Published online May 15, 2021. doi: 10.4239/wjd.v12.i5.590
Peer-review started: January 4, 2021
First decision: January 25, 2021
Revised: January 29, 2021
Accepted: April 7, 2021
Article in press: April 7, 2021
Published online: May 15, 2021
Compelling pieces of evidence derived from both clinical and experimental research has demonstrated the crucial role of the receptor for advanced-glycation end-products (RAGE) in orchestrating a plethora of proinflammatory cellular responses leading to many of the complications and end-organ damages reported in patients with diabetes mellitus (DM). During the coronavirus disease 2019 (COVID-19) pandemic, many clinical reports have pointed out that DM increases the risk of COVID-19 complications, hospitalization requirements, as well as the overall severe acute respiratory syndrome coronavirus 2 case-fatality rate. In the present review, we intend to focus on how the basal activation state of the RAGE axis in common preexisting conditions in DM patients such as endothelial dysfunction and hyperglycemia-related prothrombotic phenotype, as well as the contribution of RAGE signaling in lung inflammation, may then lead to the increased mortality risk of COVID-19 in these patients. Additionally, the cross-talk between the RAGE axis with either another severe acute respiratory syndrome coronavirus 2 receptor molecule different of angiotensin-converting enzyme 2 or the renin-angiotensin system imbalance produced by viral infection, as well as the role of this multi-ligand receptor on the obesity-associated low-grade inflammation in the higher risk for severe illness reported in diabetes patients with COVID-19, are also discussed.
Core Tip: Compelling evidence support that diabetes mellitus increases the risk of coronavirus disease 2019 (COVID-19) complications, as well as the overall syndrome coronavirus 2 case-fatality. Different reports have suggested the putative involvement of several molecular mechanisms underlying this increased risk. We herein discuss the contribution of the activation of the receptor for advanced-glycation end-products axis to the higher risk for severe illness reported in diabetes patients with COVID-19.
- Citation: Rojas A, Lindner C, Gonzàlez I, Morales MA. Advanced-glycation end-products axis: A contributor to the risk of severe illness from COVID-19 in diabetes patients. World J Diabetes 2021; 12(5): 590-602
- URL: https://www.wjgnet.com/1948-9358/full/v12/i5/590.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i5.590
Coronavirus disease 2019 (COVID-19) is an infectious disease, where the etiological agent is a novel coronavirus, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). This disease was initially detected and reported in December 2019 in Wuhan, China and then spread rapidly all over the world. This situation forced the World Health Organization to declare on January 30, 2020, the COVID-19 as a global pandemic, and thus leading humanity to face up an extraordinary challenge of a new viral disease.
Lung inflammation is the main cause of life-threatening respiratory disorders at the COVID-19 severe stage[1,2], and where lower respiratory tract symptoms and low oxygen saturation in the blood resembling acute respiratory distress syndrome (ARDS) as well as the requirement of invasive mechanical ventilation.
In addition to the lungs, SARS-CoV-2 may also infect the gastrointestinal tract, cardiovascular system, as well as central nervous system[3-5].
SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE2) molecule as the receptor for viral cell entry[6]. ACE2 plays an important role in renin-angiotensin system (RAS), and the imbalance between ACE/angiotensin II (Ang II)/angiotensin II receptor type 1 (AT1R) pathway and ACE2/Ang (1–7)/Mas receptor pathway in the RAS system will lead to multisystem inflammation[7]. The activation of the AT1R by Ang II may trigger the activation of proinflammatory signals such as oxidative and nitrosative stresses, the induction of cytokines, cell adhesion molecules, as well as the activation transcription factors such nuclear factor kappa B[8-11]. Therefore, ACE2/Ang-(1-7)/Mas receptor, has been pointed out as a counter-regulator of the deleterious effects of Ang II[12].
During the pandemic, it has been shown that DM increases the risk of COVID-19 complications. Data from different studies have pointed out that increased hospitalizations, longer and repeated hospital stays as well as the overall SARS-CoV-2 case-fatality rate are significantly higher in diabetes patients who have poorly controlled glycemia when compared to patients without DM[13-16]. Although the huge amount of compelling clinical data supporting COVID-19 complications in people with diabetes, the molecular mechanisms underlying this association are not fully understood.
The receptor for advanced-glycation end-products (RAGE) was discovered as a receptor for advanced glycation endproducts (AGEs), which are accelerated formed in hyperglycemia. Afterward, RAGE emerged as a multi-ligand receptor able to interact with a diverse myriad of non-AGE ligands and being implicated in diverse chronic inflammatory states[17,18].
In the present review, we will discuss the possible contribution of the activation of the RAGE axis to the higher risk for severe illness in diabetes patients infected with COVID-19.
RAGE was initially reported in 1992, as a membrane-associated molecule that can bind AGEs[19]. AGEs are a heterogeneous group of molecules formed from the non-enzymatic reaction of reducing sugars with free amino groups of proteins, lipids, and nucleic acids to form a freely reversible Schiff base, which spontaneously rearranges itself into an Amadori product, as is the case of the well-known hemoglobin A1c[20]. Hemoglobin A1c is an important indicator of long-term glycemic control with the ability to reflect the cumulative glycemic history of the preceding two to three months[21].
The formation of AGEs is thought to be the major cause of different diabetic complications in large part through their interactions with RAGE. Of note, AGEs may also contribute to diabetic complications through the formation of cross-links between key molecules in the basement membrane of the extracellular matrix, and thus altering the constitution of the matrix and increases stiffness[22-25].
RAGE is a single-pass transmembrane protein, which belongs to the immu
RAGE is found in human airways with high basal levels of RAGE expressed in pulmonary tissue[29]. It is also found on vascular cells, neurons, cardiomyocytes, adipocytes, glomerular epithelial cells, or podocytes[30], as well as on pro-inflammatory and immuno-competent cells such as neutrophils, monocytes, macrophages, and T and B lymphocytes[31].
Besides AGEs, RAGE can recognize many other ligands including the alarmin high mobility group box 1 protein (HMGB1), members of the S100 protein family, glycosaminoglycans, and amyloid β peptides[32].
As a consequence of RAGE engagement by its ligands, multiple signaling pathways are triggered, including reactive oxygen species, p21ras, erk1/2 (p44/p42) MAP kinases, p38 and SAPK/JNK MAP kinases, rhoGTPases, phosphoinositol-3 kinase, and the JAK/STAT pathway, having crucial downstream inflammatory consequences such as activation of nuclear factor kappa B, AP-1 and Stat-3[33].
Endogenous formation of AGEs is markedly increased in diabetes as the result of hyperglycemia and increased oxidative stress. At present, an increasing prevalence of diabetes and its complications is reported worldwide. Elevated levels of circulating AGEs are believed to play a major role in the pathogenesis of macrovascular and microvascular disease in diabetes mellitus.
Additionally, it has been demonstrated that dietary AGEs also play a major role in maintaining a high body pool of AGEs in diabetes[34].
The diabetic condition is a chronic systemic low-grade inflammation[18], and consequently, other RAGE ligands are bioavailable as is the case of some members of the S100 family and HMGB1, which can be either passively released from damaged cells or actively secreted by immune cells. A compelling body of evidence demonstrates that both AGEs and non-AGEs ligands accumulate in the plasma/serum of human subjects with diabetes[35,36].
Compelling data derived from both clinical and experimental studies support the crucial contribution of RAGE activation in vascular complications in diabetes[37].
Endothelial cells actively regulate cellular adhesion, thromboresistance, smooth muscle cell proliferation, and vessel wall inflammation. Therefore, dysfunction of the vascular endothelium is considered as an important factor in the pathogenesis of the micro-and macro-angiopathies observed in diabetes patients, and where the activation of the RAGE axis is an important contributor to this dysfunctional state[38-40].
DM has been associated with platelet hyper-reactivity, which plays a central role in the hyperglycemia-related pro-thrombotic phenotype[41,42]. In this sense, the activation of the RAGE axis has been pointed out as an important contributor to the development of a pro-thrombotic state, by its capacity to activate platelets[43,44].
DM is associated with increased disease severity and a higher risk of mortality in patients with COVID-19, who can rapidly progress to ARDS, septic shock, and multiple organ dysfunction syndrome[13-16].
Several mechanisms have been claimed for explaining the exacerbating effect of diabetes on COVID-19. These mechanisms include those directly related to hyperglycemia and the associated imbalances in pathways involved in virus entry into the cell as well as in the immune and inflammatory response. At present, the role of RAGE axis activation has been demonstrated in different animal models of ARDS and where RAGE inhibition attenuated lung injury (LI) and restored alveolar fluid clearance[45,46].
In this context, it is important to highlight that the release of the RAGE ligand HMGB1 is increased under hyperglycemic conditions[47,48], as well as the crucial role of HMGB1 in lung inflammation in diabetes[49-51].
Additionally, the contribution to LI by HMGB1-mediated RAGE signaling is well- documented in other viral diseases of the respiratory tract, as reported for the influenza virus[52].
Considering the abundance of RAGE in the lungs, the robust proinflammatory signaling triggering after the engagement, as well the relatively high expression levels in RAGE in diabetes patients[53], the activation of the RAGE axis may be an important contributor in exacerbating clinical complications in COVID-19 patients with diabetes. In this sense, it is important to highlight the contribution of RAGE axis activation in preexisting conditions such as endothelial dysfunction as well as the hyperglycemia-related prothrombotic phenotype, which increases the mortality risk of COVID-19 in DM patients.
Noteworthy, the RAGE ligand S100A12 is overexpressed in COVID-19, as recently reported[54]. This molecule is also closely related to the pathogenesis of sepsis-induced ARDS[55].
The association of the RAS with the endocrine system is particularly illustrated by the prominent role of Ang II in diabetes and metabolic syndrome. RAS has been extensively described to be involved in the onset and progress of hypertension, retinopathy, nephropathy, and cardiovascular disease in DM patients. RAS is considered an important pharmacological target in the management of micro-and macrovascular complications for these patients[56-59].
Of particular importance, individuals with diabetes have a reduced ACE2 expression. This enzyme is found in multiple organs including the lungs. ACE2 plays an important role in the RAS, and the imbalance between ACE/Ang II/AT1R pathway and ACE2/Ang (1–7)/ Mas receptor pathway in the RAS system will lead to multisystem inflammation. This reduced expression confers to individuals with diabetes an increased risk of severe LI as well as ARDS if infected by COVID-19[60].
As already mentioned SARS-CoV-2 uses ACE2 molecule as the receptor for viral cell entry[6]. ACE2 is a key counter-regulatory element in the pathway of the renin-angiotensin system, which acts to opposite the actions of Ang II by generating Ang-(1–7), and thus reducing inflammation and fibrosis and mitigate end-organ damage[61].
Strikingly, SARS-CoV-2 hijacks ACE2 to invade and damage cells, downregulating ACE2, reducing its protective effects, and exacerbating injurious Ang II effects[62].
Considering the facts that diabetes patients have a reduced expression of ACE-2, as well as the capacity of SARS-CoV-2 to hijacks ACE2, ACE2 exhaustion will be produced in patients with diabetes during infection and, thus reducing its capacity to fully function as a counterbalancing element of RAS through the ACE2/Ang-(1-7)/mas receptor pathway.
Decades of research have demonstrated that the activation of ATR1 by Ang II, triggers a robust inflammatory response involving the recruitment and activation of inflammatory cells, as well as apoptosis of both alveolar epithelial cells and pulmonary microvascular endothelial cells, and consequently, a marked increased microvascular permeability and loss of epithelial and endothelial integrity[63].
RAGE axis is an important contributor to the pathophysiology of lung inflammation because the use of different inhibition strategies can increase arterial oxygenation, reduce alveolar inflammation, and improve lung damage in acute lung inflammation[46,64].
Strikingly, a novel ligand-independent mechanism for RAGE transactivation has been recently reported to occur following activation of the AT1R by Ang II and thus leading to nuclear factor kappa B dependent expression of pro-inflammatory mediators[65]. This novel mechanism is expected to continuously fuel the lung inflammatory environment in diabetes patients during SARS-CoV-2 infection, considering both the high expression of RAGE and the reduced levels of ACE-2 in the lungs[66].
Increased infiltration and accumulation of macrophages is a common process in many of the complications of diabetes patients[67].
CD147, originally described in tumor cells, is a highly glycosylated 58-kDa transmembrane protein belonging to the immunoglobulin superfamily and also known as extracellular matrix metalloproteinase functions as a matrix metalloproteinases (MMPs) inducer, predominantly MMP-2 and MMP-9. Of note, the expression of this protein is markedly increased by AGEs by a RAGE-dependent mechanism[68].
Degradation of protein components in the alveolar epithelial–endothelial unit by both MMP-2 and MMP-9 is considered a central process in the pathogenesis of ALI/ARDS[69-71]. Strikingly, SARS-CoV-2 spike protein may bind also to CD147 glycoprotein[72], and thus mediating viral invasion. Due to the high expression levels of this protein in diabetes, this condition may then increase the accessibility of virus to tissue in patients with diabetes. A recent report demonstrates the Meplazumab, a humanized anti-CD147 antibody efficiently improved the recovery of patients with SARS-CoV-2 pneumonia with a favorable safety profile[73].
Thrombotic microangiopathy is reported as a frequent event in COVID-19[74]. In patients with diabetes, endothelial dysfunction is a very common condition, and events such as enhanced vasoconstriction, platelet hyperactivity and thrombus formation are activated due to the metabolic milieu, and where the activation of the RAGE axis is continuously fueled by hyperglycemia, insulin resistance, and the oxidative stress seen in diabetes[75]. Noteworthy, platelets can be activated by a RAGE-dependent mechanism[43].
The dysfunctional state of the endothelium is linked to an impairment of nitric oxide production and activity, which may then affect not only the vasodilator tone and platelet activity but also the recruitment of endothelial progenitor cells, which directly contribute to the homeostasis and repair of the endothelial layer in blood vessels[76-78].
Very recently, clinical findings suggest that SARS-CoV-2 infection facilitates the induction of endotheliitis in several organs as a direct consequence of viral infection[79]. However, these data have generated controversy about the nature of the viral-type particles reported because of endoplasmatic reticulum may mimic SARS-CoV-2 particles on electron microscopy[80,81]. Additionally, other pieces of evidence show the absence of viral ribonucleic acid inside endothelial cells, suggesting that indirect effects rather than direct viral infection might trigger endothelial damage[82]. On the other hand, SARS-CoV-2 spike protein may bind also to CD147 glycoprotein which is upregulated by hyperglycemia and by RAGE activation[68]. CD147 expression is significantly upregulated in activated endothelial cells[83]. Therefore, these findings raise the intriguing possibility that RAGE activation may play a role also in viral invasion to host cells.
The activation of the RAGE axis has been widely documented to be crucial to prime proinflammatory mechanisms and rendering endothelial cells into an activation state and thereby amplifying proinflammatory mechanisms in many chronic inflammatory disorders[84-86]. Thus, preexisting blood vessel damage may put people with COVID-19 at heightened risk of complications from the infection.
A dysfunctional endothelium as observed in diabetes, leading to detrimental shifts in the vascular equilibrium towards vasoconstriction, inflammation, and a pro-coagulant state resulting in thrombosis, constitute a much more proper condition to fuel inflammation in the blood vessel wall and then putting diabetes patients with COVID-19 at heightened risk of complications from the infection.
More than 90% of patients with type 2 diabetes have obesity or overweight[87]. In the context of the COVID-19 outbreak, many reports highlight that obesity and type 2 diabetes as comorbidities of SARS development in COVID-19 patients[88-90].
Both obesity and type 2 diabetes are associated with a chronic low-grade inflammatory state, and this particular basal state could then aggravate the inflammatory response to SARS-CoV-2 infection observed in severe COVID-19 cases.
In this context, there are shreds of evidence suggesting a key role of RAGE axis activation in fat tissue inflammation, and thus contributing to the obesity-associated low-grade inflammation, as well as to the reported dysregulation of adipokines[91,92].
Furthermore, many RAGE ligands such as AGEs, HMGB1, and S100/calgranulins, accumulate in adipose tissue in many models of obesity as well as in obese subjects[93-96], where they can trigger a robust proinflammatory secretion profile, which in turn, establishes a vicious loop, and thus rendering more inflammation[97].
The low-grade inflammation in adipose tissue is characterized, in addition to the robust secretion of proinflammatory cytokines, by the recruitment of leukocytes, mainly macrophages in this tissue. The accumulation of macrophage into adipose tissue correlates to both the degree of adiposity as well as the production of monocyte chemoattractant protein-1, which in turn, recruit more macrophages and thereby promote the chronicity of inflammation[98].
Furthermore, macrophages infiltrated in adipose tissue undergo a polarization process towards a spectrum of different phenotypes where two extremes are represented by the classically activated type 1 macrophages and the alternative activated type-2 macrophages[99]. Noteworthy, RAGE ligands accumulation and macrophage type 1 macrophages polarization are much more prevalent in perivascular adipose tissues[100] and thus, adding more inflammation to the vascular system.
During this pandemic, some alerts have been raised on side effects of some widely used drugs on diabetic COVID-19 patients, particularly lactic acidosis and ketoacidosis (DKA) for metformin and sodium-glucose cotransporter 2 inhibitors, res
The RAGE axis has been recently suggested to be a crucial contributor to the acute inflammatory insult during the medical crisis and treatment of DKA and thus acting as a constant source of subclinical inflammation leading to chronic diabetic vascular complications, including those of the heart[103].
Additionally, 3-deoxyglucosone is significantly elevated before and during the treatment of DKA[104]. 3-Deoxyglucosone is a dicarbonyl species that may lead to the formation of AGEs, and then fueling inflammation by RAGE engagement[105].
One mechanism by which metformin increases plasma lactate levels relates to the inhibition of mitochondrial respiration responsible for lactate removal[106,107], which correlate with the inhibition of mitochondrial oxidative phosphorylation[108].
The activation of the RAGE axis is known to increase cytosolic reactive oxygen species production which, in turn, facilitates mitochondrial superoxide production in hyperglycemic environments, and thus rendering a mitochondrial dysfunctional state[109,110]. This particular dysfunctional state could be a particular life-threatening condition in diabetic COVID-19 patients[111].
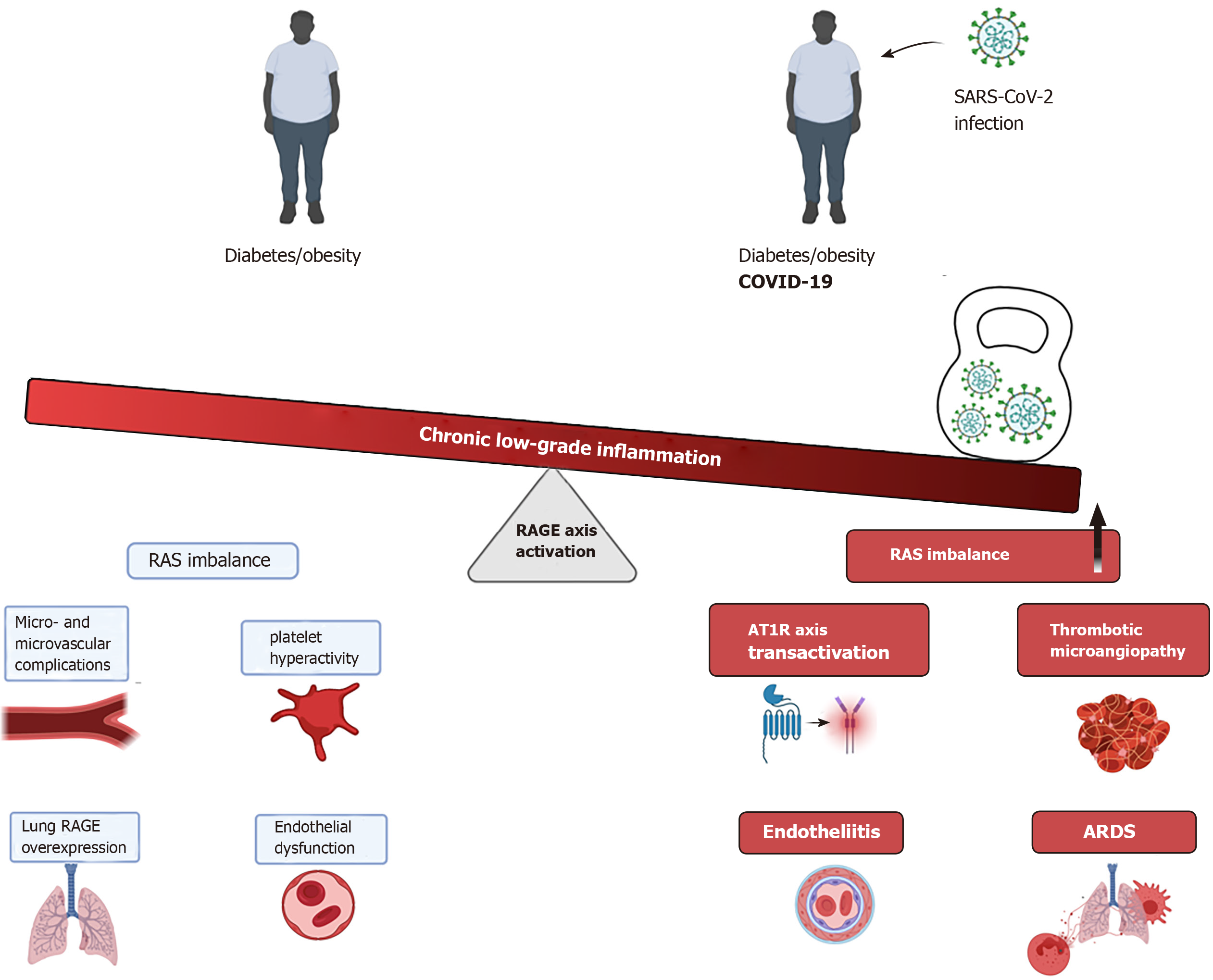
At present, a compelling body of evidence supports the crucial role of the RAGE axis in the pathophysiology of diabetes, being a key contributor in the onset and sustainment of low-grade and chronic inflammation state observed in patients with diabetes, and consequently, marked impairment of endothelial functions. Thus, this basal hyper-activated state of the RAGE axis, as occurs in diabetes patients may represent a crucial element in many clinical complications in diabetes patients who develop COVID-19 (Figure 1).
Furthermore, the novel ligand-independent transactivation of the RAGE axis by AT1R/Ang II further strengthens the hyperactivation state of the axis and consequently, fueling a robust pro-inflammatory environment particularly in the low respiratory tract, where the high expression of RAGE and AT1R receptors plays an essential role in the pathophysiology of the lung inflammation observed in those diabetic patients.
In summary, in light of what is known about the poor clinical outcomes of diabetic patients who develop COVID-19, the RAGE axis seems to be one of the key players in the enhanced inflammatory response and the high mortality rates of these patients. While the precise mechanisms by which the RAGE axis activation contributes to the higher risk of severe illness in diabetes patients infected with SARS-CoV-2 remain to be fully understood, it is important to strengthen future clinical research in this area.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mrzljak A, Ulaşoğlu C S-Editor: Zhang L L-Editor: A P-Editor: Li X
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32663] [Cited by in F6Publishing: 28514] [Article Influence: 7128.5] [Reference Citation Analysis (3)] |
| 2. | Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2988] [Cited by in F6Publishing: 3001] [Article Influence: 750.3] [Reference Citation Analysis (0)] |
| 3. | Grassia R, Testa S, Pan A, Conti CB. SARS-CoV-2 and gastrointestinal tract: The dark side of the pandemic. Dig Liver Dis. 2020;52:700-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 653] [Cited by in F6Publishing: 793] [Article Influence: 198.3] [Reference Citation Analysis (0)] |
| 5. | Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rößler L, Goebel HH, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Körtvélyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 799] [Cited by in F6Publishing: 801] [Article Influence: 267.0] [Reference Citation Analysis (0)] |
| 6. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11946] [Cited by in F6Publishing: 13083] [Article Influence: 3270.8] [Reference Citation Analysis (0)] |
| 7. | Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29:367-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 313] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 8. | Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008;264:224-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 390] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 9. | Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 489] [Cited by in F6Publishing: 484] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | Husain K, Hernandez W, Ansari RA, Ferder L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J Biol Chem. 2015;6:209-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 216] [Cited by in F6Publishing: 215] [Article Influence: 23.9] [Reference Citation Analysis (2)] |
| 11. | Capettini LS, Montecucco F, Mach F, Stergiopulos N, Santos RA, da Silva RF. Role of renin-angiotensin system in inflammation, immunity and aging. Curr Pharm Des. 2012;18:963-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Egido J. Modulation of angiotensin II effects, A potential novel approach to inflammatory and immune diseases. Curr Med Chem. 2003;2:379-394. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11409] [Cited by in F6Publishing: 10972] [Article Influence: 2743.0] [Reference Citation Analysis (0)] |
| 14. | Barone MTU, Ngongo B, Harnik SB, Oliveira LX, Végh D, de Luca PV, Pedrosa HC, Giraudo F, Cardona-Hernandez R, Chaudhury N, Menna-Barreto L. COVID-19 associated with diabetes and other noncommunicable diseases led to a global health crisis. Diabetes Res Clin Pract. 2021;171:108587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17476] [Cited by in F6Publishing: 17250] [Article Influence: 4312.5] [Reference Citation Analysis (0)] |
| 16. | Bloomgarden ZT. Diabetes and COVID-19. J Diabetes. 2020;12:347-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 17. | Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2014;25:15-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | Ramasamy R, Yan SF, Schmidt AM. The diverse ligand repertoire of the receptor for advanced glycation endproducts and pathways to the complications of diabetes. Vascul Pharmacol. 2012;57:160-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987-14997. [PubMed] [Cited in This Article: ] |
| 20. | Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 930] [Cited by in F6Publishing: 898] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 21. | Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976;295:417-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 888] [Cited by in F6Publishing: 819] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 22. | Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1436] [Cited by in F6Publishing: 1496] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 23. | Bansode S, Bashtanova U, Li R, Clark J, Müller KH, Puszkarska A, Goldberga I, Chetwood HH, Reid DG, Colwell LJ, Skepper JN, Shanahan CM, Schitter G, Mesquida P, Duer MJ. Glycation changes molecular organization and charge distribution in type I collagen fibrils. Sci Rep. 2020;10:3397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Neff LS, Bradshaw AD. Cross your heart? Cell Signal. 2021;79:109889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Bansode SB, Gacche RN. Glycation-induced modification of tissue-specific ECM proteins: A pathophysiological mechanism in degenerative diseases. Biochim Biophys Acta Gen Subj. 2019;1863:129411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Ibrahim ZA, Armour CL, Phipps S, Sukkar MB. RAGE and TLRs: relatives, friends or neighbours? Mol Immunol. 2013;56:739-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 27. | Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 28. | Kierdorf K, Fritz G. RAGE regulation and signaling in inflammation and beyond. J Leukoc Biol. 2013;94:55-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 29. | Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol. 2006;19:1437-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Ramasamy R, Shekhtman A, Schmidt AM. The multiple faces of RAGE--opportunities for therapeutic intervention in aging and chronic disease. Expert Opin Ther Targets. 2016;20:431-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | González I, Romero J, Rodríguez BL, Pérez-Castro R, Rojas A. The immunobiology of the receptor of advanced glycation end-products: trends and challenges. Immunobiology. 2013;218:790-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Bucciarelli LG, Wendt T, Rong L, Lalla E, Hofmann MA, Goova MT, Taguchi A, Yan SF, Yan SD, Stern DM, Schmidt AM. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell Mol Life Sci. 2002;59:1117-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 222] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Rojas A, Delgado-López F, González I, Pérez-Castro R, Romero J, Rojas I. The receptor for advanced glycation end-products: a complex signaling scenario for a promiscuous receptor. Cell Signal. 2013;25:609-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Garay-Sevilla ME, Beeri MS, de la Maza MP, Rojas A, Salazar-Villanea S, Uribarri J. The potential role of dietary advanced glycation endproducts in the development of chronic non-infectious diseases: a narrative review. Nutr Res Rev. 2020;33:298-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Biscetti F, Rando MM, Nardella E, Cecchini AL, Pecorini G, Landolfi R, Flex A. High Mobility Group Box-1 and Diabetes Mellitus Complications: State of the Art and Future Perspectives. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Zimmer DB, Chessher J, Wilson GL, Zimmer WE. S100A1 and S100B expression and target proteins in type I diabetes. Endocrinology. 1997;138:5176-5183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Fowler MJ. Microvascular and Macrovascular Complications of Diabetes. Clin Diabetes. 2008;26:77-82. [DOI] [Cited in This Article: ] [Cited by in Crossref: 947] [Cited by in F6Publishing: 954] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 38. | Sena CM, Pereira AM, Seiça R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832:2216-2231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 522] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 39. | Rojas A, Morales MA. Advanced glycation and endothelial functions: a link towards vascular complications in diabetes. Life Sci. 2004;76:715-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Rojas A, Romay S, González D, Herrera B, Delgado R, Otero K. Regulation of endothelial nitric oxide synthase expression by albumin-derived advanced glycosylation end products. Circ Res. 2000;86:E50-E54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 84] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 41. | Hess K, Grant PJ. Inflammation and thrombosis in diabetes. Thromb Haemost. 2011;105 Suppl 1:S43-S54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Vazzana N, Ranalli P, Cuccurullo C, Davì G. Diabetes mellitus and thrombosis. Thromb Res. 2012;129:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 43. | Fuentes E, Rojas A, Palomo I. Role of multiligand/RAGE axis in platelet activation. Thromb Res. 2014;133:308-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Ahrens I, Chen YC, Topcic D, Bode M, Haenel D, Hagemeyer CE, Seeba H, Duerschmied D, Bassler N, Jandeleit-Dahm KA, Sweet MJ, Agrotis A, Bobik A, Peter K. HMGB1 binds to activated platelets via the receptor for advanced glycation end products and is present in platelet rich human coronary artery thrombi. Thromb Haemost. 2015;114:994-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Audard J, Godet T, Blondonnet R, Joffredo JB, Paquette B, Belville C, Lavergne M, Gross C, Pasteur J, Bouvier D, Blanchon L, Sapin V, Pereira B, Constantin JM, Jabaudon M. Inhibition of the Receptor for Advanced Glycation End-Products in Acute Respiratory Distress Syndrome: A Randomised Laboratory Trial in Piglets. Sci Rep. 2019;9:9227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Blondonnet R, Audard J, Belville C, Clairefond G, Lutz J, Bouvier D, Roszyk L, Gross C, Lavergne M, Fournet M, Blanchon L, Vachias C, Damon-Soubeyrand C, Sapin V, Constantin JM, Jabaudon M. RAGE inhibition reduces acute lung injury in mice. Sci Rep. 2017;7:7208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Wu H, Chen Z, Xie J, Kang LN, Wang L, Xu B. High Mobility Group Box-1: A Missing Link between Diabetes and Its Complications. Mediators Inflamm. 2016;2016:3896147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Kim J, Sohn E, Kim CS, Jo K, Kim JS. The role of high-mobility group box-1 protein in the development of diabetic nephropathy. Am J Nephrol. 2011;33:524-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Boteanu RM, Uyy E, Suica VI, Antohe F. High-mobility group box 1 enhances the inflammatory process in diabetic lung. Arch Biochem Biophys. 2015;583:55-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 51. | Hamada N, Maeyama T, Kawaguchi T, Yoshimi M, Fukumoto J, Yamada M, Yamada S, Kuwano K, Nakanishi Y. The role of high mobility group box1 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:440-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 52. | van Zoelen MA, van der Sluijs KF, Achouiti A, Florquin S, Braun-Pater JM, Yang H, Nawroth PP, Tracey KJ, Bierhaus A, van der Poll T. Receptor for advanced glycation end products is detrimental during influenza A virus pneumonia. Virology. 2009;391:265-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Hudson BI, Lippman ME. Targeting RAGE Signaling in Inflammatory Disease. Annu Rev Med. 2018;69:349-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 54. | Arunachalam PS, Wimmers F, Mok CKP, Perera RAPM, Scott M, Hagan T, Sigal N, Feng Y, Bristow L, Tak-Yin Tsang O, Wagh D, Coller J, Pellegrini KL, Kazmin D, Alaaeddine G, Leung WS, Chan JMC, Chik TSH, Choi CYC, Huerta C, Paine McCullough M, Lv H, Anderson E, Edupuganti S, Upadhyay AA, Bosinger SE, Maecker HT, Khatri P, Rouphael N, Peiris M, Pulendran B. Systems biological assessment of immunity to mild vs severe COVID-19 infection in humans. Science. 2020;369:1210-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 661] [Cited by in F6Publishing: 759] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 55. | Zhang Z, Han N, Shen Y. S100A12 promotes inflammation and cell apoptosis in sepsis-induced ARDS via activation of NLRP3 inflammasome signaling. Mol Immunol. 2020;122:38-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum 2018. Am J Kidney Dis. 2018;71:884-895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 463] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 57. | Cao Z, Cooper ME. Efficacy of renin-angiotensin system (RAS) blockers on cardiovascular and renal outcomes in patients with type 2 diabetes. Acta Diabetol. 2012;49:243-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Behl T, Kotwani A. Potential of angiotensin II receptor blockers in the treatment of diabetic retinopathy. Life Sci. 2017;176:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Semeraro F, Morescalchi F, Cancarini A, Russo A, Rezzola S, Costagliola C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019;45:517-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 60. | Cuschieri S, Grech S. COVID-19 and diabetes: The why, the what and the how. J Diabetes Complications. 2020;34:107637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 61. | Sparks MA, South AM, Badley AD, Baker-Smith CM, Batlle D, Bozkurt B, Cattaneo R, Crowley SD, Dell'Italia LJ, Ford AL, Griendling K, Gurley SB, Kasner SE, Murray JA, Nath KA, Pfeffer MA, Rangaswami J, Taylor WR, Garovic VD. Severe Acute Respiratory Syndrome Coronavirus 2, COVID-19, and the Renin-Angiotensin System: Pressing Needs and Best Research Practices. Hypertension. 2020;76:1350-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Chung MK, Karnik S, Saef J, Bergmann C, Barnard J, Lederman MM, Tilton J, Cheng F, Harding CV, Young JB, Mehta N, Cameron SJ, McCrae KR, Schmaier AH, Smith JD, Kalra A, Gebreselassie SK, Thomas G, Hawkins ES, Svensson LG. SARS-CoV-2 and ACE2: The biology and clinical data settling the ARB and ACEI controversy. EBioMedicine. 2020;58:102907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 63. | Wang D, Chai XQ, Magnussen CG, Zosky GR, Shu SH, Wei X, Hu SS. Renin-angiotensin-system, a potential pharmacological candidate, in acute respiratory distress syndrome during mechanical ventilation. Pulm Pharmacol Ther. 2019;58:101833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 64. | Oczypok EA, Perkins TN, Oury TD. All the "RAGE" in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev. 2017;23:40-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 65. | Pickering RJ, Tikellis C, Rosado CJ, Tsorotes D, Dimitropoulos A, Smith M, Huet O, Seeber RM, Abhayawardana R, Johnstone EK, Golledge J, Wang Y, Jandeleit-Dahm KA, Cooper ME, Pfleger KD, Thomas MC. Transactivation of RAGE mediates angiotensin-induced inflammation and atherogenesis. J Clin Invest. 2019;129:406-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 66. | Rojas A, Gonzalez I, Morales MA. SARS-CoV-2-mediated inflammatory response in lungs: should we look at RAGE? Inflamm Res. 2020;69:641-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 67. | Kanter JE, Hsu CC, Bornfeldt KE. Monocytes and Macrophages as Protagonists in Vascular Complications of Diabetes. Front Cardiovasc Med. 2020;7:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 68. | Bao W, Min D, Twigg SM, Shackel NA, Warner FJ, Yue DK, McLennan SV. Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: possible role in diabetic complications. Am J Physiol Cell Physiol. 2010;299:C1212-C1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Corbel M, Belleguic C, Boichot E, Lagente V. Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell Biol Toxicol. 2002;18:51-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Davey A, McAuley DF, O'Kane CM. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. Eur Respir J. 2011;38:959-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 71. | Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002;3:409-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 72. | Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P, Wei D, Zhang Y, Sun XX, Gong L, Yang X, He L, Zhang L, Yang Z, Geng JJ, Chen R, Zhang H, Wang B, Zhu YM, Nan G, Jiang JL, Li L, Wu J, Lin P, Huang W, Xie L, Zheng ZH, Zhang K, Miao JL, Cui HY, Huang M, Zhang J, Fu L, Yang XM, Zhao Z, Sun S, Gu H, Wang Z, Wang CF, Lu Y, Liu YY, Wang QY, Bian H, Zhu P, Chen ZN. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5:283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 658] [Article Influence: 164.5] [Reference Citation Analysis (0)] |
| 73. | Bian H, Zheng ZH, Wei D, Zhang Z, Kang WZ, Hao CQ, Dong K, Kang W, Xia JL, Miao JL, Xie RH, Wang B, Sun XX, Yang XM, Lin P, Geng JJ, Wang K, Cui HY, Zhang K, Chen XC, Tang H, Du H, Yao N, Liu SS, Liu LN, Gao ZW, Nan G, Wang QY, Lian JQ, Chen ZN, Zhu P. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. medRxiv 2020. [DOI] [Cited in This Article: ] |
| 74. | Gavriilaki E, Brodsky RA. Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily. Br J Haematol. 2020;189:e227-e230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 75. | Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17:121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 323] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 76. | Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 77. | Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 383] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 78. | Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1024] [Cited by in F6Publishing: 986] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 79. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4227] [Cited by in F6Publishing: 4261] [Article Influence: 1065.3] [Reference Citation Analysis (0)] |
| 80. | Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395:e99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 81. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel A, Mehra MR, Scholkmann F, Schüpbach R, Ruschitzka F, Moch H. Electron microscopy of SARS-CoV-2: a challenging task - Authors' reply. Lancet. 2020;395:e100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 82. | Stahl K, Bräsen JH, Hoeper MM, David S. Absence of SARS-CoV-2 RNA in COVID-19-associated intestinal endothelialitis. Intensive Care Med. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Chen Y, Zhang H, Gou X, Horikawa Y, Xing J, Chen Z. Upregulation of HAb18G/CD147 in activated human umbilical vein endothelial cells enhances the angiogenesis. Cancer Lett. 2009;278:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 85. | Hori O, Yan SD, Ogawa S, Kuwabara K, Matsumoto M, Stern D, Schmidt AM. The receptor for advanced glycation end-products has a central role in mediating the effects of advanced glycation end-products on the development of vascular disease in diabetes mellitus. Nephrol Dial Transplant. 1996;11 Suppl 5:13-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Guo ZJ, Niu HX, Hou FF, Zhang L, Fu N, Nagai R, Lu X, Chen BH, Shan YX, Tian JW, Nagaraj RH, Xie D, Zhang X. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid Redox Signal. 2008;10:1699-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 87. | Bramante CT, Lee CJ, Gudzune KA. Treatment of Obesity in Patients With Diabetes. Diabetes Spectr. 2017;30:237-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Drucker DJ. Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications. Endocr Rev. 2020;41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 265] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 89. | Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 526] [Cited by in F6Publishing: 460] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 90. | Orioli L, Hermans MP, Thissen JP, Maiter D, Vandeleene B, Yombi JC. COVID-19 in diabetic patients: Related risks and specifics of management. Ann Endocrinol (Paris). 2020;81:101-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 91. | Monden M, Koyama H, Otsuka Y, Morioka T, Mori K, Shoji T, Mima Y, Motoyama K, Fukumoto S, Shioi A, Emoto M, Yamamoto Y, Yamamoto H, Nishizawa Y, Kurajoh M, Yamamoto T, Inaba M. Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2. Diabetes. 2013;62:478-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 92. | Ueno H, Koyama H, Shoji T, Monden M, Fukumoto S, Tanaka S, Otsuka Y, Mima Y, Morioka T, Mori K, Shioi A, Yamamoto H, Inaba M, Nishizawa Y. Receptor for advanced glycation end-products (RAGE) regulation of adiposity and adiponectin is associated with atherogenesis in apoE-deficient mouse. Atherosclerosis. 2010;211:431-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 93. | Gaens KH, Stehouwer CD, Schalkwijk CG. The Nε-(carboxymethyl)lysine-RAGE axis: putative implications for the pathogenesis of obesity-related complications. Expert Rev Endocrinol Metab. 2010;5:839-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Michetti F, Dell'Anna E, Tiberio G, Cocchia D. Immunochemical and immunocytochemical study of S-100 protein in rat adipocytes. Brain Res. 1983;262:352-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 86] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Son KH, Son M, Ahn H, Oh S, Yum Y, Choi CH, Park KY, Byun K. Age-related accumulation of advanced glycation end-products-albumin, S100β, and the expressions of advanced glycation end product receptor differ in visceral and subcutaneous fat. Biochem Biophys Res Commun. 2016;477:271-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Zhang J, Zhang L, Zhang S, Yu Q, Xiong F, Huang K, Wang CY, Yang P. HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol Cell Endocrinol. 2017;454:103-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 97. | Fujiya A, Nagasaki H, Seino Y, Okawa T, Kato J, Fukami A, Himeno T, Uenishi E, Tsunekawa S, Kamiya H, Nakamura J, Oiso Y, Hamada Y. The role of S100B in the interaction between adipocytes and macrophages. Obesity (Silver Spring). 2014;22:371-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 98. | Yu R, Kim CS, Kwon BS, Kawada T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring). 2006;14:1353-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 99. | Castoldi A, Naffah de Souza C, Câmara NO, Moraes-Vieira PM. The Macrophage Switch in Obesity Development. Front Immunol. 2015;6:637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 269] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 100. | Oh S, Ahn H, Park H, Lee JI, Park KY, Hwang D, Lee S, Son KH, Byun K. The attenuating effects of pyridoxamine on adipocyte hypertrophy and inflammation differ by adipocyte location. J Nutr Biochem. 2019;72:108173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 101. | Cheng X, Liu YM, Li H, Zhang X, Lei F, Qin JJ, Chen Z, Deng KQ, Lin L, Chen MM, Song X, Xia M, Huang X, Liu W, Cai J, Zhang XJ, Zhou F, Zhang P, Wang Y, Ma X, Xu Q, Yang J, Ye P, Mao W, Xia J, Zhang BH, Guo J, Zhu L, Lu Z, Yuan Y, Wei X, She ZG, Ji YX. Metformin Is Associated with Higher Incidence of Acidosis, but Not Mortality, in Individuals with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab 2020; 32: 537-547. e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 102. | Vitale RJ, Valtis YK, McDonell ME, Palermo NE, Fisher NDL. Euglycemic Diabetic Ketoacidosis with COVID-19 Infection in Patients with Type 2 Diabetes taking SGLT2 Inhibitors. Clin Case Rep. 2020;In press. [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 103. | Hoffman WH, Ishikawa T, Blum J, Tani N, Ikeda T, Artlett CM. Soluble Receptor for Glycation End-products Concentration Increases Following the Treatment of Severe Diabetic Ketoacidosis. J Clin Res Pediatr Endocrinol. 2020;160-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Hoffman WH, Kappler F, Passmore GG, Mehta R. Diabetic ketoacidosis and its treatment increase plasma 3-deoxyglucosone. Clin Biochem. 2003;36:269-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 289] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 106. | Protti A, Russo R, Tagliabue P, Vecchio S, Singer M, Rudiger A, Foti G, Rossi A, Mistraletti G, Gattinoni L. Oxygen consumption is depressed in patients with lactic acidosis due to biguanide intoxication. Crit Care. 2010;14:R22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 107. | El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1009] [Cited by in F6Publishing: 1035] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 108. | Wang DS, Kusuhara H, Kato Y, Jonker JW, Schinkel AH, Sugiyama Y. Involvement of organic cation transporter 1 in the lactic acidosis caused by metformin. Mol Pharmacol. 2003;63:844-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 109. | Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP, Brownlee M, Bierhaus A, Cooper ME, Forbes JM. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 110. | Yu Y, Wang L, Delguste F, Durand A, Guilbaud A, Rousselin C, Schmidt AM, Tessier F, Boulanger E, Neviere R. Advanced glycation end products receptor RAGE controls myocardial dysfunction and oxidative stress in high-fat fed mice by sustaining mitochondrial dynamics and autophagy-lysosome pathway. Free Radic Biol Med. 2017;112:397-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 111. | Shenoy S. Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm Res. 2020;69:1077-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |