Published online Jul 15, 2020. doi: 10.4239/wjd.v11.i7.309
Peer-review started: December 30, 2019
First decision: March 24, 2020
Revised: May 8, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: July 15, 2020
Processing time: 196 Days and 10.4 Hours
Diabetic polyneuropathy is a very common complication of diabetes. Numerous studies are available in terms of pathogenesis. But examination methods with low reliability are still not standardized and generally time consuming. High-sensitive, easy-to-access methods are expected. Biochemical markers are one of the subjects of research. We aimed to discover a potential biomarker that can be used for this purpose in patients with diabetes who have not yet developed symptoms of neuropathy.
To determine the place and availability of visfatin and thiol-disulfide homeostasis in this disorder.
A total of 392 patients with type 2 diabetes mellitus were included in the study. The polyneuropathy clinical signs were evaluated with the Subjective Peripheral Neuropathy Screen Questionnaire and Michigan Neuropathy Screening Instrument questionnaire and examination. The biochemical parameters, oxidative stress markers, visfatin, and thiol-disulfide homeostasis were analyzed and correlated with each other and clinical signs.
Subjective Peripheral Neuropathy Screen Questionnaire and Michigan Neuropathy Screening Instrument questionnaire with examination scores were correlated with each other and diabetes duration (P < 0.005). Neuropathy related symptoms were present in 20.7% of the patients, but neuropathy related findings were observed in 43.9% of the patients. Serum glucose, glycated hemoglobin, and visfatin were positively correlated with each other. Also, these parameters were positively correlated with the total oxidative stress index. Total and native thiol was positively correlated with total antioxidant status and negatively with oxidant status. Inversely thiol-disulfide positively correlated with higher glucose and oxidant status and negatively with total antioxidant status (P < 0.005). There was no correlation between visfatin and thiol-disulphide (P = 0.092, r = 0.086). However, a significant negative correlation was observed between visfatin and total with native thiol (P < 0.005, r = -0.338), (P < 0.005, r = -0.448).
Diagnosis of neuropathy is one of the issues studied in patients with diabetes. Visfatin and thiol-disulfide balance were analyzed for the first time in this study with inspiring results.
Core tip: Early diagnosis and management of micro and macrovascular complications are vital in patients with diabetes. Many algorithms and early diagnostic tools have been developed for this purpose. Yet it is still difficult to identify neuropathy because of the prolonged preclinical phase. This patient group has uncontrolled blood sugar and hypertension, accelerated renal replacement need, and life-threatening cardiac or cerebral macrovascular complications. With this study, we wanted to emphasize that screening of neuropathy should not be ignored in the follow-up of these cases. As an early diagnostic tool, many parameters that are responsible for pathogenesis should be investigated.
- Citation: Buyukaydin B, Guler EM, Karaaslan T, Olgac A, Zorlu M, Kiskac M, Kocyigit A. Relationship between diabetic polyneuropathy, serum visfatin, and oxidative stress biomarkers. World J Diabetes 2020; 11(7): 309-321
- URL: https://www.wjgnet.com/1948-9358/full/v11/i7/309.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i7.309
Type 2 diabetes mellitus (DM) is a global epidemic and a highly complex disease. The incidence is estimated to be 366 million between the years 2000 and 2030[1]. Diabetic polyneuropathy (DN) is the most common complication along with a 50% lifetime prevalence[2]. DM leads to various peripheral neuronal damages, but the most common type is the bilaterally symmetric, distal to the proximal severity of nerve damage known as stocking-glove neuropathy. Although 50% of the patients are asymptomatic, the progressive nerve damage results in instability, falls, and numb, insensate feet. DN negatively affects the quality of life and increases health expenses. The yearly medical expense for diabetes is $6632 per patient. However, the presence of DN doubles this amount, and the presence of severe neuropathy quadruples the amount[3].
For the early diagnosis, practical and reliable methods are essential[4]. Monofilament tests for superficial and vibratory stimulus for deep sensation may provide early data for neuropathy. Nerve conduction studies and skin biopsy are more detailed and useful but not practical methods[5,6]. The Toronto Consensus Panel defined diagnostic guidelines based on these methods[7]. Subjective Peripheral Neuropathy Screen (SPNS) and Michigan Neuropathy Screening Instrument (MNSI) are other reliable procedures that are simple and easily self-completed methods applied by the patient or the physician[8,9].
In the pathogenesis of neuropathy, the metabolic redox state, extreme production of mitochondrial, and cytosolic reactive oxygen species of dorsal root ganglia and Schwann cells are the major determinants[10]. Reactive oxygen species are one of the major determinants in the pathophysiology of many diseases[11]. Increased reactive oxygen species are one of the factors leading to programmed cell death of neurons. Oxidative stress is an oxidant/antioxidant imbalance, and oxidative stress index (OSI) is the ratio of total oxidant status (TOS) to total antioxidant status (TAS)[12]. Thiols, functional SH groups, have been identified as one of the main antioxidants. The plasma thiols are albumin, cysteine, cysteinylglycine, glutathione, homocysteine, and γ-glutamylcysteine. Under oxidative status, thiols transform into their reversible oxidized disulfide forms. These disulfide bonds are not covalent. Thiols may return the reduced form, and thiol-disulfide balance is maintained. Thiol-disulfide provides homeostatic redox status, and it has been associated with many clinical disorders including DM[13,14]. Visfatin, a 52 kDa protein, is produced by visceral adipose tissue. It induces cytokine production and is accepted as a proinflammatory adipokine. In the clinical studies, the relationship between visfatin and intracerebral hemorrhage, acute ischemic stroke, acute pancreatitis, and myocardial infarction have been identified[15,16]. Although it was generally associated with long-term unfavorable outcomes, it has been shown to have a regulatory effect in myocardium, neurons, and even mitochondria[17,18]. Further studies are necessary to define the precise effect of this adipocytokine in critically ill patients.
In this study, patients with diabetes were evaluated using the Subjective Peripheral Neuropathy Screen and MNSI questionnaires and MNSI examination. For all patients, oxidative status, visfatin, and thiol-disulfide balance were determined. The measured parameters were compared to each other and differences of parameters between patients with and without DN clinical signs were compared.
This study was performed at the Bezmialem Vakif University Internal Medicine and Endocrinology Department policlinics. In these clinics, our patients with diabetes come for routine control every 3 mo with an appointment. Between October 2018 and April 2019, we randomly included this group of patients in the study with informed consent. A total of 392 patients with neuropathy examinations were included in the study. Exclusion criteria were as follows: (1) Patients with acute infection or other lymphoproliferative and chronic infection like human immunodeficiency virus; (2) Patients with monoclonal gammopathy, vasculitis, alcoholism, chronic renal failure, sarcoidosis, Sjogren disease, amyloidosis, neoplasms, and paraneoplastic syndromes; and (3) Patients with a certain diagnosis for hereditary, demyelinating or multifocal neuropathies, radiculopathy, mononeuritis, cerebrovascular diseases, and chronic renal or hepatic failure. For the other possible macro and microvascular complications, we did not apply any exclusion. The age of the patients and diabetes duration was registered. Although the diabetic medications and other related disorders were registered, we were not able to group and compare the possible effect of related disorders and using agent drugs including vitamin B12.
The study was performed based on the Helsinki Declaration, and ethical consent was obtained from Bezmialem Clinical Research Ethics Committee (Number 2016/14823). Informed voluntary consent form was received from all patients.
The patients were examined on a stretcher in policlinic conditions. The researchers were educated and used the same instructions. Complaints of the patients were evaluated using the Subjective Peripheral Neuropathy Screen Questionnaire (SPNSQ) and MNSI[8,9]. SPNSQ contains 15 questions about the symptoms of neuropathy. The total score is obtained by counting the yes answers. The sum of the scores range from 0 to 15 and determine the cases from no neuropathic symptoms to the severe neuropathic symptoms. MNSI questionnaire contains 15 “yes/no” questions regarding neuropathy. For the questions 1-3, 5-6, 8-9, 11-12, 14-15, “yes” answers and for the questions 7 and 13 “no” answers were scored as one point. Questions 4 and 10 were not included in the published scoring algorithm. The sum of the questionnaire score of 7 ≥ was accepted as abnormal[9]. MNSI examination was performed by the physicians participating in the study. Each foot was examined for dryness, fissures, ulcers, and infections. The detected abnormality was scored with 1 point.
The feet were also inspected for ulcers and each foot with an ulcer received 1 point. The Achilles reflex was evaluated. If it was absent, Jendrassic maneuver was performed. If it is present, the reflex was scored as 0.5 points for each foot. If the reflex was absent with the maneuver, it was scored as 1 point. Vibration sensation was evaluated using a tuning fork placed on the dorsal face of the big toe. With covered vision, the vibration was scored according to the duration of the sensation. Below 10 s, the sensation was accepted as reduced and scored as 0.5 points. The patient with no perception was scored as 1 point for each foot. For monofilament evaluation, the feet were rested on a flat, warm surface. The filament was applied perpendicularly and briefly (< 1 s) with 10 gr pressure to ten designated spots for each foot. Eight correct answers were considered normal. One to seven correct answers were considered decreased. No correct answers were considered a loss of sensation. The total MNSI score was over 10 points, and the score ≥ 2.5 was accepted as abnormal[19].
Fasting venous blood samples were provided from the antecubital vein. The samples were centrifuged at 3000 × g for 10 min to dissociate the serum. The serum samples were aliquoted and kept at -80 °C until further analysis.
The biochemical parameters serum glucose, glycated hemoglobin (HbA1c), serum triglycerides, low density lipoprotein cholesterol, serum creatinine, alanine aminotransferase, aspartate aminotransferase, and vitamin B12 were analyzed using commercial assay kits.
Serum visfatin levels were analyzed using an enzyme-linked immunosorbent assay kit (Elabscience, Houston, TX, United States). Results were obtained by spectro-photometric method according to the manufacturer‘s directions and specified in pg/mL.
Serum TAS was evaluated with the method based by Erel et al[20]. The method encompasses the formation of hydroxyl radicals, which is a potent reactive substance. A ferrous ion solution (reagent 1) is stirred with hydrogen peroxide (reagent 2). The antioxidative capacity of a sample can be measured in terms of the inhibition of free radical reactions initiated by the generation of the hydroxyl radical. The change in assay data was very low (< 3%), and results were in mmol Trolox Eq./L.
For TOS analysis, the oxidants formed in the serum oxidize the ferrous ion of an o-dianisidine compound to the ferric ion. For the calibration of the analysis, hydrogen peroxide was used, and the results were presented with micromole hydrogen peroxide equivalents per liter (μmol H2O2 Eq./L).
OSI was calculated as OSI (arbitrary unit) = (TOS, μmol H2O2 Eq./L) / (TAS, mmol Trolox Eq./L)[21].
For the evaluation, dynamic disulfide bonds (–S–S–) in the serum sample were reduced to native thiol groups (–SH) by NaBH4. The total thiol ingredient was measured using a derivative of Ellman reagent. Native thiol was subtracted from the total thiol, and half of the obtained difference gave the disulfide bond amount. Biomarkers were measured using a spectrophotometer (Varioskan Flash Multimode Reader; Thermo, Waltham, MA, United States)[13]. After the measurement of native thiol and disulfide concentrations in the samples, the disulfide/native thiol ratio (-S-S-/-SH) was calculated as dynamic thiol-disulfide homeostasis (TDH).
All of the statistical analyses were performed using the IBM SPSS 22.0. Mean standard deviation and percentages are presented as descriptive statistics. When comparing the groups for categorical data MNSI neuropathy score categories, the chi-square test was used. For the cuts of averages, the independent sample t-test was used. The Pearson correlation coefficient was calculated for the relationship between variables. For all data, P < 0.05 was accepted as significant.
A total of 392 patients were evaluated (271 female, 121 male). The mean age of the patients was 57.5 ± 9.0 years. The mean diabetes period was 12.00 ± 7.29 years. For all patients the mean SPNSQ score was 5.6 ± 3.6, the mean MNSI questionnaire score was 4.5 ± 2.3, and the mean MNSI exam score was 2.4 ± 2.0 points. SPNSQ, MNSI questionnaire, and MNSI exam scores were correlated with each other (P < 0.005). Between the disease duration and SPNSQ, MNSI questionnaire, and MNSI examination, significant positive correlation was observed (P < 0.005, r = 0.275, P < 0.005, r = 0.242, P = 0.027, r = 0.119). However, there was no correlation between the disease duration and all other measured parameters.
MNSI questionnaire score was less than seven points in 311 patients (79.3%). In 81 patients it was more than seven points (20.7%). MNSI examination score of less than 2.5 points was observed in 220 patients (56.1%), and it was more than 2.5 points in 172 patients (43.9%). In females, the disease duration was much longer (P = 0.003), and both questionnaire scores were much higher than males (P = 0.001 and P = 0.044). But in terms of MNSI examination, there was no difference between males and females (P = 0.059).
There was no correlation between questionnaire results and the biochemical parameters, visfatin, oxidative stress biomarkers, and TDH. There was a positive linear relationship between MNSI examination scores and HbA1c, visfatin, TOS, and OSI. The same correlation was observed in the divided MNSI score analysis. These correlations with detailed information are presented in Tables 1 and 2.
| SPNSQ score, 5.66 ± 3.64 | MNSI q, 4.5 ± 2.28 | MNSI exam, 2.42 ± 1.99 | |
| Glucose, mg/dL, 154.0 ± 57.5 | P = 0.099, r = 0.084 | P = 0.110, r = 0.081 | P = 0.110, r = 0.081 |
| HbA1c, 7.68 ± 1.52 | P = 0.099, r = 0.084 | P = 0.062, r = 0.094 | bP < 0.01, r = 0.170 |
| Creatinine, mg/dL, 0.81 ± 0.13 | P = 0.017, r = -0.121 | P = 0.053, r = -0.098 | P = 0.143, r = 0.074 |
| ALT, U/L, 22.3 ± 12.8 | P = 0.177, r = 0.000 | P = 0.518, r = 0.033 | P = 0.585, r = 0.028 |
| AST, U/L, 18.6 ± 7.8 | P = 0.587, r = 0.028 | P = 0.676, r = 0.021 | P = 0.810, r = -0.012 |
| LDL cholesterol, mg/dL, 118.12 ± 28.00 | P = 0.077, r = 0.090 | P = 0.151, r = 0.073 | P = 0.868, r = 0.008 |
| Triglycerides, mg/dL, 160.3 ± 85.0 | P =0.123, r = 0.078 | P = 0.355, r = 0.047 | P = 0.994, r = 0.000 |
| Vitamin B12, pg/mL, 425.5 ± 32.0 | aP < 0.05, r = 0.132 | P = 0.062, r = 0.095 | P = 0.049, r = 0.100 |
| Visfatin, pg/mL, 19.34 ± 8.07 | P = 0.579, r = 0.028 | P = 0.330, r = 0.049 | aP < 0.05, r = 0.122 |
| TOS, μmol H2O2/L, 12.91 ± 1.92 | P = 0.417, r = 0.041 | P = 0.340, r = 0.048 | aP < 0.05, r = 0.155 |
| TAS, mmol Trolox Eq./L, 0.87 ± 0.14 | P = 0.385, r = 0.044 | P = 0.160, r = -0.071 | aP < 0.05, r = -0.127 |
| OSI (Arbitrary units), r = 0.20, 15.29 ± 4.12 | P = 0.283, r = 0.054 | P = 0.170, r = 0,069 | bP < 0.01, P = 0.000 |
| Total thiol, mmol/L, 0.51 ± 0.05 | P = 0.070, r = -0.092 | P = 0.053, r = -0.098 | P = 0.251, r = -0.05 |
| Native thiol, mmol/L, 0.35 ± 0.05 | P = 0.822, r = 0.011 | P = 0.903, r = -0.006 | P = 0.864, r = -0.009 |
| Thiol-disulfide, mmol/L, 0.08 ± 0.03 | P = 0.059, r = -0.096 | P = 0.069, r = -0.092 | P = 0.270, r = -0.056 |
| MNSI q < 7 | MNSI q ≥ 7 | P value | MNSI exam < 2.5 | MNSI exam ≥ 2.5 | P value | |
| Visfatin | 19.15 ± 7.93 | 20.08 ± 8.62 | 0.357 | 18.32 ± 6.97 | 20.64 ± 9.16 | < 0.01 |
| TOS | 12.88 ± 1.90 | 13.04 ± 2.01 | 0.486 | 12.64 ± 1.63 | 13.26 ± 2.20 | < 0.05 |
| TAS | 0.88 ± 0.14 | 0.85 ± 0.13 | 0.102 | 0.88 ± 0.12 | 0.85 ± 0.15 | < 0.05 |
| OSI | 15.14 ± 4.09 | 15.84 ± 4.23 | 0.178 | 14.62 ± 3.45 | 16.64 ± 4.72 | < 0.01 |
| Total thiol | 0.51 ± 0.00 | 0.51 ± 0.05 | 0.385 | 0.52 ± 0.05 | 0.51 ± 0.05 | 0.228 |
| Native thiol | 0.35 ± 0.05 | 0.34 ± 0.06 | 0.570 | 0.35 ± 0.05 | 0.35 ± 0.06 | 0.638 |
| Thiol-disulfide | 0.08 ± 0.03 | 0.08 ± 0.03 | 0.850 | 0.08 ± 0.03 | 0.08 ± 0.03 | 0.399 |
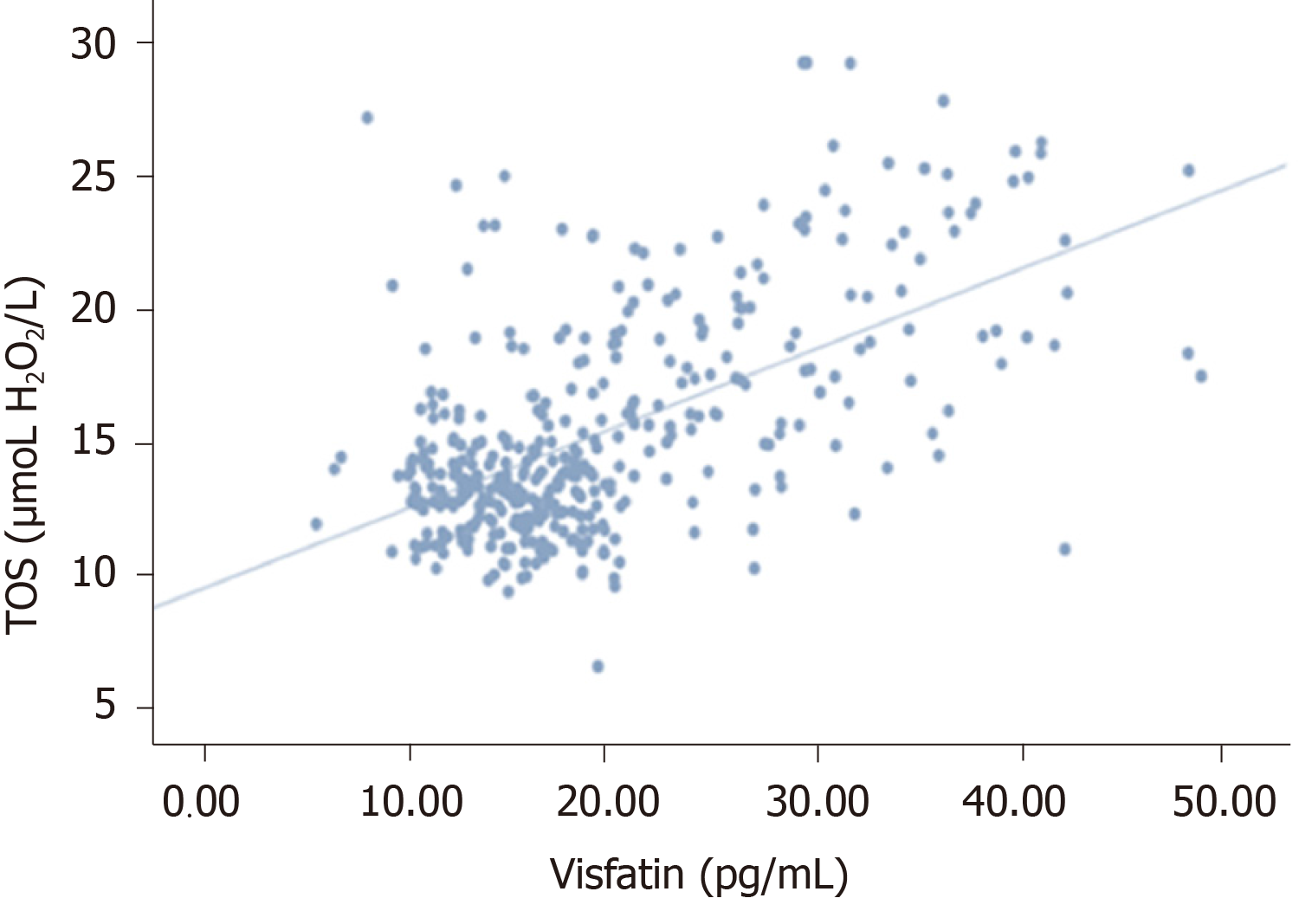
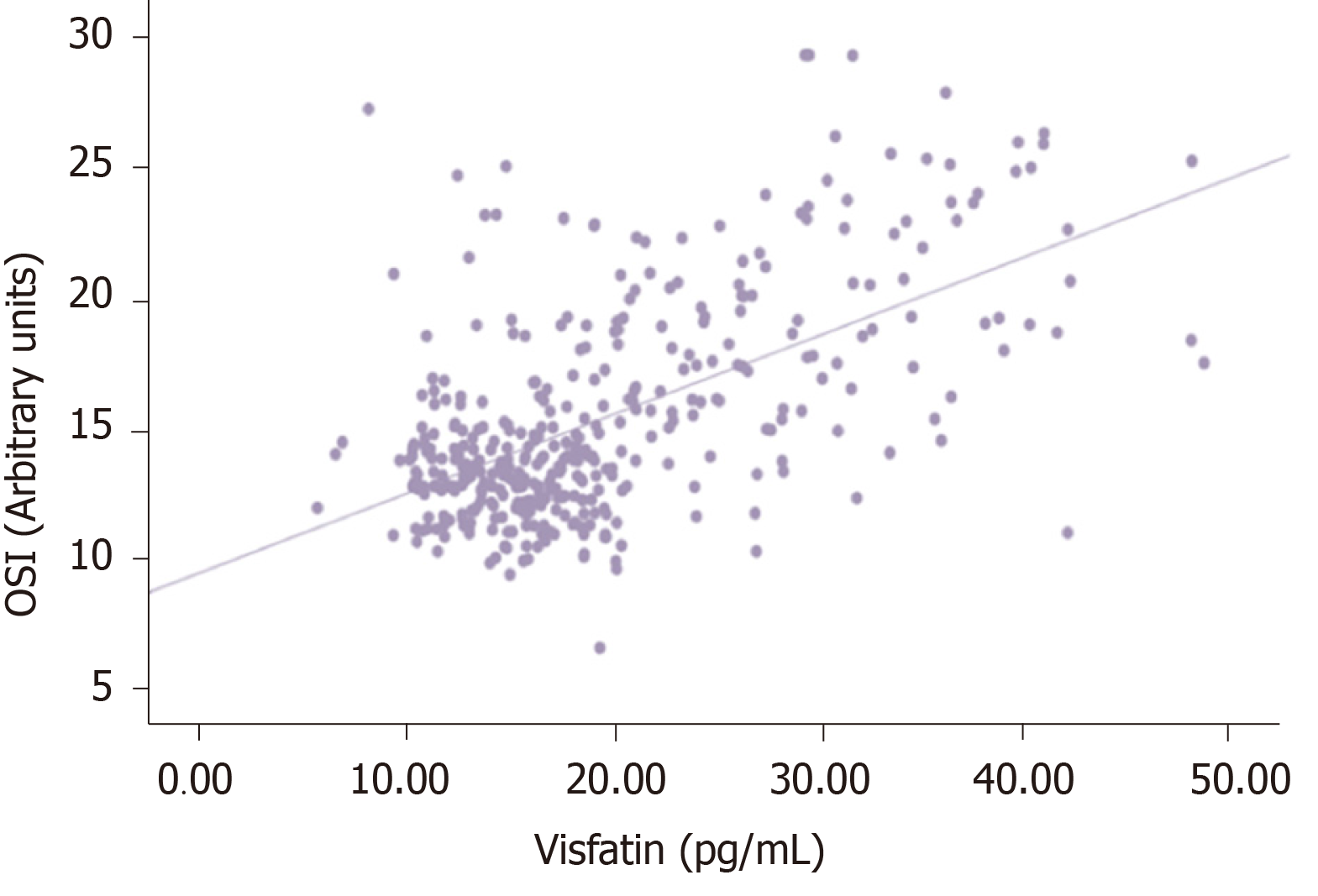
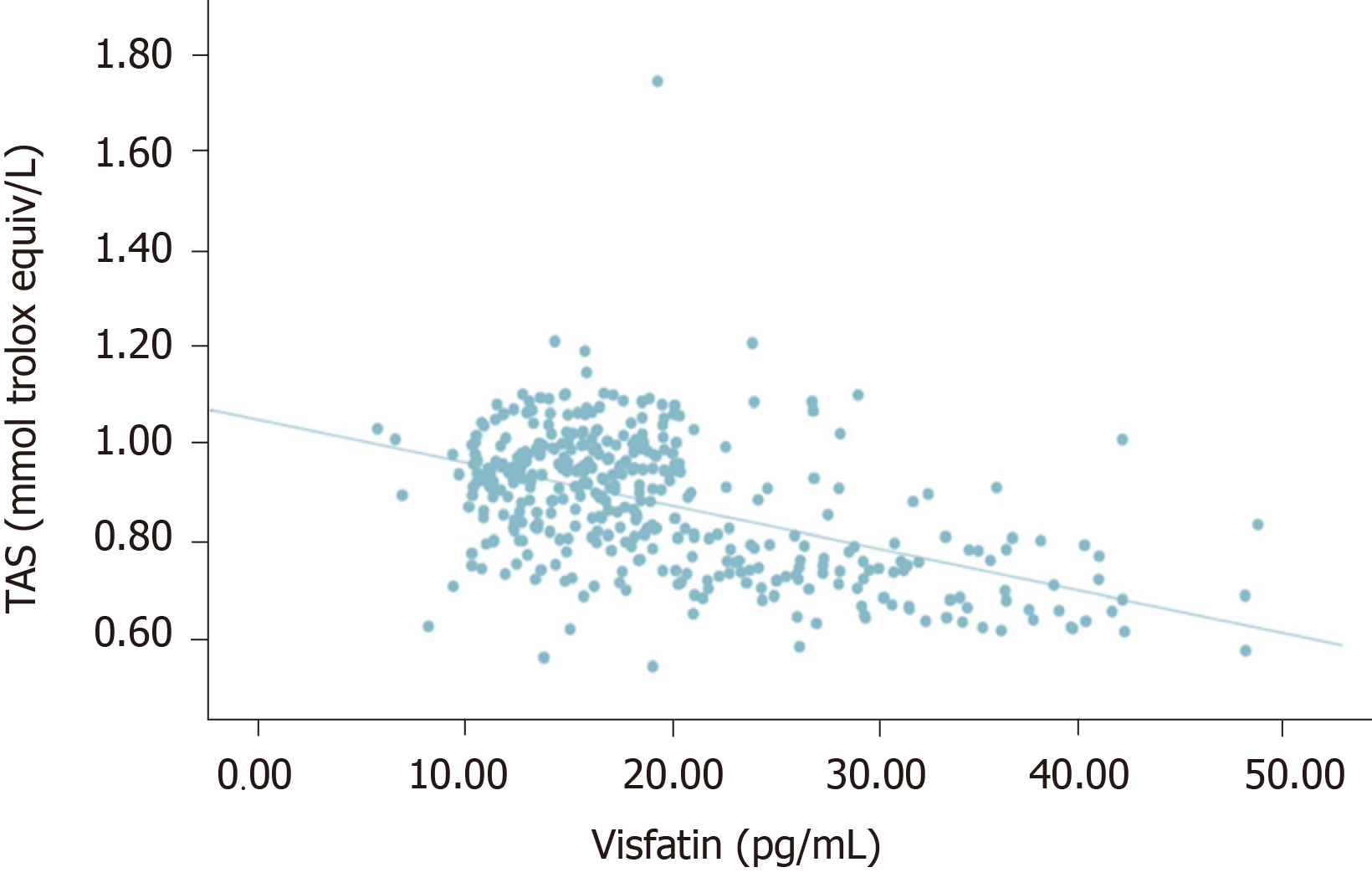
Serum HbA1c was correlated with TOS and OSI in the same direction and oppositely with TAS (P < 0.005, r = 0.503, r = 0.702, r = -0.593). Visfatin was positively correlated with higher glucose, HbA1c, TOS, and OSI (P < 0.005, r = 0.537, r = 0.753, r = 0.407, r = 0.587), and it was negatively correlated with TAS (r = -0.499).
Total and native thiol were negatively correlated with glucose, HbA1c, TOS, and OSI, but it was positively correlated with TAS. There were oppositely directed correlations between thiol-disulfide and the same parameters. Detailed results and correlations of all these parameters are presented in Table 3.
| Visfatin, 19.34 ± 8.07 | Total thiol, 0.51 ± 0.05 | Native thiol, 0.35 ± 0.05 | Thiol-disulfide, 0.08 ± 0.03 | |
| Glucose, 154.0 ± 57.5 | P < 0.05, r = 0.537 | P < 0.01, r = -0.2041 | P < 0.051, r = -0.4521 | P < 0.01, r = 0.206 |
| HbA1c, 7.68 ± 1.52 | P < 0.05, r = 0.753 | P < 0.01, r = -0.3511 | P < 0.051, r = -0.5971 | P < 0.01, r = 0.194 |
| TOS, 12.91 ± 1.92 | P < 0.05, r = 0.407 | P < 0.05, r = -0.2361 | P < 0.051, r = -0.3521 | P < 0.01, r = 0.094 |
| TAS, 0.87 ± 0.14 | P < 0.05, r = -0.4991 | P < 0.01, r = 0.243 | P < 0.05, r = 0.408 | P < 0.01, r = -0.1341 |
| OSI, 15.29 ± 4.12 | P < 0.05, r = 0.587 | P < 0.05, r = -0.3221 | P < 0.051, r = -0.4851 | P < 0.01, r = 0.132 |
| Visfatin | P < 0.01, r = -0.3381 | P < 0.011, r = -0.4481 | P = 0.092 |
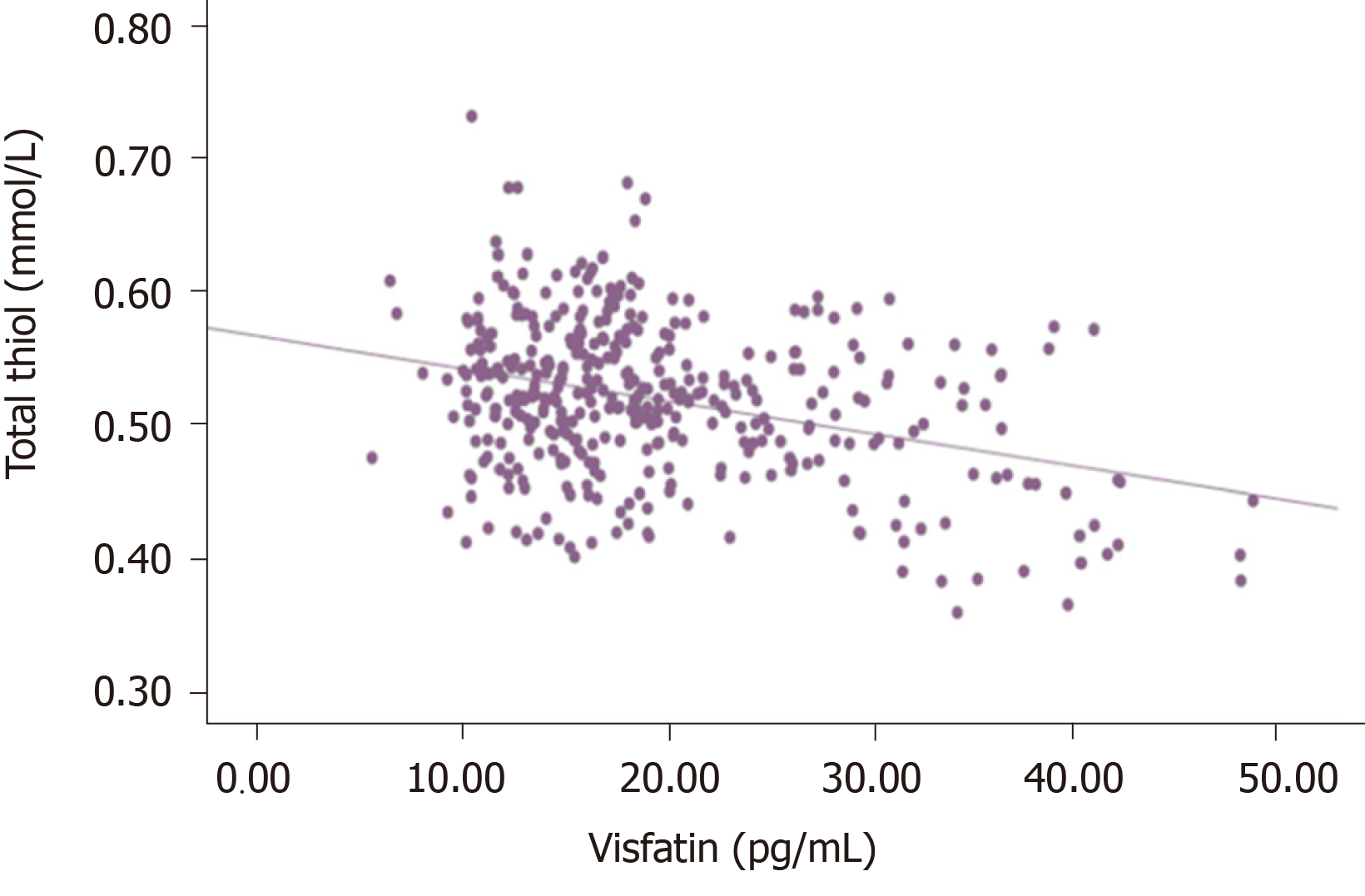
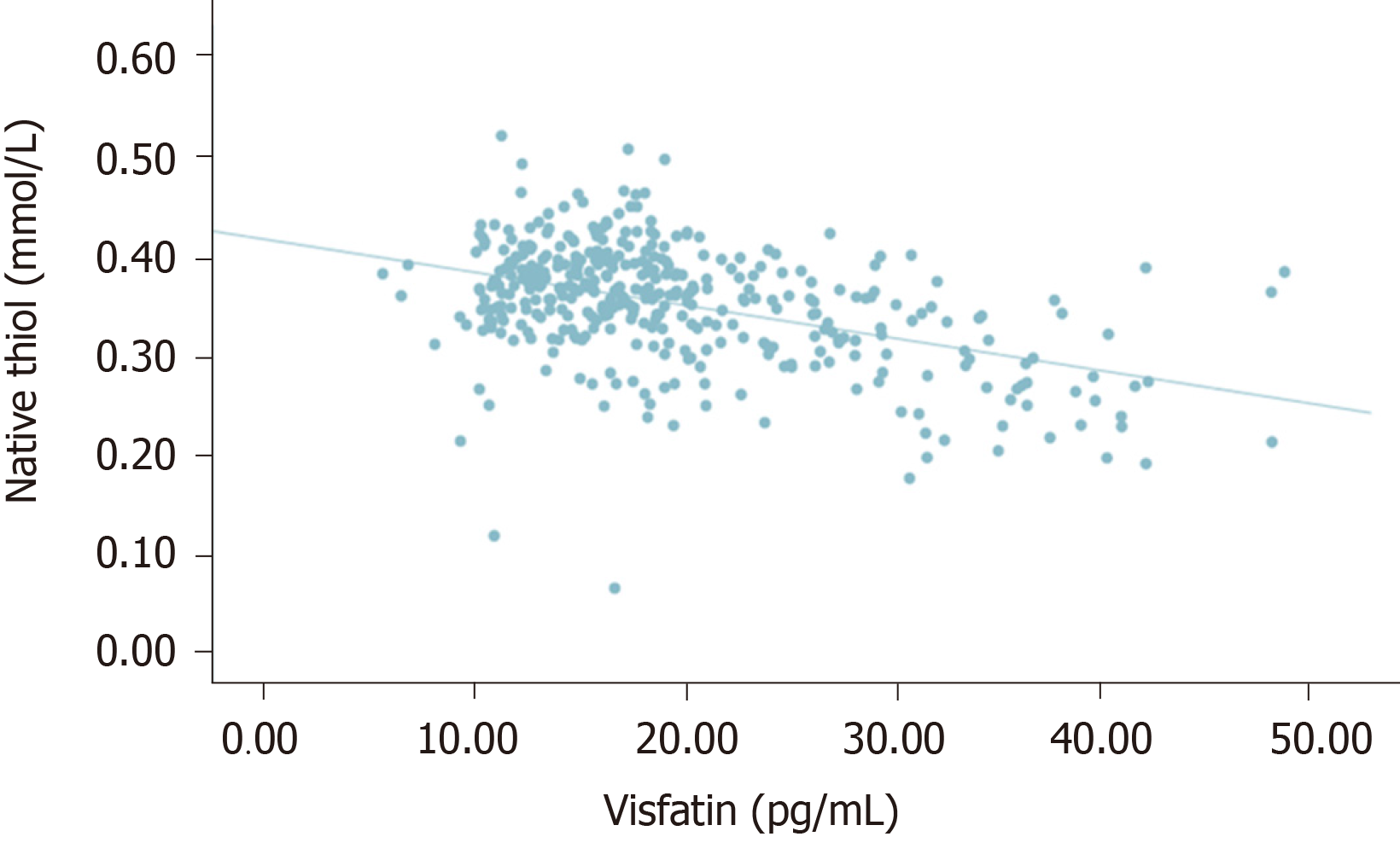
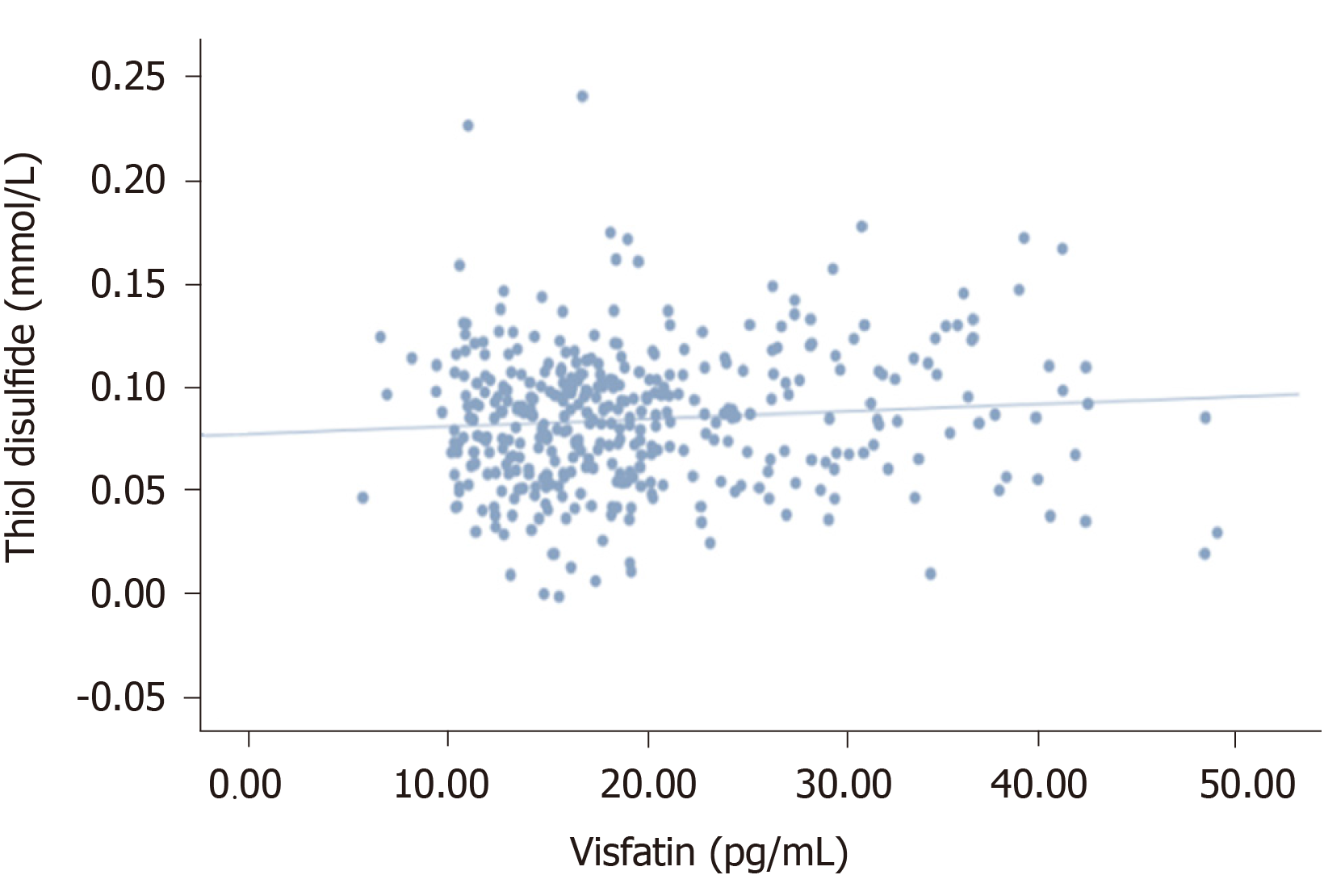
There was no significant correlation between visfatin and thiol-disulfide (P = 0.092, r = 0.086). However, a statistically significant negative correlation was detected between visfatin and total with native thiol (P < 0.005, r = - 0.338), (P < 0.005, r = -0.448). All correlations between visfatin and oxidative stress biomarkers and TDH were presented in Figures 1-6.
Type 2 DM is a major health problem worldwide. Along with increased cardiovascular risk, severe chronic complications, retinopathy, nephropathy, and neuropathy are associated with morbidity and mortality. DN is a common complication and affects more than one-third of patients. Although there are routine protocols for early diagnosis of retinopathy and nephropathy, there is no practical method with proven reliability for DN.
Microalbuminuria is controlled quarterly, and retinopathy is examined yearly if there are no additional problems. However, there is no standardized follow-up protocol and reliable methods for DN. The sensitivity of the surveys used is weak. Also in our patients, SPNSQ average score was 5.6 ± 3.6 over fifteen questions. SPNSQ is simple and easy to apply method. This survey was used to screen neuropathy in patients with human immunodeficiency virus and presented with 70% positive predictivity and 67% diagnostic efficacy[9]. This survey was compared with the Neuropathy Symptoms Score and DN 4 in patients with diabetes and reported as a proposed method for neuropathy screening. But sensitivity and reliability are insufficient because of subjective property as in our patient group[22].
The sensitivity of electrophysiological studies was observed in the preclinical period of DN[23]. Among the surveys, MNSI has been reported to have a linear relationship with the electrophysiological studies[24]. In our study, along with the MNSI questionnaire, 20.7% of the patients had neuropathy related symptoms. But examination findings were detected in 43.9% of the patients. Screening only symptoms does not seem like an effective and sensitive method. Neuropathy examination appears to be part of the clinical evaluation in these patients. We detected higher positive examination findings than similar studies[25,26]. Mean diabetes duration of 12.00 ± 7.29 years was significantly correlated with questionnaires and examination scores, and we thought that long term disease duration may be partly responsible for this result.
In patients with diabetes, microvascular complications are associated with glycemic regulation. For DN, this relationship is more evident in patients with type 1 diabetes[27]. A series of systemic and cellular imbalances were responsible in this process: Increased oxidative/nitrosative stress, activation of poly-ADP ribosylation, activation of the polyol and protein kinase C pathways, endothelial dysfunction, altered Na+/K+-ATPase pump function, cyclooxygenase-2 activation, endoplasmic reticulum stress, and impaired C-peptide-related pathways. These factors lead to cytokine and chemokine generation that induce cellular oxidative/nitrosative stress and finally neuronal damage[28,29]. In a large cohort with over one thousand participants, the relationship between IL-1β, IL-6, and measures of DN was demonstrated[30]. In another study with a small number of patients, it was shown that antioxidant activity evaluated by superoxide dismutase, catalase, and glutathione peroxidase decreased in patients with DN[31]. In our patients, the examination findings associated with neuropathy were more pronounced in patients with poor glycemic control. Also, a statistically significant higher level of TOS, OSI, visfatin and lower TAS levels were observed in this patient group. These results show that oxidative balance is one of the main determinants for the development of DN, and visfatin is likely to be effective in the oxidative direction.
With this result, the place of adipose tissue dysfunction in the pathogenesis of neuropathy once again became evident. Adipokines have endocrine, autocrine, and paracrine effects and are responsible for appetite regulation, insulin resistance, lipid metabolism, immunity, inflammation, vascular homeostasis, angiogenesis, and endothelial function. Visfatin is one of these adipokines, and it induces immune cell activation by β cell maturation and leukocyte, TNF/IL-6/IL-1b, and NFkB activation. At the same time, it leads to immune cell support on endothelial cells and vascular smooth muscle[32]. Although visfatin has been studied in many chronic disorders and has been associated with long-term unfavorable clinical outcomes and disease severity, its pathogenic or protective role has not been well established, especially in cardiovascular and cerebrovascular events[15,16].
Visfatin has been shown as a potential marker of inflammation and endothelial dysfunction in patients with myocardial infarction in a clinical trial[33]. In another case-controlled study in overweight patients with diabetes, visfatin, leptin, resistin, monocyte chemoattractive protein-1, and retinol-binding protein 4 were evaluated. Visfatin was associated with higher HbA1c and HOMA-β levels[34]. Visfatin has been evaluated in the pathogenesis of diabetic nephropathy. Patients with renal failure in different stages were compared with each other, and a major difference was not observed between the patient groups[35]. However, in another study, high visfatin was associated with a decrease in kidney function in patients with diabetic nephropathy[36]. Visfatin is possibly upregulated in a dose dependent manner for the stability of pro- and anti-inflammatory cytokines. The possible place of visfatin on the pathogenesis of DN was first investigated in our study. Our patients with neuropathy findings had higher levels of visfatin. Also, it had a positive linear relationship with the oxidative status of the patients (TOS and OSI). There are studies in the literature confirming our results. Increased visfatin along with resistin was associated with increased diabetes and cardiovascular risk in obese patients[37]. In another study, higher visfatin and TOS and lower TAS levels were observed in infants born to mothers who smoked[38]. Patients with psoriasis were evaluated for the same relationship. Increased oxidative stress associated with visfatin has been associated with chronic inflammation in patients with nonsevere psoriasis[39].
One of the parameters studied on oxidative stress in recent years is TDH. TDH has been associated with several pathophysiologies[40,41]. In our study, TAS had a positive correlation with total and native thiol and a negative correlation with thiol-disulfide. We also found a negative correlation between total and native thiol and HbA1c, TOS, and OSI. TDH appears to have antioxidant properties. In the literature, there are a few studies in which TDH is studied in patients with diabetes. In a study where prediabetic patients were compared with the control, lower native and total thiol and higher disulfide ratios were observed in the prediabetic group[14]. In another study, high levels of TDH were associated with poor glycemic control in patients with diabetes[42]. We also obtained similar results in terms of TDH and glycemic control. TDH was evaluated in patients with diabetic retinopathy, and it was found that disulfide ratios were higher in the advanced stage of retinopathy[43].
There is only one study that investigated TDH in DN pathogenesis. Patients with and without diabetes who were predominantly diagnosed with axonal polyneuropathy were evaluated in terms of TDH with the control group. In that study, total and native thiol levels were lower in patients with neuropathy than the control group, but there was no significant difference between patients with and without diabetes. However, patients with DN had higher disulfide levels than patients with nondiabetic polyneuropathy. In that study, it was emphasized that, regardless of etiology, TDH may be the last common pathway in patients with axonal damage polyneuropathy[44]. Our study is the first in the literature in which TDH was evaluated with visfatin. We did not find a significant correlation between visfatin and thiol-disulfide. However, we observed a significant negative correlation between visfatin and total native thiol. When the relationship between visfatin and TDH is evaluated together with other results of our study, it becomes evident that the effect of this adipokine on oxidative stress warrants further studies.
In conclusion, DN is one of the common complications of diabetes. Although there are many studies in terms of pathogenesis, there is currently no evidence-based and practical method for early diagnosis. Surveys and clinical examinations are insufficient and time-consuming methods. Oxidative stress has an important place in the pathogenesis of DN, and our results are consistent with the literature. In this study, oxidative stress was evaluated with visfatin and TDH in patients with DN, and significant correlations were found between these markers. We believe that more comprehensive studies involving TDH and visfatin are needed in the clinical management of DN.
Diabetic polyneuropathy is the most common complication of type 2 diabetes. However, there is no standard method for clinical follow-up and early diagnosis.
Diagnosis of neuropathy is possible only with special examination methods before clinical signs and symptoms. Studies on pathogenesis continue to be conducted on the basis of evidence. However, there is a need for practical methods for early diagnosis.
With this study, we aimed to investigate the frequency of neuropathy in our patients, to test the sensitivity of the interrogation methods used, and to investigate the location of visfatin and thiol balance, which have not yet been studied in pathogenesis.
Neuropathy examinations were completed with two defined questionnaires and examination methods: Subjective Peripheral Neuropathy Screen and Michigan Neuropathy Screening Instrument (MNSI). At the same time, venous samples were taken and stored under appropriate conditions until analysis. The analysis included biochemistry panels, oxidative stress parameters, visfatin, and thiol disulfide balance. The last two parameters were evaluated for the first time specifically for this patient group.
A total of 392 patients were evaluated (271 female, 121 male). The mean age of the patients was 57.5 ± 9.0 years. The mean diabetes period was 12.00 ± 7.29 years. The mean Subjective Peripheral Neuropathy Screen Questionnaire score was 5.6 ± 3.6, the mean MNSI questionnaire score was 4.5 ± 2.3, and the mean MNSI exam score was 2.4 ± 2.0 points. Subjective Peripheral Neuropathy Screen Questionnaire, MNSI questionnaire, and MNSI exam scores were correlated with each other (P < 0.005). There was a positive linear relationship between MNSI examination scores and glycated hemoglobin, visfatin, total oxidant status, and oxidative stress index. Visfatin was positively correlated with higher glucose, glycated hemoglobin, total oxidant status and oxidative stress index (P < 0.005, r = 0.537, r = 0.753, r = 0.407, r = 0.587), and it was negatively correlated with total antioxidant status (r = -0.499). Total and native thiol was negatively correlated with glucose, glycated hemoglobin, total oxidant status, and oxidative stress index, but it was positively correlated with total antioxidant status. A statistically significant negative correlation was detected between visfatin and total with native thiol (P < 0.005, r = -0.338), (P < 0.005, r = -0.448).
The sensitivity of the survey methods is low in the diagnosis of neuropathy. The place of oxidative stress in pathogenesis is indisputable. Neuropathy complaints must be included in the clinical examination of the patient, but its reliability is low. The sensitivity of the neuropathy examination is partially higher. However, its applicability is time consuming and difficult in the internal medicine clinic. Increased oxidative stress starts nerve damage in these patients without any clinical symptoms. Visfatin and thiol disulfide balance are being investigated in the pathogenesis of many diseases. It has been shown with this study that they may have a role in the development of polyneuropathy in pathogenesis. Routine monitoring of these parameters in patients with diabetes may be a practical approach for early diagnosis. However, the sensitivity levels of these techniques should be tested together with standard methods. In addition, comparisons for these parameters between patients with different levels of neuropathy, comorbidities, glycemic regulation, and using drugs are promising studies.
With this study, we observed how often neuropathy was in patients admitted to internal medicine clinics. We found that there is a need for a practical method for early diagnosis within the clinic. The pathogenesis of neuropathy is one of the issues illuminated in many aspects. These markers, which are thought to be involved in the pathogenesis, should continue to be studied, and their practical use should be evaluated.
Special thanks to Omer Uysal and Seda Turgut for statistical analysis.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Papazafiropoulou A, Qi XS, Zhang LL S-Editor: Zhang L L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1354] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 2. | Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, Vinik AI, Boulton AJ; Toronto Expert Panel on Diabetic Neuropathy. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. 2011;27:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 255] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen EJ, Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications. 2015;29:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Vas PRJ, Edmonds ME. Early recognition of diabetic peripheral neuropathy and the need for one-stop microvascular assessment. Lancet Diabetes Endocrinol. 2016;4:723-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Bourcier ME, Ullal J, Parson HK, Dublin CB, Witherspoon CA, Ward SA, Vinik AI. Diabetic peripheral neuropathy: how reliable is a homemade 1-g monofilament for screening? J Fam Pract. 2006;55:505-508. [PubMed] |
| 6. | McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998;55:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 390] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001;24:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 370] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 8. | Venkataramana AB, Skolasky RL, Creighton JA, McArthur JC. Diagnostic utility of the subjective peripheral neuropathy screen in HIV-infected persons with peripheral sensory polyneuropathy. AIDS Read. 2005;15:341-344, 348-349, 354. [PubMed] |
| 9. | Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 903] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 10. | Feldman EL, Nave KA, Jensen TS, Bennett DLH. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron. 2017;93:1296-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 619] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 11. | Uzar E, Tamam Y, Evliyaoglu O, Tuzcu A, Beyaz C, Acar A, Aydın B, Tasdemir N. Serum prolidase activity and oxidative status in patients with diabetic neuropathy. Neurol Sci. 2012;33:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Aslan M, Sabuncu T, Kocyigit A, Celik H, Selek S. Relationship between total oxidant status and severity of diabetic nephropathy in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2007;17:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 578] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 14. | Ates I, Kaplan M, Inan B, Alisik M, Erel O, Yilmaz N, Guler S. How does thiol/disulfide homeostasis change in prediabetic patients? Diabetes Res Clin Pract. 2015;110:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Huang Q, Dai WM, Jie YQ, Yu GF, Fan XF, Wu A. High concentrations of visfatin in the peripheral blood of patients with acute basal ganglia hemorrhage are associated with poor outcome. Peptides. 2013;39:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Schäffler A, Hamer OW, Dickopf J, Goetz A, Landfried K, Voelk M, Herfarth H, Kopp A, Buechler C, Schölmerich J, Brünnler T. Admission visfatin levels predict pancreatic and peripancreatic necrosis in acute pancreatitis and correlate with clinical severity. Am J Gastroenterol. 2011;106:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Hajri T, Gharib M, Kaul S, Karpeh MS. Association between adipokines and critical illness outcomes. J Trauma Acute Care Surg. 2017;83:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Kang YS, Bae MK, Kim JY, Jeong JW, Yun I, Jang HO, Bae SK. Visfatin induces neurite outgrowth in PC12 cells via ERK1/2 signaling pathway. Neurosci Lett. 2011;504:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Herman WH, Pop-Busui R, Braffett BH, Martin CL, Cleary PA, Albers JW, Feldman EL; DCCT/EDIC Research Group. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 341] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 20. | Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 990] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 21. | Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1722] [Cited by in RCA: 2140] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 22. | Agathos E, Tentolouris A, Eleftheriadou I, Katsaouni P, Nemtzas I, Petrou A, Papanikolaou C, Tentolouris N. Effect of α-lipoic acid on symptoms and quality of life in patients with painful diabetic neuropathy. J Int Med Res. 2018;46:1779-1790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Hyllienmark L, Alstrand N, Jonsson B, Ludvigsson J, Cooray G, Wahlberg-Topp J. Early electrophysiological abnormalities and clinical neuropathy: a prospective study in patients with type 1 diabetes. Diabetes Care. 2013;36:3187-3194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Fateh HR, Madani SP, Heshmat R, Larijani B. Correlation of Michigan neuropathy screening instrument, United Kingdom screening test and electrodiagnosis for early detection of diabetic peripheral neuropathy. J Diabetes Metab Disord. 2015;15:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | D'Souza M, Kulkarni V, Bhaskaran U, Ahmed H, Naimish H, Prakash A, S T, Dahiya B, Thapar R, Mithra P, Kumar N, Holla R, Bb D, Kumar A. Diabetic Peripheral Neuropathy and its Determinants among Patients Attending a Tertiary Health Care Centre in Mangalore, India. J Public Health Res. 2015;4:450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Khawaja N, Abu-Shennar J, Saleh M, Dahbour SS, Khader YS, Ajlouni KM. The prevalence and risk factors of peripheral neuropathy among patients with type 2 diabetes mellitus; the case of Jordan. Diabetol Metab Syndr. 2018;10:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Dziemidok P, Szcześniak G, Kostrzewa-Zabłocka E, Paprzycki P, Korzon-Burakowska A. Current glycaemic control has no impact on the advancement of diabetic neuropathy. Ann Agric Environ Med. 2012;19:742-745. [PubMed] |
| 28. | Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 734] [Article Influence: 56.5] [Reference Citation Analysis (1)] |
| 29. | Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94:2157-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 30. | Herder C, Bongaerts BW, Rathmann W, Heier M, Kowall B, Koenig W, Thorand B, Roden M, Meisinger C, Ziegler D. Association of subclinical inflammation with polyneuropathy in the older population: KORA F4 study. Diabetes Care. 2013;36:3663-3670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Kasznicki J, Kosmalski M, Sliwinska A, Mrowicka M, Stanczyk M, Majsterek I, Drzewoski J. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol Biol Rep. 2012;39:8669-8678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113:1009-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 310] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 33. | Smekal A, Vaclavik J. Adipokines and cardiovascular disease: A comprehensive review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | Toan NL, Van Hoan N, Cuong DV, Dung NV, Dung PT, Hang NT, Dieu DTH, Chung DT, Son HA, Phong PX, Lenon GB, Van De D, Van Tong H. Adipose tissue-derived cytokines and their correlations with clinical characteristics in Vietnamese patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2018;10:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Cha JJ, Min HS, Kim K, Lee MJ, Lee MH, Kim JE, Song HK, Cha DR, Kang YS. Long-term study of the association of adipokines and glucose variability with diabetic complications. Korean J Intern Med. 2018;33:367-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Akbarian N, Zarghami N, Mota A, Abediazar S, Abroon S, Mihanfar A, Amanzadeh M, Darbin A, Bannazadeh Baghi H, Rahmati-Yamchi M. Correlation Between Circulating Visfatin and Nitric Oxide Metabolites Levels in Patients With Diabetic Nephropathy. Iran J Kidney Dis. 2018;12:163-168. [PubMed] |
| 37. | Indulekha K, Surendar J, Anjana RM, Geetha L, Gokulakrishnan K, Pradeepa R, Mohan V. Metabolic obesity, adipocytokines, and inflammatory markers in Asian Indians--CURES-124. Diabetes Technol Ther. 2015;17:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Chełchowska M, Ambroszkiewicz J, Gajewska J, Rowicka G, Maciejewski TM, Mazur J. Cord Blood Adiponectin and Visfatin Concentrations in relation to Oxidative Stress Markers in Neonates Exposed and Nonexposed In Utero to Tobacco Smoke. Oxid Med Cell Longev. 2016;2016:4569108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Haberka M, Bańska-Kisiel K, Bergler-Czop B, Biedroń M, Brzezińska-Wcisło L, Okopień B, Gąsior Z. Mild to moderate psoriasis is associated with oxidative stress, subclinical atherosclerosis, and endothelial dysfunction. Pol Arch Intern Med. 2018;128:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Kolgelier S, Ergin M, Demir LS, Inkaya AC, Aktug Demir N, Alisik M, Erel O. Impaired Thiol-Disulfide Balance in Acute Brucellosis. Jpn J Infect Dis. 2017;70:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Ozler S, Oztas E, Caglar AT, Uygur D, Ergin M, Erel O, Danisman N. Thiol/disulfide homeostasis in predicting adverse perinatal outcomes at 24-28 weeks of pregnancy in gestational diabetes. J Matern Fetal Neonatal Med. 2016;29:3699-3704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Ergin M, Aydin C, Yurt EF, Cakir B, Erel O. The Variation of Disulfides in the Progression of Type 2 Diabetes Mellitus. Exp Clin Endocrinol Diabetes. 2020;128:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Gulpamuk B, Tekin K, Sonmez K, Inanc M, Neselioglu S, Erel O, Yilmazbas P. The significance of thiol/disulfide homeostasis and ischemia-modified albumin levels to assess the oxidative stress in patients with different stages of diabetes mellitus. Scand J Clin Lab Invest. 2018;78:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Vural G, Bektas H, Gumusyayla S, Deniz O, Alışık M, Erel O. Impaired thiol-disulphide homeostasis in patients with axonal polyneuropathy. Neurol Res. 2018;40:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |