Published online Jul 15, 2021. doi: 10.4239/wjd.v12.i7.1057
Peer-review started: March 16, 2021
First decision: May 3, 2021
Revised: May 6, 2021
Accepted: June 4, 2021
Article in press: June 4, 2021
Published online: July 15, 2021
Processing time: 118 Days and 2.5 Hours
The presence of excess glucose in blood is regarded as a sweet hurt for patients with diabetes. Human serum albumin (HSA) is the most abundant protein in human plasma, which undergoes severe non-enzymatic glycation with glucose in patients with diabetes; this modifies the structure and function of HSA. Furthermore, the advanced glycation end products produced by glycated HSA can cause pathological damage to the human body through various signaling pathways, eventually leading to complications of diabetes. Many potential glycation sites on HSA have different degrees of sensitivity to glucose concentration. This review provides a comprehensive assessment of the in vivo glycation sites of HSA; it also discusses the effects of glycation on the structure and function of HSA. Moreover, it addresses the relationship between HSA glycation and diabetes complications. Finally, it focuses on the value of non-enzymatic glycation of HSA in diabetes-related clinical applications.
Core Tip: In the case of hyperglycemia state, the glycation level of albumin in plasma is significantly increased, which alters the structure and function of albumin. Herein we review the different glycation sites and functional changes of glycated albumin, and discuss the relationship between albumin glycation and diabetes complications. The potential application value of glycated albumin in clinical is also discussed.
- Citation: Qiu HY, Hou NN, Shi JF, Liu YP, Kan CX, Han F, Sun XD. Comprehensive overview of human serum albumin glycation in diabetes mellitus. World J Diabetes 2021; 12(7): 1057-1069
- URL: https://www.wjgnet.com/1948-9358/full/v12/i7/1057.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i7.1057
Diabetes is a metabolic disease caused by an absolute or relative deficiency of insulin in the human body related to various pathogenic etiologies; it leads to metabolic disorders involving sugars, lipids, and proteins, with severe hyperglycemia as the main clinical manifestation[1,2]. Abnormally high glucose concentrations in patients with diabetes can cause proteins in the body to undergo non-enzymatic glycation (i.e., without the involvement of glycosyltransferase), which is the initiating factor of diabetes-related complications[3,4]. Human serum albumin (HSA) is a high-abundance protein in plasma that is mainly responsible for binding and transporting various endogenous or exogenous substances (e.g., fatty acids, cholesterol, and many drugs); thus, it has a profound impact on the pharmacokinetic properties and efficacy of many drugs[5,6]. In patients with diabetes, HSA has a higher probability of glycation than other proteins, so it is regarded as an indicator of glycemic control[7]. Elevated glycation levels can lead to changes in the structure and function of HSA, thus influencing the normal physiological activities of the body[8]. The distinct distributions of multiple glycation sites on the three-dimensional structure of HSA cause different degrees of glycation under a range of glucose concentrations. A non-enzymatic glycation modification at the main drug-binding site substantially affects the ability of this region to bind drugs, thereby influencing the pharmacokinetic properties and efficacies of therapeutic drugs[9]. In this paper, seven aspects of HSA and its non-enzymatic glycation are reviewed.
Non-enzymatic glycation (sometimes described simply as glycation) is an important post-translational modification that does not involve the catalytic activity of glycosyltransferase[10]. The reaction mainly begins with a nucleophilic addition reaction between the carbonyl group of reducing sugar and the amino group of lysine, arginine, or the N-terminus of protein[11]. Fructose and lactose are important reducing sugars in food, while glucose is the main source of energy in the human body[12]. Therefore, glucose is the primary raw material for non-enzymatic glycation in the human body. The non-enzymatic glycation process is mainly divided into three steps: (1) The carbonyl group of a reducing sugar undergoes a condensation reaction with the amino group of the protein to form a thermodynamically unstable Schiff base; (2) The unstable Schiff base is converted into a relatively stable Amadori product[13,14]; and (3) Amadori product undergoes a series of spontaneous reactions (e.g., dehydration, oxidation, rearrangement, and isomerization) that can generate various carbonyl compounds, such as methylglyoxal, glyoxal, 3-deoxyglucosone, and dehydroascorbic acid[15]. These carbonyl compounds usually react more strongly than the original reducing sugars and can quickly react with proteins to form various irreversible heterostructures, which are regarded as advanced glycation end products (AGEs)[16].
HSA is a highly abundant protein in plasma; its concentration of approximately 35-50 g/L comprises approximately 60% of the total plasma protein content[17]. It is mainly responsible for the regulation of plasma osmotic pressure[18] and pH, and binding various endogenous or exogenous substances (e.g., fatty acids, cholesterol, and many drugs)[19]. Additionally, HSA serves as an antioxidant, mediates lipid metabolism, and sequesters toxins[17]. It is composed of 585 amino acids and 17 intramolecular disulfide bonds, with a molecular weight of 66437 kDa[8]. Crystal structure analysis has shown that HSA possesses a spherical "heart-shaped" structure comprising approximately 67% of α-helices, 23% of extended chains, and 10% of β-sheets. HSA contains three homology domains: I (amino acids 1-195), II (amino acids 196-383), and III (amino acids 384-585); each of these domains contains two subdomains (A and B). The A subdomains of both domains II and III constitute the major drug-binding regions of HSA; these are regarded as sites I (amino acids 196-292) and II (amino acids 384-489)[20].
Due to the high abundance of HSA, its non-enzymatic glycation represents approximately 80% of all glycation involving circulating proteins[21]. Amadori products are the main form of glycated HSA present in the body; their amounts increase as the blood glucose concentration increases in the blood of patients with diabetes[22]. The proportion of glycated HSA in healthy people is approximately 1%-10% and can increase by 2-3-fold in patients with diabetes[8,17]. Basic amino acids on HSA, specifically, 59 lysines and 24 arginines, are regarded as potential sites of glycation.
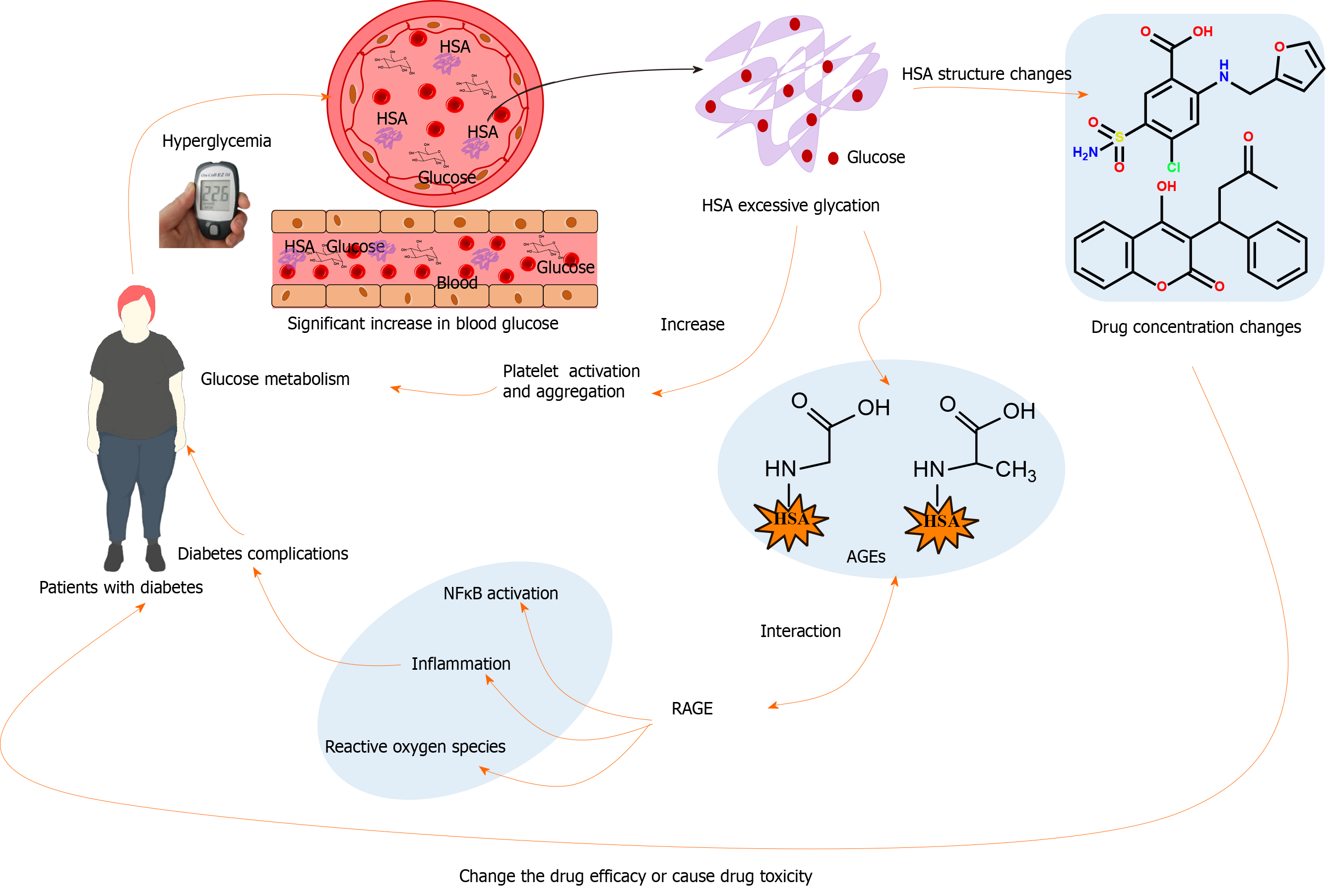
Glucose-induced modifications strongly influence HSA functional properties and have important implications for protein activity, folding, degradation, and cell function[23,24]. Although initially harmless, these modifications can become destructive and pathogenic when they become sufficiently widespread. Figure 1 shows the mechanism of the different effects of HSA glycation on the body. First, HSA glycation change the intrinsic conformations and binding efficiencies of its major binding regions, thereby changing the drug efficacy[25]. Second, the interactions of AGEs with their receptors [receptor for AGEs (RAGE)] or other macromolecules will activate various signaling pathways such as nuclear factor κB, as well as tissue damage and metabolic complications[26]. Third, glycated HSA can also stimulate platelet activation and aggregation, thereby enhancing thrombosis and inhibiting cellular uptake of glucose[27-31]. As the main drug-binding protein in plasma, HSA strongly influences drug absorption, distribution, excretion, and efficacy characteristics[32]. Changes in HSA function caused by the pathological environment can lead to unexpected types of toxicity. Drug molecules either combine with proteins and lipids in plasma or exist in a free (i.e., unbound) state in the aqueous blood environment[33]. Only free drug molecules interact with their intended targets to produce therapeutic effects[33]. In some instances, the excessive modification of HSA by non-enzymatic glycation can increase the free drug concentration, which can produce severe drug toxicity[34,35].
Glycated HSA has been used as a complementary indicator to standard assays involving glycated hemoglobin (HbA1c) or real-time glucose monitoring to assess glycemic control in patients with diabetes[10]. Notably, real-time glucose monitoring only provides a single data point concerning the glycemic status of patients with diabetes, while HbA1c provides an assessment of glycemic control over 2-3 mo and may be influenced by chronic kidney disease in some patients[36,37]. In contrast, glycated HSA provides an assessment of glycemic control over 21 d and can be used as an indicator with intermediate duration (i.e., between real-time glucose monitoring and assessment of HbA1c)[38]. Many methods have been developed to detect and quantify glycated HSA with the aim of predicting or preventing potential complications; these methods mainly involve the determination of total glycated HSA, as well as the qualitative and quantitative assessment of HSA glycation sites.
Immunoassays such as enzyme-linked immunosorbent assays and radio-immunoassays are often used to detect total glycated HSA[39,40]. In addition, other traditional methods for evaluation of glycated HSA include boronate affinity technology; thiobarbituric acid analysis; nitro-blue tetrazolium colorimetric analysis; phenylhydrazone formation reaction; fructosamine assays; ketoamine oxidase analysis; high-performance liquid chromatography (HPLC) analysis of furosine hydrolysis by strong acid; phenylborate-containing acrylamide gel electrophoresis; and the analysis of reductive activity following alkaline solution treatment, using redox indicators[41-48]. However, the above traditional methods have their own characteristics or drawbacks. For example, colorimetric analysis methods such as nitro-blue tetrazolium and thiobarbituric acid have high unspecificity[49]; fructosamine assays provide higher specificity and reliability[50]; HPLC method has a high sensitivity[41]; phenylborate-containing acrylamide gel electrophoresis method is time-consuming and not suitable for clinical measurement[51]. In recent years, electrochemical quantitative analysis methods with high sensitivity and specificity have also been developed[52]. Intact protein analyses by high resolution mass spectrometry (MS) can also be used to determine the total glycation degree of HSA[53].
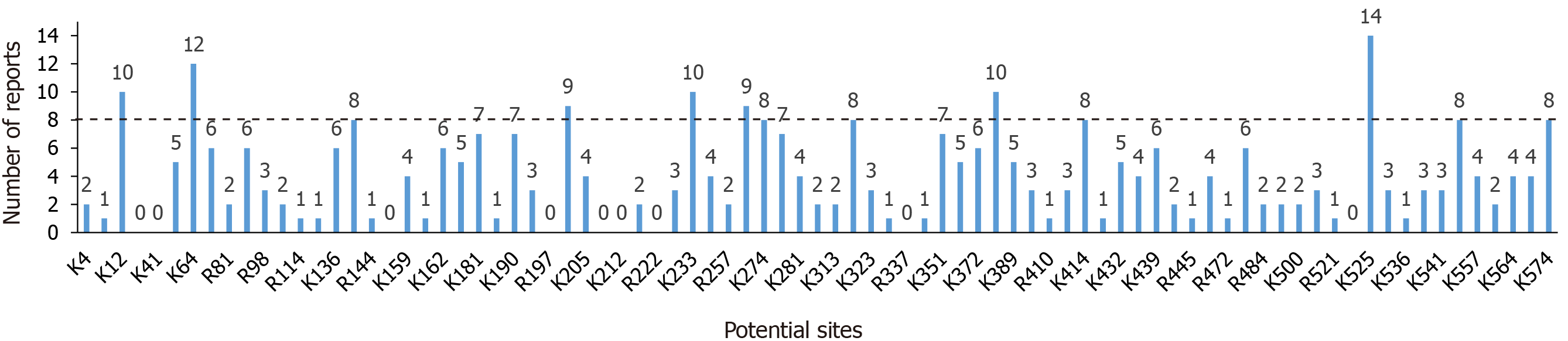
HSA is rich in basic amino acids that can undergo glycation; thus, the analysis of glycation sites on HSA mainly involves the application of high-resolution MS[54]. A “Top-Down” approach combined with tandem MS is considered a standard method to accurately assess glycation sites[55-57]. In the “Top-Down” approach, HSA is first enriched and then digested with trypsin or Lys-C[7,10]. Because of glucose steric hindrance, peptides will have missed cleavage to form peptides containing glucose modifications[58]. Thus, glycated peptides exhibit a mass shift of 162 kDa in primary MS analysis, as well as a neutral loss in tandem MS analysis, and these findings can be used to locate the accurate glycation site[12]. Many types of MS with ionization modes of matrix assisted laser desorption ionization (MALDI) or electrospray ionization (e.g., IT-TOF, LTQ-Orbitrap, Q-TOF, hybrid linear ion trap-Orbitrap, and MALDI-TOF MS) have been used to identify glycation sites[10,12,55,59,60]. For the quantitative analysis of glycated peptides, many approaches have been developed thus far[12,53,55,61]. Frolov et al[55] used the integral peak area to compare amounts of glycated peptides. In another study, isotopic labeling with 13C was performed to label native proteins, which were then digested with trypsin; the coupled 12C and 13C isotope peaks provided different types of quantitative information concerning the same glycated peptides[12]. Furthermore, 18O- and 16O-labeled H2O has been used to hydrolyze normal and glycated HSA, respectively. The 16O/18O ratios in each digested peptide were measured to compare glycation levels[61]. Furthermore, Qiu et al[53] have developed an isobaric tags for relative or absolute quantitation (iTRAQ) labeling technology combined with three-stage MS (MS3) method to compare glycation levels between healthy individuals and patients with diabetes. The iTRAQ-MS3 method makes good use of the neutral loss of glycated peptides under collision-induced dissociation in MS/MS, and high-energy collisional dissociation in MS3 fragmentation of the neutral loss ions were performed to precise quantification of the glycated peptides[53]. Table 1 shows the glycation sites that have been identified through qualitative and quantitative analyses. Notably, specific basic residues in HSA are involved in glycation in vivo[62]. Sites K525, K199, and K351 were reportedly the predominant glycation sites on HSA[62,63]. Figure 2 shows the number of reports for each potential glycation sites. Sites K12, K64, K137, K199, K233, K262, K274, K317, K378, K414, K525, K545, and K574 have been more easily identified than other sites (reported ≥ 8 times), which suggests that they are more sensitive to changes in serum glucose concentrations[7]. The underlying mechanism may be that these sites are both distributed on the HSA surface and spatially located near basic amino acids[53]. Although K199 is not completely distributed on the HSA surface, its low pKa value and spatial proximity to basic amino acids make it suitable for glycation reactions[62]. In Figure 2, we can find that some sites (e.g., K20, K41, R145, R197, R209, K212, R222, R337, and K524) had never been identified in analyses of glycation modifications, indicating that they are insensitive to changes in glucose concentrations, and further explorations of the underlying mechanism are needed to determine their roles[64-71].
| Ref. | Glycation sites reported so far | Analysis tools |
| Iberg et al[63] | HSA from a diabetic patient: Lys-12, Lys-199, Lys-233, Lys-281, Lys-317, Lys-351, Lys-439, Lys-525, Lys-534 | Amino acid analysis after hydrolysis in HCl |
| Garlick et al[64] | Freshly purified human serum albumin: Lys-525 | Cation exchange chromatography |
| Frolov et al[55] | HSA from five T2DM patients: Lys-12, Lys-51, Lys-64, Lys-162, Lys-174, Lys-181, Lys-233, Lys-262, Lys-276, Lys-351, Lys-359, Lys-378, Lys-414, Lys-475, Lys-525, Lys-545 | Q-TOF-MS |
| Kisugi et al[56] | HSA from a female diabetic patients: Lys-64/Lys-73, Lys-199, Lys-136/ Lys-137, Lys-233, Lys-274/Lys-276, Lys-317, Lys-389, Lys-439, Lys-534, Lys-525 | QSTAR Pulsar-i mass spectrometer |
| Frolov et al[57] | HSA from 5 T2DM patients and 4 healthy subjects: Lys-12, Lys-511, Lys-641, Lys-73, Lys-93, Lys-137, Lys-162, Lys-1741, Lys-1811, Lys-205, Lys-2331, Lys-2621, Lys-274, Lys-351, Lys-3591, Lys-3781, Lys-414, Lys-475, Lys-525, Lys-5451, Lys-5571 (detected only in diabetic samples), Lys-574 | Nano-ESI-LTQ Orbitrap XL MS with ETD |
| Bai et al[10] | HSA from a healthy subject and a diabetic patient: Lys-64, Lys-93, Lys-190, Lys-199, Lys-205, Lys-225, Lys-233, Lys-240, Lys-262, Lys-274, Lys-281, Lys-317, Lys-323, Lys-351, Lys-372, Lys-378, Lys-413, Lys-432, Lys-475, Lys-525, Lys-545, Lys-557, Lys-557/ Lys-560/ Lys-564, Lys-564, Lys-573/ Lys-574 | IT-TOF-MS/MS |
| Zhang et al[7] | HSA from clinical T2DM, IGT, NGT and 389 volunteers: Lys-12/ Lys-201, Arg-144, Arg-186/ Lys-1901, Arg-222/ Lys-225, Lys-240, Arg-336, Lys-372, Lys-414/ Arg-4281. (8 glucose sensitive sites) | Agilent MSD trap |
| Anguizola et al[59] | HSA from individual clinical plasma samples: Arg-10, Lys-12, Arg-10/Lys-121, Arg-981, Arg-160, Lys-162, Lys-190, Lys-199, Lys-276, Lys-281, Lys-276/Lys-2811, Lys-2861, Lys-313, Lys-317, Lys-372, Lys-428, Lys-432, Arg-484, Arg-485, Arg-484/ Arg-4851, Lys-545, Lys-557, Lys-560, Lys-5641, Lys-573/ Lys-5741 | MALDI-TOF-MS |
| Priego-Capote et al[12] | HSA from human Plasma: Lys-64, Lys-73, Lys-93, Lys-106, Lys-136, Lys-137, Lys-159, Lys-174, Lys-181, Lys-195, Arg-218, Lys-233, Lys -240, Lys-262, Lys-274, Lys-323, Lys-359, Lys-372, Lys-378, Lys-389, Lys -402, Lys-413, Lys-432, Lys-436, Lys-439, Lys-444, Lys-466, Arg-472, Lys-475, Lys-500, Lys-519, Lys -525, Lys-573 | Hybrid linear ion trap-Orbitrap MS |
| Korwar et al[65] | HSA from clinical plasma samples: Lys-12, Lys -641, Lys -136, Lys-137, Lys-1591, Lys-4021, Lys-4141, Lys-4661, Lys-5251 | Hybrid quadruple Q-Exactive Orbitrap MS |
| Zhang et al[60] | HSA from 12 NGT, 11 IGT and 8 T2DM: Lys-41, Lys-12, Lys-51, Lys-641, Lys-73, Lys-136, Lys-137, Lys-159, Lys-162, Lys-1811, Lys-1901, Lys-195, Lys-1991, Lys-205, Lys-225, Lys-2331, Lys-262, Lys-274, Lys-276, Lys-3171, Lys-351, Lys-378, Lys-414, Lys-4321, Lys-4361, Lys-475, Lys-525, Lys-538, Lys-545, Lys-5621, Lys-573, Lys-574 | Ion Trap LC-MS |
| Miyamoto et al[66] | HSA from 8 diabetic patients: Lys-51, Lys-64/ Lys-73, Lys-136/ Lys-137, Lys-159/ Lys-162, Lys-190/ Lys-195/ Lys-199/ Lys-205, Lys-233, Lys-262, Lys-274/ Lys-276, Lys-313/ Lys-317, Lys-351, Lys-378/ Lys-389, Lys-432/ Lys-436/ Lys-439, Lys-525, Lys-534/ Lys -536/ Lys-538/ Lys-541, Lys -545, Lys-573/ Lys-574 | QSTAR Pulsar-i MS |
| Brede et al[67] | HSA from plasma: Lys-12, Lys-137, Lys-414, Lys-5251 | Q-TOF MS |
| Spiller et al[68] | HSA from 48 T2DM patients and 48 non-diabetic: Lys-64, Lys-73, Lys-93, Lys-174, Lys-181, Lys-233, Lys-262, Lys-359, Lys-378, Lys-414, Lys-525, Lys-545, Lys-574 | QTRAP 4000 |
| Spiller et al[69] | HSA from 5 T2DM patients and 5 non-diabetic individuals: Lys-641, Lys-731, Lys-1811, Lys-2621, Lys-3781, Lys-5741 | ESI-QqLIT-MS (4000 |
| Takátsy et al[70] | HSA from diabetic patients and healthy individuals: Arg-81, Lys-93, Arg-98, Lys-106, Arg-114, Lys-190, Lys-199, Arg-218, Arg-257, Lys-276, Lys-317, Arg -348, Lys-372, Lys-378, Lys-389, Lys-413, Lys-436, Lys-439, Lys-444, Lys-466, Arg-484, Arg-485, Lys-500, Lys-519, Arg-521, Lys-564, Lys-536, Lys-538, Arg-445, Lys-541, Lys-560, Lys-573 | MALDI TOF MS |
| Greifenhagen et al[71] | HSA from 5 diabetic patients: Lys-12, Lys-64, Lys-137, Lys-190, Lys-199, Lys-274, Lys-276, Lys-525 | ESI-Orbitrap-MS |
| Qiu et al[53] | HSA from 4 diabetic patients and 4 healthy subjects: Lys-4, Lys-12, Lys-511, Lys-641, Lys-73, Arg-81, Lys-931, Arg-98, Arg-117, Lys-136, Lys-137, Lys-1621, Lys-174, Lys-181, Arg-186, Lys-1991, Lys-205, Lys-2331, Lys-240, Arg-257, Lys-2621, Lys-274, Lys-276, Lys-281, Lys-286, Lys-3131, Lys-317, Lys-3231, Lys-351, Lys-359, Lys-372, Lys -3781, Lys-389, Lys-4021, Lys-410, Lys-4141, Lys-436, Lys-439, Lys-4661, Lys-4751, Lys-519, Lys-5251, Lys-538, Lys-541, Lys-5451, Lys-5571, Lys-5641, Lys-573, Lys-5741 | LTQ Orbitrap Velos Pro MS |
Many functions of HSA can be attributed to its structural characteristics. The relative structural stability of HSA is mainly dependent on 17 intramolecular disulfide bonds[50]. This structural flexibility enables HSA to bind to many molecules with distinct structures[72]. The affinities of various metabolites and drugs depend on the multistage structures of binding sites, which are distributed throughout the whole HSA molecule. The major drug-binding sites of HSA are known as sites I and II[20,35,73]. Glycation contributes to various changes in HSA structure and function[74]. First, it enhances the molecular weight of HSA by attaching one or several glucose units to the basic amino acid residues of the protein. Second, glycation will change the original conformation of HSA. The intrinsic fluorescence of HSA is mainly derived from tryptophan-214 located in site I; its fluorescence is extremely sensitive to changes in the HSA environment[24,35,73]. Glycated sites located in or near Site I, such as K199, will alter the HSA structural microenvironment, thereby altering the intrinsic fluorescent characteristics of the protein. The relative fluorescence intensity of glycated HSA is reportedly reduced by 51% compared with normal HSA[75]. In addition to fluorescence chromatography, circular dichroism has also been used to study the effects of glycation on the structure of HSA[76]. Nakajou et al[75] used circular dichroism to compare different HSA molecules, which revealed that the secondary structure of HSA was altered after glycation with 50 mmol/L glucose. Third, the glycation of HSA will act as an oxidant and a pro-inflammatory mediator through different mechanisms[77].
Glycation-related changes in the structure of HSA can have varying effects on its abilities to bind a range of ligands. The main mechanisms that affect binding may involve steric hindrance of covalently bound glucose, the blockage of charged residues, or a combination of these two mechanisms[75]. Techniques used to study the binding affinity of glycated HSA include fluorescence spectroscopy, circular dichroism, HPLC with ultraviolet detection, and nuclear magnetic resonance[78-80]. Changes in the binding affinities of glycated HSA to various ligands are influenced by drug concentration and the degree of protein glycation[35,53,75] (see Table 2 [81-85]). Warfarin, tryptophan, and dansylsarcosine have often been used as probe compounds for HSA sites I and II in binding studies[75,76]. In vitro analysis has shown that HSA glycation with a range of glucose concentrations (2.5 mmol/L, 12.5 mmol/L, and 50 mmol/L) enhanced the binding of warfarin, but weakened the binding of dansylsarcosine[75]. Another study showed that both ex vivo (purified from the plasma of patients with diabetes) and in vitro glycated HSA exhibited weakened binding interactions with warfarin[35]. Joseph et al[76] proved that the binding of L-tryptophan was enhanced by 4.7-5.8 fold under glycation conditions similar to those in patients with diabetes, although the binding of warfarin remained unchanged. Notably, the above contradictory results concerning warfarin were obtained under relatively nonphysiological conditions in vitro. Qiu et al[53] found that the affinity of warfarin for HSA was greater in plasma from patients with diabetes. The level of free warfarin was also reduced in subsequent pharmacokinetic experiments[53]. Furthermore, a retrospective clinical study revealed that the anticoagulant effect of warfarin was reduced in patients with diabetes[53]. These in vivo findings may provide better reference data with respect to warfarin binding.
| Ref. | Ligands | In vivo/ vitro/ex vivo | Glycation level of HSA | Binding affinity |
| Nakajou et al[75] | Warfarin | In vitro | HSA glycated with 2.5 mmol/L, 12.5 mmol/L, and 50 mmol/L glucose | ↑ |
| Baraka-Vidot et al[35] | Warfarin | In vitro and Ex vivo | HSA purified from blood and HSA glycated with 25 mmol/L or 100 mmol/L glucose | ↓ |
| Joseph et al[76] | Warfarin | In vitro | HSA glycated with 0.5 mol/L glucose | → |
| Qiu et al[53] | Warfarin | In vivo | HSA from diabetic patients | ↑ |
| Joseph et al[76] | Tryptophan | In vitro | HSA glycated with 0.5 mol/L glucose | ↑4.7-5.8-fold |
| Nakajou et al[75] | Dansylsarcosine | In vitro | HSA glycated with 2.5 mmol/L, 12.5 mmol/L, and 50 mmol/L glucose | ↓ |
| Qiu et al[53] | Heparin | In vitro and in vivo | HSA from diabetic patients | → |
| Guerin-Dubourg et al[81] | Copper | In vivo | HSA purified from diabetic patients and control individuals | ↓16% |
| Koizumi et al[82] | Furosemide | In vitro | Prepared from HSA, and commercial HSA | ↓ |
| Okabe et al[83] | Phenylbutazone | In vitro | Each mole of HSA contains 1.94 moles of glucose | ↓ |
| Yamazaki et al[84] | Fatty acids | In vitro | HSA glycated with 100 mmol/L glucose | ↓ |
| Karp et al[85] | Diazepam | In vitro | HSA glycated with 140 mmol/L glucose | → |
| Karp et al[85] | Bilirubin | In vitro | HSA glycated with 140 mmol/L glucose | ↓30% |
| Okabe et al[83] | Ibuprofen | In vitro | Each mole of HSA contains 1.94 moles of glucose | ↓20 |
| Okabe et al[83] | Dansylproline | In vitro | Each mole of HSA contains 1.94 moles of glucose | ↓25% |
| Okabe et al[83] | Flufenamic acid | In vitro | Each mole of HSA contains 1.94 moles of glucose | ↓ |
| Koizumi et al[82] | Naproxen | In vitro | Prepared from HSA, and commercial HSA | → |
Chronic hyperglycemia is the primary condition associated with complications of diabetes. Hyperglycemia leads to excessive irreversible accumulation of AGEs on long-lived proteins, such as HSA and HbA1c. The degrees and durations of protein exposure to abnormally high levels of glucose are closely related to the degrees and rates of progression of nephropathy, stroke, neuropathy, retinopathy, and cardiovascular disease[86]. There remain questions concerning how the accumulation of AGEs promotes the development of these lesions. There are three main consequences of the formation of AGEs: (1) Cross-linking of various extracellular proteins[87]; (2) Changes in cell–matrix interactions[88,89]; and (3) Changes in DNA structure and function[90]. HSA is the main protein in blood circulation; patients with diabetes exhibit significantly greater levels of the HSA-related AGEs[91]. Interactions between AGEs and RAGEs alter cellular signals and gene expression, thereby enhancing the secretion of pro-inflammatory molecules and leading to oxidative stress reactions in patients with diabetes[92].
Glycation is a continuous process in the human body. Elevated levels of glycated proteins are associated with elevated levels of blood glucose in patients with diabetes. Thus, there is considerable interest in measuring the glycation levels in patients with diabetes; these data can be used for diagnosis, treatment, and prognosis[93,94]. For many years, HbA1c has been used for the clinical monitoring of long-term blood glucose control[95]. However, HbA1c monitoring has some limitations. Because the lifespan of HbA1c is approximately 3 mo, rapid changes in serum glucose status (e.g., treatment response) are not clearly reflected in HbA1c measurements[96,97]. In some individuals, an abnormally elevated HbA1c value may be recorded, such as patients with hemoglobin variants[96,98], patients with rapid changes in glucose control, patients with iron-deficiency anemia, patients with HIV, or pregnant patients[99-102]. In patients with reduced erythrocyte lifespan, such as those with liver cirrhosis[103], hemolytic anemia[104], chronic kidney disease, and/or hemorrhage, the recorded values of HbA1c will decrease[105,106]. HSA glycation has been suggested as an alternative clinical indicator to circumvent many limitations of HbA1c assessment. The level of HSA glycation is not affected by hemoglobin genetic variations or changes in erythrocyte lifespan[107]. Compared with HbA1c, glycated HSA has a much shorter half-life and is therefore more sensitive to changes in glycemic status. The levels of glycated HSA reflect the average plasma glucose level over a 2-wk interval[94,108]. Therefore, glycated HSA is a more dynamic indicator of glycemic control, which can be used to evaluate the drug treatment efficacy and short-term changes in glucose control. In patients with pre-diabetes, the total degree of HSA glycation does not provide all possible information regarding short-term fluctuations in plasma glucose concentrations because of the high number of possible glycation sites. Therefore, the comparison of the glycation degree of specific HSA sites sensitive to glucose (e.g., K525 and K199) can be used as clinical biomarkers for the occurrence and early diagnosis of diabetes[53,65]. However, it is noteworthy that glycated HSA levels are also influenced by hypoalbuminemic conditions such as malnutrition, nephrotic syndrome, liver cirrhosis, or other liver and renal disease[109]. Further verification is needed to determine whether and how glycated albumin can be used as an indicator of hyperglycemia under these conditions.
Hyperglycemia leads to enhanced HSA glycation in patients with diabetes; this highly non-enzymatic glycation at multiple sites can impact the function of HSA as a drug carrier. In this review, we have presented a detailed summary of non-enzymatic glycation sites identified thus far in vivo; we have also discussed the impacts of non-enzymatic glycation on the three-dimensional structure and biological functions of HSA. It would be useful to determine how modifications in HSA glycation affect drug treatments for a range of diseases. Glycated HSA may serve as a new clinical indicator for assessment of glycemic control, potentially as an alternative for the long-term indicator HbA1c. Additional in vivo studies are needed to determine the effects of glycated HSA on combinations and efficacies of various drugs, thereby providing reference data to aid in the guidance of clinical treatment for patients with diabetes.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciarambino T, Gracia-Ramos AE, Lee KS S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Huang Y. IDF Diabetes Atlas 8th Edition. International diabetes federation 2017 [cited 20 April 2021]. Available from: https://diabetestalk.net/diabetes/idf-diabetes-atlas-8th-edition. |
| 2. | Yamagishi S, Maeda S, Matsui T, Ueda S, Fukami K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta. 2012;1820:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Daneman D. Diabetes-related mortality. A pediatrician's view. Diabetes Care. 2001;24:801-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Chilelli NC, Burlina S, Lapolla A. AGEs, rather than hyperglycemia, are responsible for microvascular complications in diabetes: a "glycoxidation-centric" point of view. Nutr Metab Cardiovasc Dis. 2013;23:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol. 2005;353:38-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1354] [Cited by in RCA: 1461] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 6. | Bhat S, Jagadeeshaprasad MG, Venkatasubramani V, Kulkarni MJ. Abundance matters: role of albumin in diabetes, a proteomics perspective. Expert Rev Proteomics. 2017;14:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Zhang M, Xu W, Deng Y. A new strategy for early diagnosis of type 2 diabetes by standard-free, label-free LC-MS/MS quantification of glycated peptides. Diabetes. 2013;62:3936-3942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Shaklai N, Garlick RL, Bunn HF. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984;259:3812-3817. [PubMed] |
| 9. | Szkudlarek A, Wilk M, Maciążek-Jurczyk M. In Vitro Investigations of Acetohexamide Binding to Glycated Serum Albumin in the Presence of Fatty Acid. Molecules. 2020;25:2340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Bai X, Wang Z, Huang C, Chi L. Investigation of non-enzymatic glycosylation of human serum albumin using ion trap-time of flight mass spectrometry. Molecules. 2012;17:8782-8794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E, Koke M, Hage DS. Review: Glycation of human serum albumin. Clin Chim Acta. 2013;425:64-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 283] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 12. | Priego-Capote F, Scherl A, Müller M, Waridel P, Lisacek F, Sanchez JC. Glycation isotopic labeling with 13C-reducing sugars for quantitative analysis of glycated proteins in human plasma. Mol Cell Proteomics. 2010;9:579-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Reynolds TM. Chemistry of nonenzymic browning. I. The reaction between aldoses and amines. Adv Food Res. 1963;12:1-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 152] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Reynolds TM. Chemistry of nonenzymic browning. II. Adv Food Res. 1965;14:167-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 178] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344 Pt 1:109-116. [PubMed] |
| 16. | Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1679] [Cited by in RCA: 1647] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 17. | Bourdon E, Loreau N, Blache D. Glucose and free radicals impair the antioxidant properties of serum albumin. FASEB J. 1999;13:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 214] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Scatchard G, Batchelder AC, Brown A. Chemical, clinical, and immunological studies on the products of human plasma fractionation. vi. The osmotic pressure of plasma and of serum albumin. J Clin Invest. 1944;23:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Anguizola JA, Basiaga SB, Hage DS. Effects of Fatty Acids and Glycation on Drug Interactions with Human Serum Albumin. Curr Metabolomics. 2013;1:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Perters T. All about albumin: Biochemistry, Genetics and Medical Application. Academic Press: San Diego, 1995. |
| 21. | Schleicher ED, Mayer R, Wagner EM, Gerbitz KD. Is serum fructosamine assay specific for determination of glycated serum protein? Clin Chem. 1988;34:320-323. [PubMed] |
| 22. | Cohen MP. Intervention strategies to prevent pathogenetic effects of glycated albumin. Arch Biochem Biophys. 2003;419:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Voziyan PA, Khalifah RG, Thibaudeau C, Yildiz A, Jacob J, Serianni AS, Hudson BG. Modification of proteins in vitro by physiological levels of glucose: pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end-products through binding of redox metal ions. J Biol Chem. 2003;278:46616-46624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Mendez DL, Jensen RA, McElroy LA, Pena JM, Esquerra RM. The effect of non-enzymatic glycation on the unfolding of human serum albumin. Arch Biochem Biophys. 2005;444:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Oettl K, Stauber RE. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br J Pharmacol. 2007;151:580-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 273] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 26. | Cohen MP, Shea E, Chen S, Shearman CW. Glycated albumin increases oxidative stress, activates NF-kappa B and extracellular signal-regulated kinase (ERK), and stimulates ERK-dependent transforming growth factor-beta 1 production in macrophage RAW cells. J Lab Clin Med. 2003;141:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Miele C, Riboulet A, Maitan MA, Oriente F, Romano C, Formisano P, Giudicelli J, Beguinot F, Van Obberghen E. Human glycated albumin affects glucose metabolism in L6 skeletal muscle cells by impairing insulin-induced insulin receptor substrate (IRS) signaling through a protein kinase C alpha-mediated mechanism. J Biol Chem. 2003;278:47376-47387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Unoki H, Bujo H, Yamagishi S, Takeuchi M, Imaizumi T, Saito Y. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res Clin Pract. 2007;76:236-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Unoki H, Yamagishi S. Advanced glycation end products and insulin resistance. Curr Pharm Des. 2008;14:987-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Rubenstein DA, Yin W. Glycated albumin modulates platelet susceptibility to flow induced activation and aggregation. Platelets. 2009;20:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Doweiko JP, Bistrian BR. The effect of glycosylated albumin on platelet aggregation. JPEN J Parenter Enteral Nutr. 1994;18:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Wang S, Huang M. Structure and enzymatic activities of human serum albumin. Curr Pharm Des. 2015;21:1831-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Smith DA, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov. 2010;9:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 627] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 34. | Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Baraka-Vidot J, Guerin-Dubourg A, Bourdon E, Rondeau P. Impaired drug-binding capacities of in vitro and in vivo glycated albumin. Biochimie. 2012;94:1960-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. NDT Plus. 2011;4:368-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Neelofar K, Ahmad J. Amadori albumin in diabetic nephropathy. Indian J Endocrinol Metab. 2015;19:39-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Raghav A, Ahmad J. Glycated serum albumin: a potential disease marker and an intermediate index of diabetes control. Diabetes Metab Syndr. 2014;8:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Ikeda K, Sakamoto Y, Kawasaki Y, Miyake T, Tanaka K, Urata T, Katayama Y, Ueda S, Horiuchi S. Determination of glycated albumin by enzyme-linked boronate immunoassay (ELBIA). Clin Chem. 1998;44:256-263. [PubMed] |
| 40. | Ohe Y, Matsuura M, Nakajima Y, Shin S, Hashimoto F, Hirota M, Shima K. Radioimmunoassay of glycosylated albumin with monoclonal antibody to glucitol-lysine. Clin Chim Acta. 1987;169:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Shima K, Ito N, Abe F, Hirota M, Yano M, Yamamoto Y, Uchida T, Noguchi K. High-performance liquid chromatographic assay of serum glycated albumin. Diabetologia. 1988;31:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Dolhofer R, Wieland OH. Improvement of the thiobarbituric acid assay for serum glycosylprotein determination. Clin Chim Acta. 1981;112:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Elder E, Kennedy L. Rapid, accurate colorimetric assay of non-enzymatically glycosylated serum proteins. Diabetologia. 1983;24:70-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Baker JR, Metcalf PA, Johnson RN, Newman D, Rietz P. Use of protein-based standards in automated colorimetric determinations of fructosamine in serum. Clin Chem. 1985;31:1550-1554. [PubMed] |
| 45. | Mashiba S, Uchida K, Okuda S, Tomita S. Measurement of glycated albumin by the nitroblue tetrazolium colorimetric method. Clin Chim Acta. 1992;212:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Acharya AS, Manning JM. Amadori rearrangement of glyceraldehyde-hemoglobin Schiff base adducts. A new procedure for the determination of ketoamine adducts in proteins. J Biol Chem. 1980;255:7218-7224. [PubMed] |
| 47. | Ghiggeri GM, Candiano G, Delfino G, Queirolo C, Pallavicini G, Ginevri F. Characterisation of the phenylhydrazone derivatives of "glycated albumin" purified from diabetic sera. Carbohydr Res. 1986;153:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Kouzuma T, Uemastu Y, Usami T, Imamura S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta. 2004;346:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Vanhaeverbeek M, Brohee D, Lefevre A, Piro P, Kennes B, Neve P. Thiobarbiturate and fructosamine assays: significance and interest of the borohydride blank. Acta Diabetol. 1994;31:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Rondeau P, Bourdon E. The glycation of albumin: structural and functional impacts. Biochimie. 2011;93:645-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 51. | Morais MP, Mackay JD, Bhamra SK, Buchanan JG, James TD, Fossey JS, van den Elsen JM. Analysis of protein glycation using phenylboronate acrylamide gel electrophoresis. Proteomics. 2010;10:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Attar AM, Richardson MB, Speciale G, Majumdar S, Dyer RP, Sanders EC, Penner RM, Weiss GA. Electrochemical Quantification of Glycated and Non-glycated Human Serum Albumin in Synthetic Urine. ACS Appl Mater Interfaces. 2019;11:4757-4765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Qiu H, Jin L, Chen J, Shi M, Shi F, Wang M, Li D, Xu X, Su X, Yin X, Li W, Zhou X, Linhardt RJ, Wang Z, Chi L, Zhang Q. Comprehensive Glycomic Analysis Reveals That Human Serum Albumin Glycation Specifically Affects the Pharmacokinetics and Efficacy of Different Anticoagulant Drugs in Diabetes. Diabetes. 2020;69:760-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Niwa T. Mass spectrometry for the study of protein glycation in disease. Mass Spectrom Rev. 2006;25:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Frolov A, Hoffmann R. Identification and relative quantification of specific glycation sites in human serum albumin. Anal Bioanal Chem. 2010;397:2349-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Kisugi R, Kouzuma T, Yamamoto T, Akizuki S, Miyamoto H, Someya Y, Yokoyama J, Abe I, Hirai N, Ohnishi A. Structural and glycation site changes of albumin in diabetic patient with very high glycated albumin. Clin Chim Acta. 2007;382:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Frolov A, Blüher M, Hoffmann R. Glycation sites of human plasma proteins are affected to different extents by hyperglycemic conditions in type 2 diabetes mellitus. Anal Bioanal Chem. 2014;406:5755-5763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Lee JY, Kim JY, Cheon MH, Park GW, Ahn YH, Moon MH, Yoo JS. MRM validation of targeted nonglycosylated peptides from N-glycoprotein biomarkers using direct trypsin digestion of undepleted human plasma. J Proteomics. 2014;98:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Anguizola J, Joseph KS, Barnaby OS, Matsuda R, Alvarado G, Clarke W, Cerny RL, Hage DS. Development of affinity microcolumns for drug-protein binding studies in personalized medicine: interactions of sulfonylurea drugs with in vivo glycated human serum albumin. Anal Chem. 2013;85:4453-4460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 60. | Zhang Q, Tang N, Schepmoes AA, Phillips LS, Smith RD, Metz TO. Proteomic profiling of nonenzymatically glycated proteins in human plasma and erythrocyte membranes. J Proteome Res. 2008;7:2025-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Barnaby OS, Cerny RL, Clarke W, Hage DS. Quantitative analysis of glycation patterns in human serum albumin using 16O/18O-labeling and MALDI-TOF MS. Clin Chim Acta. 2011;412:1606-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Neelofar K, Ahmad J. An overview of in vitro and in vivo glycation of albumin: a potential disease marker in diabetes mellitus. Glycoconj J. 2017;34:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Iberg N, Flückiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem. 1986;261:13542-13545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 288] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 64. | Garlick RL, Mazer JS. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem. 1983;258:6142-6146. [PubMed] |
| 65. | Korwar AM, Vannuruswamy G, Jagadeeshaprasad MG, Jayaramaiah RH, Bhat S, Regin BS, Ramaswamy S, Giri AP, Mohan V, Balasubramanyam M, Kulkarni MJ. Development of Diagnostic Fragment Ion Library for Glycated Peptides of Human Serum Albumin: Targeted Quantification in Prediabetic, Diabetic, and Microalbuminuria Plasma by Parallel Reaction Monitoring, SWATH, and MSE. Mol Cell Proteomics. 2015;14:2150-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Miyamoto H, Kohzuma T, Ohnishi A. Changes in the albumin glycation site, plasma pentosidine and esRAGE concentrations before and after intensive diabetic treatment in patients with abnormally high glycated albumin levels. Ann Clin Biochem. 2018;55:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Brede C, Hop B, Jørgensen K, Skadberg Ø. Measurement of glycated albumin in serum and plasma by LC-MS/MS. Scand J Clin Lab Invest. 2016;76:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Spiller S, Li Y, Blüher M, Welch L, Hoffmann R. Glycated lysine-141 in haptoglobin improves the diagnostic accuracy for type 2 diabetes mellitus in combination with glycated hemoglobin HbA1c and fasting plasma glucose. Clin Proteomics. 2017;14:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Spiller S, Frolov A, Hoffmann R. Quantification of Specific Glycation Sites in Human Serum Albumin as Prospective Type 2 Diabetes Mellitus Biomarkers. Protein Pept Lett. 2017;24:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Takátsy A, Böddi K, Nagy L, Nagy G, Szabó S, Markó L, Wittmann I, Ohmacht R, Ringer T, Bonn GK, Gjerde D, Szabó Z. Enrichment of Amadori products derived from the nonenzymatic glycation of proteins using microscale boronate affinity chromatography. Anal Biochem. 2009;393:8-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Greifenhagen U, Frolov A, Blüher M, Hoffmann R. Plasma Proteins Modified by Advanced Glycation End Products (AGEs) Reveal Site-specific Susceptibilities to Glycemic Control in Patients with Type 2 Diabetes. J Biol Chem. 2016;291:9610-9616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Weber G. Energetics of ligand binding to proteins. Adv Protein Chem. 1975;29:1-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 357] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 73. | Wang Y, Yu H, Shi X, Luo Z, Lin D, Huang M. Structural mechanism of ring-opening reaction of glucose by human serum albumin. J Biol Chem. 2013;288:15980-15987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 74. | Cao H, Chen T, Shi Y. Glycation of human serum albumin in diabetes: impacts on the structure and function. Curr Med Chem. 2015;22:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Nakajou K, Watanabe H, Kragh-Hansen U, Maruyama T, Otagiri M. The effect of glycation on the structure, function and biological fate of human serum albumin as revealed by recombinant mutants. Biochim Biophys Acta. 2003;1623:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Joseph KS, Hage DS. The effects of glycation on the binding of human serum albumin to warfarin and L-tryptophan. J Pharm Biomed Anal. 2010;53:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Martinez Fernandez A, Regazzoni L, Brioschi M, Gianazza E, Agostoni P, Aldini G, Banfi C. Pro-oxidant and pro-inflammatory effects of glycated albumin on cardiomyocytes. Free Radic Biol Med. 2019;144:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Szkudlarek A, Sułkowska A, Maciążek-Jurczyk M, Chudzik M, Równicka-Zubik J. Effects of non-enzymatic glycation in human serum albumin. Spectroscopic analysis. Spectrochim Acta A Mol Biomol Spectrosc. 2016;152:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Dockal M, Carter DC, Rüker F. The three recombinant domains of human serum albumin. Structural characterization and ligand binding properties. J Biol Chem. 1999;274:29303-29310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 315] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 80. | Krenzel ES, Chen Z, Hamilton JA. Correspondence of fatty acid and drug binding sites on human serum albumin: a two-dimensional nuclear magnetic resonance study. Biochemistry. 2013;52:1559-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 81. | Guerin-Dubourg A, Catan A, Bourdon E, Rondeau P. Structural modifications of human albumin in diabetes. Diabetes Metab. 2012;38:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | Koizumi K, Ikeda C, Ito M, Suzuki J, Kinoshita T, Yasukawa K, Hanai T. Influence of glycosylation on the drug binding of human serum albumin. Biomed Chromatogr. 1998;12:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 83. | Okabe N, Hashizume N. Drug binding properties of glycosylated human serum albumin as measured by fluorescence and circular dichroism. Biol Pharm Bull. 1994;17:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Yamazaki E, Inagaki M, Kurita O, Inoue T. Kinetics of fatty acid binding ability of glycated human serum albumin. J Biosci. 2005;30:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Karp WB, Kinsley M, Subramanyam SB, Robertson AF. Binding properties of glycosylated albumin and acetaldehyde albumin. Alcohol Clin Exp Res. 1985;9:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 86. | Brownlee M. Glycosylation products as toxic mediators of diabetic complications. Annu Rev Med. 1991;42:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Tanaka S, Avigad G, Brodsky B, Eikenberry EF. Glycation induces expansion of the molecular packing of collagen. J Mol Biol. 1988;203:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 133] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Esposito C, Gerlach H, Brett J, Stern D, Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989;170:1387-1407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 260] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 89. | Vlassara H, Brownlee M, Manogue KR, Dinarello CA, Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988;240:1546-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 411] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 90. | Baker L, Dahlem S, Goldfarb S, Kern EF, Stanley CA, Egler J, Olshan JS, Heyman S. Hyperfiltration and renal disease in glycogen storage disease, type I. Kidney Int. 1989;35:1345-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Lapolla A, Piarulli F, Sartore G, Ceriello A, Ragazzi E, Reitano R, Baccarin L, Laverda B, Fedele D. Advanced glycation end products and antioxidant status in type 2 diabetic patients with and without peripheral artery disease. Diabetes Care. 2007;30:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 92. | Ramasamy R, Yan SF, Schmidt AM. Arguing for the motion: yes, RAGE is a receptor for advanced glycation endproducts. Mol Nutr Food Res. 2007;51:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol. 2008;2:1114-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 94. | Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 95. | Gillery P. A history of HbA1c through Clinical Chemistry and Laboratory Medicine. Clin Chem Lab Med. 2013;51:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 96. | Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001;47:153-163. [PubMed] |
| 97. | Jeffcoate SL. Diabetes control and complications: the role of glycated haemoglobin, 25 years on. Diabet Med. 2004;21:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 98. | Behan KJ, Merschen J. HbA1c does not always estimate average glucose. Clin Lab Sci. 2011;24:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | Panzer S, Kronik G, Lechner K, Bettelheim P, Neumann E, Dudczak R. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood. 1982;59:1348-1350. [PubMed] |
| 100. | Brooks AP, Metcalfe J, Day JL, Edwards MS. Iron deficiency and glycosylated haemoglobin A. Lancet. 1980;2:141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Kim PS, Woods C, Georgoff P, Crum D, Rosenberg A, Smith M, Hadigan C. A1C underestimates glycemia in HIV infection. Diabetes Care. 2009;32:1591-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 102. | Phelps RL, Honig GR, Green D, Metzger BE, Frederiksen MC, Freinkel N. Biphasic changes in hemoglobin A1c concentrations during normal human pregnancy. Am J Obstet Gynecol. 1983;147:651-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Jiao Y, Okumiya T, Saibara T, Park K, Sasaki M. Abnormally decreased HbA1c can be assessed with erythrocyte creatine in patients with a shortened erythrocyte age. Diabetes Care. 1998;21:1732-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Coban E, Ozdogan M, Timuragaoglu A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004;112:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 105. | Lo C, Lui M, Ranasinha S, Teede HJ, Kerr PG, Polkinghorne KR, Nathan DM, Zheng H, Zoungas S. Defining the relationship between average glucose and HbA1c in patients with type 2 diabetes and chronic kidney disease. Diabetes Res Clin Pract. 2014;104:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 106. | Seidel UK, Gronewold J, Volsek M, Todica O, Kribben A, Bruck H, Hermann DM. The prevalence, severity, and association with HbA1c and fibrinogen of cognitive impairment in chronic kidney disease. Kidney Int. 2014;85:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | Vetter SW. Glycated Serum Albumin and AGE Receptors. Adv Clin Chem. 2015;72:205-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 108. | Austin GE, Mullins RH, Morin LG. Non-enzymic glycation of individual plasma proteins in normoglycemic and hyperglycemic patients. Clin Chem. 1987;33:2220-2224. [PubMed] |
| 109. | RJ, Beck RW, Scioscia MF, Umpierrez GE, Tuttle KR. Glycemic Monitoring and management in advanced chronic kidney disease. Endocr Rev. 2020;41:756-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |