Published online Nov 15, 2021. doi: 10.4239/wjd.v12.i11.1908
Peer-review started: May 31, 2021
First decision: July 3, 2021
Revised: July 14, 2021
Accepted: September 15, 2021
Article in press: September 15, 2021
Published online: November 15, 2021
Processing time: 167 Days and 21.3 Hours
In 2017, 35000 Saudi children and adolescents were living with a type 1 diabetes (T1D) diagnosis. Diabetic complications are minimized upon strengthened glycemic regulation. The annual cost of treating diabetic patients with complications was four-fold higher than for patients without complications. The use of flash glucose monitoring (FGM) enables better diabetes treatment and thereby improves glycemic control. Understanding the factors that affect effectiveness of FGM will help enhance the device’s use and management of hospital resources, resulting in improved outcomes.
To investigate factors that affect effectiveness of the FGM system for glycated hemoglobin (HbA1c) levels/glycemic control among T1D patients.
A retrospective empirical analysis of T1D patient records from King Abdul-Aziz University Hospital and Prince Sultan Military Medical City was performed. T1D patients who began FGM between 2017 and 2019 were included.
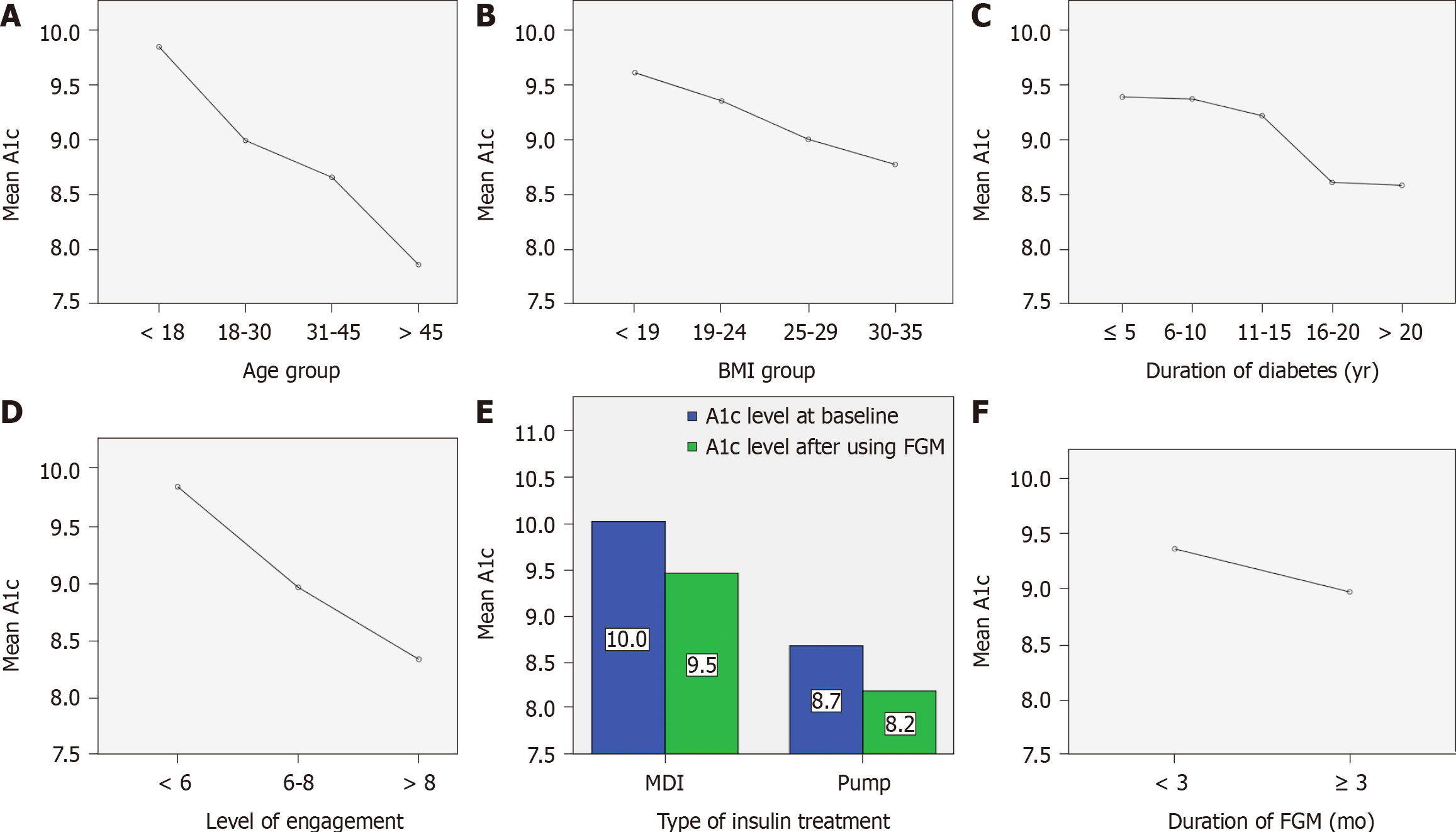
The data included 195 T1D patients (70 males and 125 females) with a mean age of 23.6 ± 8.1 years. Among them, 152 patients used multiple daily injection and 43 used an insulin pump. The difference in HbA1c level from baseline and after using FGM was -0.60 ± 2.10, with a maximum of 4.70 and a minimum of -6.30. There was a statistically significant negative correlation between the independent variables (age, duration of diabetes, level of engagement) and HbA1c. The group with the highest HbA1c mean (9.85) was 18-years-old, while the group with the lowest HbA1c mean (7.87) was 45-years-old. Patients with a low level of engagement (less than six scans per day) had the highest HbA1c mean (9.84), whereas those with a high level of engagement (more than eight scans per day) had the lowest HbA1c mean (8.33).
With proper education, FGM can help people with uncontrolled T1D over the age of 18 years to control their glucose level.
Core Tip: The factors influencing success of flash glucose monitoring are poorly understood in people with type 1 diabetes (T1D). Therefore, we retrospectively investigated the main predictor factors and their relationship with glycemic control/glycated hemoglobin (HbA1c) levels in 195 patients with T1D. Flash glucose monitoring resulted in a clinically significant reduction in HbA1c, and the uncontrolled group (baseline HbA1c > 9) had the highest reduction in HbA1c. Age and level of engagement were negatively associated with HbA1c. Patients in the age group (18-45 years) with a high level of engagement were more likely to experience a higher- reduction in HbA1c.
- Citation: Alhodaib HI, Alsulihem S. Factors influencing the effectiveness of using flash glucose monitoring on glycemic control for type 1 diabetes in Saudi Arabia. World J Diabetes 2021; 12(11): 1908-1916
- URL: https://www.wjgnet.com/1948-9358/full/v12/i11/1908.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i11.1908
In 2017 alone, 35000 Saudi children and adolescents (aged 20 years) were living with a type 1 diabetes (T1D) diagnosis, according to a study from the International Diabetes Federation, with 3900 new cases yearly[1]. Uncontrolled diabetes has an effect on nearly every organ in the body, and good glycemic control lowers the risk of diabetic complications. While the cost of treating diabetic patients with complications was US$ 11706.90 per year, this was reduced to US$ 2746.70 per year for diabetic patients without complications[2]. According to the American Diabetes Association, diabetic patients on multiple daily injections (MDIs) or insulin pumps can monitor blood glucose levels before meals, postprandially, at bedtime, before exercise, when they suspect hypoglycemia, after treating hypoglycemia, and before performing vital activities such as driving[3]. Increasing the number of times one monitors their blood glucose level is linked to lower glycated hemoglobin (HbA1c) levels and fewer complications[4].
The new continuous glucose monitoring devices and flash glucose monitoring (FGM) provide reliable glucose readings for up to 14 d after a 1 h warm-up cycle. This consists of two main components: a portable reader and a disposable, factory-calibrated sensor worn on the back of the upper arm by the patient. The reader is used by the patient to wirelessly scan the sensor and obtain glucose readings. Every minute a sensor measures the glucose concentration in the interstitial fluid. It also automatically records the glucose concentration every 15 min and saves the information in an 8-h log. The use of this technology has a beneficial influence on patient adherence to blood glucose monitoring and glycemic control[2] because it accurately measures interstitial fluid glucose within a reasonable range of error as capillary blood glucose[5-9]. FGM is more costly than standard treatment, and there are no set guidelines for which patients should use it and when they should start using it.
Understanding the factors that affect the effectiveness of FGM will help enhance the device’s use and management of hospital resources, resulting in improved outcomes. Research has shown that using continuous glucose monitoring improves glycemic control by lowering HbA1c levels, reducing the number of hypoglycemic events, increasing time in the target range and reducing the number of hospital visits due to hypoglycemia or ketoacidosis[4,10-12].
The formula of Hulley et al[13] was used to determine the sample size. Accordingly, a sample size of 195 patients was used in the current study. This study was a retrospective study involving 234 patients who had undergone FGM during the study period from the involved research centers (60 patients from King Abdulaziz University Hospital and 174 from Prince Sultan Military Medical City). Patients who were 15 years or older with T1D, used FGM for at least 1 mo, and were capable of checking and controlling their glucose levels themselves based on the data generated by the sensor were included in the study. However, 39 people requiring tighter glycemic control were excluded from the study (13 had type 2 diabetes, 6 had chronic kidney disease, 6 were under the age of 15, and 10 were new to FGM). In the present study, the dependent variable was HbA1c, while the independent variables were age, body mass index (BMI), diabetes length, duration of using FGM, degree of involvement, and type of insulin treatment. Demographic information as well as lab results were extracted from each hospital’s information system. Because each patient’s sensor data was stored in their register, the artificial intelligence was able to derive the degree of commitment, which was determined by the average number of scans per day. Ethical approval was obtained from the King Saud University ethics committee (Ref No: 19/0812/IRB) as well as access letters from both hospitals. Data collection lasted for about 4 wk.
Statistical analysis was performed using SPSS software (Version 23; IBM Corp., Armonk, NY, United States). The dependent variable (HbA1c) was tested for normality using the Kolmogorov-Smirnov and Shapiro-Wilk tests across the independent variables. Differences between groups for unevenly distributed data were analyzed using non-parametric tests (Mann-Whitney test, Kruskal-Wallis test). For the association, Spearman’s correlation coefficient was used. Independent sample t-tests were used for data with two groups (duration of using FGM and type of insulin treatment), and one-way analysis of variance was used for data with three or more groups. Pearson’s correlation coefficient was used for the relationship of data with more than two groups (duration of diabetes and level of engagement). The relationship between the type of insulin treatment (nominal data) and the dependent variable HbA1c was analyzed using cross tabulation.
The demographic characteristics of the included patients are represented in Table 1. The result shows that the average age of the patients was 23.6 years, with almost half (49.7%) of them being between the ages of 18 and 30. The majority of the participants were women (64.1%). In terms of engagement, the majority of patients (48.7%) had a low level of engagement, scanning fewer than six times per day. MDI was chosen as the type of insulin treatment by 77.9% of the participants.
Using the Wilcoxon signed rank test, FGM resulted in a statistically significant reduction in HbA1c (z = -4.535, P = 0.000) with a broad effect size (r = 0.119). From pre-FGM (median = 9.7) to post-FGM (median = 9.0), the median HbA1c score decreased. In 62% of the patients, HbA1c was reduced after FGM, and the majority of them (76.0%) had an HbA1c of more than 9. On the other hand, HbA1c levels increased in 32% of patients, and 39% of them had an HbA1c of less than 8. In 11 patients, there was no difference in HbA1c. The highest rise in HbA1c was 4.7%, while the maximum decrease was 6.3%.
The relationship between age and HbA1c was moderately negative and statistically significant (rs = -0.373, P = 0.000). Kruskal-Wallis test showed a statistically significant difference in mean HbA1c between the age groups (P = 0.001, χ2 = 17.79). The age group under 18 years had the highest HbA1c mean level (9.8 ± 1.5), while the age group over 45 years had the lowest level (7.8 ± 0.8) (Figure 1A).
Although BMI and HbA1c had a weak negative correlation (rs = -0.129, P = 0.068), the correlation was not statistically significant. There was no statistically significant difference in HbA1c between the different BMI categories according to the Kruskal-Wallis test [P = 0.141, χ2 (2) = 5.461] (Figure 1B).
There was a weak negative relationship between diabetes duration and HbA1c, which was statistically significant (rs = -0.162, P = 0.024). A one-way analysis of variance test revealed no statistically significant difference in HbA1c levels between groups of diabetes duration [P = 0.231, F (4,190) = 4.168] (Figure 1C).
The relationship between degree of involvement and HbA1c was moderately negative and statistically significant (r = -0.394, P = 0.000). One-way analysis of variance test showed a statistically significant difference in mean HbA1c between the various levels of engagement (P = 0.000, F = 17.733). The HbA1c level after FGM was significantly lower in those who scanned six to eight times per day (8.9 ± 1.5, P = 0.018) and those who scanned more than eight times per day (8.3 ± 1.3, P = 0.000) compared to those who scanned less than six times per day (9.8 ± 1.7) (Figure 1D).
The form of insulin therapy had a mild relationship with HbA1c, and the correlation was statistically significant (η2 = 0.094, P = 0.000). The MDI group and the insulin pump group had a statistically significant difference in mean HbA1c according to an independent-sample t-test. The MDI group had a higher mean HbA1c (9.5 ± 1.7) than the insulin pump group (8.2 ± 1.2) (P = 0.000, t = 4.49) (Figure 1E).
The length of FGM use and HbA1c had a weak negative relationship, but the correlation was not statistically important (r = -0.116, P = 0.107). Using an independent-sample t-test, no statistically significant difference in mean HbA1c was found between the two classes of FGM duration [t (195) = 1.57, P = 0.765]. Patients who used FGM for less than 3 mo had an HbA1c of 9.4 ± 1.7 compared to those who used it for more than 3 mo, who had an HbA1c of 9.0 ± 1.8 (Figure 1F).
Several medical associations are now taking essential steps to help patients with T1D and type 2 diabetes regulate their glycemic index. Monitoring the amount of glucose in a diabetic patient’s body is an important part of diabetes management and the treatment process. FGM is an excellent method for assessing the level of “glucose metabolic disturbance” and directing the treatment process. Diabetes affects a large number of children in Saudi Arabia. As a result, FGM instruments are currently used to calculate the amount of glucose in the blood. This is achieved by implanting a tracker under the skin for 14 d to monitor blood glucose, and the patient must check the sensor’s reader and read the blood glucose level over the previous 8 h.
FGM is expensive to use because one sensor costs $89 and test strips cost $0.75 each. The National Health Service has developed some guidelines for those who are eligible to receive funding for FGM. These individuals must be on “intensive insulin therapy” and plan to attend an education session on the topic of FGM, be able to scan their glucose levels at least six times a day, report their findings with the National Health Service clinic, and participate in a “diabetes self-management” education program[14]. Determining the effectiveness of FGM will help to justify the cost in patients who will receive the greatest benefit.
In the present study, FGM resulted in a substantial and clinically significant reduction in HbA1c. This result confirms findings from previous studies that looked at the efficacy of FGM in diabetic patients[15-17]. In the current study, HbA1c was decreased by at least 0.5 in 104 patients, with 84 of them having a baseline HbA1c of more than 9. After using FGM, the uncontrolled group had the greatest reduction in HbA1c levels at baseline, while the monitored group had no reduction or an increase in HbA1c. This is in line with the findings of Tyndall et al[14] who used FGM on 900 T1D patients and followed them for 245 d. According to the findings of their report, some patients’ HbA1c levels changed. Individuals who did not use FGM had no improvement in their HbA1c during the same time span. The change in A1c was the study’s primary outcome.
Patients under the age of 18 years had the smallest change in HbA1c levels after using FGM, out of all age groups. Our results in the present study confirm those from a previous study that showed that HbA1c levels in patients under the age of 18 worsen over time[18]. Patients in the 18-45 years age group showed the greatest decrease in HbA1c levels relative to other age groups. This was consistent with the findings from the Paris et al[19] study, which was conducted to determine the effectiveness of FGM on HbA1c in 120 T1D patients between the ages of 18-76 years. The retrospective study reported positive improvements in HbA1c, with FGM being especially helpful for patients with a baseline HbA1c of 7.5%.
According to the study by Campbell et al[20] of 76 participants, including children and adolescents, FGM supported children with T1D with an average HbA1c reduction of 0.4 (from 7.9 to 7.5), indicating that patients with T1D who used FGM had better glycemic control. Just 13 patients (6.1%) in the current study began FGM within the first year of diagnosis, which was used to compare early and late initiation of FGM after diagnosis. A significant but weak correlation between late initiation and lower HbA1c was observed, which contradicts the findings from a previous study that looked at the usability and efficacy of starting FGM within the first year of T1D diagnosis. That study observed that patients who began FGM earlier had greater glycemic regulation than those who started FGM later[11]. Anderson and his colleagues[21] contrasted the HbA1c levels between long-term and short-term usage of continuous glucose monitoring in 10 outpatient clinics over a 1.1 year period in a retrospective sample and observed that long-term users had lower HbA1c levels than short-term users, which was statistically significant. In regard to patients who were on insulin pump treatment, 41.8% had baseline HbA1c < 8 compared to only 19.7% of patients in the MDI group. This may have influenced the observed association between HbA1c levels after FGM and the insulin pump group. Over a 2.5 year duration, Mulinacci et al[11] observed in 396 new T1D patients that MDI patients with FGM had a 1.5% lower HbA1c compared to MDI patients without FGM. Patients who were treated with an insulin pump and started on FGM had a 0.7% lower HbA1c than those who were on an insulin pump but not on FGM. The study concluded that, regardless of insulin treatment type, early use of FGM was helpful in lowering blood glucose levels in T1D patients.
Because the sensor measures interstitial fluid glucose, the accuracy of FGM is inversely related to BMI, which can ultimately affect the HbA1c level[22]. Using 58 T1D patients aged 18-years-old to 64-years-old, the accuracy of interstitial glucose was compared to FGM. Two sensors were implanted in each participant, and a record was taken at 10 h, 12 h, 24 h and 72 h after the insertion. The results showed the median and mean absolute relative difference values were 9.3% and 12.8%, respectively. The study observed that FGM sensor measurements were as reliable as the venous measurements, but that BMI had a minor impact on accuracy[23]. The accuracy of the FGM sensor was not affected by BMI in the Bailey et al[24] report, which is consistent with what was observed in the current study. We did not identify any correlation between BMI and HbA1c.
High levels of interaction resulted in lower HbA1c levels, which was consistent with previous studies[19,25,26]. The 12 mo observational study involving 120 people conducted by Paris et al[19] to determine FGM use in T1D patients with frequent hypoglycemia showed that HbA1c levels improved after 3 mo of FGM use in certain patients. The study also found a clear association between HbA1c and scanning frequency, which matches the results of the current study. As a result, patient education is crucial in motivating FGM users to scan at least six times per day to collect 100% of the data (before and after meals, before and after exercise, and before sleep).
This study had some limitations. While retrospective analysis helps us to collect data over a longer period of time, it is impossible to monitor the confounding factors that can influence HbA1c levels with this type of study design. Another significant drawback of this study was the lack of FGM sensor availability during the research period. Due to registration issues with a few patients, it was not possible to obtain the most recent HbA1c readings. The number of people involved in the sample was not equal because each hospital had different requirements for starting a diabetic patient on FGM.
FGM is an acceptable technology for T1D patients over the age of 18 years who are committed to monitoring their glucose levels at least six times a day because it offers real-time information. As a result, it can assist patients in maintaining glucose regulation by making the right decisions. FGM is a secure procedure with a high level of consumer acceptance in real-life situations.
Type 1 diabetes (T1D) affects a large number of children and adolescents in Saudi Arabia, and as a result flash glucose monitoring (FGM) devices are widely used. The factors influencing the effectiveness of FGM are poorly understood in people with T1D.
FGM is more expensive than standard treatment, and there is no guideline for which patients should receive FGM or when they should start FGM. Each hospital in Saudi Arabia has different requirements for starting a diabetic patient on FGM. The effectiveness of FGM can be influenced by many factors, including age, body mass index, type of insulin treatment, duration of diabetes, duration of using FGM, and level of patient engagement.
We investigated the association between glycated hemoglobin (HbA1c) levels after using FGM and potential predictor factors in a population with T1D. The ultimate goal was to help develop national guidelines for those who are eligible to receive funding for FGM, which in turn will enhance the utilization of the device and manage hospital resources, resulting in improved outcomes.
In this retrospective cohort study of 195 T1D patients aged 15 years and above who had used FGM for at least 1 mo, demographic and clinical parameters and related data were extracted from patient records at two hospitals.
FGM in this study resulted in a clinically significant reduction in HbA1c (-0.6 ± 2.1). The uncontrolled group (baseline HbA1c > 9) had the largest reduction in HbA1c levels. There was a statistically significant moderate and negative association between age and level of engagement and HbA1c levels. Patients in the age group of 18-years-old to 45-years-old with a high level of engagement were more likely to demonstrate a large reduction in HbA1c levels. The relationships between HbA1c and other factors varied between no association to weak association.
FGM is a more effective technology for T1D patients over the age of 18 years who are committed to checking their glucose level at least six times a day.
To identify the relationships between HbA1c levels and predictor factors on the long-term use of FGM, a multicenter, prospective, large-scale study on patients with T1D should be conducted in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Su G S-Editor: Wu YXJ L-Editor: A P-Editor: Wang LYT
| 1. | Robert AA, Al-Dawish A, Mujammami M, Dawish MAA. Type 1 Diabetes Mellitus in Saudi Arabia: A Soaring Epidemic. Int J Pediatr. 2018;2018:9408370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Alramadan MJ, Magliano DJ, Alhamrani HA, Alramadan AJ, Alameer SM, Amin GM, Alkharras WA, Bayaseh NA, Billah B. Lifestyle factors and macro- and micro-vascular complications among people with type 2 diabetes in Saudi Arabia. Diabetes Metab Syndr. 2019;13:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018; 41: S55-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 591] [Article Influence: 84.4] [Reference Citation Analysis (1)] |
| 4. | Ziegler R, Heidtmann B, Hilgard D, Hofer S, Rosenbauer J, Holl R; DPV-Wiss-Initiative. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 5. | Cao B, Wang R, Gong C, Wu D, Su C, Chen J, Yi Y, Liu M, Liang X, Li W. An Evaluation of the Accuracy of a Flash Glucose Monitoring System in Children with Diabetes in comparison with Venous Blood Glucose. J Diabetes Res. 2019;2019:4845729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Ji L, Guo X, Guo L, Ren Q, Yu N, Zhang J. A Multicenter Evaluation of the Performance and Usability of a Novel Glucose Monitoring System in Chinese Adults With Diabetes. J Diabetes Sci Technol. 2017;11:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Ólafsdóttir AF, Attvall S, Sandgren U, Dahlqvist S, Pivodic A, Skrtic S, Theodorsson E, Lind M. A Clinical Trial of the Accuracy and Treatment Experience of the Flash Glucose Monitor FreeStyle Libre in Adults with Type 1 Diabetes. Diabetes Technol Ther. 2017;19:164-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Hulse A, Rai S, Prasanna Kumar KM. Evaluation of accuracy of ambulatory glucose profile in an outpatient setting in children with type 1 diabetes. Indian J Endocrinol Metab. 2016;20:643-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Edge J, Acerini C, Campbell F, Hamilton-Shield J, Moudiotis C, Rahman S, Randell T, Smith A, Trevelyan N. An alternative sensor-based method for glucose monitoring in children and young people with diabetes. Arch Dis Child. 2017;102:543-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Al Hayek AA, Robert AA, Al Dawish MA. Evaluation of FreeStyle Libre Flash Glucose Monitoring System on Glycemic Control, Health-Related Quality of Life, and Fear of Hypoglycemia in Patients with Type 1 Diabetes. Clin Med Insights Endocrinol Diabetes. 2017;10:1179551417746957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Mulinacci G, Alonso GT, Snell-Bergeon JK, Shah VN. Glycemic Outcomes with Early Initiation of Continuous Glucose Monitoring System in Recently Diagnosed Patients with Type 1 Diabetes. Diabetes Technol Ther. 2019;21:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Charleer S, Mathieu C, Nobels F, De Block C, Radermecker RP, Hermans MP, Taes Y, Vercammen C, T'Sjoen G, Crenier L, Fieuws S, Keymeulen B, Gillard P; RESCUE Trial Investigators. Effect of Continuous Glucose Monitoring on Glycemic Control, Acute Admissions, and Quality of Life: A Real-World Study. J Clin Endocrinol Metab. 2018;103:1224-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 13. | Hulley SB, Cummings SR, Browner WS, Grady D, Newman TB. Designing clinical research: an epidemiologic approach. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013: 79. |
| 14. | Tyndall V, Stimson RH, Zammitt NN, Ritchie SA, McKnight JA, Dover AR, Gibb FW. Marked improvement in HbA1c following commencement of flash glucose monitoring in people with type 1 diabetes. Diabetologia. 2019;62:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Anjana RM, Kesavadev J, Neeta D, Tiwaskar M, Pradeepa R, Jebarani S, Thangamani S, Sastry NG, Brijendra Kumar S, Ramu M, Gupta PPK, Vignesh J, Chandru S, Kayalvizhi S, Jagdish PS, Uthra SCB, Lovelena M, Jyoti S, Suguna Priya S, Kannan A, Mohan V, Unnikrishnan R. A Multicenter Real-Life Study on the Effect of Flash Glucose Monitoring on Glycemic Control in Patients with Type 1 and Type 2 Diabetes. Diabetes Technol Ther. 2017;19:533-540. [PubMed] [DOI] [Full Text] |
| 16. | Mancini G, Berioli MG, Santi E, Rogari F, Toni G, Tascini G, Crispoldi R, Ceccarini G, Esposito S. Flash Glucose Monitoring: A Review of the Literature with a Special Focus on Type 1 Diabetes. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Dover AR, Stimson RH, Zammitt NN, Gibb FW. Flash Glucose Monitoring Improves Outcomes in a Type 1 Diabetes Clinic. J Diabetes Sci Technol. 2017;11:442-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Clements MA, Foster NC, Maahs DM, Schatz DA, Olson BA, Tsalikian E, Lee JM, Burt-Solorzano CM, Tamborlane WV, Chen V, Miller KM, Beck RW; T1D Exchange Clinic Network. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes. 2016;17:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 19. | Paris I, Henry C, Pirard F, Gérard AC, Colin IM. The new FreeStyle libre flash glucose monitoring system improves the glycaemic control in a cohort of people with type 1 diabetes followed in real-life conditions over a period of one year. Endocrinol Diabetes Metab. 2018;1:e00023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Campbell FM, Murphy NP, Stewart C, Biester T, Kordonouri O. Outcomes of using flash glucose monitoring technology by children and young people with type 1 diabetes in a single arm study. Pediatr Diabetes. 2018;19:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Anderson J, Attvall S, Sternemalm L, Pivodic A, Fahlén M, Hanås R, Ekeroth G, Lind M. Effect on glycemic control by short- and long-term use of continuous glucose monitoring in clinical practice. J Diabetes Sci Technol. 2011;5:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Massa GG, Gys I, Op 't Eyndt A, Bevilacqua E, Wijnands A, Declercq P, Zeevaert R. Evaluation of the FreeStyle® Libre Flash Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. Horm Res Paediatr. 2018;89:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Weinstein RL, Schwartz SL, Brazg RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30:1125-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol Ther. 2015;17:787-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 502] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 25. | Jangam S, Dunn T, Xu Y, Hayter G, Ajjan RA. Flash glucose monitoring improves glycemia in higher risk patients: a longitudinal, observational study under real-life settings. BMJ Open Diabetes Res Care. 2019;7:e000611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Laurenzi A, Caretto A, Barrasso M, Mario BA, Molinari C, Zanardini A, Scavini M. Frequency of Flash Glucose Monitoring Scans and Hemoglobin A1c in Real Life. Diabetes. 2018;67. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |