Published online Jul 15, 2010. doi: 10.4239/wjd.v1.i3.89
Revised: June 22, 2010
Accepted: June 29, 2010
Published online: July 15, 2010
There is consistent epidemiological evidence linking low birth weight, preterm birth and adverse fetal growth to an elevated risk of the metabolic syndrome (obesity, raised blood pressure, raised serum triglycerides, lowered serum high-density lipoprotein cholesterol and impaired glucose tolerance or insulin resistance) and related disorders. This “fetal or developmental origins/programming of disease” concept is now well accepted but the “programming” mechanisms remain poorly understood. We reviewed the major evidence, implications and limitations of current hypotheses in interpreting developmental programming and discuss future research directions. Major current hypotheses to interpret developmental programming include: (1) thrifty phenotype; (2) postnatal accelerated or catch-up growth; (3) glucocorticoid effects; (4) epigenetic changes; (5) oxidative stress; (6) prenatal hypoxia; (7) placental dysfunction; and (8) reduced stem cell number. Some hypothetical mechanisms (2, 4 and 8) could be driven by other upstream “driver” mechanisms. There is a lack of animal studies addressing multiple mechanisms simultaneously and a lack of strong evidence linking clinical outcomes to biomarkers of the proposed programming mechanisms in humans. There are needs for (1) experimental studies addressing multiple hypothetical mechanisms simultaneously; and (2) prospective pregnancy cohort studies linking biomarkers of the proposed mechanisms to clinical outcomes or surrogate biomarker endpoints. A better understanding of the programming mechanisms is a prerequisite for developing early life interventions to arrest the increasing epidemic of the metabolic syndrome, type 2 diabetes and other related disorders.
- Citation: Luo ZC, Xiao L, Nuyt AM. Mechanisms of developmental programming of the metabolic syndrome and related disorders. World J Diabetes 2010; 1(3): 89-98
- URL: https://www.wjgnet.com/1948-9358/full/v1/i3/89.htm
- DOI: https://dx.doi.org/10.4239/wjd.v1.i3.89
The metabolic syndrome is commonly defined as a combination of at least three of the following five conditions: obesity, elevated blood pressure, elevated serum triglycerides, low serum high-density lipoprotein (HDL) cholesterol and impaired glucose tolerance or insulin resistance[1]. The clustering of these risk factors predisposes an individual to non-insulin-dependent (type 2) diabetes, cardiovascular morbidity and mortality. There is general consensus regarding the five components of the syndrome but definitions differ regarding cutoffs and mandatory criteria. Recently, central obesity [waist circumference > 102 cm in males or > 88 cm in females or body mass index (BMI) > 30] was proposed as a mandatory component by the International Diabetes Federation[2].
Epidemiological and experimental evidence suggest an association between an adverse prenatal environment and the risk of developing the metabolic syndrome and related disorders. This “fetal origins of disease” hypothesis was first proposed by Barker and Hales’ group to explain the associations between low birth weight (LBW < 2500 grams) and increased risk of impaired glucose tolerance and cardiovascular disease in retrospective cohort studies[3-5]. Subsequent epidemiological studies in different populations largely confirmed this “fetal origins” phenomenon[3-8]. In recent years, the term “fetal origins/programming” has been replaced by “developmental origins/programming” to accommodate the increasingly accepted concept that “programming” may continue in the early postnatal period.
A number of hypotheses have been proposed to interpret developmental programming[9-14] but none have received unanimous recognition. It is now worth reflecting on what is known about the mechanisms of developmental programming after decades of research. We critically reviewed the key evidence, implications and limitations of current hypotheses to interpret developmental programming of the metabolic syndrome and discuss the directions for future research. The evidence acquisition was based on a literature review based on a PubMed search of publications between January 1970 and February 2010.
We use the term “major hypotheses” to refer to those supported by substantial epidemiological and experimental evidence. Two competing major hypotheses have been proposed: “thrifty phenotype” and “postnatal accelerated growth”.
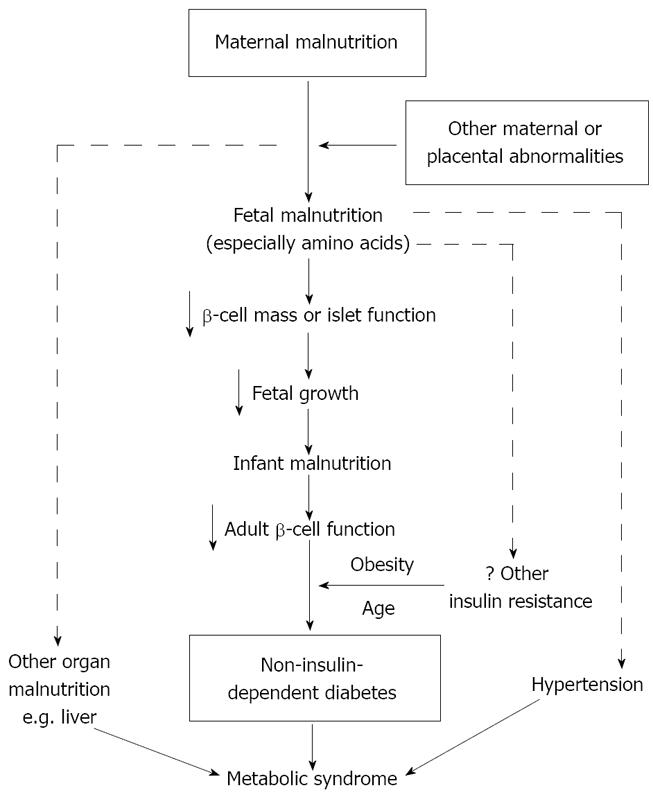
Rationale: Hales and Barker proposed the thrifty phenotype hypothesis (Figure 1)[11,12]. The hypothesis suggests that fetal and early postnatal malnutrition may induce poor development of pancreatic β-cell mass. Malnutrition may have a selective impact on the growth of different organs with protection of the most vital (e.g. the brain). Because altered growth during critical periods permanently changes the structure and functional capacity of pancreatic β-cell mass, such changes may “program” the metabolic system which increases the fetus’ chance of survival in poor nutritional environments but results in difficulty in coping with nutritional abundance later in life. The development of the metabolic syndrome following malnutrition in early life may depend on the superimposition of other postnatal risk factors, notably physical inactivity and obesity.
Epidemiological evidence: Barker and colleagues observed in 1986 that the geographical distribution of heart disease in the United Kingdom was closely related to a person’s place of birth[15], suggesting that early life events could cause permanent changes in physiology predisposing to chronic heart disease. LBW has been strongly linked with impaired glucose tolerance and type 2 diabetes in adulthood[3-5,16-18]. Reduced fetal growth was related to increased plasma concentrations of 32-33 split proinsulin, a sign of beta-cell dysfunction[19-21], and was linked to high blood pressure[22,23]. Children small in birth size may predispose to metabolic abnormalities upon exposure to postnatal environmental risk factors such as low physical activity and/or high-energy intake[24]. Prenatal exposure to famine during the Dutch Hunger Winter of 1944-1945 was associated with impaired glucose tolerance and insulin secretion in adulthood[25,26]. The Pune Maternal Nutrition Study correlated prenatal specific micronutrient (vitamin B12) deficiency with increased insulin resistance in childhood[27]. Changes resulting from fetal and early postnatal malnutrition include: (1) metabolic adaptations in hepatic enzymes[28], lipoprotein profiles[29] and clotting factors[30]; (2) anatomical adaptations that affect end-organ glucose uptake[31] and renal solute metabolism[32]; and (3) endocrine adaptations that affect the hypothalamic-pituitary-adrenal (HPA) axis[33], insulin signaling[34] and leptin levels[35]. These changes could collectively lead to the metabolic syndrome and related disorders[36].
Experimental evidence: In animal models, fetal malnutrition has been associated with marked structural and physiological alterations[37-39]. Gestational calorie restriction and protein deprivation in rats led to hypertension in adult offspring[40-42] and to altered glucose metabolism in sheep[43,44]. Malnutrition-associated changes in fetal leptin levels may alter the programming of appetite and eating behaviors leading to an increased risk of cardiovascular and metabolic diseases[45-48]. The pattern of dietary response of inbred mouse strains was similar to that expected under the thrifty phenotype hypothesis[49]. Permanent reductions in pancreatic cells and insulin secretion have been observed in protein-malnourished fetuses[50].
Implications and limitations: The thrifty phenotype is the most widely accepted hypothesis to interpret developmental programming. The hypothesis emphasizes the etiological role of poor fetal and early postnatal nutrition and implicitly advocates promoting fetal and infant nutrition and growth[51]. It may well explain the increasing prevalence of obesity and type 2 diabetes in India and South Asian countries where malnutrition was previously common but has become less so in recent decades[52]. However, in virtually all human studies, maternal and fetal nutritional status, as determined either directly by specific nutrient biomarkers or indirectly by weight gain during pregnancy, were not available for linkage to clinical outcomes. The hypothesis does not match the trends of increasing birth weights and declining LBW rates in many countries in recent decades[53-55], raising concerns as to whether poor fetal nutrition or “thrifty phenotype” is a major driver of developmental programming. Furthermore, poor fetal growth is a mere proxy for various perinatal insults. It is plausible that adverse insults may drive both poor fetal growth and developmental programming.
Rationale: The “postnatal accelerated growth” hypothesis was proposed by Drs. Singhal and Lucas to explain the association between faster early postnatal growth and surrogate endpoints in childhood and adolescence indicative of metabolic and cardiovascular risks based on follow-ups of preterm infants in two early neonatal feeding/nutritional intervention trials[13,14]. Increased infant growth rate by a nutrient enriched diet, even for only a few weeks postnatally, was associated with long-term adverse metabolic effects[6-8,56]. Fasting concentrations of 32-33 split proinsulin (a marker of insulin resistance) in adolescents born preterm were significantly elevated among those who had received a nutrient-enriched diet in early postnatal life vs. placebo[7]. The authors concluded that early postnatal accelerated growth rather than prematurity per se may be the culprit in programming insulin resistance and related disorders[14].
Cianfarani proposed a similar “catch-up growth” hypothesis[9]. At birth, infants with intrauterine growth retardation (IUGR) have low concentrations of insulin, insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein (IGFBP)-3; and high concentrations of growth hormone, IGFBP-1 and IGFBP-2. Normalization of these hormones occurs during the first trimester of postnatal life[57,58]. During this early postnatal catch-up growth period when suddenly exposed to increased concentrations of insulin and IGF-1, tissues chronically depleted of these two hormones during fetal life may counteract the hike by developing insulin resistance as a metabolic defense against developing hypoglycemia[9]. Therefore, IUGR infants who show early and complete growth recovery could be at higher risk for the occurrence of the metabolic syndrome in adulthood.
Dr. Gluckman proposed another similar hypothesis, the “predictive adaptive response”[10]. Based on the “predicted” postnatal environment, the fetus would make adaptations in utero or in the early postnatal period[10]. IUGR fetuses would thus predict a poor postnatal nutritional environment. When mismatch occurred between predicted and actual, disease would manifest[10,59].
Epidemiologic evidence: Early childhood growth acceleration has been associated with later insulin resistance[60], obesity[61] and cardiovascular disease[8]. In low birth weight infants, early growth acceleration has been associated with metabolic disturbances including dyslipidemia and elevated concentrations of insulin and IGF-1[9,62,63]. IUGR infants often experience compensatory accelerated growth after a period of poor fetal nutrition followed by the removal of such nutritional deficiency postnatally[64]. The most rapid growth occurs in early infancy[65] in the first few weeks after birth[66,67]. Factors promoting neonatal growth such as enhanced neonatal nutrition could permanently affect or program long-term health[68]. Such accelerated growth may have adverse consequences later in life[64], increase the propensity to cardiovascular disease[69] and its risk factors such as insulin resistance[70], obesity[65] and higher blood pressure[71]. Patients with impaired glucose tolerance or diabetes typically had a low BMI in infancy, an early adiposity rebound in childhood and an accelerated increase in BMI until adulthood[72].
Experimental evidence: In rats, accelerated early postnatal growth impaired glucose tolerance and shortened the lifespan[73]. Small neonatal rats temporarily overfed during the brief suckling period had permanent elevations in plasma insulin and cholesterol levels in adulthood[74]. Postnatal accelerated growth can adversely affect glucose tolerance in rats[75]. Even in rats without IUGR, overfeeding during the brief suckling period permanently increased later plasma insulin and cholesterol concentrations[76,77] with the propensity to obesity, high blood pressure and diabetes[53,77].
Implications and limitations: An important implication of the “postnatal accelerated growth” or “catch-up growth” hypothesis is that it questions the current practice of promoting postnatal catch-up growth of small babies[14]. Enhancing infant growth rate by a nutrient-enriched diet may actually do more harm than good in the long run. However, this hypothesis has been well tested only in preterm infants in Dr. Lucas’s studies. It remains unclear whether the findings hold for catch-up growth in IUGR infants born at term. The increasing birth weights in most countries in recent decades[54,78-80] indicate that there are unlikely substantial increases in the incidence of postnatal catch-up growth. Consequently, it appears difficult to explain the substantial rise in the incidence of the metabolic syndrome. Also, the hypothesis does not match the epidemiological evidence of an elevated risk of the metabolic syndrome among macrosomic infants who often show catch-down rather than catch-up growth during the early postnatal period.
We use the term “minor hypotheses” to refer to those supported by insufficient research data, especially in humans.
A product of the activation of the HPA axis, glucocorticoids have potent effects on tissue development especially the maturation of organs such as the lung[81]. One outcome of fetal malnutrition is the exposure of the fetus to excess glucocorticoids which may restrict fetal growth and program the cardiovascular, endocrine and metabolic systems[82]. Normally, fetal glucocorticoid levels are much lower than maternal levels due to the placental barrier[83,84] - the placental enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) catalyzes glucocorticoids (cortisol and corticosterone) into inert forms (cortisone, 11-dehydrocorticosterone)[80,85]. However, synthetic glucocorticoids (betamethasone, dexamethasone) commonly administered to pregnant women at risk of preterm delivery to reduce neonatal pulmonary, renal and cerebral morbidities[86], are poor substrates of 11β-HSD2. Prenatal glucocorticoid overexposure through external sources or inhibition of placental 11β-HSD-2 may induce fetal HPA axis dysfunction - a potential link between adverse fetal environment and insulin resistance and hypertension in adulthood[87].
There is strong evidence of glucocorticoid programming in animal models. Many studies have reported decreased birth weights and abnormal levels of plasma HPA-axis hormones in rats prenatally exposed to synthetic glucocorticoids or inhibition of 11β-HSD2 with increased blood pressures and glucose intolerance in adulthood[87-89]. Hypertension in rats whose mothers were fed a low-protein diet during pregnancy was preventable by chemical blockade of maternal glucocorticoid synthesis[90], suggesting that the link between maternal protein deprivation and adult-onset hypertension may be mediated by maternal glucocorticoids. However, there is weak and inconsistent evidence regarding antenatal exposure to synthetic glucocorticoids and components of the metabolic syndrome in humans. Studies have reported no change, slight increase or decrease in blood pressure[91-94]. A Cochrane meta-analysis showed no differences in adult blood pressure[95]. In contrast, a recent follow-up study of 534 adults whose mothers had participated in a randomized controlled trial reported increased insulin resistance associated with antenatal betamethasone treatment[93]. LBW adults had much higher urinary glucocorticoid[96] and plasma cortisol concentrations[97] and showed greater responsiveness to adrenocorticotropic hormone[98,99]. Prenatal glucocorticoids may be the link between LBW and increased risk of glucose intolerance and hypertension[87]. Elevated blood pressure after antenatal exposure to glucocorticoids may result from altered renal renin-angiotensin system development[100] or from epigenetic changes affecting the expression of specific transcription factors, especially the glucocorticoid receptor[87].
However, there is a lack of strong evidence of glucocorticoid programming in humans. It remains unknown whether glucocorticoids drive both IUGR and the programming of metabolic syndrome components as observed in animal models.
Experimentally, transmission to the next generation of a “programmed” phenotype has been demonstrated for birth weight, metabolic dysfunction[101-103], blood pressure and vascular dysfunction[104]. Wild type mice born to hypertensive heterozygous nitric oxide synthase-3 knockout mice displayed hypertension and vascular dysfunction[104]. Such transmission can be attributed to the fact that the programmed mother provided a deprived intrauterine environment, thus perpetuating the cycle of fetal (mal) adaptations. An alternate possibility is that epigenetic modification of the germline by stable DNA methylation or histone acetylation transmitted the prenatal experience of one generation to future generations.
Many candidate and confirmed players able to induce developmental programming can modify gene methylation. For example, Rees showed hypermethylation in the fetal liver of low protein fed dams[105]. Peroxisomal proliferator-activated receptor (PPAR) alpha and glucocorticoid receptor genes were hypomethylated and their expression increased in the liver of the offspring of protein-restricted rats[106]. Reactive oxygen species can modify methylation leading to changes in gene transcription and expression[107]. However, there are relatively little data demonstrating epigenetic changes after adverse perinatal conditions in genes closely implicated in cardiovascular and metabolic disorders. Bogdarina reported modifications in the methylation status of the angiotensin II AT1b receptor gene in the adrenal gland of the offspring of low-protein fed dams[108]. Neonatal overfeeding in rats led to permanent dysregulation of the central circuitry of food intake inhibition with resistance to signals triggered by insulin and leptin[109-111]. Circulating leptin and insulin stimulate the expression of the main anorexigenic neurohormone - proopiomelanocortin (POMC) while inhibiting the orexigenic neuropeptide Y[112,113]. Plagemann recently demonstrated hypermethylation of the hypothalamic POMC gene promoter region in neonatal overfeeding animals within two specific protein-1 (Sp-1) binding sequences. This led to blunted POMC expression despite hyperleptinemia and hyperinsulinemia and demonstrated that a nutritionally acquired alteration of the methylation pattern could modify the set point of a gene promoter critical for body weight regulation[114].
The availability of methyl donor micronutrients may affect epigenetic programming. Dietary protein res-triction in pregnancy induced and folic acid supplementation prevented epigenetic modifications of he-patic glucocorticoid receptor gene expression in rat offspring[106]. Folate supplementation of low-protein fed dams prevented elevation of blood pressure in adult offspring[115]. Restricting the supply of vitamin B12, folate and methionine even within normal physiological ranges during the periconceptional period was associated with widespread epigenetic alternations, insulin resistance and elevated blood pressure in sheep[116]. In contrast, maternal high folate status in pregnancy was associated with increased insulin resistance in children[27], indicating the need for caution in applying results from animal models to humans. Dietary methyl supplementation with folic acid and B12 may have unintended deleterious consequences on epigenetic regulation[117]. More human data are needed in this nascent research area.
It should be pointed out that epigenetic programming is a physiological process in normal fetal development; the epigenetic changes dictate cell differentiation. The question is, are some epigenetic changes “pathological” secondary to certain perinatal insults? There is a lack of human data linking perinatal “programming” insults to developmental epigenetic changes and later risk of the metabolic syndrome and related disorders. Improved understanding of epigenetic changes may be helpful in designing interventions to prevent or possibly reverse adverse programming.
Because many known or suspected causes of or conditions associated with adverse fetal growth or preterm birth have been associated with oxidative stress, it is plausible that oxidative stress may be the underlying common link to elevated risks of the metabolic syndrome[118]. Oxidative stress programming may act directly through modulation of gene expression (perhaps epigenetic) or indirectly via the effects of certain oxidized molecules. Experimental investigations have well demonstrated the role of redox balance in modulating gene expression[119,120]. There is considerable experimental evidence indicating that both the insulin function axis and blood pressure regulation could be sensitive targets to oxidative stress programming during the prenatal and early postnatal periods[121-126]. However, there remains a lack of epidemiological data relating biomarkers of perinatal oxidative stress to the metabolic syndrome. Validation of the oxidative stress hypothesis would suggest new early interventions to stem the modern epidemic of the metabolic syndrome.
There is some evidence linking prenatal hypoxia to increased vulnerability to metabolic and cardiovascular diseases[127]. Chronic hypoxia is a common insult to the fetus and reduced uteroplacental blood flow can result in fetal IUGR independently of malnutrition[128-130]. Chronic prenatal hypoxia has been shown to increase the susceptibility of the adult heart to ischemia-reperfusion injury[131]. Human studies at high altitude also suggest that prenatal hypoxia can result in LBW[132-134]. Chronic hypoxia has profoundly adverse effects on cardiac development and function in the fetus[129] and enhanced β1-adrenergic receptor signaling may induce cardiomyocyte apoptosis via a protein kinase A-dependent mechanism[132]. Animal studies show that chronic hypoxia could increase the heart-to-body weight ratio in the fetus, suggesting an asymmetric growth of the heart[133]. Chronic hypoxia significantly increased the levels of cytochrome C, a mitochondrial marker protein, in the fetal heart[134]. The increased cytochrome C levels are likely a metabolic adaptation in the myocardium during asymmetric enlargement of the heart in hypoxic fetuses. Animal experiments showed that mitochondrial biogenesis played an important role in the early stages of cardiac hypertrophy[128]. Hypoxia could induce apoptosis in cultured neonatal rat cardiomyocytes[135]. In response to chronic hypoxia during gestation, many genes related to cell signaling and survival are down- or up-regulated in the fetal heart and other tissues[127]. No epidemiological data are available as to whether these effects are transient or permanent.
It has been proposed that adult cardiovascular and metabolic diseases originate via developmental plasticity and adaptations arising from failure of the maternal-placental nutrient supply to match fetal requirements[136]. This hypothesis emphasizes the role of the placenta in fetal programming. Maternal nutrition was associated with fetal development and programming of human cardiovascular and metabolic disease[137]. Maternal body composition and nutrition intake may affect fetal development by direct effects on substrate availability to the fetus and indirect effects via changes in placental function and structure[136]. Fetal adaptations may result from alterations in placental growth and vascular resistance, altered nutrient and hormone metabolism in the placenta and changes in nutrient transfer and partitioning between mother, placenta and fetus[138-140]. Fetal cardiovascular adaptations, alterations in fetal body composition and changes in fetal endocrinology and metabolism may have long-term effects on postnatal health. However, no data are available on the epidemiological associations between pathological placental changes and the metabolic syndrome in the offspring. The hypothesis implies that improving placental function may have lifelong health benefits.
After organogenesis, the ability of cells to divide substantially for self-renewal and repair is limited although some stem cells remain in various tissues in postnatal life. The stem cell hypothesis suggests that IUGR is associated with a reduced number of tissue stem cells, leading to an early exhaustion of organ function when demands are increased greatly[141]. The time windows for stem cell proliferation may represent critical periods. Diabetes patients often have fewer β-cells prior to the onset of disease or the pancreas failed to generate more β-cells in response to an increased demand for insulin[142]. In rats, fetal and neonatal nutritional deprivation caused permanent reductions in β-cell mass and functional efficiency, resulting in glucose intolerance in adulthood[143]. IUGR induced by bilateral uterine artery ligation in pregnant rats caused postnatal glucose intolerance and insulin resistance in offspring; the β-cells mass of IUGR rats was reduced by one-third[144]. There is a lack of data on stem cell number as it relates to metabolic syndrome programming in humans.
Although most research is focused on adverse programming associated with poor fetal growth, there is evidence that maternal overnutrition or fetal overgrowth may result in an offspring phenotype susceptible to the metabolic syndrome[145]. Maternal high fat or cholesterol overfeeding during pregnancy and lactation in rodents resulted in a phenotype of the offspring that closely resembled the human metabolic syndrome[145,146]. Gestational diabetes is associated with glucose oversupply to the fetus and consequently fetal macrosomia and may have adverse metabolic programming effects[147]. The effects of gestational diabetes seem independent of genetic factors[148]. Experimental evidence indicates that overnutrition may program obesity and metabolic syndrome through epigenetic changes[114]. A meta-analysis showed a U-shaped relationship between birth weight and type 2 diabetes risk in humans[149]. The hypothesis is concordant with the increasing birth weights over recent decades and may partly explain the increasing prevalence of the metabolic syndrome. It is unclear whether the effects of fetal overgrowth programming could be largely explained by impaired maternal glucose tolerance in humans.
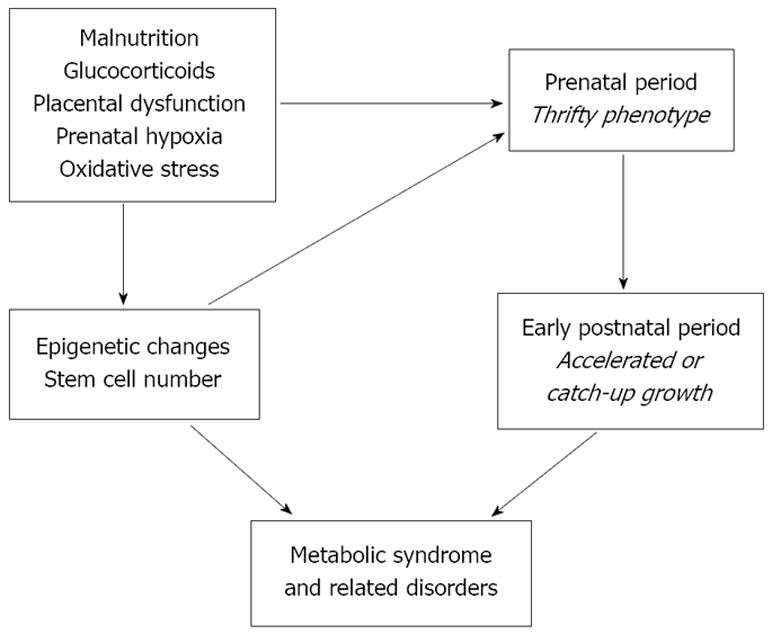
The various hypotheses for interpreting developmental programming could be interrelated, indicating the need for research to address multiple mechanisms simultaneously. Some mechanisms could be driven by other “driver” mechanisms (Figure 2). Multiple overarching drivers may exist: malnutrition, glucocorticoids, oxidative stress, prenatal hypoxia and placental dysfunction. These drivers may act alone or in combinations to induce epigenetic changes or reduce stem cell number, leading to the thrifty phenotype often followed by catch-up growth and the propensity to metabolic syndrome. Thus, adverse programming may occur in the absence of poor fetal growth.
The current prevailing theory is that fetal programming effects are magnified over the life course. However, the postnatal environment may either mask or magnify the true effects of programming - the direction of effect modifications is unknown. The strongest evidence supporting the various hypothetical mechanisms comes from animal models. However, we cannot assume that findings from animal models are applicable to humans as human pregnancy physiology is much more complex. For example, preeclampsia (gestational hypertension with proteinuria) is a gestational complication unique to humans[150]. Even the commonly used operating definitions for retarded or excessive fetal growth in humans are largely arbitrary and need re-evaluations[151]. There is a need for studies to address multiple mechanisms simultaneously in animal models and a need for prospective pregnancy cohort data linking intrauterine environmental biomarkers of the proposed programming mechanisms to clinical outcomes or surrogate biomarker endpoints in humans. A better understanding of the programming mechanisms is a prerequisite for developing early life interventions to halt the worldwide increasing epidemic of the metabolic syndrome, type 2 diabetes and other related disorders.
Peer reviewer: Christa Buechler, PhD, Department of Internal Medicine I, Regensburg University Hospital, Franz Josef Strauss Allee 11, Regensburg 93042, Germany
| 1. | Day C. Metabolic syndrome, or What you will: definitions and epidemiology. Diab Vasc Dis Res. 2007;4:32-38. |
| 2. | Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295-300. |
| 3. | Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62-67. |
| 5. | Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019-1022. |
| 6. | Singhal A, Cole TJ, Lucas A. Early nutrition in preterm infants and later blood pressure: two cohorts after randomised trials. Lancet. 2001;357:413-419. |
| 7. | Singhal A, Fewtrell M, Cole TJ, Lucas A. Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet. 2003;361:1089-1097. |
| 8. | Singhal A, Cole TJ, Fewtrell M, Lucas A. Breastmilk feeding and lipoprotein profile in adolescents born preterm: follow-up of a prospective randomised study. Lancet. 2004;363:1571-1578. |
| 9. | Cianfarani S, Germani D, Branca F. Low birthweight and adult insulin resistance: the “catch-up growth” hypothesis. Arch Dis Child Fetal Neonatal Ed. 1999;81:F71-F73. |
| 10. | Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733-1736. |
| 11. | Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595-601. |
| 12. | Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5-20. |
| 14. | Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet. 2004;363:1642-1645. |
| 15. | Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077-1081. |
| 16. | Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94:3246-3250. |
| 17. | Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50-60 years. BMJ. 1996;312:406-410. |
| 18. | Valdez R, Athens MA, Thompson GH, Bradshaw BS, Stern MP. Birthweight and adult health outcomes in a biethnic population in the USA. Diabetologia. 1994;37:624-631. |
| 19. | Cook JT, Levy JC, Page RC, Shaw JA, Hattersley AT, Turner RC. Association of low birth weight with beta cell function in the adult first degree relatives of non-insulin dependent diabetic subjects. BMJ. 1993;306:302-306. |
| 20. | Khan N, Couper JJ. Low-birth-weight infants show earlier onset of IDDM. Diabetes Care. 1994;17:653-656. |
| 21. | Persson B, Pschera H, Binder C, Efendic S, Hanson U, Hartling S, Lunell NO. Decreased beta-cell function in women with previous small for gestational age infants. Horm Metab Res. 1993;25:170-174. |
| 22. | Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815-831. |
| 23. | Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935-941. |
| 24. | Tappy L. Adiposity in children born small for gestational age. Int J Obes. 2006;30:S36-S40. |
| 25. | Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82:485-491. |
| 26. | de Rooij SR, Painter RC, Phillips DI, Osmond C, Michels RP, Godsland IF, Bossuyt PM, Bleker OP, Roseboom TJ. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care. 2006;29:1897-1901. |
| 27. | Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, Bhat DS, Naik SS, Coyaji KJ, Joglekar CV. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29-38. |
| 28. | Desai M, Crowther NJ, Ozanne SE, Lucas A, Hales CN. Adult glucose and lipid metabolism may be programmed during fetal life. Biochem Soc Trans. 1995;23:331-335. |
| 29. | Laurén L, Järvelin MR, Elliott P, Sovio U, Spellman A, McCarthy M, Emmett P, Rogers I, Hartikainen AL, Pouta A. Relationship between birthweight and blood lipid concentrations in later life: evidence from the existing literature. Int J Epidemiol. 2003;32:862-876. |
| 30. | Martyn CN, Meade TW, Stirling Y, Barker DJ. Plasma concentrations of fibrinogen and factor VII in adult life and their relation to intra-uterine growth. Br J Haematol. 1995;89:142-146. |
| 31. | McKeigue PM, Lithell HO, Leon DA. Glucose tolerance and resistance to insulin-stimulated glucose uptake in men aged 70 years in relation to size at birth. Diabetologia. 1998;41:1133-1138. |
| 32. | Woods LL. Fetal origins of adult hypertension: a renal mechanism? Curr Opin Nephrol Hypertens. 2000;9:419-425. |
| 33. | Seckl JR, Nyirenda MJ, Walker BR, Chapman KE. Glucocorticoids and fetal programming. Biochem Soc Trans. 1999;27:74-78. |
| 34. | Ozanne SE, Nave BT, Wang CL, Shepherd PR, Prins J, Smith GD. Poor fetal nutrition causes long-term changes in expression of insulin signaling components in adipocytes. Am J Physiol. 1997;273:E46-E51. |
| 35. | Phillips DI, Fall CH, Cooper C, Norman RJ, Robinson JS, Owens PC. Size at birth and plasma leptin concentrations in adult life. Int J Obes Relat Metab Disord. 1999;23:1025-1029. |
| 36. | Barker DJP. Mothers, babies and diseases in later life. London: BMJ Publishing Group 1994; . |
| 37. | Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27:1200-1204. |
| 38. | Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339-341. |
| 39. | Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes. 1991;40 Suppl 2:115-120. |
| 40. | Holemans K, Gerber R, Meurrens K, De Clerck F, Poston L, Van Assche FA. Maternal food restriction in the second half of pregnancy affects vascular function but not blood pressure of rat female offspring. Br J Nutr. 1999;81:73-79. |
| 41. | Woodall SM, Johnston BM, Breier BH, Gluckman PD. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res. 1996;40:438-443. |
| 42. | Langley-Evans SC. Critical differences between two low protein diet protocols in the programming of hypertension in the rat. Int J Food Sci Nutr. 2000;51:11-17. |
| 43. | Bloomfield FH, Oliver MH, Giannoulias CD, Gluckman PD, Harding JE, Challis JR. Brief undernutrition in late-gestation sheep programs the hypothalamic-pituitary-adrenal axis in adult offspring. Endocrinology. 2003;144:2933-2940. |
| 44. | Oliver MH, Breier BH, Gluckman PD, Harding JE. Birth weight rather than maternal nutrition influences glucose tolerance, blood pressure, and IGF-I levels in sheep. Pediatr Res. 2002;52:516-524. |
| 45. | Christou H, Serdy S, Mantzoros CS. Leptin in relation to growth and developmental processes in the fetus. Semin Reprod Med. 2002;20:123-130. |
| 46. | El-Haddad MA, Desai M, Gayle D, Ross MG. In utero development of fetal thirst and appetite: potential for programming. J Soc Gynecol Investig. 2004;11:123-130. |
| 47. | Roberts TJ, Nijland MJ, Caston-Balderrama A, Ross MG. Central leptin stimulates ingestive behavior and urine flow in the near term ovine fetus. Horm Metab Res. 2001;33:144-150. |
| 48. | McMillen IC, Muhlhausler BS, Duffield JA, Yuen BS. Prenatal programming of postnatal obesity: fetal nutrition and the regulation of leptin synthesis and secretion before birth. Proc Nutr Soc. 2004;63:405-412. |
| 49. | Cheverud JM, Pletscher LS, Vaughn TT, Marshall B. Differential response to dietary fat in large (LG/J) and small (SM/J) inbred mouse strains. Physiol Genomics. 1999;1:33-39. |
| 50. | Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate. 1990;57:107-118. |
| 51. | Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull. 2001;60:103-121. |
| 52. | Yajnik CS. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J Nutr. 2004;134:205-210. |
| 53. | Ananth CV, Wen SW. Trends in fetal growth among singleton gestations in the United States and Canada, 1985 through 1998. Semin Perinatol. 2002;26:260-267. |
| 54. | Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;104:720-726. |
| 55. | Antonisamy B, Sivaram M, Richard J, Rao PS. Trends in intra-uterine growth of single live births in southern India. J Trop Pediatr. 1996;42:339-341. |
| 56. | Singhal A, Farooqi IS, O’Rahilly S, Cole TJ, Fewtrell M, Lucas A. Early nutrition and leptin concentrations in later life. Am J Clin Nutr. 2002;75:993-999. |
| 57. | Cianfarani S, Germani D, Rossi P, Rossi L, Germani A, Ossicini C, Zuppa A, Argirò G, Holly JM, Branca F. Intrauterine growth retardation: evidence for the activation of the insulin-like growth factor (IGF)-related growth-promoting machinery and the presence of a cation-independent IGF binding protein-3 proteolytic activity by two months of life. Pediatr Res. 1998;44:374-380. |
| 58. | Leger J, Noel M, Limal JM, Czernichow P. Growth factors and intrauterine growth retardation. II. Serum growth hormone, insulin-like growth factor (IGF) I, and IGF-binding protein 3 levels in children with intrauterine growth retardation compared with normal control subjects: prospective study from birth to two years of age. Study Group of IUGR. Pediatr Res. 1996;40:101-107. |
| 59. | Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183-187. |
| 60. | Forsén T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med. 2000;133:176-182. |
| 61. | Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967-971. |
| 62. | Colle E, Schiff D, Andrew G, Bauer CB, Fitzhardinge P. Insulin responses during catch-up growth of infants who were small for gestational age. Pediatrics. 1976;57:363-371. |
| 63. | Finken MJJ, Keijzer-Veen MG, Van Montfoort AG. Early catch up growth in weight of very preterm low birth weight infants is associated with high level LDL-cholesterol and apo-B at age 19. Pediatr Res. 2003;53:32A. |
| 64. | Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol Evol. 2001;16:254-260. |
| 65. | Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. 1995;38:733-739. |
| 66. | Eriksson JG, Forsén T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427-431. |
| 67. | Fewtrell MS, Doherty C, Cole TJ, Stafford M, Hales CN, Lucas A. Effects of size at birth, gestational age and early growth in preterm infants on glucose and insulin concentrations at 9-12 years. Diabetologia. 2000;43:714-717. |
| 68. | Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ. 2001;323:1331-1335. |
| 69. | Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088-1092. |
| 70. | Hokken-Koelega AC, De Ridder MA, Lemmen RJ, Den Hartog H, De Muinck Keizer-Schrama SM, Drop SL. Children born small for gestational age: do they catch up? Pediatr Res. 1995;38:267-271. |
| 71. | Lucas A. Programming by early nutrition in men. The Childhood Environment and Adult Disease. CIBA Foundation Symposium 156 Chichester: Whiley, UK 1991; 55-83. |
| 72. | Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, Biswas SK, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865-875. |
| 73. | Hales CN, Desai M, Ozanne SE, Crowther NJ. Fishing in the stream of diabetes: from measuring insulin to the control of fetal organogenesis. Biochem Soc Trans. 1996;24:341-350. |
| 74. | Williams JP, Tanner JM, Hughes PC. Catch-up growth in male rats after growth retardation during the suckling period. Pediatr Res. 1974;8:149-156. |
| 75. | Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dörner G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999;836:146-155. |
| 76. | Plagemann A, Heidrich I, Gotz F, Rohde W, Dorner G. Obesity and enhanced diabetes and cardiovascular risk in adult rats due to early postnatal overfeeding. Exp Clin Endocrinol. 1992;99:154-158. |
| 77. | Hahn P. Effect of litter size on plasma cholesterol and insulin and some liver and adipose tissue enzymes in adult rodents. J Nutr. 1984;114:1231-1244. |
| 78. | Morken NH, Källen K, Hagberg H, Jacobsson B. Preterm birth in Sweden 1973-2001: rate, subgroups, and effect of changing patterns in multiple births, maternal age, and smoking. Acta Obstet Gynecol Scand. 2005;84:558-565. |
| 79. | Branum AM, Schoendorf KC. Changing patterns of low birthweight and preterm birth in the United States, 1981-98. Paediatr Perinat Epidemiol. 2002;16:8-15. |
| 80. | Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993;341:355-357. |
| 81. | Ward RM. Pharmacologic enhancement of fetal lung maturation. Clin Perinatol. 1994;21:523-542. |
| 82. | Bertram CE, Hanson MA. Prenatal programming of postnatal endocrine responses by glucocorticoids. Reproduction. 2002;124:459-467. |
| 83. | Campbell AL, Murphy BE. The maternal-fetal cortisol gradient during pregnancy and at delivery. J Clin Endocrinol Metab. 1977;45:435-440. |
| 84. | Murphy BE, Clark SJ, Donald IR, Pinsky M, Vedady D. Conversion of maternal cortisol to cortisone during placental transfer to the human fetus. Am J Obstet Gynecol. 1974;118:538-541. |
| 85. | Bernal AL, Flint AP, Anderson AB, Turnbull AC. 11 beta-Hydroxyteroid dehydrogenase activity (E.C. 1.1.1.146) in human placenta and decidua. J Steroid Biochem. 1980;13:1081-1087. |
| 86. | Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev. 2000;CD000065. |
| 87. | Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63-84. |
| 88. | Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR. Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia. 1996;39:1299-1305. |
| 89. | Langley-Evans SC. Intrauterine programming of hypertension by glucocorticoids. Life Sci. 1997;60:1213-1221. |
| 90. | Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens. 1997;15:537-544. |
| 91. | Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond). 2000;98:137-142. |
| 92. | Aghajafari F, Murphy K, Willan A, Ohlsson A, Amankwah K, Matthews S, Hannah M. Multiple courses of antenatal corticosteroids: a systematic review and meta-analysis. Am J Obstet Gynecol. 2001;185:1073-1080. |
| 93. | Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, Harding JE. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365:1856-1862. |
| 94. | Dessens AB, Haas HS, Koppe JG. Twenty-year follow-up of antenatal corticosteroid treatment. Pediatrics. 2000;105:E77. |
| 95. | Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. |
| 96. | Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, Barker DJ, Whorwood CB. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension. 2000;35:1301-1306. |
| 97. | Levitt NS, Lambert EV, Woods D, Hales CN, Andrew R, Seckl JR. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young south african adults: early programming of cortisol axis. J Clin Endocrinol Metab. 2000;85:4611-4618. |
| 98. | Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB, Phillips DI. Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metab. 2001;86:245-250. |
| 99. | Gardner DS, Jackson AA, Langley-Evans SC. Maintenance of maternal diet-induced hypertension in the rat is dependent on glucocorticoids. Hypertension. 1997;30:1525-1530. |
| 100. | Wyrwoll CS, Mark PJ, Waddell BJ. Developmental programming of renal glucocorticoid sensitivity and the renin-angiotensin system. Hypertension. 2007;50:579-584. |
| 101. | Zambrano E, Martínez-Samayoa PM, Bautista CJ, Deás M, Guillén L, Rodríguez-González GL, Guzmán C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225-236. |
| 102. | Nijland MJ, Ford SP, Nathanielsz PW. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20:132-138. |
| 103. | Pinheiro AR, Salvucci ID, Aguila MB, Mandarim-de-Lacerda CA. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin Sci (Lond). 2008;114:381-392. |
| 104. | Costantine MM, Ghulmiyyah LM, Tamayo E, Hankins GD, Saade GR, Longo M. Transgenerational effect of fetal programming on vascular phenotype and reactivity in endothelial nitric oxide synthase knockout mouse model. Am J Obstet Gynecol. 2008;199:250.e1-e7. |
| 105. | Rees WD, Hay SM, Brown DS, Antipatis C, Palmer RM. Maternal protein deficiency causes hypermethylation of DNA in the livers of rat fetuses. J Nutr. 2000;130:1821-1826. |
| 106. | Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382-1386. |
| 107. | Cerda S, Weitzman SA. Influence of oxygen radical injury on DNA methylation. Mutat Res. 1997;386:141-152. |
| 108. | Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100:520-526. |
| 109. | Davidowa H, Plagemann A. Insulin resistance of hypothalamic arcuate neurons in neonatally overfed rats. Neuroreport. 2007;18:521-524. |
| 110. | Davidowa H, Plagemann A. Decreased inhibition by leptin of hypothalamic arcuate neurons in neonatally overfed young rats. Neuroreport. 2000;11:2795-2798. |
| 111. | Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dörner G. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol. 1999;11:541-546. |
| 112. | Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661-671. |
| 113. | Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571-578. |
| 114. | Plagemann A, Harder T, Brunn M, Harder A, Roepke K, Wittrock-Staar M, Ziska T, Schellong K, Rodekamp E, Melchior K. Hypothalamic proopio–melanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol. 2009;587:4963-4976. |
| 115. | Torrens C, Brawley L, Anthony FW, Dance CS, Dunn R, Jackson AA, Poston L, Hanson MA. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension. 2006;47:982-987. |
| 116. | Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, Maloney CA. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA. 2007;104:19351-19356. |
| 117. | Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293-5300. |
| 118. | Luo ZC, Fraser WD, Julien P, Deal CL, Audibert F, Smith GN, Xiong X, Walker M. Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med Hypotheses. 2006;66:38-44. |
| 119. | Turpaev KT. Reactive oxygen species and regulation of gene expression. Biochemistry (Mosc). 2002;67:281-292. |
| 120. | Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001;29:345-350. |
| 121. | Linning KD, Tai MH, Madhukar BV, Chang CC, Reed DN Jr, Ferber S, Trosko JE, Olson LK. Redox-mediated enrichment of self-renewing adult human pancreatic cells that possess endocrine differentiation potential. Pancreas. 2004;29:e64-e76. |
| 122. | Wang X, Li H, De Leo D, Guo W, Koshkin V, Fantus IG, Giacca A, Chan CB, Der S, Wheeler MB. Gene and protein kinase expression profiling of reactive oxygen species-associated lipotoxicity in the pancreatic beta-cell line MIN6. Diabetes. 2004;53:129-140. |
| 123. | Franco MC, Dantas AP, Akamine EH, Kawamoto EM, Fortes ZB, Scavone C, Tostes RC, Carvalho MH, Nigro D. Enhanced oxidative stress as a potential mechanism underlying the programming of hypertension in utero. J Cardiovasc Pharmacol. 2002;40:501-509. |
| 124. | Racasan S, Braam B, van der Giezen DM, Goldschmeding R, Boer P, Koomans HA, Joles JA. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension. 2004;44:83-88. |
| 125. | Yzydorczyk C, Comte B, Cambonie G, Lavoie JC, Germain N, Ting Shun Y, Wolff J, Deschepper C, Touyz RM, Lelièvre-Pegorier M. Neonatal oxygen exposure in rats leads to cardiovascular and renal alterations in adulthood. Hypertension. 2008;52:889-895. |
| 126. | Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Lê NL, Pladys P, Gauthier C, Lahaie I, Abran D. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1236-R1245. |
| 127. | Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig. 2005;12:2-13. |
| 128. | Unger C, Weiser JK, McCullough RE, Keefer S, Moore LG. Altitude, low birth weight, and infant mortality in Colorado. JAMA. 1988;259:3427-3432. |
| 129. | Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health. 1997;87:1003-1007. |
| 130. | Moore LG. Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol. 2003;4:141-156. |
| 131. | Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10:265-274. |
| 132. | Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329-1334. |
| 133. | Miller SL, Green LR, Peebles DM, Hanson MA, Blanco CE. Effects of chronic hypoxia and protein malnutrition on growth in the developing chick. Am J Obstet Gynecol. 2002;186:261-267. |
| 134. | Xiao D, Ducsay CA, Zhang L. Chronic hypoxia and developmental regulation of cytochrome c expression in rats. J Soc Gynecol Investig. 2000;7:279-283. |
| 135. | Yaniv G, Shilkrut M, Lotan R, Berke G, Larisch S, Binah O. Hypoxia predisposes neonatal rat ventricular myocytes to apoptosis induced by activation of the Fas (CD95/Apo-1) receptor: Fas activation and apoptosis in hypoxic myocytes. Cardiovasc Res. 2002;54:611-623. |
| 136. | Godfrey KM. The role of the placenta in fetal programming-a review. Placenta. 2002;23 Suppl A:S20-S27. |
| 137. | Godfrey KM, Breier BH, Cooper C. Constraint of the maternal placental supply of nutrients: causes and consequences. London: Royal College of Obstetricians and Gynaecologists 1999; 283-298. |
| 138. | Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611-624. |
| 139. | Gunn a, Gatehouse AG. The influence of larval phase on metabolic reserves, fecundity and life-span of the African army worm, Spodoptera exempta (Walker). Bull Entomol Res. 1987;77:6451-6460. |
| 140. | O’Brien PMS, Wheeler T, Barker DJP. Fetal Programming influences on development and disease in later life. London: RCOG Press 1999; . |
| 141. | Cianfarani S. Foetal origins of adult diseases: just a matter of stem cell number? Med Hypotheses. 2003;61:401-404. |
| 143. | Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, Hill DJ. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology. 1999;140:4861-4873. |
| 144. | Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279-2286. |
| 145. | Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3-8. |
| 146. | Langley-Evans SC. Intrauterine programming of hypertension in the rat: nutrient interactions. Comp Biochem Physiol A Physiol. 1996;114:327-333. |
| 147. | Fetita LS, Sobngwi E, Serradas P, Calvo F, Gautier JF. Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab. 2006;91:3718-3724. |
| 148. | Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care. 2010;33:402-404. |
| 149. | Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165:849-857. |
| 150. | Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447-1451. |
| 151. | Xu H, Simonet F, Luo ZC. Optimal birth weight percentile cut-offs in defining small- or large-for-gestational-age. Acta Paediatr. 2010;99:550-555. |