Published online Aug 15, 2021. doi: 10.4239/wjd.v12.i8.1312
Peer-review started: December 31, 2020
First decision: January 18, 2021
Revised: January 18, 2021
Accepted: April 25, 2021
Article in press: April 25, 2021
Published online: August 15, 2021
Processing time: 221 Days and 3.4 Hours
The alarming rise in the worldwide prevalence of obesity is paralleled by an increasing burden of type 2 diabetes mellitus (T2DM). Metabolic surgery is the most effective means of obtaining substantial and durable weight loss in individual obese patients with T2DM. There are randomized trials that justify the inclusion of metabolic surgery into the treatment algorithm for patients with T2DM, but remission rates of T2DM after metabolic surgery can display great variability.
To discuss the most commonly used surgical options including vertical sleeve gastrectomy, adjustable gastric banding, Roux-en-Y gastric bypass, and biliopancreatic diversion with duodenal switch.
We also report from observational and randomized controlled studies on rate of remission of T2DM after the surgical procedures.
In light of the recent findings, metabolic surgery is a safe and effective treatment option for obese patient with T2DM, but further studies are needed to clarify better the rate of diabetes remission.
In light of the recent findings, metabolic surgery is a safe and effective treatment option for obese patients with T2DM, but further studies are needed to clarify better the rate of diabetes remission.
Core Tip: There are randomized trials that justify the inclusion of metabolic surgery into the treatment algorithm for patients with type 2 diabetes mellitus (T2DM), but remission rates of T2DM after metabolic surgery can display great variability. Here, we discuss the most commonly used surgical options, including vertical sleeve gastrectomy, adjustable gastric banding, Roux-en-Y gastric bypass, and biliopancreatic diversion with duodenal switch, and clarify the unknown issues of metabolic surgery and remission criteria of T2DM.
- Citation: Akkus G, Tetiker T. Which predictors could effect on remission of type 2 diabetes mellitus after the metabolic surgery: A general perspective of current studies? World J Diabetes 2021; 12(8): 1312-1324
- URL: https://www.wjgnet.com/1948-9358/full/v12/i8/1312.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i8.1312
The prevalence of type 2 diabetes mellitus (T2DM) throughout the world has been increasing at alarming rates. It has been reported to affect more than 400 million people, with an estimation of 650 million cases by 2040. In addition, obesity is a main critical risk factor for the development of T2DM. Both obesity and T2DM are associated with insulin resistance[1,2]. Regarding physiological status, pancreatic islet â-cells increase insulin release to overcome reduced efficiency of insulin action, thus normal glucose levels are maintained[3]. Since obesity and insulin resistance are associated with T2DM, islet â-cells are unable to compensate fully for decreased insulin sensitivity[4]. Diabetes and insulin resistance have a close relationship; this close relationship is called new paradigm “diabesity”, meaning that a majority of individuals with diabetes are overweight or obese. Although intensive lifestyle modification, with diet-induced weight loss, exercise, and intensive medical therapy, can play a major role to achieve blood glucose regulation, a majority of obese patients with T2DM could not achieve glycemic control. In addition, intensification of medical therapy can cause hypoglycemia or weight gain.
Metabolic surgery has become as an alternative treatment option for appropriate candidates with inadequate control of T2DM and a body mass index (BMI) > 30 kg/m2[5]. Inclusion of surgery among standard diabetes therapies represents a significant improvement of glycemic control[6]. The exact psychopathological mechanism of metabolic surgery is still not clearly understood, but it has played a prominent role for the gut in glucose homeostasis[7]. Therefore, surgical weight loss provides T2DM remission with rates from 24% to 95% for nearly 2 years, depending on the types of surgical treatment[8]. While numerous randomized-controlled trials have declared the efficacy of metabolic surgery in treating T2DM compared to medical management and lifestyle changes, metabolic surgery is still not a widely accepted method due to surgical complications or risk of regaining weight[9,10].
Herein we review evidence regarding the effects of metabolic surgery in patients with T2DM. In addition, the concept of what defines a cure or remission of T2DM will be critically discussed in this review.
As we know, adipose tissue modulates to produce non-esterified fatty acids (NEFAs) and many cytokines such as leptin, adiponectin, and retinol-binding protein-4[11]. In particular, retinol-binding protein-4 causes an increase in insulin resistance by reducing phosphatidylinositol-3-OH-kinase signaling pathway in muscles; therefore, it aggregates the expression of gluconeogenic enzyme phosphoenolpyruvate carboxykinase in the liver through retinol depending mechanism[12]. On the other hand, adiponectin plays the role of an insulin sensitizer by stimulating fatty acid oxidation[13]. In addition, adipose tissue releases other proinflammatory cytokines including tumor necrosis factor alpha, interleukin 6, and monocyte chemoattractant protein-1; so these substrates trigger the occurrence of insulin resistance[14-16]. NEFAs are responsible for insulin resistance in obese patients. Elevated levels of NEFAs in T2DM causes severe muscle and liver insulin resistance and inhibits insulin secretion[17]. These are responsible for impairment in glucose oxidation/glycogen synthesis and decrease glucose transport/phosphorylation. Intramyocellular toxic NEFA metabolites (LCFA-CoA, diacylglycerol, ceramide) can lead to the activation of protein kinase C, and the insulin signaling cascade is damaged in the early steps in this pathway[17]. Since insulin sensitivity modulates â-cell function, over time, â-cell function is impaired. As a result of â-cell dysfunction and inadequate insulin secretion, an increase in postprandial and fasting glucose levels takes place. Additionally, hepatic glucose production is suppressed, and liver and muscle glucose uptake becomes inadequate. Increased plasma glucose levels are commonly related with glucotoxic effect on the â-cells and harmful effects on insulin sensitivity, and it might contribute to the progression of the disease[15,18].
Metabolic surgery is accepted as a potential treatment for both morbid obesity and overweight type 2 diabetic patients without glycemic control. In 2016, the second diabetes surgery summit declared recommendations, which were supported by 45 pioneer medical and scientific societies worldwide, to consider bariatric surgery as a treatment for type 2 DM[19]. Metabolic surgery is currently approved with the following recommendations: (1) Adults with class III obesity (BMI ≥ 40) regardless of glycemic control or complexity of glucose lowering regimens; (2) Patients with class II obesity (BMI: 35.0-39.9) with inadequately controlled hyperglycemia despite the optimal medical therapy; and (3) Diabetic patients with class I obesity (BMI: 30.0-34.9) and inadequately controlled hyperglycemia despite optimal medical treatment by oral or injectable medications (including insulin).
The history of metabolic surgery can often be subdivided by categorizing procedures by their presumed mechanisms of action in promoting weight loss[20]. This includes nutrient malabsorption, gastric restriction, hormonal manipulation, or any combination of these mechanisms. Currently, the most frequently performed surgical modalities are the vertical sleeve gastrectomy (VSG), adjustable gastric banding (AGB), Roux-en-Y gastric bypass (RYGB), and biliopancreatic diversion with duodenal switch (BPD-DS).
VSG consists of two parts involving the resection of the major proportion of the fundus and the corpus of the stomach, leaving tube-shaped gastric residue[21]. It was originally performed as the first part of the approach for biliopancreatic diversion of high risk individuals[22]. VSG is a technically easier operation to perform and post-operative results are comparable to RYGB with fewer complications. Although VSG has been primarily developed as a restrictive method, it has become a hormonal component to the weight loss effect due to decreasing levels of ghrelin[23].
VSG can have a very prominent effect on weight loss, and resolution of obesity related comorbidities can be solved in the short term. Initially, it reduces the number of calories through volume restriction, resulting in decreased overall caloric intake and weight loss[24]. The rate of major complications after VSG is between 0% and 6%. Early complications are generally leaking, bleeding, symptomatic stenosis, deep vein thrombosis/pulmonary embolism, especially portomesenteric venous thrombosis, and dehydration. Late complications such as stricture, weight regain, and malnutrition can be seen[25]. Along with the decreasing size of gastric residue, performing VSG also increases the intraluminal pressure of the stomach and can lead to gastro-esophageal reflux diseases (GERD). While some studies have shown GERD improvement after VSG, some indicated progressive or de novo post-operative reflux in patients. Therefore, the use of sleeve gastrectomy in GERD patients remains controversial[26,27]. However, in view of the outcomes of VSG, this procedure is greatly encouraged for obese diabetic individuals.
Adjustable gastric banding is an almost restrictive method, whereas the other procedures can lead to profound modifications in the digestive processes, secretion of gastrointestinal (GI) peptides, and nutrient sensing[28]. It appears to be an advantageous surgical technique as it does not involve any anastomosis or resection; it is reversible, there are very few life-threatening complications, and it is a minimally invasive intervention[29]. Restrictive operations were developed to promote weight loss by having the patient experience early satiety during food intake by partitioning the stomach and creating a smaller capacity chamber to store the consumed food bolus[26,30]. Placement of an adjustable silicon band around the upper part of the stomach is a major part of this method. The size of the band and thus the degree of restriction can be adjusted by adding or removing saline solution through a subcutaneously inserted port[31]. AGB has the lowest perioperative risk compared to RYGB, VSG, and BPD-DS. Most common complications of this method can be listed as pouch dilatation and port malfunction, persistent GERD, port infection, and stomal obstruction[32].
RYGB includes creating a small-volume gastric pouch that is anastomosed to the distal part of jejunum. The limb carrying biliopancreatic secretion is anastomosed typically 150 cm distal to gastro-jejunostomy[33,34]. RYGB is considered a technically more challenging operation, but it induces weight loss through a combination of restrictive and malabsorptive mechanisms. In addition, RYGB operation bypasses nearly 95% of stomach upper GI tract[35]. The rest of the stomach and the proximal intestine remain in the body and maintain nutrient flow, but the distal portion of the duodenum is reattached further down within the jejunum to allow bile acids and digestive enzymes to reach nutrients[36]. Except for the restrictive mechanism, the other basic physiological change is that the large postprandial increases significant gut-secreted peptides. Many of these peptides play a regulating role in appetite, energy expenditure, and glucose and lipid metabolism[37]. During this procedure, a large mesenteric defect can be created which can result in internal herniation of the small bowel or colon in the post-operative phase of care[30,38]. Intraluminal post-operative bleeding, extraluminal post-operative bleeding, anastomotic leak, dumping syndrome, and development of marginal ulcer at the anastomosis area are the major complications of RYGB[39,40].
BPD-DS is also a mixed restrictive/malabsorptive procedure that was originally introduced in 1979 by Scopinaro et al[41]. It encompasses partial horizontal gastrectomy and anastomosis of the gastric remnant in the distal 250 cm of the small intestine (alimentary limb), while the diverted proximal intestine carries biliopancreatic secretions[42]. The posterior part of the intestine is anastomosed to the alimentary limb at a varying distance from the ileocecal valve; thus it determines the degree of malabsorption[43]. BPD-DS is almost exclusively performed laparoscopically with a low conversion rate of open surgery. Although BPD-DS can promote very high success rates regarding weight loss and metabolic improvement among all bariatric modalities, its technical difficulties and increased rates of post-operative complications restrict its application on all obese or type 2 diabetic patients[44]. It can be used for patients with massive obesity (BMI > 50 kg/m2).
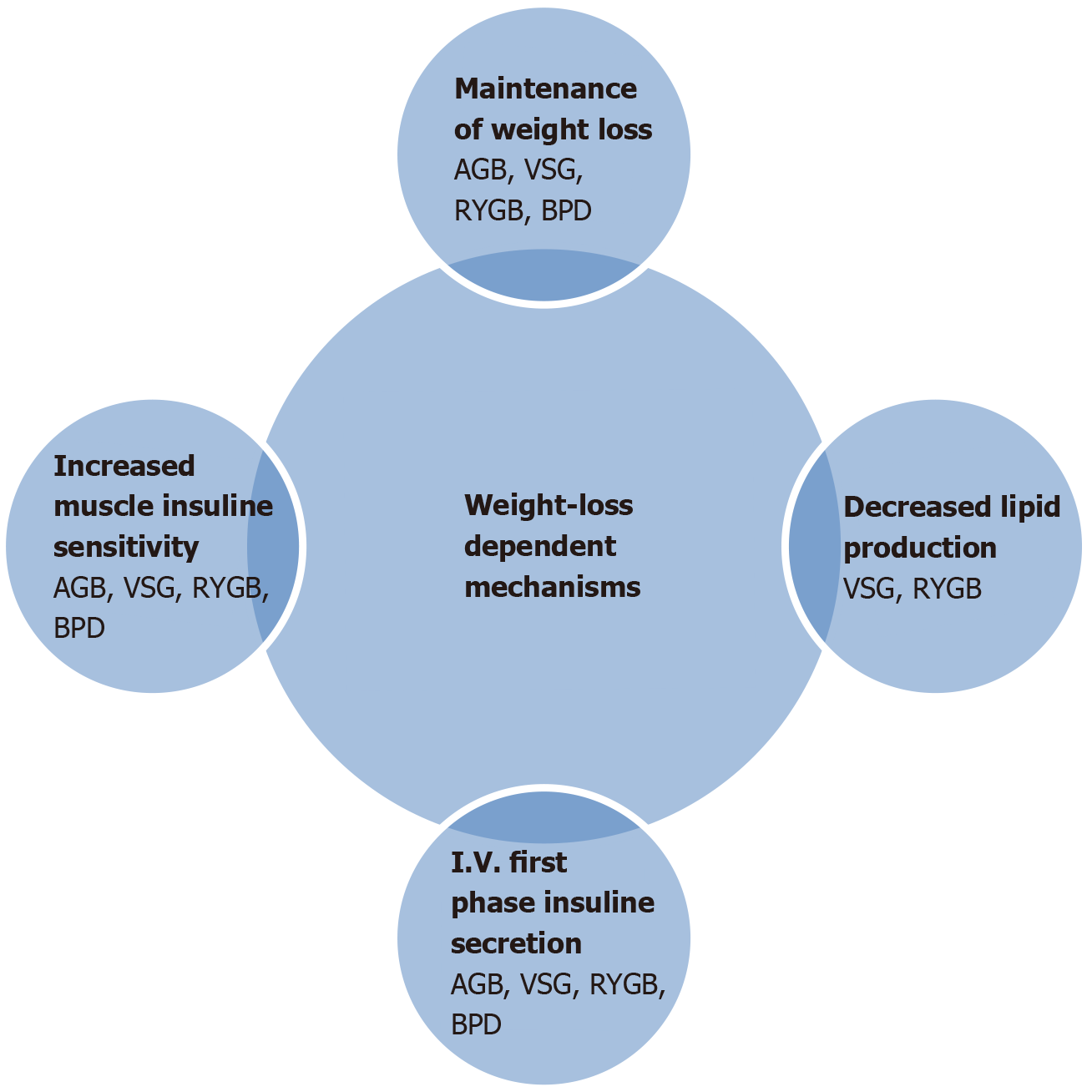
Peripheral insulin sensitivity, hepatic insulin sensitivity, and the disposition index improve during dynamic weight loss or after weight stabilization. Fasting plasma glucose levels and oral glucose tolerance are also improved a few days to a few weeks after metabolic surgery in relation to the weight loss independent effect of glucose homeostasis[45]. Metabolic parameters in humans demonstrate that hepatic glucose output is dramatically reduced quickly after bariatric surgery, but peripheral (muscle) insulin sensitivity measured by euglycemic-hyperinsulinemic clamp can be normalized only after significant weight loss[46]. Related to the variability accompanying type of surgery, insulin sensitivity is rapidly normalized in BPD-DS compared to other procedures, and it is attributed to lipid malabsorption. RYGB exerts its main effect via decreasing hepatic glucose production and increasing insulin secretion, while BPD-DS acts essentially by normalizing insulin sensitivity[47]. RYGB significantly increases glucose uptake and utilization related with increased gut metabolism[48]. Absence of hypertrophic jejunal mucosa and reduced glucose absorption have been shown after VSG as a different phenotype[49]. Intestinal glucose metabolism is a major contributor to weight-loss independent resolution of T2DM. The incretin effect is triggered by the secretion of gut hormones including glucose-dependent insulinotropic peptide and cholecystokinin, glucagon-like peptide (GLP)-1, GLP-2, oxyntomodulin, and also peptide YY[50]. Gut hormones can cause an innervation of afferent neurons in the GI tract to signal to the caudal brainstem or enteric neurons, and the other process of these hormones enter the circulation to act on peripheral glucose and lipid metabolism[48,51]. After bariatric surgery, some of gut peptides including GLP-1 are secreted largely as a result of rapid nutrient delivery further down in the GI tract, where the majority of L-cells are located[52]. Therefore, intestinal physiology can be adapted to rapid nutrient entry by increasing the number of enteroendocrine cells or increasing nutrient sensitivity of the existing enteroendocrine cell population[53]. Related with increased levels of GLP-1, insulin levels are exaggerated and peripheral glucose absorption is increased as well.
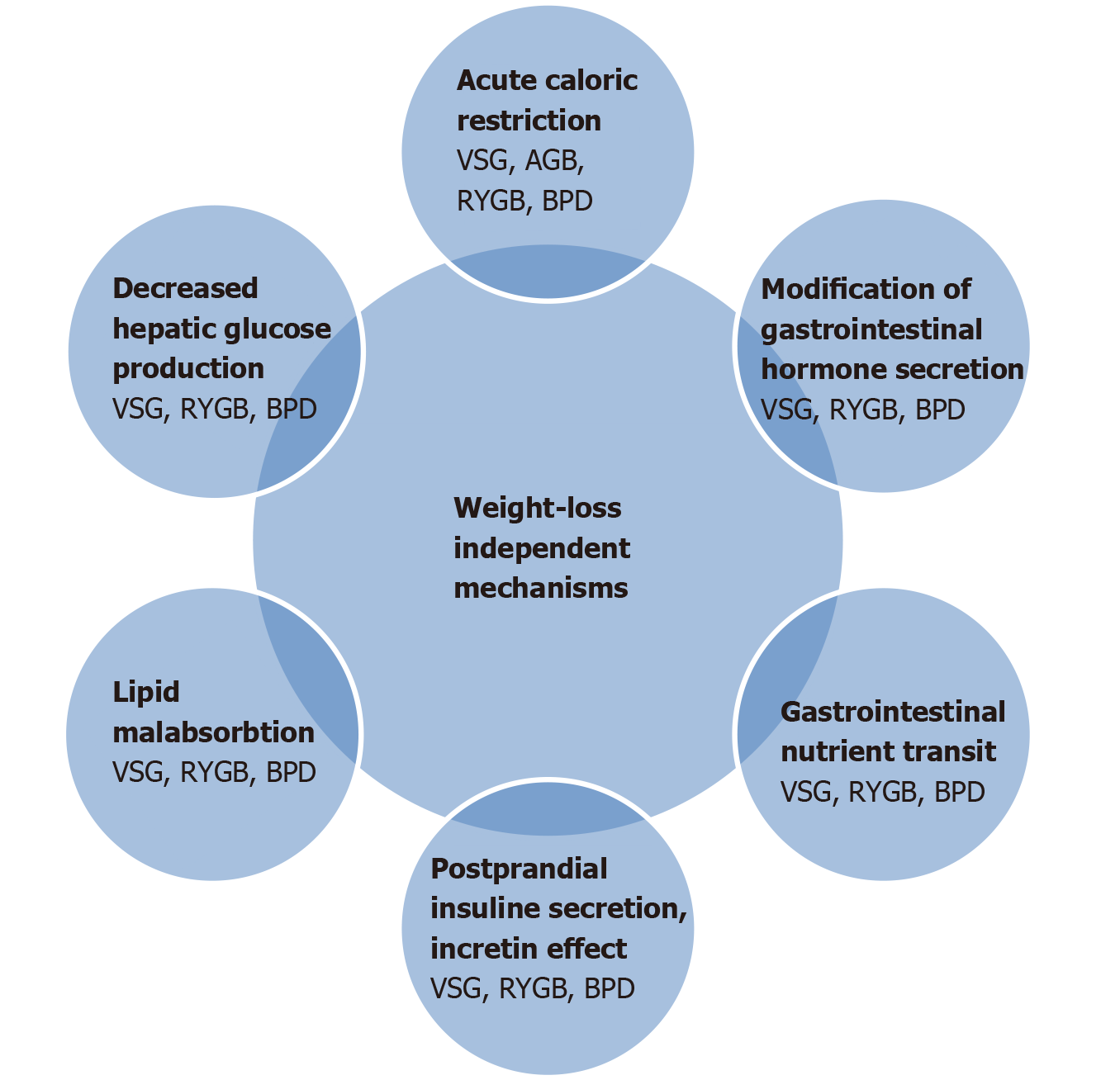
Another reported mechanism is plasma bile acid elevation after RYGB and VSG operations. As we know, bile acids can also act on rapid nutrient delivery further down the GI tract[54]. In addition, acute drastic caloric restriction in the post-operative period can also contribute to these mechanisms. The magnitude of weight loss is the cornerstone of T2DM remission after metabolic surgery[55], but weight-loss-independent mechanisms are more related with the improvement of beta cell function and decreased hepatic glucose production[56]. Modification of gut hormone secretion is certainly important in the long term maintenance of glucose regulation. Mechanisms of T2DM remission are demonstrated in detail in Figures 1 and 2.
It is widely believed that T2DM is a chronic progressive disease that at best can be controlled but never cured and that once treatment with glucose lowering medication is initiated, it needs to be increased over time. However, growing evidence from studies have indicated the remission of T2DM in patients with performed metabolic surgery[57,58]. Current definition of remission criteria is based on an American Diabetes Association multidisciplinary expert panel consensus in June 2009[59]. This panel declared a definition based on three criteria: Glycemic index below normal reference range, absence of treatment, and sustainability over time. In particular, there is no need for a drug treatment. A remission can be characterized as partial or complete; it is partial when glycemic indices fall into the pre-diabetic range for at least 1 year [hemoglobin A1c (HbA1c) 5.7%-6.4%, fasting plasma glucose (FPG) 100-125 mg/dL], whereas complete remission indicates the return to “normal” measures of glucose metabolism (HbA1c < 5.7%, FPG < 100 mg/dL). Prolonged remission is complete remission that lasts far more than 5 years.
This panel also recommended regular screening for the microvascular complications of the disease including nephropathy and retinopathy. Nevertheless, there is no consensus for how long monitoring is required for these patients.
Among the studies reporting T2DM remission rates after medical or surgical therapy, there is a considerable bias regarding the criteria used to define remission. Two important published studies highlighted the improvement of fasting plasma glucose and HbA1c during the long term follow-up. In 1995, Pories et al[60] published a paper titled ‘’Who would have thought it?” An operation proves to be the most effective therapy for adult onset diabetes mellitus. They documented that blood glucose levels normalized and the need for insulin therapy markedly diminished within 24 h of an RYGB. In addition, Scopinaro et al[61] reported BPD-DS accounted for glycemic control, and permanent serum cholesterol normalization was seen in 100% of operated patients. İn several initiated confirmatory clinical case series and trial studies it was reported that remission of T2DM took place in patients treated surgically[62,63]. PubMed research indicates that from 2004 to the present time, 70 clinical trials and randomized controlled trials have been referenced under bariatric surgery and remission of T2DM patients.
Mingrone et al[64] declared that complete remission of diabetes at 2 years was achieved in 75% of RYGB and 95% of BPD-DS patients. In another prominent randomized controlled study by Courcoulas et al[65] it was reported that partial and complete remission rates of T2DM were 50% and 17%, respectively, in the RYGB group, 27% and 23%, respectively, in the AGB group.
One of most quoted studies titled ”Calorie Reduction or Surgery: Seeking to Reduce Obesity and Diabetes Study randomized controlled study’’ compared the patients with RYGB vs intensive life style medical intervention[66]. Diabetes remission at 1 year was 60% with RYGB and 5.9% with medical intervention. The HbA1c decline was modestly more after RYGB (from 7.7 ± 1.0% to 6.4 ± 1.6%) compared to the medical intervention group (from 7.3 ± 0.9% to 6.9 ± 1.3%). The superiority of bariatric surgery to achieve diabetes remission compared to conservative management has been demonstrated in subjects within overweight range. Wentworth et al[67] evaluated the metabolic effects of AGB when added to multidisciplinary diabetes care in overweight individuals with T2DM. At two years, remission rates were 52% in the surgically treated group, whereas it was 8% in the control group receiving conservative treatment only.
The other cohort study that included patients with T2DM and BMI > 35 kg/m2 who received bariatric surgery (n = 8546) declared that complete remission of T2DM was in 58.2% (n = 2090) at 2 years and 46.6% at 5 years (n = 681)[68]. The study also indicated that remission of T2DM after bariatric surgery was inversely associated with the duration of diabetes and was highest among patients with recent onset and those not receiving insulin treatment. Although there is an agreement that remission rates have been closely related with types of surgery, patients’ characteristics such as age, diabetes duration, pre-operative glycemic control, and absence of insulin treatment were identified as predictive factors.
Another landmark randomized and controlled study titled ‘’Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently’’ reported that poorly controlled diabetic patients who were treated surgically (VSG, RYGB) significantly achieved more complete remission compared to patients in the medically treated group. The primary end point at 12 mo was HbA1c level of 6% or less. Across RYGB, VSG, and control groups, the primary end point occurred in 42%, 37%, and 12%, respectively, at the end of the first year. In addition, shorter diabetes duration was indicated as a better predictive factor for the remission of T2DM[69]. We know that higher pre-operative HbA1c does not necessarily correlate to reduced beta cell function, but it shows poorer glycemic control and greater severity of disease, factors known to reduce the remission of diabetes. Pre-operative insulin treatment shows reduced beta cell reserve that may not fully respond to the increase in incretin secretion after bariatric surgery. Purnell et al[70] investigated post-bariatric diabetes remission in 1868 obese participants. After 3 years, 68.7% of RYGB and 30.2 % LAGB participants were in diabetes remission. Baseline factors were related with diabetes remission including greater fasting C-peptide, lower HbA1c, and without need for insulin treatment. As mentioned above, improvement in glycemic indices was observed as early as within a few days post-operatively before any clinically significant weight loss was achieved, suggesting the presence of weight loss independent mechanisms of amelioration following bariatric surgery. Two nationwide meta-analyses showed that bariatric surgery was associated with greater short term (< 2 years) weight loss and better glucose outcomes related with metabolic surgery compared to medical treatment. Two recent meta-analyses that followed patients up to 5 years also indicated that T2DM patients receiving bariatric surgery had significantly higher remission rates ranging from 5.7% to 76.9%. Mingrone et al[64] presented 5 year follow-up data from their randomized controlled trial and partial remission rates at 2 years in patients treated with RYGB and BPD were 75% and 95%, respectively. In addition, partial remission rates at 5 years in patients treated with RYGB and BPD were 37% and 63%, respectively[48]. Chen et al[71] declared that after 10 years follow-up, RYGB compared with nonsurgical treatment resulted in significantly greater weight loss and reduction in HbA1c and use of antidiabetic medications in 173 obese patients with T2DM.
To date, a number of scoring systems have been developed with the purpose of better clarifying patient related factors associated with the probability of T2DM remission into prognostic models. Lee et al[72] proposed a diabetes surgical age, BMI, C-peptide level, and duration of diabetes (ABCD) score based on the results of a large prospective study. These prognostic factors were used to construct a simple scaling system ranging from 0 to 10. Patients with a higher score are more likely to achieve diabetes remission after surgery. This original scoring system went through some modifications to enhance predictive power with very low scores from the lower BMI population. The modified ABCD scoring system consisted of 510 patients from different hospitals across Asia and demonstrated very good predictability of diabetes remission from 5.9% to 93.3%. This simple scoring system provides helpful and novel information for identifying the best candidates for metabolic surgery, and it also indicates that the type of surgery has a significant influence on glycemic control.
Another scoring system called ‘’DiaRem’’ involves four clinical variables (use of insulin, age, HbA1c and type of antidiabetic agent), and these variables were sufficient to develop an algorithm that produces a T2DM remission. Using DiaRem, a complete and partial remission rate of 63% in 690 patients with performed RYGB was found. DiaRem score, however, was developed only for RYGB surgery, and it is limited in differentiating people with severe diseases. The DiaRem score is a practical clinical tool and has undergone external validation[73,74].
Aminian et al[75] recently published a novel scoring system to construct and validate remission of T2DM after metabolic surgery. Individualized Metabolic Surgery Score: Procedure Selection Based on Diabetes Severity (IMS) score based on large patient sample (n = 659) who underwent RYGB or VSG and included four independent predictors of type 2 diabetes remission. The IMS score variables include the number of diabetes medications, insulin use, duration of diabetes, and HbA1c levels. This score classified diabetes severity as mild, moderate, and severe. Patients with milder severity have been predicted to have a higher probability of T2DM remission after metabolic surgery.
An ideal prediction model for diabetes remission after metabolic surgery will guide clinicians and patients to make the optimal decision for diabetes treatment by balancing surgical risks against potential benefits. It should be able to select suitable candidates for metabolic surgery among those with diabetes and should be consistently reproducible in patients with baseline characteristics. Three mentioned scoring systems (ABCD, DiaRem, IMS) are models that are used to describe remission of diabetes and are efficient systems to identify patients during pre- and post-operative periods. But, we know that there was a considerable geographic difference of three cohorts (e.g., DiaRem and IMS include patients form United States, ABCD include from Asian population), and the baseline of BMI was shown to have variability. These prediction scoring systems should be validated according to various ethnicities to ensure universal applicability. The heterogeneity of results in all mentioned trials can be related with the description of T2DM remission. In general, the remission end points of most studies include the restoration of glycemia non-diabetic levels in the absence of active pharmacotherapy, but even this is not universally the case, as in the Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently trial. The cut-off values for FPG and HbA1c used in different studies also vary considerably. In addition, different durations of follow-up or differences in study populations may also affect the reported rates of T2DM remission in different studies. The peak weight loss effects of bariatric surgery are typically observed at more than 12 mo[76]. Therefore, types of surgical intervention, follow-up period, or study population for prediction of T2DM remission should be highlighted. Remission rates and criteria of T2DM after metabolic surgery in mentioned studies are shown in Table 1.
| Ref. | Study population | Study design | Surgical intervention | Remission end point of T2DM | Prediction factor of remission |
| Pories et al[60], 1995 | Morbidly obese, prediabetes, T2DM | Retrospective | RYGB | Normal levels of FPG and HbA1c | Shorter duration time of T2DM, younger age |
| Scopinaro et al[61], 2005 | Obese, T2DM | Retrospective | BPD | FPG < 125 mg/dL | Types of surgical intervention |
| Mingrone et al[64], 2015 | Patients with BMI > 35 kg/m2, T2DM | Prospective | RYGB, BPD | FPG < 5.6 mmol/L HbA1c < 6% | Types of surgical intervention |
| Courcoulas et al[65], 2014 | BMI 30-40 kg/m2 | RCT | AGB, RYGB | FPG < 100 mg/dL HbA1c < 5.7% | - |
| Cummings et al[66], 2016 (CROSSROADS) | BMI 30-45 kg/m2 | RCT | RYGB | HbA1c < 6% | Age, sex, baseline BMI, diabetes duration, insulin therapy |
| Wentworth et al[67], 2014 | BMI 25-30 kg/m2 | RCT | AGB | FPG < 7 mmol/L | Baseline measures of glycemia |
| Jans et al[68], 2019 | BMI > 35 kg/m2, T2DM | Retrospective | VSG, RYGB | Free from diabetes medication | Duration of diabetes, insulin treatment, age, baseline HbA1c |
| Schauer et al[69], 2012 (STAMPEDE) | BMI > 30 kg/m2, T2DM | RCT | VSG, RYGB | HbA1c < 6% | Age, sex, insulin treatment, baseline BMI, HbA1c |
| Purnell et al[70], 2016 | BMI > 30 kg/m2 | Prospective | AGB, RYGB | HbA1c < 6.5% FPG 6.9 mmol/L | Baseline weight, insulin treatment |
| Chen et al[71], 2016 | Obese type 2 diabetic patients | Retrospective | RYGB | HbA1c < 6% FPG < 100 mg/dL | Duration of diabetes, baseline HbA1c, insulin treatment |
| Lee et al[72], 2014 (ABCD score) | Morbid obese patients T2DM | RCT | VSG | HbA1c < 6% | Age, BMI, c peptide, duration of diabetes |
| Aminian et al[73], 2014 (DiaRem score) | Obese diabetic patients | Retrospective | RYGB | HbA1c < 6% Off medication | Duration of diabetes |
| Aminian et al[75], 2017 (IMS score) | Obese patients T2DM | RCT | RYGB, VSG | HbA1c < 6.5% | Duration of diabetes, insulin treatment, glycemic control |
In summary, the available evidence suggests that metabolic surgery can led to significant improvement in glycemic control and a substantial decrease in the rate of chronic diabetic complications. However, the identification of pre-operative patient level characteristics that signify the highest probability of remission is more important than the most convenient type of surgery. An individual therapeutic scheme for metabolic surgery should aim to minimize the long-term complications and improve the quality of life of affected individuals.
Metabolic surgery has achieved effective glycemic control in individuals with diabetes and obesity. However, long-term data are limited to show recurrence or remission of hyperglycemia years after the procedure. Moreover, there is limited data on the predictors of remission criteria on type 2 diabetes mellitus (T2DM) after metabolic surgery.
The medical management of T2DM is based on lifestyle modifications and specific glucose-lowering medications. The main purpose is targeted at maintaining glucose levels within an acceptable range, while the former purpose was to achieve weight loss through diet, increased physical activity, and behavioral therapy in order to modulate beneficially the underlying pathophysiology of T2DM. Although most individuals benefit from these conservative approaches in the short term, achieving a sustainable and clinically significant weight loss and its associated metabolic improvement is difficult. The significant effects of bariatric surgery regarding sustained weight loss and metabolic amelioration have gradually drawn attention and highlighted the potential of surgery to serve as a therapeutic modality for T2DM.
In this review, the research objective was to summarize type 2 diabetic patients treated with metabolic surgery. Moreover, all randomized controlled trials studying metabolic surgery were included in the research objectives.
All randomized controlled trials, case control trials, or multicenter studies were included in this review. These research studies were evaluated in detail in terms of patients’ demographic characteristics, types of surgical methods, duration times of diabetes, and the period after metabolic surgery.
The potential benefits of a wide-scale integration of bariatric surgery in standard diabetes care is hindered by the poor penetration that surgical therapeutic options share in T2DM management. However, there is still a lack of data derived from populations of individuals with T2DM. The identification of pre-operative patient-level characteristics may show the highest probability of being refractory or leading to a remission condition after metabolic surgery.
The number of metabolic surgeries used to treat obesity and T2DM will only increase in the coming years. More studies are needed to test the efficacy and safety of these surgical methods for T2DM. Clinicians should be cognizant of the long term effects on T2DM after metabolic surgery.
Understanding pathophysiology of durable remission and late relapse could aid patients and the procedure selection process. Further research is needed to study the potential effects bariatric surgery might have on the subsequent remission of diabetes mellitus.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feizi A, Tentolouris N, Vazquez-Jimenez JG S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LYT
| 1. | American Diabetes Association. 7. Obesity Management for the Treatment of Type 2 Diabetes. Diabetes Care. 2017;40:S57-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Bastien M, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56:369-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 769] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 3. | Persson PB, Bondke Persson A. Metabolism, obesity and the metabolic syndrome. Acta Physiol (Oxf). 2018;223:e13096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66 Suppl 2:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 455] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 5. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric surgery vs intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1179] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 6. | Kheniser KG, Kashyap SR. Diabetes management before, during, and after bariatric and metabolic surgery. J Diabetes Complications. 2018;32:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Grenier-Larouche T, Carreau AM, Carpentier AC. Early Metabolic Improvement After Bariatric Surgery: The First Steps Toward Remission of Type 2 Diabetes. Can J Diabetes. 2017;41:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Arterburn DE, Bogart A, Sherwood NE, Sidney S, Coleman KJ, Haneuse S, O'Connor PJ, Theis MK, Campos GM, McCulloch D, Selby J. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 304] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 10. | Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1262] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 11. | Chen M, Bergman RN, Porte D Jr. Insulin resistance and beta-cell dysfunction in aging: the importance of dietary carbohydrate. J Clin Endocrinol Metab. 1988;67:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 100] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1539] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 13. | Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 462] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Akash MSH, Rehman K, Liaqat A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J Cell Biochem. 2018;119:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 408] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 15. | Townsend LK, Medak KD, Peppler WT, Meers GM, Rector RS, LeBlanc PJ, Wright DC. High-saturated-fat diet-induced obesity causes hepatic interleukin-6 resistance via endoplasmic reticulum stress. J Lipid Res. 2019;60:1236-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2023] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 17. | Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. 2014;220:T1-T23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 347] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 18. | Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1276] [Cited by in RCA: 1368] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 19. | Cohen RV, Shikora S, Petry T, Caravatto PP, Le Roux CW. The Diabetes Surgery Summit II Guidelines: a Disease-Based Clinical Recommendation. Obes Surg. 2016;26:1989-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å, Bouchard C, Carlsson B, Karason K, Lönroth H, Näslund I, Sjöström E, Taube M, Wedel H, Svensson PA, Sjöholm K, Carlsson LM. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 741] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 21. | Chung AY, Thompson R, Overby DW, Duke MC, Farrell TM. Sleeve Gastrectomy: Surgical Tips. J Laparoendosc Adv Surg Tech A. 2018;28:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Du J, Tian J, Ding L, Trac C, Xia B, Sun S, Schones DE, Huang W. Vertical sleeve gastrectomy reverses diet-induced gene-regulatory changes impacting lipid metabolism. Sci Rep. 2017;7:5274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Navarro García MI, González-Costea Martínez R, Torregrosa Pérez N, Romera Barba E, Periago MJ, Vázquez Rojas JL. Fasting ghrelin levels after gastric bypass and vertical sleeve gastrectomy: An analytic cohort study. Endocrinol Diabetes Nutr. 2020;67:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Cottam D, Qureshi FG, Mattar SG, Sharma S, Holover S, Bonanomi G, Ramanathan R, Schauer P. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 452] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 25. | Parikh M, Issa R, McCrillis A, Saunders JK, Ude-Welcome A, Gagner M. Surgical strategies that may decrease leak after laparoscopic sleeve gastrectomy: a systematic review and meta-analysis of 9991 cases. Ann Surg. 2013;257:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 26. | Daes J, Jimenez ME, Said N, Daza JC, Dennis R. Laparoscopic sleeve gastrectomy: symptoms of gastroesophageal reflux can be reduced by changes in surgical technique. Obes Surg. 2012;22:1874-1879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Himpens J, Dapri G, Cadière GB. A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg. 2006;16:1450-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 494] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 28. | Furbetta N, Cervelli R, Furbetta F. Laparoscopic adjustable gastric banding, the past, the present and the future. Ann Transl Med. 2020;8:S4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 920] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 30. | Aung L, Lee WJ, Chen SC, Ser KH, Wu CC, Chong K, Lee YC, Chen JC. Bariatric Surgery for Patients With Early-Onset vs Late-Onset Type 2 Diabetes. JAMA Surg. 2016;151:798-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Pujol Rafols J, Al Abbas AI, Devriendt S, Guerra A, Herrera MF, Himpens J, Pardina E, Peinado-Onsurbe J, Ramos A, Ribeiro RJDS, Safadi B, Sanchez-Aguilar H, de Vries C, Van Wagensveld B. Roux-en-Y gastric bypass, sleeve gastrectomy, or one anastomosis gastric bypass as rescue therapy after failed adjustable gastric banding: a multicenter comparative study. Surg Obes Relat Dis. 2018;14:1659-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Furbetta N, Gragnani F, Flauti G, Guidi F, Furbetta F. Laparoscopic adjustable gastric banding on 3566 patients up to 20-year follow-up: Long-term results of a standardized technique. Surg Obes Relat Dis. 2019;15:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Rasheid S, Banasiak M, Gallagher SF, Lipska A, Kaba S, Ventimiglia D, Anderson WM, Murr MM. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg. 2003;13:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Hao Z, Townsend RL, Mumphrey MB, Morrison CD, Münzberg H, Berthoud HR. RYGB Produces more Sustained Body Weight Loss and Improvement of Glycemic Control Compared with VSG in the Diet-Induced Obese Mouse Model. Obes Surg. 2017;27:2424-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Jørgensen NB, Dirksen C, Bojsen-Møller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved â-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62:3044-3052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 36. | Shah M, Law JH, Micheletto F, Sathananthan M, Dalla Man C, Cobelli C, Rizza RA, Camilleri M, Zinsmeister AR, Vella A. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes. 2014;63:483-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 37. | Gagner M, Rogula T. Laparoscopic reoperative sleeve gastrectomy for poor weight loss after biliopancreatic diversion with duodenal switch. Obes Surg. 2003;13:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Praveenraj P, Gomes RM, Kumar S, Perumal S, Senthilnathan P, Parthasarathi R, Rajapandian S, Palanivelu C. Comparison of weight loss outcomes 1 year after sleeve gastrectomy and Roux-en-Y gastric bypass in patients aged above 50 years. J Minim Access Surg. 2016;12:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Lee WJ, Chong K, Aung L, Chen SC, Ser KH, Lee YC. Metabolic Surgery for Diabetes Treatment: Sleeve Gastrectomy or Gastric Bypass? World J Surg. 2017;41:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, Dallal GE, Saltzman E. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Scopinaro N, Gianetta E, Adami GF, Friedman D, Traverso E, Marinari GM, Cuneo S, Vitale B, Ballari F, Colombini M, Baschieri G, Bachi V. Biliopancreatic diversion for obesity at eighteen years. Surgery. 1996;119:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 205] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Sethi M, Chau E, Youn A, Jiang Y, Fielding G, Ren-Fielding C. Long-term outcomes after biliopancreatic diversion with and without duodenal switch: 2-, 5-, and 10-year data. Surg Obes Relat Dis. 2016;12:1697-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Scopinaro N. Thirty-five years of biliopancreatic diversion: notes on gastrointestinal physiology to complete the published information useful for a better understanding and clinical use of the operation. Obes Surg. 2012;22:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Topart PA, Becouarn G. Revision and reversal after biliopancreatic diversion for excessive side effects or ineffective weight loss: a review of the current literature on indications and procedures. Surg Obes Relat Dis. 2015;11:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Gulliford MC, Booth HP, Reddy M, Charlton J, Fildes A, Prevost AT, Khan O; King’s Bariatric Surgery Study Group. Effect of Contemporary Bariatric Surgical Procedures on Type 2 Diabetes Remission. A Population-Based Matched Cohort Study. Obes Surg. 2016;26:2308-2315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Zhao L, Zhu L, Su Z, Liu Y, Li P, Yang X, Li W, Tan L, Sun X, Zhu S. Using the hyperinsulinemic euglycemic clamp to assess insulin sensitivity at 3 months following Roux-en-Y gastric bypass surgery in type 2 diabetes patients with BMI <35 kg/m2 in China. Int J Surg. 2017;38:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, Gastaldelli A, Chambers KT, Su X, Okunade A, Patterson BW, Klein S. Gastric bypass and banding equally improve insulin sensitivity and â cell function. J Clin Invest. 2012;122:4667-4674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 48. | Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab. 2013;98:4391-4399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 49. | le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, Ghatei MA, Patel A, Bloom SR, Aylwin SJ. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Laferrère B. Diabetes remission after bariatric surgery: is it just the incretins? Int J Obes (Lond). 2011;35 Suppl 3:S22-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 586] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 52. | Nicoletti CF, Morandi Junqueira-Franco MV, dos Santos JE, Marchini JS, Salgado W Jr, Nonino CB. Protein and amino acid status before and after bariatric surgery: a 12-month follow-up study. Surg Obes Relat Dis. 2013;9:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Staak M. [Morbidity of the drug addict]. Versicherungsmedizin. 1990;42:106-109. [PubMed] |
| 54. | Zhang M. [Preparation on monoclonal antibodies against laminin receptors and their inhibitory effects on attachment and spreading of tumor cells]. Zhonghua Yi Xue Za Zhi. 1990;70:311-314, 22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 55. | Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery vs hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479-2485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 501] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 56. | Nosso G, Griffo E, Cotugno M, Saldalamacchia G, Lupoli R, Pacini G, Riccardi G, Angrisani L, Capaldo B. Comparative Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Glucose Homeostasis and Incretin Hormones in Obese Type 2 Diabetic Patients: A One-Year Prospective Study. Horm Metab Res. 2016;48:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1882] [Cited by in RCA: 1682] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 58. | Cho YM. A gut feeling to cure diabetes: potential mechanisms ofdiabetes remission after bariatric surgery. Diabetes Metab J. 2014;38:406-1532. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, McLaughlin S, Phillips GL 2nd, Robertson RP, Rubino F, Kahn R, Kirkman MS. How do we define cure of diabetes? Diabetes Care. 2009;32:2133-2135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 729] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 60. | Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM. Who would have thought it? Ann Surg. 1995;222:339-50; discussion 350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1456] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 61. | Scopinaro N, Marinari GM, Camerini GB, Papadia FS, Adami GF. Specific effects of biliopancreatic diversion on the major components of metabolic syndrome: a long-term follow-up study. Diabetes Care. 2005;28:2406-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 62. | Aminian A, Vidal J, Salminen P, Still CD, Nor Hanipah Z, Sharma G, Tu C, Wood GC, Ibarzabal A, Jimenez A, Brethauer SA, Schauer PR, Mahawar K. Late Relapse of Diabetes After Bariatric Surgery: Not Rare, but Not a Failure. Diabetes Care. 2020;43:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 63. | Coleman KJ, Haneuse S, Johnson E, Bogart A, Fisher D, O'Connor PJ, Sherwood NE, Sidney S, Theis MK, Anau J, Schroeder EB, O'Brien R, Arterburn D. Long-term Microvascular Disease Outcomes in Patients With Type 2 Diabetes After Bariatric Surgery: Evidence for the Legacy Effect of Surgery. Diabetes Care. 2016;39:1400-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 64. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery vs conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 887] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 65. | Courcoulas AP, Belle SH, Neiberg RH, Pierson SK, Eagleton JK, Kalarchian MA, DeLany JP, Lang W, Jakicic JM. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA Surg. 2015;150:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 283] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 66. | Cummings DE, Arterburn DE, Westbrook EO, Kuzma JN, Stewart SD, Chan CP, Bock SN, Landers JT, Kratz M, Foster-Schubert KE, Flum DR. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. 2016;59:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 67. | Wentworth JM, Playfair J, Laurie C, Ritchie ME, Brown WA, Burton P, Shaw JE, O'Brien PE. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 68. | Jans A, Näslund I, Ottosson J, Szabo E, Näslund E, Stenberg E. Duration of type 2 diabetes and remission rates after bariatric surgery in Sweden 2007-2015: A registry-based cohort study. PLoS Med. 2019;16:e1002985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 69. | Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery vs intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 1590] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 70. | Purnell JQ, Selzer F, Wahed AS, Pender J, Pories W, Pomp A, Dakin G, Mitchell J, Garcia L, Staten MA, McCloskey C, Cummings DE, Flum DR, Courcoulas A, Wolfe BM. Type 2 Diabetes Remission Rates After Laparoscopic Gastric Bypass and Gastric Banding: Results of the Longitudinal Assessment of Bariatric Surgery Study. Diabetes Care. 2016;39:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 71. | Chen Y, Corsino L, Shantavasinkul PC, Grant J, Portenier D, Ding L, Torquati A. Gastric Bypass Surgery Leads to Long-term Remission or Improvement of Type 2 Diabetes and Significant Decrease of Microvascular and Macrovascular Complications. Ann Surg. 2016;263:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 72. | Lee WJ, Almulaifi A, Tsou JJ, Ser KH, Lee YC, Chen SC. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: predicting the success by ABCD score. Surg Obes Relat Dis. 2015;11:991-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 73. | Aminian A, Brethauer SA, Kashyap SR, Kirwan JP, Schauer PR. DiaRem score: external validation. Lancet Diabetes Endocrinol. 2014;2:12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Lee WJ, Chong K, Chen SC, Zachariah J, Ser KH, Lee YC, Chen JC. Preoperative Prediction of Type 2 Diabetes Remission After Gastric Bypass Surgery: a Comparison of DiaRem Scores and ABCD Scores. Obes Surg. 2016;26:2418-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 75. | Aminian A, Brethauer SA, Andalib A, Nowacki AS, Jimenez A, Corcelles R, Hanipah ZN, Punchai S, Bhatt DL, Kashyap SR, Burguera B, Lacy AM, Vidal J, Schauer PR. Individualized Metabolic Surgery Score: Procedure Selection Based on Diabetes Severity. Ann Surg. 2017;266:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 76. | Dicker D, Golan R, Aron-Wisnewsky J, Zucker JD, Sokolowska N, Comaneshter DS, Yahalom R, Vinker S, Clément K, Rudich A. Prediction of Long-Term Diabetes Remission After RYGB, Sleeve Gastrectomy, and Adjustable Gastric Banding Using DiaRem and Advanced-DiaRem Scores. Obes Surg. 2019;29:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |