoclonal rabbit anti-PKCζ Ab, monoclonal mouse anti-insulin Ab, polyclonal rabbit anti-glucagon Ab, polyclonal rabbit Ab and polyclonal rabbit anti-Foxo1 Ab | Pancreata were harvested and fixed in 4% buffered formaldehyde. Islets were incubated over a period of 60 min in 1 mL; Krebs-Ringer bicarbonate Hepes buffer (KRBH, 140 mMNaCl, 3.6 mM KCl, 0.5 mM NaH2PO4, 0.5 mM MgSO4,1.5 mM CaCl2, 2 mM NaHCO3, 10 mM Hepes (pH 7.4), and 0.25% BSA) containing 2.8 mmol/L glucose or 16.7 mmol/L glucose | Immuno | Animal experiment; C57BLKS/J-Leprdb/Leprdb (db/db) and C57BLKS/J-Leprdb/m (db/m) male mice from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences (SLAC, CAS) | Not applied | Not applied | Glucose tolerance tests; Serum insulin | This study demonstrated that Glut-2, MafA, Pdxl and Nkx6.1 were deactivated during B cell dedifferentiation; consequently the identification of small molecules that increase the expression of these factors may be useful in treatment of T2DM patients | No other biomarkers were associated | β-cell function |
| Spagnuolo et al[23], 2010 | Italy | Systemic and bowel inflammation markers | C-reactive protein; NO; Fecal calprotectin | Fecal concentration of calprotectin → (ELISA) test (Calprest Eurospital, SpA, Trieste, Italy | No pretreatment was specified | ELISAs | Sample size: 34 children (25 males; median age 10.8 ± 3.4 yr) with severe obesity; Groups: Normal glucose tolerance (n = 10), Impaired glucose tolerance (n = 24) and T2DM (n = 7) | Inflammatory bowel diseases and systemic or intestinal infections were excluded during the evaluation. | Not specified | Age, pubertal stage, BMI, oral glucose tolerance test; anthropo | This study demonstrated a positive correlation between obesity and glucose abnormalities with the elevation of biomarkers demonstrating intestinal inflammation in the pediatric population | C- reactive protein; Nitric oxide; Plasma insulin; HOMA-IR | Pancreatic reserve |
| Stansfield et al[28], 2016 | United States | Leptin; Adiponectin; C-reactive protein | Leptin; Adiponectin; C-reactive protein | Leptin → ELISA (R&D Systems, Minneapolis, Minnesota); Adiponectin → ELISA (Linco Research, St. Charles, Missouri); CRP → high-sensitive ELISA (ALPCO Diagnostics, Salem, New Hampshire) | No pretreatment was specified | ELISAs | Sample size: 575 adolescents aged 14-18 years (52% female, 46% black population) | If they were taking medications or had any medical conditions that could affect growth, maturation, physical activity, nutritional status or metabolism. | Yes | Age, sex, race, Tanner stage, BMI percentile, BMI category, physical activity and socioeconomic status and BP. Fasting serum glucose, HOMA-IR, plasma triglycerides, plasma total cholesterol, plasma HDL cholesterol, plasma LDL cholesterol serum leptin, plasma adiponectin and plasma C-reactive protein | This study demonstrated that there was no association between birth weight and the development of greater visceral adiposity and elevation of biomarkers implicated in insulin resistance once they reach an adolescent age | Birth weight, fasting blood samples were measured for glucose, insulin, lipids, adiponectin, leptin, and C-reactive protein | T2DM prediction |
| Walker et al[25], 2014 | Italy | WAT inflammation biomarkers; Liver fibrosis biomarkers | C-reactive protein, TNF-a, IL-6; Crown like structures in SAT | CRP → high sensitivity latex agglutination method on HITACHI 911 Analyzer (Sentinel Ch., Milan). TNF-a and IL-6 → sandwich ELISA (R&D System Europe Ltd, Abingdon, United Kingdom) | Biopsies were routinely processed (i.e. formalin-fixed and paraffin-embedded) and sections of liver tissue were stained with hematoxylin-eosin, Van Gieson, Periodic acid-Schiff diastase and Prussian blue stain | High sensitivity latex agglutination; Sandwich ELISAs | Sample size: 33 children (mean BMI 28.1 ± 5.1 kg/m2 and mean age 11.6 ± 2.2 yr) with confirmed NAFLD.Biopsy samples of abdominal (SAT) and liver were simulta | Adipose samples from 7 of the 40 children biopsied were not viable, and these participants were excluded from formal analysis. | Yes | Anthropo | This study demonstrated that the presence of crown like structures in liver biopsies were related to liver fibrosis but independent of BMI and this may contribute to diabetes risk by reducing insulin secretion. Liver fibrosis was associated with the presence of white adipose tissue inflammation, and this was strongly associated with obesity | HOMA; AST, ALT, total triglycerides, LDL, HDL, Adiponectin | T2DM prediction |
| Xu et al[16], 2017 | China | Antioxidant biomarkers; NF-KB, Bax, cleaved Caspase-3 and Bcl2 | Level of antioxidant SOD, MDA and GSP levels; Beta cell function was measured (HOMA). | SOD, MDA and GSP → kits Nanjing Jiancheng Biotechnology Institute (Nanjing, China). Antibodies for Bax, Bcl-2, cleaved Caspase-3 and NF-KB → Santa Cruz Biotechnology (Santa Cruz, CA, United States). Nuclear and cytoplasmic protein → ProteoJETTM Cytoplasmic and Nuclear Protein Extraction kit; (Fermentas International Inc., Burlington, ON, Canada). Protein content → BCA protein assay kit (Pierce Biotechnology, Rockford, United States) | Cells were scraped, pelleted by centrifugation at 250 g for 5 min, mixed with cell lysis buffer, homogenized, and set on ice for 10 min. Then, themixture was centrifuged at 500 g for 7 min at 4 C, and then the supernatant was further centrifuged at 20000 g for 15 min at 4 °C | Immunochemistry; Western Blot; ELISAs | Animal experiment; Male Sprague–Daw | Not exclusion criteria were specified | Not applied | Not applied | This study demonstrated that treatment with ginseng oligopeptides partially reversed abnormal oral glucose test tolerance in rats induced with T2DM and demonstrated an amelioration of the pancreatic damage and increased insulin content | Oral glucose tolerance, plasma glucose, serum insulin, ALT, AST, serum urea nitrogen, total cholesterol, triglycerides | T2DM prediction |
In a global view of the articles, all analyze a variety of biomarkers that may translate into an early β-cell failure. Target populations were similar across the articles. Most of them concerned pediatric subjects (children and adolescents) with either T1DM, T2DM, MS or at high risk of presenting whichever of this metabolic complications mainly due to the presence of obesity in any of its stages. A few of them involved older subjects (young adults in the third decade of life), and only one of them included an adult population (Dentelli et al[27]). Sample size was heterogeneous across the review. The largest one involved 575 adolescents (Stansfield et al[28]), whilst the smallest one involved only 9 children and adolescents (Cazeau et al[29]).
Then we classified the β-cell failure biomarkers according to the type of diabetes (Tables 1-3).
| Ref. | Setting | Biomarker | Insulin resistance | Beta cell mass | Insulin sensitivity |
| Type 1 diabetes | |||||
| Abdel-Moneim, et al[17], 2020 | Egypt | MDA; NO | IR was studied by measuring HbA1c and fasting glucose plasma levels, which were higher in the studied population | Not analyzed | Not reported |
| Babar et al[18], 2011 | United States | hs-CRP, Homocysteine | IR was analyzed indirectly by measuring the flow-mediated dilatation of the brachial artery, which had a positive relation with higher levels of Hb1A | Not analyzed | Not reported |
| Cabrera et al[39], 2018 | United States | Innate immunity activity (circulating activated regulatory T cells (CD4+/CD45RA−/FOXP3high) | IR was indirectly analyzed by the rate of C-peptide decline | Not analyzed | Not reported |
| Cazeau et al[29], 2016 | United States | Endothelial function/ dysfunction: (ECFCs: CD34+, CD133+, CD45-). Oxidative stress: TAOC, hs-CRP, EPCs | IR was analyzed by indirect biomarkers that relate to high levels of HbA1c such as adiponectin and hs-CRP | Not analyzed | Not reported |
| Cree-Green et al[41], 2018 | United States | hs-CRP, adiponectin, leptin and C-peptide | IR was assessed by insulin sensitivity four-phase HE clamp - glucose and glycerol rate of appearance, rate of disappearance and metabolic clearance rate over the last 30 min of each phase of the clamp | Not analyzed | IS was assessed with a four-phase HE clamp: a bolus of 4.5 mg/kg 6,6-2; H2-glucose followed by a continuous infusion at 0.03 mg/kg/min 6,6-2H2-glucose was paired with a primed (1.6 mmol/kg) then constant (0.11 mmol/kg/min), infusion of 2H5-glycerol. During the last 30 min of each of the four clamp phases, four samples, each 10 min apart, were drawn for glucose, glycerol, free fatty acid and insulin concentrations |
| Elbarbary et al[33], 2018 | Egypt | Neopterin | IR was indirectly analyzed with biomarkers related to higher levels of HbA1c such as dyslipidemia, hs-CRP and also fasting blood glucose | Not analyzed | Not reported |
| Lakhter et al[35], 2018 | United States | miR-21-5pin Beta cell extracellular vesicle | Not reported | Not analyzed | Not reported |
| Vorobjova et al[32], 2019 | Estonia | Inflammation markers; Association with Enterovirus infection; Immunity | IR was analyzed by indirect biomarkers that relate to high levels of HbA1c such as leptin | Not analyzed | Not reported |
| Type 1 and 2 diabetes | |||||
| Aburawi et al[30], 2016 | United Arab Emirates | sICAM-1, sVCAM-1, IL-6, TNF-α, hs-CRP | IR was analyzed by indirect biomarkers that relate to high levels of HbA1c such as adiponectin | Not analyzed | Not reported |
| Type 2 diabetes | |||||
| Arenaza et al[36], 2017 | Spain | miRNA expression in circulating exosomes and in peripheral blood mononuclear cells | IR was measured by the HOMA, which has been shown to be a reliable method in pediatric population. | Not analyzed | IS was indirectly measured by the amount of total, visceral and abdominal as well as hepatic and pancreatic adiposity. |
| Bacha et al[40], 2014 | United States | Leptin, IL-6, VCAM-1, ICAM-1, E-selectin | IR was measured by OGTT (1.75 g/kg, maximum 75 g Trutol), which was performed with glucose, insulin and C-peptide determination at 215, 0, 15, 30, 60 and 120 min | Not analyzed | IS analysis was measured using intravenous crystalline insulin, which was infused at a constant rate of 80 mU/m2/min, and plasma glucose was clamped at 100 mg/dL (5.6 mmol/L) with a variable rate infusion of 20% dextrose |
| Barbosa-Cortés et al[31], 2017 | México | Leptin, adiponectin, VCAM, ICAM | IR was measured directly measured based on the fasting insulin and glucose concentrations using the Homeostatic Model Assessment of Insulin Resistance [HOMA-IR = (fasting insulin (μU/ml) × fasting glucose (mmol/L)/22.5)] and indirectly by measuring serum levels of leptin and adiponectin | Not analyzed | Not reported |
| Carlbom et al[34], 2017 | Sweden | Functional Beta cell mass | IR was measured using the HOMA-IR | Intravenous arginine test and a glucose potentiated arginine test, which are considered the gold standard to assess the functional B-cell mass; Beta cell mass was analyzed using [11C]5-hydroxytryptophan positron emission tomography | IS was assessed using the HOMA2-S calculator previously calibrated so that 100% represents values obtained from young healthy adults |
| Dentelli et al[27], 2013 | Germany | Adipose-derived stem cell pluripoten | Not reported | Not analyzed | Not reports |
| Kim-Muller et al[38], 2016 | United States | Impaired mitochondrial function (Aldehyde dehydro | Not reported | Not analyzed | Not reported |
| Sheng et al[37], 2016 | China | B-cell dedifferentiation (Glut2, Pdx1, Nkx6.1, MafA, Foxo1, GLP-1, PKC) | IR was assessed by glucose tolerance tests. The mice were fasted for 12 h then they were injected intraperitoneally with 1 g kg−1 glucose. The glucose measurements were taken up to 2 h after injection | Pancreatic sections were analyzed by immunohistochemistry | Not reported |
| Spagnuolo et al[23], 2010 | Italy | C-reactive protein, NO, Fecal calprotectin | OGTT was performed with a load of 1.75 g/kg/body weight of glucose (maximum 75 g) after a 12 h overnight fast | Not analyzed | IS was estimated by the HOMA-IR index from fasting glucose and insulin concentrations according to the following formula: insulin (mU/L) × glucose (mmol/L)/22.5 |
| Stansfield et al[28], 2016 | United States | Leptin, adiponectin, C-reactive protein | IR was assessed using HOMA-IR. It was calculated by use of the formula: insulin (pmol/L) × glucose (mmol/L)/22.5.25 | Not analyzed | Not reported |
| Walker et al[25], 2014 | Italy | C-reactive protein, TNF-a, IL-6, Crown like structures (CLS) in SAT | IR was assessed by standard OGTT performed with 1.75 g of glucose per kg of body weight (up to 75 g), and glucose and insulin were measured at 0, 30, 60, 90 and 120 min | Not analyzed | IS was estimated by the HOMA |
| Xu et al[16], 2017 | China | Superoxide dismutase activity, MDA and glycated serum protein levels | IR was assessed performing OGTT and HOMA-IR model | Beta cell mass was analyzed with histopathology. For light microscopy, the fixed tissue samples were dehydrated through a graded ethanol series, embedded in paraffin and cut into 7 μm-thick sections with HE stain using a routine protocol | Not reported |
The majority of the selected papers (61%) corresponded to research studies regarding T2DM, whereas 35% of research protocols regarded T1DM. A striking result is that the inflammation biomarkers are shown predominantly in T1DM studies. The role of the biomarkers in T2DM is wider encompassing inflammatory, oxidative stress, endothelial dysfunction processes, insulin resistance and β-cell mass markers among others (Tables 2 and 3).
Our approach was to focus on the early and late damage of the β-cell failure in order to address the physiopathology of diabetes and the evolution of the complications and comorbidities in the pediatric population.
This review involved both experimental (clinical trials, cohort studies, among others) and observational studies (including 9 reviews); most of them implicated human clinical trials. There were only three pure animal experiments and one human and animal experiment considered for this review. Exclusion criteria for the studies was heterogeneous but mainly consisted in the presence of infectious diseases, other chronic comorbidities, rheumatologic diseases and the use of certain drugs. Informed consent was signed in all of the studies concerning human beings, either by patients (if they were > 18-years-old) or by parents or tutors for adolescents and younger children. The respective ethics committee in accordance with the Declaration of Helsinki had previously approved all of the articles included in this review.
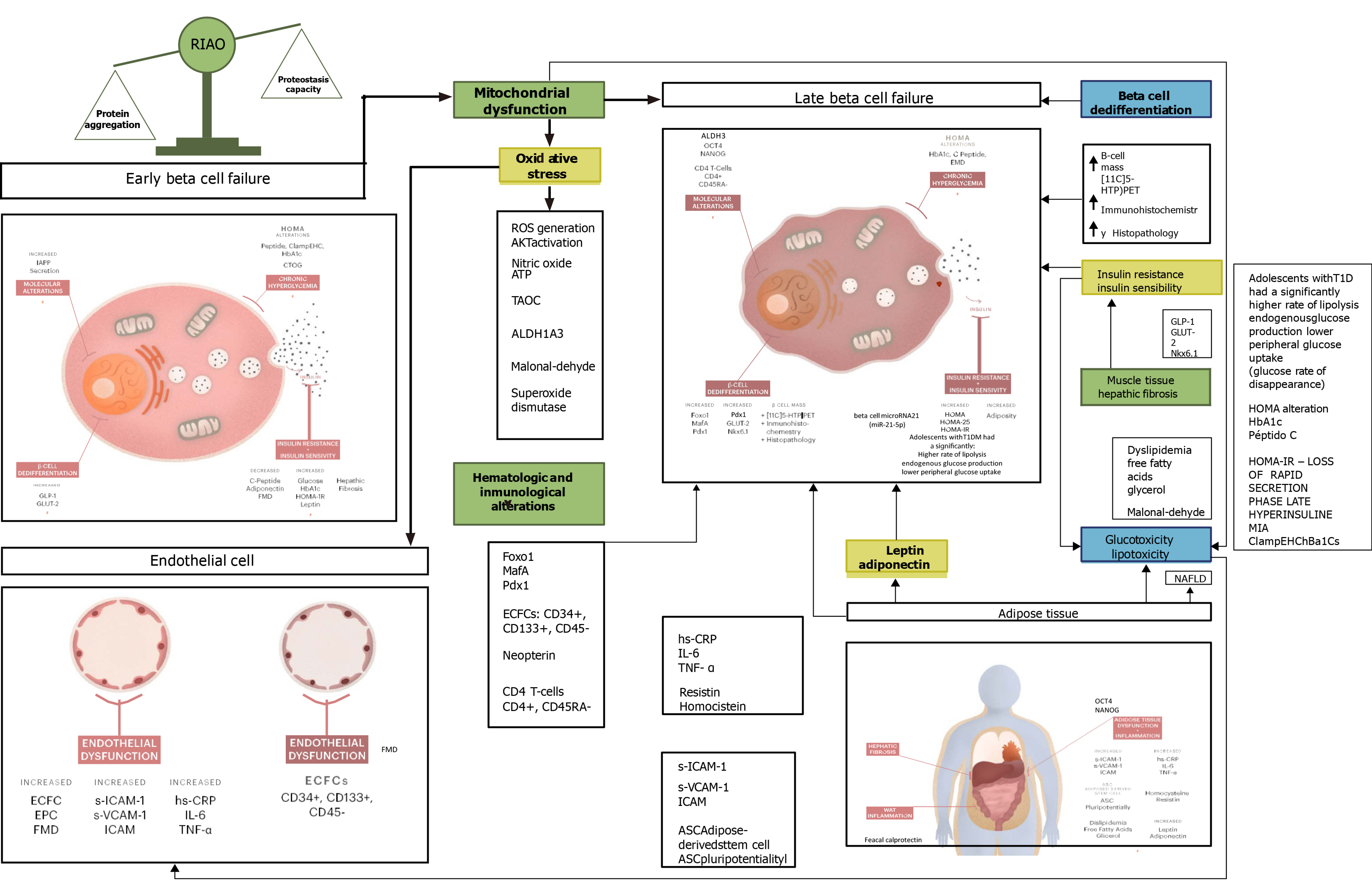
Whilst analyzing the articles considered for the final revision, it was widely evident that most of the biomarkers of β-cell failure currently being used and/or studied in pediatric populations are those that concern local or systemic inflammation processes and oxidative stress as well as those related to endothelial dysfunction processes. This correlates to the fact that most of the subjects enrolled for the different studies presented with elevated BMI, and they were classified as either overweight or obese in its different stages, thus, they sojourn a chronic systemic inflammation state. The main results are resumed in Table 2 and Figure 2.
The two most common biomarkers found in this review were leptin (Tables 1-3), a hormone produced by the adipose tissue and enterocytes, which has a characteristic decreased sensitivity on its receptors at the central nervous system level and adiponectin, an adipokine and protein hormone mainly produced in adipose tissue that reduces free fatty acid levels and promotes lipid metabolism[30] and is also known to modulate endothelial function. The excess of adipose tissue in conditions such as obesity alters the release of these adipokines, which are known to increase insulin sensitivity and vascular functionality, thereby when decreased increasing the risk of metabolic complications[31].
The second most analyzed biomarkers were the ones referring to endothelial inflammation and dysfunction (Tables 1-3), such as cytoadhesive molecules [(soluble Intracellular cell adhesion molecule (ICAM-1) and soluble vascular cell adhesion molecule] and systemic inflammation markers such as interleukin-6 (IL-6), tumor necrosis factor α, high-sensitivity C-reactive protein, homocysteine and E-selectin. ICAM-1 is a glycoprotein involved in tissue adhesion and is expressed in response to the release of cytokines. Therefore, it has been used as a biomarker for inflammation. Its soluble form may estimate its tissue concentration from a blood sample. A wide cross-sectional study involving 79 patients with type 1 diabetes, 55 patients with type 2 diabetes and 47 controls with mean age 20 ± 6 years demonstrated that patients with diabetes had higher levels of cytoadhesive molecules (soluble ICAM-1 and soluble vascular cell adhesion molecule-1, P < 0.001), adiponectin (P < 0.001) and haptoglobin (P = 0.023). Furthermore, hemoglobin A1c (HbA1c) > 8.0% (estimated average blood glucose > 10 mmol/L) was associated with higher adiponectin (P < 0.001), and obesity was associated with lower adiponectin (P < 0.001). As expected, obesity had a positive correlation with soluble ICAM-1 (P = 0.015) and also presented with higher levels of inflammation markers such as tumor necrosis factor α, IL-6 and high-sensitivity C-reactive protein[27] (Table 2).
Endothelial dysfunction and early atherosclerosis has been shown to be common among patients with DM (Tables 1-3). One study[18] analyzed a sample of 21 type 1 children with T1DM and 15 group-matched healthy siblings. Flow-mediated dilatation of the brachial artery and carotid intima-media thickness were measured. The results showed that children with T1DM had lower flow-mediated dilatation (%) than control children (7.1% ± 0.8% vs 9.8% ± 1.1%; P = 0.04) thus displaying evidence of low-intensity vascular inflammation, which translates long-term into an increased cardiovascular risk.
Chronic inflammation represents an important factor to be considered in the context of patients with DM. T1DM being an autoimmune disease has been associated with other autoimmune diseases. One study[32] studied the association between T1DM and Celiac disease (CD). They analyzed a sample of seventy two patients (median age 10.1 years) who had undergone small bowel biopsy from which 24 patients with CD (median age 6.5 years), 9 patients with CD + T1DM (median age 7.0 years), 2 patients with T1DM (median age 8.5 years), and 37 patients (median age 14.0 years) with functional gastrointestinal disorders and a normal small bowel mucosa as controls. Their most relevant finding was the significant increase of several proinflammatory cytokines and chemokines like IL-15, IL-8, IL-17F, IP-10 and MIP-1β in CD patients and its highly significant correlation with the grade of small bowel mucosa atrophy, which plays a major role as a sign of small bowel mucosa inflammation and damage. CD patients with coexisting T1DM showed particularly higher levels of IL-15, IL-17F, MIP-1β and sIL-2Rα compared to the control group.
Another cross-sectional study included 60 children and adolescents with T1DM (aged ≤ 18 years with at least 5 years disease duration), and they studied a particular biomarker of inflammation and cellular immune response, Neopterin. It is elevated in conditions of T cell or macrophage activation, such as diabetic peripheral neuropathy (DPN), which is a condition associated to both inflammation and immune processes. The participants were subjected to a neurological assessment using nerve conduction studies and the neuropathy disability score. Frequency of DPN in patients with T1DM according to neuropathy disability score was 40 (66.7%) patients out of 60, while nerve conduction studies confirmed that only 30 of those 40 patients presented this complication. Neopterin levels were significantly higher in patients with T1DM compared to the control group[33].
Another commonly measured biomarker was the lever of oxidative stress, which was measured by different molecules such as NO, malondialdehyde (MDA), total plasma antioxidant capacity and superoxide dismutase (Tables 1-3)[17]. The authors demonstrated a positive relation between NO and MDA and hematologic abnormalities such as decreased levels of erythrocyte and platelets counts. Moreover, a depletion in hemoglobin and hematocrit index contrasted with an increased leukocyte count in children with T1DM.
In one study[23], a cohort of 34 children (25 males; median age 10.8 ± 3.4 years) with severe obesity (BMI > 95%) was screened for diabetes with oral glucose tolerance test, of 1.75 g/kg/body weight of glucose (maximum 75 gr) after a 12 h overnight fast, and acute biomarker of systemic inflammation with C-reactive protein (CRP) and gut inflammation were determined. They found that fecal calprotectin was increased in 47% of patients (mean 77 ± 68 μg/g) and in 50% of children with abnormal glucose metabolism (mean 76 ± 68 μg/g), with a correlation with increasing BMI z-score. NO was pathological in 88% and in 87.5% of glucose impairment (mean 6.8 ± 5 μmol/L).
In the context of oxidative stress, there was one study that analyzed a small cohort composed of 9 children and adolescents aged 8-15 years (mean age 12.9 ± 0.9) with a mean T1DM duration of 5.0 ± 2.5 years. The authors hypothesized that antioxidant vitamin therapy could decrease oxidative damage thereby improving endothelial function and vascular repair capacity. Notwithstanding their hypothesis proved to be incorrect as they showed no changes in post occlusive forearm vascular resistance (FVR), hs-CRP, total antioxidant capacity (TAC), adiponectin or endothelial colony-forming cells[29].
Functional beta cell mass was another common biomarker measured in the articles included in this review using the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), which is a way of assessing insulin resistance. This implicates an indirect way of evaluating β-cell function by using two laboratory parameters: fasting levels of glucose and insulin. The HOMA-IR, in one study of Mexican children survivors of acute lymphoblastic leukemia and lymphoma, was the only predictive factor of developing MS at 6 mo. It showed that a higher HOMA correlated with an increase of 5.1 mg of fasting glucose, 2.5 cm of abdominal perimeter and higher levels of leptin[31] (Tables 2 and 3). On another cross-sectional study analyzing patients at different stages of T2DM, two different HOMA indexes were used, HOMA2-B as an estimate of the β-cell steady-state function and HOMA2-S as an estimate of insulin sensitivity, both of them reported as percentage (assuming 100% corresponds to young healthy adults). Both were significantly decreased in the different study groups compared to the control group[32]. HOMA-IR and HOMA-B were analyzed as well on a pure animal study that analyzed the potential beneficial effects of Panax-ginseng treatment. Their results showed that treatment with Panax-ginseng oligopeptides may decrease HOMA-IR, whereas it could increase HOMA-B values in T2DM-induced rats[16] (Tables 2 and 3).
A few articles studied molecular biomarkers such as microRNA (miR) expression in circulating exosomes and miR-21-5 in β-cell extracellular vesicles (EV)[34,35]. They carried out a human and animal experiment in which they hypothesized that the inflammatory milieu of developing diabetes may also increase miR-21-5p in β-cell EV cargo and that circulating EV miR-21-5p would be increased during type 1 diabetes development. Their results showed that β-cell EV miR-21-5p cargo is increased in response to treatment with inflammatory cytokines. This increase was predominantly due to cytokine-induced effects on β-cell exosome miR-21-5p and was only partially blocked by inhibition of apoptosis. They found this to be a promising biomarker in the early development of T1DM. Additionally their findings highlight that, for certain miRNAs, total circulating miRNA levels are distinct from circulating EV miRNA content (Tables 2 and 3).
Analogously in the PREDIKID project (Arenaza et al[36]), a cohort of 84 children, aged 8-12 years, with a high risk of T2DM was randomly assigned to control group (n = 42) and intervention (n = 42). Both groups were submitted to a lifestyle education and psycho-educational program (2 d/mo), while the study group underwent an extra exercise program (3 d/wk, 90 min per session including warm-up, moderate-to-vigorous aerobic activities and strength exercises). miRNA species were assessed before and after interventions. This project aimed to identify miRNAs potentially associated with early onset of insulin resistance syndrome in overweight/obese children (Tables 2 and 3).
Two studies in particular evaluated β-cell dedifferentiation. The first one[37,38], which was an animal model study, used Glut2, Pdx1, Nkx6.1, MafA, Foxo1, GLP-1 and PKC as target molecules, and the second one[35] used aldehyde dehydro
One study[39] analyzed the relationship between T1DM in a pediatric population and innate immunity using as target molecules CD4 T lymphocytes (CD4+/ CD45RA−/FOXP3 high). They aimed to determine the existence of different subtypes of T1DM based on immunoregulatory profiles at clinical onset of the disease. They concluded from their data that multiple subtypes of T1DM exist, and they are characterized from their baseline innate inflammatory response as well as C-peptide decline rate obtained. Lower innate inflammatory bias at the time of clinical onset was more likely to present slower rates of decline in residual insulin secretion (Tables 2 and 3).
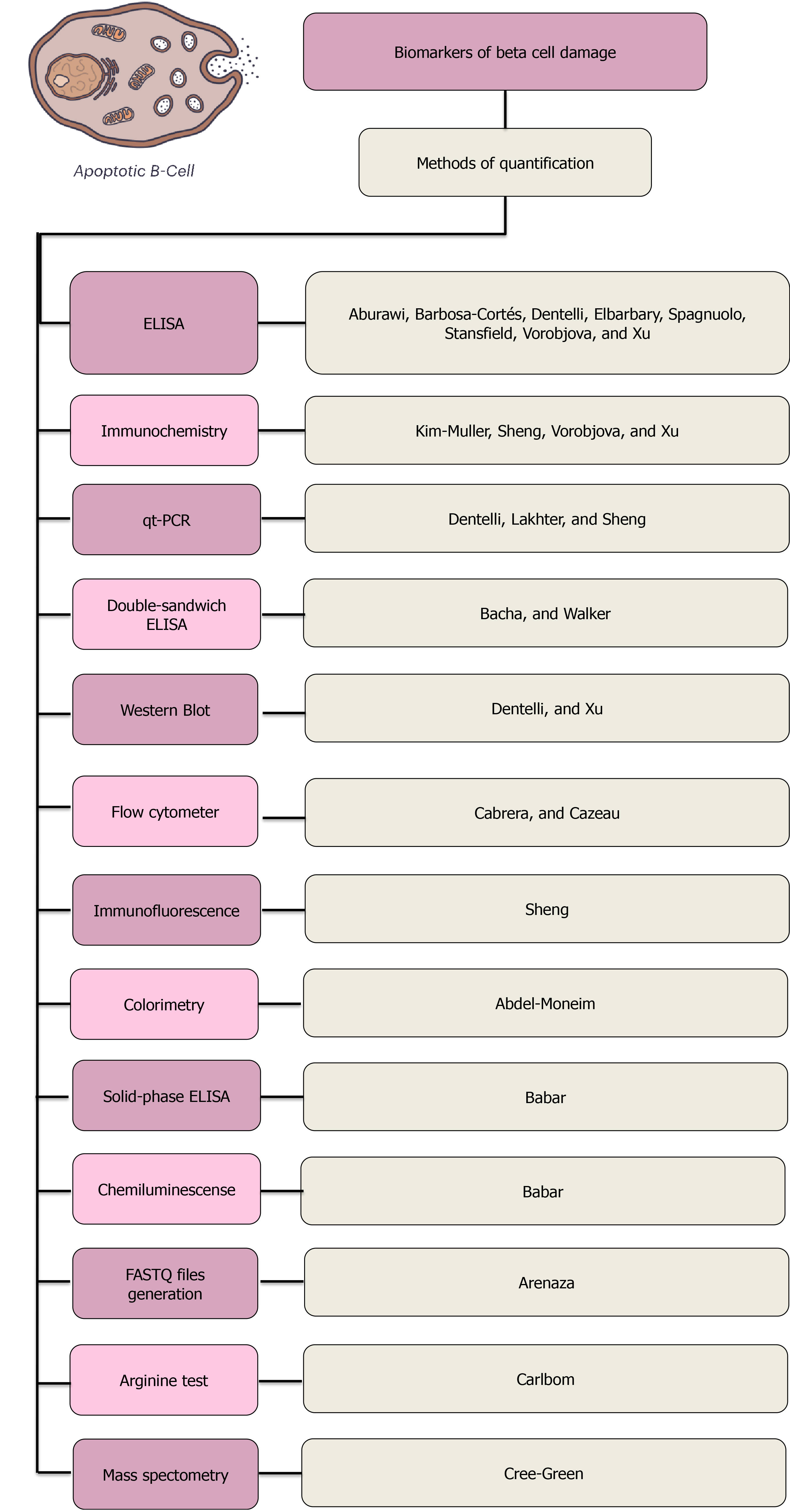
The most common method of quantifying biomarkers found on this review was the enzyme-linked immunosorbent assay, commonly known as ELISA, a common analytical biochemistry assay that detects the presence of a ligand that is usually a protein using antibodies against the protein to be measured. The type of ELISAs used were diverse, from simple forms of the assays to solid-phase and double-sandwich ELISAs. The second method most commonly used was immunochemistry and then quantitative PCR analysis. Less commonly used methods were colorimetry, chemiluminescence, LSR flow cytometer, arginine test and glucose-potentiated arginine test, gas chromatography-mass spectrometry, Western Blot and latex agglutination assays, which were found in either one or two studies. Sixty percent (n = 12) of the studies evaluated in this review analyzed their corresponding biomarkers after applying a pretreatment to the samples used. Most of them used corporal fluids such as peripheral venous blood, plasma and serum collected previously from patients. The majority of them were taken under fasting conditions of the subjects. Three studies used tissue obtained from biopsies, two from pancreatic tissue and one from hepatic tissue[27]. Though they did not analyze an exclusively pediatric population, they studied a cohort of patients with DM who underwent abdominal surgery. They analyzed the effects of high glucose-mediated effects upon adipose-derived stem cells (ASCs). In this study, they compared DASCs (diabetic participants) with N-ASCs (non-diabetic participants). ASC availability was first evaluated, and an average (mean ± standard error) of 9.16 × 104 ± 9.20 × 104 and 5.95 × 104 ± 7.50 × 104 ASCs per gram of visceral adipose tissue were isolated from patients and controls, respectively. The biomarkers used were levels of reactive oxygen species in ASCs.
The diagnostic methods used in the articles analyzed for this review are summarized in Figure 3.
As mentioned before, most of the biomarkers analyzed in the different studies reviewed here may be assessed or measured using widely standardized tests such as ELISA.
One of the fundamental aspects when dealing with a pediatric population, which is of utmost importance, is to be able to perform tests with the least possible invasion. Hence, most of the biomarkers analyzed in the studies presented for this review have the advantage that they can be measured by simply obtaining a peripheral blood sample, which is not only a minimally invasive procedure but also a low-cost one. This is also the case for markers of systemic and intestinal inflammation[23], which are rectal NO and fecal calprotectin. They are non-invasive, reproducible and non-discomfortable tests, highly suitable for the pediatric population.
One study[25] that showed a positive correlation between subcutaneous adipose inflammation and the extent of liver fibrosis in children is quite promising due to the fact that it may be a way of estimating the degree of inflammation and liver fibrosis without the need for a liver biopsy in patients at risk of presenting metabolic complications.
Most of the biomarkers analyzed in the articles included for this review were measured in humans, and its replicability is wide and approved for research in children.
One of the main limitations in a global view of this research is the possibility to extrapolate most of the results to the Mexican population because only one of the articles found was performed in México[31]. By itself, this represents a significant bias due to the fact that it studies a very restricted population. It analyzes the risk of acute lymphoblastic leukemia and lymphoma of developing metabolic abnormalities such as obesity, insulin resistance, diabetes and MS. Although most of the protocols included subjects with either overweight or obese patients, it should be highlighted that the risk between Hispanic and other populations is different due to the fact that being Hispanic represents an independent risk factor for DM.
Some of the biomarkers analyzed, which bear to be very promising on identifying patients at most risk of developing complications secondary to obesity such as insulin resistance syndrome and/or DM, are still under study. Such is the case of miRNA in circulating exosomes[36]. In the PREDIKID protocol, a cohort of children at high risk of developing T2DM was analyzed. They measured the levels of miRNA before and after a multidisciplinary program involving interventions such as regular physical activity, dietary assessment and changes in sedentary behaviors. Despite the fact that this marker has been proposed as very promising, their study is still underway, and further analysis should be performed in diverse populations.
When it comes to studies regarding pediatric populations, something that must be taken into consideration is the degree of invasiveness of the procedures. Even though it was shown that neopterin is a potential early marker of DPN[33], further studies are needed and the point of comparison should be the gold standard, electromyography, which represents a painful procedure and thus difficult to perform on a pediatric population.
The heterogeneity of the sample population among the different protocols may be a significant bias for this review too because we included protocols with small sample sizes, and its statistical significance may be questionable[29]. Reports also stated that a short study duration was a limitation. For example, a study that used vitamin C and E supplementation for 6 wk reported that the short study may have been inadequate to significantly decrease oxidative damage, improve endothelial function and increase vascular repair capacity.
Specifically, one study presented an important limitation regarding the main purposes of this review. They analyzed a sample of 25 patients with T2DM and 15 non-diabetic participants who underwent abdominal surgery. Thus most of the studied population did not involve pediatric patients[27].
Three pure animal experiments were taken into account for this review, thus further studies in humans are needed to validate the applicability of the biomarkers studied. Such is the case of the study that observed the striking enrichment in the expression of ALDH1A3 is crucial as β-cells become dedifferentiated[38]. Another study that analyzed β-cell dedifferentiation was conducted in mice. Results showed that chronic euglycemia was maintained in the mice group with long-term caloric-restriction intervention. β-cell dedifferentiation was significantly reduced, while the expression of Glut2, Pdx1 and Nkx6.1 was reversed. On the other hand, MafA expression was significantly increased with long-term caloric restriction[37]. The third animal experiment[16] aimed to determine whether treatment with ginseng oligopeptides could modulate hyperglycemia related to T2DM in rats induced by high-fat diet and low doses of alloxan. Another important limitation is that treatment with ginseng is not yet approved for use in pediatric populations.
One of the main results shown by this review (Table 2) was that subclinical inflammation and endothelial dysfunction are indubitably common among young patients with diabetes as shown by Aburawi et al[30]. They particularly demonstrated a positive correlation within obesity and high levels of soluble ICAM-1 and inflammatory biomarkers such as tumor necrosis factor α, IL-6 and high-sensitivity C-reactive protein, whilst high levels of adiponectin were shown in patients with poor control of diabetes presenting HbA1c > 8%. An intriguing result shown by this study was the possible damping effect that obesity has with levels of adiponectin, which is usually high during hyperglycemia but showed a negative correlation with obesity whilst this condition was associated with higher levels of inflammatory biomarkers. There was one study[18] that demonstrated in a cross-sectional study that vascular homeostasis was disrupted in T1DM even in early stages of the disease. They studied a pediatric population with a mean duration of DM of 4.3 years. They proposed in this study that although high-sensitivity CRP is an important vascular inflammation marker, it is a low intensity marker inasmuch as its elevation has a wide differential diagnosis (Table 2 and Figure 2).
Most of the studies found and taken into account for this research have a solid focus on finding biomarkers that could aid into the treatment but most likely into the secondary prevention of patients with T1DM and T2DM who are known to have an elevated risk of presenting cardiovascular disease (CVD) and other chronic comorbidities such as diabetic kidney failure and peripheral neuropathies[30]. Inflammation biomarkers as an independent predictor of adverse effects in diabetic patients emphasize the need of early interventions, such as weight loss and regular exercise in patients at risk. This was actually followed up in the PREDIKID project[36], where they aimed to demonstrate that early interventions involving positive changes in lifestyles of patients at risk have a positive impact in preventing all type of complications associated with diabetes. The biomarker used was the expression of different species of miRNA measured before and after the interventions. Thus far, the involvement of these RNA particles in the regulation of insulin resistance is still under study (Tables 2 and 3).
There was one study[40] that demonstrated in a larger cohort of adolescents with obesity the presence of increased cardiovascular risk, and they associated subclinical atherosclerosis manifesting as coronary artery calcifications with obesity rather than hyperglycemia. There was another study[18] that despite the fact that their cohort was significatively minor, they demonstrated that flow-mediated dilatation of the brachial artery represented a marker of early endothelium-dependent vasodilation, whereas it correlated positively with higher levels of HbA1c, thus representing an increase in cardiovascular risk. As mentioned above, many of these studied are aimed at prevention. Whether primary or secondary[31], they studied a cohort of children survivors of acute lymphoblastic leukemia and lymphoma who had been in remission for about 4 years. This population was of particular interest due to their highest risk of developing MS. They were evaluated two times, at 0 mo and 13 mo. They mainly evaluated predictors of MS such as leptin, adiponectin, insulin resistance and adiposity. The odds of developing MS at the first assessment were higher in those with a body fat percentage (% adiposity) [odds ratio (OR) 5.1, 95% confidence interval: 1.9-22.0], leptin level (OR 4.8, 95% confidence interval: 1.4-17.2) and leptin/adiponectin ratio (OR 5.2, 95% confidence interval: 1.2-22.6) in the highest tertile, whereas the lowest tertile of adiponectin was associated with a protective but not significant effect.
Aside from the important cardiovascular risk that patients with DM present, other complications and/or comorbidities have been associated with it, such is the case of DPN[33]. This study analyzed a cohort of 60 pediatric patients presenting with at least 5 years of disease duration. They were subjected to neurological assessment by neuropathy disability score and nerve conduction studies for median, ulnar, posterior tibial and common peroneal nerves. At the same time, they evaluated neopterin levels, which they concluded that neopterin could be used as an early reliable serum biomarker for DPN in pediatric patients with T1DM with a cutoff value of 32 nmol/L. This could differentiate patients with and without DPN with 100% sensitivity and 96.7% specificity and a positive predictive value 96.8% and negative predictive value of 100%. Neopterin levels (OR, 2.976) were significantly higher in patients with DPN than those without [median (interquartile range), 53.5 (35-60) nmol/L vs 17 (13-32) nmol/L] and healthy controls [5.0 (3.2-7.0) nmol/L] (P < 0.001). Another interesting finding in this protocol was that levels of neopterin were positively correlated with latency and negatively correlated with amplitude and/or velocity of the affected nerves. This translates to the proinflammatory processes in nerve tissues in patients that present with symptoms of neuropathy(Tables 2 and 3).
Early changes in biomarkers such as oxidative stress molecules may be translated into clinical alterations such as hematologic abnormalities, which according to these findings[17] could be used as early clinical detection of patients with known T1DM who are at risk of complications. This is explained according to the phenomenon caused by hyperglycemia and chronic oxidation status, which decreases deformability and changes the mechanical characteristics of erythrocytes. Therefore, it reduces red blood cell life span transducing to decreased hemoglobin and hematocrit indices. This study also showed a positive relationship between T1DM patients and higher levels of MDA. Furthermore, there is a positive correlation with micro albuminuria and thus with early diabetic kidney dysfunction and in consequence an elevated cardiovascular risk. Another study[29] hypothesized that combined therapy with antioxidant vitamins C and E might improve endothelial function and vascular repair capacity in patients with T1DM. Nonetheless, their results showed no difference between the study and the control group, proving their initial hypothesis to be wrong. However, the sample size used for this study was relatively small and duration of therapy was short. Thus, they suggest that longer term studies may be needed to determine the effects of antioxidant treatment. Based on their conclusions, another study[17] suggested that antioxidant supplementation may be required for children with T1DM to reduce oxidative stress and delay or even prevent diabetic complications.
Actual evidence demonstrated that reactive oxygen species are crucial to promote ASC dedifferentiation[27]. It was demonstrated in vitro that the percentage of HbA1c diabetic ASCs and thus NOX activation correlated with ASC dedifferentiation. This involved AKT activation and OCT4/NANOG production, which resulted in the activation of other proinflammation markers such as IL-6 and IL-8.
It was demonstrated that β-cell EV miR-21-5p cargo was increased in response to treatment with inflammatory cytokines[35]. This increase was predominantly due to cytokine-induced effects on beta cell exosome miR-21-5p and was only partially blocked by inhibition of apoptosis. A promising finding in their study showed that circulating EV miR-21-5p may be a biomarker of developing type 1 diabetes, in that progressive elevations in serum EV miR-21-5p preceded hyperglycemia in NOD mice and were present in children with new-onset T1DM (Table 2).
It was shown that the prevalence of glucose abnormalities in obese children is higher than in other series[23]. Furthermore, a correlation was present between markers of systemic and intestinal inflammation and glucose abnormalities (NO and fecal calprotectin). They indirectly evaluated the intestinal status of children with severe obesity, and further research in this field is required. Stansfield et al[28] determined the association of birth weight with abdominal fat distribution and the presence of markers known to represent an increased CVD risk as well as T2DM. Interestingly, they found that both low and high birth weights were associated with greater visceral adiposity and presence of insulin resistance and inflammation-associated biomarkers (Tables 2 and 3).
As the incidence and prevalence of DM continues to raise worldwide, both in adults and children, the urge to find new and more effective premature diagnostic methods has resulted in a global public health issue.
Our analysis of the most relevant literature in biomarkers of T1DM revealed that the inflammation and endovascular dysfunction process (Tables 1-3 and Figure 2) are the key factors triggering all the complications. T-cell driven autoimmune disease resulting in the loss of tolerance towards pancreatic β-cell is increasing alarmingly, and its causes are yet to be identified[20]. T1DM is characterized not only by insulin deficiency but by a 10-fold increase in all-cause mortality. Specifically, CVD represents the leading cause in morbidity and mortality. Actual evidence demonstrated that CVD develops in childhood, and insulin resistance is not due to obesity alone. Remarkably pediatric patients presenting with higher insulin sensitivity relative to other youth with T1DM have a CVD risk profile more similar to that of controls without diabetes[41].
T2DM is caused by insulin resistant pancreatic islet β-cell failure (Tables 1-3 and Figure 2). The coaction between environmental and genetic factors that lead to β-cell impairment is the reason why in T2DM we can find a great variety of biomarkers (Tables 2 and 3). Insufficient insulin secretion in T2DM has been attributed to β-cell dysfunction due to various insulin resistance mechanisms, among them dedifferentiation[42]. Furthermore, the incidence of T2DM in the pediatric population has been increasing in close relation to the increasing rates of obesity in this population[43]. Obesity is a well-known risk factor for the development of insulin resistance that may potentially lead to several disorders such as T2DM[44]. IR is associated to T2DM in the pediatric population, whilst obesity and MS are traditional risk factors for IR and CVD[41].
The goal of this review was to determine which biomarkers of β-cell failure, both early and late damage, have been analyzed and used over the past decade around the world and classified according to each type of diabetes (Tables 1-3). Table 1 opens the epistemic landscape to recognize immediately which biomarkers appear predominantly to each type of diabetes (T1DM or T2DM). This may serve as a guide to the endocrinologist in order to identify the onset and progression of the disease and potentially the prognosis too (Figure 2 and Tables 2 and 3). Furthermore this review analyzed the β-cell failure biomarkers and identified the markers that are characterized in the different steps of the natural history of the diabetes (Figure 2 and Tables 2 and 3). Regardless of the type of diabetes, the essential goal was to identify the mechanism of damage to understand the physiopathology of the disease and to be able to control and prevent complications in the short, medium and long term[45,46]. The real challenge is to identify a novel molecule that could simulta
Early identification of patients at highest risk of developing metabolic complications such as T1DM or T2DM is essential as morbidity and mortality due to these causes continue to rise and represents one of the main issues of the health systems worldwide. A lot is yet to be studied and analyzed as new clinical and scientific horizons emerge. The priority of medical and scientific efforts should be directed to the prevention of the development of T2DM in patients at risk and at the same time to the secondary prevention of complications derived from both T2DM and T1DM.
In recent years different techniques have been developed to assess peripheral insulin sensitivity. At the present time, the euglycemic hyperinsulinemic clamp is the gold standard for each rating. However, this technique is complex, expensive and not without risk, specifically in children. In general, the euglycemic clamp is accepted as a better method of measuring insulin sensitivity, whereas the hyperglycemic clamp is a measure of insulin secretion. At the present time, it is only used in research, thus other simpler and more practical techniques are being used in the clinical practice (such as fasting insulin and HOMA index). Fasting insulinemia has been so far the most used in epidemiologic studies to assess the situation of insulin resistance due to its simplicity and its good correlation with the HOMA index. It has been described in both adults and children that the situation of hyperinsulinism is present years before alterations on insulin secretion occur[47].
Other indices of insulin sensitivity/resistance using the data from the oral glucose tolerance test have been proposed in the last 20 years. The HOMA index such as QUIKI and Matsuda, which are suitable for clinical purposes have been proposed, while HES, McAuley, Belfiore, Cederholm, Avignon and Stumvoll index are more suitable for epidemiological/research purposes[47]. In regard to this review, the only index found was HOMA-IR as well as some of its variations.
Understanding glucose homeostasis has been one of the main points of effort between the medical and scientific community. Glucose homeostasis is maintained by the balance between insulin sensitivity and secretion. Impaired insulin sensitivity and secretion are thought to be the two main components in the pathogenesis of T2DM. For some years now, scientific efforts have shown that fasting insulin and glucose levels could be used as valuable surrogate estimates of insulin sensitivity and pancreatic β-cell function in nondiabetic children presenting with high risk factors of developing T2DM such as obesity[48].
In Table 2 we demonstrated that the majority of biomarkers in T1DM play a role as predictors of chronic complications (MDA, NO, miRNA, innate immunity), whereas in T2DM are several to predict or prevent T2DM (leptin, adiponectin, vascular cell adhesion molecule-1).
Among the main limitations we found in this review, one that we must take into account while analyzing the different studies is the one regarding the lack of studies related to pediatric patients in the Hispanic population. Given the fact that some studies have shown race-related differences (for instance, insulin sensitivity measured with the clamp technique demonstrated the known race-related differences between White and African American subjects) in insulin sensitivity in children detected by fasting insulin levels or direct measures of insulin sensitivity. Nonetheless, QUICKI-estimated and HOMA-estimated race-related differences only approached statistical significance. Another important factor to take into consideration while analyzing results is that it must be cautioned that these estimates cannot be applied uniformly across laboratories because of poor comparability of glucose and, more specifically, insulin measurements among various laboratories[48,49].
The new diabetes epistemic landscape as a protein conformational disease is highly promising but also challenging. hIAPP aggregation-oligomerization and amyloid fibril formation kills insulin-producing islet cells in patients with diabetes. Several studies have shown that oligomers are more highly toxic than monomers or fibers[14,50]. The successful knowledge translation of basic research of the molecular homo- and hetero-oligomeric assemblies of hIAPP using real sera samples to the clinical field is an important step. However, given the complexity of the real phenomenon taking place such as the polymorphism of the molecular aggregates, the co-aggregation between several proteins, the co-aggregation networks, etc. is like opening Pandora’s box[45,46].
Recently our research group demonstrated that the hIAPP oligomers acquired from sera from patients with T1DM, T2DM and obesity were of different protein compositions and size distributions and evolved rapidly to form fibrils. We offered crucial new information regarding the physical properties of hIAPP oligomers using transmission electron microscopy, Western blot immunoassays, circular dichroism spectroscopy, ThT and size exclusion chromatography[45].
Furthermore, we studied the interesting correlations between the oligomeric aggregates of hIAPP (RIAO) in sera and childhood obesity and diabetes and demonstrated the potential application of RIAO level as a biomarker of early β-cell damage[46]. We carried out an integral study of the clinical data and used an immunoassay to quantify the presence of hIAPP oligomers in the sera of children. We found elevated levels of RIAO and a significant difference between the control group and the other groups (P < 0.001 in all the pairs). RIAO level can be a diagnostic tool, a tool for better classification and a therapeutic target. Additionally, the study of hIAPP in pediatric patients changes the way in which conformational diseases are thought of because it extends the focus from Alzheimer’s disease and other ageing diseases towards the pediatric implications of cytotoxic oligomers that are present since childhood and can manifest as DM among others[45,46].
The isolation of real hIAPP opens the door to new studies that can change the way in which protein conformational disease are studied, diagnosed and treated. The immunoassay is not only simple — when coupled with a well-taken patient’s medical history — but also cost-effective and liable to being used for diagnosis in an open population and be able to substitute several laboratory studies. We are closed to the task of having a single molecule that works as a reliable biomarker and therapeutic target.
This review makes widely evident that most biomarkers currently used as early signs of β-cell failure are those that concern local or systemic inflammation processes and oxidative stress as well as those related to endothelial dysfunction processes. Landing in the clinical practice we propose that RIAO is good for identifying patients with β-cell damage and potentially could substitute many biomarkers.
The study of biomarkers in diabetes is quite advanced, especially in its relation to inflammatory diseases and vascular dysfunction. The study of these markers as they relate to immune diseases is quite advanced but never have been classified in early and late β-cell failure in diabetes mellitus (DM) patients. The challenge until now has been finding a key biomarker as a novel and targeted molecule for early diagnosis and therapeutic approach of DM.
An earlier study carried out by our group revealed that the diagnostic tools for DM are either not cost-effective, far too invasive or both. Furthermore, the diagnosis of DM is based on the golden rule of tolerance to glucose, and it becomes positive when 60% of the pancreas must have already been destroyed, meaning that the diagnosis comes too late.
We aimed to investigate the main biomarkers used in pediatric populations as early signs of pancreatic β-cell failure in patients presenting with risk factors such as being overweight, obesity, diabetes and metabolic syndrome.
We performed a systematic review of the literature based on the PRISMA and PICO approaches. PubMed, BIREME and Web of Science databases were searched for studies on DM and biomarkers of β-cell failure. Studies were selected in several steps and quality evaluation.
In this systematic review, we took into consideration 78 articles from which, after a selection process, we considered 20 articles for further analysis. We demonstrated that the β-cell-failure biomarkers are of two types: early and late β-cell damage. It is very useful in order to identify the evolution of the disease and separate the biomarkers in relation to the types of diabetes. The biomarkers of the early steps of β-cell failure are those that concern local or systemic inflammation processes and oxidative stress as well as those related to endothelial dysfunction processes. Finally, we explored the novelties of diabetes as a protein conformational disease and the novel biomarker called real human islet amyloid polypeptide amyloid oligomers (RIAO). Different types of biomarkers in type 1 and type 2 diabetes should be used in order to assess the role they play in the progress of diabetes in pediatric patients.
We carried out an integral study of the clinical data and the biomarkers of β-cell failure in DM in childhood. We found in the analyzed papers a significant difference between the control group and the patients.
The further value of this study lies in the promise that real human islet amyloid polypeptide amyloid oligomers level shows as a diagnostic tool, as a tool for better classification and as a therapeutic target.
The authors would like to acknowledge the experimental support and fruitful discussions provided by Dr. de la Chesnaye E. We also wish to thank Dr. Gonzalez J, Dr. González C and Dr. Torres J for their support. The contributions of the assigned pre-graduate research fellows at Facultad de Medicina, UNAM are greatly appreciated. We are also thankful for the contributions of Perla Sueiras, who proofread the manuscript and Alexa Guadalupe Bernal Grosvenor and Rogelio Ezequiel for the artwork.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Crenn PP S-Editor: Gao CC L-Editor: Filipodia P-Editor: Xing YX
| 1. | Suzuki Y, Brender JR, Hartman K, Ramamoorthy A, Marsh EN. Alternative pathways of human islet amyloid polypeptide aggregation distinguished by (19)f nuclear magnetic resonance-detected kinetics of monomer consumption. Biochemistry. 2012;51:8154-8162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Brender JR, Heyl DL, Samisetti S, Kotler SA, Osborne JM, Pesaru RR, Ramamoorthy A. Membrane disordering is not sufficient for membrane permeabilization by islet amyloid polypeptide: studies of IAPP(20-29) fragments. Phys Chem Chem Phys. 2013;15:8908-8915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Riek R, Eisenberg DS. The activities of amyloids from a structural perspective. Nature. 2016;539:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 366] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 4. | Eisenberg DS, Sawaya MR. Structural Studies of Amyloid Proteins at the Molecular Level. Annu Rev Biochem. 2017;86:69-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 386] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 5. | Brender JR, Lee EL, Cavitt MA, Gafni A, Steel DG, Ramamoorthy A. Amyloid fiber formation and membrane disruption are separate processes localized in two distinct regions of IAPP, the type-2-diabetes-related peptide. J Am Chem Soc. 2008;130:6424-6429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Brender JR, Salamekh S, Ramamoorthy A. Membrane disruption and early events in the aggregation of the diabetes related peptide IAPP from a molecular perspective. Acc Chem Res. 2012;45:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 7. | Tomasello MF, Sinopoli A, Pappalardo G. On the Environmental Factors Affecting the Structural and Cytotoxic Properties of IAPP Peptides. J Diabetes Res. 2015;2015:918573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Montemurro C, Nomoto H, Pei L, Parekh VS, Vongbunyong KE, Vadrevu S, Gurlo T, Butler AE, Subramaniam R, Ritou E, Shirihai OS, Satin LS, Butler PC, Tudzarova S. IAPP toxicity activates HIF1α/PFKFB3 signaling delaying β-cell loss at the expense of β-cell function. Nat Commun. 2019;10:2679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Shigihara N, Fukunaka A, Hara A, Komiya K, Honda A, Uchida T, Abe H, Toyofuku Y, Tamaki M, Ogihara T, Miyatsuka T, Hiddinga HJ, Sakagashira S, Koike M, Uchiyama Y, Yoshimori T, Eberhardt NL, Fujitani Y, Watada H. Human IAPP-induced pancreatic β cell toxicity and its regulation by autophagy. J Clin Invest. 2014;124:3634-3644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Landau M, Sawaya MR, Faull KF, Laganowsky A, Jiang L, Sievers SA, Liu J, Barrio JR, Eisenberg D. Towards a pharmacophore for amyloid. PLoS Biol. 2011;9:e1001080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 470] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 12. | Chiti F, Dobson CM, Dikic I, Hartl FU, Sontag EM, Samant RS, Frydman J, Eisenberg DS, Sawaya MR. Structural Studies of Amyloid Proteins at the Molecular Level. Annu Rev Biochem. 2017;86:193-224. |
| 13. | Clark A, Nilsson MR. Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 2004;47:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Raleigh D, Zhang X, Hastoy B, Clark A. The β-cell assassin: IAPP cytotoxicity. J Mol Endocrinol. 2017;59:R121-R140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 15. | Clark A, Cooper GJ, Lewis CE, Morris JF, Willis AC, Reid KB, Turner RC. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987;2:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 277] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Xu M, Sun B, Li D, Mao R, Li H, Li Y, Wang J. Beneficial Effects of Small Molecule Oligopeptides Isolated from Panax ginseng Meyer on Pancreatic Beta-Cell Dysfunction and Death in Diabetic Rats. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Abdel-Moneim A, Zanaty MI, El-Sayed A, Khalil RG, Rahman HA. Relation Between Oxidative Stress and Hematologic Abnormalities in Children With Type 1 Diabetes. Can J Diabetes. 2020;44:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Babar GS, Zidan H, Widlansky ME, Das E, Hoffmann RG, Daoud M, Alemzadeh R. Impaired endothelial function in preadolescent children with type 1 diabetes. Diabetes Care. 2011;34:681-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Wołoszyn-Durkiewicz A, Myśliwiec M. The prognostic value of inflammatory and vascular endothelial dysfunction biomarkers in microvascular and macrovascular complications in type 1 diabetes. Pediatr Endocrinol Diabetes Metab. 2019;25:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Thorsen SU, Eising S, Mortensen HB, Skogstrand K, Pociot F, Johannesen J, Svensson J; Danish Childhood Diabetes Registry. Systemic levels of CCL2, CCL3, CCL4 and CXCL8 differ according to age, time period and season among children newly diagnosed with type 1 diabetes and their healthy siblings. Scand J Immunol. 2014;80:452-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Blum B, Roose AN, Barrandon O, Maehr R, Arvanites AC, Davidow LS, Davis JC, Peterson QP, Rubin LL, Melton DA. Reversal of β cell de-differentiation by a small molecule inhibitor of the TGFβ pathway. Elife. 2014;3:e02809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | White MG, Marshall HL, Rigby R, Huang GC, Amer A, Booth T, White S, Shaw JA. Expression of mesenchymal and α-cell phenotypic markers in islet β-cells in recently diagnosed diabetes. Diabetes Care. 2013;36:3818-3820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Spagnuolo MI, Cicalese MP, Caiazzo MA, Franzese A, Squeglia V, Assante LR, Valerio G, Merone R, Guarino A. Relationship between severe obesity and gut inflammation in children: what's next? Ital J Pediatr. 2010;36:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Walsh JJ, D'Angiulli A, Cameron JD, Sigal RJ, Kenny GP, Holcik M, Doucette S, Alberga AS, Prud'homme D, Hadjiyannakis S, Gunnell K, Goldfield GS. Changes in the Brain-Derived Neurotrophic Factor Are Associated with Improvements in Diabetes Risk Factors after Exercise Training in Adolescents with Obesity: The HEARTY Randomized Controlled Trial. Neural Plast. 2018;2018:7169583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Walker RW, Allayee H, Inserra A, Fruhwirth R, Alisi A, Devito R, Carey ME, Sinatra F, Goran MI, Nobili V. Macrophages and fibrosis in adipose tissue are linked to liver damage and metabolic risk in obese children. Obesity (Silver Spring). 2014;22:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Tomsa A, Klinepeter Bartz S, Krishnamurthy R, Bacha F. Endothelial Function in Youth: A Biomarker Modulated by Adiposity-Related Insulin Resistance. J Pediatr. 2016;178:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Dentelli P, Barale C, Togliatto G, Trombetta A, Olgasi C, Gili M, Riganti C, Toppino M, Brizzi MF. A diabetic milieu promotes OCT4 and NANOG production in human visceral-derived adipose stem cells. Diabetologia. 2013;56:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Stansfield BK, Fain ME, Bhatia J, Gutin B, Nguyen JT, Pollock NK. Nonlinear Relationship between Birth Weight and Visceral Fat in Adolescents. J Pediatr. 2016;174:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Cazeau RM, Huang H, Bauer JA, Hoffman RP. Effect of Vitamins C and E on Endothelial Function in Type 1 Diabetes Mellitus. J Diabetes Res. 2016;2016:3271293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Aburawi EH, AlKaabi J, Zoubeidi T, Shehab A, Lessan N, Al Essa A, Yasin J, Saadi H, Souid AK. Subclinical Inflammation and Endothelial Dysfunction in Young Patients with Diabetes: A Study from United Arab Emirates. PLoS One. 2016;11:e0159808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Barbosa-Cortés L, López-Alarcón M, Mejía-Aranguré JM, Klünder-Klünder M, Del Carmen Rodríguez-Zepeda M, Rivera-Márquez H, de la Vega-Martínez A, Martin-Trejo J, Shum-Luis J, Solis-Labastida K, López-Aguilar E, Matute-González G, Bernaldez-Rios R. Adipokines, insulin resistance, and adiposity as a predictors of metabolic syndrome in child survivors of lymphoma and acute lymphoblastic leukemia of a developing country. BMC Cancer. 2017;17:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Vorobjova T, Tagoma A, Oras A, Alnek K, Kisand K, Talja I, Uibo O, Uibo R. Celiac Disease in Children, Particularly with Accompanying Type 1 Diabetes, Is Characterized by Substantial Changes in the Blood Cytokine Balance, Which May Reflect Inflammatory Processes in the Small Intestinal Mucosa. J Immunol Res. 2019;2019:6179243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Elbarbary NS, Ismail EAR, El-Hilaly RA, Ahmed FS. Role of neopterin as a biochemical marker for peripheral neuropathy in pediatric patients with type 1 diabetes: Relation to nerve conduction studies. Int Immunopharmacol. 2018;59:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Carlbom L, Espes D, Lubberink M, Martinell M, Johansson L, Ahlström H, Carlsson PO, Korsgren O, Eriksson O. [11C]5-hydroxy-tryptophan PET for Assessment of Islet Mass During Progression of Type 2 Diabetes. Diabetes. 2017;66:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Lakhter AJ, Pratt RE, Moore RE, Doucette KK, Maier BF, DiMeglio LA, Sims EK. Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia. 2018;61:1124-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 36. | Arenaza L, Medrano M, Amasene M, Rodríguez-Vigil B, Díez I, Graña M, Tobalina I, Maiz E, Arteche E, Larrarte E, Huybrechts I, Davis CL, Ruiz JR, Ortega FB, Margareto J, Labayen I. Prevention of diabetes in overweight/obese children through a family based intervention program including supervised exercise (PREDIKID project): study protocol for a randomized controlled trial. Trials. 2017;18:372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Sheng C, Li F, Lin Z, Zhang M, Yang P, Bu L, Sheng H, Li H, Qu S. Reversibility of β-Cell-Specific Transcript Factors Expression by Long-Term Caloric Restriction in db/db Mouse. J Diabetes Res. 2016;2016:6035046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. |
Kim-Muller JY, Fan J, Kim YJ, Lee SA, Ishida E, Blaner WS, Accili D. Aldehyde dehydro |
| 39. | Cabrera SM, Engle S, Kaldunski M, Jia S, Geoffrey R, Simpson P, Szabo A, Speake C, Greenbaum CJ; Type 1 Diabetes TrialNet CTLA4-Ig (Abatacept) Study Group; Chen YG, Hessner MJ. Innate immune activity as a predictor of persistent insulin secretion and association with responsiveness to CTLA4-Ig treatment in recent-onset type 1 diabetes. Diabetologia. 2018;61:2356-2370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Bacha F, Edmundowicz D, Sutton-Tyrell K, Lee S, Tfayli H, Arslanian SA. Coronary artery calcification in obese youth: what are the phenotypic and metabolic determinants? Diabetes Care. 2014;37:2632-2639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Cree-Green M, Stuppy JJ, Thurston J, Bergman BC, Coe GV, Baumgartner AD, Bacon S, Scherzinger A, Pyle L, Nadeau KJ. Youth With Type 1 Diabetes Have Adipose, Hepatic, and Peripheral Insulin Resistance. J Clin Endocrinol Metab. 2018;103:3647-3657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Solimena M, Schulte AM, Marselli L, Ehehalt F, Richter D, Kleeberg M, Mziaut H, Knoch KP, Parnis J, Bugliani M, Siddiq A, Jörns A, Burdet F, Liechti R, Suleiman M, Margerie D, Syed F, Distler M, Grützmann R, Petretto E, Moreno-Moral A, Wegbrod C, Sönmez A, Pfriem K, Friedrich A, Meinel J, Wollheim CB, Baretton GB, Scharfmann R, Nogoceke E, Bonifacio E, Sturm D, Meyer-Puttlitz B, Boggi U, Saeger HD, Filipponi F, Lesche M, Meda P, Dahl A, Wigger L, Xenarios I, Falchi M, Thorens B, Weitz J, Bokvist K, Lenzen S, Rutter GA, Froguel P, von Bülow M, Ibberson M, Marchetti P. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia. 2018;61:641-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 43. | Janem WF, Scannapieco FA, Sabharwal A, Tsompana M, Berman HA, Haase EM, Miecznikowski JC, Mastrandrea LD. Salivary inflammatory markers and microbiome in normoglycemic lean and obese children compared to obese children with type 2 diabetes. PLoS One. 2017;12:e0172647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Garanty-Bogacka B, Syrenicz M, Goral J, Krupa B, Syrenicz J, Walczak M, Syrenicz A. Changes in inflammatory biomarkers after successful lifestyle intervention in obese children. Endokrynol Pol. 2011;62:499-505. [PubMed] |
| 45. | Altamirano-Bustamante MM, Altamirano-Bustamante NF, Larralde-Laborde M, Lara-Martínez R, Leyva-García E, Garrido-Magaña E, Rojas G, Jiménez-García LF, Revilla-Monsalve C, Altamirano P, Calzada-León R. Unpacking the aggregation-oligomerization-fibrillization process of naturally-occurring hIAPP amyloid oligomers isolated directly from sera of children with obesity or diabetes mellitus. Sci Rep. 2019;9:18465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Altamirano-Bustamante NF, Garrido-Magaña E, Morán E, Calderón A, Pasten-Hidalgo K, Castillo-Rodríguez RA, Rojas G, Lara-Martínez R, Leyva-García E, Larralde-Laborde M, Domíguez G, Murata C, Margarita-Vazquez Y, Payro R, Barbosa M, Valderrama A, Montesinos H, Domínguez-Camacho A, García-Olmos VH, Ferrer R, Medina-Bravo PG, Santoscoy F, Revilla-Monsalve C, Jiménez-García LF, Morán J, Villalobos-Alva J, Villalobos MJ, Calzada-León R, Altamirano P, Altamirano-Bustamante MM. Protein-conformational diseases in childhood: Naturally-occurring hIAPP amyloid-oligomers and early β-cell damage in obesity and diabetes. PLoS One. 2020;15:e0237667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015;19:160-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 48. | Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 339] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 49. | Gungor N, Arslanian S. Progressive beta cell failure in type 2 diabetes mellitus of youth. J Pediatr. 2004;144:656-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Akter R, Cao P, Noor H, Ridgway Z, Tu LH, Wang H, Wong AG, Zhang X, Abedini A, Schmidt AM, Raleigh DP. Islet Amyloid Polypeptide: Structure, Function, and Pathophysiology. J Diabetes Res. 2016;2016:2798269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 51. | Cummings LAM, Clarke A, Sochett E, Daneman D, Cherney DZ, Reich HN, Scholey JW, Dunger DB, Mahmud FH. Social Determinants of Health Are Associated with Markers of Renal Injury in Adolescents with Type 1 Diabetes. J Pediatr. 2018;198:247-253.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Heier M, Margeirsdottir HD, Gaarder M, Stensæth KH, Brunborg C, Torjesen PA, Seljeflot I, Hanssen KF, Dahl-Jørgensen K. Soluble RAGE and atherosclerosis in youth with type 1 diabetes: a 5-year follow-up study. Cardiovasc Diabetol. 2015;14:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Ho J, Reimer RA, Doulla M, Huang C. Effect of prebiotic intake on gut microbiota, intestinal permeability and glycemic control in children with type 1 diabetes: study protocol for a randomized controlled trial. Trials. 2016;17:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Hoffman RP, Dye AS, Huang H, Bauer JA. Glycemic variability predicts inflammation in adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2016;29:1129-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Kingery SE, Wu YL, Zhou B, Hoffman RP, Yu CY. Gene CNVs and protein levels of complement C4A and C4B as novel biomarkers for partial disease remissions in new-onset type 1 diabetes patients. Pediatr Diabetes. 2012;13:408-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Marchand L, Jalabert A, Meugnier E, Van den Hende K, Fabien N, Nicolino M, Madec AM, Thivolet C, Rome S. miRNA-375 a Sensor of Glucotoxicity Is Altered in the Serum of Children with Newly Diagnosed Type 1 Diabetes. J Diabetes Res. 2016;2016:1869082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 57. | Marek-Trzonkowska N, Myśliwiec M, Iwaszkiewicz-Grześ D, Gliwiński M, Derkowska I, Żalińska M, Zieliński M, Grabowska M, Zielińska H, Piekarska K, Jaźwińska-Curyłło A, Owczuk R, Szadkowska A, Wyka K, Witkowski P, Młynarski W, Jarosz-Chobot P, Bossowski A, Siebert J, Trzonkowski P. Factors affecting long-term efficacy of T regulatory cell-based therapy in type 1 diabetes. J Transl Med. 2016;14:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 58. | Redondo MJ, Rodriguez LM, Haymond MW, Hampe CS, Smith EO, Balasubramanyam A, Devaraj S. Serum adiposity-induced biomarkers in obese and lean children with recently diagnosed autoimmune type 1 diabetes. Pediatr Diabetes. 2014;15:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Ryba-Stanisławowska M, Rybarczyk-Kapturska K, Myśliwiec M, Myśliwska J. Elevated levels of serum IL-12 and IL-18 are associated with lower frequencies of CD4(+)CD25 (high)FOXP3 (+) regulatory t cells in young patients with type 1 diabetes. Inflammation. 2014;37:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Singh H, Yu Y, Suh MJ, Torralba MG, Stenzel RD, Tovchigrechko A, Thovarai V, Harkins DM, Rajagopala SV, Osborne W, Cogen FR, Kaplowitz PB, Nelson KE, Madupu R, Pieper R. Type 1 Diabetes: Urinary Proteomics and Protein Network Analysis Support Perturbation of Lysosomal Function. Theranostics. 2017;7:2704-2717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 61. | Şiraz ÜG, Doğan M, Hatipoğlu N, Muhtaroğlu S, Kurtoğlu S. Can Fetuin-A Be a Marker for Insulin Resistance and Poor Glycemic Control in Children with Type 1 Diabetes Mellitus? J Clin Res Pediatr Endocrinol. 2017;9:293-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Zhang B, Kumar RB, Dai H, Feldman BJ. A plasmonic chip for biomarker discovery and diagnosis of type 1 diabetes. Nat Med. 2014;20:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 63. | Krapfenbauer K. Identification of beta cell dysfunction at the pre-symptomatic stage of diabetes mellitus by novel analytical system: liquid biopsy measurements in femtograms. EPMA J. 2017;8:35-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Roberts K, Jaffe A, Verge C, Thomas PS. Noninvasive monitoring of glucose levels: is exhaled breath the answer? J Diabetes Sci Technol. 2012;6:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Arslanian S, El Ghormli L, Young Kim J, Bacha F, Chan C, Ismail HM, Levitt Katz LE, Levitsky L, Tryggestad JB, White NH; TODAY Study Group. The Shape of the Glucose Response Curve During an Oral Glucose Tolerance Test: Forerunner of Heightened Glycemic Failure Rates and Accelerated Decline in β-Cell Function in TODAY. Diabetes Care. 2019;42:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 66. | Berezin AE. Diabetes mellitus related biomarker: The predictive role of growth-differentiation factor-15. Diabetes Metab Syndr. 2016;10:S154-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Burke SJ, Batdorf HM, Burk DH, Noland RC, Eder AE, Boulos MS, Karlstad MD, Collier JJ. db/db Mice Exhibit Features of Human Type 2 Diabetes That Are Not Present in Weight-Matched C57BL/6J Mice Fed a Western Diet. J Diabetes Res. 2017;2017:8503754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 68. | Can U, Buyukinan M, Guzelant A, Ugur A, Karaibrahimoglu A, Yabancıun S. Investigation of the inflammatory biomarkers of metabolic syndrome in adolescents. J Pediatr Endocrinol Metab. 2016;29:1277-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Cui X, You L, Zhu L, Wang X, Zhou Y, Li Y, Wen J, Xia Y, Ji C, Guo X. Change in circulating microRNA profile of obese children indicates future risk of adult diabetes. Metabolism. 2018;78:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 70. | DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition. 2013;29:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 71. | Fanjul M, Gmyr V, Sengenès C, Ratovo G, Dufresne M, Lefebvre B, Kerr-Conte J, Hollande E. Evidence for epithelial-mesenchymal transition in adult human pancreatic exocrine cells. J Histochem Cytochem. 2010;58:807-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Garnett SP, Baur LA, Noakes M, Steinbeck K, Woodhead HJ, Burrell S, Chisholm K, Broderick CR, Parker R, De S, Shrinivasan S, Hopley L, Hendrie G, Ambler GR, Kohn MR, Cowell CT. Researching Effective Strategies to Improve Insulin Sensitivity in Children and Teenagers - RESIST. A randomised control trial investigating the effects of two different diets on insulin sensitivity in young people with insulin resistance and/or pre-diabetes. BMC Public Health. 2010;10:575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Hansen JB, Moen IW, Mandrup-Poulsen T. Iron: the hard player in diabetes pathophysiology. Acta Physiol (Oxf). 2014;210:717-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 74. | Ishida E, Kim-Muller JY, Accili D. Pair Feeding, but Not Insulin, Phloridzin, or Rosiglitazone Treatment, Curtails Markers of β-Cell Dedifferentiation in db/db Mice. Diabetes. 2017;66:2092-2101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 75. | Jaberi-Douraki M, Liu SW, Pietropaolo M, Khadra A. Autoimmune responses in T1DM: quantitative methods to understand onset, progression, and prevention of disease. Pediatr Diabetes. 2014;15:162-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Kaya A, Koçyiğit C, Çatlı G, Özkan EB, Dündar BN. The Relationship Between Glycemic Variability and Inflammatory Markers in Obese Children with Insulin Resistance and Metabolic Syndrome. J Clin Res Pediatr Endocrinol. 2017;9:202-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Kim JY, Michaliszyn SF, Nasr A, Lee S, Tfayli H, Hannon T, Hughan KS, Bacha F, Arslanian S. The Shape of the Glucose Response Curve During an Oral Glucose Tolerance Test Heralds Biomarkers of Type 2 Diabetes Risk in Obese Youth. Diabetes Care. 2016;39:1431-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Kuo T, Kim-Muller JY, McGraw TE, Accili D. Altered Plasma Profile of Antioxidant Proteins as an Early Correlate of Pancreatic β Cell Dysfunction. J Biol Chem. 2016;291:9648-9656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Mastrangelo A, Martos-Moreno GÁ, García A, Barrios V, Rupérez FJ, Chowen JA, Barbas C, Argente J. Insulin resistance in prepubertal obese children correlates with sex-dependent early onset metabolomic alterations. Int J Obes (Lond). 2016;40:1494-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Neelankal John A, Morahan G, Jiang FX. Incomplete Re-Expression of Neuroendocrine Progenitor/Stem Cell Markers is a Key Feature of β-Cell Dedifferentiation. J Neuroendocrinol. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 81. | Park SH, Ha E, Hong YS, Park H. Serum Levels of Persistent Organic Pollutants and Insulin Secretion among Children Age 7-9 Years: A Prospective Cohort Study. Environ Health Perspect. 2016;124:1924-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Rachdi L, Kariyawasam D, Aïello V, Herault Y, Janel N, Delabar JM, Polak M, Scharfmann R. Dyrk1A induces pancreatic β cell mass expansion and improves glucose tolerance. Cell Cycle. 2014;13:2221-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Santoro N, Caprio S, Giannini C, Kim G, Kursawe R, Pierpont B, Shaw MM, Feldstein AE. Oxidized fatty acids: A potential pathogenic link between fatty liver and type 2 diabetes in obese adolescents? Antioxid Redox Signal. 2014;20:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Serbis A, Giapros V, Challa A, Chaliasos N, Siomou E. Elevated 1-hour post-load plasma glucose identifies obese youth with abnormal glucose metabolism and an unfavourable inflammatory profile. Clin Endocrinol (Oxf). 2018;89:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 85. | Tenenbaum A, Fisman EZ. Balanced pan-PPAR activator bezafibrate in combination with statin: comprehensive lipids control and diabetes prevention? Cardiovasc Diabetol. 2012;11:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |