Published online Apr 15, 2020. doi: 10.4239/wjd.v11.i4.137
Peer-review started: October 15, 2019
First decision: November 19, 2019
Revised: January 19, 2020
Accepted: February 17, 2020
Article in press: February 17, 2020
Published online: April 15, 2020
Processing time: 174 Days and 0.2 Hours
Previous studies have shown that patients with diabetes mellitus (DM) respond poorly to clopidogrel treatment.
To systematically evaluate the efficacy of clopidogrel for the treatment of acute coronary syndromes or ischemic stroke in patients with or without DM.
PubMed, the Cochrane Central Register of Controlled Trials, and EMBASE were searched from 1980 on 27 June 2019 to identify relevant randomized controlled trials that compared the effect of a combination of clopidogrel and aspirin with aspirin alone. A random-effects meta-analysis was used to estimate the hazard ratio (HR) and its 95% confidence interval (CI). Sensitivity analysis was performed using a fixed-effect model. The I2 statistic was used to evaluate the heterogeneity of the study data.
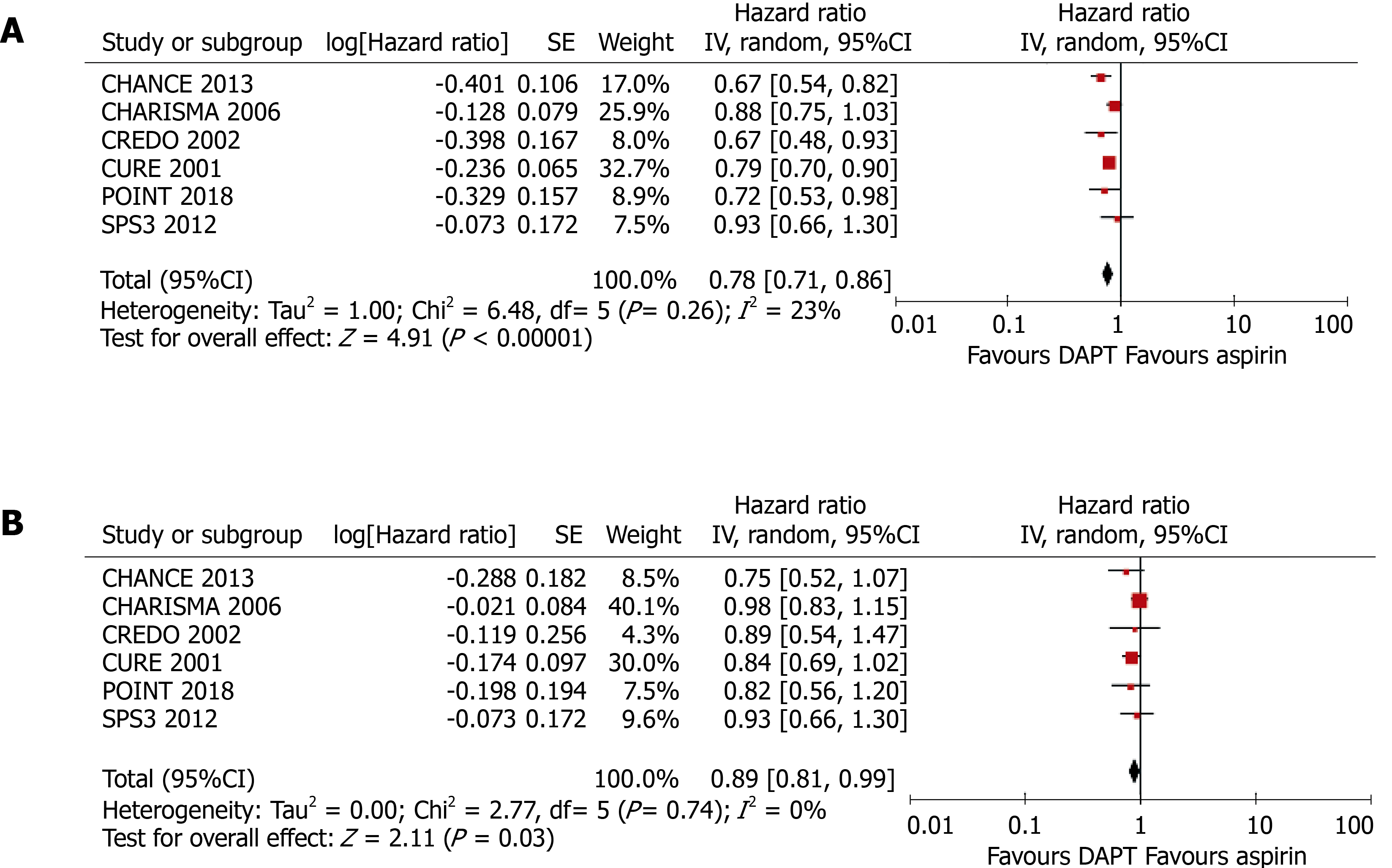
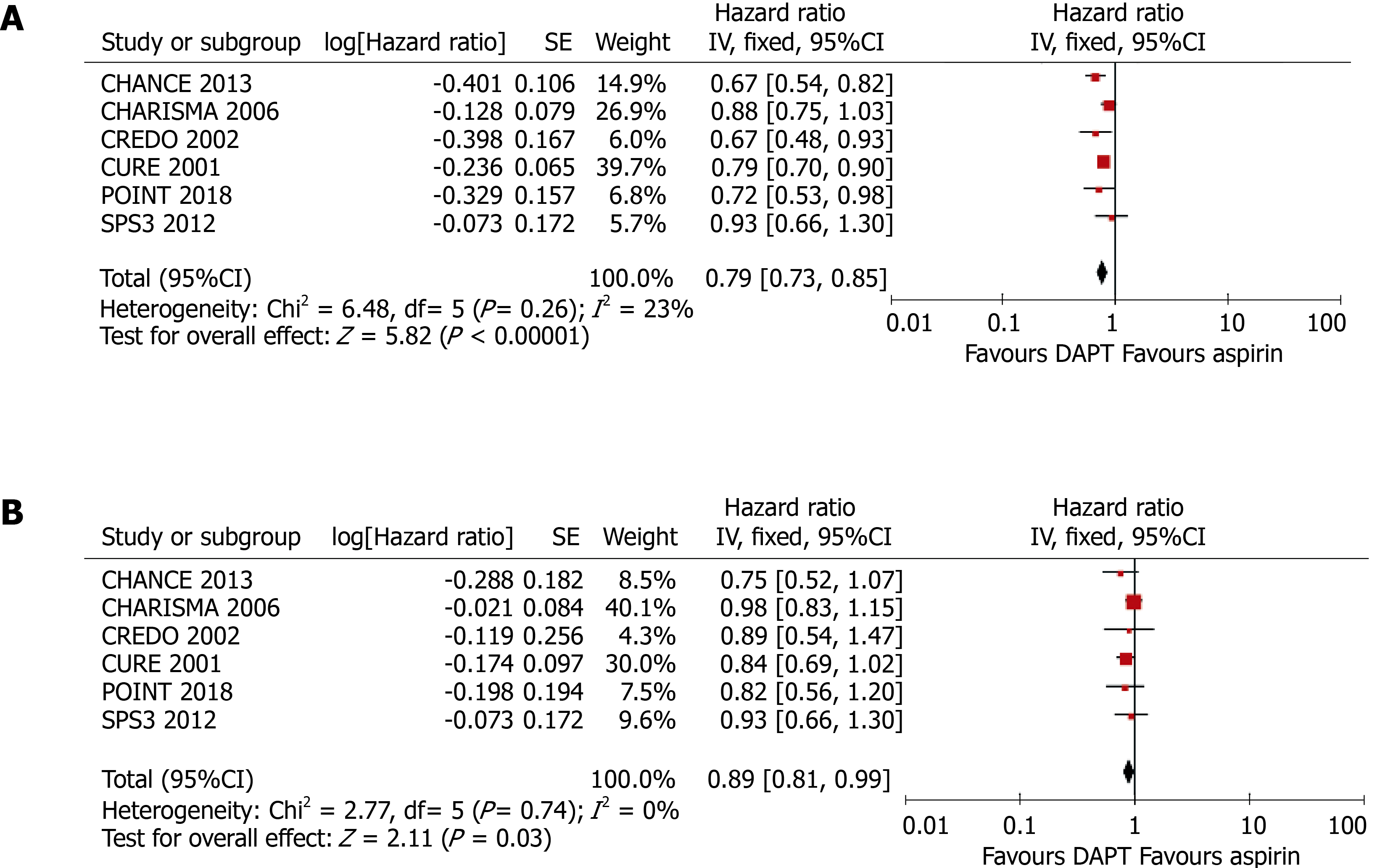
Six randomized controlled trials, comprising 43352 participants (13491 with and 29861 without DM) who had received antiplatelet therapy for ≥ 3 mo, were included in the meta-analysis. Compared with aspirin alone, a combination of clopidogrel and aspirin significantly reduced the risk of any cardiovascular event in patients without DM (HR = 0.78, 95%CI: 0.71–0.86, P < 0.001; I2 = 23%, P = 0.26). Clopidogrel plus aspirin also significantly reduced cardiovascular risk in patients with DM, although the effect was smaller (HR = 0.89, 95%CI: 0.81–0.99, P = 0.030; I2 = 0%, P = 0.74). Nevertheless, there was no significant difference in the efficacy of clopidogrel at reducing the risk of cardiovascular events in patients with DM vs those without (P for interaction = 0.062).
Thus, the present study shows that the addition of clopidogrel to aspirin significantly lowers cardiovascular risk in patients with or without DM who have experienced ischemic cardiovascular disease. The beneficial effect of the addition of clopidogrel to aspirin for patients with DM was lower than that in patients without DM, although the modifying effect of DM did not reach significance.
Core tip: The long-term effects of clopidogrel in patients with and without diabetes mellitus (DM) have not been systematically reviewed. The present meta-analysis firstly investigated the modifying effect of DM on the efficacy of long-term clopidogrel treatment in patients with ischemic cardiovascular disease. Although the analysis showed that the hazard ratio reduction in patients with DM was less than that in those without, this difference was not significant. The efficacy of dual antiplatelet therapy with clopidogrel and aspirin appeared to be slightly lower in patients with DM, emphasizing the need for individualized antiplatelet treatment for patients with DM after myocardial infarction or ischemic stroke.
- Citation: Liang LR, Ma Q, Feng L, Qiu Q, Zheng W, Xie WX. Long-term effect of clopidogrel in patients with and without diabetes: A systematic review and meta-analysis of randomized controlled trials. World J Diabetes 2020; 11(4): 137-149
- URL: https://www.wjgnet.com/1948-9358/full/v11/i4/137.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i4.137
Ischemic cardiovascular disease remains the leading cause of mortality globally[1,2]. Early initiation of dual antiplatelet therapy with a P2Y12 receptor antagonist plus aspirin is recommended for patients with ischemic cardiovascular disease in the current guidelines[3,4]. Clopidogrel remains the most widely prescribed P2Y12 receptor antagonist and is recommended by the latest guidelines for the management of ischemic stroke and acute coronary syndromes (ACS)[5,6]. Studies have shown that patients with diabetes mellitus (DM) exhibit a poorer response to clopidogrel than patients without DM[7,8], leading to a significant difference in the incidence of recurrence of cardiovascular events and mortality associated with DM[9].
Previous research has shown that hyperglycemia increases platelet reactivity, and the underlying mechanisms include higher platelet receptor expression, intracellular downstream signaling abnormalities, and P2Y12 mutation and expression level[7,10]. The findings of the OPTIMUS trial, which compared the efficacy of a 150-mg maintenance dose of clopidogrel with 75 mg in 40 patients with both DM and coronary artery disease, showed that the higher maintenance dose of clopidogrel is associated with greater antiplatelet effects[11]. Hence, it is reasonable to hypothesize that clopidogrel treatment of ischemic vascular disease would be associated with a less substantial reduction in the risk of cardiovascular events in patients with DM than in those without.
Subgroup analyses of data from several randomized controlled trials (RCTs) comparing the efficacy of a combination of clopidogrel and aspirin with aspirin alone have shown that DM reduces the effect of clopidogrel on the incidence of recurrence of cardiovascular events and all-cause mortality, although the interactions were not significant[12-14]. A large-scale cohort study also showed that DM was associated with a lower efficacy of clopidogrel for a reduction in 1-year cardiovascular mortality in patients with myocardial infarction[9]. However, the long-term effects of clopidogrel in patients with and without DM have not been systematically reviewed. Therefore, we hypothesized that the presence of DM modifies the long-term efficacy of clopidogrel for a reduction in cardiovascular risk, and we performed a systematic review and meta-analysis to test this hypothesis.
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[15].
The literature search was conducted on 27 June 2019 using the Cochrane Library database for trials, EMBASE, and PubMed. The search strategy was: (1) PubMed (from 1980) 2019/06/27, (clopidogrel[Title/Abstract]) AND aspirin[Title/Abstract], article types: Clinical Trial; (2) EMBASE (from 1980) 2019/06/27, #2 #1 AND 'randomized controlled trial'/de, #1 'clopidogrel':ab,ti AND 'aspirin':ab,ti; and (3) Cochrane Central Register of Controlled Trials (CENTRAL, from 1980) 2019/06/27, keywords: (“clopidogrel” AND “aspirin”); search limits: Trials.
The inclusion criteria for RCTs were double-blind design, clopidogrel and aspirin vs aspirin for the treatment of ischemic cardiovascular disease, age ≥ 18 years, subgroup analysis according to the presence of diabetes (if not reported, the corresponding author was contacted to obtain the data)[16], and a duration of treatment ≥ 3 mo[17]. We also included the CHARISMA trial, because the percentage of patients with documented vascular disease in the trial was high (78%)[14].
The outcomes assessed in the meta-analysis were fatal and non-fatal cardiovascular events. Table 1 summarizes the definitions of the primary outcomes of the included RCTs.
| Trial | Definition of primary efficacy outcome |
| CHANCE 2013 | Stroke recurrence (ischemic or hemorrhagic). |
| CHARISM 2006 | The composite of myocardial infarction, stroke, or death from cardiovascular causes. |
| CREDO 2002 | The composite of death, myocardial infarction, and stroke. |
| CURE 2001 | The composite of myocardial infarction, stroke, or death from cardiovascular causes. |
| POINT 2018 | The composite of ischemic stroke, myocardial infarction, or death from ischemic vascular causes. |
| SPS3 2012 | Stroke recurrence (ischemic or hemorrhagic). |
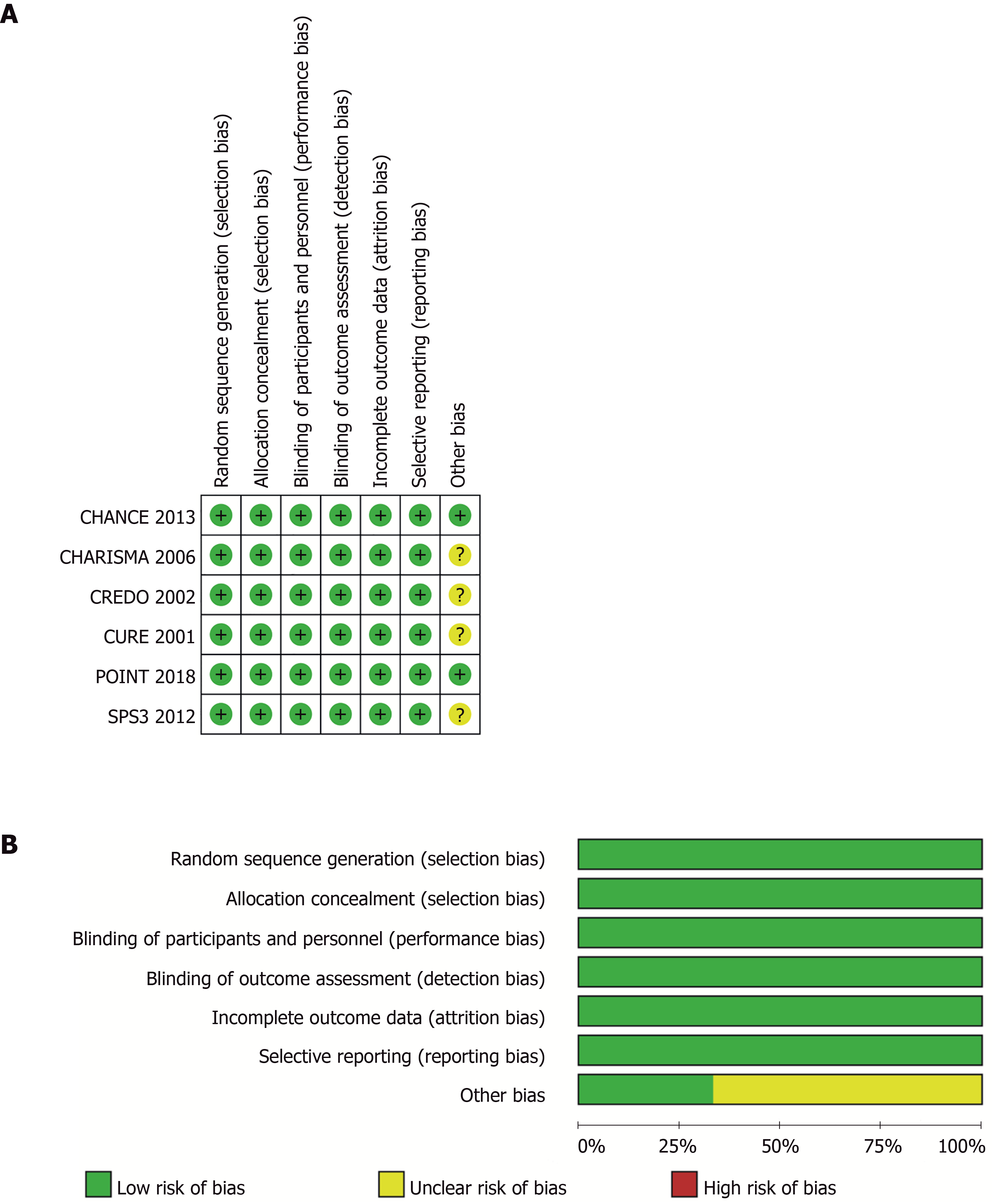
Two researchers (Liang LR and Ma Q) used a specially designed questionnaire to extract data independently. Consensus was reached by discussion. The Cochrane Collaboration’s risk-of-bias table was used to assess the quality of the included RCTs independently by two authors (Feng L and Xie WX)[18]. Figure 1 shows the risk of bias in the RCTs.
Review Manager 5.3 (Nordic Cochrane Center, Copenhagen, Denmark) was used to conduct the meta-analysis within random-effect models to estimate the pooled hazard ratio (HR) and its 95% confidence interval (CI). Sensitivity analysis was also performed using fixed-effect models. To evaluate the modifying effect of DM on the efficacy of clopidogrel, we used the Z-test to compare the difference between the two pooled HRs from the subgroup analyses, using the method proposed by Altman and Bland[19]. We used the I2 statistic to estimate the heterogeneity of the included RCTs, and the Egger test and funnel plots to estimate the level of publication bias in STATA (version 11; Stata Corp, College Station, TX). All analyses were two-sided, and an alpha value of 0.05 was used as the threshold for statistical significance.
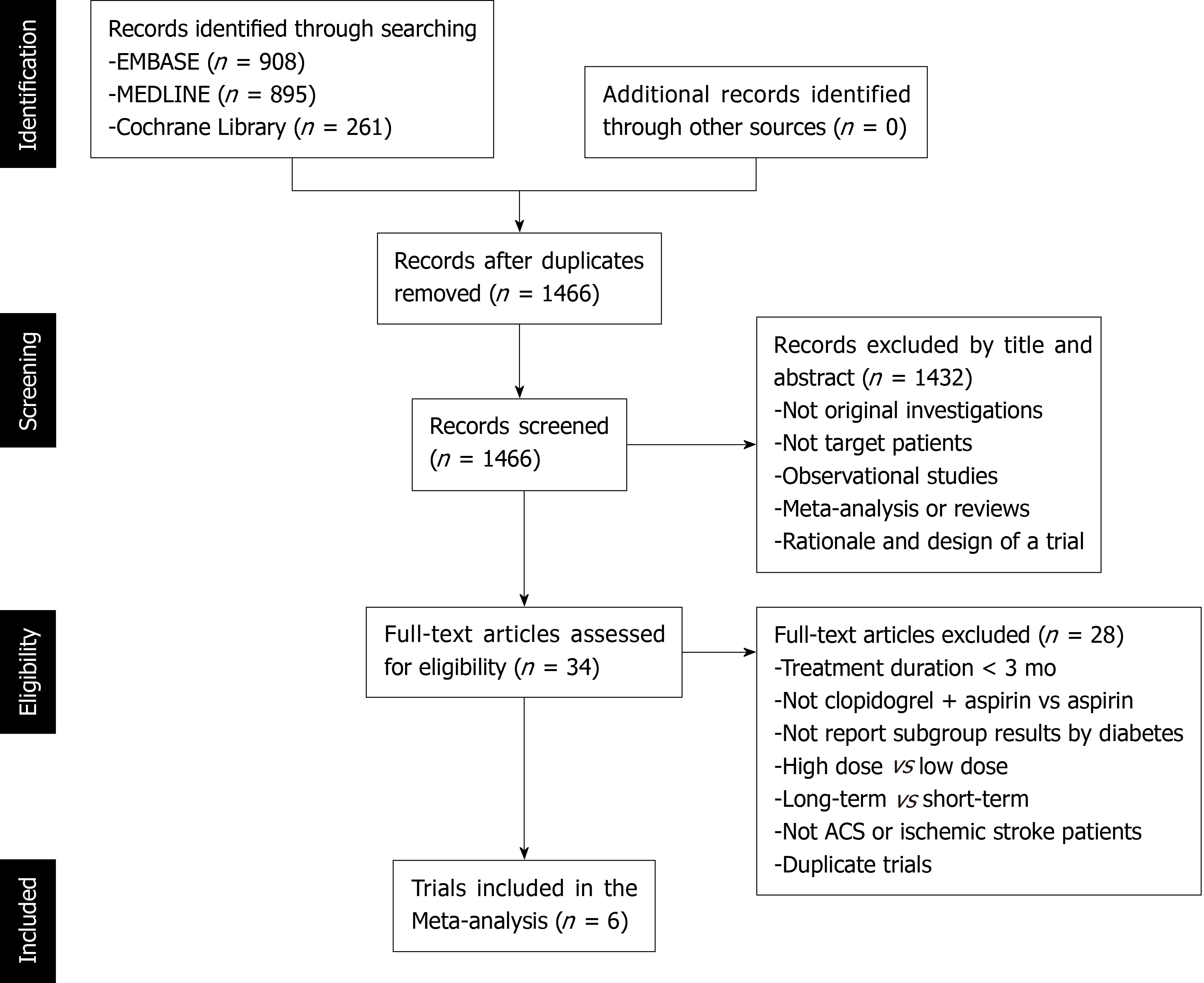
Figure 2 shows the study selection process, which was conducted according to the PRISMA statement. Two thousand and sixty-four publications were identified in the initial search, from which 598 duplicates were excluded, and a further 1432 articles were removed after reviewing their title and abstract. The full text of the remaining 34 articles was then reviewed independently by two researchers, and 28 of these were excluded because of a treatment duration < 3 mo, a lack of comparison between clopidogrel plus aspirin and aspirin alone, a lack of subgroup analysis according to the presence of DM, a long-term vs short-term analysis, a high-dose vs low-dose analysis, ACS or ischemic stroke patients were not studied, or duplication (Table 2). Therefore, six RCTs, comprising 43352 participants (13491 with and 29861 without DM), were eligible for the present analysis[12-14,16,20,21]. Table 3 presents the characteristics of the included RCTs, of which three had recruited patients who had experienced an ischemic stroke or transient ischemic attack, two had recruited ACS patients, and one had recruited patients with cardiovascular disease or multiple risk factors. Table 4 summarizes the antiplatelet treatments and daily doses used in these trials.
| Title | Reason for exclusion |
| A randomised, blinded, trial of clopidogrel vs aspirin in patients at risk of ischaemic events (CAPRIE) | Not clopidogrel + aspirin vs aspirin |
| Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation | Treatment duration < 3 mo; Not report subgroup results |
| Addition of clopidogrel to aspirin in 45852 patients with acute myocardial infarction: Randomised placebo-controlled trial | Treatment duration < 3 mo; Not report subgroup results |
| Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): Randomised, double-blind, placebo-controlled trial | Not clopidogrel + aspirin vs aspirin |
| Aspirin and extended-release dipyridamole vs clopidogrel for recurrent stroke | Not clopidogrel + aspirin vs aspirin |
| Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery | Treatment duration < 3 mo |
| Aspirin plus clopidogrel vs aspirin alone after coronary artery bypass grafting: The clopidogrel after surgery for coronary artery disease (CASCADE) Trial | Not report subgroup results |
| Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin vs aspirin alone | Duplicate trials |
| Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: One year outcomes | Duplicate trials |
| Dose comparisons of clopidogrel and aspirin in acute coronary syndromes | High dose vs low dose |
| Duration of Dual Antiplatelet Therapy after Implantation of Drug-Eluting Stents | Long-term vs short-term |
| Efficacy and safety outcomes of ticagrelor compared with clopidogrel in elderly Chinese patients with acute coronary syndrome | Not clopidogrel + aspirin vs aspirin |
| Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics | Treatment duration < 3 mo |
| Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study | Duplicate trials |
| Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): A randomised controlled pilot trial | Not report subgroup results |
| Prasugrel or double-dose clopidogrel to overcome clopidogrel low-response – The TAILOR randomized trial | Not clopidogrel + aspirin vs aspirin |
| Prasugrel vs clopidogrel for acute coronary syndromes without revascularization | Not clopidogrel + aspirin vs aspirin |
| Prasugrel vs clopidogrel in patients with acute coronary syndromes | Not clopidogrel + aspirin vs aspirin |
| PROCLAIM: Pilot study to examine the effects of clopidogrel on inflammatory markers in patients with metabolic syndrome receiving low-dose aspirin. | Treatment duration < 3 mo |
| Randomized clinical trial of the antiplatelet effects of aspirin-clopidogrel combination vs aspirin alone after lower limb angioplasty | Treatment duration < 3 mo |
| Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention | Not clopidogrel + aspirin vs aspirin |
| Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial | Not report subgroup results |
| Ticagrelor vs aspirin in acute stroke or transient ischemic attack | Not clopidogrel + aspirin vs aspirin |
| Ticagrelor vs clopidogrel in patients with acute coronary syndromes | Not clopidogrel + aspirin vs aspirin |
| Ticagrelor vs clopidogrel after fibrinolytic therapy in patients with ST-elevation myocardial infarction | Not clopidogrel + aspirin vs aspirin |
| Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome | Not clopidogrel + aspirin vs aspirin |
| Twelve or 30 mo of dual antiplatelet therapy after drug-eluting stents | Long-term vs short-term |
| Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation | Not ACS or ischemic stroke patients |
| Trial | Country | Centers | Patients | Sample size | Male % | Mean age Y | Diabetes% | Treatment duration M | Lost to follow-up % | Blinding | ITT analysis |
| CHANCE 2013 | China | 114 | Minor ischemic stroke or high-risk TIA | 5170 | 66 | 63 | 21 | 3 | 0.7 | Double-blind | Yes |
| CHARI-SM 2006 | Worldwide | 768 | CVD or multiple risk factors | 15603 | 70 | 64 | 42 | 28 | 0.5 | Double-blind | Yes |
| CREDO 2002 | United States and Canada | 99 | Those would undergo elective PCI | 2116 | 71 | 62 | 26 | 12 | 1.1 | Double-blind | Yes |
| CURE 2001 | Worldwide | 482 | ACS without ST-segment elevation | 12562 | 62 | 64 | 23 | 12 | 0.1 | Double-blind | Yes |
| POINT 2018 | Worldwide | 269 | Acute ischemic stroke or high-risk TIA | 4881 | 55 | 65 | 27 | 3 | 4.1 | Double-blind | Yes |
| SPS3 2012 | Worldwide | 82 | Recent symptomatic lacunar infarcts | 3020 | 63 | 63 | 36 | 41 | 2.0 | Double-blind | Yes |
| Trial | Drug (daily doses, mg) | |
| Clopidogrel + Aspirin | Aspirin | |
| CHANCE 2013 | Clopidogrel (day 1: 300; day 2-90: 75) + Aspirin (day 1: 75-300; day 2-21: 75; day 22-90: placebo) | Aspirin (day 1: 75-300; day 2-90: 75) |
| CHARISM 2006 | Clopidogrel (75) + Aspirin (75-162) | Aspirin (75-162) |
| CREDO 2002 | Clopidogrel (day 1: 300; day 2 to 12 mo: 75) + Aspirin (day 1: 325; day 2 to 12 mo: 81-325) | Clopidogrel (day 2-28: 75) + Aspirin (day 1: 325; day 2 to 12 mo: 81-325) |
| CURE 2001 | Clopidogrel (day 1: 300, and then 75) + Aspirin (75-325) | Aspirin (75-325) |
| POINT 2018 | Clopidogrel (day 1: 600, and then 75) + Aspirin (50-325) | Aspirin (50-325) |
| SPS3 2012 | Clopidogrel (75) + Aspirin (325) | Aspirin (325) |
As shown in Figure 3, compared with aspirin alone, the addition of clopidogrel to aspirin significantly reduced the risk of any cardiovascular event in patients without DM (HR = 0.78, 95%CI: 0.71–0.86, P < 0.001; I2 = 23%, P = 0.26) and also in patients with DM, although the benefit was less (HR = 0.89, 95%CI: 0.81-0.99, P = 0.030; I2 = 0%, P = 0.74). The presence of DM was associated with an 11% higher HR, although the difference in the efficacy of clopidogrel in patients with and without DM did not reach significance (P for interaction = 0.062).
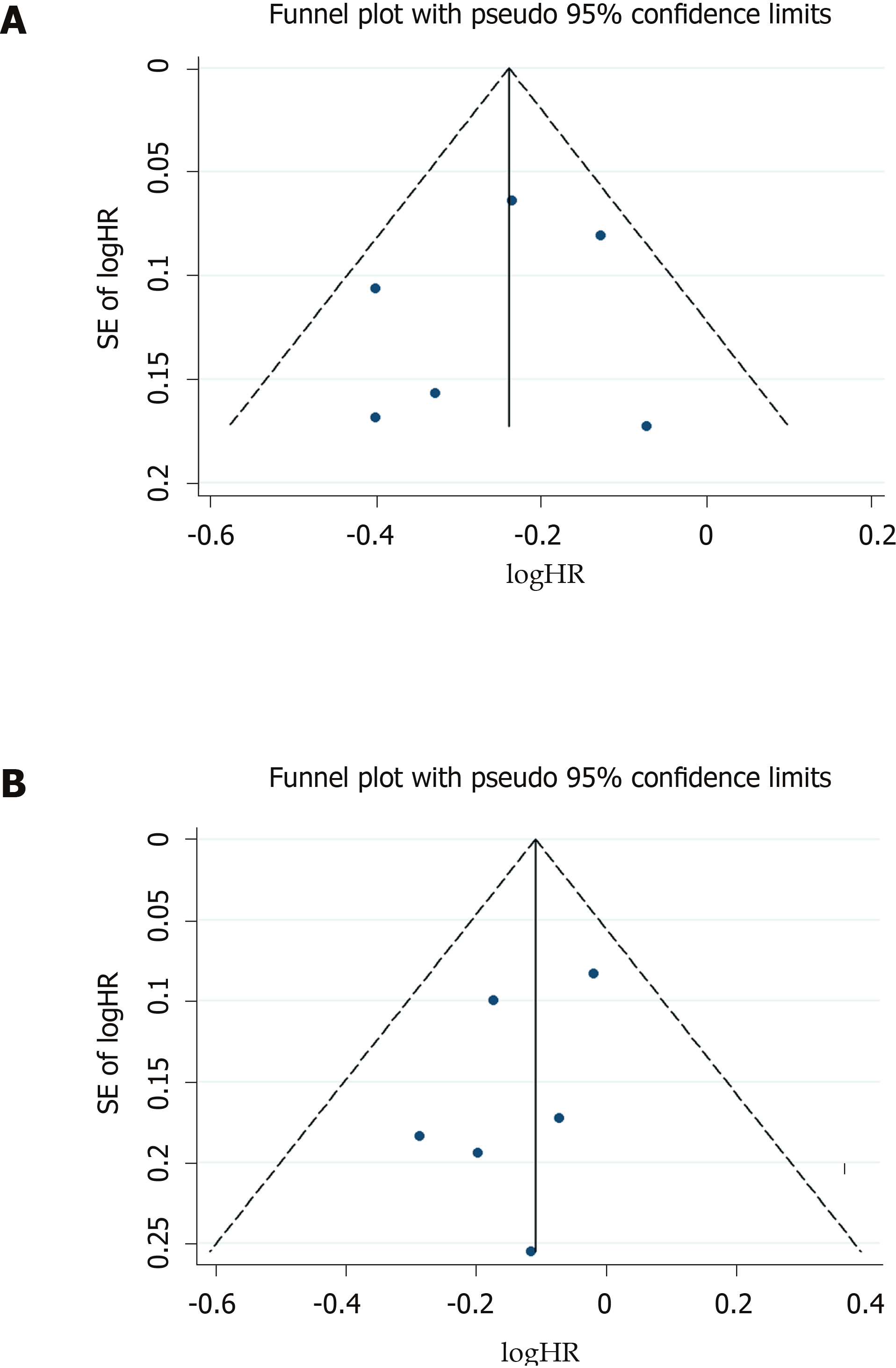
Both funnel plots (Figure 4) and Egger tests (P = 0.592 for patients without DM, and P = 0.296 for patients with DM) showed no evidence of publication bias.
The pooled results of a meta-analysis using fixed-effect models was consistent with the principal findings of random-effect models (Figure 5). Clopidogrel treatment was associated with relative risk reductions of 11% (HR = 0.89, 95%CI: 0.81–0.99, P = 0.030) and 21% (HR = 0.79, 95%CI: 0.73-0.85, P < 0.001) for any cardiovascular event in patients with and without DM, respectively. However, the modifying effect of DM on the incidence of cardiovascular events was not significant (P for interaction = 0.064).
Our systematic review and meta-analysis found that the addition of clopidogrel to aspirin significantly reduced the risk of any cardiovascular event in patients with or without DM. In addition, the results show that the presence of DM is associated with an increase of 11% in the HR, although this difference was not significant. To our knowledge, this is the first meta-analysis to evaluate the efficacy of long-term clopidogrel treatment in patients with ischemic cardiovascular disease, according to the presence or absence of DM.
The latest evidence provided by head-to-head RCTs has led to the current guidelines for the management of ACS stating that clopidogrel is inferior to ticagrelor and prasugrel as a P2Y12 inhibitor for patients with ST-segment elevation myocardial infarction or non-STE ACS[3,4]. Recently, subgroup analyses of these RCTs have shown that the effects of ticagrelor and prasugrel are not modified by the presence of DM[22,23]. Evidence from previous trials and the present meta-analysis indicate that it is reasonable to use ticagrelor or prasugrel in preference to clopidogrel for the treatment of ACS patients, and especially those with DM. However, it should be noted that both ticagrelor and prasugrel are associated with higher risks of bleeding than clopidogrel; therefore, clopidogrel can still be used if ticagrelor and prasugrel are not available or are contraindicated[3,4]. Furthermore, data regarding the clinical efficacy and safety of ticagrelor or prasugrel for the treatment of ischemic stroke are limited. The SOCRATES trial, which compared the efficacy and safety of ticagrelor and aspirin in 13199 patients who had experienced a mild ischemic stroke or high-risk transient ischemic attack, found that ticagrelor was no more effective than aspirin at lowering cardiovascular risk over 90 days[24]. Therefore, clopidogrel remains the first choice of P2Y12 inhibitor for the treatment of ischemic stroke[6]. In the present study, a weaker effect of clopidogrel was identified in patients with DM, which implies that a new antiplatelet agent is needed for ischemic stroke patients with DM.
A prospective cohort study of 58851 patients who had experienced a myocardial infarction confirmed this hypothesis[9]. Andersson and colleagues reported that clopidogrel treatment was associated with lesser reductions in 1-year all-cause mortality and cardiovascular mortality in patients with DM, compared to those without[9]. The results of the large-scale cohort study were consistent with the present findings, and we consider that one of the most important reasons why the present meta-analysis did not demonstrate a modifying effect of DM was the relatively small number of RCTs included. Therefore, a second meta-analysis should be performed when data from additional RCTs become available.
Several potential mechanisms that might explain the variability in the response to clopidogrel have been suggested. Firstly, a pharmacokinetic study showed that variability in intestinal absorption is the principal cause[25]. Second, polymorphisms in hepatic cytochrome (CYP) P450 3A, including in CYP3A4 and CYP3A5, may also be important: An inverse correlation has been shown between the percentage platelet aggregation and CYP3A4 activity in clopidogrel users[26]. In particular, of the multiple polymorphisms of CYP3A4, the IVS10 + 12G>A variant has been shown to regulate clopidogrel responsiveness[27]. Furthermore, a CYP3A5 polymorphism has been shown to be associated with atherothrombosis after clopidogrel treatment[28], although there is new evidence that challenges the existence of the association between CYP3A5 genotype and clopidogrel response[29]. Third, genetic platelet receptor polymorphisms have been implicated. Single-nucleotide polymorphisms of platelet receptors, such as GP IIIa L33P (= PlA1/2) and GP Ia 807 C/T have been suggested to be risk factors for thrombosis[30]. Fourth, the use of concurrent medication, such as calcium channel blockers, has recently been demonstrated to be a predictor of atherothrombotic events after clopidogrel treatment[29]. These mechanisms might also explain the effect of DM on the clopidogrel response.
Our study had several strengths: The inclusion of high-quality RCTs, large samples, no significant heterogeneity among the studies, and no evidence of publication bias. However, the study also had some limitations. First, the primary outcomes of the included RCTs were not entirely consistent, and secondary outcome data cannot be collected, because publications describing RCTs only show subgroup data for the primary outcomes. The present study only pooled the results of the primary outcomes of the included trials, which may have caused bias. Second, we estimated the pooled HRs on the basis of trial-level data, whereas pooled HRs derived from individual-level data would be more accurate. Third, although we conducted a thorough literature search to identify relevant RCTs, it is possible that some appropriate RCTs were not included in this study, implying the possibility of selection bias.
In conclusion, the present study found that the addition of clopidogrel to aspirin significantly reduced cardiovascular risk in patients with and without DM who had experienced ischemic cardiovascular disease. The beneficial effect of a combination of clopidogrel and aspirin for patients with DM appeared to be lower than that for patients without DM, although this difference did not reach significance.
Clopidogrel remains the most widely prescribed P2Y12 receptor antagonist and is recommended by the latest guidelines for the management of ischemic stroke and acute coronary syndromes. Studies have shown that patients with diabetes mellitus (DM) exhibit a poorer response to clopidogrel than patients without DM, leading to a significant difference in the incidence of recurrence of cardiovascular events and mortality associated with DM. However, the long-term effects of clopidogrel in patients with and without DM have not been systematically reviewed.
We hypothesized that the presence of DM modifies the long-term efficacy of clopidogrel for a reduction in cardiovascular risk. To our knowledge, this is the first meta-analysis to evaluate the efficacy of long-term clopidogrel treatment in patients with ischemic cardiovascular disease, according to the presence or absence of DM.
This study aimed to systematically evaluate the efficacy of clopidogrel for the treatment of acute coronary syndromes or ischemic stroke in patients with or without DM.
A systematic review and meta-analysis of randomized controlled trials.
Six randomized controlled trials, comprising 43,352 participants (13,491 with and 29,861 without DM) who had received antiplatelet therapy for ≥ 3 mo, were included in the meta-analysis. Compared with aspirin alone, a combination of clopidogrel and aspirin significantly reduced the risk of any cardiovascular event in patients without DM (HR = 0.78, 95%CI: 0.71–0.86, P < 0.001; I2 = 23%, P = 0.26). Clopidogrel plus aspirin also significantly reduced cardiovascular risk in patients with DM, although the effect was smaller (HR = 0.89, 95%CI: 0.81–0.99, P = 0.030; I2 = 0%, P = 0.74). Nevertheless, there was no significant difference in the efficacy of clopidogrel at reducing the risk of cardiovascular events in patients with DM vs those without (P for interaction = 0.062).
The present study found that the addition of clopidogrel to aspirin significantly reduced cardiovascular risk in patients with and without DM who had experienced ischemic cardiovascular disease. The beneficial effect of the addition of clopidogrel to aspirin for patients with DM was lower than that in patients without DM, although the modifying effect of DM did not reach significance. In the present study, a weaker effect of clopidogrel was identified in patients with DM, which implies that a new antiplatelet agent is needed for ischemic stroke patients with DM. We hypothesized that the presence of DM modifies the long-term efficacy of clopidogrel for a reduction in cardiovascular risk. Cardiologist and neurologist should pay attention to the presence of DM when provide clopidogrel for patients with ischemic cardiovascular disease.
A systematic review and meta-analysis of randomized controlled trials to summarize the evidence on this topic is important. A new antiplatelet agent is needed for ischemic stroke patients with DM.
We thank Prof. S. Claiborne Johnston, MD, PhD, from the Dean’s Office, Dell Medical School, University of Texas at Austin, Austin, US, for providing the subgroup data according to diabetes status from the POINT trial. We also thank Mark Cleasby, PhD, from Liwen Bianji, Edanz Group China (http://www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eleazu C, García-Mayor RV, Koch T S-Editor: Wang YQ L-Editor: A E-Editor: Liu MY
| 1. | Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67-e492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4163] [Cited by in RCA: 4811] [Article Influence: 687.3] [Reference Citation Analysis (1)] |
| 2. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9582] [Article Influence: 737.1] [Reference Citation Analysis (0)] |
| 3. | Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4045] [Cited by in RCA: 4400] [Article Influence: 440.0] [Reference Citation Analysis (0)] |
| 4. | Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7073] [Cited by in RCA: 6652] [Article Influence: 950.3] [Reference Citation Analysis (0)] |
| 5. | Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1582] [Cited by in RCA: 1697] [Article Influence: 212.1] [Reference Citation Analysis (0)] |
| 6. | Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2924] [Cited by in RCA: 3739] [Article Influence: 534.1] [Reference Citation Analysis (0)] |
| 7. | Hu L, Chang L, Zhang Y, Zhai L, Zhang S, Qi Z, Yan H, Yan Y, Luo X, Zhang S, Wang Y, Kunapuli SP, Ye H, Ding Z. Platelets Express Activated P2Y12 Receptor in Patients With Diabetes Mellitus. Circulation. 2017;136:817-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Schuette C, Steffens D, Witkowski M, Stellbaum C, Bobbert P, Schultheiss HP, Rauch U. The effect of clopidogrel on platelet activity in patients with and without type-2 diabetes mellitus: a comparative study. Cardiovasc Diabetol. 2015;14:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Andersson C, Lyngbæk S, Nguyen CD, Nielsen M, Gislason GH, Køber L, Torp-Pedersen C. Association of clopidogrel treatment with risk of mortality and cardiovascular events following myocardial infarction in patients with and without diabetes. JAMA. 2012;308:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123:798-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, Zenni MM, Guzman LA, Bass TA, Costa MA. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation. 2007;115:708-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 350] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 12. | Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4702] [Cited by in RCA: 4492] [Article Influence: 187.2] [Reference Citation Analysis (0)] |
| 13. | Steinhubl SR, Berger PB, Mann JT, Fry ET, DeLago A, Wilmer C, Topol EJ; CREDO Investigators. Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2349] [Cited by in RCA: 2222] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 14. | Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2037] [Cited by in RCA: 1861] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47194] [Article Influence: 2949.6] [Reference Citation Analysis (0)] |
| 16. | Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N Engl J Med. 2018;379:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 843] [Article Influence: 120.4] [Reference Citation Analysis (0)] |
| 17. | Palacio S, Hart RG, Pearce LA, Benavente OR. Effect of addition of clopidogrel to aspirin on mortality: systematic review of randomized trials. Stroke. 2012;43:2157-2162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: www.cochrane-handbook.org. |
| 19. | Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 2389] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SC; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1226] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 21. | SPS3 Investigators, Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 504] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 22. | Dalby AJ, Gottlieb S, Cyr DD, Magnus Ohman E, McGuire DK, Ruzyllo W, Bhatt DL, Wiviott SD, Winters KJ, Fox KAA, Armstrong PW, White HD, Prabhakaran D, Roe MT; TRILOGY ACS Investigators. Dual antiplatelet therapy in patients with diabetes and acute coronary syndromes managed without revascularization. Am Heart J. 2017;188:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Bhatt DL, Bonaca MP, Bansilal S, Angiolillo DJ, Cohen M, Storey RF, Im K, Murphy SA, Held P, Braunwald E, Sabatine MS, Steg PG. Reduction in Ischemic Events With Ticagrelor in Diabetic Patients With Prior Myocardial Infarction in PEGASUS-TIMI 54. J Am Coll Cardiol. 2016;67:2732-2740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Jonasson J, Minematsu K, Molina CA, Wang Y, Wong KS; SOCRATES Steering Committee and Investigators. Ticagrelor versus Aspirin in Acute Stroke or Transient Ischemic Attack. N Engl J Med. 2016;375:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 374] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 25. | Taubert D, Kastrati A, Harlfinger S, Gorchakova O, Lazar A, von Beckerath N, Schömig A, Schömig E. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thromb Haemost. 2004;92:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Lau WC, Gurbel PA, Watkins PB, Neer CJ, Hopp AS, Carville DG, Guyer KE, Tait AR, Bates ER. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation. 2004;109:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 331] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Cavallari U, Trabetti E, Sabaté M, Hernández R, Moreno R, Escaned J, Alfonso F, Bañuelos C, Costa MA, Bass TA, Pignatti PF, Macaya C. Contribution of gene sequence variations of the hepatic cytochrome P450 3A4 enzyme to variability in individual responsiveness to clopidogrel. Arterioscler Thromb Vasc Biol. 2006;26:1895-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Suh JW, Koo BK, Zhang SY, Park KW, Cho JY, Jang IJ, Lee DS, Sohn DW, Lee MM, Kim HS. Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. CMAJ. 2006;174:1715-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 29. | Rodríguez-González F, Martínez-Quintana E, Saavedra P, Medina-Gil JM, Riaño M, Garay-Sánchez P, Tugores A. CYP2C19 or CYP3A5 Genotyping Does Not Predict Clinical Response to Clopidogrel. J Clin Pharmacol. 2018;58:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |