Published online Nov 15, 2010. doi: 10.4239/wjd.v1.i5.153
Revised: August 30, 2010
Accepted: September 6, 2010
Published online: November 15, 2010
AIM: To assess the efficacy and safety of vildagliptin/pioglitazone combination therapy in Korean patients with type 2 diabetes mellitus (T2DM).
METHODS: This was a post hoc analysis in Korean patients, from a 24-wk, randomized, active-controlled, double-blind, parallel-group, multicenter study. Eligible patients were aged between 18 and 80 years, drug naive, and had been diagnosed with T2DM [hemoglobin A1c (HbA1c): 7.5%-11.0% and fasting plasma glucose (FPG): < 270 mg/dL (< 15 mmol/L)]. Patients were randomized (1:1:1:1) to receive the vildagliptin/pioglitazone combination at 100/30 mg q.d. (high-dose) or 50/15 mg q.d. (low-dose), vildagliptin 100 mg q.d., or pioglitazone 30 mg q.d. monotherapies. The primary outcome measure was change in HbA1c from baseline to endpoint.
RESULTS: The distribution of baseline demographic and clinical parameters was well balanced between treatment groups. The overall mean age, body mass index, HbA1c, FPG, and duration of disease were 50.8 years, 24.6 kg/m2, 8.6%, 10.1 mmol/L, and 2.2 years, respectively. Adjusted mean changes (± standard error) in HbA1c from baseline (~8.7%) to week 24 endpoint were -2.03% ± 0.16% (high-dose, N = 34), -1.88% ± 0.15% (low-dose, N = 34), -1.31% ± 0.21% (vildagliptin, N = 36), and -1.52% ± 0.16% (pioglitazone, N = 36). The high-dose combination therapy demonstrated greater efficacy than monotherapies [vildagliptin (P = 0.029) and pioglitazone (P = 0.027)]. Percentage of patients achieving HbA1c < 7% and ≤ 6.5% was the highest in the high-dose group (76% and 68%) followed by low-dose (58% and 47%), vildagliptin (59% and 37%), and pioglitazone (53% and 28%) groups. The overall incidence of adverse events was comparable.
CONCLUSION: In Korean patients, first-line treatment with high-dose combination therapy improved glycemic control compared to pioglitazone and vildagliptin monotherapies, consistent with results published for the overall study population.
- Citation: Kim SW, Baik SH, Yoon KH, Lee HW, Filozof C. Efficacy and safety of vildagliptin/pioglitazone combination therapy in Korean patients with diabetes. World J Diabetes 2010; 1(5): 153-160
- URL: https://www.wjgnet.com/1948-9358/full/v1/i5/153.htm
- DOI: https://dx.doi.org/10.4239/wjd.v1.i5.153
Diabetes is emerging as a global epidemic, imposing enormous humanitarian and economic burdens on society. According to a WHO projection, by 2030, approximately 190 million people will be affected in the Asia-Pacific region alone. Over 3 million of these people are expected to be from Korea[1].
Evidence suggests that patients with type 2 diabetes mellitus (T2DM) increasingly require multiple pharmacological combinations to reach treatment goals. This is specially relevant for diabetic patients in Korea, where only 37.3% achieve glycosylated hemoglobin A1c(HbA1c) < 7% with the currently available therapies[2]. Clinical inertia, with failure to advance therapy despite persistently elevated HbA1c levels above target, has become a major problem for the stepwise approach to treatment[3]. Initial combination therapy using two oral anti-diabetic drugs (OAD) with complementary mechanisms of action is an alternative approach that may provide better or more sustained glycemic control. It may also allow the use of lower doses of the component OADs and thus minimize any dose-related adverse events (AEs).
Probably because of different genetic characteristics, diet habits and lifestyle, diabetic patients from Korea show a different clinical profile than Caucasians[4,5]. More than 70% to 80% of Korean patients with T2DM are non-obese, with a body mass index (BMI) below 25 kg/m2. This finding is in sharp contrast to the patients from the West, where diabetes and obesity are frequently concurrent in the population[5,6]. Although impairment of early phase insulin secretion is reported to be the initial abnormality in the development of glucose intolerance in Koreans, the contribution of increased insulin resistance in the pathophysiology of diabetes is also becoming significant[7].
Vildagliptin is a potent and selective dipeptidyl peptidase-4 (DPP-4) inhibitor that improves pancreatic islet function, evidenced from improved ability of α-cells and β-cells to sense and respond to glucose after treatment[8]. In addition, vildagliptin inhibits hepatic glucose production during meals as well as during the overnight post-absorptive period. DPP-4 inhibition by vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration[9]. On the other hand, pioglitazone is an agonist for peroxisome proliferator-activated receptors (PPARs) in target tissues for insulin action that regulates transcription of insulin-responsive genes involved in controlling glucose production, transportation, and utilization, thereby enhancing tissue sensitivity to insulin[10]. We recently reported results of a multi-center, international study which indicate that first-line treatment of diabetic patients with vildagliptin/pioglitazone combination therapy provides better glycemic control than the corresponding monotherapies and has a comparable tolerability profile[11].A large proportion of the study population were Korean providing an opportunity to conduct an exploratory analysis to evaluate the efficacy and tolerability of vildagliptin/pioglitazone combination therapy compared with corresponding monotherapies in Korean patients with T2DM. Results of this analysis are presented here.
This is a Korean sub-group analysis of a 24-wk, randomized, active-controlled, double-blind, double-dummy, parallel-group study conducted at 145 centers in eight countries, the results of which have been published previously[11]. Participating centers were located in the United States, Europe, and Asia (Korea, India, and Taiwan). In Korea, the study was conducted at 15 centers.
The study was conducted in accordance with the ICH-Good Clinical Practice Guidelines, the Declaration of Helsinki, and the local laws and regulations. The protocol was approved by the independent ethics committee/institutional review board at each study site.
Eligible patients were aged between 18 and 80 years and had been diagnosed with T2DM (HbA1c: 7.5%-11.0%, BMI: 22-45 kg/m2, and fasting plasma glucose (FPG): < 270 mg/dL (< 15 mmol/L). The patients had not received any treatment for at least 12 wk prior to screening and no oral anti-diabetic agent for more than three consecutive months in the past.
Patients were excluded if they had a history of type 1 or secondary forms of diabetes, acute metabolic diabetic complications, myocardial infarction, unstable angina, or coronary artery bypass surgery within the previous 6 mo, congestive heart failure, liver disease such as cirrhosis or chronic active hepatitis, or any contraindications and warnings according to the country-specific label for pioglitazone. Patients with any of the following laboratory abnormalities were also excluded: alanine aminotransferase or aspartate aminotransferase > 2.5 times the upper limit of normal (ULN); direct bilirubin > 1.3 times the ULN; serum creatinine levels > 220 mmol/L, clinically significant abnormal TSH, or fasting triglycerides (TGs) > 7.9 mmol/L.
Eligible patients were randomized to receive vildagliptin/pioglitazone combination therapy at 100/30 mg q.d. (high-dose, N = 34) or 50/15 mg q.d. (low-dose, N = 34), or monotherapy with vildagliptin 100 mg q.d. (N = 36) or pioglitazone 30 mg q.d. (N = 36). All the participants provided written informed consent.
The efficacy variables were change from baseline to endpoint in (1) HbA1c; (2) FPG; (3) post-prandial plasma glucose (PPG), glucagon-like peptide-1 (GLP-I), glucagon and insulin levels after a meal test; (4) fasting pro-insulin, pro-insulin/insulin ratio, homeostasis model assessment of β-cell function (HOMA-B), insulinogenic index, HOMA of insulin resistance (HOMA-IR); and (5) changes in body weight. HbA1c reductions in Korean patients were also compared to the non-Korean (all patients from the overall study population, excluding the Korean patients) and non-Asian (all patients from the overall study population, excluding the Indian, Taiwanese, and Korean patient sub-populations) subgroups of patients, and the overall study population.
A standard breakfast meal (500 kcal; 60% carbohydrates, 30% fat, and 10% protein) was provided at baseline (week 0) and at week 24 to the subset of patients (44.6%) who had volunteered to participate in the meal challenge test. Samples for measurement of PPG, insulin, glucagon, and active GLP-1 levels were obtained at 20 min pre-meal and 0, 15, 30, 60, 90, 120, and 240 min post-meal (provided immediately after the 0-min sample and consumed within 15 min). The areas under curve (AUC) for PPG, insulin, and GLP-1 were calculated with the trapezoidal method.
Standard hematology and biochemistry laboratory assessments were made at each visit except for week 16. HbA1c was quantified using ion exchange high-performance liquid chromatography. All laboratory assessments were made by a central laboratory (Covance-US, Indianapolis, IN, USA) with standardized and validated procedures according to Good Laboratory Practice.
Safety and tolerability were evaluated by physical examination, measurement of vital signs, recording of electrocardiograms, and safety laboratory measurements. All AEs were monitored throughout the study and evaluated by the investigators for level of severity, duration, outcome, and relationship to study drug.
Data for this post hoc analysis were summarized with respect to demography, efficacy, and safety variables. All efficacy analyses were performed on the intent-to-treat (ITT) population (randomized patients who received at least one dose of study medication and for whom a baseline and at least one post-baseline efficacy assessment was available) using last observation carried forward for patients who discontinued early.
The mean change in each efficacy variable from baseline to endpoint was analyzed using an analysis of covariance (ANCOVA) model, with treatment and pooled center as factors and baseline as the covariate. All ANCOVAs were performed on the set of patients within both treatment arms being compared. Data are presented as mean ± standard error (SE) unless otherwise stated. Statistical significance for subgroup analyses was set at 0.05.
The demographic and clinical parameters of patients included in this sub-group analysis are summarized in Table 1. These characteristics were generally comparable across treatment arms. The overall mean age, BMI, HbA1c, FPG, and duration of disease were 50.8 years, 24.6 kg/m2, 8.6%, 10.1 mmol/L, and 2.2 years, respectively.
| Mean±SD | Vilda/Pio 100/30 mg q.d. | Vilda/Pio 50/15 mg q.d. | Vilda 100 mg q.d. | Pio 30 mg q.d. |
| N | 34 | 34 | 36 | 36 |
| Males, n (%) | 23 (67.6) | 25 (73.5) | 32 (88.9) | 30 (83.3) |
| Age (years) | 52.9 ± 10.6 | 51.3 ± 10.3 | 49.8 ± 10.0 | 49.2 ± 9.4 |
| BMI (kg/m2) | 24.7 ± 2.8 | 24.7 ± 2.5 | 24.7 ± 2.2 | 24.3 ± 2.3 |
| HbA1c (%) | 8.4 ± 0.8 | 8.8 ± 0.9 | 8.6 ± 0.9 | 8.7 ± 0.9 |
| FPG (mmol/L) | 9.4 ± 2.0 | 10.1 ± 2.0 | 10.9 ± 2.6 | 9.8 ± 2.6 |
| Duration of T2DM (years) | 3.1 ± 3.6 | 1.6 ± 2.4 | 1.9 ± 2.5 | 2.1 ± 2.7 |
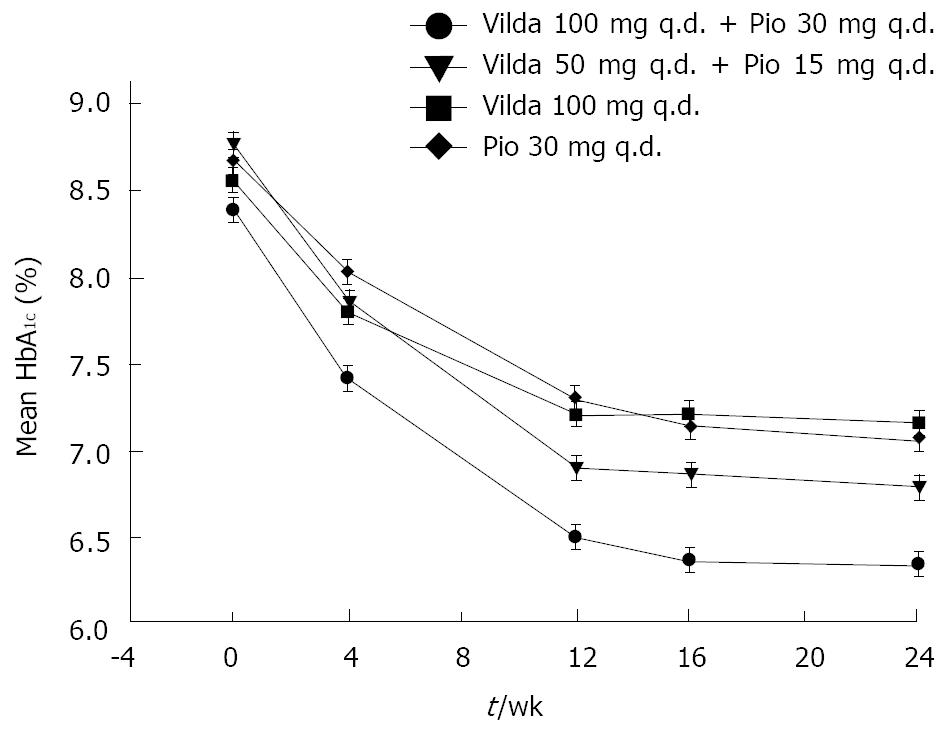
Figure 1 represents the time-course of mean HbA1c during a 24-wk treatment period in the four treatment arms. Near-maximal efficacy was reached by week 16, and maintained up to 24 wk, for all treatment groups. The adjusted mean change (AM∆) in HbA1c from baseline to endpoint was similar between the high-dose and low-dose combination groups (-2.03% ± 0.16% and -1.88% ± 0.15%, respectively); however, the change was greater in patients receiving high-dose combination compared with those receiving monotherapy with vildagliptin (-1.31% ± 0.21%) or pioglitazone (-1.52% ± 0.16%), and the difference between these treatments (high-dose combination vs monotherapies) was significant (P < 0.05). The mean HbA1c reduction was numerically larger for the low-dose combination compared with pioglitazone monotherapy; however, the difference was not statistically significant (between-treatment difference, -0.30% ± 0.21%, P = 0.156).
A sub-analysis of the primary endpoint was also performed on the basis of initial HbA1c level. In all treatment groups, patients with high HbA1c at baseline (> 8% and > 9%) showed consistently larger reductions in HbA1c than patients with lower HbA1c at baseline. In patients with baseline HbA1c > 9.0% receiving the high-dose combination, the mean change from baseline HbA1c was -3.14% ± 0.32%. In those receiving the low-dose combination, vildagliptin monotherapy, or pioglitazone monotherapy, the mean changes from baseline HbA1c were -2.20% ± 0.31%, -1.48% ± 0.50%, and -2.01% ± 0.28%, respectively.
The percentage of patients achieving target HbA1c level of < 7% and ≤ 6.5%, respectively, was highest in the high-dose combination group (75.8% and 67.6%) compared with the low-dose combination (57.6% and 47.1%), vildagliptin (58.8% and 37.1%), and pioglitazone (52.8% and 27.8%) groups. Treatment difference between high-dose combination and pioglitazone monotherapy was statistically significant (< 7%, P = 0.047; ≤ 6.5%, P < 0.001). The overall ability of the different therapies to bring about ≥ 1% reduction in HbA1c was comparable for high-dose and low-dose combination therapies (82.4% and 85.3%), and it was lower for the component monotherapies (vildagliptin, 68.6%; pioglitazone, 69.4%).
The adjusted mean change in HbA1c from baseline to endpoint was similar between the high-dose combination groups in Korean (-2.03% ± 0.16%), non-Korean (-1.92% ± 0.11%), non-Asian (-1.93% ± 0.13%), and the overall study population (-1.93% ± 0.09%). However, numerically larger reductions were observed in Korean patients receiving the low-dose combination (-1.88% ± 0.15%) compared with non-Korean (-1.61% ± 0.11%), non-Asian (-1.51% ± 0.14%), and the overall study population (-1.67% ± 0.09%).
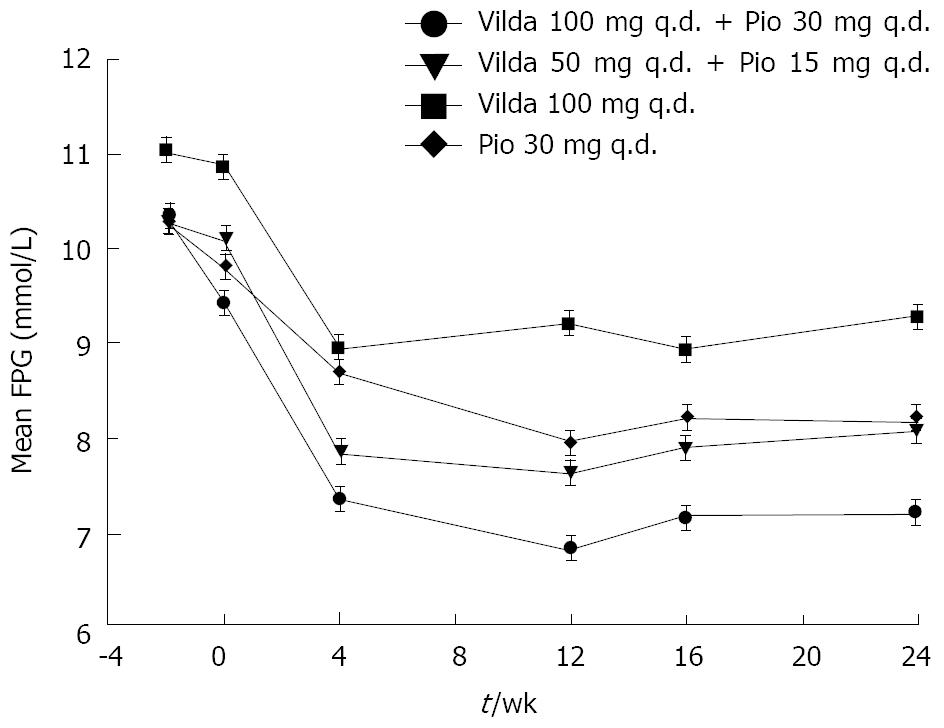
As shown in Figure 2, reduction in the FPG level was observed during the 24-wk treatment period in all the treatment arms. Near-maximal reductions (mmol/L) were observed after 4 wk of initiation of therapy, and were maintained over the next 20 wk (mean reductions of -2.30 ± 0.27, -1.93 ± 0.27, -1.13 ± 0.36, and -1.60 ± 0.26 in the high-dose combination, low-dose combination, vildagliptin, and pioglitazone groups, respectively).
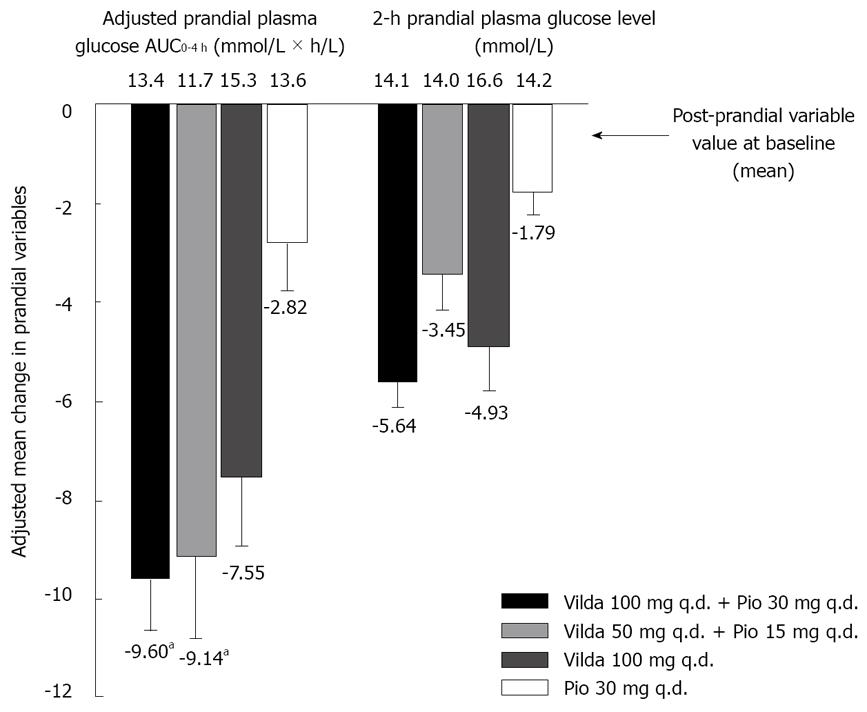
Sixty-two patients (44.6%) volunteered for the standard meal challenge tests (15, 12, 18, and 17 patients in the high-dose and low-dose combinations, vildagliptin, and pioglitazone groups, respectively). Figure 3 shows the AM∆ in the indicated prandial variables across the four treatment groups for these patients.
The adjusted post-prandial plasma glucose AUC0-4 h (mmol × h/L) was greatly reduced with the high-dose (-9.60 ± 1.03) and low-dose (-9.14 ± 1.66) combinations as well as with vildagliptin monotherapy (-7.55 ± 1.37), in comparison to the reduction with pioglitazone monotherapy (-2.82 ± 0.97). The difference between treatments was statistically significant for combination therapy vs pioglitazone monotherapy (high-dose, P < 0.001; low-dose, P = 0.005). Similar reductions were seen in 2-h PPG levels, with the largest reductions observed in the high-dose combination therapy group.
AM∆ in post-prandial active GLP-1 AUC0-2 h (pmol × h/L) were 14.56 ± 2.87, 6.87 ± 0.99, 4.24 ± 4.80, and -7.95 ± 3.49 in the high-dose combination, low-dose combination, vildagliptin, and pioglitazone groups, respectively. The difference in mean change was statistically significantly higher in both the combination groups (high-dose, 22.51 ± 5.22, P = 0.012; low-dose, 11.18 ± 1.35, P = 0.014), compared with pioglitazone monotherapy.
AM∆ in post-prandial glucagon AUC0-2 h (pmol × h/L) were -7.84 ± 1.78, -3.32 ± 1.92, -5.06 ± 2.57, and -3.02 ± 1.67 in the high-dose combination, low-dose combination, vildagliptin, and pioglitazone groups, respectively. The difference in mean change was numerically higher in both the combination groups (high-dose, -4.82 ± 2.44, P = 0.058; low-dose, -0.23 ± 2.52, P = 0.928), compared with pioglitazone monotherapy.
AM∆ in post-prandial plasma insulin AUC0-4 h (pmol× h/L) varied substantially between the treatment groups (high-dose, -30.07 ± 50.02; low-dose, 4.09 ± 53.54; vildagliptin, 144.56 ± 42.37; and pioglitazone, -10.24 ± 46.97).
Fasting pro-insulin levels decreased across all treatment groups. The reductions (pmol/L) were numerically higher and comparable between the high-dose and low-dose combination groups (-9.45 ± 1.74 and -8.68 ± 1.46) than with vildagliptin (-2.43 ± 2.38) or pioglitazone (-7.21 ± 1.64) monotherapy. Correspondingly, small changes in fasting pro-insulin/insulin ratio (-0.19 to -0.27) were also observed across all treatment groups.
HOMA-B increased across all treatment groups. The largest increase was observed in the high-dose combination group, followed by vildagliptin monotherapy, low-dose combination, and pioglitazone monotherapy groups (12.09 ± 2.83, 8.06 ± 2.51, 4.86 ± 1.58, and 4.57 ± 2.84, respectively). However, the difference between combination therapies (high-dose and low-dose) and pioglitazone was not significant.
In both the high-dose and the low-dose combination therapy groups, an increase in insulinogenic index (0-min peak glucose) was observed (0.15 ± 0.06 and 0.42 ± 0.18, respectively) compared with the change in vildagliptin (0.08 ± 0.02) and pioglitazone (0.01 ± 0.07) monotherapy groups.
HOMA-IR decreased for all treatment groups except vildagliptin monotherapy (high-dose combination, -0.45 ± 0.14; low-dose combination, -0.56 ± 0.09; vildagliptin, 0.18 ± 0.18; and pioglitazone, -0.64 ± 0.14). The between-treatment differences in reduction were not significant.
Increase in weight from baseline to end of study was observed for three treatment groups (2.39 ± 0.49 kg, 1.59 ± 0.47 kg, and 1.54 ± 0.48 kg for high-dose and low-dose combinations, and pioglitazone monotherapy, respectively). No weight gain was observed with vildagliptin monotherapy (0.64 ± 0.30 kg).
During the 24-wk study, all treatments were generally well tolerated. The overall incidence of AEs was comparable across treatment groups (summarized in Table 2). The majority of reported AEs were mild to moderate in severity. The most frequently reported AEs in the study were nasopharyngitis, dizziness, and headache.
| Vilda/Pio 100/30 mg q.d. (N = 34) n (%) | Vilda/Pio 50/15 mg q.d. (N = 34) n (%) | Vilda 100 mg q.d. (N = 35) n (%) | Pio 30 mg q.d. (N = 36) n (%) | |
| Any primary system organ class AE | 13 (38.2) | 15 (44.1) | 13 (37.1) | 13 (36.1) |
| Common AEs | ||||
| Nasopharyngitis | 0 | 4 (11.8) | 2 (5.7) | 3 (8.3) |
| Dizziness | 2 (5.9) | 1 (2.9) | 2 (5.7) | 3 (8.3) |
| Headache | 3 (8.8) | 1 (2.9) | 0 | 0 |
| Upper respiratory tract infection | 0 | 0 | 2 (5.7) | 2 (5.6) |
| Asthenia | 1 (2.9) | 1 (2.9) | 0 | 2 (5.6) |
| Constipation | 0 | 0 | 2 (5.7) | 1 (2.8) |
| AEs leading to discontinuations | 2 (5.9) | 2 (5.9) | 1 (2.9) | 1 (2.8) |
| Headache | 1 (2.9) | 0 | 0 | 0 |
| Hepatitis | 1 (2.9) | 0 | 0 | 0 |
| Cerebral hemorrhage | 0 | 1 (2.9) | 0 | 0 |
| Colon cancer | 0 | 1 (2.9) | 1 (2.9) | 0 |
| Generalized edema | 0 | 0 | 0 | 1 (2.8) |
| SAEs | 1 (2.9) | 2 (5.9) | 1 (2.9) | 0 |
| Thermal burn | 1 (2.9) | 0 | 0 | 0 |
| Cerebral hemorrhage | 0 | 1 (2.9) | 0 | 0 |
| Colon cancer | 0 | 1 (2.9) | 1 (2.9) | 0 |
The proportion of AEs suspected by the investigators to be study drug-related were relatively higher in the high-dose combination group (17.6%) than in other groups (11.8% in low-dose combination, 2.9% in vildagliptin monotherapy, and 8.3% in the pioglitazone monotherapy group). However, no clustering of specific AEs assessed as drug-related was observed in any treatment group. No hypoglycemic events were reported in this population. AE-related discontinuations were infrequent in all treatment groups, with no meaningful relation to treatment or dose of combination treatment.
A total of 3 AEs were adjudicated by the adjudication committees, with one report each of stroke (low-dose combination), angioedema (vildagliptin monotherapy), and generalized edema (pioglitazone monotherapy).
The overall incidence of other clinically significant AEs was higher with high-dose combination therapy (11.7%) than with other treatments (5.6%-8.8%), primarily because of the higher incidence of mild headache (8.8%) in this group.
No deaths occurred during the study period. Overall, SAEs were observed in 4 patients during the course of the treatment, with no apparent relation to treatment or dose of combination therapy. No major changes or consistent trends were observed for any hematological, biochemical, or urinanalysis parameter or vital signs, and the frequency of treatment-emergent ECG abnormalities was low and comparable in all treatment groups.
This post hoc analysis suggests that in Korean patients with T2DM, first-line treatment with a vildagliptin/pioglitazone high-dose combination provides, as in the overall population, better glycemic control than the individual component monotherapies. The adjusted mean change in HbA1c from baseline to endpoint was comparable between the high-dose and low-dose combination groups in Korean patients, both numerically larger than that reported in the overall population[11].
In this post hoc analysis, 76% of patients achieved the recommended target HbA1c of < 7% in the high-dose combination arm, which is numerically higher than the 65% reported in the overall study population[11]. This may be attributed to pathophysiological differences between Asian and Caucasian diabetic populations. There is some evidence suggesting that small increases in insulin resistance uncover a greater degree of β-cell dysfunction in Asian populations than in non-Asians[7,12]; the therapeutic effect of vildagliptin may then be intensified in patients with greater degrees of β-cell dysfunction.
In the current study, in the sub-population of patients participating in the meal test, treatment with the combination therapy demonstrated substantial reduction from baseline to endpoint in PPG AUC0-4 h and 2-h PPG (≈ -4 mmol/L ), as compared to the reduction with pioglitazone monotherapy. The decrease in PPG levels was associated with concomitantly improved post-meal GLP-1 levels and reduced post-prandial glucagon levels. In addition there was a tendency towards improvement in markers of β-cell function (HOMA-B and insulinogenic index) and insulin resistance (HOMA-IR). All these differences and trends are consistent with previous studies with vildagliptin and its principal mechanism of action to increase the sensitivity of the α- and β-cells to glucose. In keeping with this mechanism is the finding that there was no increased risk of hypoglycemia when vildagliptin was administered in combination with pioglitazone.
Both the combination therapies were well tolerated with an overall similar incidence of AEs and SAEs across treatment groups, and with no discernible relationship with treatment and dose of combination therapy.
The main limitation of this study is that as a post hoc analysis, it was not adequately powered to provide answers to some questions. Nevertheless, even with limited number of patients per treatment group the significant difference in the treatment arms indicates the usefulness of the combination therapy in Korean patients, and clears the way for a dedicated study in this specific patient population.
In conclusion, this sub-study indicates that the overall outcomes in Korean patients with T2DM are consistent with results seen in the overall study population. Treatment with vildagliptin and pioglitazone (high-dose combination) improved glycemic control with substantial reductions in HbA1c, PPG, FPG, and large HbA1c responder rates at study endpoint compared with that of pioglitazone monotherapy. Though the results were only statistically significant with the high-dose combination, the trend towards relatively large reductions with the low-dose combination should be explored further in a study specifically designed to investigate this trend in this sub-population. Furthermore, studies comparing the effect of low-dose combination therapy in different ethnic populations are warranted.
The authors take full responsibility for the content of the paper and thank Dr. Shruti Agarwal and Dr. Ashish Agarwal (Novartis) for medical writing support, editorial assistance and collation and incorporation of comments from all authors.
Evidence suggests that patients with type 2 diabetes mellitus (T2DM) increasingly require multiple pharmacological combinations to reach treatment goals. However, clinical inertia in up-titration of treatment dose and initiation of add-on therapies may contribute to sub-optimal glycemic control rates. Initial combination therapy using two oral anti-diabetic drugs with complementary mechanisms of action is an alternative approach that may provide better or more sustained glycemic control, and allow use of lower doses of the component therapies. Diabetic patients from Korea have differences in genetic characteristics, diet habits and lifestyle, resulting in differences in the clinical profile, as compared to Caucasian patients. Only 37.3% of Korean diabetic patients achieve glycosylated hemoglobin (HbA1c) < 7% with the currently available therapies. Thus, specific studies and analyses assessing the efficacy and safety of newer therapies in Korean patients are warranted.
Vildagliptin is a potent and selective dipeptidyl peptidase-4 (DPP-4) inhibitor that improves pancreatic islet function, evidenced from improved ability of α-cells and β-cells to sense and respond to glucose after treatment. In addition, vildagliptin inhibits hepatic glucose production during meals as well as during overnight post-absorptive period. Pioglitazone is an agonist for peroxisome proliferator-activated receptors in target tissues for insulin action, which enhances tissue sensitivity to insulin. We previously reported results from a multi-center, international study which indicated that first-line treatment of diabetic patients with vildagliptin/pioglitazone combination therapy provides better glycemic control than the corresponding monotherapies and has a comparable tolerability profile. Drug combinations of vildagliptin/pioglitazone 100/30 mg q.d. (high-dose) or 50/15 mg q.d. (low-dose), or component monotherapies (vildagliptin 100 mg q.d. or pioglitazone 30 mg q.d.) were evaluated in this study. Data from an exploratory analysis of these study results in Korean patients with T2DM, to evaluate the efficacy and tolerability of vildagliptin/pioglitazone combination therapy compared with corresponding monotherapies, are presented here.
Results from this post hoc analysis suggest that in Korean patients with T2DM, first-line treatment with vildagliptin/pioglitazone high-dose combination provides, as in the overall population, better glycemic control than the individual component monotherapies. Percentage of patients achieving HbA1c < 7% and ≤ 6.5% was highest in the high-dose group (76% and 68%) followed by low-dose (58% and 47%), vildagliptin (59% and 37%), and pioglitazone (53% and 28%) groups. Combination therapy (high-dose) also provided substantial reductions in PPG, FPG, and large HbA1c responder rates at study endpoint compared with that of pioglitazone monotherapy. The combinations were well tolerated with an overall similar incidence of AEs and SAEs across treatment groups, with no added risk of hypoglycemia, and with no discernible relationship with treatment and dose of combination therapy.
Evidence suggests that patients with T2DM increasingly require multiple pharmacological combinations to reach treatment goals. An early combination, using two oral anti-diabetic drugs with complementary mechanisms of action is a rational approach that may provide better or more sustained glycemic control with better tolerability. This sub-study indicates that the overall outcomes in Korean patients with T2DM are consistent with results seen in the overall study population. Our approach of earlier aggressive treatment with vildagliptin/pioglitazone combination is a potential treatment option for effective management of diabetes in Korean patients.
Glucagon-like peptide-1 (GLP-1) has been shown to increase insulin secretion and suppress glucagon release in a glucose-dependent manner. However, active circulating GLP-1 has a half-life of approximately 1 to 2 min and is rapidly degraded and inactivated by dipeptidyl peptidase-4 (DPP-4). Inhibition of DPP-4 with vildagliptin, a selective DPP-4 inhibitor, results in enhanced active GLP-1 levels in vivo.
This manuscript presents data on the Korean subgroup of an international study investigating the effect and safety of the combination therapy of vildagliptin and pioglitazone in comparison to monotherapies. This is a well written manuscript, focusing on the subgroup of one country. The rational for this analysis is that Korean patients with type 2 diabetes have a different phenotype compared with type 2 diabetics of other nationalities, as they are typically non-obese (BMI less than 25 kg/m2). Therefore, there is a reasonable case for undertaking this sub-set analysis. Overall, the study design is sound and the question is important in the context of the growing burden of type 2 diabetes in Korea.
Peer reviewers: Harald Sourij, MD, Department of Internal Medicine, Division of Endocrionology and Nuclear Medicine, Medical University of Graz, Auenbruggerpl. 15, Graz 8036, Austria; Beverly Sara Muhlhausler, PhD, NHMRC Peter Doherty Postdoctoral Fellow, Health Sciences, School of Pharmacy and Medical Science/Sansom Institute, 283 Military Road, Semaphore, SA 5019, Australia; John Gaylord Teeter, MD, Clinical Assistant Professor of Medicine, Yale University School of Medicine, 50 Pequot Avenue, A4223, New Haven, CT 06320, United States; Mark A Sperling, MD, Children's Hospital of Pittsburgh of UPMC, One Children's Hospital Drive 4401 Penn Avenue, 3rd Floor, Pittsburgh, PA 15224, United States
S- Editor Zhang HN L- Editor Hughes D E- Editor Liu N
| 1. | Lee CM, Huxley RR, Lam TH, Martiniuk AL, Ueshema H, Pan WH, Welborn T, Woodward M. Prevalence of diabetes mellitus and population attributable fractions for coronary heart disease and stroke mortality in the WHO South-East Asia and Western Pacific regions. Asia Pac J Clin Nutr. 2007;16:187-192. |
| 2. | Noh JH, Kim SK, Cho YJ, Nam HU, Kim IJ, Jeong IK, Choi MG, Yoo HJ, Ahn YH, Bae HY. Current status of diabetes management in elderly Koreans with diabetes. Diabetes Res Clin Pract. 2007;77 Suppl 1:S71-S75. |
| 3. | Knecht LA, Gauthier SM, Castro JC, Schmidt RE, Whitaker MD, Zimmerman RS, Mishark KJ, Cook CB. Diabetes care in the hospital: is there clinical inertia? J Hosp Med. 2006;1:151-160. |
| 4. | Kang HW, Kim DJ, Lee MS, Kim KW, Lee MK. Pathophysiologic heterogeneity in the development of type 2 diabetes mellitus in Korean subjects. Diabetes Res Clin Pract. 2005;69:180-187. |
| 5. | Kim DJ, Song KE, Park JW, Cho HK, Lee KW, Huh KB. Clinical characteristics of Korean type 2 diabetic patients in 2005. Diabetes Res Clin Pract. 2007;77 Suppl 1:S252-S257. |
| 6. | Lee TH. Prevalence of obesity in Korean non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1996;32:71-80. |
| 7. | Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001;50:590-593. |
| 8. | He YL, Wang Y, Bullock JM, Deacon CF, Holst JJ, Dunning BE, Ligueros-Saylan M, Foley JE. Pharmacodynamics of vildagliptin in patients with type 2 diabetes during OGTT. J Clin Pharmacol. 2007;47:633-641. |
| 9. | Balas B, Baig MR, Watson C, Dunning BE, Ligueros-Saylan M, Wang Y, He YL, Darland C, Holst JJ, Deacon CF. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007;92:1249-1255. |
| 10. | Ravikumar B, Gerrard J, Dalla Man C, Firbank MJ, Lane A, English PT, Cobelli C, Taylor R. Pioglitazone decreases fasting and postprandial endogenous glucose production in proportion to decrease in hepatic triglyceride content. Diabetes. 2008;57:2288-2295. |
| 11. | Rosenstock J, Kim SW, Baron MA, Camisasca RP, Cressier F, Couturier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:175-185. |
| 12. | Suzuki H, Fukushima M, Usami M, Ikeda M, Taniguchi A, Nakai Y, Matsuura T, Kuroe A, Yasuda K, Kurose T. Factors responsible for development from normal glucose tolerance to isolated postchallenge hyperglycemia. Diabetes Care. 2003;26:1211-1215. |