Published online Apr 15, 2020. doi: 10.4239/wjd.v11.i4.126
Peer-review started: October 23, 2019
First decision: December 12, 2019
Revised: December 20, 2019
Accepted: February 8, 2020
Article in press: February 8, 2020
Published online: April 15, 2020
Processing time: 165 Days and 10.8 Hours
Obesity is a disease state with serious adverse metabolic complications, including glucose intolerance and type 2 diabetes that currently has no cure. Identifying and understanding roles of various modulators of body composition and glucose homeostasis is required for developing effective cures. Syndecan-1 (Sdc1) is a member of the heparan sulfate proteoglycan family that has mainly been investigated for its role in regulating proliferation and survival of epithelia and tumor cells, but little is known about its roles in regulating obesity and glucose homeostasis.
To examine the role of Sdc1 in regulating body fat and glucose metabolism.
We used female wild type and Sdc1 knockout (Sdc1 KO) mice on BALB/c background and multiple methods. Metabolic measurements (rates of oxygen consumption, carbon dioxide production, respiratory exchange ratio and energy expenditure) were performed using an open-flow indirect calorimeter with additional features to measure food intake and physical activity. Glucose intolerance and insulin resistance were measured by established tolerance test methods.
Although our primary goal was to investigate the effects of Sdc1 deficiency on body fat and glucose homeostasis, we uncovered that Sdc1 regulates multiple metabolic parameters. Sdc1KO mice have reduced body weight due to significant decreases in fat and lean masses under both chow and high fat diet conditions. The reduced body weight was not due to changes in food intakes, but Sdc1 KO mice exhibited altered feeding behavior as they ate more during the dark phase and less during the light phase than wild type mice. In addition, Sdc1 KO mice suffered from high rate of energy expenditure, glucose intolerance and insulin resistance.
These results reveal critical multisystem and opposing roles for Sdc1 in regulating normal energy balance and glucose homeostasis. The results will have important implications for targeting Sdc1 to modulate metabolic parameters. Finally, we offer a novel hypothesis that could reconcile the opposing roles associated with Sdc1 deficiency.
Core tip: Obesity is a disease state with serious adverse metabolic complications that currently has no cure. Identifying key modulators is required for developing effective cures. Here we investigated Syndecan-1 (Sdc1), a member of the heparan sulfate proteoglycan family for its roles in regulating obesity and glucose homeostasis. Sdc1 knockout (Sdc1 KO) mice have reduced body weight with decreases in fat and lean masses under both chow and high fat diet conditions. The reduced body weight was not due to changes in food intakes, but Sdc1 KO mice exhibited altered feeding behavior as they ate more during the dark phase and less during the light phase than wild type mice. In addition, Sdc1 KO mice suffered from high rate of energy expenditure, glucose intolerance and insulin resistance. Our results reveal critical multisystem and opposing roles for Sdc1 in regulating normal energy balance and glucose homeostasis.
- Citation: Jaiswal AK, Sadasivam M, Aja S, Hamad ARA. Lack of Syndecan-1 produces significant alterations in whole-body composition, metabolism and glucose homeostasis in mice. World J Diabetes 2020; 11(4): 126-136
- URL: https://www.wjgnet.com/1948-9358/full/v11/i4/126.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i4.126
Obesity is a major risk factor for metabolic diseases, including insulin resistance, type 2 diabetes and cardiovascular diseases[1,2]. Attempts to control obesity by adjusting lifestyle produce incremental or no improvement in most individuals[3]. Significant efforts have therefore been directed towards alternative strategies to combat obesity and its complications. A major focus has been on the roles of specific cell types and molecules. Among these are different immune cells, which are being investigated for their roles in regulating adipogenesis, and chronic low-grade inflammation that fuels metabolic syndromes[4,5]. Other cell types and molecules have received less attention, including members of the heparan sulfate proteoglycans (HSPG) family. These are glycoproteins with one or more covalently attached glycosaminoglycan[6]. HSPG are located in the extracellular matrix, in secretory vesicles[6], and at cell membranes where they can regulate ligand-receptor interactions. Syndecans are major components of HSPG family. The syndecan family is comprised of four transmembrane HSPGs[7]. These four HSPGs are syndecan-1, -2, -3, and -4. There is some evidence of involvement of sdc3 and sdc4 in regulating body fat and metabolism. Syndecan-4 is implicated in angiogenesis and wound healing[8-10]. Syndecan-3 has been implicated in regulating feeding behavior by modulating hypothalamic melanocortin activity[11], and syndecan-3 knock out mice are resistant to diet-induced obesity[12].
However, there is limited information about role of Sdc1 in body metabolism. Sdc1 is expressed in epithelial and other non-hematopoietic cell types[13,14], and serves as a marker for plasma cells and developing B lymphocytes[15]. We have recently shown that Sdc1 regulates homeostasis of natural-killer T-cells (NKT) and gamma-delta T-cells that produce interleukin-17[13,16]. Sdc1 has also been investigated for its role in mediating hepatic clearance of triglyceride-rich lipoproteins[17] and regulating lipoprotein metabolism[18,19]. Little is yet known about the role of Sdc1 to regulate body fat and glucose homeostasis. However, in one study Kasza et al[20] reported that loss of Sdc1 in mice caused depletion of intradermal fat, making those more susceptible to cold stress[20].
In this study, we show that deficiency of Sdc1 significantly reduces body weight and adiposity, and lean mass. These effects of sdc-1 deficiency exist under normal diet and obesogenic high-fat diet conditions. Using indirect calorimetry, we show that Sdc1 knockout (Sdc1 KO) mice have increased rates of energy expenditure. Despite the decreased adiposity of Sdc1 mice, they have impaired glucose tolerance and insulin sensitivity as compared to wild type (WT) control, whether fed normal chow or high-fat diet (HFD). These results reveal critical multi-system roles for Sdc1 in regulating normal energy balance and glucose homeostasis, and inform as to potential uses, and risks, of Sdc1 immunotherapy.
Sdc1 KO BALB/c mice were a gift from Stepp et al[21] (George Washington University). Wild-type female BALB/c mice were purchased from the Jackson Laboratory. Mice were bred and housed in approved vivaria on the Johns Hopkins University School of Medicine campus. Unless noted otherwise, mice between the ages of 8 and 10 wk were used. Except for pre-test food deprivations associated with glucose and insulin tolerance tests, mice were maintained on ad libitum normal diet (Teklad 2018, Envigo) or switched to HFD with 60% kcals from fat (D12492, Research Diets) at age of 4 wk. Experiments were conducted under protocols approved by the Johns Hopkins University Animal Care and Use Committee, in compliance with the Animal Welfare Act and principles set forth in the Guide for the Care and Use of Laboratory Animals.
Masses of fat and lean tissue, along with total body water, were measured in awake mice with an Echo-MRI-100TM QMR analyzer (Echo Medical Systems, Houston, TX, United States). Group sizes were at least n = 12/group, Sdc1 KO vs WT, for both the studies with normal diet and with HFD. QMR measurements were performed at the Johns Hopkins University Phenotyping Core facility.
After QMR for body composition, mice were moved to the Center for Metabolism and Obesity Research core facility for indirect calorimetry studies. Studies utilized an open-flow instrument [Comprehensive Lab Animal Monitoring System (CLAMS), Columbus Instruments]. Mice acclimated to the facility for one week before study. Mice were then monitored in the CLAMS for three days to confirm acclimation as indicated by stable daily body weights, food and fluid intakes, and photoperiod fluctuations of metabolic data. Data from the fourth day are presented. These calorimetry data were collected in two experiments (n = 12 Sdc1 KO, n = 12 WT per experiment), one utilizing normal diet, and the other utilizing mice made obese on HFD. The CLAMS reported rates of oxygen consumption (VO2, mL/kg/h) and carbon dioxide production (VCO2), respiratory exchange ratio (RER), and rate of energy expenditure (EE, kcal/kg/h) for each mouse chamber every 25 min throughout the studies. Respiratory exchange ratio (RER = VCO2/VO2) was calculated by Oxymax software (v.4.02) to estimate relative oxidation of carbohydrate (RER = 1.0) vs fat (RER approaching 0.7), not accounting for protein oxidation. Energy expenditure was calculated as EE = VO2 × [3.815 + (1.232 × RER)][22]. Average values for VO2, VCO2, RER and EE were calculated per subject for 24 h, and for the corresponding 12 h dark and 12 h light periods. Additional CLAMS feature permitted measurements of diet intake (powdered food in cups, on scales) and physical activity (x-axis infrared beam array). These data were also reported every 25 min throughout the studies, and are presented as 24-h sums, and sums for the 12-h light and dark phases.
Glucose tolerance tests (GTT) were performed with 8-wk-old Sdc1 KO and WT mice maintained on normal diet or switched to HFD at 4 wk of age. Mice were fasted for 4 h and then D-glucose (1 g/kg and 5 g/kg body weight in 200 μL saline) was injected intraperitoneally. Glucose levels were measured by tail-snip technique with a hand-held glucometer (Bayer Contour TS, Abbott Laboratories, Abbott Park, IL) at baseline and at 15, 30, 60 and 120 min after glucose injection.
To determine the insulin sensitivity, insulin tolerance tests were performed with in Sdc1 KO and WT mice of similar ages. After 2 h of fasting, mice were injected with 0.5 IU/kg insulin (Humulin R, Eli Lilly, Indianapolis, IN, United States) intraperitoneally. Glucose levels were measured as in GTT at baseline, and at 15, 30, 60- and 120 min post-injection.
Statistical tests were performed using Prism 6 (Graph Pad). Data were expressed as mean ± SE. Statistical significance was evaluated using two-tailed unpaired Student’s t-test. One-way ANOVA was utilized to analyze body weights and body composition during the long-term diet studies (at 8 and 10 wk, respectively, on the HF diet). Statistical significance was determined as P < 0.05.
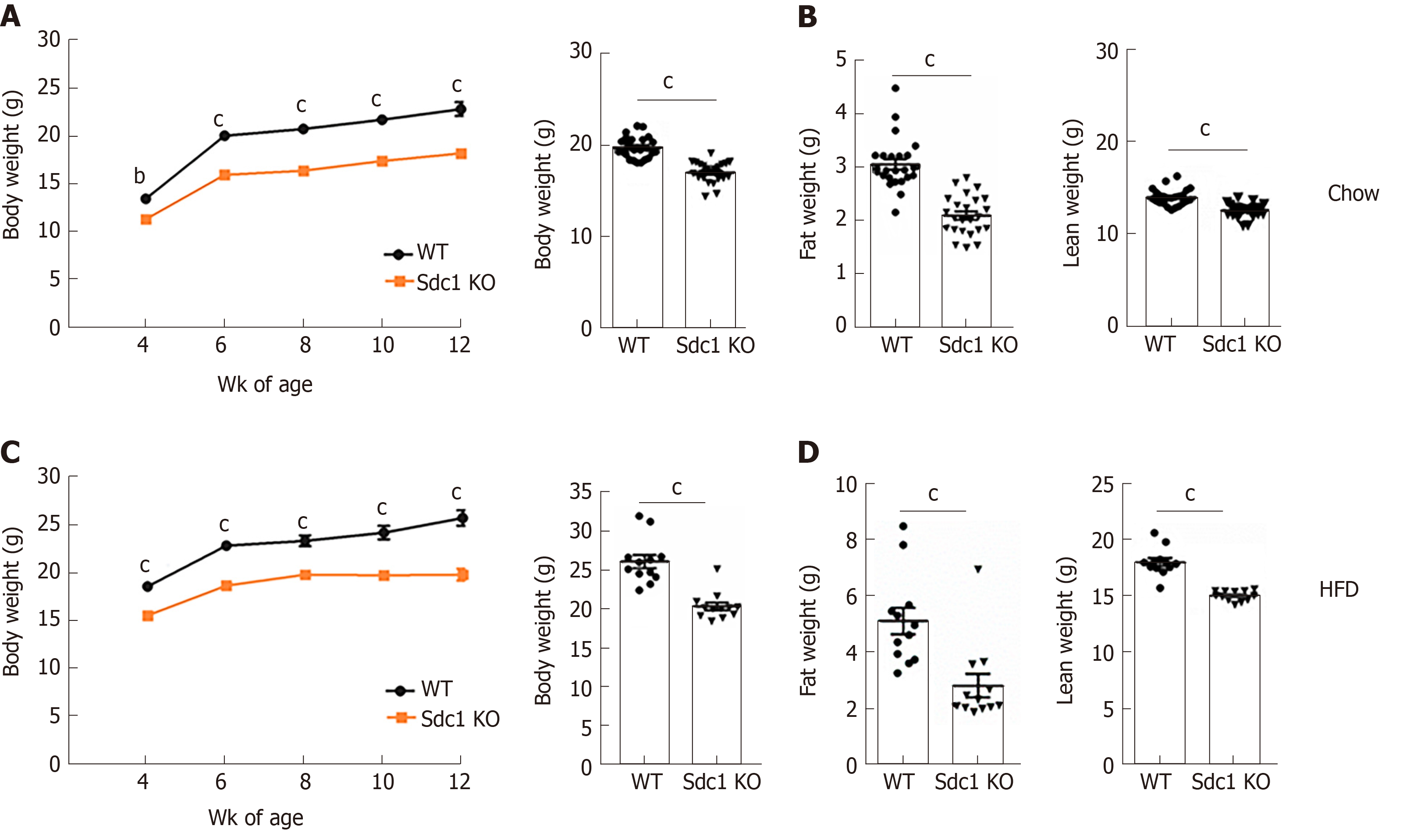
We first evaluated the effect of Sdc1 deficiency on body weight and composition using Sdc1 KO and WT BALB/c female mice that were fed normal diet between the ages of 4-12 wk. Sdc1 KO female mice had significantly reduced body weight compared with WT mice at all ages examined (Figure 1A). QMR analysis of body composition at 10 wk of age showed that Sdc1 KO mice had decreases in both fat and lean masses (Figure 1B). Next, we monitored effects of Sdc1 deficiency under HFD condition. The Sdc1 KO female BALB/c mice gained less weight than WT controls during the HFD feeding period (Figure 1C). QMR analysis at 12 wk of age (8 wk on HFD) showed that HFD-fed Sdc1 KO mice had reduced body fat and lean mass as compared to HFD-fed WT mice (Figure 1D). Thus, the systemic effects of sdc-1 deficiency on body composition and weight are maintained under both normal diet and HFD conditions. We also measured body weights of male BALB/c mice; Sdc1 KO males on a normal diet had lower body weights than the WT controls, similar to results in female mice (Supplementary Figure S1).
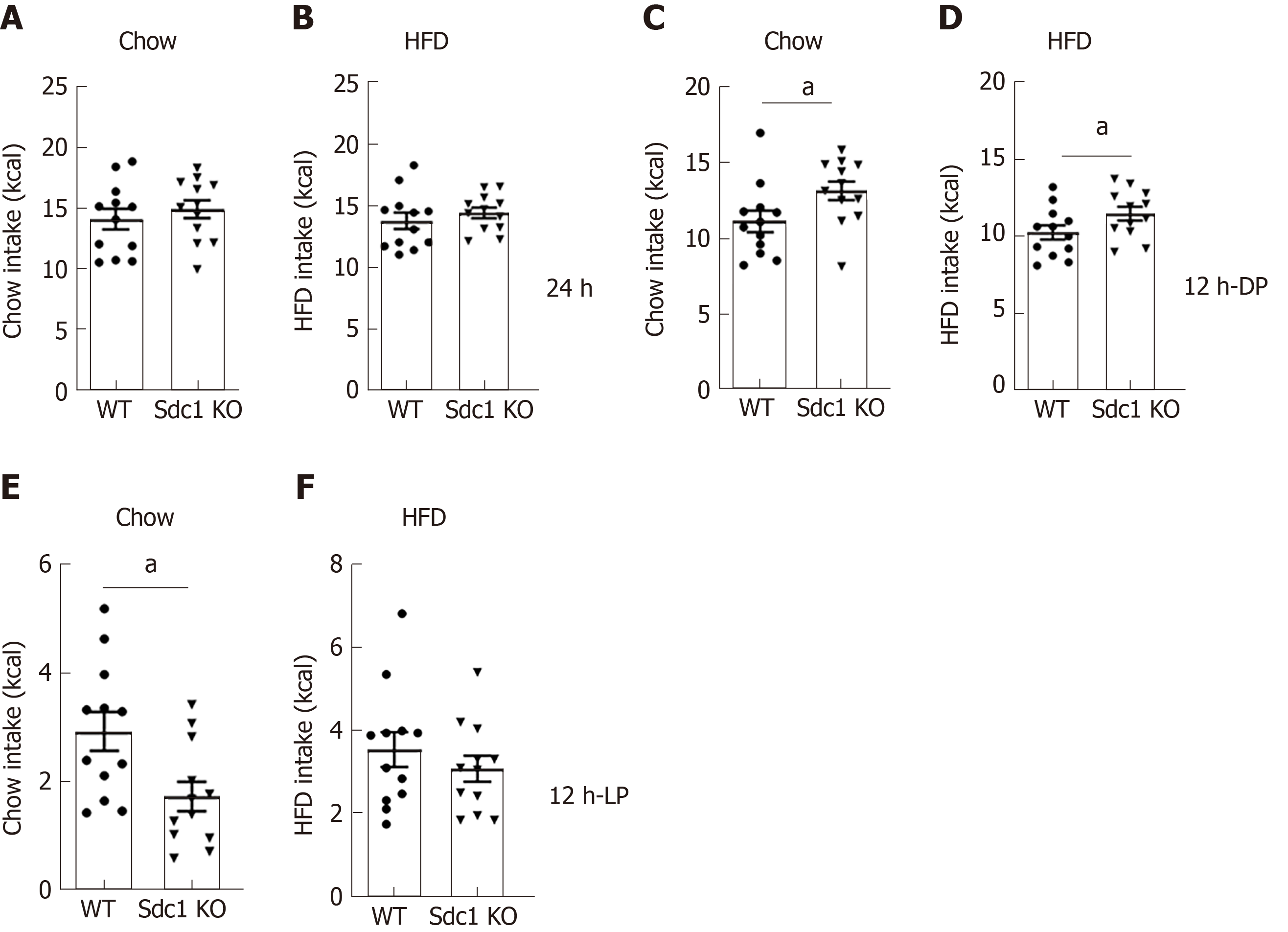
To determine whether reduced weight is due to less food intake, we measured food consumption of Sdc1 KO and WT mice fed chow diet or HFD during the indirect calorimetry trials (Figure 2). Under both conditions, the daily caloric intakes by Sdc1 KO mice were similar to those of WT mice (Figure 2A and B). However, although total food intake over a 24 h period was not different between the two genotypes, the pattern of food intake was altered. As compared to WT mice, Sdc1 KO mice ate more food during the dark phase when fed chow or HFD (Figure 2C and D). Conversely, WT mice ate more during the light phase, although the difference was statistically significant only under chow diet (Figure 2E and F). Taken together, these results exclude daily food intake reduction as a cause of the decreased body weight of Sdc1 KO mice. However, they uncovered a significant role for Sdc1 in regulating feeding patterns of mice.
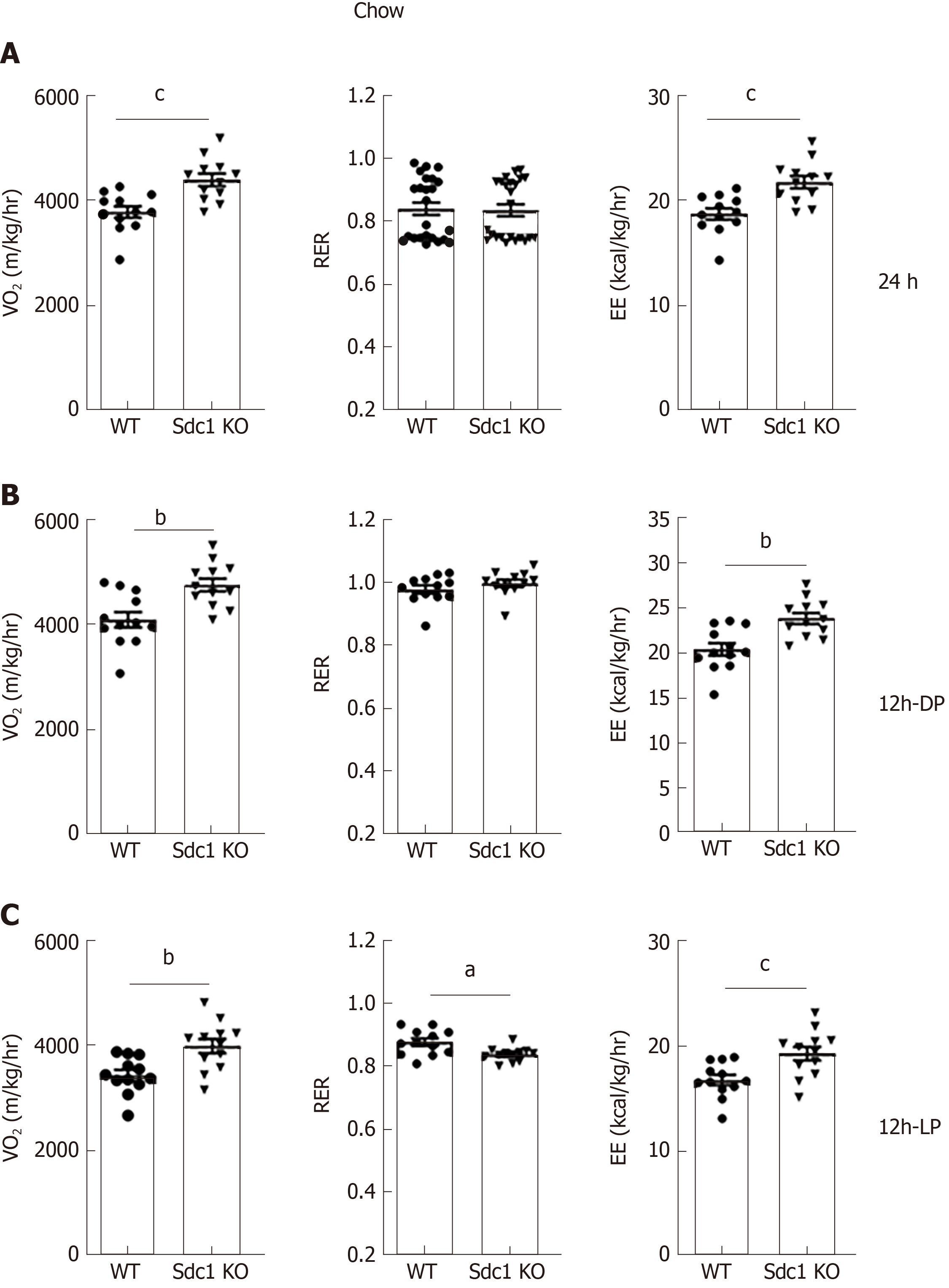
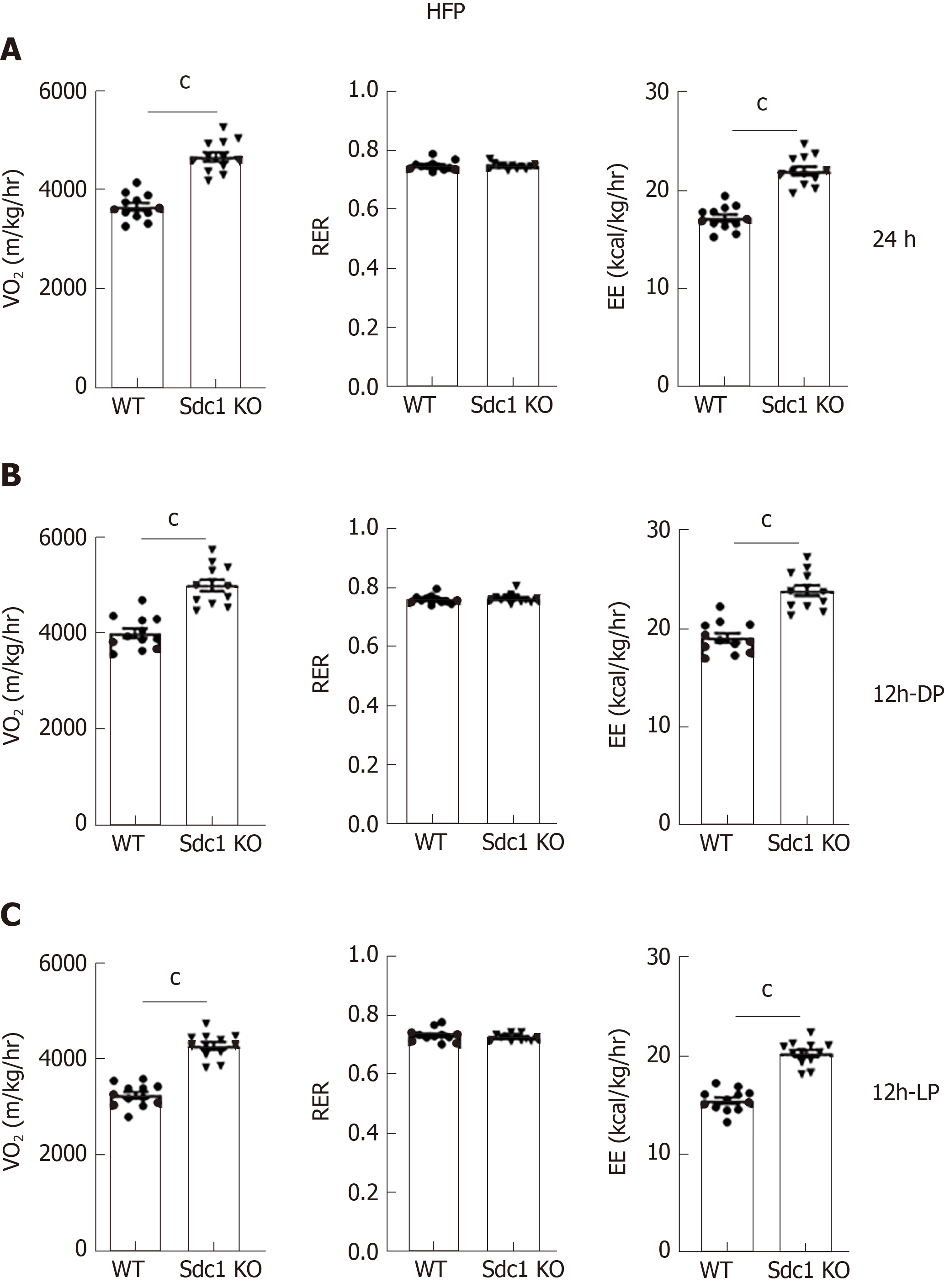
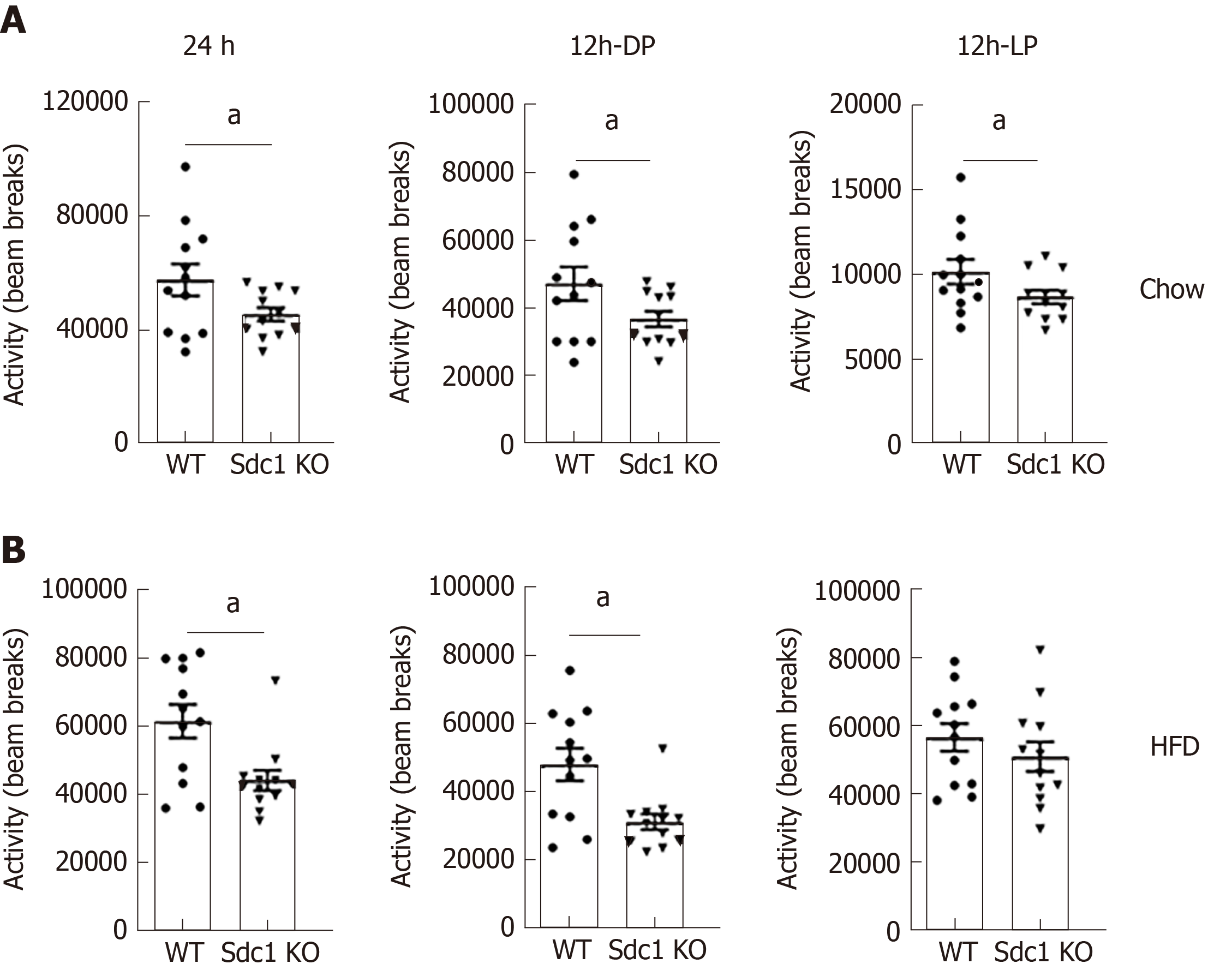
Having excluded differences in daily food consumption as a cause of decreased body weight in Sdc1 KO mice, we analyzed the effect of Sdc1 deficiency on VO2, RER (VCO2/VO2 ratio) and EE. Sdc1 KO mice had significantly elevated VO2 compared with WT mice (Figure 3A). The RER was not significantly different between the genotypes for 24-h or for the 12-h dark phase and was in ranges expected for intake of the low-fat/high-carbohydrate diet (Figure 3B). During the light phase, the RER of Sdc1 KO mice was significantly lower than for WT mice, consistent with the decreased food intake by Sdc1 KO mice compared with WT during this period, and an increased expected reliance on body fat stores to fuel metabolism (Figure 3C). Overall, the increased VO2 in Sdc1 KO mice drove a significant increase in the energy expenditure compared with WT (Figure 3C), and this is likely what drives the loss of body weight. Infrared beam crossing was monitored during the indirect calorimetry study to assess physical activity of WT and Sdc1 KO mice. We did similar analysis under HFD using indirect calorimetry and CLAMS. Sdc1 KO and WT mice were placed on HFD for 8 wk and then subjected to indirect calorimetry. Like Sdc1 KO mice fed chow diet, Sdc1 KO mice fed HFD showed reduced fat mass and increased energy expenditure (Figure 4A-C). Despite the overall increase in whole-body energy expenditure in Sdc1 KO mice fed either chow or HFD, their physical activity was significantly lower throughout the photoperiod compared with WT mice (Figure 5A and B).
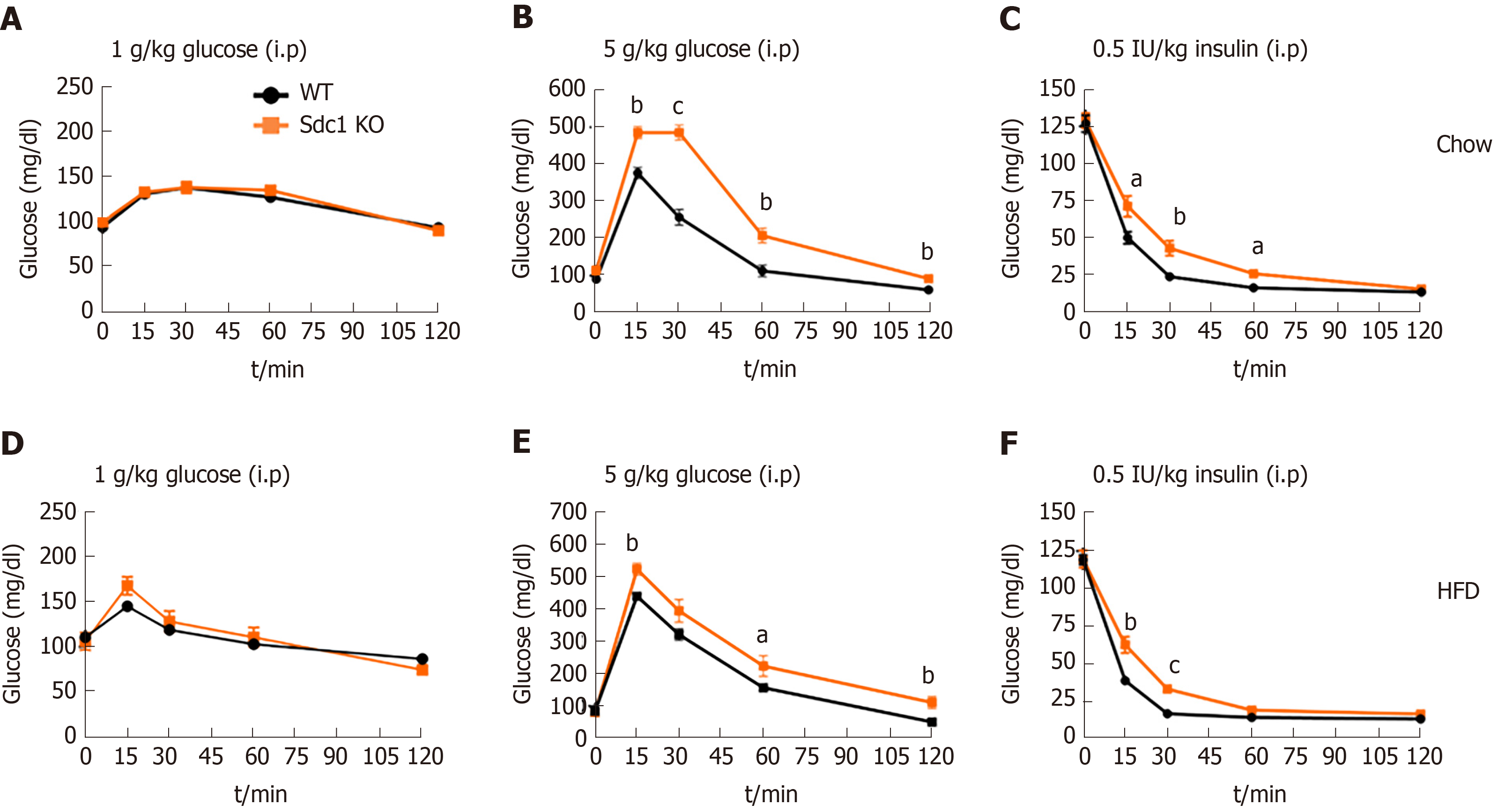
Our body composition studies showed that Sdc1 KO mice had lower fat masses than WT mice, whether maintained on chow or HFD (Figure 1A and C). No previous work, to our knowledge, has examined the role of Sdc1 in regulating glucose homeostasis. We performed GTT on 12-wk-old female BALB/c mice that were fed chow or HFD. After 4 h of fasting, we subjected WT and Sdc1 KO mice to GTT using increasing doses of glucose. Both WT and Sdc1 KO cleared 1g/kg doses of glucose at comparable rates regardless of the diet type (Figure 6A and D). They also cleared 2 g/kg to similar extent (data not shown). However, when we increased the glucose challenge to a 5 g/kg, Sdc1 KO mice, contrary to our expectation, showed diminished capacity to clear this large glucose dose as compared to WT mice, under both normal diet and HFD conditions (Figure 6B and E). We also determined whether Sdc1 deficiency alters insulin tolerance, we performed insulin tolerance tests in 12-wk-old Sdc1 KO and WT mice under normal diet and HFD conditions. Sdc1 KO mice showed significant insulin resistance under both normal diet and HFD conditions (Figure 6C and F). Thus, reduced body fat in Sdc1 KO is not associated with enhanced glucose or insulin tolerance, but rather a limited capacity to clear large dose of glucose and impaired insulin tolerance.
Our results reveal critical multisystem and opposing roles for Sdc1 in regulating normal energy balance and glucose homeostasis. The apparently contradictory effects are unusual but fit well with the properties of Sdc1. In general, cell surface receptors on each have one corresponding ligand and their expression is limited to cell types that perform similar functions. Contrary to this general rule, Sdc1 is expressed by cell types that carry non-overlapping biological functions such as epithelial cells and B cells. Furthermore, Sdc1, like other members of the HSPG family, has multiple ligands that include cytokines, chemokines, and growth factors, hence impacts different functions based on the ligand engaged[6]. These unique characteristics of Sdc1 show that it plays multiple diverse functions which are reflected in the wide range and multisystem effects its deletion has on key biological functions, including body fat, lean mass, feeding behavior, energy expenditure and physical activity.
We hypothesize that the effect of Sdc1 deficiency could be due to a critical role for Sdc1 in regulating homeostasis of different cell types regardless of their specific function. Consistent with this hypothesis, our recent results show that Sdc1 plays a critical role in negatively regulating homeostasis of IL-17-producing subsets of NKT cells (NKT17) and IL-17-producing γδT cells, Tγδ17 cells[13,16]. Consequently, sdc1 KO mice have higher frequency of NKT17 and Tγδ17 cells. These unconventional cell types reside in different non-lymphoid tissues. For example, Tγδ17 cells reside mainly in subcutaneous tissues, whereas NKT17 cells reside in liver and white adipose tissues. IL-17 is anti-adipogenic factors and increased IL-17 production has been linked to glucose intolerance[23]. Therefore, it is plausible that reduced intradermal fat reported by Kasza et al[20] and reduced body fat described here are linked to increased IL-17 production by unconventional T cells. On the other hand, impaired glucose tolerance could be due to high production of IL-17 by NKT cells localized in liver and white adipose tissue[24].
Our results uncover a previously unexpected role for Sdc1 in regulating feeding pattern and links it to the circadian rhythm[25]. This is indicated by the observation that Sdc1 KO mice ate more during the day than at night clocks with no significant change in total food consumption. Given that Sdc1 is ubiquitously expressed on intestinal epithelia and hepatocytes, whether alteration in feeding pattern is dependent on its expression on any of these cell types is currently unknown. Interestingly, lineage specification of IL-17-producing T helper cells varies diurnally by a mechanism that is linked to circadian clocks[26]. Interestingly, Sdc1 when expressed ectopically led to hyperphagia[27], but the study did not examined its effect on feeding behavior. Therefore, our results identify for the first time a role for Sdc1 in regulating feeding behavior. Future studies should determine whether the role of Sdc1 in feeding behavior is linked to its role in regulating homeostasis of NKT17 and Tγδ17 cells.
In summary, our results uncover a complex role for Sdc1 in regulating key metabolic pathways and systems. The results lay the ground for future studies and analyses of role of Sdc1 in a cell-specific mechanism that could have important implications for developing strategies to target Sdc1 to manipulate specific metabolic parameters and obesity.
Obesity is a disease state with serious adverse metabolic complications that currently has no cure. Attempts to control obesity by altering lifestyle has no significant improvement in most individuals. Therefore, it is necessary to discover alternative strategies to combat obesity and its complications.
Syndecan-1 (Sdc1) is a member of the heparan sulfate proteoglycan family that has mainly been investigated for its role in regulating proliferation and survival of epithelia and tumor cells, but its roles in regulating obesity and glucose homeostasis are not well study.
The objective of this study is to examine the role of Sdc1 in regulating body fat and glucose metabolism.
We used female wild type and Sdc1 knockout (Sdc1 KO) mice on BALB/c background and multiple methods. Metabolic measurements were performed using an open-flow indirect calorimeter with additional features to measure food intake and physical activity. Glucose intolerance and insulin resistance were measured by established tolerance test methods.
Although our primary goal was to investigate the effects of Sdc1 deficiency on body fat and glucose homeostasis, we uncovered that Sdc1 regulates multiple metabolic parameters. Sdc1 KO mice have reduced body weight due to significant decreases in fat and lean masses under both chow and high fat diet conditions. The reduced body weight was not due to changes in food intakes, but Sdc1 KO mice exhibited altered feeding behavior as they ate more during the dark phase and less during the light phase than wild type mice. In addition, Sdc1 KO mice suffered from high rate of energy expenditure, glucose intolerance and insulin resistance.
These results reveal critical multisystem and opposing roles for Sdc1 in regulating normal energy balance and glucose homeostasis.
The results provide important insights that will guide future strategies to target syndecan-1 for immunotherapy for obesity.
We thank to Dr. Mary Ann Stepp of George Washington University for providing Sdc1 knockout mice.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Serhiyenko VA S-Editor: Zhang L L-Editor: A E-Editor: Ma YJ
| 1. | Olufadi R, Byrne CD. Clinical and laboratory diagnosis of the metabolic syndrome. J Clin Pathol. 2008;61:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14:1225-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 372] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 3. | Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, Bain CE, Wang CY, Blackburn GL, McTiernan A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring). 2012;20:1628-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 4. | de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 553] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 5. | Liu R, Nikolajczyk BS. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front Immunol. 2019;10:1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 6. | Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 1109] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 7. | Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2114] [Cited by in RCA: 2116] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 8. | Li R, Xie J, Wu H, Li G, Chen J, Chen Q, Wang L, Xu B. Syndecan-4 shedding impairs macrovascular angiogenesis in diabetes mellitus. Biochem Biophys Res Commun. 2016;474:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9-R14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 349] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, Yanada M, Yamamoto K, Matsushita T, Nishimura M, Kusugami K, Saito H, Muramatsu T. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J Biol Chem. 2001;276:47483-47488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Reizes O, Benoit SC, Strader AD, Clegg DJ, Akunuru S, Seeley RJ. Syndecan-3 modulates food intake by interacting with the melanocortin/AgRP pathway. Ann N Y Acad Sci. 2003;994:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Strader AD, Reizes O, Woods SC, Benoit SC, Seeley RJ. Mice lacking the syndecan-3 gene are resistant to diet-induced obesity. J Clin Invest. 2004;114:1354-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Jaiswal AK, Sadasivam M, Archer NK, Miller RJ, Dillen CA, Ravipati A, Park PW, Chakravarti S, Miller LS, Hamad ARA. Syndecan-1 Regulates Psoriasiform Dermatitis by Controlling Homeostasis of IL-17-Producing γδ T Cells. J Immunol. 2018;201:1651-1661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Teng YH, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012;31:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Jaiswal AK, Sadasivam M, Hamad ARA. Syndecan-1-coating of interleukin-17-producing natural killer T cells provides a specific method for their visualization and analysis. World J Diabetes. 2017;8:130-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Dai H, Rahman A, Saxena A, Jaiswal AK, Mohamood A, Ramirez L, Noel S, Rabb H, Jie C, Hamad AR. Syndecan-1 identifies and controls the frequency of IL-17-producing naïve natural killer T (NKT17) cells in mice. Eur J Immunol. 2015;45:3045-3051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Leonova EI, Sadovnikova ES, Shaykhutdinova ER, Galzitskaya OV, Murashev AN, Solonin AS. Hepatic and Aortic Arch Expression and Serum Levels of Syndecan-1 in ApoE-/- Mice. Open Biochem J. 2017;11:77-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest. 2009;119:3236-3245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Stanford KI, Wang L, Castagnola J, Song D, Bishop JR, Brown JR, Lawrence R, Bai X, Habuchi H, Tanaka M, Cardoso WV, Kimata K, Esko JD. Heparan sulfate 2-O-sulfotransferase is required for triglyceride-rich lipoprotein clearance. J Biol Chem. 2010;285:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Kasza I, Suh Y, Wollny D, Clark RJ, Roopra A, Colman RJ, MacDougald OA, Shedd TA, Nelson DW, Yen MI, Yen CL, Alexander CM. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet. 2014;10:e1004514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci. 2002;115:4517-4531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Shin MK, Drager LF, Yao Q, Bevans-Fonti S, Yoo DY, Jun JC, Aja S, Bhanot S, Polotsky VY. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1α. PLoS One. 2012;7:e46562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Zúñiga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 298] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 24. | Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. 2018;18:559-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 272] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 25. | Blancas-Velazquez A, Mendoza J, Garcia AN, la Fleur SE. Diet-Induced Obesity and Circadian Disruption of Feeding Behavior. Front Neurosci. 2017;11:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, Rothman PB, Takahashi JS, Hooper LV. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 337] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 27. | Reizes O, Lincecum J, Wang Z, Goldberger O, Huang L, Kaksonen M, Ahima R, Hinkes MT, Barsh GS, Rauvala H, Bernfield M. Transgenic expression of syndecan-1 uncovers a physiological control of feeding behavior by syndecan-3. Cell. 2001;106:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |