Published online Jul 15, 2021. doi: 10.4239/wjd.v12.i7.1102
Peer-review started: January 21, 2021
First decision: March 16, 2021
Revised: April 5, 2021
Accepted: June 4, 2021
Article in press: June 4, 2021
Published online: July 15, 2021
Processing time: 171 Days and 19 Hours
Uncarboxylated osteocalcin (GluOC) has been reported to improve glucose metabolism, prevent type 2 diabetes, and decrease the severity of obesity in mice with type 2 diabetes. GluOC can increase glucose uptake in a variety of cells. Glucose metabolism is the main source of energy for osteoblast proliferation and differentiation. We hypothesized that decarboxylated osteocalcin (dcOC), a kind of GluOC, can increase glucose uptake in MG63 cells (osteoblast-like osteosar
To investigate the effects of dcOC on glucose uptake in human osteoblast-like osteosarcoma cells and the possible signaling pathways involved.
MG63 cells (human osteoblast-like osteosarcoma cells) were treated with dcOC (0, 0.3, 3, 10, or 30 ng/mL) for 1 and 72 h, and glucose uptake was measured by flow cytometry. The effect of dcOC on cell proliferation was measured with a CCK-8 assay, and alkaline phosphatase (ALP) enzyme activity was measured. PI3K was inhibited with LY294002, and hypoxia-inducible factor 1 alpha (HIF-1α) was silenced with siRNA. Then, GPRC6A (G protein-coupled receptor family C group 6 subtype A), total Akt, phosphorylated Akt, HIF-1α, and glucose transporter 1 (GLUT1) levels were measured by Western blot to elucidate the possible pathways by which dcOC modulates glucose uptake.
The glucose uptake of MG63 cells was significantly increased compared with that of the paired control cells after short-term (1 h) treatment with dcOC at different concentrations (0.3, 3, and 10 ng/mL groups, P < 0.01; 30 ng/mL group, P < 0.05). Glucose uptake of MG63 cells was significantly increased compared with that of the paired control cells after long-term (72 h) treatment with dcOC at different concentrations (0.3, 3, and 10 ng/mL groups, P < 0.01; 30 ng/mL group, P < 0.05). DcOC triggered Akt phosphorylation in a dose-dependent manner, and the most effective stimulatory concentration of dcOC for short-term (1 h) was 3 ng/mL (P < 0.01). LY294002 abolished the dcOC-mediated (1 h) promotion of Akt phosphorylation and glucose uptake without affecting GLUT1 protein expression. Long-term dcOC stimulation triggered Akt phosphorylation and increased the protein levels of HIF-1α, GLUT1, and Runx2 in a dose-dependent manner. Inhibition of HIF-1α with siRNA abolished the dcOC-mediated glucose uptake and substantially decreased GLUT1 protein expression. DcOC interven
Short- and long-term dcOC treatment can increase glucose uptake and affect proliferation and ALP activity in MG63 cells. This effect may occur through the PI3K/Akt, HIF-1α, and GLUT1 signaling factors.
Core Tip: Uncarboxylated osteocalcin (GluOC) has been reported to improve glucose metabolism and prevent type 2 diabetes. GluOC can increase the glucose uptake in a variety of cells. In this study, MG63 cells were treated with different concentrations of decarboxylated osteocalcin (dcOC) for 1 h and 72 h to observe the changes in glucose uptake, proliferation, and alkaline phosphatase (ALP) activity, as well as possible signaling pathway. Short- or long-term intervention with dcOC in vitro can increase glucose uptake and promote the proliferation and ALP activity of MG63 cells. This effect may occur through the PI3K/Akt, hypoxia-inducible factor 1 alpha, and glucose transporter 1 signaling factors.
- Citation: Jin S, Chang XC, Wen J, Yang J, Ao N, Zhang KY, Suo LN, Du J. Decarboxylated osteocalcin, a possible drug for type 2 diabetes, triggers glucose uptake in MG63 cells. World J Diabetes 2021; 12(7): 1102-1115
- URL: https://www.wjgnet.com/1948-9358/full/v12/i7/1102.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i7.1102
Osteocalcin (OC), synthesized by mature osteoblasts, is one of the most abundant noncollagen proteins in bone[1]. In the circulation, the concentration of OC is a measure of bone formation[2]. In osteoblasts, after protein translation in the endoplasmic reticulum, OC is posttranslationally modified by the addition of carboxyl groups at glutamyl (Glu) residues at positions 17, 21, and 24 via γ-Glu carboxylase, thereby facilitating its binding to hydroxyapatite in the bone matrix[1,3]. However, OC containing one or more uncarboxylated Glu residues (most commonly Glu17) is referred to as undercarboxylated OC (ucOC). Carboxylated OC can also be decar
Previous studies indicated that GluOC can improve glucose metabolism, prevent type 2 diabetes, and decrease the severity of obesity in mice with type 2 diabetes[8,9]. GluOC has great potential to become a new drug for the treatment of type 2 diabetes in the future. GluOC was also shown to promote glucose uptake in adipocytes, C2C12 myotubes, and muscles[10-13]. However, both the direct effects of GluOC on glucose uptake in bone tissues and the underlying mechanisms remain unexplored.
A previous study showed that the energy required for osteoblast proliferation and differentiation is produced mainly via glucose metabolism[14], and glucose transporter 1 (GLUT1) is the major glucose transporter[15]. GLUT1-mediated glucose uptake in osteoblasts occurs independently of insulin[14]. Moreover, GLUT1 may participate in osteosarcoma biology[16,17]. Activation of the PI3K/Akt and hypoxia-inducible factor 1 alpha (HIF-1α) signaling pathways is closely related to glucose uptake mediated by GLUT1[18,19]. Idelevich et al[20] found that the glucose uptake rate of mouse chondrocyte precursor cells (ADTC5 cells) overexpressing OC was increased. The siRNA-mediated silencing of HIF-1α abolished the effect of OC in upregulating the protein and mRNA expression of GLUT1, suggesting that HIF-1α mediates the effect of OC on upregulating the expression of GLUT1 in ADTC5 cells[20].
Herein, we treated MG63 cells, a human osteoblast-like osteosarcoma cell line, with dcOC for 1 h and 72 h in vitro in the absence of insulin to confirm whether dcOC affects glucose uptake and to verify whether PI3K/Akt and HIF-1α are involved in this process.
MG63, HEK-293, and Jurkat cells were purchased from American Type Culture Collection. Synthetic human dcOC was purchased from Creative BioMart (United States). Antibodies specific for p-AKT (Ser473), AKT, HIF-1α, GLUT1, and Runx2 were purchased from Cell Signaling Technology.
Synthetic human dcOC was freshly resuspended and diluted in phosphate-buffered saline to the desired final concentrations before the experiments. MG63, HEK-293, and Jurkat cells were cultured in low-glucose Dulbecco’s modified Eagle’s medium (L-DMEM; 1000 mg/L glucose, HyClone, United States), high-glucose Dulbecco’s modified Eagle’s medium (H-DMEM; 4500 mg/L glucose, HyClone), and complete RPMI 1640 medium (HyClone), respectively. All media were supplemented with 10% fetal bovine serum (FBS, HyClone, United States) and 1% Pen-Strep, and cultures were maintained at 37 °C in a humidified atmosphere with 5% CO2. When the cells were 70% confluent, the medium was replaced with L-DMEM containing 4% FBS and different concentrations of dcOC (0, 0.3, 3, 10, and 30 ng/mL). When MG63 cells were treated with different concentrations of dcOC solution for 72 h, the culture medium was changed daily. After cells were treated for 1 h or 72 h, glucose uptake and Western blot analyses were performed to determine the optimal concentration of dcOC.
For the PI3K inhibition assay, cells were treated with the PI3K inhibitor LY294002 (10 µmol/L, Cayman Chemical Inc., Michigan, United States) or 0.1% dimethyl sulfoxide as the vehicle control for 20 min and then treated with dcOC for 1 h.
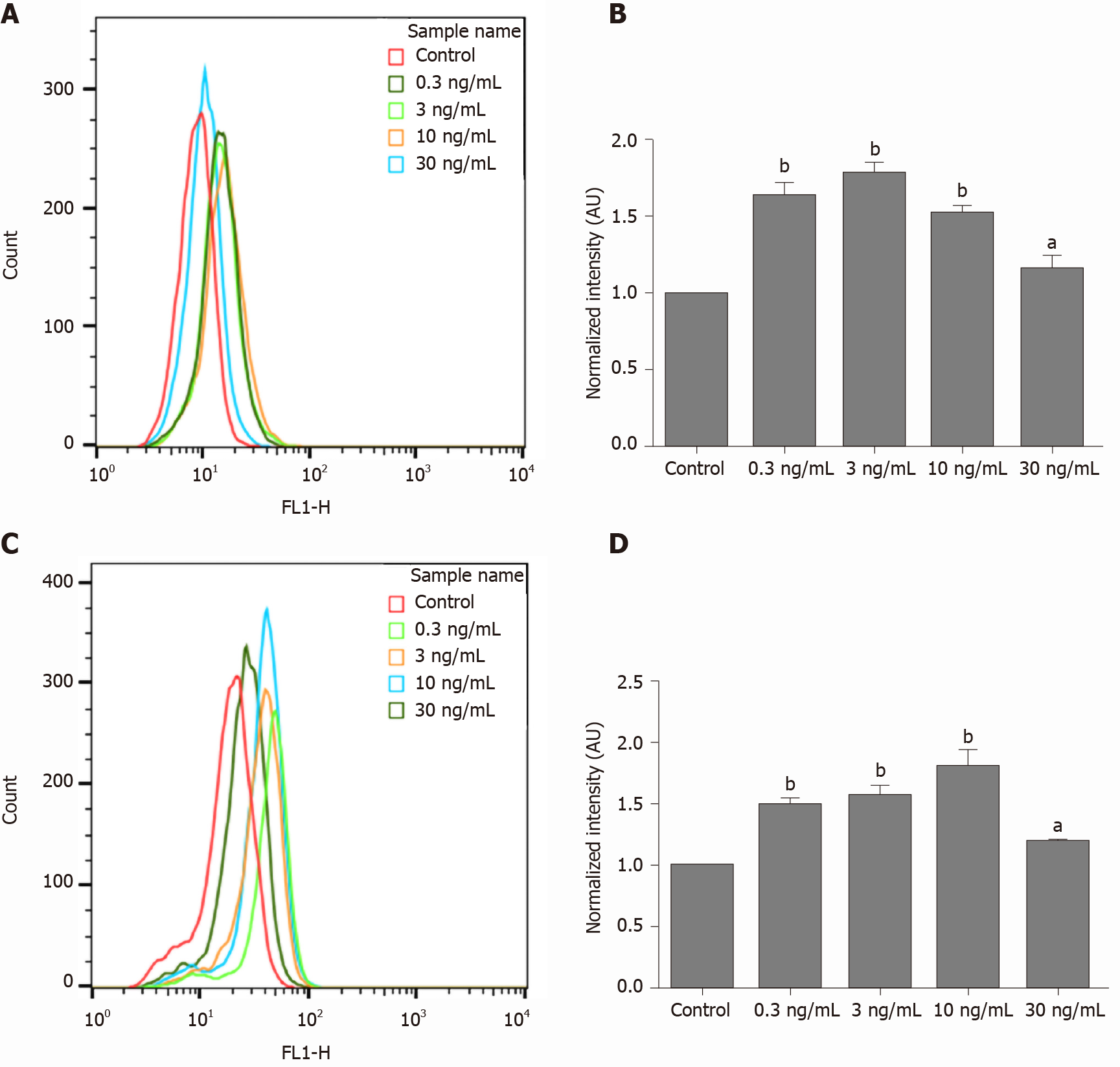
A glucose uptake assay was performed according to a previously described method with minor modifications[21]. MG63 cells were plated at a density of 1 × 106 cells per well (for the 1 h dcOC treatment) or 1 × 105 cells per well (for the 72 h dcOC treatment) in 6-well plates and used at subconfluence. After 24 h of incubation, the culture medium was replaced with 2.5 mL of culture medium containing various concentrations of dcOC (0, 0.3, 3, 10, or 30 ng/mL) and incubated for 1 h or 72 h. The medium was then removed, and the cells were washed twice with precooled Krebs Ringer buffer (KRB). Fresh culture medium containing 100 μmol/L 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-glucose (2-NBDG, Creative BioMart Inc., New York, NY, United States), a fluorescent glucose analog, was added to the cells and incubated at 37 °C for 30 min in a humidified atmosphere with 5% CO2. 2-NBDG uptake was stopped by removal of the incubation medium, and the cells were washed twice with precooled KRB. The cells were trypsinized and resuspended in 2.0 mL of precooled fresh growth medium. For each measurement, data from 10000 single-cell events were recorded by flow cytometry (FACSCalibur, BD, United States). Second, cells were plated at a density of 1 × 105 cells per well in 6-well plates and used at subconfluence after 24 h of incubation. The culture medium was then removed from each well and replaced with 2.5 mL of culture medium containing dcOC (0, 0.3, 3, 10, or 30 ng/mL) for 72 h of preincubation. The subsequent steps were the same as those listed above. The optimal concentration at dcOC affected glucose uptake was determined by these experiments. The experiment was performed in triplicate, and the experimental data were analyzed using FlowJo software. The geometric mean was used to determine the mean fluorescence intensity (MFI). The average fluorescence intensity of each group was compared with that of the control group to obtain the normalized fluorescence intensity (AU), which indicated the glucose uptake ability of MG63 cells.
MG63 cells were grown and transfected with either HIF-1α siRNA (Sangon Biotech, Shanghai, China) or negative control siRNA using the transfection reagent Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. After transfection, the medium was replaced with regular medium, and the cells were treated with dcOC for 72 h. Glucose uptake and Western blot assays were then performed.
MG63 cells were treated with the PI3K inhibitor LY294002 (10 µmol/L) for 30 min and then with dcOC for 1 h. Then, glucose uptake and Western blot assays were performed in triplicate. The glucose uptake data were analyzed using FlowJo software. The geometric mean was used to determine the MFI.
The levels of GPRC6A, p-AKT (Ser473), AKT, HIF-1α, GLUT1, and Runx2 in MG63 cells were determined. For analysis of GPRC6A expression, HEK-293 cells were used as the negative control[22], and Jurkat cells were used as the positive control. Cell samples were homogenized on ice in lysis buffer containing protease and phosphatase inhibitors. A bicinchoninic acid protein assay was used to determine the protein concentration according to the manufacturer’s instructions (Beyotime Institute of Biotechnology, China). All steps were performed as previously described[23]. Immunoblotting was performed using primary antibodies against GPRC6A (Absin Bioscience Inc., China) and horseradish peroxidase-conjugated anti-rabbit and anti-mouse secondary antibodies (Cell Signaling Technology). Immunoreactions were visualized with enhanced chemiluminescence reagents (GE Healthcare, UK, Ltd.). The band intensities were determined using ImageJ analysis software (NIH).
A Cell Counting Kit-8 (CCK-8, Tongren, China) assay was used to assess the effects of dcOC on MG63 cell proliferation. In brief, cells were plated in 96-well plates (4000 cells per well) in L-DMEM supplemented with 4% serum containing normal (5.5 mmol/L) levels of glucose and treated with various concentrations of dcOC (0, 0.3, 3, 10, and 30 ng/mL) for 24, 48, or 72 h. The absorbance values were read at 450 nm using an automated microplate reader (Power Waves XS, BioTek, United States).
The rate of p-nitrophenyl phosphate (Sigma) hydrolysis was analyzed to measure alkaline phosphatase (ALP) activity. Cells were plated in 24-well plates at a density of 1 × 105 cells per well and incubated overnight. Then, the cells were incubated with solutions containing various concentrations of dcOC for 72 h. The medium was removed, and 500 μL of 0.1% Triton X-100 (Sigma) was added to each well. Cells were frozen at -70 °C, thawed at 37 °C, and centrifuged at 14000 g and 4 °C for 10 min. The supernatant was collected for ALP activity measurement. The absorbance values at 405 nm were read on a microplate reader (Power Waves XS, BioTek, United States). The Bradford method was used to determine the protein concentration.
Every experiment was performed at least three times. Data are presented as the mean ± SD. The results were analyzed by one-way analysis of variance (ANOVA) followed by the LSD method for multiple comparisons using the statistical software SPSS 22.0. A P value of < 0.05 was considered statistically significant.
Previous studies have indicated that GPRC6A is expressed in bones and osteoblasts[24] of mice. We examined the expression of GPRC6A in MG63 cells by Western blot. Because previous experiments confirmed that HEK-293 cells lack GPRC6A expression[5], they were selected as the negative control, while Jurkat cells served as the positive control. Western blot analysis confirmed that MG63 cells expressed the GPRC6A receptor (Figure 1), thereby indicating that the roles of dcOC in MG63 cells could be examined.
The normalized fluorescence intensity (AU) of MG63 cells was significantly increased compared with that of the paired control cells after short-term (1 h) treatment with dcOC at different concentrations (0.3, 3, and 10 ng/mL groups, P < 0.01; 30 ng/mL group, P < 0.05; Figure 2A and B). In addition, 3 ng/mL was determined to be the optimal concentration for short-term (1 h) stimulation. Treatment with 3 ng/mL dcOC (1 h) increased glucose intake by 79% compared with that in the control group (P < 0.01; Figure 2B). In addition, the normalized fluorescence intensity (AU) of MG63 cells was significantly increased compared with that of paired control cells after long-term (72 h) treatment with dcOC at different concentrations (0.3, 3, and 10 ng/mL groups, P < 0.01; 30 ng/mL group, P < 0.05; Figure 2C and D). Additionally, 10 ng/mL was determined to be the optimal concentration for long-term (72 h) stimulation. Treatment with 10 ng/mL dcOC (72 h) increased glucose intake by 81% compared with that in the control group (P < 0.01; Figure 2D).
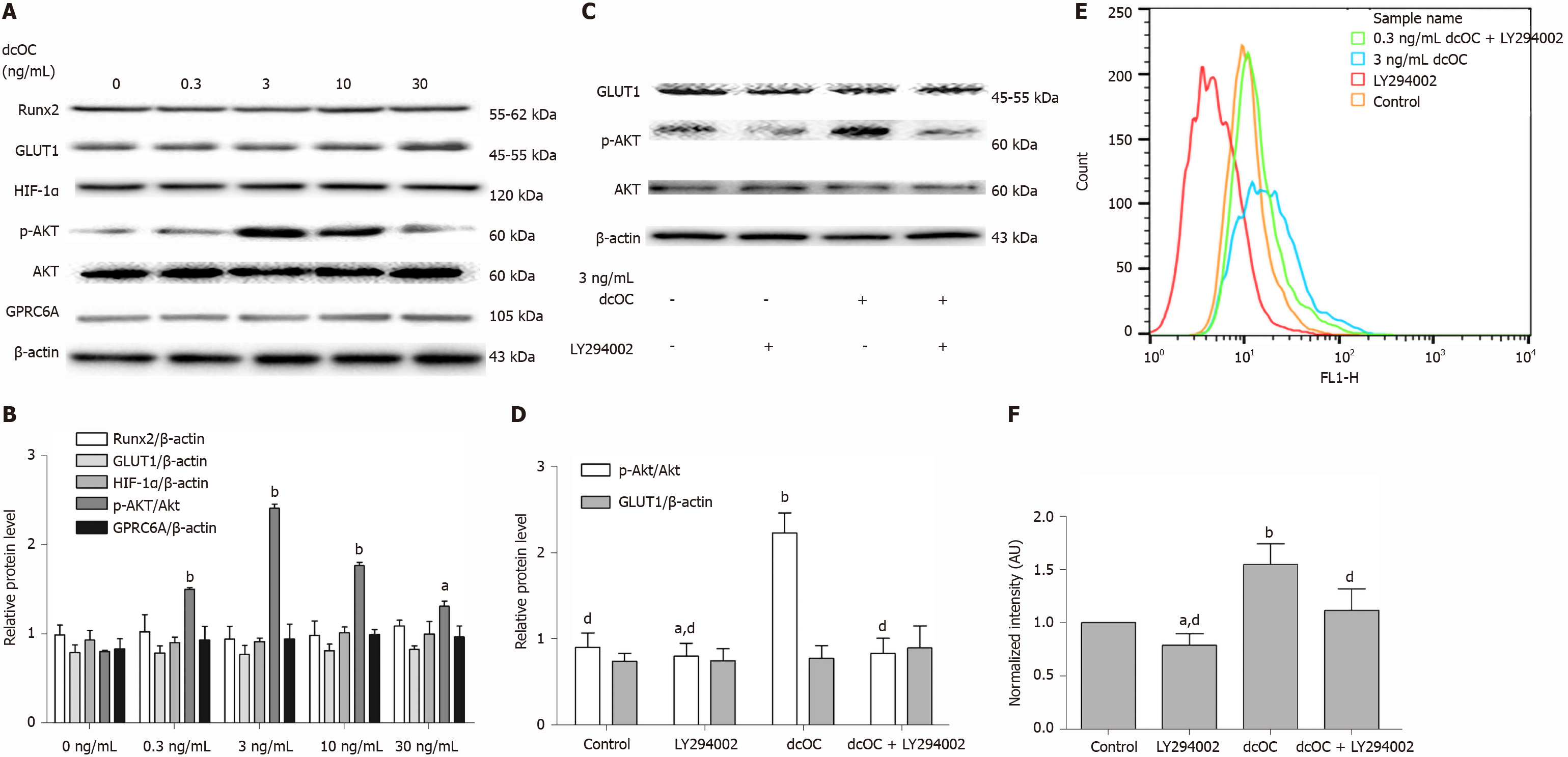
As shown in Figure 3A and B, dcOC triggered Akt phosphorylation in a dose-dependent manner, and the most effective stimulatory concentration was 3 ng/mL in the short term (1 h) (P < 0.01). The protein expression of p-Akt (Ser473) in the 3 ng/mL group was increased by 2.99-fold compared to that in the 0 ng/mL group, as assessed relative to the level of Akt, after 1 h of dcOC stimulation (P < 0.01; Figure 3B). However, no significant changes were observed in the expression levels of GPRC6A, HIF-1α, GLUT1, or Runx2 as assessed relative to the level of β-actin (Figure 3B). Moreover, inhibition of PI3K with LY294002 abolished the dcOC-mediated promotion of Akt phosphorylation (Figure 3C and D) and 2-NBDG uptake (Figure 3E and F) without affecting GLUT1 protein expression (Figure 3C and D), indicating that PI3K/Akt signaling is involved in the enhancement of dcOC-induced glucose uptake for a short period (1 h).
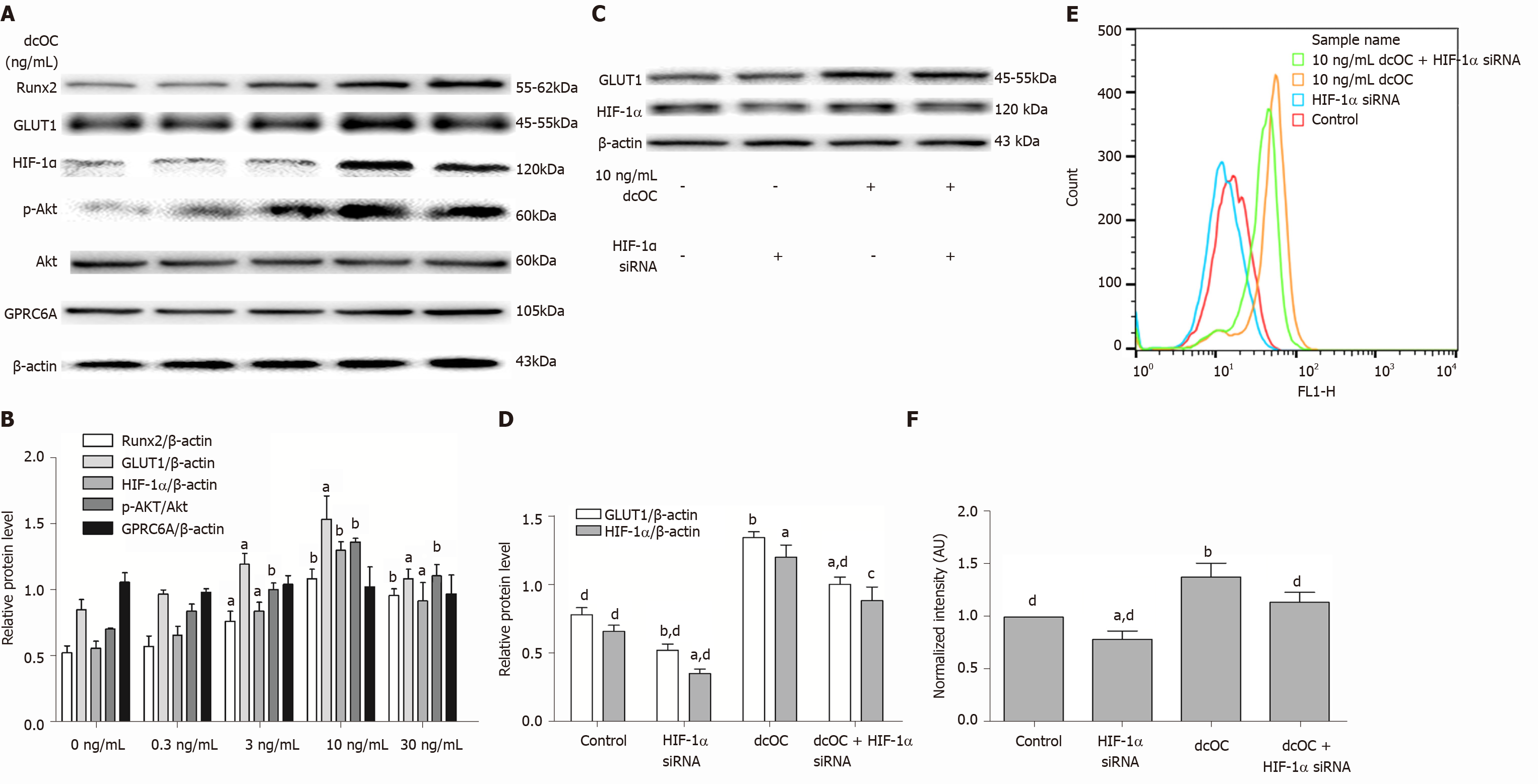
Long-term dcOC stimulation not only triggered Akt phosphorylation but also increased the protein levels of HIF-1α, GLUT1, and Runx2 in a dose-dependent manner (Figure 4B), whereas no increase in the expression level of GPRC6A was observed (Figure 4A and B). The most effective stimulatory concentration of dcOC was 10 ng/mL. The Western blot results indicated that the protein expression of p-Akt in the 10 ng/mL group was increased by 1.93-fold compared to that in the 0 ng/mL group, as assessed relative to the level of Akt, after 72 h of dcOC stimulation (P < 0.01, Figure 4B). The protein expression of HIF-1α, GLUT1, and Runx2 in the 10 ng/mL group was increased by 2.32-fold (P < 0.01, Figure 4B), 1.40-fold (P < 0.05, Figure 4B), and 2.06-fold (P < 0.01, Figure 4B), respectively, compared to that in the 0 ng/mL group, as assessed relative to the level of β-actin. Then, HIF-1α siRNA was used to decrease the HIF-1α protein levels in MG63 cells (P < 0.05, vs the control group, Figure 4D). Inhibition of HIF-1α with siRNA abolished dcOC-mediated 2-NBDG uptake (Figure 4E and F) and substantially decreased the GLUT1 protein expression (Figure 4C and D), indicating that HIF-1α signaling is involved in the promotional effect of dcOC on glucose uptake via GLUT1 modulation.
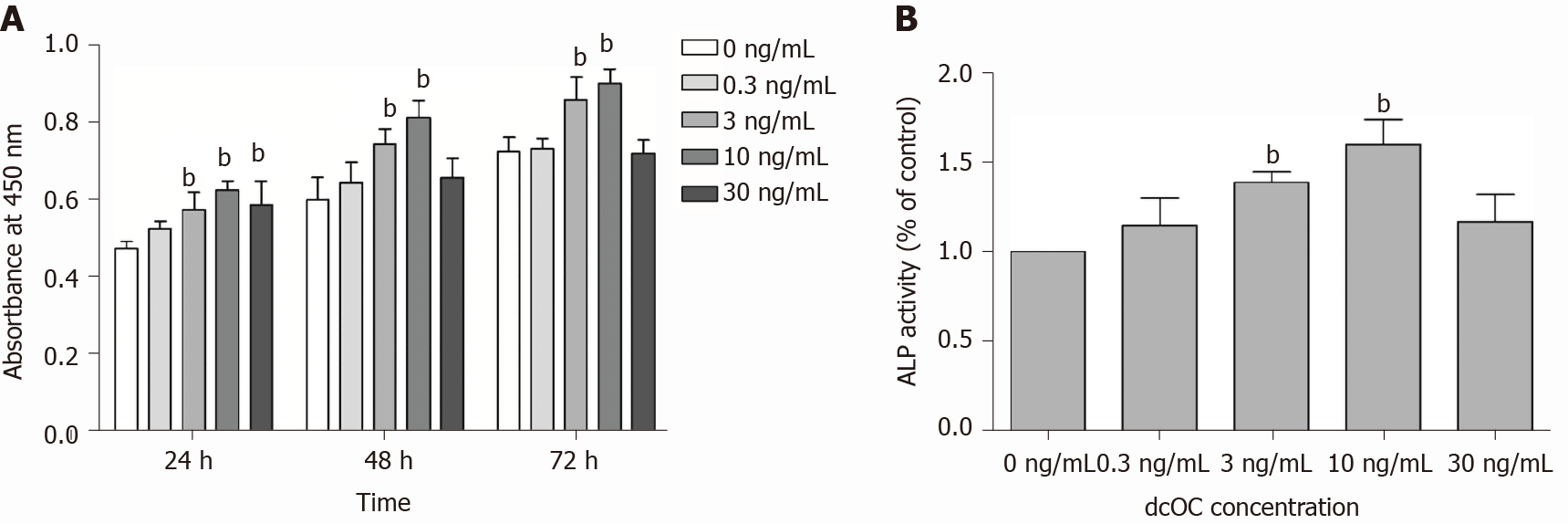
According to the cell proliferation assay results, the average cell counts for each day were plotted as a histogram. In the presence of dcOC, the number of cells was increased at 24, 48, and 72 h (Figure 5A). These results demonstrate that dcOC enhances MG63 cell proliferation in a time- and dose-dependent manner. DcOC had the most marked effect on proliferation at a concentration of 10 ng/mL (P <0.01, Figure 5A). The ALP activity assay results showed that treatment with either 3 ng/mL or 10 ng/mL dcOC affected ALP activity in MG63 cells after 72 h (P < 0.01, Figure 5B).
An increasing number of studies have shown that GluOC is closely related to energy metabolism[25,26]. In vitro experiments showed that different doses of GluOC could increase glucose uptake in various cells with or without insulin[11,12]. Glucose uptake is mediated by transmembrane GLUT proteins in cells[27]. Among these transporters, GLUT1 is closely related to the energy metabolism of tumor cells. The increase in glucose uptake by tumor cells is closely related to their proliferation[28]. Previous studies have shown that the expression of GLUT1 affects the prognosis of patients with osteosarcoma[29,30]. In addition, Fan et al[31] reported that interfering with GLUT1 expression may inhibit the proliferation of MG63 cells in vitro. Our study explored the relationship between dcOC, a type of GluOC, and glucose uptake in osteoblastic sarcoma cells in vitro and the possible association of this effect with GLUT1. Although MG63 cells synthesize GluOC, the level of GluOC synthesized by MG63 cells without retinoic acid intervention and lα,25-dihydroxycholecalciferol [lα,25(OH)2D], as shown in previous studies[32], was negligible compared with the level of exogenous dcOC in our study. In addition, the concentration of dcOC used in our study was confirmed to increase glucose uptake in the previous study[11].
As the receptor for GluOC, GPRC6A plays an important role in the regulation of glucose metabolism by GluOC in histiocytes[6,10]. The expression of GPRC6A in human osteoblasts was confirmed by scholars as early as 1998[33]. In the present study, we confirmed the expression of GPRC6A in MG63 cells (Figure 1), which provided a basis to study the effect of dcOC on the metabolic response. In our study, both short- and long-term dcOC intervention altered the glucose uptake by MG63 cells but did not change the protein level of GPRC6A. We speculate that dcOC may affect the signaling pathway downstream of GPRC6A by altering the protein conformation of GPRC6A, thereby affecting the glucose uptake of MG63 cells. However, further investigation is needed to confirm this hypothesis.
Hill et al[11] showed that short-term intervention with carboxylated osteocalcin in adipocytes could increase the glucose uptake and transport in a cell-based system (in an insulin-independent manner). GLUT1 is the main glucose transporter in osteoblasts[15]. Activation of the PI3K/Akt signaling pathway has been confirmed to be necessary for GLUT1 translocation, cell glucose uptake, and maintenance of cell surface GLUT1 Levels[18]. Application of a PI3K inhibitor can effectively inhibit the expression of GLUT1 on the cell membrane, thereby affecting the uptake of glucose in cells[34]. A previous study showed that ucOC could increase the phosphorylation of Akt in adipocytes in an insulin-independent manner[12]. In our study, short-term incubation (1 h) of MG63 cells with dcOC promoted glucose uptake under physiological glucose conditions in an insulin-independent manner (Figure 2A and B). In addition, the phosphorylation of AKT was increased significantly in MG63 cells incubated with dcOC for a short time, although the GLUT1 expression was not affected (Figure 3A and B), suggesting that short-term dcOC treatment increases the glucose uptake rate, probably by regulating GLUT1 activity and transport via PI3K/Akt rather than by increasing the protein expression of GLUT1. Then, we used the PI3K inhibitor LY294002 to reduce the phosphorylation of Akt and found that the effect of dcOC on increasing glucose uptake was abolished (Figure 3C-F). This result confirmed that the PI3K/Akt signaling pathway participates in dcOC, promoting glucose uptake in MG63 cells. In addition to GLUT1, GLUT3 and GLUT4 have also been reported to be expressed in osteoblasts[15]. The increase in Akt phosphorylation is related to increases in GLUT3 and GLUT4 translocation and protein expression levels[35,36]. DcOC may increase glucose uptake by increasing the GLUT3 or GLUT4 translocation or protein expression in MG63 cells after short-term intervention, but further research is needed to confirm this hypothesis. Moreover, the expression of GPRC6A was not altered by short-term treatment with dcOC, suggesting that short-term dcOC intervention induces glucose uptake in MG63 cells by altering the activity or configuration of the receptor GPRC6A rather than its expression.
Next, we validated the effect of glucose uptake on MG63 cells with long-term dcOC intervention (72 h). Similar to the short-term stimulation, the long-term stimulation of MG63 cells with dcOC also promoted glucose uptake under physiological glucose conditions in an insulin-independent manner (Figure 2C and D), accompanied by a significantly increased p-AKT level (Figure 4A and B). However, long-term stimulation significantly increased the protein expression levels of HIF-1α and GLUT1 in MG63 cells treated with different concentrations of dcOC, suggesting that dcOC can increase the glucose uptake rate, probably by regulating GLUT1 protein expression after incubation for an extended duration (Figure 4A and B). Then, we silenced HIF-1α via siRNA and found that the effect of dcOC on increasing glucose uptake was abolished, with decreased expression of HIF-1α and GLUT1 (Figure 4C-F). HIF-1 is a transcription factor that mediates the adaptive response, which enhances the energy metabolism of cancer cells. HIF-1 is composed of two subunits, HIF-1α and HIF-1β, among which HIF-1α determines the activity of HIF-1. HIF-1α is closely related to GLUT1 in tumor cells. For example, HIF-1α can upregulate the protein expression of GLUT1[37], and silencing or inhibiting HIF-1α in tumor cells can decrease the expression of GLUT1[38,39]. Previous studies have shown that HIF-1α plays an important role in the growth of osteosarcoma[40,41]. The results of our study suggested that dcOC promotes the glucose uptake in MG63 cells after incubation for an extended duration by upregulating the protein expression of GLUT1. Using HIF-1α siRNA, we demonstrated that GLUT1 expression induced by dcOC is dependent on HIF-1α in MG63 cells. Therefore, these results support a new perspective on the modulation of GLUT1 expression by HIF-1α in response to dcOC in MG63 cells.
Runx2 is an essential transcription factor for osteoblast differentiation, matrix production, and mineralization during bone formation[42]. It controls the expression of bone formation-related genes such as OCN, ALP, BMP2, and BMP4[43]. In this study, short-term intervention with dcOC in MG63 cells did not change the protein expression of Runx2 (Figure 3A and B), while long-term dcOC intervention signifi
In our study, dcOC promoted the proliferation of MG63 cells (Figure 5A). Bone formation and osteoblast proliferation are energy-expensive processes, and the energy for osteoblast proliferation is generated mainly via glucose metabolism[14]. Because GLUT1 is the key glucose transporter regulating glucose uptake[46], we speculated that the ability of dcOC to increase MG63 cell proliferation is closely related to its ability to improve glucose metabolism.
ALP is a major enzyme expressed during the early maturation of osteoblasts. UcOC has been reported to increase ALP activity in bone marrow mesenchymal stem cells (BMSCs)[47]. In our study, the ALP activity in MG63 cells was increased after dcOC treatment for 72 h (Figure 5B), consistent with the results of a previous study. Moreover, previous studies have shown that increased serum ALP concentrations are indicative of a worse prognosis in osteosarcoma, correlating with shorter survival times and disease-free intervals[48-50]. The results of our study indicated that dcOC might affect the prognosis of osteosarcoma patients by increasing the ALP activity in osteoblast-like osteosarcoma cells. However, the specific mechanism by which dcOC increases ALP activity is not clear and needs to be further investigated.
GluOC has been suggested to be closely related to energy metabolism and can increase glucose uptake in various cells[10-13]. In animal experiments, GluOC has been proven to reduce blood glucose levels and visceral fat in mice with type 2 diabetes[8,9]. GluOC is considered a potential agent for the treatment of type 2 diabetes and insulin resistance. The present study clearly showed that dcOC, a type of GluOC, promoted glucose uptake in MG63 cells in vitro. dcOC may affect the invasion and migration of MG63 cells and the prognosis of osteosarcoma patients by affecting the ALP activity and Runx2 expression in osteoblastic sarcoma cells. Thus, while considering dcOC as a potential treatment for type 2 diabetes, it is also necessary to be aware of its possible adverse effects on osteoblastic osteosarcoma.
DcOC can promote glucose uptake in MG63 cells in vitro. DcOC may affect the invasion and migration of MG63 cells and the prognosis of osteosarcoma patients by affecting ALP activity and Runx2 expression.
Uncarboxylated osteocalcin (GluOC) has been reported to improve glucose metabolism, prevent type 2 diabetes, and decrease the severity of obesity in mice with type 2 diabetes. GluOC can increase glucose uptake in a variety of cells. GluOC has great potential to become a new drug for the treatment of type 2 diabetes in the future.
Glucose metabolism is the main source of energy for osteoblast proliferation and differentiation. However, both the direct effects of GluOC on glucose uptake in bone tissues and the underlying mechanisms remain unexplored.
To investigate the effects of decarboxylated osteocalcin (dcOC), a kind of GluOC, on glucose uptake in human osteoblast-like osteosarcoma cells and the possible signaling pathways involved.
MG63 cells (human osteoblast-like osteosarcoma cells) were treated with dcOC (0, 0.3, 3, 10, or 30 ng/mL) for 1 and 72 h, and glucose uptake was measured by flow cytometry. The effect of dcOC on cell proliferation was measured with a CCK-8 assay, and alkaline phosphatase (ALP) enzyme activity was measured. PI3K was inhibited with LY294002, and hypoxia-inducible factor 1 alpha (HIF-1α) was silenced with siRNA. Then, the G protein-coupled receptor family C group 6 subtype A, total Akt, phosphorylated Akt, HIF-1α, and glucose transporter 1 (GLUT1) levels were measured by Western blot to elucidate the possible pathways by which dcOC modulates glucose uptake.
The glucose uptake of MG63 cells was significantly increased compared with that of the paired control cells after short-term (1 h) treatment and long-term (72 h) treatment with dcOC at different concentrations. LY294002 abolished the dcOC-mediated (1 h) promotion of Akt phosphorylation and glucose uptake without affecting GLUT1 protein expression. Long-term dcOC stimulation triggered Akt phosphorylation and increased the protein levels of HIF-1α, GLUT1, and Runx2 in a dose-dependent manner. Inhibition of HIF-1α abolished the dcOC-mediated glucose uptake and substantially decreased GLUT1 protein expression. DcOC intervention promoted cell proliferation in a time- and dose-dependent manner. Treatment with dcOC affected the ALP activity in MG63 cells.
DcOC can promote glucose uptake in MG63 cells in vitro. DcOC may affect the invasion and migration of MG63 cells and the prognosis of osteosarcoma patients by affecting ALP activity and Runx2 expression.
DcOC can promote glucose uptake in human osteoblast-like osteosarcoma cells. It is necessary to be aware of its possible adverse effects on osteoblastic osteosarcoma while considering dcOC as a potential treatment for type 2 diabetes.
The authors would like to acknowledge Zhao YY and Bai BW for skillful technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Evans RW S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Neve A, Corrado A, Cantatore FP. Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol. 2013;228:1149-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 2. | Booth SL, Centi A, Smith SR, Gundberg C. The role of osteocalcin in human glucose metabolism: marker or mediator? Nat Rev Endocrinol. 2013;9:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 849] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 4. | Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 910] [Cited by in RCA: 824] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 5. | Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26:1680-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Wei J, Hanna T, Suda N, Karsenty G, Ducy P. Osteocalcin promotes β-cell proliferation during development and adulthood through Gprc6a. Diabetes. 2014;63:1021-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Liu J, Yang J. Uncarboxylated osteocalcin inhibits high glucose-induced ROS production and stimulates osteoblastic differentiation by preventing the activation of PI3K/Akt in MC3T3-E1 cells. Int J Mol Med. 2016;37:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 9. | Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266-5270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 678] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 10. | Tsuka S, Aonuma F, Higashi S, Ohsumi T, Nagano K, Mizokami A, Kawakubo-Yasukochi T, Masaki C, Hosokawa R, Hirata M, Takeuchi H. Promotion of insulin-induced glucose uptake in C2C12 myotubes by osteocalcin. Biochem Biophys Res Commun. 2015;459:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Hill HS, Grams J, Walton RG, Liu J, Moellering DR, Garvey WT. Carboxylated and uncarboxylated forms of osteocalcin directly modulate the glucose transport system and inflammation in adipocytes. Horm Metab Res. 2014;46:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Guedes JAC, Esteves JV, Morais MR, Zorn TM, Furuya DT. Osteocalcin improves insulin resistance and inflammation in obese mice: Participation of white adipose tissue and bone. Bone. 2018;115:68-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Lin X, Parker L, Mclennan E, Zhang X, Hayes A, McConell G, Brennan-Speranza TC, Levinger I. Uncarboxylated Osteocalcin Enhances Glucose Uptake Ex Vivo in Insulin-Stimulated Mouse Oxidative But Not Glycolytic Muscle. Calcif Tissue Int. 2018;103:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, Takarada T, Lezaki T, Pessin JE, Hinoi E, Karsenty G. Glucose Uptake and Runx2 Synergize to Orchestrate Osteoblast Differentiation and Bone Formation. Cell. 2015;161:1576-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 15. | Zoidis E, Ghirlanda-Keller C, Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem. 2011;348:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Chen R, Lin J, Yan W, Chen D. miR-522-3p Promotes Osteosarcoma Cell Growth By Regulating Glucose Uptake And GLUT1 Expression. Onco Targets Ther. 2019;12:9053-9058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Lin S, Zhu B, Huang G, Zeng Q, Wang C. Microvesicles derived from human bone marrow mesenchymal stem cells promote U2OS cell growth under hypoxia: the role of PI3K/AKT and HIF-1α. Hum Cell. 2019;32:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 460] [Cited by in RCA: 456] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Heydarzadeh S, Moshtaghie AA, Daneshpoor M, Hedayati M. Regulators of glucose uptake in thyroid cancer cell lines. Cell Commun Signal. 2020;18:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Idelevich A, Rais Y, Monsonego-Ornan E. Bone Gla protein increases HIF-1alpha-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler Thromb Vasc Biol. 2011;31:e55-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Wang MS, Luo Z, Nitin N. Rapid assessment of drug response in cancer cells using microwell array and molecular imaging. Anal Bioanal Chem. 2014;406:4195-4206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Pi M, Parrill AL, Quarles LD. GPRC6A mediates the non-genomic effects of steroids. J Biol Chem. 2010;285:39953-39964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Guo Q, Li H, Xu L, Wu S, Sun H, Zhou B. Undercarboxylated osteocalcin reverts insulin resistance induced by endoplasmic reticulum stress in human umbilical vein endothelial cells. Sci Rep. 2017;7:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201-40209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 25. | Lacombe J, Karsenty G, Ferron M. In vivo analysis of the contribution of bone resorption to the control of glucose metabolism in mice. Mol Metab. 2013;2:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Yasutake Y, Mizokami A, Kawakubo-Yasukochi T, Chishaki S, Takahashi I, Takeuchi H, Hirata M. Long-term oral administration of osteocalcin induces insulin resistance in male mice fed a high-fat, high-sucrose diet. Am J Physiol Endocrinol Metab. 2016;310:E662-E675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235:1492-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 584] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974;355:77-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 61] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Endo M, Tateishi U, Seki K, Yamaguchi U, Nakatani F, Kawai A, Chuman H, Beppu Y. Prognostic implications of glucose transporter protein-1 (glut-1) overexpression in bone and soft-tissue sarcomas. Jpn J Clin Oncol. 2007;37:955-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004;64:2627-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 565] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 31. | Fan J, Zhou JQ, Yu GR, Lu DD. Glucose transporter protein 1-targeted RNA interference inhibits growth and invasion of the osteosarcoma cell line MG63 in vitro. Cancer Biother Radiopharm. 2010;25:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Szulc P, Delmas PD. Influence of vitamin D and retinoids on the gammacarboxylation of osteocalcin in human osteosarcoma MG63 cells. Bone. 1996;19:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Divieti P, Lanske B, Kronenberg HM, Bringhurst FR. Conditionally immortalized murine osteoblasts lacking the type 1 PTH/PTHrP receptor. J Bone Miner Res. 1998;13:1835-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Makinoshima H, Takita M, Saruwatari K, Umemura S, Obata Y, Ishii G, Matsumoto S, Sugiyama E, Ochiai A, Abe R, Goto K, Esumi H, Tsuchihara K. Signaling through the Phosphatidylinositol 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Axis Is Responsible for Aerobic Glycolysis mediated by Glucose Transporter in Epidermal Growth Factor Receptor (EGFR)-mutated Lung Adenocarcinoma. J Biol Chem. 2015;290:17495-17504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 35. | Zaulkffali AS, Md Razip NN, Syed Alwi SS, Abd Jalil A, Abd Mutalib MS, Gopalsamy B, Chang SK, Zainal Z, Ibrahim NN, Zakaria ZA, Khaza'ai H. Vitamins D and E Stimulate the PI3K-AKT Signalling Pathway in Insulin-Resistant SK-N-SH Neuronal Cells. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Griffith CM, Macklin LN, Cai Y, Sharp AA, Yan XX, Reagan LP, Strader AD, Rose GM, Patrylo PR. Impaired Glucose Tolerance and Reduced Plasma Insulin Precede Decreased AKT Phosphorylation and GLUT3 Translocation in the Hippocampus of Old 3xTg-AD Mice. J Alzheimers Dis. 2019;68:809-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Song K, Li M, Xu XJ, Xuan L, Huang GN, Song XL, Liu QF. HIF-1α and GLUT1 gene expression is associated with chemoresistance of acute myeloid leukemia. Asian Pac J Cancer Prev. 2014;15:1823-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Askenazi DJ, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, Mehta RL, Ambalavanan N. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res. 2011;70:302-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN, Reginato MJ. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 2014;54:820-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 40. | Guo M, Cai C, Zhao G, Qiu X, Zhao H, Ma Q, Tian L, Li X, Hu Y, Liao B, Ma B, Fan Q. Hypoxia promotes migration and induces CXCR4 expression via HIF-1α activation in human osteosarcoma. PLoS One. 2014;9:e90518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Zhang Y, Cheng H, Li W, Wu H, Yang Y. Highly-expressed P2X7 receptor promotes growth and metastasis of human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3β/β-catenin and mTOR/HIF1α/VEGF signaling. Int J Cancer. 2019;145:1068-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 42. | Liu TM, Lee EH. Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Eng Part B Rev. 2013;19:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 43. | Molagoda IMN, Karunarathne WAHM, Choi YH, Park EK, Jeon YJ, Lee BJ, Kang CH, Kim GY. Fermented Oyster Extract Promotes Osteoblast Differentiation by Activating the Wnt/β-Catenin Signaling Pathway, Leading to Bone Formation. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Sadikovic B, Thorner P, Chilton-Macneill S, Martin JW, Cervigne NK, Squire J, Zielenska M. Expression analysis of genes associated with human osteosarcoma tumors shows correlation of RUNX2 overexpression with poor response to chemotherapy. BMC Cancer. 2010;10:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | Xie Y, Sun W, Deng Z, Zhu X, Hu C, Cai L. MiR-302b Suppresses Osteosarcoma Cell Migration and Invasion by Targeting Runx2. Sci Rep. 2017;7:13388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Li W, Deng Y, Feng B, Mak KK. Mst1/2 Kinases Modulate Glucose Uptake for Osteoblast Differentiation and Bone Formation. J Bone Miner Res. 2018;33:1183-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Liu Z, Yang J. Uncarboxylated osteocalcin promotes osteogenic differentiation of mouse bone marrow-derived mesenchymal stem cells by activating the Erk-Smad/β-catenin signalling pathways. Cell Biochem Funct. 2020;38:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Garzotto CK, Berg J, Hoffmann WE, Rand WM. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. J Vet Intern Med. 2000;14:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 441] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 50. | Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1405] [Article Influence: 61.1] [Reference Citation Analysis (0)] |