Published online Aug 15, 2021. doi: 10.4239/wjd.v12.i8.1292
Peer-review started: November 11, 2020
First decision: June 5, 2021
Revised: June 15, 2021
Accepted: July 13, 2021
Article in press: July 13, 2021
Published online: August 15, 2021
Processing time: 270 Days and 19.4 Hours
In addition to insulin resistance, impaired insulin secretion has recently been identified as a crucial factor in the pathogenesis of type 2 diabetes mellitus (T2DM). Scarce clinical data exist for pediatric T2DM.
To investigate the association of β-cell function and insulin resistance with pediatric T2DM in the first Chinese multicenter study.
This multicenter cross-sectional study included 161 newly diagnosed T2DM children and adolescents between January 2017 and October 2019. Children with normal glycemic levels (n = 1935) were included as healthy control subjects. The homeostasis models (HOMAs) were used to assess the β-cell function (HOMA2-%B) and insulin resistance (HOMA2-IR) levels. The HOMA index was standardized by sex and age. We performed logistic regression analysis to obtain odds ratios (ORs) for T2DM risk using the standardized HOMA index, adjusted for confounding factors including sex, Tanner stage, T2DM family history, body mass index z-score, and lipid profile.
The male-female ratio of newly diagnosed T2DM patients was 1.37:1 (OR = 2.20, P = 0.011), and the mean ages of onset for boys and girls were 12.5 ± 1.9 years and 12.3 ± 1.7 years, respectively. The prevalence of related comorbidities including obesity, elevated blood pressure, and dyslipidemia was 58.2%, 53.2%, and 80.0%, respectively. The T2DM group had lower HOMA2-%B levels (P < 0.001) and higher HOMA2-IR levels (P < 0.001) than the control group. Both the decrease in HOMA2-%B z-score (OR = 8.40, 95%CI: 6.40–11.02, P < 0.001) and the increase in HOMA2-IR z-score (OR = 1.79, 95%CI: 1.60–2.02, P < 0.001) were associated with a higher risk of T2DM, and the decrease in HOMA2-%B z-score always had higher ORs than the increase in HOMA2-IR z-score after adjusting for confounding factors.
Besides insulin resistance, β-cell function impairment is also strongly associated with Chinese pediatric T2DM. Gender difference in susceptibility and high comorbidities warrant specific T2DM screening and prevention strategies in Chinese children.
Core Tip: This multicenter cross-sectional study described the clinical characteristics of Chinese type 2 diabetes (T2DM) children in detail, and characterized the association of β-cell function and insulin resistance with T2DM among children and adolescents. This study showed that β-cell dysfunction, compared with insulin resistance, is a stronger risk factor associated with pediatric T2DM among Chinese children, regardless of obesity status. Our results suggested that pediatricians should pay more attention to children with relatively low β-cell function regardless of insulin resistance during pediatric T2DM screening and emphasize the protection and improvement of β-cell function in T2DM children in clinical practice.
- Citation: Xu ZR, Du HW, Cui LW, Zheng RX, Li GM, Wei HY, Lu FY, Chen LL, Wu CS, Zhang SX, Zhang SL, Liu F, Zhang MY, Pei Z, Sun CJ, Wu J, Luo FH. Association of β-cell function and insulin resistance with pediatric type 2 diabetes among Chinese children. World J Diabetes 2021; 12(8): 1292-1303
- URL: https://www.wjgnet.com/1948-9358/full/v12/i8/1292.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i8.1292
Over the past two decades, pediatric type 2 diabetes mellitus (T2DM) has emerged in both developed and underdeveloped countries, mainly driven by the increasing prevalence of obesity in children and adolescents[1-4]. China’s estimated annual incidence of T2DM is 1.96/100000 people[5]. With higher risks of complications and comorbidities than adult T2DM and pediatric T1DM, pediatric T2DM imposes an increasing burden on both patients and society[6,7].
Insulin resistance and impaired insulin secretion are two crucial factors in the pathogenesis of T2DM[8]. However, for T2DM patients of different sexes, ages, and ethnicities, these factors affect the pathogenesis of T2DM differently. The United States Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) Trial found that impairment of β-cell function in children with T2DM was more serious than that in adults at the time of diagnosis[9], and occurred as early as prediabetes[10,11]. The β-cell function deteriorated more rapidly in children and adolescents than in adults[12,13]. When comparing with the United States population, it was indicated that β-cell dysfunction played a more important role than insulin resistance in the occurrence of T2DM among the Japan population[14]; whereas in the latest study, Wang et al[15] found that insulin resistance was more strongly associated with incident diabetes among Chinese adults, and in the Caucasian population, the high insulin resistance level in females during puberty made girls more prone to T2DM[16]. Therefore, it is still inconsistent among different studies regarding the insulin resistance and β-cell function dysfunction in different population and age groups about T2DM pathogenesis.
In natural human development, insulin resistance increases significantly with the progression of puberty development but decreases to near prepubertal levels at Tanner stage 5[17,18], thus the ever-increasing insulin resistance if accompanied by β-cell dysfunction during puberty development may increase the T2DM risk in adolescents. So far, study especially multi-center study in the Chinese pediatric population is still scarce. We thus aimed to systematically describe the clinical characteristics of Chinese children and adolescents with T2DM in the first Chinese multicenter pediatric T2DM cohort and assess the relationship between sex and pediatric T2DM. We used homeostasis models (HOMAs) to investigate the association between islet function and pediatric T2DM, as well as to assess the effects of sex, obesity, and puberty on β-cell function and insulin resistance.
This multicenter cross-sectional study collected data from six centers in China located in Changchun, Harbin, Jinan, Shanghai, Tianjin, and Zhengzhou. These included four tertiary hospitals and two pediatric hospitals (Supplementary Figure 1). Between January 2017 and October 2019, data were collected from all patients newly diagnosed with T2DM who were under 18 years old. Cases were validated using the criteria of the International Society for Pediatric and Adolescent Diabetes (ISPAD)[19,20]. Cases were excluded if the patient had positive diabetes-associated autoantibodies (including islet-cell antibody, insulin autoantibody, serum glutamate decarboxylase antibody, protein tyrosine phosphatase antibody, and zinc transporter-8 antibody), inherited disorders, other endocrine diseases, or other serious chronic disorders. Ultimately, 168 newly diagnosed T2DM patients were collected from six centers during the study period; seven cases were excluded due to positive diabetes-associated autoantibodies (Supplementary Figure 2). This study was approved by the medical ethics committees of the six hospitals.
Control subjects were enrolled from primary school and middle school. Informed consent was obtained from the guardians of the students. Children aged below 7 years or over 18 years, those who did not complete a medical examination, and those who might have had glucose metabolism disorders (history of diabetes, fasting blood glucose ≥ 7.0 mmol/L, or HbA1c ≥ 6.5%) were excluded. Cases with data missing for the HOMA index, sex, or age were also excluded. Ultimately, 1935 children and adolescents were enrolled as the control population (Supplementary Figure 2). The recruitment of the control group was approved by the medical ethics committee of Children’s Hospital of Fudan University.
From medical records, clinical characteristics of T2DM patients were collected, including name, sex, age of onset, birth weight, family history of T2DM, height, weight, blood pressure, Tanner stage, fasting serum glucose, fasting serum insulin, HbA1c, diabetes-associated autoantibodies, total triglycerides, total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol. Biochemical data were measured simultaneously at diagnosis of disease on admission in the central laboratory in the local center. Data from the control population were obtained from physical examinations and questionnaires. Fasting blood samples of the control population were collected and measured in the central laboratory in Shanghai.
Body mass index (BMI) was calculated, and BMI z-scores were computed using the Chinese standard[21]. Obesity and elevated blood pressure were diagnosed according to the Chinese standards for children and adolescents[22,23]. Dyslipidemia was diagnosed according to the National Heart, Lung, and Blood Institute guidelines for children and adolescents[24].
HOMAs were used to evaluate islet function. The homeostasis model assessment of β-cell function (HOMA2-%B) and homeostasis model assessment of insulin resistance (HOMA2-IR) were computed using the HOMA2 Calculator v2.2.3 (Diabetes Trials Unit, University of Oxford, United Kingdom).
We also performed a literature review to identify the clinical characteristics of T2DM in children from different countries, especially the male-female ratio (Supplementary material and Supplementary Figure 3).
Data are described as numbers (proportions) for categorical variables and the mean ± SD or medians (IQRs) for continuous variables. We used χ² tests to compare categorical variables. For normally distributed continuous variables, Student's t test was used to compare the differences between two groups, and the Kruskal-Wallis test was used if variables were not consistent with a normal distribution.
Since insulin resistance level and β-cell function change during puberty, the HOMA index was standardized to sex- and age-specific z-scores [the (index-mean)/SD, mean, and SD were calculated from the control population].
We examined the associations of the HOMA2-%B z-score and the HOMA2-IR z-score with pediatric T2DM using univariate and multivariate binary logistic regression analyses. Confounding factors were adjusted, including sex, Tanner stage, T2DM family history, BMI z-score, and lipid profile. Regression analyses were also done in different subgroups based on sex, obese status, and pubescent status. We also conducted three sensitivity analyses. To avoid the influence of glucotoxicity effect on β-cell function, we removed the cases with diabetic ketoacidosis (DKA) and did the multivariate binary logistic regression analysis again[25]. We further analyzed T2DM association with different groups of age (≤ 12 years and > 12 years) and BMI z-scores, which were used as another diagnostic criterion for childhood obesity (< 2 and ≥ 2). Binary logistic regression analysis was also used to estimate the OR for pediatric T2DM in Chinese boys. A two-sided P value of less than 0.05 was considered significant. All analyses were performed using IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, United States).
Among the newly diagnosed T2DM patients, 93 (57.8%) were male (male-female ratio: 1.37: 1). The mean age of onset was 12.4 ± 1.8 (range: 6.6 to 16.7) years, and 15 (9.3%) patients were diagnosed before 10 years of age (Table 1). The age of onset in Chinese boys was similar to that in Chinese girls (12.5 ± 1.9 vs 12.3 ± 1.7, P = 0.50) (Supplementary Table 1). Most of the patients (76.2%) were pubescent; however, nearly 30.9% of the male patients were diagnosed with T2DM before puberty, and 73.3% of those < 10 years old were at the Tanner I stage. Furthermore, 26.2% of cases had a birth history of macrosomia.
| T2DM (n = 161) | Control population (n = 1935) | P value | |
| Gender | 0.068 | ||
| Male | 93/161 (57.8%) | 973/1935 (50.3%) | |
| Female | 68/161 (42.2%) | 962/1935 (49.7%) | |
| Age, yr | 12.4 ± 1.8 | 11.4 ± 2.5 | < 0.0001c |
| BMI z-score | 2.22 ± 0.91 | 0.33 ± 1.10 | < 0.0001c |
| Birth weight, kg | 3.52 ± 0.74 | 3.32 ± 0.46 | < 0.0001c |
| Obesity status | |||
| Normal weight | 19/153 (12.4%) | 1558/1935 (80.5%) | < 0.0001c |
| Overweight | 45/153 (29.4%) | 232/1935 (12.0%) | < 0.0001c |
| Obesity | 89/153 (58.2%) | 145/1935 (7.5%) | < 0.0001c |
| Stage of puberty | |||
| Tanner I | 24/101 (23.8%) | 960/1841 (52.1%) | < 0.0001c |
| Tanner II | 26/101 (25.7%) | 231/1841 (12.5%) | < 0.0001c |
| Tanner III | 20/101 (19.8%) | 221/1841 (12.0%) | 0.021a |
| Tanner IV | 14/101 (13.9%) | 319/1841 (17.3%) | 0.37 |
| Tanner V | 17/101 (16.8%) | 110/1841 (6.0%) | < 0.0001c |
| Family history of T2DM | 75/159 (47.2%) | 324/1871 (17.3%) | < 0.0001c |
| Fasting serum glucose (mmol/L) | 10.6 (7.1-15.2) | 4.6 (4.4-4.9) | < 0.0001c |
| Fasting serum insulin (mIU/L) | 13.0 (7.5-25.9) | 10.0 (6.9-14.1) | < 0.0001c |
| HbA1c (%) | 11.5 (8.9-13.0) | 5.2 (5.0-5.4) | < 0.0001c |
| Total triglycerides (mmol/L) | 1.7 (1.11-2.63) | 0.67 (0.51-0.90) | < 0.0001c |
| Total cholesterol (mmol/L) | 4.70 (3.94-5.40) | 3.86 (3.45-4.30) | < 0.0001c |
| LDL-C (mmol/L) | 3.01 (2.45-3.47) | 2.27 (1.93-2.70) | < 0.0001c |
| HDL-C (mmol/L) | 1.10 (0.92-1.27) | 1.91 (1.64-2.19) | < 0.0001c |
| Dyslipidemia | 108/135 (80.0%) | 245/1935 (12.7%) | < 0.0001c |
| Elevated blood pressure | 59/111 (53.2%) | 231/1862 (12.4%) | < 0.0001c |
The onset of pediatric T2DM was insidious. About 30% of the pediatric T2DM patients did not report any discomfort or present characteristic clinical manifestations, and were diagnosed accidentally. Children and adolescents with T2DM tended to have other metabolically related comorbidities, including obesity, elevated blood pressure, and dyslipidemia; the comorbidities of the above diseases were 58.2%, 53.2%, and 80.0%, respectively.
Metformin and insulin injection were the two most commonly used medications for Chinese pediatric T2DM patients, with 63.4% of the patients using only metformin.
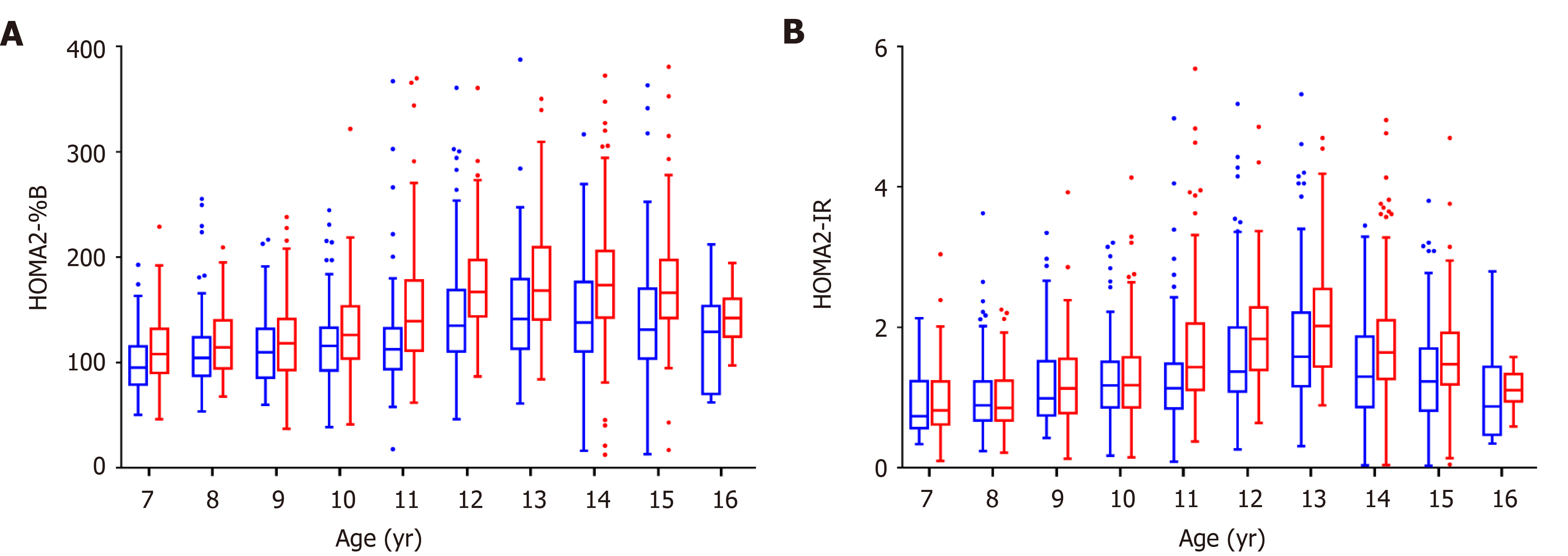
The variation trend of islet function in Chinese children and adolescents of different ages were categorized by sex, using the control population. Both boys and girls showed a gradual increase in insulin resistance and did not show a downward trend until age 14 (Figure 1). The level of insulin resistance was similar between boys and girls before age 10; while older girls had higher insulin resistance levels than did boys in the same age group. Changes in β-cell function followed the trend of insulin resistance in compensation.
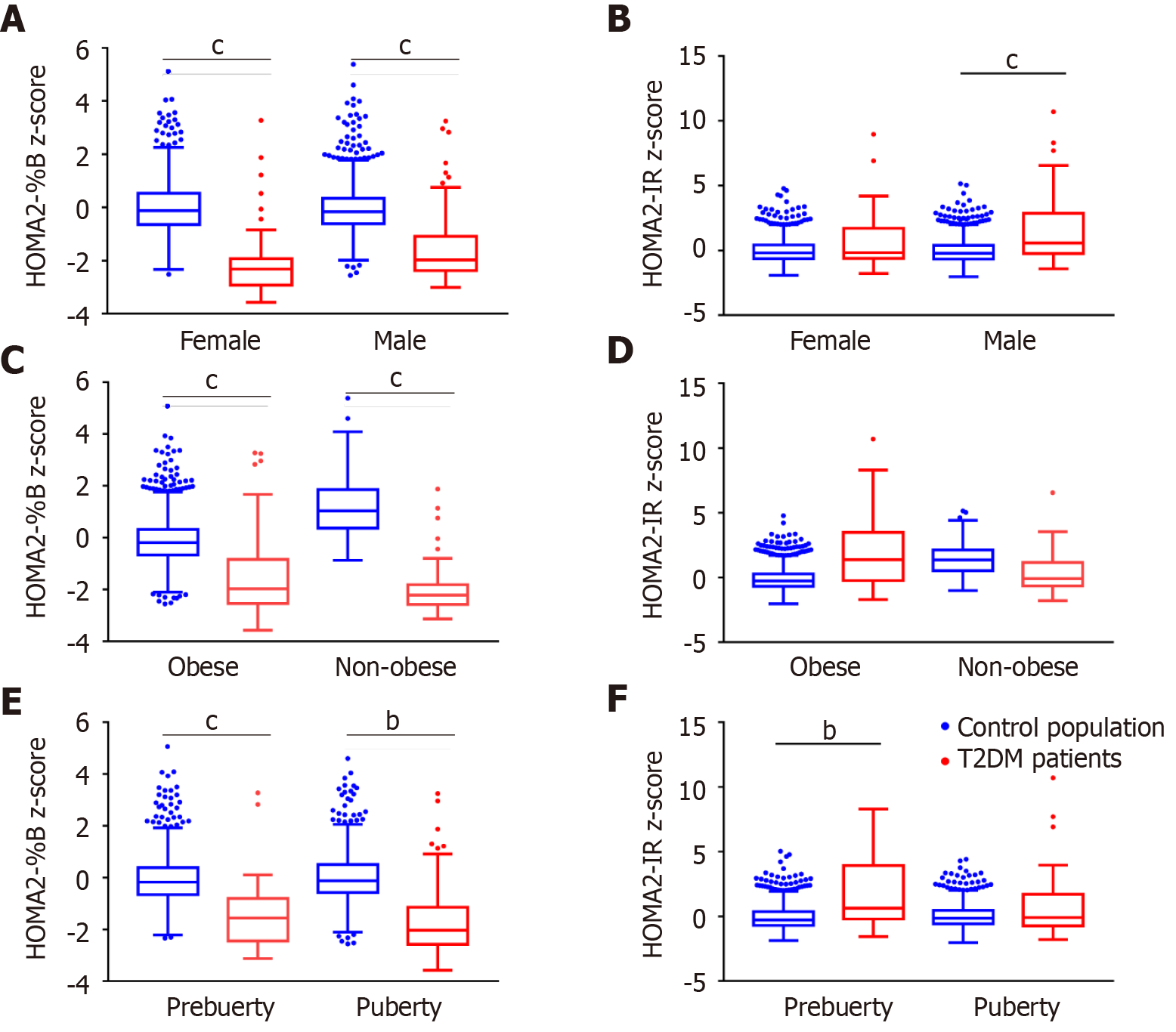
Newly diagnosed children and adolescents with T2DM had impaired β-cell function and serious insulin resistance. The HOMA2-%B levels of T2DM patients were significantly lower than those of the control population [34.7 (16.7–87.1) vs 126.9 (98.9–165.2), P < 0.0001], while the HOMA2-IR levels were significantly higher [2.23 (1.25–4.37) vs 1.31 (0.90–1.87), P < 0.0001]. However, in our stratification analysis after using z-score standardization to adjust for the effects of age and sex, T2DM children always had lower HOMA2-%B z-score than the control population, but we found that there was no significant difference in insulin resistance levels between T2DM patients and the control population in girls, obese and non-obese children, and pubescent children (Figure 2 and Supplementary Figure 4).
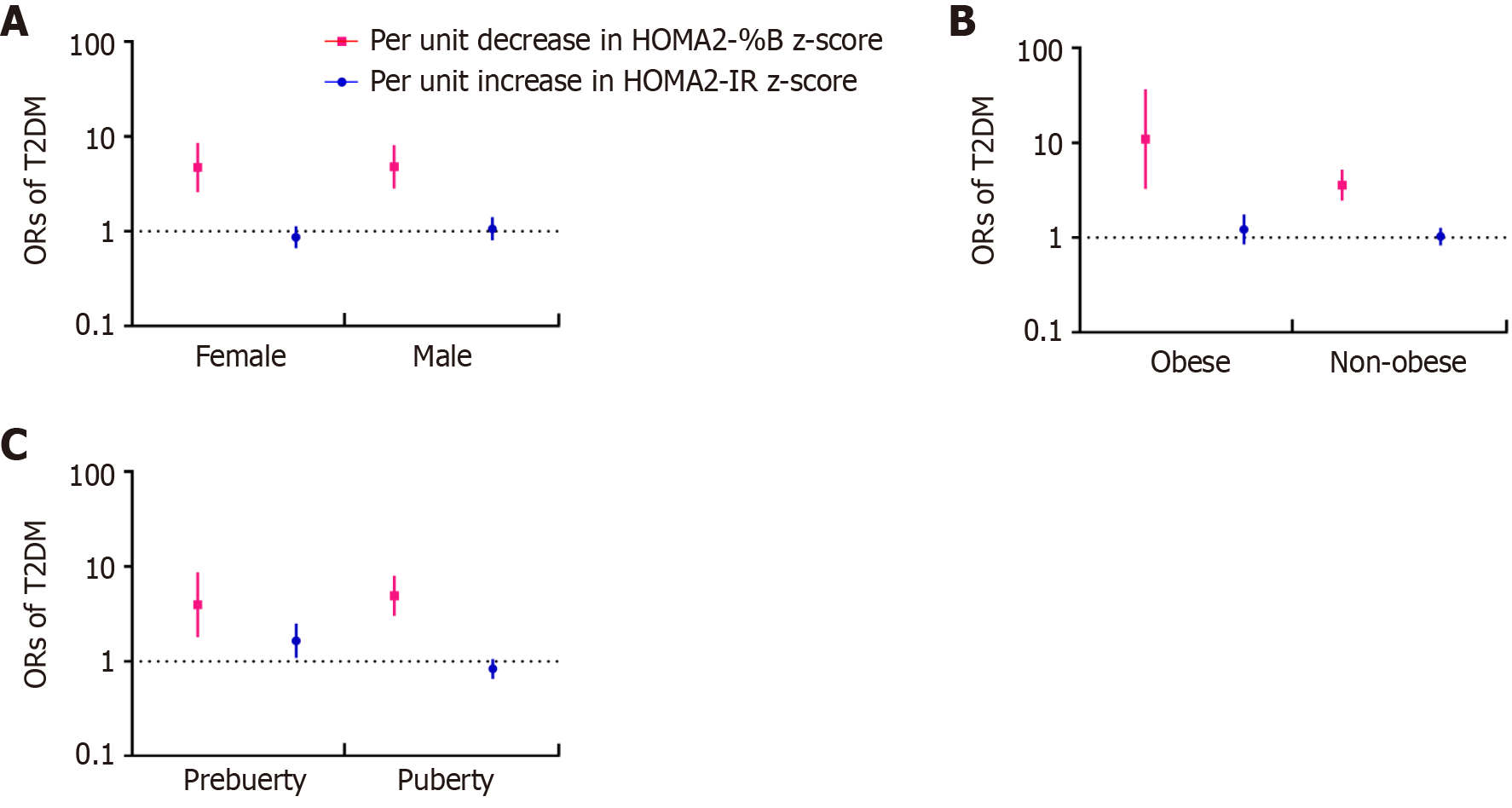
Both the decrease in HOMA2-%B and the increase in HOMA2-IR were associated with pediatric T2DM. The ORs for T2DM were 8.40 (95%CI: 6.40–11.02) per unit decrease in the z-score of HOMA2-%B and 1.79 (95%CI: 1.60–2.02) per unit increase in the z-score of HOMA2-IR according to the univariate regression. A decrease in HOMA2-%B z-score always had higher ORs than an increase in HOMA2-IR z-score in different models (Supplementary Table 2). Furthermore, after adjusting for sex, age, BMI z-score, T2DM family history, Tanner stage, and serum lipid, only HOMA2-%B showed a close relationship with T2DM, with an OR of 4.78 (95%CI: 3.22–7.11), while HOMA2-IR did not have a close association with T2DM (OR = 0.93, 95%CI: 0.77–1.11). Further stratified analysis of sex, obesity status, and puberty status revealed that the increase in HOMA2-IR z-score was only closely related to T2DM among prepubescent children (OR = 1.65, 95%CI: 1.08–2.50), while HOMA2-%B consistently had a strong association with pediatric T2DM (Figure 3).
Our sensitivity analyses produced the same results. A decrease in HOMA2-%B z-score, rather than an increase in HOMA2-IR z-score, was significantly associated with T2DM across different BMI z-score and age groups (Supplementary Figure 5). Furthermore, when excluding T2DM cases with DKA to avoid the influence of glucotoxicity effect on β-cell function, the decrease in HOMA2-%B z-score still had higher ORs than the increase in HOMA2-IR z-score [per unit decrease in the z-score of HOMA2-%B: 4.58 (3.06–6.84); per unit increase in the z-score of HOMA2-IR: 0.96 (0.80–1.15)].
Chinese boys had a higher risk of T2DM than did girls, with an OR (95%CI) of 2.20 (1.20–4.04) after adjusting for age, T2DM family history, BMI z-score, Tanner stage, and dyslipidemia. After adding the HOMA index to the model, boys still had a higher risk of T2DM, with an OR (95%CI) of 5.12 (1.48–17.65). The high ORs and male-female ratio of 1.37:1 suggested that Chinese males are more susceptible to pediatric T2DM.
This nationwide cross-sectional study produced a detailed portrayal of Chinese T2DM children and adolescents and analyzed the association between pediatric T2DM and islet function in terms of age and sex. A decrease in HOMA2-%B and an increase in HOMA2-IR were both related to the onset of pediatric T2DM, and β-cell impairment played an important role in addition to insulin resistance. Furthermore, we identified high rates of susceptibility to T2DM in male Chinese children and adolescents after adjusting for other related factors.
Obesity is known to be one of the main causes of insulin resistance and T2DM. Other factors such as genetics, a sedentary lifestyle, and puberty are also associated with insulin resistance[26]. T2DM children and adolescents have been shown to have high levels of HOMA2-IR[9,10]. However, previous studies often did not account for the effects of age and sex on insulin resistance when comparing insulin resistance levels between children with T2DM and healthy children. In this study, we adjusted the HOMA2-IRs by using age- and sex-specific z-score standardization according to the control population and found that there was no significant difference between the HOMA2-IRs of T2DM patients and those of the control population, for both obese and non-obese children. Furthermore, decreases in HOMA2-IR z-scores were not closely associated with pediatric T2DM after the adjustment for other related factors. While we did not discount insulin resistance as a key feature of T2DM, our results suggested that most Chinese children had a relatively high insulin resistance level during puberty and thus β-cell impairment was of great importance in the development of pediatric T2DM in the context of a high level of insulin resistance.
The β-cell function impairment of T2DM patients was closely related to genetic susceptibility, which thus might lead to ethnic differences[27,28]. Chinese T2DM children and adolescents had significant decreases in HOMA2-%B in our stratification analyses even after z-score standardization. Our findings supported studies that have indicated that children and East Asian T2DM patients tend to have a more fragile β-cell function[10,14]. For instance, the TODAY study found that the pediatric T2DM involved a rapid deterioration of β-cell function, and our previous single-center study of Chinese children found a notable decrease in the β-cell function of newly diagnosed T2DM children[11,29]. Additional studies have provided evidence that β-cell function is crucial to the pathogenesis of T2DM, and Weir et al[30] noted that deficient β-cell mass was essential for the pathogenesis of T2DM.
However, Wang et al[15] found a stronger association between insulin resistance and T2DM, with an attributable risk percentage of 24% among Chinese adults. The results of our stratified analysis partially explained why the study by Wang et al [15] produced results that differed from those of the other studies, by highlighting the differences between Chinese children and adults. Boys and prepubescent patients had higher HOMA2-IR z-scores than control patients, and prepubescent children with high HOMA2-IR z-scores were at a higher risk for T2DM, with an OR of 1.65 (95%CI: 1.08–2.50). Puberty is known to cause an increase in insulin resistance that is more significant in girls than in boys[18], as was the case for the Chinese adolescents in our study, who experienced a significant increase in insulin resistance after puberty; even children with euglycemia were unable to fully compensate for this effect. Therefore, in the context of high levels of insulin resistance, the inability of the β-cells to compensate for increased insulin resistance had a larger effect than increased insulin resistance alone on the pathogenesis of pediatric T2DM. Prepubescent children had relatively low insulin resistance levels; therefore, a high insulin resistance level was associated with the occurrence of T2DM among prepubescent children.
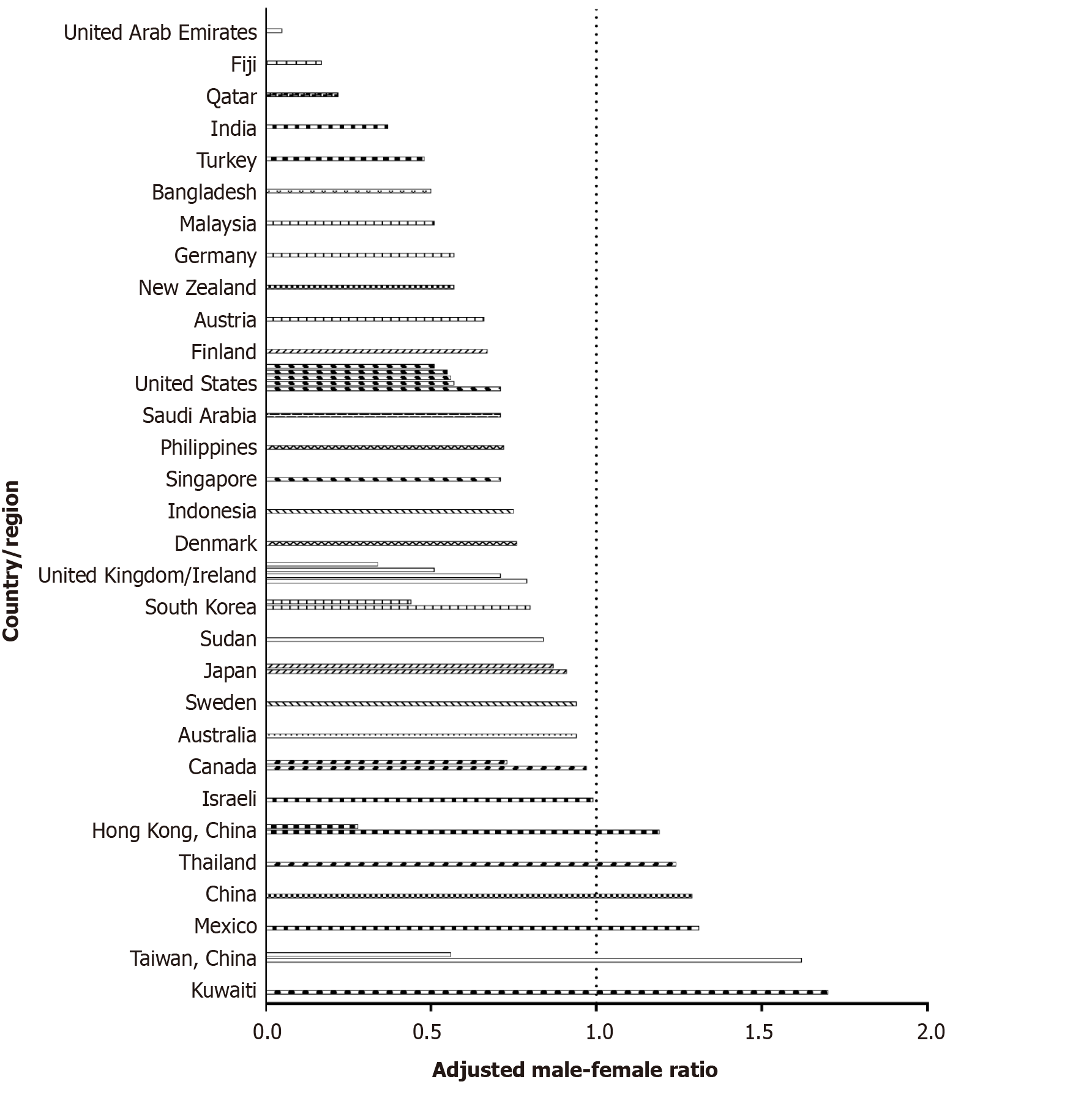
This study also revealed some unique characteristics of Chinese pediatric T2DM patients, including higher male susceptibility rates, relatively early ages of onset (especially in boys), and slightly low comorbidity of obesity. In most Western countries, there are more female patients with pediatric T2DM; the SEARCH study found that the male-female ratio of incidence rate ranged from 0.58 to 0.81[1]. The present study reported a male-female ratio of 1.37:1; other studies of the Chinese population also reported increased male susceptibility to pediatric T2DM[4,5]. In order to show the sex composition of pediatric T2DM patients in different regions, we review the relevant literature. With the exception of China, only Thailand, Mexico, and Kuwait have reported male-female ratios over 1.00 after adjusting the population male-female ratio according to the literature review (Figure 4 and Supplementary Table 3). One possible hypothesis for the increased male susceptibility is that the obesity incidence rate in Chinese boys is higher than that in girls[31]. However, other factors, including genetic polymorphisms, different lifestyles, and dietary habits, might account for the high rates of male susceptibility in Chinese children.
Aside from male susceptibility, the age of onset in Chinese boys was close to that in girls, contrary to the former belief that the age of onset in boys was 1 year later than that in girls[20]. The increase in insulin resistance caused by puberty was identified as an important cause of T2DM; females start puberty earlier than males, which explains why the age of onset in females was earlier than that in males. The similar ages of onset between male and female Chinese patients further confirmed that the increased levels of insulin resistance during puberty only had a limited effect on the pathogenesis of T2DM. Furthermore, only 58% of patients with T2DM were obese in this study, and the obesity rate in China is significantly lower than that in Europe[32]. It was noticed that even little weight gain could cause insulin resistance and increased risk of diabetes among the Chinese population[15]. Although some patients suffered from weight loss before diagnosis, Chinese patients were relatively thinner than European patients, which also indicated the different pathogenesis of T2DM among Chinese children.
The findings of this study may provide a basis for the prevention, screening, and control of pediatric T2DM in China. The ISPAD and the American Diabetes Association recommend screening at-risk obese youth for T2DM in a clinical setting after either age 10 or the onset of puberty[19]. However, based on the results of our study, it may be necessary to pay special attention to boys and non-obese children (even those younger than 10 years or before puberty) who have high-risk factors other than obesity. Additionally, patients with low levels of β-cell function in our study were at risk for T2DM even though their insulin resistance levels were within the normal range. Therefore, during screening, pediatricians should determine whether β-cell function is at the lower limits for the patient’s age and whether it is relatively deficient with regard to the patient’s insulin resistance level.
This study had several limitations. First, the sample size of pediatric T2DM patients was limited. Despite its increasing incidence rate, T2DM is still a relatively rare disease among Chinese children and adolescents, even much rarer than type 1 diabetes[5]. However, this multicenter cross-sectional study covered regions with different climates, lifestyles, and economic development levels in China, and each center was the main provider of medical services for local pediatric diabetes patients; therefore, the data are representative. Second, part of the T2DM cases in this study did not have molecular genetic testing to exclude monogenic diabetes. However, since monogenic diabetes only accounted for about 1% to 6% of pediatric diabetes patients[33], the number of patients in our population who might have monogenic diabetes and its impact on outcomes were limited. Third, we used HOMA to evaluate the β-cell function and insulin resistance level. Though not the gold standard, HOMA is the most widely used and the most feasible method, and has been validated among children and adolescents[34]. What’s more, we established the normal range of the HOMA index of different genders and ages through the control population. The islet function of T2DM patients was evaluated according to these standards, which was more reasonable than comparing the indicators directly without considering the influences of gender and puberty.
In conclusion, β-cell function impairment is strongly associated with pediatric T2DM among Chinese children and adolescents in addition to insulin resistance. This finding, along with the high level of male susceptibility to pediatric T2DM, indicates that special attention should be paid to boys in specific T2DM screening and prevention strategies for Chinese children.
β-cell dysfunction and insulin resistance are two crucial factors in the pathogenesis of type 2 diabetes mellitus (T2DM), but the effects may vary with ages, sexes, and ethnicities.
The study of the association β-cell function and insulin resistance with pediatric T2DM is still scarce.
To investigate the association of β-cell function and insulin resistance with pediatric T2DM among Chinese children and adolescents.
We applied a multicenter cross-sectional study in China between 2017 to 2019, including 161 children and adolescents with newly diagnosed T2DM, and 1935 healthy control subjects. Homeostasis models (HOMAs) were used to evaluate β-cell function and insulin resistance, and HOMA index were standardized for sex and age.
The decrease in homeostasis model assessment of β-cell function (HOMA2-%B) z-score and the increase in homeostasis model assessment of insulin resistance (HOMA2-IR) z-score were associated with a higher risk of T2DM, and the decrease in HOMA2-%B z-score always had higher odd ratios than the increase in HOMA2-IR z-score after adjusting for confounding factors.
β-cell dysfunction, compared with insulin resistance, is a stronger risk factor associated with pediatric T2DM among Chinese children, regardless of obesity status.
Future cohort studies with larger samples and mechanism researches would help to better understand the specific pathogenesis of T2DM among Chinese children.
We would like to thank the parents and children for participating in the study. We thank the doctors and nursing staff of these centers for their detailed assessment and dedicated care of these young patients.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L; SEARCH for Diabetes in Youth Study. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. 2017;376:1419-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1042] [Article Influence: 130.3] [Reference Citation Analysis (0)] |
| 2. | Fazeli Farsani S, van der Aa MP, van der Vorst MM, Knibbe CA, de Boer A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia. 2013;56:1471-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep. 2014;14:508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 4. | Fu JF, Liang L, Gong CX, Xiong F, Luo FH, Liu GL, Li P, Liu L, Xin Y, Yao H, Cui LW, Shi X, Yang Y, Chen LQ, Wei HY. Status and trends of diabetes in Chinese children: analysis of data from 14 medical centers. World J Pediatr. 2013;9:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Wu H, Zhong J, Yu M, Wang H, Gong W, Pan J, Fei F, Wang M, Yang L, Hu R. Incidence and time trends of type 2 diabetes mellitus in youth aged 5-19 years: a population-based registry in Zhejiang, China, 2007 to 2013. BMC Pediatr. 2017;17:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Constantino MI, Molyneaux L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, Twigg SM, Yue DK, Wong J. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863-3869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 7. | Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014;37:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 8. | Druet C, Tubiana-Rufi N, Chevenne D, Rigal O, Polak M, Levy-Marchal C. Characterization of insulin secretion and resistance in type 2 diabetes of adolescents. J Clin Endocrinol Metab. 2006;91:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Chernausek SD, Arslanian S, Caprio S, Copeland KC, El ghormli L, Kelsey MM, Koontz MB, Orsi CM, Wilfley D. Relationship Between Parental Diabetes and Presentation of Metabolic and Glycemic Function in Youth With Type 2 Diabetes: Baseline Findings From the TODAY Trial. Diabetes Care. 2016;39:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care. 2005;28:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Xu ZR, Zhang MY, Ni JW, Cheng RQ, Zheng ZQ, Xi L, Luo FH. Clinical characteristics and beta-cell function of Chinese children and adolescents with type 2 diabetes from 2009 to 2018. World J Pediatr. 2019;15:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Gungor N, Arslanian S. Progressive beta cell failure in type 2 diabetes mellitus of youth. J Pediatr. 2004;144:656-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Bacha F, Gungor N, Lee S, Arslanian SA. Progressive deterioration of β-cell function in obese youth with type 2 diabetes. Pediatr Diabetes. 2013;14:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Ahuja V, Kadowaki T, Evans RW, Kadota A, Okamura T, El Khoudary SR, Fujiyoshi A, Barinas-Mitchell EJ, Hisamatsu T, Vishnu A, Miura K, Maegawa H, El-Saed A, Kashiwagi A, Kuller LH, Ueshima H, Sekikawa A; ERA JUMP Study Group. Comparison of HOMA-IR, HOMA-β% and disposition index between US white men and Japanese men in Japan: the ERA JUMP study. Diabetologia. 2015;58:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Wang T, Lu J, Shi L, Chen G, Xu M, Xu Y, Su Q, Mu Y, Chen L, Hu R, Tang X, Yu X, Li M, Zhao Z, Chen Y, Yan L, Qin G, Wan Q, Dai M, Zhang D, Gao Z, Wang G, Shen F, Luo Z, Qin Y, Huo Y, Li Q, Ye Z, Zhang Y, Liu C, Wang Y, Wu S, Yang T, Deng H, Zhao J, Lai S, Bi Y, DeFronzo RA, Wang W, Ning G; China Cardiometabolic Disease and Cancer Cohort Study Group. Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol. 2020;8:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 16. | Mittendorfer B. Insulin resistance: sex matters. Curr Opin Clin Nutr Metab Care. 2005;8:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Moran A, Jacobs DR Jr, Steinberger J, Hong CP, Prineas R, Luepker R, Sinaiko AR. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 590] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 18. | Kelsey MM, Zeitler PS. Insulin Resistance of Puberty. Curr Diab Rep. 2016;16:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 19. | Mayer-Davis EJ, Kahkoska AR, Jefferies C, Dabelea D, Balde N, Gong CX, Aschner P, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19 Suppl 27:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 377] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 20. | Zeitler P, Arslanian S, Fu J, Pinhas-Hamiel O, Reinehr T, Tandon N, Urakami T, Wong J, Maahs DM. ISPAD Clinical Practice Consensus Guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19 Suppl 27:28-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Li H, Ji CY, Zong XN, Zhang YQ. [Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years]. Zhonghua Er Ke Za Zhi. 2009;47:487-492. [PubMed] |
| 22. | Group of China Obesity Task Force. [Body mass index reference norm for screening overweight and obesity in Chinese children and adolescents]. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:97-102. [PubMed] |
| 23. | Mi J, Wang TY, Meng LH, Zhu GJ, Han SM, Zhong Y, Liu GS, Wan YP, Xiong F, Shi JP, Yan WL, Zhou PM. Development of blood pressure reference standards for Chinese children and adolescents. Chin J Evid Based Pediatr. 2010;5:4-14. [DOI] [Full Text] |
| 24. | Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart; Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213-S256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1578] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 25. | Redondo MJ, Rodriguez LM, Escalante M, O'Brian Smith E, Balasubramanyam A, Haymond MW. Beta cell function and BMI in ethnically diverse children with newly diagnosed autoimmune type 1 diabetes. Pediatr Diabetes. 2012;13:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Kaufman FR. Type 2 diabetes in children and youth. Rev Endocr Metab Disord. 2003;4:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Carayol J, Hosking J, Pinkney J, Marquis J, Charpagne A, Metairon S, Jeffery A, Hager J, Martin FP. Genetic Susceptibility Determines β-Cell Function and Fasting Glycemia Trajectories Throughout Childhood: A 12-Year Cohort Study (EarlyBird 76). Diabetes Care. 2020;43:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 28. | Krentz NAJ, Gloyn AL. Insights into pancreatic islet cell dysfunction from type 2 diabetes mellitus genetics. Nat Rev Endocrinol. 2020;16:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 29. | TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013;36:1749-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 30. | Weir GC, Gaglia J, Bonner-Weir S. Inadequate β-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet Diabetes Endocrinol. 2020;8:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 31. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5072] [Cited by in RCA: 4754] [Article Influence: 594.3] [Reference Citation Analysis (2)] |
| 32. | Klingensmith GJ, Lanzinger S, Tamborlane WV, Hofer SE, Cheng P, de Beaufort C, Gal RL, Reinehr T, Kollman C, Holl RW. Adolescent type 2 diabetes: Comparing the Pediatric Diabetes Consortium and Germany/Austria/Luxemburg Pediatric Diabetes Prospective registries. Pediatr Diabetes. 2018;19:1156-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Hattersley AT, Greeley SAW, Polak M, Rubio-Cabezas O, Njølstad PR, Mlynarski W, Castano L, Carlsson A, Raile K, Chi DV, Ellard S, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2018: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2018;19 Suppl 27:47-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 201] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 34. | Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 339] [Article Influence: 16.1] [Reference Citation Analysis (0)] |