Published online Jan 15, 2021. doi: 10.4239/wjd.v12.i1.69
Peer-review started: May 18, 2020
First decision: September 24, 2020
Revised: November 13, 2020
Accepted: December 22, 2020
Article in press: December 22, 2020
Published online: January 15, 2021
Processing time: 234 Days and 7.4 Hours
In spite of an increase in the incidence and prevalence of diabetes mellitus (DM) and Alzheimer’s disease (AD) in the aging population worldwide, limited attention has been paid to their potential association.
To investigate the association of DM and cardiometabolic syndrome (CMS, a precursor to DM) with risk of incident AD among postmenopausal women.
Postmenopausal women aged 50-79 (n = 63117) who participated in the U.S. Women’s Health Initiative Observational Study (WHIOS), recruited in 1993-1998, without baseline AD and followed up through March 1, 2019, were analyzed. AD was classified by participant-reported history of doctor-diagnosis of incident AD in the WHIOS. DM was defined by participant-report or treated because of diabetes or serum glucose concentrations ≥ 126 mg/dL. CMS was defined as having ≥ 3 of five CMS components: large waist circumference, high blood pressure, elevated triglycerides, elevated glucose, and low high-density lipoprotein cholesterol. The associations of DM and CMS with AD were analyzed using Cox’s proportional hazards regression analysis.
During a median follow-up of 20 years (range: 3.36 to 23.36 years), of 63117 participants, 8340 developed incident AD. Women with DM had significantly higher incidence of AD [8.5, 95% confidence interval (CI): 8.0-9.0 per 1000 person-years (PY)] than those without DM (7.1, 95%CI: 6.9-7.2 per 1000 PY). Multivariate Cox’s regression analysis indicated that women with DM or CMS had a significantly higher risk of AD than those without DM or CMS. The corresponding hazard ratios [HR (95%CI)] were 1.22 (1.13-1.31, P < 0.001) in subjects with DM, and 1.18 (1.09-1.27, P < 0.001) in subjects with CMS. The HRs diminished with age and became non-significant in the oldest age group.
During a median follow-up of 20 years, DM and CMS were significantly associated with the risk of AD among postmenopausal women. More specifically, women aged 50-69 with DM or CMS vs those without these conditions had significantly higher relative risks of AD than the relative risks of AD in those aged 70-79 with DM or CMS vs those without DM or CMS.
Core Tip: Data from population-based studies on the association of diabetes and cardiometabolic syndrome (CMS) with risk of Alzheimer’s disease (AD) was limited. This study, using data from one of the largest population-based cohort studies in the United States women aged 50-79 at baseline to test a hypothesis that diabetes and CMS are significantly associated with the risk of AD. This analysis is one of the first studies to prospectively test this hypothesis using a large-scale longitudinal cohort data. Findings from the study add new evidence to the body of research literature and provide new insights into the prevention of AD through control of diabetes and CMS.
- Citation: Liu L, Gracely EJ, Yin X, Eisen HJ. Impact of diabetes mellitus and cardiometabolic syndrome on the risk of Alzheimer’s disease among postmenopausal women. World J Diabetes 2021; 12(1): 69-83
- URL: https://www.wjgnet.com/1948-9358/full/v12/i1/69.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i1.69
Diabetes mellitus (DM) and Alzheimer's disease (AD, an irreversible, progressive brain disorder, which is the most common type of dementia among older adults) pose serious public health problems in the United States and worldwide. Today, about 34.1 million adults aged 18 years or older have DM (13.0% of United States adults). The prevalence rate of DM is 4.2% in United States adults aged 18-44 years old, 17.5% in adults aged 45-64, and 26.8% among the elderly aged 65 and older[1]. Similar to DM, increased incidence and prevalence of AD have been observed in the United States. An estimated 5.4 million Americans suffered from AD in 2016 and that number is likely to increase to 13.8 million by 2050[2-4]. AD affects one in nine people aged ≥ 65. The risk of AD is one out of three in ages ≥ 85. Meanwhile, about two thirds of patients diagnosed with AD are women, which is partly attributable to women having a longer life expectancy than men[5]. Furthermore, an estimated 121000 Americans died due to AD in 2017[3]. AD is the nation’s sixth leading cause of death. In 2015, the direct cost of treating the disease totaled $236 billion, and economists estimate that the unpaid labor provided by caregivers to AD’s patients amounted to another $220 billion in 2015[3,4]. AD mainly affects older people and it was traditionally viewed as a predominately inherited disease for many years. However, recent studies suggest that cardiovascular and metabolic disorders including hypertension, dysglycemia, central adiposity and dyslipidemia may play a pivotal role in the development of AD[6,7]. The cluster of these risk factors is commonly called cardiometabolic syndrome (CMS or called metabolic syndrome)[8,9]. DM and CMS are the established risk factors for cardiovascular diseases (CVD)[10-14]. Consequently, whether AD or AD related dementia are similar to CVD as a brain complication of diabetes has been hypothesized and studied in the recent years[6,7,15,16]. Most of these results of the association of DM and CMS with risk of AD or AD related dementia were reported from hospital-based or small sample size of population-based observational studies[4,6,7,16-21]. Given the complex status of disease in participants in hospital-based studies and small sample sizes of the previous population-based studies, selection bias due to nonrepresentative participants and potential misclassification attributable to multiple comorbidities may occur using data from hospital-based studies and loss of statistical power in hypothesis testing from studies with small sample sizes. Furthermore, although two-thirds of patients with AD are women, only few studies were conducted in women. Studies of the associations between cardiometabolic disorders and AD are in their infancy compared to other areas of studies of diabetic complications. In the study, we hypothesize that women with DM or CMS have significantly higher risk of AD than those without DM or CMS. To test this hypothesis, we have two specific aims: (1) To examine the long-term effect of DM and CMS on the risk of incident AD, and (2) To examine whether there is a potential modifying effect of age on AD risk using data from a large-scale population-based observational cohort study among women aged 50 to 79 years.
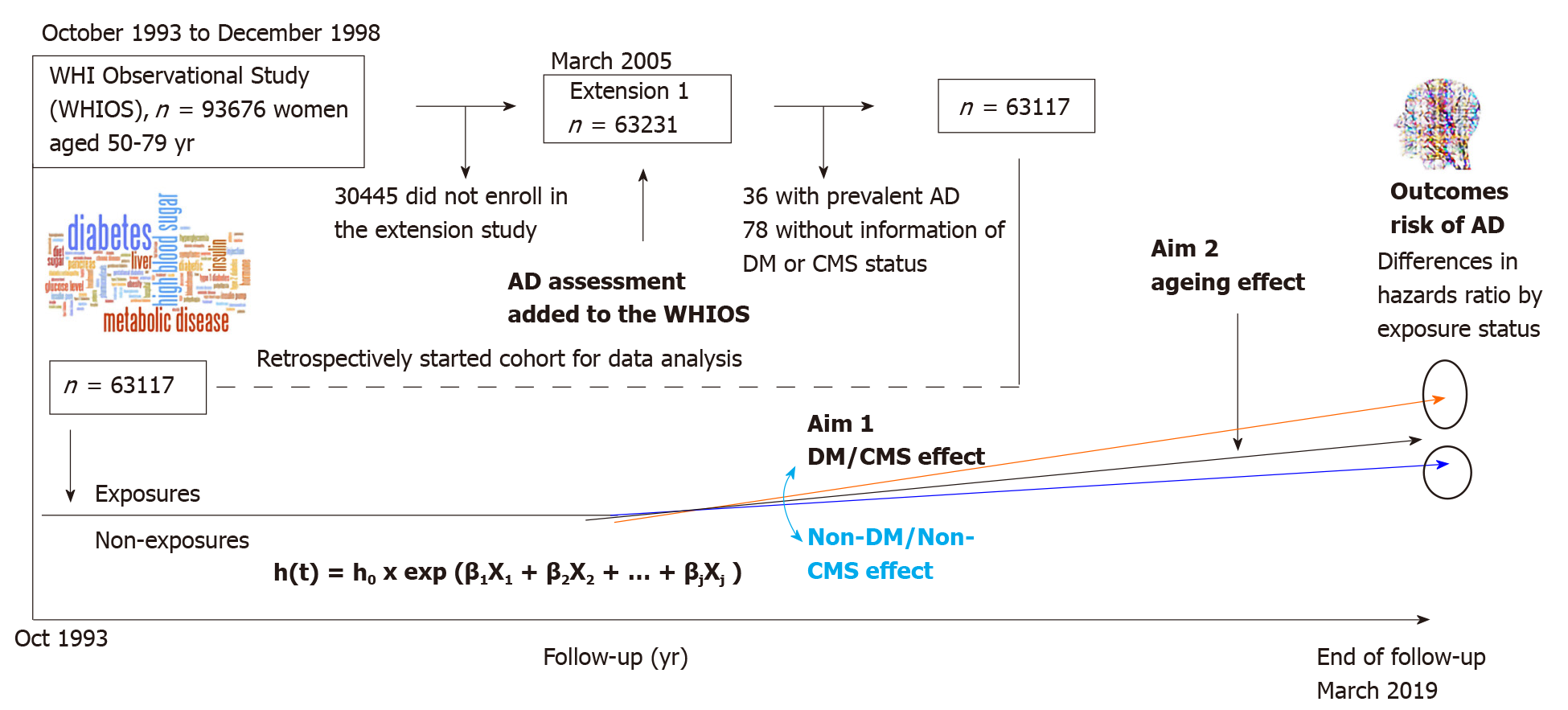
The study used data from the U.S. Women’s Health Initiative Observational Study (WHIOS) with a prospective analysis design[22-24]. The WHIOS, launched in 1993 is a longitudinal population-based observational study. The study design and materials were approved by institutional review boards at each survey center. Details of the scientific rationale, eligibility requirements and survey designs have been published[25]. In brief, a total of 93676 postmenopausal women aged 50-79 were enrolled from October 1, 1993 to December 31, 1998 at 40 United States clinical centers nationwide and participated in the study to examine risk factors for cardiovascular disease, cancers, diabetes and osteoporotic fractures. The inclusion criteria of the participants were those who planned to reside in the survey area for at least 3 years and had medical conditions predictive of survival more than 3 years, and no complicating conditions such as alcoholism, drug dependency and no baseline AD and dementia. Participants received annual follow-ups via mailings sent from the Clinical Coordinating Center (CCC) and were invited to have Clinical Center visits at year 3 after enrollment to update selected baseline data, obtain additional risk factor data, and collect a blood specimen. The annual mailing follow-ups consisted of a cover letter, a self-administered medical history update (i.e., health outcomes) and assessment of exposures to risk factors. Non-responders received two additional mailings and telephone contacts by clinical center staff. When these efforts were not successful, clinical center staff contacted proxies to determine the location and status of the participant and to collect information on health outcomes. The initial WHIOS ended in March 2005. Then it continued to follow up the study participants who stay in the survey cohort, that it is designed as the WHIOS extension studies, with its Extension 1 from 2005 to 2010, and Extension 2 from 2010 to 2020. In the Extension studies, participants’ health outcomes are followed by mails using self-administrated survey questionnaires. The assessments of incident AD started from the WHIOS Extension 1 and continued to its Extension 2[23,24]. Starting from the Extension 1, there were 63231 participants who had the assessments of AD status using a self-administered survey instrument. In the analysis, we used the recently available ended-follow-up data through March 1, 2019. This study was reviewed and approved by Drexel University Institutional Review Board and data was obtained from the U.S. National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository. In the analysis, of 63231 participants, 36 with prevalent AD and 78 without information on DM or CMS were excluded. The final analysis sample size is 63117 in the report (listed in the rectangular box of Figure 1).
In the WHIOS, AD and chronic conditions, including hypertension, hyper-cholesteremia, diabetes, heart attack, coronary heart disease, heart failure and AD were classified by self-administered standard survey instruments[23,24]. These chronic conditions were determined by the WHIOS participants' self-reported answers to questions “have you been diagnosed or treated because of the disease?” An answer “yes” was classified as having the disease. The survey assessment of incident AD started from WHIOS Extension 1 (2005) and ongoing. Annual survey self-administered questionnaires were mailed to the WHIOS participants’ households to collect major health outcomes data through participants self-report or a family member or those who knew the study participants’ health status. The assessment of AD was classified by a question that asked, “has a doctor told you for the first time that you have moderate or severe memory problems, for example, dementia or Alzheimer’s?” Of the total classified incident AD cases, 40% were reported by the WHIOS participants (i.e., self-report), 13% by a family member or friend of the WHIOS study participants, 2% by a healthcare provider of the WHIOS study participants, and 45% by any others who knew the participants’ AD status. Duration (days) of follow-up for each participant was calculated from the participant’s enrollment in the survey to the date of their first physician-diagnosis of AD or to the date of the end of the follow-up for those who had no AD or for those who ended the survey earlier due to any other reasons, whatever which came first[26].
Diabetes was determined by WHIOS participants' self-reported answer to, “have you been diagnosed or treated because of diabetes?” The answer “yes” was classified as having diabetes. In addition, subjects who had a blood sample with fasting glucose ≥ 126 mg/dL or those using glucose-lowering medication were also classified as DM. For CMS, there are several definitions[8,9,27,28]. We used the criteria proposed by the U.S. National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) with a minor modification by the American Heart Association (AHA), because this definition is most frequently used in the United States[28]. The AHA defines CMS as having ≥ 3 of the following five components: (1) Central adiposity (assessed by waist circumference > 88 cm in women); (2) Elevated fasting blood triglyceride (TG ≥ 150 mg/dL); (3) Reduced blood high-density lipoprotein cholesterol (HDL-C < 50 mg/dL in women); (4) Elevated blood pressure (systolic blood pressure ≥ 130 or diastolic blood pressure ≥ 85 mmHg) or those who are taking antihypertensive medications; and (5) Elevated blood fasting glucose (≥ 110 mg/dL) or those who had self-reports of physician-diagnosis of pre-diabetes or who have glucose-lowering medication use. In the WHIOS participants with AD status assessments, blood TG, HDL-C and glucose were tested in a subsample of 4443 participants. To estimate the total prevalent CMS, we applied the WHIOS participants’ self-reports of physician-diagnosis of hypercho-lesterolemia as a surrogate for the estimate of elevated TG among those who had no blood sample measures of TG[29,30]. Meanwhile, the WHIOS participants’ self-reported angina was applied to serve as a surrogate for the estimate of reduced HDL-C for those who had no blood sample measures of HDL-C because there is a well-established association between reduced HDL-C and angina risk[29]. This method of using a surrogate to estimate CMS has been applied and accepted by several large-scale observational population-based studies[9,29,30].
Covariates included participants’ age (years), race/ethnicity (self-reported, White, African American, the other race/ethnicity group), current marital status (never or separated, widowed, or married), educational attainments (grouped as ≤ high school, > higher school or completed associate degree, and ≥ college), average annual family income (grouped as < 35000, 35000-49999, 50000-74999, and ≥ 75000 US $/year), health insurance status (including Medicare and pre-paid private health insurance), hormone therapy use (yes/no), smoking (defined as never, former (irrespective of the time since quitting) and current)), and self-reported physician-diagnosis of chronic conditions (myocardial infarction, stroke and peripheral artery disease).
To test the study hypothesis, we conducted a serial analysis. In the first analysis, baseline characteristics of participants were described by DM and CMS status. Differences in demographic characteristics, socioeconomic status, smoking, and medical history of chronic conditions by DM and CMS status were tested using Student t-tests for continuous variables, and Chi-square tests for categorical variables. In the second analysis, the incidence rates of AD per 1000 person-years of follow-up, and 95% confidence intervals (95%CI) of the incidence rates, were estimated using SAS Proc GEMOND. Differences in incident AD rates between participants with DM or with CMS and those without DM or without CMS were tested using Wald Chi-square tests[31,32]. In the third analysis, multivariate-adjusted Cox’s proportional hazards (PH) regression was applied to estimate the associations of DM and CMS with the risk of AD. Cox’s PH assumption were tested by using Kaplan-Meier curves [i.e., assessed by the graph of the log(-log(survival) vs log of survival time)], and including time dependent covariates in the Cox models[33,34]. Hazard ratios (HRs) and their 95%CI of DM and CMS associated with the risk of incident AD were estimated in three multivariate-adjusted models for key covariates because these covariates are strongly correlated with both the study exposures and the outcomes. Model 1 adjusted for age, race/ethnicity, and marital status. Model 2 adjusted for covariates that were included in Model 1 plus education, family income, health insurance, smoking, and history of hormone therapy use. Model 3 adjusted for covariates that were included in Model 2 plus medical history of myocardial infarction, stroke, and peripheral arterial disease.
To test whether there are modifying effects of age differences on the associations of DM and CMS with risks of AD, stratification analyses were performed by three 10-year age groups (50-59, 60-69, and 70-79). Interaction effects between age stratum (the 3 10-year groups), DM and CMS (age × DM and age × CMS) on the risk of AD were examined based on a 1-degree-of-freedome test using maximum likelihood estimates using the total combined sample dataset. The analysis framework is shown in Figure 1.
Sensitivity analysis: We performed sensitivity analyses to take into consideration the accuracy of the assessment of AD by repeating the analysis separately for the data sources who completed the self-administered survey questionnaire: (1) For those who completed the survey questionnaire themselves, (2) For those with surveys completed by a family member or a friend of the WHIOS study participants, (3) For those with surveys completed by a health care provider for the WHIOS study participants, and (4) For those with surveys completed by others who knew the participant’s health conditions. Of them, groups 2 and 3 had relatively small number of AD reports.
All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC 2018). A two-sided P value ≤ 0.05 was considered statistically significant.
Of 63117 participants, Table 1 shows that participants with DM (n = 7255) had a significantly higher mean age than those without DM (n = 55862), (ages 63.2 vs 62.8 years old, P < 0.001). African Americans had the highest prevalence of DM (24.4%) followed by the "other" race/ethnicity group (16.5%) and White (10.3%) (P < 0.001). Married participants, those who had educational attainments of college or higher, or those who had the highest family average incomes had the lowest prevalence of DM compared to their counterparts (P < 0.001). Participants with Medicare (a federal health insurance program for adults aged 65 and older in the United States) had higher prevalence of DM than those without Medicare (12.2% vs 11.1%, P < 0.001). Women with current hormone therapy use had the lowest prevalence of DM compared to those who never used or used before. Participants with hypertension, myocardial infarction, angina, stroke or peripheral artery disease had significantly higher prevalence of DM than those without these conditions. Similar to DM, there were significant differences in mean age and the other factors between participants with CMS and those without CMS (Table 2).
| By DM status | |||||
| Non-DM | DM | ||||
| n | mean, % | n | mean, % | P value | |
| Age, mean (SD), yr | 55862 | 62.9 (7.2) | 7255 | 63.2 (7.0) | < 0.001 |
| Age group, No., % | < 0.001 | ||||
| 50-59 | 19398 | 89.4 | 2295 | 10.6 | |
| 60-69 | 25068 | 87.9 | 3467 | 12.2 | |
| 70-79 | 11396 | 88.4 | 1493 | 11.6 | |
| Race/ethnicity, No., % | < 0.001 | ||||
| White | 49927 | 89.7 | 5745 | 10.3 | |
| Africa American | 2706 | 75.7 | 871 | 24.4 | |
| Others | 3229 | 83.5 | 639 | 16.5 | |
| Marital status1, No., % | < 0.001 | ||||
| Never or separated | 10645 | 87.1 | 1579 | 12.9 | |
| Widowed | 8139 | 86.3 | 1296 | 13.7 | |
| Presently married | 36869 | 89.5 | 4341 | 10.5 | |
| Education level2, No., % | < 0.001 | ||||
| ≤ High School | 9484 | 84.7 | 1707 | 15.3 | |
| > HS and Associate | 19398 | 87.1 | 2873 | 12.9 | |
| ≥ College | 26589 | 91.1 | 2608 | 8.9 | |
| Annual family income (US$), No., % | < 0.001 | ||||
| < 35000 | 17099 | 84.8 | 3073 | 15.2 | |
| 35000-49999 | 10809 | 88.5 | 1401 | 11.5 | |
| 50000-74999 | 11685 | 89.9 | 1306 | 10.1 | |
| ≥ 75000 | 12902 | 92.4 | 1055 | 7.6 | |
| Medicare, No., % | < 0.001 | ||||
| No | 35403 | 88.9 | 4402 | 11.1 | |
| Yes | 20034 | 87.8 | 2787 | 12.2 | |
| Pre-paid private insurance, No., % | 0.13 | ||||
| No | 32403 | 88.4 | 4269 | 11.6 | |
| Yes | 23034 | 88.7 | 2920 | 11.3 | |
| Hormone usage status, No., % | < 0.001 | ||||
| Never used | 20552 | 86.6 | 3180 | 13.4 | |
| Past user | 7901 | 87.7 | 1104 | 12.3 | |
| Current user | 27368 | 90.2 | 2963 | 9.8 | |
| Smoking status, No., % | 0.46 | ||||
| Never | 28078 | 88.6 | 3609 | 11.4 | |
| Past | 24222 | 88.5 | 3140 | 11.5 | |
| Current | 2908 | 87.9 | 401 | 12.1 | |
| Medical history, No., % | |||||
| Hypertension | 20494 | 81.8 | 4566 | 18.2 | < 0.001 |
| Myocardial infarction | 1746 | 78.9 | 467 | 21.1 | < 0.001 |
| Angina | 1080 | 79.1 | 285 | 20.9 | < 0.001 |
| Stroke | 1802 | 81.1 | 421 | 18.9 | < 0.001 |
| Peripheral artery disease | 766 | 75.6 | 247 | 24.4 | < 0.001 |
| By CMS status | |||||
| Non-CMS | CMS | ||||
| n | mean, % | n | mean, % | P value | |
| Age, mean (SD), yr | 55485 | 62.8 (7.2) | 7632 | 63.7 (6.9) | < 0.001 |
| Age group, No., % | < 0.001 | ||||
| 50-59 | 19522 | 90.0 | 2171 | 10.0 | |
| 60-69 | 24771 | 86.8 | 3764 | 13.2 | |
| 70-79 | 11192 | 86.8 | 1697 | 13.2 | |
| Race/ethnicity, No., % | < 0.001 | ||||
| White | 50415 | 90.6 | 5257 | 9.4 | |
| Africa American | 1996 | 55.8 | 1581 | 44.2 | |
| Others | 3074 | 79.5 | 794 | 20.5 | |
| Marital status1, No., % | < 0.001 | ||||
| Never or separated | 10472 | 85.7 | 1752 | 14.3 | |
| Widowed | 7946 | 84.2 | 1489 | 15.8 | |
| Presently married | 36852 | 89.4 | 4358 | 10.6 | |
| Education level2, No., % | < 0.001 | ||||
| ≤ High School | 9207 | 82.3 | 1984 | 17.7 | |
| > HS and Associate | 19253 | 86.4 | 3018 | 13.6 | |
| ≥ College | 26641 | 91.2 | 2556 | 8.8 | |
| Annual family income (US$), No., % | < 0.001 | ||||
| < 35000 | 16660 | 82.6 | 3512 | 17.4 | |
| 35000-49999 | 10710 | 87.7 | 1500 | 12.3 | |
| 50000-74999 | 11734 | 90.3 | 1257 | 9.7 | |
| ≥ 75000 | 13056 | 93.5 | 901 | 6.5 | |
| Medicare, No., % | < 0.001 | ||||
| No | 35329 | 88.8 | 4476 | 11.2 | |
| Yes | 19741 | 86.5 | 3080 | 13.5 | |
| Pre-paid private insurance, No., % | 0.084 | ||||
| No | 32178 | 87.7 | 4494 | 12.3 | |
| Yes | 22892 | 88.2 | 3062 | 11.8 | |
| Hormone usage status, No., % | < 0.001 | ||||
| Never used | 20136 | 84.8 | 3596 | 15.2 | |
| Past user | 7802 | 86.6 | 1203 | 13.4 | |
| Current user | 27502 | 90.7 | 2829 | 9.3 | |
| Smoking status, No., % | 0.027 | ||||
| Never | 27885 | 88.0 | 3802 | 12.0 | |
| Past | 24092 | 88.0 | 3270 | 12.0 | |
| Current | 2861 | 86.5 | 448 | 13.5 | |
| Medical history, No., % | |||||
| Diabetes | 2740 | 37.8 | 4515 | 62.2 | < 0.001 |
| Hypertension | 18928 | 75.5 | 6142 | 24.5 | < 0.001 |
| Myocardial infarction | 1695 | 76.6 | 518 | 23.4 | < 0.001 |
| Angina | 629 | 46.1 | 736 | 53.9 | < 0.001 |
| Stroke | 1758 | 79.1 | 465 | 20.9 | < 0.001 |
| Peripheral artery disease | 721 | 71.2 | 292 | 28.8 | < 0.001 |
With a median follow-up of 20 years, of 63117 participants who had no AD at baseline, 8340 women reported that they had incident AD. The cumulative incidence rate (95%CI) of AD was 8.5 (8.0-9.0) per 1000 person-years in participants with DM and 7.1 (6.9-7.2) per 1000 person-years in those without DM (P < 0.001). Similar to DM, women with CMS had significantly higher incidence rates of AD (8.6 per 1000 person-years, 95%CI: 8.1-9.1) than those without CMS (7.0 per 1000 person-years, 95%CI: 6.9-7.2), P < 0.001.
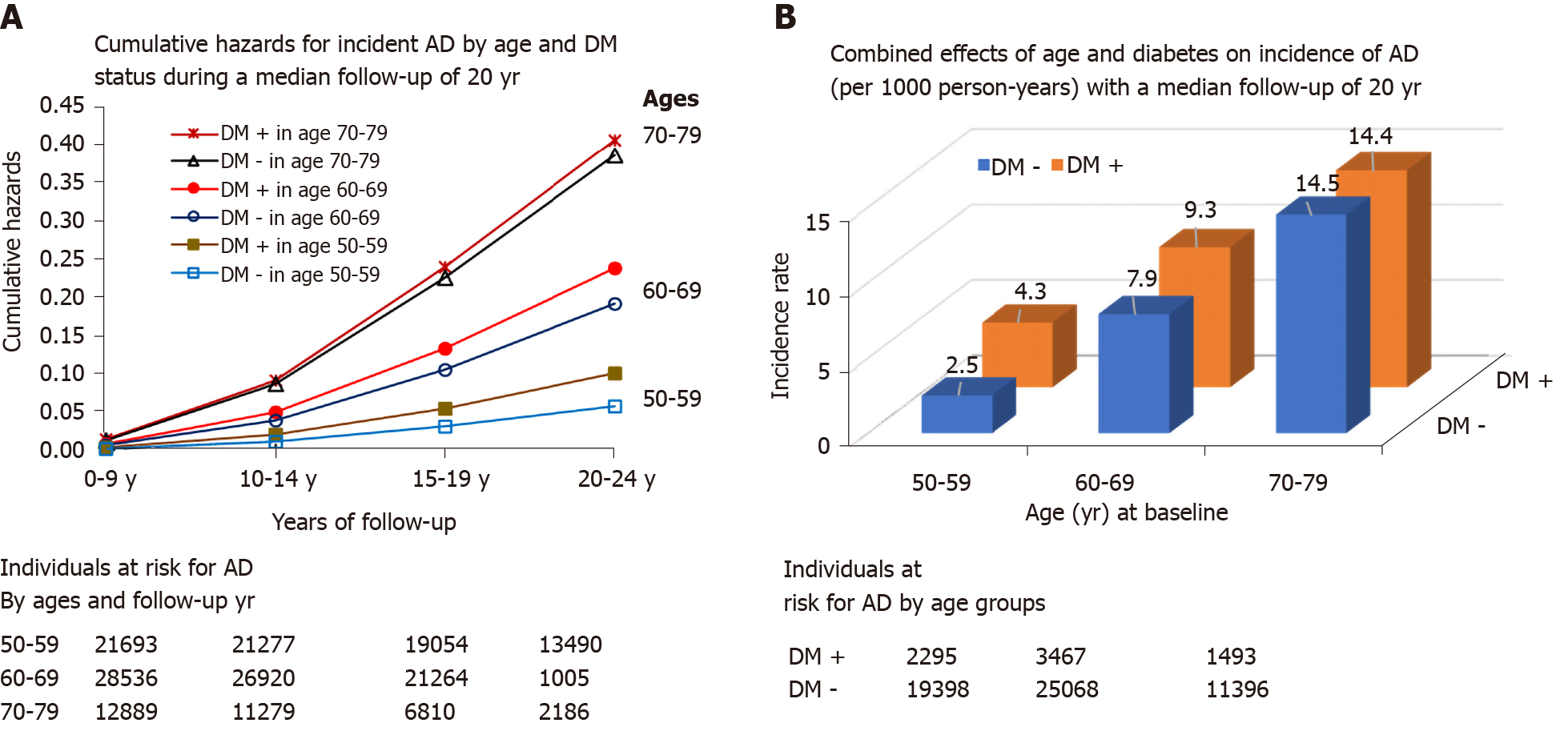
The cumulative incidence rates (95%CI) of AD were 2.7 (2.5-2.9), 8.1 (7.8-8.3), and 14.5 (14.0-15.0) per 1000 person-years among women ages 50-59, 60-69, and 70-79 respectively (age differences in AD rates, P < 0.001).
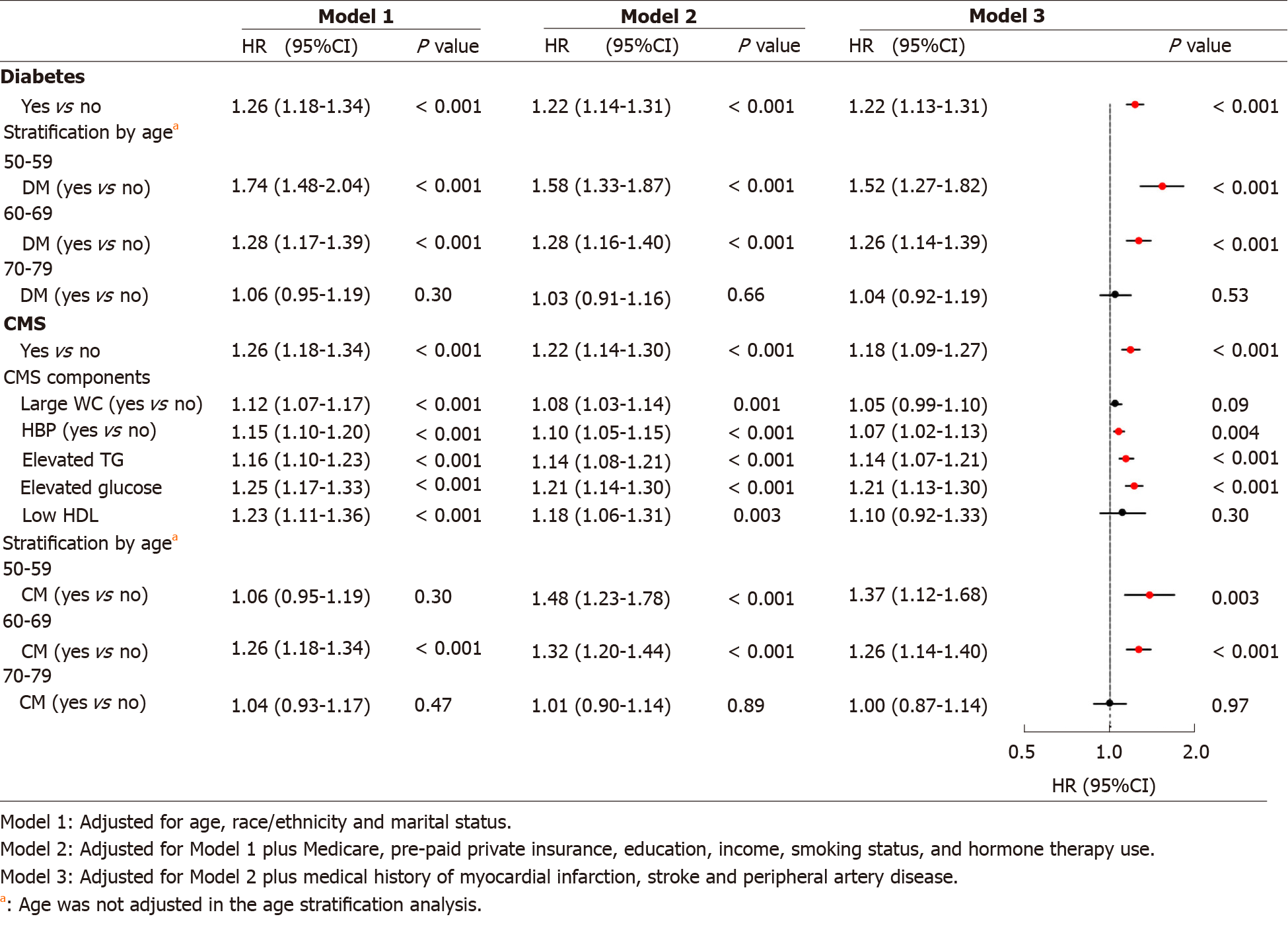
Figure 2 shows that DM was significantly associated with risk of AD. After adjusting for all the study covariates, Model 3 indicates that women with DM had 22% higher risk of AD than those without DM. The hazard ratio (95%CI) was 1.22 (1.13-1.31, P < 0.001). Women aged 50-59 at baseline had the largest hazard ratio (HR) of DM vs absence of DM for AD (HR = 1.52, 95%CI: 1.27-1.82, P < 0.001), followed by 1.26 (1.14-1.39, P < 0.001) and 1.04 (0.92-1.19, P = 0.53) in those aged 60-69 and 70-79. Women with CMS had 18% higher risk of AD than those without CMS, with corresponding HR (95%CI) of 1.18 (1.09-1.27, P < 0.001). Of five CMS components, Model 3 indicates that high BP (HR = 1.07, 95%CI: 1.02-1.13, P = 0.004), elevated TG (1.14, 1.07-1.21, P < 0.001), and elevated glucose concentrations (1.21, 1.13-1.30, P < 0.001) were significantly associated with increased risks of AD. Similar to DM, the relative risk of CMS vs absence of CMS for AD were greater in the younger age groups than that in the older. No significant association between CMS and AD risk was observed in the oldest age group (ages 70-79), with HR (95%CI) of 1.00 (0.87-1.14, P = 0.97). No significant interaction effects of age with DM, and age with CMS on the risk of AD were observed.
Figure 3 shows that overall increase in age was significantly associated with an increased incidence rate of AD in individuals with or without DM. Individuals with diabetes had significantly higher relative risk (i.e., hazards) of AD in those aged 50-59 and 60-69 as compared to their corresponding counterparts without diabetes (Figure 3A). The incidence rates (95%CI) of AD for DM vs non-DM individuals were 4.3 (3.7-4.8) vs 2.5 (2.4-2.8) per 1000 person-years, P < 0.001 in those aged 50-59, and 9.3 (8.6-10.1) vs 7.9 (7.7-8.2) per 1000 person-years, P < 0.001 in those aged 60-69. This difference in AD by DM status was not significant among those aged 70-79 [14.4 (12.9-16.0) vs 14.5 (13.9-15.0) per 1000 person-years, P = 0.88, Figure 3B)]. Similar to DM, the relative risks of those with CMS vs non-CMS for incident AD were higher among the younger age groups (data not shown).
Supplementary Table 1 shows that the HRs (95%CI) remained statistically significant for those who completed the survey instrument on AD conditions by themselves (HR = 1.33, 95%CI: 1.19-1.49, P < 0.001, Model 3) and those whose AD status was reported by others (1.14, 1.02-1.28, P = 0.022). Similar to DM, subjects with CMS had a significantly increased risk of AD among these two subgroups (Model 3). The corresponding HRs (95%CI) are 1.19 (1.05-1.34, P = 0.006) and 1.19 (1.07-1.34, P = 0.002). However, the associations were statistically non-significant among the other two subgroups who completed the survey questionnaire on behalf of the study participants. The corresponding HRs (95%CI) of DM associated with AD are 1.14 (0.93-1.39, P = 0.20) and 1.57 (0.96-2.56, P = 0.07) among those whose survey questionnaires were answered by a family member or friend, or a healthcare provider. The corresponding HRs (95%CI) of CMS associated with AD are 1.09 (0.89-1.33, P = 0.41) and 1.36 (0.82-2.27, P = 0.24) in the two subgroups. It should be noted that restricting to these subgroups greatly decreased the available sample size for these analyses. The resulting estimated HRs had wider 95%CI's, suggesting that further studies with increased sample sizes are needed (Supplementary Table 1).
To the best of our knowledge, this study is one of the first reports on the association of DM and CMS with AD risk among postmenopausal women. The main findings indicate that with a median follow-up of 20-years, postmenopausal women aged 50-79 with DM or CMS had an overall significantly higher incidence rate of AD than their corresponding counterparts in the total study participants. Although the absolute risk of AD increased with age, the relative risks of AD with DM or CMS among younger women aged 50-69 with DM or CMS vs those without DM or CMS were much stronger than the relative risk of AD among older women aged 70-79.
AD is an irreversible and progressive brain disorder that slowly destroys memory and thinking skills, and eventually the ability to carry out the simplest tasks. Therefore, early prevention of this disease becomes important if any preventable risk factors prior to the development of AD can be identified. In recent decades a rapidly increasing number of studies have examined the association of diabetes, obesity, hypertension, dyslipidemia and dysglycemia with risk of cognitive impairment and AD[20]. For example, the Rotterdam study observed a significant association of diabetes vs non-DM with AD risk (relative risk, RR = 1.9, 95%CI: 1.2-3.1) in a prospective population-based cohort study of 6,370 adults aged 55 and older during an average of 2.1 years of follow-up[16,35]. Findings from the Rochester study, a prospective cohort study of 1455 subjects aged 20 and older, indicated a significant association between adult onset diabetes and risk of AD in men (relative risk: 2.27, 95%CI: 1.55-3.31), and a positive but nonsignificant association among women (RR = 1.37, 95%CI: 0.94-2.01)[36]. Findings from the Canadian Study of Health and Aging (CSHA) in participants (n = 5574) aged 65 and over in a 5-year follow-up indicate that the relative risk of DM vs non-DM for incident AD was 1.30 (0.83-2.03) in their combined sample of both genders (results are not presented by gender in their report)[16]. Our results are in general consistent with these previous reports, with a 22% higher risk of AD in those with DM vs those without DM in women aged 50 and older (HR = 1.22, 95%: 1.13-1.31, P < 0.001). Our study extends the previous studies by using a larger sample size and addressing AD risk by age groups. Of individual CMS components, findings from Nägga et al[21] study indicate a significant association between increased midlife triglycerides and risk of beta-amyloid (Aβ) and tau pathology. Several review articles have also examined the associations of CMS and diabetes with the development of AD[20,37,38]. However, studies on the development of AD were limited. Data from the WHIOS fills the gap to assess the metabolic disorders and risk of AD among postmenopausal women. Findings of this study contribute evidence to the body of literature by demonstrating a significant association of DM and CMS with AD. Given the potentially preventable risk of DM and CMS, we expect that the risk of AD can be reduced if we focus on the control and prevention of DM and CMS as early as possible.
Although the mechanisms by which DM or CMS play a role in the development of AD remain poorly understood, some possible pathways have been proposed. Of these, insulin resistance, an impaired response of the body to insulin resulting in elevated levels of glucose in the blood, has been proposed to be an important cause of DM and its relationship to the development of AD, because severe neuropathological changes are common to diabetes and AD. For example, hyperglycemia in patients with DM can result in glycosylation of various receptors, leading to the formation of “receptors for advanced glycation end products” (RAGE). An affinity between RAGE and beta-amyloid (Aβ) peptides can trigger and propagate chronic brain inflammation[39]. Dyslipidemia and hypertension are also proposed as important risk factors associated with risk of AD. In a longitudinal study of 318 elderly individuals with normal cognition, the investigators found that higher fasting triglyceride levels in midlife were associated with increased risk of brain Aβ and tau pathology 20 years later. This association was independent of age, sex, APOE ε4, and vascular risk factors[21]. The potential pathophysiologic effect of triglycerides on Aβ pathology remains under study. In transgenic AD mouse models blood triglyceride concentrations have been shown to increase prior to Aβ deposition, indicating a direct association between triglycerides and Aβ homeostasis[40]. Lipids may influence membrane fluidity, which could directly affect secretase-mediated Aβ[41]. In a meta-analysis of 18 prospective studies examining the relationship of total cholesterol with risk for AD and vascular dementia, midlife total cholesterol levels were consistently associated with an increased risk of AD and all dementia, whereas no increased risk was observed for late-life total cholesterol[42]. In our study, significant associations of central adiposity (assessed by large waist circumference) and low HDL-C with AD were observed after adjustment for age, race/ethnicity, marital status, insurance, education, family income, smoking and hormone therapy use (Models 1 and 2 of Figure 2). The associations of large WC and low HDL-C with risk of AD became nonsignificant after a further adjustment by including myocardial infarction (MI), stroke and peripheral arterial disease (PAD) (Model 3 of Figure 2). These attenuated associations in model 3 are possibly attributable to an over-adjustment because these cardiovascular conditions are likely on the pathway of the development of AD (large WC and dyslipi-demia—cardiovascular diseases—AD). Several studies have indicated that MI, stroke, and PAD predict the risk of cognitive impairment and AD[43-46]. The association between hypertension and AD is complex and may involve direct and indirect effects with other risk factors, such as the fact that hypertension increases risk of arterial stiffness, Aβ accumulation, and has interaction effect with insulin resistance[42,47,48]. Increased ages are significantly associated with increased risks of AD. However, it should be noted that in our study the relative risks (i.e., HRs) of AD in younger women aged 50-69 with DM or CMS vs those without DM or CMS were higher compared to the relative risks of AD in older adults aged 70-79 without DM or CMS vs those without DM or CMS. The relative risks of DM and CMS vs those without DM or CMS for AD became nonsignificant in the older age group. Similar nonsignificant associations between those with and those without DM among the elderly were reported from previous studies as well[21,49]. The potential mechanisms are unclear. One of the explanations is that this nonsignificance in the older age group is possibly attributable to survivor bias. Individuals aged 70-79 may have better healthy conditions than those who died before their ages 70-79. The relative effect of DM or CMS on AD risk may become weaker in the older age group. Meanwhile, individuals aged 70-79 have a higher proportion of comorbidities, such as stroke and hypertension than the youngers. Other, currently-unknown, age-related causes of AD may become more important in older age groups as well. Therefore, DM may have a relatively smaller impact on AD risk in the older patients. Further studies among the older age groups are warranted.
That there are several limitations when interpreting the results. First, the WHIOS included only women who were postmenopausal, which limits the generalizability to women earlier in their life cycle. Second, the classification of AD was based on a self-administered survey questionnaire. We had no access to more definitive measures such as imaging of brain structures (i.e., magnetic resonance imaging and com-puterized tomography) or measures of the burden of amyloid deposits, and of neurofibrillary tangles in brain (such as Amyloid PET and Tau Pet imaging). Although it is common to define a disease status using a pre-designed self-administered questionnaire in most large-scale observational epidemiological studies, possible information bias may have occurred, which may lead to under- or over-estimate of the incidence of AD. In addition, based on the survey instrument on the question on AD, we thought that the classification of AD in the analysis might have included these with AD or AD related dementia. In our sensitivity analysis, we tested DM-AD and CMS-AD associations separately for subgroups based on who completed the self-administered questionnaire. It shows that AD reported by a family member or friend or a healthcare provider of the study participant had nonsignificant associations with DM and CMS. However, this subsample analysis is questionable by their much smaller sample sizes (AD cases) reported by these two groups, which is also demonstrated by their wide 95% confidence intervals. Certainly, given the limitation that using a self-administered instrument is subject to bias, findings from this study call for further studies with detailed clinical measures and evaluations. Third, in the analysis, CMS was included for the purpose of examining the associations of CMS and its components with risk of AD. We had to estimate missing values of TG and HDL using their corresponding surrogates. These estimations may lead to under- or overestimate of their true values, which would introduce bias. Nevertheless, as consistent findings of CMS and DM were observed in the study and compared with the others, we would say that the bias due to the estimated approach is minimized. Finally, residual confounding cannot be completely eliminated from epidemiological studies, which could be attributable to the factors that the survey did not include.
Our study also has several strengths. First, the WHIOS is one of the largest observational prospective study among postmenopausal women. With an increase in life expectancy in the nation and worldwide, findings from the study add important evidence to the research literature. Second, of all reported studies related to DM and AD risk, the WHIOS has the longest duration of follow-up, which offers an unique opportunity to test the long-term effect of DM and CMS on the risk of AD. Third, a significantly higher relative risk of AD in younger women with DM or CMS vs those without DM or CMS than that in the older age group not only calls for further etiological studies, but also adds evidence to the prevention of AD risk in younger adults with DM or CMS.
During a median follow-up of 20 years, DM and CMS were significantly associated with the risk of AD among postmenopausal women. More specifically, women aged 50-69 with DM or CMS vs those without these conditions had significantly higher relative risks of AD than the relative risks of AD in those aged 70-79 with DM or CMS vs those without DM or CMS.
In spite of an increase in the incidence and prevalence of diabetes mellitus (DM) and Alzheimer’s disease (AD) in the aging population, limited attention has been paid to investigate their associations.
To investigate the association of DM and cardiometabolic syndrome with risk of AD among the United States older adults.
To examine the association of DM and cardiometabolic syndrome (CMS, a precursor to DM) with risk of incident AD among postmenopausal women.
Postmenopausal women aged 50-79 (n = 63117) who participated in the U.S. Women’s Health Initiative Observational Study (WHIOS), recruited in 1993-1998 without baseline AD and followed up through March 1, 2019 were analyzed. AD was classified by participants-reported history of doctor-diagnosis of first-listed AD. DM was defined by participant-report or serum glucose concentrations or those anti-diabetic medication use. CMS was defined as having ≥ 3 of five CMS components: large waist circumference, high blood pressure, elevated triglycerides, elevated glucose, and low high-density lipoprotein cholesterol. The associations of DM and CMS with AD were analyzed using Cox’s proportional hazards regression analysis.
Within a median follow-up of 20 years (range: 3.36 to 23.36 years), of 63117 participants, 8340 had incident AD. Women with DM had significantly higher incidence of AD [8.5, 95% confidence interval (CI): 8.0-9.0 per 1000 person-years (PY)] than those without DM (7.1, 95%CI: 6.9-7.2 per 1000 PY). Multivariate Cox’s regression analysis indicates that women with DM or CMS had significantly higher risk of AD than those without DM or CMS. The corresponding hazard ratios [HR (95%CI)] were 1.22 (1.13-1.31, P < 0.001) in subjects with DM, and 1.18 (1.09-1.27, P < 0.001) in subjects with CMS. The HRs of AD in those with DM or MS vs those without DM or CMS diminished with age and became non-significant in the oldest age group.
Diabetes and cardiometabolic syndrome were significantly associated with risk of Alzheimer's disease.
Further studies are needed to investigate the mechanisms by which DM and CMS may cause the development of AD.
This manuscript was prepared using WHIOS and WHICT Research Materials obtained from the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center (RMDA# 6820) and does not necessarily reflect the opinions or views of the WHIOS, WHICT or the NHLBI.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang Y, Qi XS S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
| 1. | Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020. [cited May 8, 2020]. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.Pdf. |
| 2. | Centers for Disease Control and Prevention. Alzheimer's Disease. [cited August 16, 2019]. Available from: https://www.cdc.gov/chronicdisease/resources/publications/aag/alzheimers.htm. |
| 3. | National Institute on Aging. Alzheimer's Disease & Related Dementias. [cited August 11, 2019]. Available from: https://www.nia.nih.gov/health/alzheimers. |
| 4. | Alzheimer’s Association: 2018 Alzheimer's disease facts and figures. Alzheimer's and Dementia 2018; 14: 367-429, [cited August 11, 2019]. Available from: https://www.alz.org/media/homeoffice/facts%20and%20figures/facts-and-figures.pdf. |
| 5. | Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 699] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 6. | Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer's disease. Nat Clin Pract Neurol. 2006;2:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer's disease. J Alzheimers Dis. 2009;16:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Castro JP, El-Atat FA, McFarlane SI, Aneja A, Sowers JR. Cardiometabolic syndrome: pathophysiology and treatment. Curr Hypertens Rep. 2003;5:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Liu L, Núñez AE. Cardiometabolic syndrome and its association with education, smoking, diet, physical activity, and social support: findings from the Pennsylvania 2007 BRFSS Survey. J Clin Hypertens (Greenwich). 2010;12:556-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 587] [Cited by in RCA: 722] [Article Influence: 72.2] [Reference Citation Analysis (13)] |
| 11. | Nass CM, Reck K. Clinical challenges: the intersection of diabetes, chronic kidney disease, and cardiovascular disease. Curr Diab Rep. 2004;4:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, Nesto RW, Wilson PW, Vasan RS. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 388] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 13. | Liu L, Simon B, Shi J, Mallhi AK, Eisen HJ. Impact of diabetes mellitus on risk of cardiovascular disease and all-cause mortality: Evidence on health outcomes and antidiabetic treatment in United States adults. World J Diabetes. 2016;7:449-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Liu L, Yin X, Chen M, Jia H, Eisen HJ, Hofman A. Geographic Variation in Heart Failure Mortality and Its Association With Hypertension, Diabetes, and Behavioral-Related Risk Factors in 1,723 Counties of the United States. Front Public Health. 2018;6:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Raffaitin C, Gin H, Empana JP, Helmer C, Berr C, Tzourio C, Portet F, Dartigues JF, Alpérovitch A, Barberger-Gateau P. Metabolic syndrome and risk for incident Alzheimer's disease or vascular dementia: the Three-City Study. Diabetes Care. 2009;32:169-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer's disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | McClean PL, Gault VA, Harriott P, Hölscher C. Glucagon-like peptide-1 analogues enhance synaptic plasticity in the brain: a link between diabetes and Alzheimer's disease. Eur J Pharmacol. 2010;630:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Panza F, Frisardi V, Capurso C, Imbimbo BP, Vendemiale G, Santamato A, D'Onofrio G, Seripa D, Sancarlo D, Pilotto A, Solfrizzi V. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;21:691-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Caulcrick M, Eisen H, Liu L. Cardiometabolic Risk Factors and Cognitive Function Among Different Racial/Ethnic Populations in the United States. August 16, Circulation 2008; 118: S-759. [cited November 18, 2019]. Available from: https://www.ahajournals.org/doi/10.1161/circ.118.suppl_18.S_759-b. |
| 20. | Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 397] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 21. | Nägga K, Gustavsson AM, Stomrud E, Lindqvist D, van Westen D, Blennow K, Zetterberg H, Melander O, Hansson O. Increased midlife triglycerides predict brain β-amyloid and tau pathology 20 years later. Neurology. 2018;90:e73-e81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Study TWsHI. Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1916] [Cited by in RCA: 2031] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 23. | Women’s Health Initiatives. WHI Extension Study Protocol Outline. [cited November 18, 2019]. Available from: https://www.whi.org/researchers/studydoc/Consents/Protocol%202010-2015.pdf. |
| 24. | Women’s Health Initiatives. WHI Extension Study 2010-2020. [cited November 18, 2019]. Available from: https://www.whi.org/about/SitePages/WHI%20Extension%202010-2020.aspx. |
| 25. | Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107-S121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 524] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 26. | Women’s Health Initiatives. Data Preparation and Use of WHI Investigator Datasets - Updated. [cited October 1, 2019]. Available from: https://www.whi.org/researchers/data/Documents/WHI%20Data%20Preparation%20and%20Use.pdf. |
| 27. | Vasudevan AR, Ballantyne CM. Cardiometabolic risk assessment: an approach to the prevention of cardiovascular disease and diabetes mellitus. Clin Cornerstone. 2005;7:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C; American Heart Association; National Heart; Lung; and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3603] [Cited by in RCA: 3668] [Article Influence: 174.7] [Reference Citation Analysis (0)] |
| 29. | Reppert A, Steiner BF, Chapman-Novakofski K. Prevalence of metabolic syndrome and associated risk factors in Illinois. Am J Health Promot. 2008;23:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Montana D. Metabolic syndrome and its components in Montana. [cited June 1, 2019]. Available from: https://dphhs.mt.gov/publichealth/diabetes/dpp. |
| 31. | Tabachnick BG and Fidell LS: Using Multivariate Statistics. ISBN-13: 9780205459384. Boston, MA: Allyn and Bacon, 2007. |
| 32. | Szklo M, Nieto FJ. Epidemiology Beyond the Basics. ISBN-13: 978-1284116595 Sudbury, MA: Jones and Bartlett, 2007. |
| 33. | Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 647] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 34. | Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 386] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 35. | Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1486] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 36. | Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O'Brien PC, Palumbo PJ. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 428] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 37. | Goldwaser EL, Acharya NK, Sarkar A, Godsey G, Nagele RG. Breakdown of the Cerebrovasculature and Blood-Brain Barrier: A Mechanistic Link Between Diabetes Mellitus and Alzheimer's Disease. J Alzheimers Dis. 2016;54:445-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Klimova B, Kuca K, Maresova P. Global View on Alzheimer's Disease and Diabetes Mellitus: Threats, Risks and Treatment Alzheimer's Disease and Diabetes Mellitus. Curr Alzheimer Res. 2018;15:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Taguchi A. Vascular factors in diabetes and Alzheimer's disease. J Alzheimers Dis. 2009;16:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Ghiso J, Shayo M, Calero M, Ng D, Tomidokoro Y, Gandy S, Rostagno A, Frangione B. Systemic catabolism of Alzheimer's Abeta40 and Abeta42. J Biol Chem. 2004;279:45897-45908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 41. | Jaeger S, Pietrzik CU. Functional role of lipoprotein receptors in Alzheimer's disease. Curr Alzheimer Res. 2008;5:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 43. | Honig LS, Tang MX, Albert S, Costa R, Luchsinger J, Manly J, Stern Y, Mayeux R. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 45. | Marfany A, Sierra C, Camafort M, Doménech M, Coca A. High blood pressure, Alzheimer disease and antihypertensive treatment. Panminerva Med. 2018;60:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Ottaviani C. Brain-heart interaction in perseverative cognition. Psychophysiology. 2018;55:e13082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 47. | Sierra C, Vicario A, Escoda O. Hypertension, Cognitive Decline, and Dementia Hypertension and Brain Damage: Springer, Cham 2016: 197-211. |
| 48. | Carnevale D, Perrotta M, Lembo G, Trimarco B. Pathophysiological Links Among Hypertension and Alzheimer's Disease. High Blood Press Cardiovasc Prev. 2016;23:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Gustavsson AM, van Westen D, Stomrud E, Engström G, Nägga K, Hansson O. Midlife Atherosclerosis and Development of Alzheimer or Vascular Dementia. Ann Neurol. 2020;87:52-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |