Published online Jun 15, 2021. doi: 10.4239/wjd.v12.i6.685
Peer-review started: December 28, 2020
First decision: January 11, 2021
Revised: January 22, 2021
Accepted: March 18, 2021
Article in press: March 18, 2021
Published online: June 15, 2021
Processing time: 157 Days and 16.6 Hours
Diabetic cardiomyopathy (DCM) is commonly defined as cardiomyopathy in patients with diabetes mellitus in the absence of coronary artery disease and hypertension. As DCM is now recognized as a cause of substantial morbidity and mortality among patients with diabetes mellitus and clinical diagnosis is still inappropriate, various expert groups struggled to identify a suitable biomarker that will help in the recognition and management of DCM, with little success so far. Hence, we thought it important to address the role of biomarkers that have shown potential in either human or animal studies and which could eventually result in mitigating the poor outcomes of DCM. Among the array of biomarkers we thoroughly analyzed, long noncoding ribonucleic acids, soluble form of suppression of tumorigenicity 2 and galectin-3 seem to be most beneficial for DCM detection, as their plasma/serum levels accurately correlate with the early stages of DCM. The combination of relatively inexpensive and accurate speckle tracking echocardiography with some of the highlighted biomarkers may be a promising screening method for newly diagnosed diabetes mellitus type 2 patients. The purpose of the screening test would be to direct affected patients to more specific confirmation tests. This perspective is in concordance with current guidelines that accentuate the importance of an interdisciplinary team-based approach.
Core Tip: Diabetic cardiomyopathy (DCM), which affects 12% of diabetics, is an under-recognized and lethal complication of diabetes. Thus, there is an urgent need for reliable and available biomarkers for DCM detection. To date, none of the conducted studies have been successful in identifying such biomarkers. Hence, in concordance with current guidelines that accentuate the importance of an interdisciplinary team-based approach, we propose the combination of speckle tracking echocardiography and a few novel biomarkers as a screening method for DCM in patients with new onset diabetes mellitus type 2.
- Citation: Kumric M, Ticinovic Kurir T, Borovac JA, Bozic J. Role of novel biomarkers in diabetic cardiomyopathy. World J Diabetes 2021; 12(6): 685-705
- URL: https://www.wjgnet.com/1948-9358/full/v12/i6/685.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i6.685
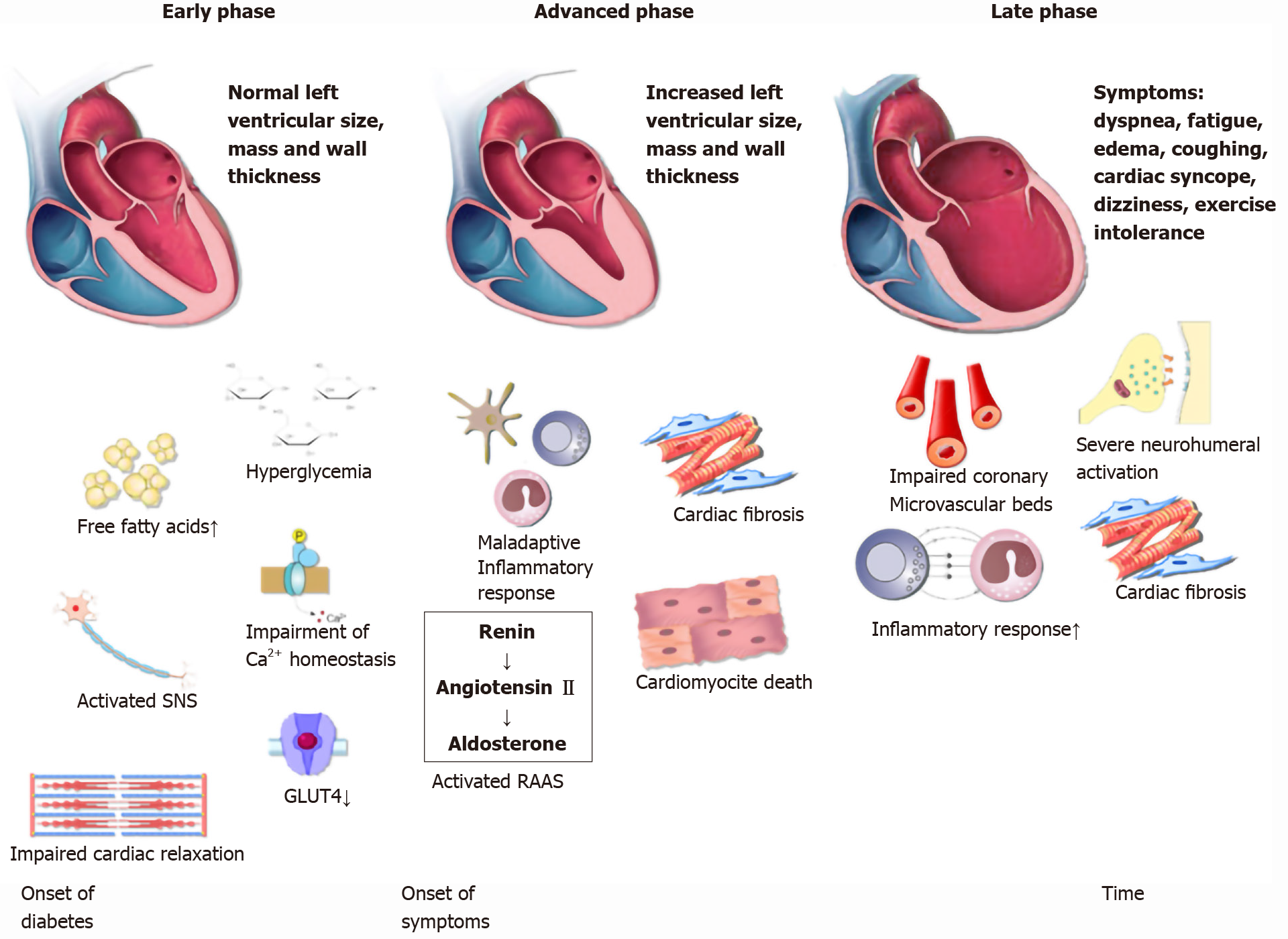
The first records of diabetic cardiomyopathy (DCM) date back to 1972[1], when it was first observed in the post-mortem analysis of diabetics who died of heart failure (HF), having no evidence of coronary artery pathology or any other pathology that could explain the observed structural changes. These findings were supported by the Framingham study in which HF was five times more common among patients with diabetes mellitus (DM)[2], even after the adjustment for hypertension and coronary heart disease. DCM is now commonly defined as cardiomyopathy in patients with DM in the absence of coronary artery disease, valvular disease, and hypertension, or any other conventional cardiovascular risk factor for that matter[3]. Diagnostic criteria include left ventricular diastolic dysfunction and/or reduced left ventricular ejection fraction, pathological left ventricle hypertrophy and interstitial fibrosis[4]. However, timely and appropriate diagnosis is still fairly challenging in everyday clinical practice[5]. The reason behind the exigent diagnosis of this clinical entity lies in the long asymptomatic phase of the disease. DCM initially presents with clinically covert myocardial fibrosis, dysfunctional cardiac remodeling and associated diastolic dysfunction, later progressing to systolic dysfunction, and eventually to overt HF. The changes that lead to DCM are triggered by hyperinsulinemia and increased insulin resistance, whereas the underlying molecular changes that are involved in the pathophysiologic development of DCM include: Abnormalities in the adenosine monophosphate-activated protein kinase, nuclear factor κ-light-chain-enhancer of activated B cells (NFκB), nuclear factor erythroid 2–related factor 2, mitogen-activated protein kinase (MAPK), cyclic adenosine 5′-monophosphate-responsive element modulator, peroxisome proliferator-activated receptors (PPARs), O-linked N-acetylglucosamine, protein kinase C (PKC), micro ribonucleic acid (microRNA) and exosome pathways[4]. As DCM is now recognized as a cause of substantial morbidity and mortality among patients with diabetes mellitus, affecting 12% of patients with diabetes, various expert groups struggle to identify a suitable biomarker that will help in the recognition and management of DCM[6,7]. The rising burden of DM, estimated to afflict 592 million people by 2035[8], calls attention to this matter even more. Similarly, the prevalence of DM in HF could be over 40%, while in patients with DM, the prevalence of HF ranges from 10% to 22%[9,10]. Unfortunately, so far none of the conducted studies have resulted in the implementation of either conventional cardiac biomarkers or new diagnostic tools in DCM management, yet the current guidelines accentuate the importance of an interdisciplinary team-based approach[11]. Therefore, in this study we sought to address the role of certain biomarkers that have shown potential in either human or animal studies and which could eventually result in mitigating the poor outcomes of DM by participating in the prevention and/or treatment of DCM.
So far, most of the underlying pathophysiological mechanisms leading to DCM have been disclosed[12]. The pathogenesis of DCM is complex and consists of the following systemic and cardiac processes triggered by hyperinsulinemia and increased insulin resistance: impaired coronary microcirculation, dysregulation of the sympathetic nervous system activity and the renin-angiotensin-aldosterone system (RAAS), inappropriate immune response, metabolic disequilibrium of the myocardium and abnormalities of the sub-cellular components. Underlying these pathophysiological events, a role for several proteins and signaling pathways has emerged: adenosine monophosphate-activated protein kinase, PPARs, O-linked N-acetylglucosamine, Sodium-Glucose Cotransporter 2 (SGLT2), PKC, MAPK, NFκB, erythroid 2–related factor 2, microRNA and exosomes[4,13]. Other important mediators implicated in almost every step of DCM development are reactive oxygen species. It is important to point out that these processes are not independent, instead they mutually interact and result in HF. In this review, we highlight some of the above-mentioned pathways relevant for comprehension of the role of biomarkers, as greater details of DCM pathophysiology are beyond the scope of this review. The development of HF in DM is gradual and consists of three distinct phases.
Insulin cell signaling is comprised of two major transduction pathways. The first being phosphatidylinositol 3-kinase-protein kinase B (PI3K-AKT) and the other being MAPK[13]. The PI3K-AKT pathway mainly exerts the metabolic functions of insulin, most important being glucose transporter-4 (GLUT4) cell-surface expression and endothelial nitric oxide (NO) synthase (eNOS) expression. In contrast, the MAPK pathway promotes growth, hypertrophy and remodeling[14]. In an insulin resistant state, these pathways are imbalanced in favor of the MAPK pathway, creating a base for DCM development[15]. Attenuation of the PI3K-AKT and up-regulation of the MAPK pathway are a result of complex interactions between ROS, overfeeding, RAAS activity and many other components which we will discuss further.
Coronary microcirculation seems to be impaired in DM, mediated by multiple pathophysiological processes[16]. Stiffness of small blood vessels is commonly observed among patients with DM, driven by hyperinsulinemia-induced vascular smooth muscle cells differentiation to an osteoblast-like phenotype[17]. In an insulin-resistant state, owing to reduced eNOS levels, NO synthesis is reduced, whereas its degradation is accelerated as a consequence of a heightened state of oxidative stress[18,19]. As it promotes vasodilation via guanylyl cyclase activation, a negative balance of NO leads to coronary vasoconstriction[20]. Recent studies suggest a role of the endothelial-to-mesencyhmal transition (EndoMT) in this setting. EndoMT is a mechanistic phenomenon that explains the loss of normal vascular phenotype of endothelial cells, increased cardiac fibroblast content and cardiac fibrosis in the diabetic heart[21]. Importantly, Widyantoro et al[22] showed that cardiac fibrosis in the diabetic myocardium is due to stimulation of the EndoMT pathway. It seems that this detrimental cascade which is translated from vasculature onto myocardium could be an important contributor to the onset of HF with preserved ejection fraction (HFpEF)[23].
Altered sympathetic nervous system activity is one of the established hallmarks of DM[24]. The over-expression of β1-adrenergic receptors has been shown to promote myocyte hypertrophy, interstitial fibrosis and myocyte apoptosis[25]. Conversely, sympathetic denervation as a part of cardiac autonomic neuropathy (CAN) is also an important feature of DM. Interestingly, myocardial regions of persistent sympathetic innervation exhibit the greatest deficits of vasodilator reserve[26], thus indicating an association between CAN and impaired myocardial blood flow.
It has been shown that hyperglycemia increases the transcription of angio
Low-grade systemic inflammation and increased polarization towards the pro-inflammatory M1 macrophages and TH1 lymphocytes is fairly common among obese patients and in an insulin-resistant state[15,34,35]. Although regulatory T cells attenuate inflammation in the myocardium, it has been proposed that the secreted pro-inflammatory cytokines, chemokines and growth factors could result in increased cardiac fibrosis and impaired diastolic relaxation[36,37].
An influx of glucose to the myocardial cells is mainly exerted via insulin-mediated GLUT4, whereas free fatty acids (FFA) uptake depends on fatty acid translocase (CD36) expression[14,38]. Under physiological circumstances, the heart can use both glucose and FFA as a source of energy. However, in an insulin-resistant state, the expression of GLUT4 diminishes, whereas CD36 expression on plasma membrane is up-regulated. Moreover, elevated levels of intracellular FFA stimulate PPAR-alpha expression, which leads to an increased uptake and oxidation of FFA. Hence, myocardial metabolism shifts from glucose to FFA oxidation, making the myocardium less energy-efficient[39]. As DCM progresses, the expression of genes regulating beta-oxidation of fatty acids is down-regulated, thereby further mitigating the metabolic efficiency of the myocardium[40]. Hyperglycemia leads to the accumulation of the advanced glycation end-products (AGE) via non-enzymatic glycation. The AGE induce extracellular matrix cross-linking thus promoting myocardial fibrosis and impaired passive relaxation[13]. Additionally, AGE can stimulate a pro-inflammatory state by binding to the receptors for AGE[41,42]. It should be noted that a relatively novel group of anti-diabetic agents, the SGLT2 inhibitors, have been shown to attenuate hyperglycemia-induced cardiac dysfunction in lipodystrophic mice[43]. In con
Mitochondrial damage is one of the pivotal pathophysiological mechanisms that contribute to DCM. Substrate overflow induces mitochondrial ROS production and impaired oxidative phosphorylation. Consequently, this leads to altered mitochondrial Ca2+ handling, which prolongs diastolic relaxation time (diastolic dysfunction) and in later stages leads to cell death[46-48]. Apart from mitochondrial damage, excessive ROS also impair post-translational protein modifications that occur in the endoplasmic reticulum and interfere with insulin signaling pathways. Endoplasmic reticulum stress further stimulates ROS production and favors myocyte apoptosis.
MicroRNAs, small non-coding RNAs, take part in the regulation of mitochondrial function, ROS production, Ca2+ handling, apoptosis, autophagy and fibrosis, all of which are regarded as important mechanisms in diabetes induced HF[13]. These microRNAs are transported in exosomes, recently recognized extracellular vesicles involved in cell-to-cell communication[49].
The development of DCM can be divided in three distinct phases (Figure 1)[13]. In the initial phase, there are no obvious changes in the myocardium tissue and systolic function is preserved[50]. However, using echocardiography and magnetic resonance imaging (MRI) in rodents, authors observed subtle anomalies that indicate impaired diastolic relaxation. MRI findings that pointed to the impaired diastolic relaxation were slow initial and peak filling rates, whereas abnormal myocardial performance index, long period of isovolumic relaxation and impaired septal annular wall motion were the observed echocardiographic diastolic parameters[51,52,15]. In humans, early DCM is characterized by increased cardiac stiffness and impaired cardiac relaxation with consequent reduction in early diastolic filling and an increase in atrial filling [50,53]. In addition, another hallmark is a decrease in the myocardial blood-flow reserve that can be detected by various imaging techniques[54]. Needless to say, the whole initial phase is completely asymptomatic. As underlying pathophysiological mechanisms continue to exhibit their deleterious cellular effects on cardiac tissue, DCM becomes more and more evident. In the advanced phase, as myocardium becomes hypertrophic and increasingly permeated with fibrous tissue, left ventricular mass and wall thickness both increase and hence, diastolic dysfunction becomes clinically apparent. In this phase, patients may notice first symptoms which correspond to the symptoms observed in HFpEF, the most prominent being exercise intolerance[55,56]. In the late phase of DCM development, except for diastolic dysfun
Established cardiac biomarkers used for detection in patients with HF have failed to timely recognize DCM. Brain natriuretic peptide (BNP) correlation with HF is blunted owing to the association between BNP and insulin resistance[57]. In contrast, N-terminal pro-BNP and ANP have been able to predict HF in experimental DCM rat models[58,59]. Furthermore, both natriuretic peptides successfully demonstrated diastolic dysfunction in diabetics and in conjunction with 2D echocardiography [60,61]. However, their value as a biomarker was limited to symptomatic patients, those with pseudo-normalized mitral flow pattern and those with a restrictive filling pattern[60]. There was no correlation of these natriuretic peptides with diastolic dysfunction among asymptomatic patients and those with relaxation abnormalities. Additional studies also demonstrated a lack of correlation among asymptomatic patients with diastolic dysfunction and overall poor correlation with most of the echocardiography parameters[62,63]. In conclusion, the utility of natriuretic peptides in pre-clinical DCM detection is limited; however, BNP seems to be an independent predictor of poor outcomes in this cardiomyopathy[64-66].
Another family of entrenched cardiac markers are the troponins, a set of proteins that control the calcium-mediated interaction between actin and myosin. This multiprotein complex consists of troponin C which binds calcium, troponin T (TnT) which binds to tropomyosin and troponin I (TnI) which prevents actin-myosin interaction[67]. Cardiac troponin I and T are commonly used in routine clinical practice due to their high sensitivity and specificity for the detection of myocardial injury[68]. Both human and animal studies suggest that TnI and TnT are constitutively phosphorylated in diabetes via PKC, leading to depressed myofilament function and Ca2+ responsiveness[69,70]. Of note, losartan, an At II receptor blocker seems to abrogate TnI phosphorylation[71]. Although it is well-known that TnT and TnI are elevated among patients with diabetes, especially among those with concomitant coronary artery disease, to our knowledge no studies have investigated the difference in troponin plasma levels between diabetics with DCM and diabetics without DCM[72,73]. Taken together, established laboratory biomarkers measuring myocardial injury and mechanical hemodynamic overload of the ventricles are not specific markers of DCM.
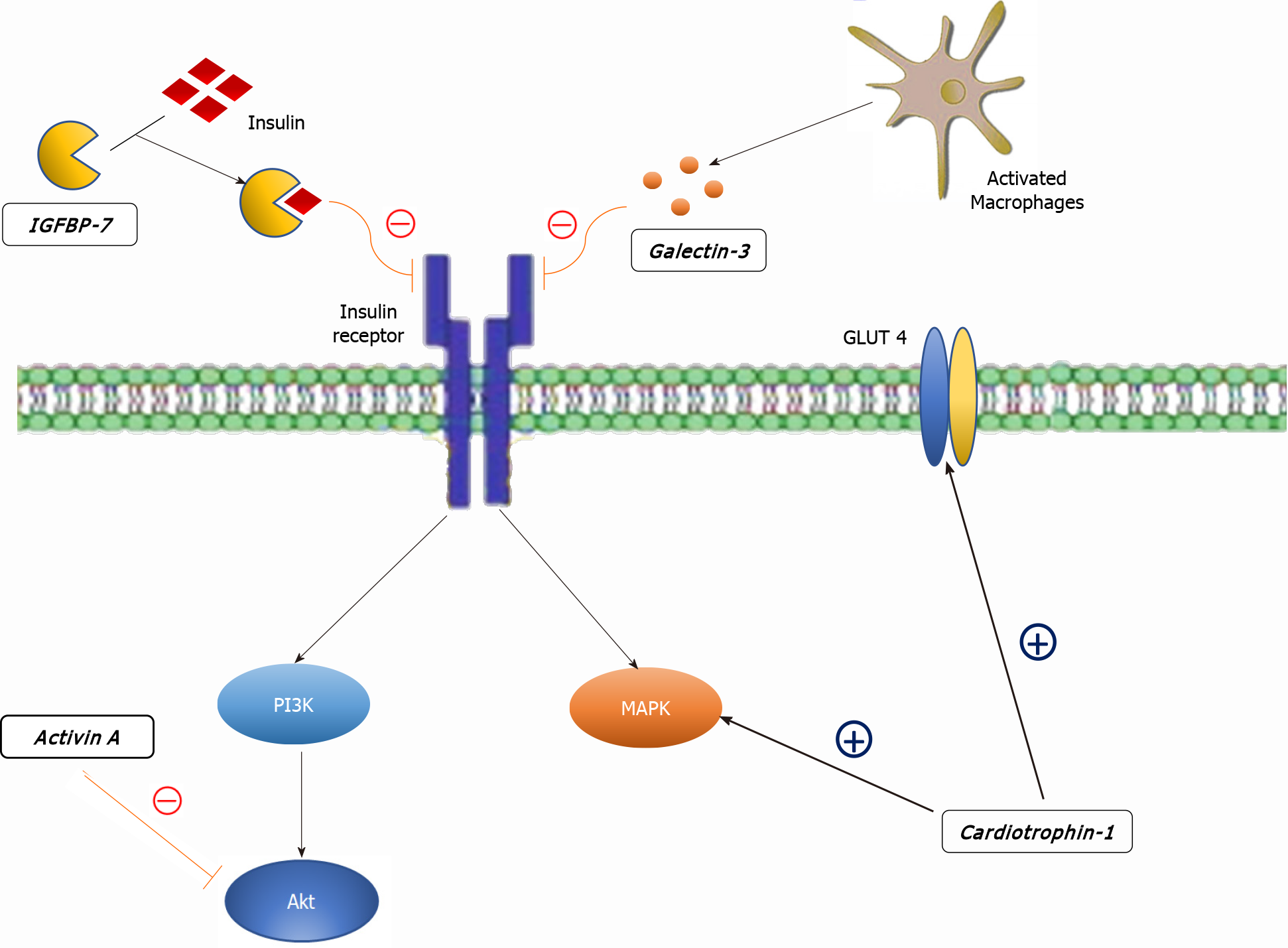
Cardiotrophin-1 (CT-1), a member of the glycoprotein 130 family, is a potent inducer of cardiomyocyte hypertrophy in vitro[74]. CT-1 secretion is stimulated by various triggers: mechanical stretch of cardiomyocytes, hypoxic stress, ROS, At II, aldosterone, urocortin, glucose, insulin and fibroblast growth factor-2[75-82]. Triggered by any of the above-mentioned, CT-1 modulates myocardial contractility, fibrosis and cardiac conduction via activation of the JAK/STAT and MAPK pathways (Figure 2)[83,84]. Apart from its effects on heart remodeling, CT-1 also takes part in cardiac glucose metabolism by increasing insulin-stimulated glucose uptake[85,86]. In line with this, plasma CT-1 levels are positively correlated with basal glycemia and left ventricular hypertrophy[87]. Other studies showed elevated plasma levels of CT-1 in recently diagnosed diabetics and neonates exposed to maternal diabetes[88], but interestingly, reduced levels in obese non-diabetics[89,90]. Moreover, low CT-1 plasma levels seem to be associated with decreased risk of both metabolic syndrome and DM type 2 in obese subjects[91]. Although CT-1 is to a great extent implicated in DCM, there are two major setbacks that prevent CT-1 implementation in the DCM diagnostic algorithm[92]. Firstly, CT-1 is also expressed by various tissues such as liver, lung, kidney and skeletal muscle[93]. Secondly, CT-1 plasma level alterations are also associated with other types of cardiomyopathies, including ischemic, making it less specific[84].
Insulin-like growth factor binding protein 7 (IGFBP7) is a part of the IGFBP superfamily of homogenous proteins which regulate the IGF signaling pathway by binding with insulin and IGFs[94]. Unlike IGFBP 1-6, IGFBP7 has low binding affinity to IGF but high affinity to insulin[95]. Owing to its high binding affinity to insulin, IGFB7 may interfere with the biological response of insulin, subsequently inducing insulin resistance and is involved in the development of diabetes, as shown by multiple studies (Figure 2)[96,97]. Apart from its role in insulin signaling, IGFBP7 is associated with multiple processes including fibrogenesis and tumor development [98,99]. IGFBP7 has also been implicated in HF where it serves as a novel prognostic biomarker for heart failure with reduced ejection fraction and shows a significant correlation with the presence and severity of the echocardiographic parameters of abnormal diastolic function[100]. In a recent study, the potential of IGFBP7 in improving the diagnosis of acute HF has been highlighted[101]. The most important evidence of IGFBP7 utility in the setting of DCM was provided by Shaver et al[102] who tested the potential of various serum biomarkers in a West Virginian population. The authors compared plasma levels between controls and diabetics (DM group), but more importantly, between diabetics with diastolic dysfunction (DM, DD+ group) and diabetics without diastolic dysfunction (DM, DD- group). IGFBP7 plasma levels were significantly higher in the DM, DD+ group in comparison to the DM, DD- group. Given their role in insulin resistance, fibrogenesis, HF development and the results presented by Shaver et al[102], we argue that further research of IGFBP7 in this manner is valuable as it could be a candidate for early detection of DCM.
Another important finding by Shaver et al[102] is in regards to transforming growth factor-β (TGF-β), a ubiquitous fibrogenic cytokine that promotes extracellular matrix accumulation[103]. As a result of increased ROS production, TGF-β is up-regulated in patients with diabetes[104]. Additionally, TGF-β correlates with the degree of cardiac fibrosis[105]. Of note, although most of the TGF-β-induced cardiac fibrosis is exerted by modulating the fibroblast phenotype and function[106], an additional mechanism that may contribute to fibrosis is TGF-β-mediated induction of EndoMT[107,108], a deleterious process implicated in HFpEF pathophysiology[23]. Shaver et al[102] reported higher plasma levels of TGF-β in patients with both DM and DD in comparison to the other two groups, respectively. Therefore, TGF-β could serve as a marker in DCM management. This is in line with previous studies conducted on this topic. By using FT23, an orally active anti-fibrotic compound, Tan et al[109] success
Activin A, a protein secreted by epicardial adipose tissue (EAT) is another member of the TGF-β superfamily that seems to be involved in the development of DCM[112]. Greulich et al[113] demonstrated that excessive activation of Activin A-signaling results in contractile dysfunction and insulin resistance in high fat diet fed guinea pigs. The underlying mechanism seems to be inhibition of insulin-mediated phosphory
Considering the importance of ROS overproduction in DCM pathophysiology and the well-known ROS-induced inflammatory response, multiple authors have tested the potential of inflammatory markers in this setting. A recent study on core gene biomarkers in patients with DCM addressed the vital role of interleukin-6 in DCM pathophysiology[122]. Furthermore, Shaver et al[102] found that both interleukin-6 and tumor necrosis factor-alpha are more increased in patients with both DM and DD in contrast to patients with DM exclusively. Nevertheless, owing to the low specificity of the two, it seems that growth differentiation factor-15 (GDF-15), another inflammatory marker, has a much better chance of being implemented in DCM diagnosis [123]. GDF-15, another member of the TGF-β superfamily is produced in response to oxidative stress and inflammation by multiple cell types, including macrophages, adipocytes, and cardiovascular cells[123]. Elevated plasma levels of GDF-15 seem to be associated with increased risk in fatal and non-fatal cardiovascular events of community-dwelling subjects and patients with cardiovascular disease, as shown by multiple studies[125-127]. Interestingly, in these studies GDF-15 levels were higher among patients with established DM type 2. Additionally, several studies addressed the contribution of GDF-15 in diastolic dysfunction[128,129]. As demon
Galectin-3 is a lectin family protein that has been associated with fibrosis and inflammation in cardiac, kidney and liver diseases[134,135]. Galectin-3 levels correlate with accumulation of AGE, oxidative stress products and pro-apoptotic pathways which directly promote endothelial dysfunction[136,137]. Perhaps the most important role of galectin-3 is its role in HF, where galectin-3 is an important mediator by which multiple molecules, such as At II and aldosterone, exert their pro-fibrotic activity and where it is able to promote oxidative stress with well-known repercussions[138-143]. The first evidence to support these findings were provided by Sharma et al[144] in a study which demonstrated that galectin-3 was the strongest differentially regulated gene associated with HF. Subsequently, a number of authors produced abundant evidence that successfully associated galectin-3 with HF in both animal models and in human studies, leading to the Food and Drug Administration approval of galectin-3 as a novel biomarker for predicting cardiovascular adverse events in 2010[145-149]. It is important to note that inhibition of galectin-3 could be an important target molecule in the HF therapeutic approach, based on its potential to undermine cardiac fibrosis and mitigate poor outcomes of HF. Multiple studies have highlighted the link between DM type 2 and galectin-3. The Dallas Heart Study associated galectin-3 with diabetes prevalence and incidence even after adjustment for conventional metabolic and cardiovascular risk factors[150]. Furthermore, in young obese patients without known cardiovascular disease, galectin-3 is associated with the presence of left ventricular diastolic dysfunction and elevated pulmonary artery systolic pressure, indicating its possible role in screening for preclinical metabolic heart disease[151]. On the other hand, in patients with HF, galectin-3 plasma levels were higher among those with impaired glucose metabolism (Figure 2)[152]. Finally, the possible role of galectin-3 in the DCM diagnostic approach was evaluated in a recent study by Flores-Ramírez et al[153]. The study showed that galectin-3 is elevated in diabetic patients with mild depressed ejection fraction and is associated with a diminished global longitudinal strain, an easy and reproducible echocardiographic tool in the evaluation and follow-up of DCM[154].
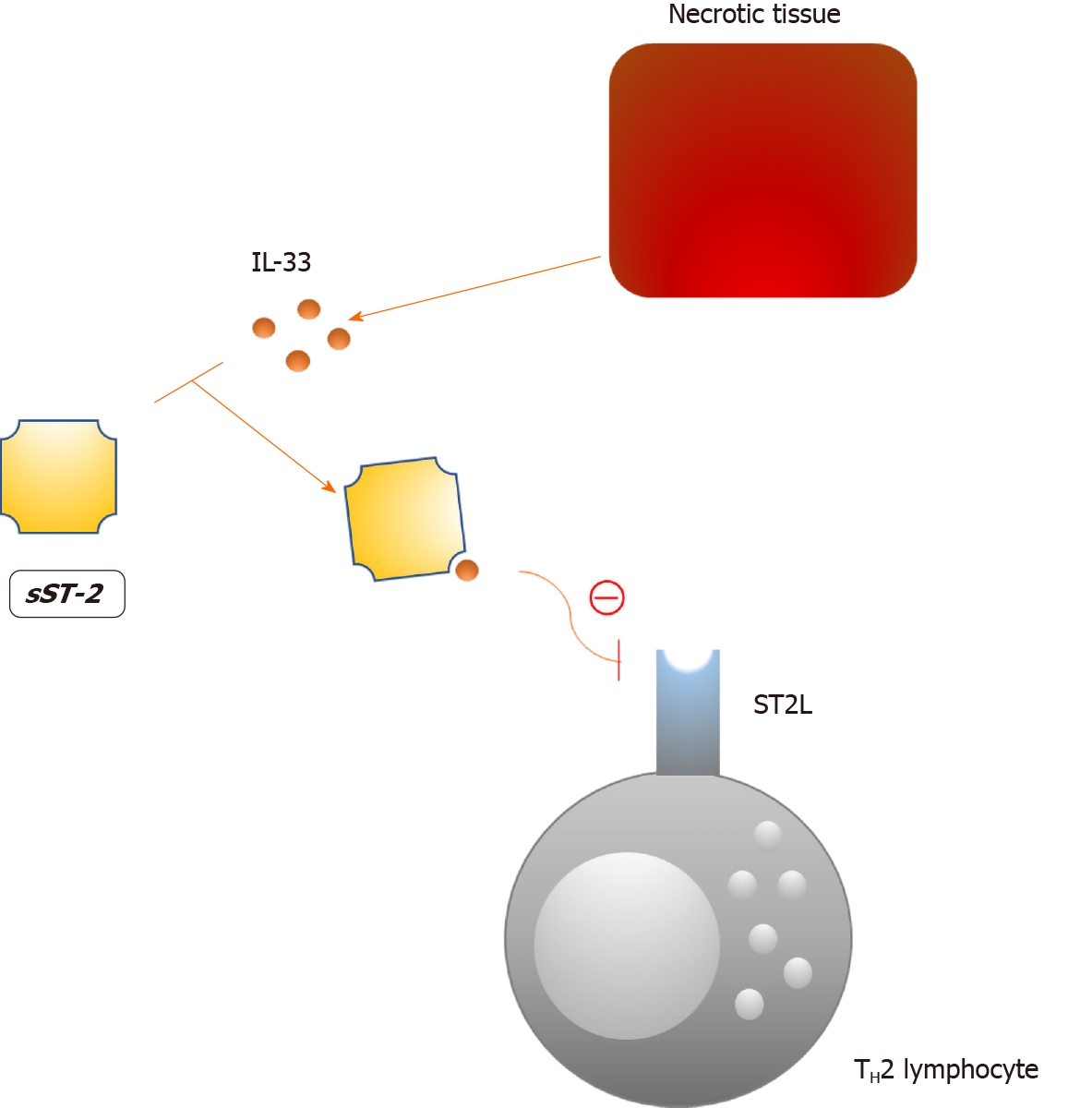
The soluble form of suppression of tumorigenicity 2 (sST2) is a interleukin-33 (IL-33) decoy receptor that tones down the Th2 inflammatory response via the IL-33/ST2/sST2 axis (Figure 3)[155]. Consequently, the protective effects of IL-33 in atherosclerosis and cardiac remodeling are mitigated, as this axis is an important component of the autocrine/paracrine mechanism that prevents tissue injury[156,157]. Increased plasma concentrations of sST2 are not specific for a single disorder in humans which undermines its value as a biomarker[158]. However, increased plasma levels of sST2 have been linked to a worse prognosis in numerous diseases, the most important being HF[159-162]. In line with this, sST2 is now included in the 2017 ACCF/AHA guidelines for additive risk stratification of patients with acute and chronic HF[163]. In the case of diabetes, Fousteris et al[164] demonstrated higher plasma concentrations of sST2 among patients with DM type 2 in comparison to healthy controls. More importantly, authors observed even higher levels of sST2 in patients with both DM type 2 and grade I left ventricular diastolic dysfunction, an early finding in DCM[165]. The presented data suggest a possible association between sST2 and the early stages of DCM; however, a larger body of evidence is needed to support these findings.
Long noncoding RNAs (lncRNAs) are a diverse subgroup of noncoding RNAs comprised of sequences longer than 200 nucleotides that act as epigenetic regulators of gene expression[166]. There is a large body of evidence indicating that lncRNAs are implicated in cardiac development, function and diseases[167,168]. Recent studies suggest that circulating lncRNAs could serve as diagnostic and prognostic biomarkers of cardiac remodeling and survival in cardiovascular diseases[169,170]. Both in vitro and in vivo studies showed that lncRNAs are involved in the pathophysiology of diabetes and its complications[171-173]. The most important study that addressed the potential of multiple lncRNAs as early DCM biomarkers was conducted by de Gonzalo-Calvo et al[174]. These authors compared a panel of lncRNAs that are directly involved in either diabetic conditions or cardiovascular disease and attempted to determine their relationship with MRI indices of cardiac dimensions and function. Long intergenic non-coding RNA predicting cardiac remodeling (LIPCAR) was inversely associated with E/A peak flow, an established indicator of diastolic dysfunction. In addition, LIPCAR serum levels positively correlated with grade I diastolic dysfunction. However, although LIPCAR was also correlated with waist circumference, plasma fasting insulin, subcutaneous fat volume and HDL-C, which could seemingly undermine LIPCAR value as a specific biomarker of cardiac impairment, the observed correlation with cardiac dysfunction was independent of the aforementioned. On the other hand, smooth muscle and endothelial cell-enriched migration/differentiation-associated long noncoding RNA (SENCR) and myocardial infarction-associated transcript (MIAT) lncRNAs serum levels were both associated with left ventricular mass to volume ratio, a marker of cardiac remodeling, even after adjustment for possible confounding factors. Notably, the highest left ventricular mass to volume ratios were observed in patients with the highest MIAT and SENCR expression. It is also important to point out that neither SENCR nor MIAT levels correlated with other clinical, biochemical, or metabolic parameters, which supports the hypothesized utility of these lncRNAs as biomarkers of left ventricular remodeling.
MicroRNAs are small noncoding RNA molecules which regulate gene expression by post-transcriptional mechanisms[175]. These molecules control around 30% of all protein-coding genes of the mammalian genome[176]. Additionally, microRNAs are also paracrine mediators of cell-to-cell communication transported via exosomes, a mechanism which has lately become an emerging research field for understanding the development of cardiac pathology[177]. The release of circulating exosomes filled with microRNA in the bloodstream from cardiomyocytes, driven by oxidative stress or hypoxia/reoxygenation, as well as stable microRNA-protein complex transport, makes microRNA an attractive target for analytical studies[178-182]. Recent pre-clinical level studies identified several distinct microRNAs which have been involved in DCM pathophysiology. Among many, we highlighted those we thought most suitable for DCM diagnosis based on their pathophysiologic role in DCM: microRNA-223 which regulates Glut4 receptor expression and cardiomyocyte glucose uptake and microRNA-133a which is implicated in cardiac hypertrophy and myocardial matrix remodeling[183-185]. Despite their potential, there are currently no ongoing clinical trials regarding the role of microRNAs in this manner. Perhaps the biggest setback in using microRNAs as markers is discordance between human and animal serum microRNAs associated with DCM[186]. The only exceptions are microRNA-34a, a regulator of high glucose-induced apoptosis and microRNA-30d, a molecule involved in the process of cardiomyocyte pyroptosis[187,188].
Despite substantial efforts to establish appropriate diagnostic biomarkers of DCM, this entity is not even diagnosed among clinicians, mainly due to the absence of agreement among experts[4]. Hence, new strategies must be applied in order to ameliorate poor outcomes of diabetes-related HF. In an ideal setting, DCM would be recognized in the early asymptomatic phase, before irreversible myocardial damage occurs. Different imaging approaches such as Phase-MRI, Speckle tracking echocardiography (STE) and nuclear imaging have been successful in the recognition of early metabolic myocardial changes in both animal and human studies[189-194]. However, most of these are limited by price and availability, whereas STE, although promising, can have reduced accuracy in irregular ventricular remodeling and wall thinning[6]. Importantly, global longitudinal strain, an echocardiographic measurement, seems to be more impaired in DM vs healthy controls whereas among diabetics, it is more impaired in patients with albuminuria in comparison to patients without it[195]. In addition, patients with uncomplicated DM type II show a similar time-dependent pattern of global longitudinal strain change, altogether indicating subclinical systolic dysfunction in patients with diabetes that is associated with duration and extent of the disease[196]. Of the aforementioned biomarkers, we believe that lncRNA, sST2 and galectin-3 will be the most beneficial for DCM detection, as their plasma/serum levels accurately correlate with the early stages of DCM.
In addition, there are several molecules which are rarely debated in this manner and which we find valuable for further research based on their functional properties. Catestatin, a pleiotropic cardioprotective peptide that counterbalances the negative effects of the sympathetic nervous system, is implicated in both the metabolic syndrome and HF[197]. Specifically, alongside sST2, our recent study suggested that catestatin plasma levels reflect myocardial fibrosis and sympathetic overactivity during the acute worsening of HF[198]. With regard to diabetes, catestatin has been shown to increase glucose uptake and up-regulate GLUT4 plasma expression in rat cardiomyocytes[199], as well as improve insulin sensitivity in mice with diet-induced obesity[200].
To sum up, further research is needed to improve DCM approach strategies. The combination of relatively inexpensive and accurate STE with some of the highlighted biomarkers seems promising (Table 1); however, well-designed studies with long-term follow-up and validation are obligatory for implementation in everyday clinical practice. With the exception of “What to test?”, rather more important questions are “When and whom to test”. Given that DCM affects around 12% of diabetics, we need a predictive scoring system to establish that a patient is at risk of DCM development, as they all are. Thus, screening methods should be applied for all newly-diagnosed type 2 DM patients. In DM type 1, due to the discrepancy in certain pathophysiological aspects in respect to DM type 2, further research is needed to reach proper conclusions[201,202]. With regard to “When to test?”, as DCM progression deteriorates heart function stepwise and as new therapeutic strategies that specifically target early phase mechanisms emerge, it will be vital to detect DCM as soon as possible. Finally, we argue that an effort must be made to create an easy and reproducible algorithm which will, by using a combination of STE and biomarkers, direct affected patients to confirmation tests such as Phase-MRI. Consequently, in patients with validated DCM, new specific therapies that target early phase mechanisms could be applied. This type of approach is needed to stratify patients because most of the new therapies will be very costly.
| Biomarker | Pathophysiological pathway | Supporting evidence |
| LncRNA (LIPCAR, MIAT, SENCR) | Epigenetic regulation of multiple genes involved in diabetes and cardiac dysfunction | Liu et al[171]; Yan et al[172]; Carter et al[173]; de Gonzalo-Calvo et al[174] |
| sST-2 | IL-33 decoy receptor that tones down Th2 inflammatory response via the IL-33/ST2/sST2 axis | Fousteris et al[164]; Kiencke et al[165] |
| TGF-β | The main pro-fibrotic factor in heart failure: it modulates the fibroblast phenotype and function and mediates induction of EndoMT | Shaver et al[102]; Iglesias-De La Cruz et al[104]; Asbun et al[105] |
| Galectin-3 | Mediator by which multiple molecules (e.g. angiotensin II and aldosterone) exert their pro-fibrotic activity and promote oxidative stress | Ho et al[146]; Ueland et al[147]; Sharma et al[148] |
| GDF-15 | Regulator of inflammatory pathways involved in regulation of apoptosis, cell repair and cell growth | Berezin[123]; Dominguez-Rodriguez et al[130] |
The figures were kindly provided by Zrinka Miocic, M.Arch.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shen XC, Zhang DM S-Editor: Zhang L L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1267] [Article Influence: 23.9] [Reference Citation Analysis (1)] |
| 2. | Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1457] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 3. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-e239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4116] [Cited by in RCA: 4655] [Article Influence: 387.9] [Reference Citation Analysis (1)] |
| 4. | Lee MMY, McMurray JJV, Lorenzo-Almorós A, Kristensen SL, Sattar N, Jhund PS, Petrie MC. Diabetic cardiomyopathy. Heart. 2019;105:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10348] [Cited by in RCA: 9360] [Article Influence: 1040.0] [Reference Citation Analysis (3)] |
| 6. | Berezin AE, Berezin AA. Circulating Cardiac Biomarkers in Diabetes Mellitus: A New Dawn for Risk Stratification-A Narrative Review. Diabetes Ther. 2020;11:1271-1291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Lorenzo-Almorós A, Tuñón J, Orejas M, Cortés M, Egido J, Lorenzo Ó. Diagnostic approaches for diabetic cardiomyopathy. Cardiovasc Diabetol. 2017;16:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 8. | Tao Z, Shi A, Zhao J. Epidemiological Perspectives of Diabetes. Cell Biochem Biophys. 2015;73:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 9. | Greenberg BH, Abraham WT, Albert NM, Chiswell K, Clare R, Stough WG, Gheorghiade M, O'Connor CM, Sun JL, Yancy CW, Young JB, Fonarow GC. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 2007; 154: 277.e1-277. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 586] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 11. | Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, Deswal A, Dickson VV, Kosiborod MN, Lekavich CL, McCoy RG, Mentz RJ, Piña IL; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and the Heart Failure Society of America. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140:e294-e324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 391] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 12. | Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res. 2018;122:624-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1248] [Article Influence: 208.0] [Reference Citation Analysis (0)] |
| 13. | Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 684] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 14. | Dhalla NS, Pierce GN, Innes IR, Beamish RE. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol. 1985;1:263-281. [PubMed] |
| 15. | Jia G, Habibi J, Bostick BP, Ma L, DeMarco VG, Aroor AR, Hayden MR, Whaley-Connell AT, Sowers JR. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension. 2015;65:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Factor SM, Minase T, Cho S, Fein F, Capasso JM, Sonnenblick EH. Coronary microvascular abnormalities in the hypertensive-diabetic rat. A primary cause of cardiomyopathy? Am J Pathol. 1984;116:9-20. [PubMed] |
| 17. | Olesen P, Nguyen K, Wogensen L, Ledet T, Rasmussen LM. Calcification of human vascular smooth muscle cells: associations with osteoprotegerin expression and acceleration by high-dose insulin. Am J Physiol Heart Circ Physiol. 2007;292:H1058-H1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Sharma A, Sellers S, Stefanovic N, Leung C, Tan SM, Huet O, Granville DJ, Cooper ME, de Haan JB, Bernatchez P. Direct Endothelial Nitric Oxide Synthase Activation Provides Atheroprotection in Diabetes-Accelerated Atherosclerosis. Diabetes. 2015;64:3937-3950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, Demarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 2010;55:880-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Hayden MR, Habibi J, Joginpally T, Karuparthi PR, Sowers JR. Ultrastructure Study of Transgenic Ren2 Rat Aorta - Part 1: Endothelium and Intima. Cardiorenal Med. 2012;2:66-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Li Q, Yao Y, Shi S, Zhou M, Zhou Y, Wang M, Chiu JJ, Huang Z, Zhang W, Liu M, Wang Q, Tu X. Inhibition of miR-21 alleviated cardiac perivascular fibrosis via repressing EndMT in T1DM. J Cell Mol Med. 2020;24:910-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, Yagi K, Miyagawa K, Rikitake Y, Suzuki T, Kisanuki YY, Yanagisawa M, Hirata K. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 23. | Elgendy IY, Pepine CJ. Heart Failure With Preserved Ejection Fraction: Is Ischemia Due to Coronary Microvascular Dysfunction a Mechanistic Factor? Am J Med. 2019;132:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Thackeray JT, Beanlands RS, Dasilva JN. Altered sympathetic nervous system signaling in the diabetic heart: emerging targets for molecular imaging. Am J Nucl Med Mol Imaging. 2012;2:314-334. [PubMed] |
| 25. | Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, Roden R, Asano K, Blaxall BC, Wu SC, Communal C, Singh K, Colucci W, Bristow MR, Port DJ. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol. 2000;32:817-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 185] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Stevens MJ, Dayanikli F, Raffel DM, Allman KC, Sandford T, Feldman EL, Wieland DM, Corbett J, Schwaiger M. Scintigraphic assessment of regionalized defects in myocardial sympathetic innervation and blood flow regulation in diabetic patients with autonomic neuropathy. J Am Coll Cardiol. 1998;31:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Lee MH, Song HK, Ko GJ, Kang YS, Han SY, Han KH, Kim HK, Han JY, Cha DR. Angiotensin receptor blockers improve insulin resistance in type 2 diabetic rats by modulating adipose tissue. Kidney Int. 2008;74:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2726] [Cited by in RCA: 3088] [Article Influence: 162.5] [Reference Citation Analysis (0)] |
| 29. | Zhou MS, Schulman IH, Zeng Q. Link between the renin-angiotensin system and insulin resistance: implications for cardiovascular disease. Vasc Med. 2012;17:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res. 2004;94:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137-35146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | Tirosh A, Garg R, Adler GK. Mineralocorticoid receptor antagonists and the metabolic syndrome. Curr Hypertens Rep. 2010;12:252-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Nigro J, Osman N, Dart AM, Little PJ. Insulin resistance and atherosclerosis. Endocr Rev. 2006;27:242-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 34. | Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 361] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 35. | Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. 2017;113:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 36. | Yu Q, Vazquez R, Zabadi S, Watson RR, Larson DF. T-lymphocytes mediate left ventricular fibrillar collagen cross-linking and diastolic dysfunction in mice. Matrix Biol. 2010;29:511-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Cao Y, Xu W, Xiong S. Adoptive transfer of regulatory T cells protects against Coxsackievirus B3-induced cardiac fibrosis. PLoS One. 2013;8:e74955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Schaffer SW. Cardiomyopathy associated with noninsulin-dependent diabetes. Mol Cell Biochem. 1991;107:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Nagoshi T, Yoshimura M, Rosano GM, Lopaschuk GD, Mochizuki S. Optimization of cardiac metabolism in heart failure. Curr Pharm Des. 2011;17:3846-3853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Tuunanen H, Knuuti J. Metabolic remodelling in human heart failure. Cardiovasc Res. 2011;90:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Bando YK, Murohara T. Diabetes-related heart failure. Circ J. 2014;78:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Thomas MC, Iyngkaran P. Forensic interrogation of diabetic endothelitis in cardiovascular diseases and clinical translation in heart failure. World J Cardiol. 2020;12:409-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Joubert M, Jagu B, Montaigne D, Marechal X, Tesse A, Ayer A, Dollet L, Le May C, Toumaniantz G, Manrique A, Charpentier F, Staels B, Magré J, Cariou B, Prieur X. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Cardiomyopathy in a Diabetic Lipodystrophic Mouse Model. Diabetes. 2017;66:1030-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 44. | Arow M, Waldman M, Yadin D, Nudelman V, Shainberg A, Abraham NG, Freimark D, Kornowski R, Aravot D, Hochhauser E, Arad M. Sodium-glucose cotransporter 2 inhibitor Dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc Diabetol. 2020;19:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 45. | Alkagiet S, Tziomalos K. Role of sodium-glucose co-transporter-2 inhibitors in the management of heart failure in patients with diabetes mellitus. World J Diabetes. 2020;11:150-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Zhao J, Randive R, Stewart JA. Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J Diabetes. 2014;5:860-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 47. | Wendt T, Bucciarelli L, Qu W, Lu Y, Yan SF, Stern DM, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep. 2002;4:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Dhalla NS, Liu X, Panagia V, Takeda N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res. 1998;40:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res. 2016;109:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 50. | Falcão-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012;17:325-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 51. | Bostick B, Habibi J, Ma L, Aroor A, Rehmer N, Hayden MR, Sowers JR. Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity. Metabolism. 2014;63:1000-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 52. | Bostick B, Habibi J, DeMarco VG, Jia G, Domeier TL, Lambert MD, Aroor AR, Nistala R, Bender SB, Garro M, Hayden MR, Ma L, Manrique C, Sowers JR. Mineralocorticoid receptor blockade prevents Western diet-induced diastolic dysfunction in female mice. Am J Physiol Heart Circ Physiol. 2015;308:H1126-H1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Westermeier F, Riquelme JA, Pavez M, Garrido V, Díaz A, Verdejo HE, Castro PF, García L, Lavandero S. New Molecular Insights of Insulin in Diabetic Cardiomyopathy. Front Physiol. 2016;7:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 54. | Ernande L, Derumeaux G. Diabetic cardiomyopathy: myth or reality? Arch Cardiovasc Dis. 2012;105:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Harper AR, Patel HC, Lyon AR. Heart failure with preserved ejection fraction. Clin Med (Lond). 2018;18:s24-s29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Borghetti G, von Lewinski D, Eaton DM, Sourij H, Houser SR, Wallner M. Diabetic Cardiomyopathy: Current and Future Therapies. Beyond Glycemic Control. Front Physiol. 2018;9:1514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 57. | Inoue Y, Kawai M, Minai K, Ogawa K, Nagoshi T, Ogawa T, Yoshimura M. The impact of an inverse correlation between plasma B-type natriuretic peptide levels and insulin resistance on the diabetic condition in patients with heart failure. Metabolism. 2016;65:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Howarth FC, Al-Shamsi N, Al-Qaydi M, Al-Mazrouei M, Qureshi A, Chandranath SI, Kazzam E, Adem A. Effects of brain natriuretic peptide on contraction and intracellular Ca2+ in ventricular myocytes from the streptozotocin-induced diabetic rat. Ann N Y Acad Sci. 2006;1084:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Howarth FC, Adem A, Adeghate EA, Al Ali NA, Al Bastaki AM, Sorour FR, Hammoudi RO, Ghaleb NA, Chandler NJ, Dobrzynski H. Distribution of atrial natriuretic peptide and its effects on contraction and intracellular calcium in ventricular myocytes from streptozotocin-induced diabetic rat. Peptides. 2005;26:691-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Dahlström U. Can natriuretic peptides be used for the diagnosis of diastolic heart failure? Eur J Heart Fail. 2004;6:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Betti I, Castelli G, Barchielli A, Beligni C, Boscherini V, De Luca L, Messeri G, Gheorghiade M, Maisel A, Zuppiroli A. The role of N-terminal PRO-brain natriuretic peptide and echocardiography for screening asymptomatic left ventricular dysfunction in a population at high risk for heart failure. The PROBE-HF study. J Card Fail. 2009;15:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Dencker M, Stagmo M, Dorkhan M. Relationship between natriuretic peptides and echocardiography parameters in patients with poorly regulated type 2 diabetes. Vasc Health Risk Manag. 2010;6:373-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Valle R, Bagolin E, Canali C, Giovinazzo P, Barro S, Aspromonte N, Carbonieri E, Milani L. The BNP assay does not identify mild left ventricular diastolic dysfunction in asymptomatic diabetic patients. Eur J Echocardiogr. 2006;7:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Brunner-La Rocca HP, Sanders-van Wijk S. Natriuretic Peptides in Chronic Heart Failure. Card Fail Rev. 2019;5:44-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 65. | Romano S, Di Mauro M, Fratini S, Guarracini L, Guarracini F, Poccia G, Penco M. Early diagnosis of left ventricular diastolic dysfunction in diabetic patients: a possible role for natriuretic peptides. Cardiovasc Diabetol. 2010;9:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Rahimi K, Bennett D, Conrad N, Williams TM, Basu J, Dwight J, Woodward M, Patel A, McMurray J, MacMahon S. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail. 2014;2:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 67. | Gomes AV, Potter JD, Szczesna-Cordary D. The role of troponins in muscle contraction. IUBMB Life. 2002;54:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Russell NE, Higgins MF, Amaruso M, Foley M, McAuliffe FM. Troponin T and pro-B-type natriuretic Peptide in fetuses of type 1 diabetic mothers. Diabetes Care. 2009;32:2050-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Malhotra A, Kang BP, Cheung S, Opawumi D, Meggs LG. Angiotensin II promotes glucose-induced activation of cardiac protein kinase C isozymes and phosphorylation of troponin I. Diabetes. 2001;50:1918-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Hange-Dickerson PA. Oncology nurse practitioner provides continuity of care. Nurse Pract. 1992;17:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Malhotra A, Reich D, Nakouzi A, Sanghi V, Geenen DL, Buttrick PM. Experimental diabetes is associated with functional activation of protein kinase C epsilon and phosphorylation of troponin I in the heart, which are prevented by angiotensin II receptor blockade. Circ Res. 1997;81:1027-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Eggers KM, Al-Shakarchi J, Berglund L, Lindahl B, Siegbahn A, Wallentin L, Zethelius B. High-sensitive cardiac troponin T and its relations to cardiovascular risk factors, morbidity, and mortality in elderly men. Am Heart J. 2013;166:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Segre CA, Hueb W, Garcia RM, Rezende PC, Favarato D, Strunz CM, Sprandel Mda C, Roggério A, Carvalho AL, Maranhão RC, Ramires JA, Kalil Filho R. Troponin in diabetic patients with and without chronic coronary artery disease. BMC Cardiovasc Disord. 2015;15:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Lopez-Andres N, Fortuno MA, Diez J, Zannad F, Lacolley P, Rossignol P. Vascular effects of cardiotrophin-1: a role in hypertension? J Hypertens. 2010;28:1261-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Hishinuma S, Funamoto M, Fujio Y, Kunisada K, Yamauchi-Takihara K. Hypoxic stress induces cardiotrophin-1 expression in cardiac myocytes. Biochem Biophys Res Commun. 1999;264:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Robador PA, San José G, Rodríguez C, Guadall A, Moreno MU, Beaumont J, Fortuño A, Díez J, Martínez-González J, Zalba G. HIF-1-mediated up-regulation of cardiotrophin-1 is involved in the survival response of cardiomyocytes to hypoxia. Cardiovasc Res. 2011;92:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Ateghang B, Wartenberg M, Gassmann M, Sauer H. Regulation of cardiotrophin-1 expression in mouse embryonic stem cells by HIF-1alpha and intracellular reactive oxygen species. J Cell Sci. 2006;119:1043-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Fukuzawa J, Booz GW, Hunt RA, Shimizu N, Karoor V, Baker KM, Dostal DE. Cardiotrophin-1 increases angiotensinogen mRNA in rat cardiac myocytes through STAT3: an autocrine loop for hypertrophy. Hypertension. 2000;35:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | López-Andrés N, Iñigo C, Gallego I, Díez J, Fortuño MA. Aldosterone induces cardiotrophin-1 expression in HL-1 adult cardiomyocytes. Endocrinology. 2008;149:4970-4978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Janjua S, Lawrence KM, Ng LL, Latchman DS. The cardioprotective agent urocortin induces expression of CT-1. Cardiovasc Toxicol. 2003;3:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 81. | Liu J, Liu Z, Huang F, Xing Z, Wang H, Li Z. Pioglitazone inhibits hypertrophy induced by high glucose and insulin in cultured neonatal rat cardiomyocytes. Pharmazie. 2007;62:925-929. [PubMed] |
| 82. | Jiang ZS, Jeyaraman M, Wen GB, Fandrich RR, Dixon IM, Cattini PA, Kardami E. High- but not low-molecular weight FGF-2 causes cardiac hypertrophy in vivo; possible involvement of cardiotrophin-1. J Mol Cell Cardiol. 2007;42:222-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Ruiz-Hurtado G, Gómez-Hurtado N, Fernández-Velasco M, Calderón E, Smani T, Ordoñez A, Cachofeiro V, Boscá L, Díez J, Gómez AM, Delgado C. Cardiotrophin-1 induces sarcoplasmic reticulum Ca(2+) leak and arrhythmogenesis in adult rat ventricular myocytes. Cardiovasc Res. 2012;96:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Jougasaki M. Cardiotrophin-1 in cardiovascular regulation. Adv Clin Chem. 2010;52:41-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | García-Cenador MB, Lopez-Novoa JM, Díez J, García-Criado FJ. Effects and mechanism of organ protection by cardiotrophin-1. Curr Med Chem. 2013;20:246-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Moreno-Aliaga MJ, Romero-Lozano MA, Castaño D, Prieto J, Bustos M. Role of cardiotrophin-1 in obesity and insulin resistance. Adipocyte. 2012;1:112-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Gamella-Pozuelo L, Fuentes-Calvo I, Gómez-Marcos MA, Recio-Rodriguez JI, Agudo-Conde C, Fernández-Martín JL, Cannata-Andía JB, López-Novoa JM, García-Ortiz L, Martínez-Salgado C. Plasma Cardiotrophin-1 as a Marker of Hypertension and Diabetes-Induced Target Organ Damage and Cardiovascular Risk. Medicine (Baltimore). 2015;94:e1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Briana DD, Germanou K, Boutsikou M, Boutsikou T, Athanasopoulos N, Marmarinos A, Gourgiotis D, Malamitsi-Puchner A. Potential prognostic biomarkers of cardiovascular disease in fetal macrosomia: the impact of gestational diabetes. J Matern Fetal Neonatal Med. 2018;31:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | van den Brom CE, Huisman MC, Vlasblom R, Boontje NM, Duijst S, Lubberink M, Molthoff CF, Lammertsma AA, van der Velden J, Boer C, Ouwens DM, Diamant M. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovasc Diabetol. 2009;8:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 90. | Hung HC, Lu FH, Ou HY, Wu HT, Wu JS, Yang YC, Chang CJ. Increased cardiotrophin-1 in subjects with impaired glucose tolerance and newly diagnosed diabetes. Int J Cardiol. 2013;169:e33-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Pennica D, King KL, Shaw KJ, Luis E, Rullamas J, Luoh SM, Darbonne WC, Knutzon DS, Yen R, Chien KR. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci USA. 1995;92:1142-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 404] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 92. | Rendo-Urteaga T, García-Calzón S, Martínez-Ansó E, Chueca M, Oyarzabal M, Azcona-Sanjulián MC, Bustos M, Moreno-Aliaga MJ, Martínez JA, Marti A. Decreased cardiotrophin-1 Levels are associated with a lower risk of developing the metabolic syndrome in overweight/obese children after a weight loss program. Metabolism. 2013;62:1429-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Gritman K, Van Winkle DM, Lorentz CU, Pennica D, Habecker BA. The lack of cardiotrophin-1 alters expression of interleukin-6 and leukemia inhibitory factor mRNA but does not impair cardiac injury response. Cytokine. 2006;36:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 285] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 95. | López-Bermejo A, Khosravi J, Fernández-Real JM, Hwa V, Pratt KL, Casamitjana R, Garcia-Gil MM, Rosenfeld RG, Ricart W. Insulin resistance is associated with increased serum concentration of IGF-binding protein-related protein 1 (IGFBP-rP1/MAC25). Diabetes. 2006;55:2333-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Kutsukake M, Ishihara R, Momose K, Isaka K, Itokazu O, Higuma C, Matsutani T, Matsuda A, Sasajima K, Hara T, Tamura K. Circulating IGF-binding protein 7 (IGFBP7) levels are elevated in patients with endometriosis or undergoing diabetic hemodialysis. Reprod Biol Endocrinol. 2008;6:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Liu Y, Wu M, Ling J, Cai L, Zhang D, Gu HF, Wang H, Zhu Y, Lai M. Serum IGFBP7 Levels associate with insulin resistance and the risk of metabolic syndrome in a Chinese population. Sci Rep. 2015;5:10227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 98. | Vizioli MG, Sensi M, Miranda C, Cleris L, Formelli F, Anania MC, Pierotti MA, Greco A. IGFBP7: an oncosuppressor gene in thyroid carcinogenesis. Oncogene. 2010;29:3835-3844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Guo XH, Liu LX, Zhang HY, Zhang QQ, Li Y, Tian XX, Qiu ZH. Insulin-like growth factor binding protein-related protein 1 contributes to hepatic fibrogenesis. J Dig Dis. 2014;15:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Gandhi PU, Gaggin HK, Sheftel AD, Belcher AM, Weiner RB, Baggish AL, Motiwala SR, Liu PP, Januzzi JL Jr. Prognostic usefulness of insulin-like growth factor-binding protein 7 in heart failure with reduced ejection fraction: a novel biomarker of myocardial diastolic function? Am J Cardiol. 2014;114:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 101. | Kalayci A, Peacock WF, Nagurney JT, Hollander JE, Levy PD, Singer AJ, Shapiro NI, Cheng RK, Cannon CM, Blomkalns AL, Walters EL, Christenson RH, Chen-Tournoux A, Nowak RM, Lurie MD, Pang PS, Kastner P, Masson S, Gibson CM, Gaggin HK, Januzzi JL Jr. Echocardiographic assessment of insulin-like growth factor binding protein-7 and early identification of acute heart failure. ESC Heart Fail. 2020;7:1664-1675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Shaver A, Nichols A, Thompson E, Mallick A, Payne K, Jones C, Manne ND, Sundaram S, Shapiro JI, Sodhi K. Role of Serum Biomarkers in Early Detection of Diabetic Cardiomyopathy in the West Virginian Population. Int J Med Sci. 2016;13:161-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 103. | Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA. 2000;97:8015-8020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 715] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 104. | Iglesias-De La Cruz MC, Ruiz-Torres P, Alcamí J, Díez-Marqués L, Ortega-Velázquez R, Chen S, Rodríguez-Puyol M, Ziyadeh FN, Rodríguez-Puyol D. Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells. Kidney Int. 2001;59:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 105. | Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 371] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 106. | Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 478] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 107. | Wermuth PJ, Carney KR, Mendoza FA, Piera-Velazquez S, Jimenez SA. Endothelial cell-specific activation of transforming growth factor-β signaling in mice induces cutaneous, visceral, and microvascular fibrosis. Lab Invest. 2017;97:806-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 108. | Pardali E, Sanchez-Duffhues G, Gomez-Puerto MC, Ten Dijke P. TGF-β-Induced Endothelial-Mesenchymal Transition in Fibrotic Diseases. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 109. | Tan SM, Zhang Y, Wang B, Tan CY, Zammit SC, Williams SJ, Krum H, Kelly DJ. FT23, an orally active antifibrotic compound, attenuates structural and functional abnormalities in an experimental model of diabetic cardiomyopathy. Clin Exp Pharmacol Physiol. 2012;39:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 110. | Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116:2127-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 299] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 111. | Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, Gonzalez-Quesada C, Rai V, Dobaczewski M, Lee DW, Wang XF, Frangogiannis NG. Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice. Circ Heart Fail. 2015;8:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 112. | Greulich S, Maxhera B, Vandenplas G, de Wiza DH, Smiris K, Mueller H, Heinrichs J, Blumensatt M, Cuvelier C, Akhyari P, Ruige JB, Ouwens DM, Eckel J. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation. 2012;126:2324-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 113. | Greulich S, de Wiza DH, Preilowski S, Ding Z, Mueller H, Langin D, Jaquet K, Ouwens DM, Eckel J. Secretory products of guinea pig epicardial fat induce insulin resistance and impair primary adult rat cardiomyocyte function. J Cell Mol Med. 2011;15:2399-2410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Ouwens DM, Diamant M. Myocardial insulin action and the contribution of insulin resistance to the pathogenesis of diabetic cardiomyopathy. Arch Physiol Biochem. 2007;113:76-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 115. | Blumensatt M, Greulich S, Herzfeld de Wiza D, Mueller H, Maxhera B, Rabelink MJ, Hoeben RC, Akhyari P, Al-Hasani H, Ruige JB, Ouwens DM. Activin A impairs insulin action in cardiomyocytes via up-regulation of miR-143. Cardiovasc Res. 2013;100:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Chen WJ, Greulich S, van der Meer RW, Rijzewijk LJ, Lamb HJ, de Roos A, Smit JW, Romijn JA, Ruige JB, Lammertsma AA, Lubberink M, Diamant M, Ouwens DM. Activin A is associated with impaired myocardial glucose metabolism and left ventricular remodeling in patients with uncomplicated type 2 diabetes. Cardiovasc Diabetol. 2013;12:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 117. | Yndestad A, Ueland T, Øie E, Florholmen G, Halvorsen B, Attramadal H, Simonsen S, Frøland SS, Gullestad L, Christensen G, Damås JK, Aukrust P. Elevated levels of activin A in heart failure: potential role in myocardial remodeling. Circulation. 2004;109:1379-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 118. | Ueland T, Aukrust P, Aakhus S, Smith C, Endresen K, Birkeland KI, Gullestad L, Johansen OE. Activin A and cardiovascular disease in type 2 diabetes mellitus. Diab Vasc Dis Res. 2012;9:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 119. | Weigert J, Neumeier M, Wanninger J, Schober F, Sporrer D, Weber M, Schramm A, Wurm S, Stögbauer F, Filarsky M, Schäffler A, Aslanidis C, Schölmerich J, Buechler C. Adiponectin upregulates monocytic activin A but systemic levels are not altered in obesity or type 2 diabetes. Cytokine. 2009;45:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 120. | Wu H, Wu M, Chen Y, Allan CA, Phillips DJ, Hedger MP. Correlation between blood activin levels and clinical parameters of type 2 diabetes. Exp Diabetes Res. 2012;2012:410579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 121. | Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clément K, Hatem SN. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2015;36:795-805a. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 429] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 122. | Li N, Wu H, Geng R, Tang Q. Identification of Core Gene Biomarkers in Patients with Diabetic Cardiomyopathy. Dis Markers. 2018;2018:6025061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 123. | Berezin AE. Diabetes mellitus related biomarker: The predictive role of growth-differentiation factor-15. Diabetes Metab Syndr. 2016;10:S154-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 124. | Dostálová I, Roubícek T, Bártlová M, Mráz M, Lacinová Z, Haluzíková D, Kaválková P, Matoulek M, Kasalicky M, Haluzík M. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol. 2009;161:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 125. | Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: the Rancho Bernardo Study. Circulation. 2011;123:2101-2110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 126. | Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, Olofsson S, Venge P, Larsson A, Hulthe J, Elmgren A, Wollert KC. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur Heart J. 2009;30:2346-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 127. | Schnabel RB, Schulz A, Messow CM, Lubos E, Wild PS, Zeller T, Sinning CR, Rupprecht HJ, Bickel C, Peetz D, Cambien F, Kempf T, Wollert KC, Benjamin EJ, Lackner KJ, Münzel TF, Tiret L, Vasan RS, Blankenberg S. Multiple marker approach to risk stratification in patients with stable coronary artery disease. Eur Heart J. 2010;31:3024-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 128. | Baessler A, Strack C, Rousseva E, Wagner F, Bruxmeier J, Schmiedel M, Riegger G, Lahmann C, Loew T, Schmitz G, Fischer M. Growth-differentiation factor-15 improves reclassification for the diagnosis of heart failure with normal ejection fraction in morbid obesity. Eur J Heart Fail. 2012;14:1240-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 129. | Stahrenberg R, Edelmann F, Mende M, Kockskämper A, Düngen HD, Lüers C, Binder L, Herrmann-Lingen C, Gelbrich G, Hasenfuss G, Pieske B, Wachter R. The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur J Heart Fail. 2010;12:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 130. | Dominguez-Rodriguez A, Abreu-Gonzalez P, Avanzas P. Usefulness of growth differentiation factor-15 Levels to predict diabetic cardiomyopathy in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2014;114:890-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 131. | Lu J, Zhang Y, Dong X, Lu J, Zhang C, Liu J, Yu Q, Teng H, Yao Q, Yin J, Qin L. Association between MIC-1 and Type 2 Diabetes: A Combined Analysis. Dis Markers. 2019;2019:7284691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 132. | Tang M, Luo M, Lu W, Wang S, Zhang R, Liang W, Gu J, Yu X, Zhang X, Hu C. Serum growth differentiation factor 15 is associated with glucose metabolism in the third trimester in Chinese pregnant women. Diabetes Res Clin Pract. 2019;156:107823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 133. | Tsai VWW, Husaini Y, Sainsbury A, Brown DA, Breit SN. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018;28:353-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 134. | Suthahar N, Meijers WC, Silljé HHW, Ho JE, Liu FT, de Boer RA. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics. 2018;8:593-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 135. | Tan KCB, Cheung CL, Lee ACH, Lam JKY, Wong Y, Shiu SWM. Galectin-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Diabetologia. 2018;61:1212-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 136. | Luís C, Costa R, Rodrigues I, Castela Â, Coelho P, Guerreiro S, Gomes J, Reis C, Soares R. Xanthohumol and 8-prenylnaringenin reduce type 2 diabetes-associated oxidative stress by downregulating galectin-3. Porto Biomed J. 2019;4:e23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 137. | Berezin A. Metabolic memory phenomenon in diabetes mellitus: Achieving and perspectives. Diabetes Metab Syndr. 2016;10:S176-S183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 138. | Song X, Qian X, Shen M, Jiang R, Wagner MB, Ding G, Chen G, Shen B. Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim Biophys Acta. 2015;1853:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 139. | Azibani F, Benard L, Schlossarek S, Merval R, Tournoux F, Fazal L, Polidano E, Launay JM, Carrier L, Chatziantoniou C, Samuel JL, Delcayre C. Aldosterone inhibits antifibrotic factors in mouse hypertensive heart. Hypertension. 2012;59:1179-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 140. | Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, López-Andrés N. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 276] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 141. | Ibarrola J, Sádaba R, Garcia-Peña A, Arrieta V, Martinez-Martinez E, Alvarez V, Fernández-Celis A, Gainza A, Santamaría E, Fernández-Irigoyen J, Cachofeiro V, Fay R, Rossignol P, López-Andrés N. A role for fumarate hydratase in mediating oxidative effects of galectin-3 in human cardiac fibroblasts. Int J Cardiol. 2018;258:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 142. | Ibarrola J, Arrieta V, Sádaba R, Martinez-Martinez E, Garcia-Peña A, Alvarez V, Fernández-Celis A, Gainza A, Santamaría E, Fernández-Irigoyen J, Cachofeiro V, Zalba G, Fay R, Rossignol P, López-Andrés N. Galectin-3 down-regulates antioxidant peroxiredoxin-4 in human cardiac fibroblasts: a new pathway to induce cardiac damage. Clin Sci (Lond). 2018;132:1471-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 143. | He J, Li X, Luo H, Li T, Zhao L, Qi Q, Liu Y, Yu Z. Galectin-3 mediates the pulmonary arterial hypertension-induced right ventricular remodeling through interacting with NADPH oxidase 4. J Am Soc Hypertens 2017; 11: 275-289. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 144. | Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, André S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 680] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 145. | Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, van der Harst P, Pitt B, Goldstein IJ, Koerts JA, van Veldhuisen DJ, Bank RA, van Gilst WH, Silljé HH, de Boer RA. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013;6:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 146. | Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 456] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 147. | Ueland T, Aukrust P, Broch K, Aakhus S, Skårdal R, Muntendam P, Gullestad L. Galectin-3 in heart failure: high levels are associated with all-cause mortality. Int J Cardiol. 2011;150:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 148. | Sharma U, Rhaleb NE, Pokharel S, Harding P, Rasoul S, Peng H, Carretero OA. Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am J Physiol Heart Circ Physiol. 2008;294:H1226-H1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 149. | Keng BMH, Gao F, Ewe SH, Tan RS, Teo LLY, Xie BQ, Koh WP, Koh AS. Galectin-3 as a candidate upstream biomarker for quantifying risks of myocardial ageing. ESC Heart Fail. 2019;6:1068-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 150. | Vora A, de Lemos JA, Ayers C, Grodin JL, Lingvay I. Association of Galectin-3 With Diabetes Mellitus in the Dallas Heart Study. J Clin Endocrinol Metab. 2019;104:4449-4458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 151. | Gopal DM, Ayalon N, Wang YC, Siwik D, Sverdlov A, Donohue C, Perez A, Downing J, Apovian C, Silva V, Panagia M, Kolachalama V, Ho JE, Liang CS, Gokce N, Colucci WS. Galectin-3 Is Associated With Stage B Metabolic Heart Disease and Pulmonary Hypertension in Young Obese Patients. J Am Heart Assoc. 2019;8:e011100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 152. | Holmager P, Egstrup M, Gustafsson I, Schou M, Dahl JS, Rasmussen LM, Møller JE, Tuxen C, Faber J, Kistorp C. Galectin-3 and fibulin-1 in systolic heart failure - relation to glucose metabolism and left ventricular contractile reserve. BMC Cardiovasc Disord. 2017;17:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 153. | Flores-Ramírez R, Azpiri-López JR, González-González JG, Ordaz-Farías A, González-Carrillo LE, Carrizales-Sepúlveda EF, Vera-Pineda R. Global longitudinal strain as a biomarker in diabetic cardiomyopathy. A comparative study with Gal-3 in patients with preserved ejection fraction. Arch Cardiol Mex. 2017;87:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 154. | Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 662] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 155. | Altara R, Ghali R, Mallat Z, Cataliotti A, Booz GW, Zouein FA. Conflicting vascular and metabolic impact of the IL-33/sST2 axis. Cardiovasc Res. 2018;114:1578-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 156. | Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2564] [Cited by in RCA: 2893] [Article Influence: 144.7] [Reference Citation Analysis (0)] |
| 157. | Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. 2015;115:3B-7B. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 158. | Pusceddu I, Dieplinger B, Mueller T. ST2 and the ST2/IL-33 signalling pathway-biochemistry and pathophysiology in animal models and humans. Clin Chim Acta. 2019;495:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 159. | Benoit JL, Hicks CW, Engineer RS, Hart KW, Lindsell CJ, Peacock WF. ST2 in emergency department patients with noncardiac dyspnea. Acad Emerg Med. 2013;20:1207-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 160. | Haider T, Simader E, Hacker P, Ankersmit HJ, Heinz T, Hajdu S, Negrin LL. Increased serum concentrations of soluble ST2 are associated with pulmonary complications and mortality in polytraumatized patients. Clin Chem Lab Med. 2018;56:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 161. | Vocca L, Di Sano C, Uasuf CG, Sala A, Riccobono L, Gangemi S, Albano GD, Bonanno A, Gagliardo R, Profita M. IL-33/ST2 axis controls Th2/IL-31 and Th17 immune response in allergic airway diseases. Immunobiology. 2015;220:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 162. | Ruiz-Castilla M, Bosacoma P, Dos Santos B, Baena J, Guilabert P, Marin-Corral J, Masclans JR, Roca O, Barret JP. Soluble Suppression Of Tumorigenicity-2 Predicts Hospital Mortality in Burn Patients: An Observational Prospective Cohort Pilot Study. Shock. 2019;51:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 163. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137-e161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1590] [Cited by in RCA: 1982] [Article Influence: 247.8] [Reference Citation Analysis (0)] |
| 164. | Fousteris E, Melidonis A, Panoutsopoulos G, Tzirogiannis K, Foussas S, Theodosis-Georgilas A, Tzerefos S, Matsagos S, Boutati E, Economopoulos T, Dimitriadis G, Raptis S. Toll/interleukin-1 receptor member ST2 exhibits higher soluble levels in type 2 diabetes, especially when accompanied with left ventricular diastolic dysfunction. Cardiovasc Diabetol. 2011;10:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 165. | Kiencke S, Handschin R, von Dahlen R, Muser J, Brunner-Larocca HP, Schumann J, Felix B, Berneis K, Rickenbacher P. Pre-clinical diabetic cardiomyopathy: prevalence, screening, and outcome. Eur J Heart Fail. 2010;12:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 166. | Devaux Y, Zangrando J, Schroen B, Creemers EE, Pedrazzini T, Chang CP, Dorn GW 2nd, Thum T, Heymans S; Cardiolinc network. Long noncoding RNAs in cardiac development and ageing. Nat Rev Cardiol. 2015;12:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 282] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 167. | Thum T, Condorelli G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ Res. 2015;116:751-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 168. | Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res. 2015;116:737-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 602] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 169. | Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J, Holzmann A, Lemesle G, de Groote P, Pinet F, Thum T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res. 2014;114:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 506] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 170. | Vausort M, Wagner DR, Devaux Y. Long noncoding RNAs in patients with acute myocardial infarction. Circ Res. 2014;115:668-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 410] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 171. | Liu JY, Yao J, Li XM, Song YC, Wang XQ, Li YJ, Yan B, Jiang Q. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5:e1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 358] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 172. | Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, Tao ZF, Song YC, Chen Q, Jiang Q. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116:1143-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 501] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 173. | Carter G, Miladinovic B, Patel AA, Deland L, Mastorides S, Patel NA. Circulating long noncoding RNA GAS5 Levels are correlated to prevalence of type 2 diabetes mellitus. BBA Clin. 2015;4:102-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 174. | de Gonzalo-Calvo D, Kenneweg F, Bang C, Toro R, van der Meer RW, Rijzewijk LJ, Smit JW, Lamb HJ, Llorente-Cortes V, Thum T. Circulating long-non coding RNAs as biomarkers of left ventricular diastolic function and remodelling in patients with well-controlled type 2 diabetes. Sci Rep. 2016;6:37354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 175. | Ambros V. The functions of animal microRNAs. Nature. 2004;431:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7919] [Cited by in RCA: 8601] [Article Influence: 409.6] [Reference Citation Analysis (0)] |
| 176. | Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3661] [Cited by in RCA: 3994] [Article Influence: 234.9] [Reference Citation Analysis (0)] |
| 177. | Ong SG, Wu JC. Exosomes as potential alternatives to stem cell therapy in mediating cardiac regeneration. Circ Res. 2015;117:7-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 178. | Wong LL, Wang J, Liew OW, Richards AM, Chen YT. MicroRNA and Heart Failure. Int J Mol Sci. 2016;17:502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 179. | LeBoeuf RA, Kerckaert GA, Aardema MJ, Gibson DP. Multistage neoplastic transformation of Syrian hamster embryo cells cultured at pH 6.70. Cancer Res. 1990;50:3722-3729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 180. | Vasudevan S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip Rev RNA. 2012;3:311-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 354] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 181. | Voelter-Mahlknecht S. Epigenetic associations in relation to cardiovascular prevention and therapeutics. Clin Epigenetics. 2016;8:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 182. | Zhou Q, Lv D, Chen P, Xu T, Fu S, Li J, Bei Y. MicroRNAs in diabetic cardiomyopathy and clinical perspectives. Front Genet. 2014;5:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 183. | Thrainsdottir IS, Aspelund T, Thorgeirsson G, Gudnason V, Hardarson T, Malmberg K, Sigurdsson G, Rydén L. The association between glucose abnormalities and heart failure in the population-based Reykjavik study. Diabetes Care. 2005;28:612-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 184. | Feng B, Chen S, George B, Feng Q, Chakrabarti S. miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes Metab Res Rev. 2010;26:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 185. | Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170-178, 6p following 178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 685] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 186. | León LE, Rani S, Fernandez M, Larico M, Calligaris SD. Subclinical Detection of Diabetic Cardiomyopathy with MicroRNAs: Challenges and Perspectives. J Diabetes Res. 2016;2016:6143129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 187. | Zhao F, Li B, Wei YZ, Zhou B, Wang H, Chen M, Gan XD, Wang ZH, Xiong SX. MicroRNA-34a regulates high glucose-induced apoptosis in H9c2 cardiomyocytes. J Huazhong Univ Sci Technolog Med Sci. 2013;33:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 188. | Li X, Du N, Zhang Q, Li J, Chen X, Liu X, Hu Y, Qin W, Shen N, Xu C, Fang Z, Wei Y, Wang R, Du Z, Zhang Y, Lu Y. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5:e1479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 189. | Enomoto M, Ishizu T, Seo Y, Kameda Y, Suzuki H, Shimano H, Kawakami Y, Aonuma K. Myocardial dysfunction identified by three-dimensional speckle tracking echocardiography in type 2 diabetes patients relates to complications of microangiopathy. J Cardiol. 2016;68:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 190. | Ernande L, Bergerot C, Girerd N, Thibault H, Davidsen ES, Gautier Pignon-Blanc P, Amaz C, Croisille P, De Buyzere ML, Rietzschel ER, Gillebert TC, Moulin P, Altman M, Derumeaux G. Longitudinal myocardial strain alteration is associated with left ventricular remodeling in asymptomatic patients with type 2 diabetes mellitus. J Am Soc Echocardiogr. 2014;27:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 191. | Liu JH, Chen Y, Yuen M, Zhen Z, Chan CW, Lam KS, Tse HF, Yiu KH. Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 192. | Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, Fang ZY, Prins JB, Stanton T. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. 2015;101:1061-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 193. | Sasso FC, Rambaldi PF, Carbonara O, Nasti R, Torella M, Rotondo A, Torella R, Mansi L. Perspectives of nuclear diagnostic imaging in diabetic cardiomyopathy. Nutr Metab Cardiovasc Dis. 2010;20:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 194. | Tillquist MN, Maddox TM. Update on diabetic cardiomyopathy: inches forward, miles to go. Curr Diab Rep. 2012;12:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 195. | Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, Nochioka K, Biering-Sørensen T. Global Longitudinal Strain Is a Superior Predictor of All-Cause Mortality in Heart Failure With Reduced Ejection Fraction. JACC Cardiovasc Imaging. 2015;8:1351-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 282] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 196. | Liu X, Yang ZG, Gao Y, Xie LJ, Jiang L, Hu BY, Diao KY, Shi K, Xu HY, Shen MT, Ren Y, Guo YK. Left ventricular subclinical myocardial dysfunction in uncomplicated type 2 diabetes mellitus is associated with impaired myocardial perfusion: a contrast-enhanced cardiovascular magnetic resonance study. Cardiovasc Diabetol. 2018;17:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 197. | Liu L, Ding W, Li R, Ye X, Zhao J, Jiang J, Meng L, Wang J, Chu S, Han X, Peng F. Plasma levels and diagnostic value of catestatin in patients with heart failure. Peptides. 2013;46:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 198. | Borovac JA, Glavas D, Susilovic Grabovac Z, Supe Domic D, Stanisic L, D'Amario D, Kwok CS, Bozic J. Circulating sST2 and catestatin levels in patients with acute worsening of heart failure: a report from the CATSTAT-HF study. ESC Heart Fail. 2020;7:2818-2828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 199. | Ying W, Mahata S, Bandyopadhyay GK, Zhou Z, Wollam J, Vu J, Mayoral R, Chi NW, Webster NJG, Corti A, Mahata SK. Catestatin Inhibits Obesity-Induced Macrophage Infiltration and Inflammation in the Liver and Suppresses Hepatic Glucose Production, Leading to Improved Insulin Sensitivity. Diabetes. 2018;67:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 200. | Gallo MP, Femminò S, Antoniotti S, Querio G, Alloatti G, Levi R. Catestatin Induces Glucose Uptake and GLUT4 Trafficking in Adult Rat Cardiomyocytes. Biomed Res Int. 2018;2018:2086109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 201. | Kanamori H, Takemura G, Goto K, Tsujimoto A, Mikami A, Ogino A, Watanabe T, Morishita K, Okada H, Kawasaki M, Seishima M, Minatoguchi S. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy. 2015;11:1146-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 202. | Hölscher ME, Bode C, Bugger H. Diabetic Cardiomyopathy: Does the Type of Diabetes Matter? Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |