Copyright
©The Author(s) 2015.
World J Diabetes. Apr 15, 2015; 6(3): 380-390
Published online Apr 15, 2015. doi: 10.4239/wjd.v6.i3.380
Published online Apr 15, 2015. doi: 10.4239/wjd.v6.i3.380
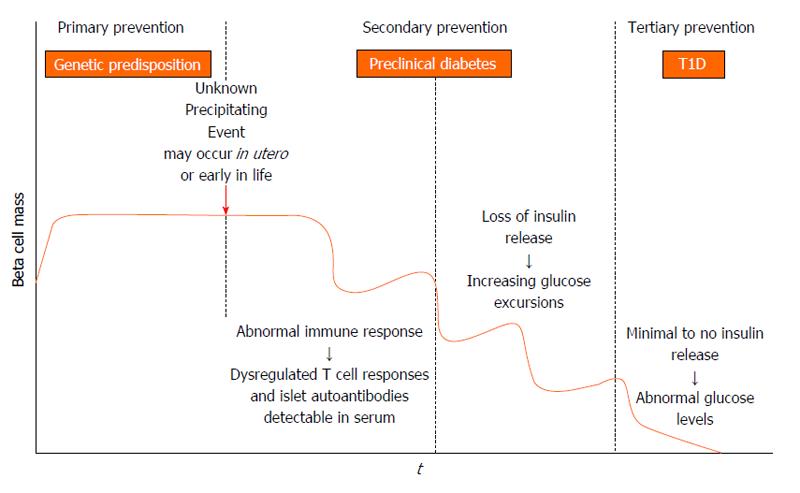
Figure 1 Stages in the development of type 1 diabetes adapted from the initial model proposed by George Eisenbarth.
In genetically at risk individuals an unknown trigger, presumably environmental, initiates an autoimmune response that results in loss of beta cell mass. Before metabolic disturbances occur, islet autoantibodies (insulin, glutamic decarboxylase, islet antigen 2, zinc transporter 8) are measureable in serum. As beta cell mass decreases, potentially in a relapsing-remitting manner, there is loss of endogenous insulin release and ensuing hyperglycemia. Within this model, there are opportunities for type 1 diabetes (T1D) prevention in genotypically high risk individuals (primary prevention) and in autoantibody positive individuals (secondary prevention). Interventions to preserve remaining beta cell mass at diagnosis are also possible (tertiary prevention).
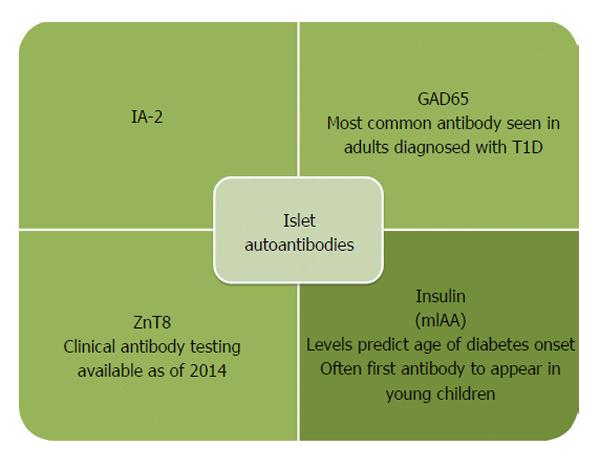
Figure 2 Insulin autoantibodies are often the first antibody to develop in young children.
In contrast, adults most often are GAD65 and IA-2 autoantibody positive at diagnosis. The ZnT8 antibody is the most recently identified autoantibody with commercial testing now available. T1D: Type 1 diabetes; GAD65: Glutamic decarboxylase; IA-2: Islet antigen 2; ZnT8: Zinc transporter 8.
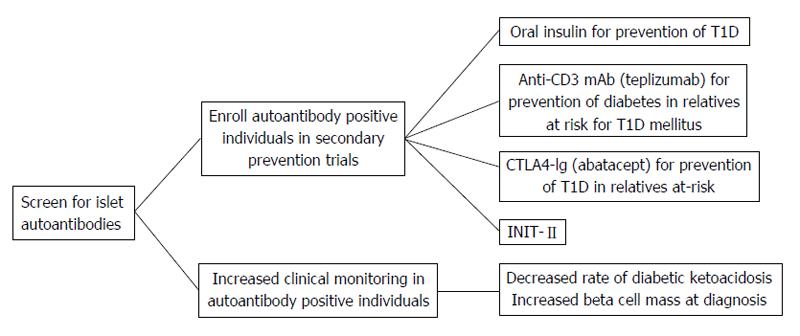
Figure 3 The measurement of serum islet autoantibodies has made type 1 diabetes a predictable disease.
Early identification of islet autoantibody positive individuals leads to improved clinical outcomes by decreasing the risk for diabetic ketoacidosis and potentially preserving beta cell mass through clinical prevention trials. T1D: Type 1 diabetes; INIT-II: Intranasal Insulin Trial.
- Citation: Simmons KM, Michels AW. Type 1 diabetes: A predictable disease. World J Diabetes 2015; 6(3): 380-390
- URL: https://www.wjgnet.com/1948-9358/full/v6/i3/380.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i3.380