Published online Oct 15, 2020. doi: 10.4239/wjd.v11.i10.447
Peer-review started: April 30, 2020
First decision: May 24, 2020
Revised: June 2, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: October 15, 2020
Processing time: 167 Days and 7.6 Hours
Bariatric surgery is one of most effective long-term treatments for morbid obesity. However, post-bariatric surgery anemia is identified as a common adverse effect and remains a challenge nowadays.
To estimate the risk of post-bariatric surgery anemia and to stratify the association between age, gender, and types of surgery.
This study is a population-based cohort study. We conducted this nationwide study using claims data from National Health Insurance Research Database in Taiwan. There were 4373 morbidly obese patients in this study cohort.
Among patients who were diagnosed with morbid obesity, 2864 received bariatric surgery. All obesity-associated comorbidities decreased in the surgical group. Increasing risk of post-bariatric surgery anemia among obese patients was found by Cox proportional hazards regression [adjusted hazard ratio (HR): 2.36]. Also, we found significantly increasing cumulative incidence rate of anemia among patients receiving bariatric surgery by log-rank test. After adjusting for age and gender, the increasing incidence of post-bariatric surgery anemia was found among women (adjusted HR: 2.48), patients in the 20–29-year-old group (adjusted HR: 3.83), and patients in the 30-64-year-old group (adjusted HR: 2.37). Moreover, malabsorptive and restrictive procedures had significantly higher adjusted HRs, 3.18 and 1.55, respectively.
Bariatric surgery give rise to anemia risk among obese patients, specifically in women, young- and middle-aged patients, and patients undergoing malabsorptive procedures in our population-based cohort study in Taiwan.
Core Tip: Based on a population-based cohort study in Taiwan, this study demonstrated that obese patients receiving bariatric surgery had significantly higher risk of anemia than patients who did not receive bariatric surgery. After adjusting for gender and age, women, young-aged (20-29 years) and middle-aged (30-64 years), had significantly higher incidence of post-bariatric surgery anemia. Both malabsorptive procedures and restrictive procedures increased the incidence of anemia. However, malabsorptive procedures had a higher hazard ratio of post-bariatric surgery anemia than restrictive procedures.
- Citation: Wang TY, Huang HH, Hsieh MS, Chen CY. Risk of anemia in morbidly obese patients after bariatric surgery in Taiwan. World J Diabetes 2020; 11(10): 447-458
- URL: https://www.wjgnet.com/1948-9358/full/v11/i10/447.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i10.447
The incidence of obesity has increased rapidly and has tripled over the past decades[1], significantly threatening public health. Bariatric surgery has been shown to be the most effective long-term treatment for morbidly obese patients[1-4] considering that this surgery results in significant and sustainable weight loss and improves comorbidities, long-term mortality, and patients’ quality of life[1-8]. Although bariatric surgery is considered safe and has beneficial effects, the development of anemia after bariatric surgery remains a concern. Anemia due to micronutrient deficiencies is identified as a common adverse effect, specifically among patients without regular nutrient supplementation postoperatively.
Post-bariatric surgery anemia can influence as many as two-thirds of patients undergoing bariatric surgery[9]. During these years, several efforts were made to decrease the incidence rate of post-bariatric surgery anemia. First, considering the metabolic sequelae of bariatric surgery, lifelong micronutrient supplementation was considered mandatory[10]. Moreover, the quality and sustainability of medical follow-up consultation became important issues[4]. Some studies revealed that the incidence of anemia is lower in sleeve gastrectomy than that in Roux-en-Y gastric bypass[11-13]. Furthermore, a cohort study in France suggested that the increasing popularity of sleeve gastrectomy is another reason why the incidence of anemia has reduced[4,11]. However, recently, the prevalence of post-bariatric surgery anemia is still considered nonnegligible. In France, 5% of patients were diagnosed with anemia after bariatric surgery between 2008 and 2016[11]. Additionally, if a patient did not receive an outpatient follow-up, the prevalence of anemia could even be 57% 10 years after Roux-en-Y gastric bypass[14].
Postoperative anemia may develop as a result of several factors. First, absorption of folate and iron mainly happens in the proximal jejunum and duodenum. Malabsorptive procedures, like intestinal bypass, may cause deficiencies of folate, iron, and vitamin B12 and lead to anemia[11,15]. Restrictive procedures, like sleeve gastrectomy, may also reduce the intrinsic factor, gastric acid, and food gastric passing time and subsequently reduce the bioavailability and digestion of nutrients[16]. Furthermore, the net effects observed as a result of the adaptation of bariatric surgery may synergistically affect the hemoglobin level. These complicated factors include reduction of inflammation, adaptation of micronutrient absorption, limited meat intake, attenuated energy intake, and menstruation[12,13,17,18].
A large cohort study from France revealed the long-term anemia incidence among patients who receiving a bariatric procedure[11]. Studies assessing the long-term incidence of post-bariatric surgery anemia have not been conducted yet. This study used the nationwide data [Taiwanese National Health Insurance Research Database (NHIRD)] with large sample size. This study aimed to estimate and compare the long-term incidence of anemia between morbidly obese patients who underwent bariatric surgery vs patients who did not undergo bariatric surgery and to stratify the association between gender, age, and types of bariatric surgery in morbidly obese patients who received bariatric surgery or not.
This study is a population-based cohort study in which data were obtained from the NHIRD. The National Health Insurance (NHI) provided coverage for approximately 99.2% of the Taiwan population (more than 23.03 million residents). The NHI is managed by the National Health Insurance Administration (NHIA) since 1995.
The National Health Research Institute (NHRI) obtained the identification-encrypted data from NHIA and established the NHIRD. The Longitudinal Health Insurance Database 2000 (LHID2000), which was used in this study, comprised 1 million randomly sampled medical information from the registry of all beneficiaries in 2000. There was no significant difference in age- and gender-distributions between the data in the LHID2000 and the original NHIRD. The diagnosis code of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used.
These data of NHIRD were all de-identified by obfuscating the information about patients and medical facilities to ensure privacy. Moreover, data confidentiality is maintained in accordance with the data regulations of NHIA and NHRI. Because the NHIRD comprises de-identified secondary data for research, informed consent from subjects was waived because anonymous data were used. Furthermore, the Institutional Review Board of China Medical University (CMUH104-REC2-115) approved this study.
Patients who were diagnosed with morbid obesity (ICD-9-CM, 278.01) with age ≥ 20 years and < 100 years and received bariatric surgery (ICD-9-CM, 44.99, 44.95, 44.68, 44.39, 44.38, 44.31, 43.89, and 43.82) between study period January 1, 2000 to December 31, 2010 were recruited. Furthermore, we excluded morbidly obese patients who receiving surgery before obesity diagnosed data, patients who received surgery out of period between 2000 to 2010, patients with diagnosed date of anemia before the index date, those aged < 20 years or ≥ 100 years, and those with missing information of gender or age. Patients who were diagnosed with anemia (ICD-9-CM, 280 and 281.0) and patients who were not diagnosed were analyzed. Morbidly obese patients were identified by the ICD-9-CM codes, with at least one diagnosis in admission during the whole study period. The data from the Registry for Catastrophic Illness Patient Database, a subset of the NHIRD, confirmed the diagnostic accuracy of morbid obesity[19,20].
By referring to the ICD-9-CM codes, the potential confounding factors for morbid obesity were systematically identified among the data from NHIRD. Age, gender, comorbidities, insurance premium, occupation, medications, and level of urbanization were identified as confounding factors. Hyperlipidemia (ICD-9-CM, 272), diabetes mellitus (ICD-9-CM, 250, 366.41, 357.2, 362.01-362.02, and 357.2), hypertension (ICD-9-CM, 401–405), coronary artery disease (ICD-9-CM, 411–414), congestive heart failure (ICD-9-CM, 428), stroke (ICD-9-CM, 430–438), asthma (ICD-9-CM, 493), chronic obstructive pulmonary disease (ICD-9-CM, 490–492, 494, and 496), peripheral arterial occlusive disease (ICD-9-CM, 440–444), and chronic kidney disease (ICD-9-CM, 285.21, 250.4, 403-404, and 581–588) were found to be the comorbidities associated with major adverse cardiovascular events. We applied multivariate logistic regression with baseline covariates to calculate propensity scores. The baseline characteristics of study (with bariatric surgery) cohort and comparison (without bariatric surgery) cohort were compared. Furthermore, both cohorts were matched by standardized mean differences, calculated as the difference in proportions or means of a variable divided by a pooled estimate of the standard deviation of the variable.
Demographic characteristics differences and comorbidities differences between the study cohort (receiving bariatric surgery) and comparison (without surgery) cohort were analyzed. We conducted the chi-squared test for noncontinuous variables and the two-sample t-test for continuous variables. Cox proportional hazards regression was performed to calculat the hazard ratios (HRs) with 95% confidence intervals (CIs) for each variable. Differences in the incidence of major adverse cardiovascular events between the study cohort and comparison cohort were estimated using the Kaplan-Meier curves by performing the log-rank test. Statistical Analysis System (SAS) version 9.4 statistical package (SAS Institute Inc., Cary, NC, United States) was used for statistical analyses. The level of significance was set at 0.05.
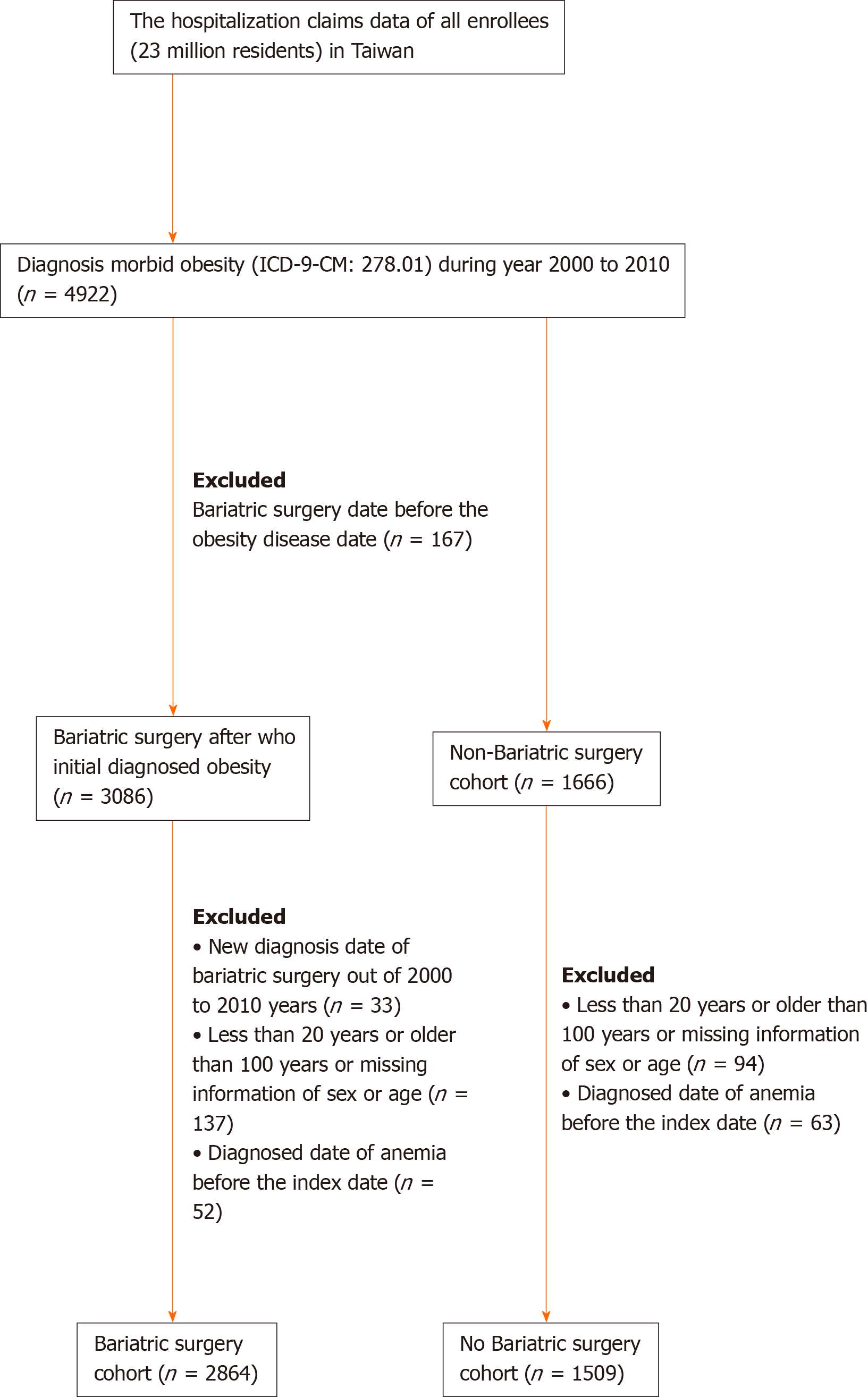
A total of 4922 adult patients were hospitalized for morbid obesity during the study period between 2000 to 2010. Of these, 3086 patient received bariatric surgery, and 1666 patients did not receive bariatric surgery. To reduce the influence of pre-surgery anemia, our study group was recruited and analyzed after the exclusion of those who were diagnosed anemia before the index date. Finally, 2864 patients who were diagnosed with morbid obesity and received bariatric surgery in the study cohort and 1509 patients who were diagnosed morbid obesity and did not receive bariatric surgery in the comparison cohort were recruited. (Figure 1). There were significantly more female patients in the study cohort than that in the comparison cohort (64.8% vs 54.2%, respectively, P < 0.0001) (Table 1). The mean age of the study cohort was significant younger than that of the comparison cohort (33.1 ± 9.1 vs 44.3 ± 15.3, respectively, P < 0.0001). Compared to the comparison cohort, some demographic characteristics, like hypertension, hyperlipidemia, coronary artery disease, congestive heart failure, stroke, peripheral arterial occlusive disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, gastrointestinal ulcer, and gastrointestinal hemorrhage were significantly lower (P < 0.0001) in the study cohort. The means (median) of the follow-up period were 5.68 (5.31) years for the study cohort and 4.21 (3.84) years for the comparison cohort.
| Variable | Receiving bariatric surgery | P value1 | |||
| No [n = 1509 (34.51%)] | Yes [n = 2864 (65.49%)] | ||||
| n | % | n | % | ||
| Malabsorptive procedures | - | - | 1773 | 61.91 | - |
| Restrictive procedures | - | - | 2465 | 86.07 | - |
| Sex | < 0.0001 | ||||
| Female | 818 | 54.21 | 1855 | 64.77 | |
| Male | 691 | 45.79 | 1009 | 35.23 | |
| Age at baseline, yr | < 0.0001 | ||||
| 20-29 | 302 | 20.01 | 1305 | 45.57 | |
| 30-64 | 1034 | 68.52 | 1558 | 54.4 | |
| 65-100 | 173 | 11.46 | 1 | 0.03 | |
| mean ± SD | 44.30 ± 15.25 | 33.09 ± 9.07 | < 0.00012 | ||
| Comorbidities | |||||
| Hypertension | 751 | 49.77 | 254 | 8.87 | < 0.0001 |
| Hyperlipidemia | 314 | 20.81 | 110 | 3.84 | < 0.0001 |
| Diabetes mellitus | 525 | 34.79 | 174 | 6.08 | < 0.0001 |
| Coronary artery disease | 261 | 17.3 | 69 | 2.41 | < 0.0001 |
| Congestive heart failure | 243 | 16.1 | 39 | 1.36 | < 0.0001 |
| Stroke | 123 | 8.15 | 37 | 1.29 | < 0.0001 |
| Chronic kidney disease | 124 | 8.22 | 22 | 0.77 | < 0.0001 |
| Asthma | 186 | 12.33 | 75 | 2.62 | < 0.0001 |
| Chronic obstructive pulmonary disease | 145 | 9.61 | 34 | 1.19 | < 0.0001 |
| Peripheral arterial occlusive disease | 29 | 1.92 | 3 | 0.1 | < 0.0001a |
| Gastrointestinal ulcer | 185 | 12.26 | 73 | 2.55 | < 0.0001 |
| Gastrointestinal bleeding | 44 | 2.92 | 12 | 0.42 | < 0.0001 |
In Table 2, asthma, female sex, and gastrointestinal ulcer increased the risk of anemia significantly according to the univariate analyses. Receiving bariatric surgery increased the rate of anemia significantly (HR = 1.71; 95%CI: 1.2-2.44; P = 0.003). Receiving bariatric surgery also caused a significantly increased rate of anemia after adjusting for the potential confounding factors of gender, age, and all comorbidities in multivariate analyses (adjusted HR = 2.36; 95%CI: 1.52-3.65; P = 0.0001).
| Variable | Anemia | Crude1 | Adjusted2 | ||||
| No. (n = 221) | HR | (95%CI) | P value | HR | (95%CI) | P value | |
| Receiving bariatric surgery | |||||||
| No | 38 | 1.00 | reference | 1.00 | reference | ||
| Yes | 183 | 1.71 | (1.2-2.44) | 0.003 | 2.36 | (1.52-3.65) | 0.0001 |
| Gender | |||||||
| Female | 186 | 1.00 | reference | 1.00 | reference | ||
| Male | 35 | 0.32 | (0.22-0.46) | < 0.0001 | 0.33 | (0.23-0.48) | < 0.0001 |
| Age | |||||||
| 20-29 years | 88 | 1.00 | reference | 1.00 | reference | ||
| 30-64 years | 126 | 1.08 | (0.82-1.41) | 0.6028 | 1.06 | (0.79-1.41) | 0.6956 |
| 65-100 years | 7 | 1.33 | (0.61-2.88) | 0.4724 | 1.33 | (0.54-3.3) | 0.5391 |
| Comorbidities (ref = non-) | |||||||
| Hypertension | 41 | 1.07 | (0.76-1.51) | 0.6837 | 1.43 | (0.9-2.27) | 0.1349 |
| Hyperlipidemia | 18 | 1.06 | (0.65-1.71) | 0.8249 | 1.14 | (0.64-2.01) | 0.6595 |
| Diabetes mellitus | 26 | 0.95 | (0.63-1.44) | 0.812 | 0.96 | (0.59-1.57) | 0.8826 |
| Coronary artery disease | 15 | 1.23 | (0.73-2.08) | 0.441 | 1.17 | (0.61-2.25) | 0.6403 |
| Congestive heart failure | 10 | 1.02 | (0.54-1.93) | 0.9436 | 0.91 | (0.42-1.94) | 0.8027 |
| Stroke | 4 | 0.63 | (0.23-1.7) | 0.3611 | 0.52 | (0.18-1.5) | 0.2286 |
| Chronic kidney disease | 7 | 1.32 | (0.62-2.8) | 0.4752 | 1.47 | (0.66-3.28) | 0.3504 |
| Asthma | 17 | 1.74 | (1.06-2.85) | 0.0293 | 1.85 | (1.05-3.25) | 0.034 |
| Chronic obstructive pulmonary disease | 6 | 0.93 | (0.41-2.08) | 0.8514 | 0.56 | (0.22-1.43) | 0.2267 |
| Peripheral arterial occlusive disease | 1 | 0.99 | (0.14-7.05) | 0.9905 | 1.47 | (0.2-10.88) | 0.705 |
| Gastrointestinal ulcer | 20 | 2.17 | (1.37-3.44) | 0.001 | 2.25 | (1.34-3.77) | 0.0021 |
| Gastrointestinal bleeding | 5 | 2.64 | (1.09-6.42) | 0.0321 | 1.83 | (0.7-4.78) | 0.2204 |
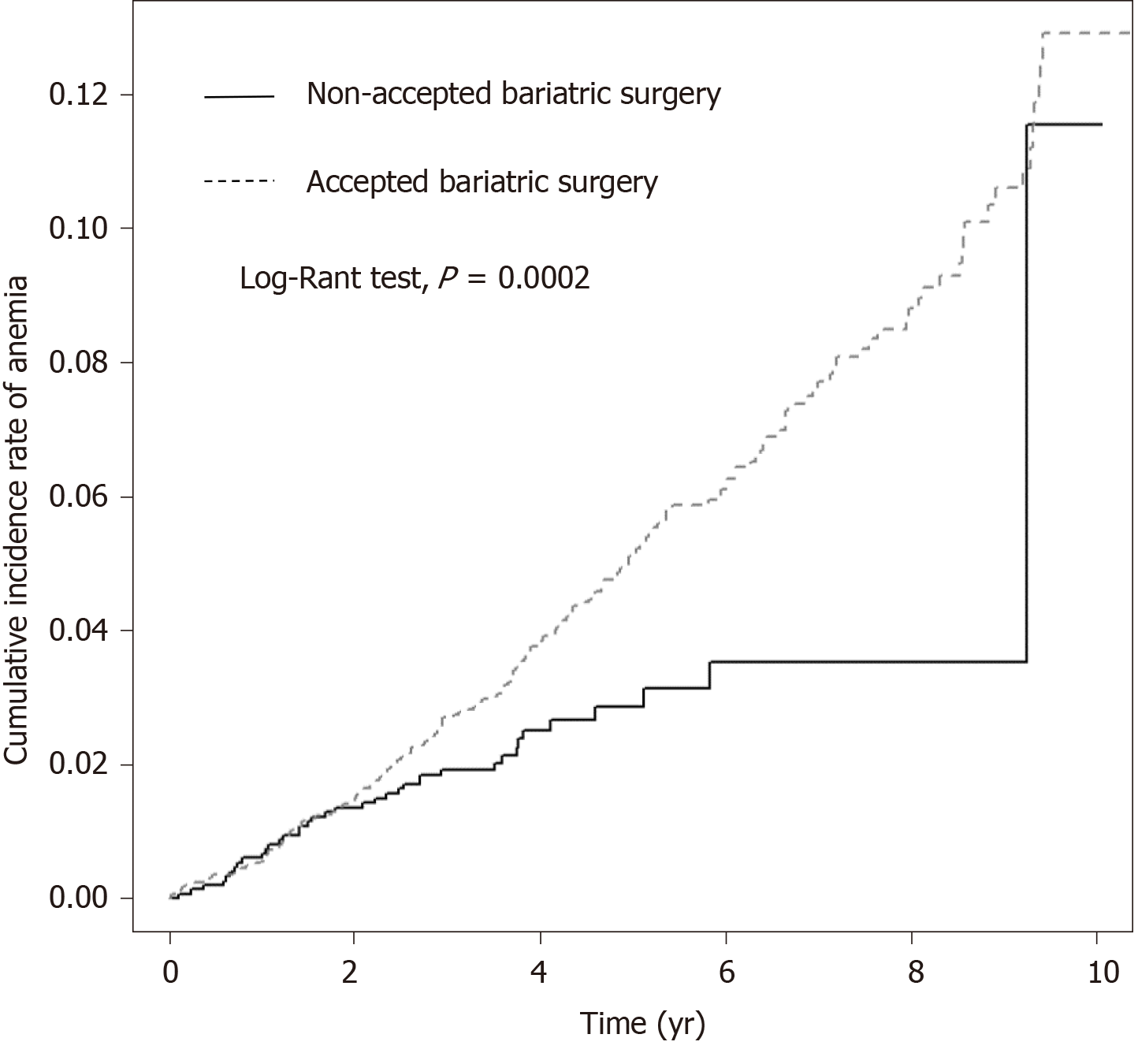
The Kaplan-Meier curves of the both cohorts suggested significantly increasing cumulative incidence of anemia in the study cohort (with bariatric surgery) (P = 0.0002) (Figure 2).
In Table 3, the HR of post-bariatric surgery anemia and the incidence rate of anemia were stratified by age and gender. Female, age of 20-29 and 30-64 years had significantly higher adjusted HRs (3.83 and 2.87, respectively).
| Variable | Receiving bariatric surgery | Receiving vs not-receiving bariatric surgery | ||||||
| No | Yes | Crude HR | Adjusted HR2 | |||||
| Event | Person years | IR1 | Event | Person years | IR1 | |||
| Total | 38 | 6359 | 5.98 | 183 | 16277 | 11.24 | 1.71(1.20-2.44)b | 2.36(1.52-3.65)c |
| Gender | ||||||||
| Female | 27 | 3455 | 7.82 | 159 | 10688 | 14.88 | 1.67(1.10-2.53)a | 2.48(1.50-4.09)c |
| Male | 11 | 2905 | 3.79 | 24 | 5588 | 4.29 | 1.24(0.60-2.55) | 2.04(0.89-4.70) |
| Age group, yr | ||||||||
| 20-29 yr | 4 | 1386 | 2.89 | 84 | 7825 | 10.73 | 3.11(1.14-8.54)a | 3.83(1.13-12.99)a |
| 30-64 yr | 27 | 4341 | 6.22 | 99 | 8446 | 11.72 | 1.81(1.18-2.79)b | 2.37(1.45-3.88)c |
| 65-100 yr | 7 | 632 | 11.07 | 0 | 6 | 0 | - | - |
In Table 4, HR of post-bariatric surgery anemia and the incidence rate were stratified by malabsorptive and restrictive procedures. Malabsorptive and restrictive procedures had significantly higher adjusted HRs (3.18 and 1.55, respectively).
Our study demonstrated that patients who were diagnosed with morbid obesity and received bariatric surgery had significantly higher risk of anemia than patients who did not receive bariatric surgery. After stratification by gender and age, female sex, young-aged (20-29 years) and middle-aged (30-64 years) patients had significantly higher HRs than male sex and older-aged patients. Malabsorptive procedures had a higher HR of post-bariatric surgery anemia than restrictive procedures.
Currently, bariatric surgery is considered a promising treatment strategy because of its long-term benefits for morbidly obese patients. These benefits include the following: Sustainable body weight loss; improvement in comorbidities; reduction in medicine use; and improvement in patients’ quality of life[1-8]. However, post-bariatric surgery anemia, which mainly results from micronutrient deficiencies, is recognized as the most common adverse effect[3-5]. The metabolic sequela, such as anemia, was identified before the 1990s[21,22]. Moreover, recently, post-bariatric surgery anemia remains a challenge. The large cohort study from France revealed that 5% were diagnosed with anemia after bariatric surgery. Furthermore, the overall risk rate of diagnosing anemia postoperatively was 7.8%[11]. In our study, we demonstrate that obese patients who received bariatric surgery significantly had 2.36-fold higher risk of anemia than obese patients who did not receive bariatric surgery.
Post-bariatric surgery anemia is considered mainly due to micronutrient deficiency. Deficiencies of vitamin B12, iron, and vitamin D were commonly observed postoperatively[17,23]. Additionally, oral supplementation of these nutrients was described in the updated guidelines[10]. Other beneficial postoperative physiological changes regarding anemia were already determined. Weight loss after bariatric surgery reduces inflammation, consequently reducing anemia. Chronic inflammation has been recognized as a characteristic feature of morbid obesity[24,25]. Decreasing inflammatory markers such as ferritin, C-reactive protein, haptoglobin, and white blood cell count were noted after bariatric surgery[18,26,27]. However, the positive effects of post-bariatric surgery anemia are less observed than the negative effects in the long-term.
This study also proposed that females had a greater risk of post-bariatric surgery anemia than males among obese patients. Women are nearly 3-fold more likely to have a diagnosis of anemia postoperatively compared with men. The results are similar to the result of a study conducted in France[11]. von Drygalski et al[18] revealed that Roux-en-Y bypass surgery increased the percentage of anemia from 12% to 23%. During the postoperative period, premenopausal women have greater prevalence of anemia than postmenopausal women and men. According to the National Health and Nutrition Examination Survey studies, women in their childbearing years generally have a greater percentage of anemia than men (12.2% vs 1.5%, respectively), and the gender differences were no longer observed after the age of 50 years[28]. However, data from our study revealed that, after adjusting age and other confounding factors, the risk of post-bariatric surgery anemia is still higher in women than in men. Obesity is associated with an irregular menstruation cycle[29,30]. After bariatric surgery, a more consistent menstrual cycle rather than amenorrhea or irregular menstruation cycle is observed. Hence, premenopausal women are highly diagnosed with anemia postoperatively. Another study suggested that women are at a higher risk of eating less than men, although with limited evidence, which may add other risk for iron deficiency anemia[12].
We also found that young-aged (20-29 years) and middle-aged (30–64 years) patients had significantly higher HRs post-bariatric surgery anemia than older-aged patients. A study conducted in France revealed that patients aged less than 52 years have a 50% higher risk of developing post-bariatric surgery anemia than elderly patients[11]. Groups were classified according to the age of 52 years because menstruation was taken into consideration[11]. Thus, considering that premenopausal women are at an increased risk of developing anemia, a French cohort did not consider the effect of age. The following factors may potentially explain the effect of age on anemia after bariatric surgery, although with insufficient evidence: Young-aged individuals consume less calories and nutrients than required and have limited compliance to medical follow-up and limited adherence to micronutrient supplement consumption. Furthermore, the small sample size of older-aged patients, that is, only six older-aged obese patients underwent bariatric surgery, must be taken into consideration.
The risks of post-bariatric surgery anemia vary among different types of surgery. Our study revealed that obese patients who underwent malabsorptive procedures have 3.18-fold higher risk of developing anemia than patients who did not undergo malabsorptive procedures. Moreover, obese patients who underwent restrictive procedures also have 1.55-fold higher risk of developing anemia than patients who did not undergo restrictive procedures. Previous studies also demonstrated that malabsorptive procedures have greater risk of anemia than restrictive procedures. The French cohort showed that the risk rates of developing anemia due to micronutrient deficiency were 13.0% after gastric bypass, 5.6% after sleeve gastrectomy, and 4.0% after adjustable gastric banding. Both malabsorptive and restrictive procedures lessen the volume of gastric pouch and also reduce hydrochloric acid production. Gut hypoacidity reduces transit time; consequently, early satiety and decreased intake amount contribute to the risk of micronutrient deficiency anemia after bariatric surgery. In addition to stomach reduction, malabsorptive procedures (e.g., Roux-en-Y bypass surgery) further bypass the main sites of iron absorption (duodenum and a portion of the jejunum). Hence, malabsorptive procedures theoretically result in more iron deficiency. However, patients who underwent malabsorptive procedures are possibly able to adapt iron absorption[5,31]. Therefore, postoperative iron deficiency is insignificantly different between the two types of surgery. One meta-analysis also revealed that sleeve gastrectomy and Roux-en-Y bypass surgery are comparable regarding the risk of postoperative iron deficiency[5].
On the contrary, malabsorptive procedures have significant effects on vitamin B12 absorption. Roux-en-Y bypass surgery reduces acid secretion, affects intrinsic factor function, and limits the mixing of food with pancreatic secretions. Consequently, vitamin B12 maldigestion and malabsorption lead to higher risk anemia postoperatively[5,32]. One meta-analysis revealed that Roux-en-Y bypass surgery had 3.55-fold higher risk of postoperative vitamin B12 deficiency than sleeve gastrectomy[5]. Recent studies demonstrate that malabsorptive procedures result in excess weight loss at midterm, but the difference was not statistically significant. The morbidity rates of the two procedures were statistically insignificant[33,34].
Our study has the following strength: The nationwide cohort with large sample size, paying careful attention to post-bariatric surgery anemia in morbidly obese patients. However, there are some limitations in our study. First, the Taiwan NHIRD did not comprise information regarding hemoglobin level, body weight, and height. Therefore, the associations between body mass index changes, body weight changes, and post-bariatric surgery anemia could not be analyzed. To reduce the influence of pre-surgery anemia, our study group was analyzed after excluding who had anemia diagnosis before index date. Second, due to the retrospective cohort design of the study, the evidence of this study was lower in statistical quality than randomized trials. Third, regimens for micronutrient supplementation were not assessed in this study. Thus, we could not further analyze the micronutrient deficiency between groups.
In conclusion, our study demonstrated that morbidly obese patients who received bariatric surgery had a significantly higher risk of developing anemia than patients who did not receive bariatric surgery. After the stratification of confounding factors, female sex, young-aged (20-29 years) and middle-aged (30-64 years) patients, and patients who underwent malabsorptive procedures had significantly higher HRs than male sex, older-aged patients, and patients who did not undergo malabsorptive procedures.
Bariatric surgery is considered to be the most effective long-term treatment for morbidly obese patients. However, post-bariatric surgery anemia is identified as a common adverse effect and remains a challenge nowadays. This study revealed the long-term incidence of anemia in morbidly obese patients who received a bariatric procedure in large cohorts.
Although post-bariatric surgery anemia is identified as a common adverse effect, there are insufficient population-based cohort studies to demonstrate the long-term incidence of anemia and the risk of post-bariatric surgery anemia.
To estimate the risk of post-bariatric surgery anemia and to stratify the association between sex, age, and type of surgery.
This study is a population-based cohort study. We conducted this nationwide study using claims data from National Health Insurance Research Database (NHIRD) in Taiwan. There were 4373 morbidly obese patients in this study cohort.
There were 4373 patients in the cohort. Among patients who were diagnosed with morbid obesity, 2864 received bariatric surgery. All obesity- and obesity-associated comorbidities decreased in the surgical group. Increasing risk of post-bariatric surgery anemia among obese patients was found by Cox proportional hazards regression [adjusted hazard ratio(HR): 2.36]. Also, we found significantly increasing cumulative incidence rate of anemia among patients receiving bariatric surgery by log-rank test. After adjusting for age and sex, the increasing incidence of post-bariatric surgery anemia was found among women (adjusted HR: 2.48), patients in the 20-29-year-old group (adjusted HR: 3.83) and patients in 30–64-year-old group (adjusted HR: 2.37). Moreover, malabsorptive and restrictive procedures had significantly higher adjusted HRs, 3.18 and 1.55, respectively.
We demonstrated the long-term incidence of post-bariatric surgery anemia and the risk of post-bariatric surgery anemia via a population-based cohort study in which data were obtained from the Taiwan NHIRD. Bariatric surgery increases the risk of anemia among obese patients, specifically in women, young- and middle-aged patients, and patients undergoing malabsorptive procedures. Malabsorptive procedures have a higher risk of anemia than restrictive procedures. Bariatric surgery increases the long-term risk of anemia. Considering the risk of post-bariatric surgery anemia, lifelong micronutrient supplementation was considered mandatory. Moreover, the quality and sustainability of medical follow-up consultation became an important consideration of bariatric surgery.
A population-based database, like the Taiwan NHIRD, could provide the evidence of long-term risk. The data could also provide information for further analysis of the associated risks. A prospective cohort study or randomized trial could provide better statistical quality.
We would like to thank the Clinical Research Core Laboratory of Taipei Veterans General Hospital for providing experimental space and facilities.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farhat S, Zhu SH S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LL
| 1. | Cheng J, Gao J, Shuai X, Wang G, Tao K. The comprehensive summary of surgical versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomized controlled trials. Oncotarget. 2016;7:39216-39230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416-23; discussion 423-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 859] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 3. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 1902] [Article Influence: 237.8] [Reference Citation Analysis (0)] |
| 4. | Thereaux J, Lesuffleur T, Païta M, Czernichow S, Basdevant A, Msika S, Millat B, Fagot-Campagna A. Long-term follow-up after bariatric surgery in a national cohort. Br J Surg. 2017;104:1362-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 941] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 6. | Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lönroth H, Narbro K, Näslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1154] [Cited by in RCA: 1126] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 7. | Huang HH, Lee WJ, Chen SC, Chen TF, Lee SD, Chen CY. Bile Acid and Fibroblast Growth Factor 19 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Sleeve Gastrectomy. J Clin Med. 2019;8:815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Wang W, Fann CSJ, Yang SH, Chen HH, Chen CY. Weight loss and metabolic improvements in obese patients undergoing gastric banding and gastric banded plication: A comparison. Nutrition. 2019;57:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Brolin RE, LaMarca LB, Kenler HA, Cody RP. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg. 2002;6:195-203; discussion 204-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis. 2017;13:727-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 448] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 11. | Bailly L, Schiavo L, Sebastianelli L, Fabre R, Pradier C, Iannelli A. Anemia and Bariatric Surgery: Results of a National French Survey on Administrative Data of 306,298 Consecutive Patients Between 2008 and 2016. Obes Surg. 2018;28:2313-2320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Kheniser KG, Kashyap SR, Schauer PR, Lam ETC, Kullman ES. Prevalence of Anemia in Subjects Randomized into Roux-en-Y Gastric Bypass or Sleeve Gastrectomy. Obes Surg. 2017;27:1381-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Kwon Y, Kim HJ, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. Anemia, iron and vitamin B12 deficiencies after sleeve gastrectomy compared to Roux-en-Y gastric bypass: a meta-analysis. Surg Obes Relat Dis. 2014;10:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Chen GL, Kubat E, Eisenberg D. Prevalence of Anemia 10 Years After Roux-en-Y Gastric Bypass in a Single Veterans Affairs Medical Center. JAMA Surg. 2018;153:86-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Malinowski SS. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci. 2006;331:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, Gonzalez P, Papapietro K. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;19:1515-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Chou JJ, Lee WJ, Almalki O, Chen JC, Tsai PL, Yang SH. Dietary Intake and Weight Changes 5 Years After Laparoscopic Sleeve Gastrectomy. Obes Surg. 2017;27:3240-3246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | von Drygalski A, Andris DA, Nuttleman PR, Jackson S, Klein J, Wallace JR. Anemia after bariatric surgery cannot be explained by iron deficiency alone: results of a large cohort study. Surg Obes Relat Dis. 2011;7:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Huang HH, Hsieh MS, Chen CY. Risk of cholecystectomy in morbidly obese patients after bariatric surgery in Taiwan. Obes Res Clin Pract. 2019;13:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Chang CM, Hsieh MS, Yang TC, Hsieh VC, Chiang JH, Huang HH, How CK, Hu SY, Yen DH. Selective serotonin reuptake inhibitors and the risk of hepatocellular carcinoma in hepatitis B virus-infected patients. Cancer Manag Res. 2017;9:709-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Amaral JF, Thompson WR, Caldwell MD, Martin HF, Randall HT. Prospective hematologic evaluation of gastric exclusion surgery for morbid obesity. Ann Surg. 1985;201:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 113] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Ponsky TA, Brody F, Pucci E. Alterations in gastrointestinal physiology after Roux-en-Y gastric bypass. J Am Coll Surg. 2005;201:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Gletsu-Miller N, Wright BN. Mineral malnutrition following bariatric surgery. Adv Nutr. 2013;4:506-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Cottam DR, Mattar SG, Barinas-Mitchell E, Eid G, Kuller L, Kelley DE, Schauer PR. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg. 2004;14:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Chen CY, Fujimiya M, Laviano A, Chang FY, Lin HC, Lee SD. Modulation of ingestive behavior and gastrointestinal motility by ghrelin in diabetic animals and humans. J Chin Med Assoc. 2010;73:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Agrawal V, Krause KR, Chengelis DL, Zalesin KC, Rocher LL, McCullough PA. Relation between degree of weight loss after bariatric surgery and reduction in albuminuria and C-reactive protein. Surg Obes Relat Dis. 2009;5:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Anty R, Dahman M, Iannelli A, Gual P, Staccini-Myx A, Amor IB, Luciani N, Saint-Paul MC, Huet PM, Sadoul JL, Srai SK, Unwin R, Gugenheim J, Le Marchand-Brustel Y, Tran A, Bekri S. Bariatric surgery can correct iron depletion in morbidly obese women: a link with chronic inflammation. Obes Surg. 2008;18:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 911] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 29. | Chang PJ, Chen PC, Hsieh CJ, Chiu LT. Risk factors on the menstrual cycle of healthy Taiwanese college nursing students. Aust N Z J Obstet Gynaecol. 2009;49:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Wei S, Schmidt MD, Dwyer T, Norman RJ, Venn AJ. Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obesity (Silver Spring). 2009;17:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Schümann K, Elsenhans B, Forth W, Schroeder P. Intestinal iron transfer after ileojejunal transposition. Digestion. 1991;50:182-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Smith CD, Herkes SB, Behrns KE, Fairbanks VF, Kelly KA, Sarr MG. Gastric acid secretion and vitamin B12 absorption after vertical Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 1993;218:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Salminen P, Helmiö M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, Hurme S, Soinio M, Nuutila P, Victorzon M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA. 2018;319:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 714] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 34. | Shoar S, Saber AA. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surg Obes Relat Dis. 2017;13:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |