Published online Mar 15, 2016. doi: 10.4251/wjgo.v8.i3.289
Peer-review started: June 27, 2015
First decision: July 28, 2015
Revised: October 25, 2015
Accepted: December 17, 2015
Article in press: December 18, 2015
Published online: March 15, 2016
Processing time: 254 Days and 23.2 Hours
The most frequent cause of treatment failure following surgery for gastric cancer is peritoneal dissemination, mainly caused by the seeding of free cancer cells from the primary gastric cancer, which is the most common type of spread. Unfortunately, there is no standard modality of intraperitoneal free cancer cells detection to predict peritoneal metastasis until now. We reviewed English literature in PubMed was done using the MeSH terms for gastric cancer, peritoneal wash, and reverse transcriptase polymerase chain reaction. All the articles were reviewed and core information was tabulated for reference. After a comprehensive review of all articles, the data was evaluated by clinical implication and predictive value of each marker for peritoneal recurrence. There are still many limitations to overcome before the genetic diagnosis for free cancer cells detection can be considered as routine assay. To make it a reliable diagnostic tool for detecting free cancer cells, the process and method of genetic detection with peritoneal washes should be standardized, and the development of simple diagnostic devices and easily available kits are necessary. Herein, we reviewed the past, present and future perspectives of the peritoneal lavage for the detection of intraperitoneal free cancer cells in patients with gastric cancer.
Core tip: The most common cause of treatment failure after gastric cancer surgery is peritoneal metastasis, mainly caused by free cancer cells from primary cancer. Genetic detection using reverse transcriptase polymerase chain reaction analysis has been used for the detection of free cancer cells. The process and method of genetic detection with peritoneal washes should be standardized, and the development of simple diagnostic devices and easily available kits are necessary in the future. In this article, we summarize the current evidence of genetic detection in peritoneal washes from gastric cancer patient.
- Citation: Chae HD. Role of genetic detection in peritoneal washes with gastric carcinoma: The past, present and future. World J Gastrointest Oncol 2016; 8(3): 289-296
- URL: https://www.wjgnet.com/1948-5204/full/v8/i3/289.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i3.289
Although the incidence of gastric cancer has been declining in western countries, gastric cancer is still the fourth most common cancer and the second leading cause of cancer deaths worldwide[1]. Peritoneal dissemination of gastric cancer, which is caused by free cancer cell seeding from the primary tumor, is the most common cause of treatment failure after surgery. In general, the frequency of peritoneal dissemination is increased with depth of invasion of the gastric wall[2]. Intraperitoneal free cancer cells detected in peritoneal washes from gastric cancer patients have been reported to be significant and independent prognostic factor for recurrence and survival after surgery.
To predict peritoneal metastasis, several methods have been used for many studies, which are conventional cytology[3,4], ThinPrep[5], and molecular markers[4,6-8]. Conventional cytology had been regarded as the gold standard for detecting cancer cells in peritoneal washes. However, the usefulness of conventional cytology for prediction of peritoneal metastasis has controversy because of its low sensitivity[3,4,7,9].
Recently, reverse transcriptase polymerase chain reaction (RT-PCR) analysis has been used for the genetic detection of free cancer cells[10-12]. Several target genes have been used, which are carcinoembryonic antigen (CEA), heparanase, matrix metalloproteinase-7 (MMP-7), cytokeratin 20 (CK-20), telomerase, and melanoma-associated gene (MAGE)[2,13]. The sensitivity of RT-PCR is higher than that of conventional cytology[14-16]. Several studies reported that the results of RT-PCR analysis from peritoneal washes correlate strongly with peritoneal recurrence and prognosis after curative surgery in patients with advanced gastric cancer[6,14-20].
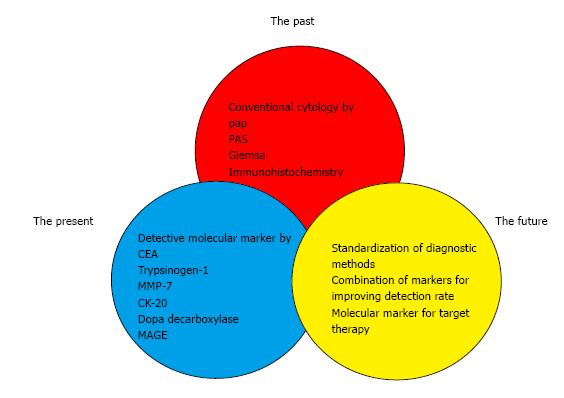
In this reason, we reviewed the present status and future perspectives of peritoneal lavage for the detection of intraperitoneal free cancer cells in patients with gastric cancer (Figure 1).
Conventional cytology from the peritoneal washes had been widely used to detect free cancer cells and to predict peritoneal metastasis[3,9], based on several studies reporting a correlation between peritoneal recurrence and presence of intraperitoneal free cancer cells[21-24].
In patients with serosal involvement, 50% of patients develop peritoneal recurrence even if curative resection is performed[25,26]. Bando et al[3] reported that 24% of positive cytology from 1297 gastric cancer patients. This result presented higher positive rate than other studies, probably because many patients with advanced stage of gastric cancer were included in the study (296 patients had peritoneal metastasis). Ribeiro et al[27] reported much higher incidence of positive cytology (41%) from their study from 49 patients with advanced gastric cancer including metastatic disease. Other studies that evaluated patients with gastric cancer underwent curative resection showed approximately 5% of positive rate from peritoneal washes cytology[3,8-10,25,27,28].
In many past studies, intraperitoneal free cancer cells detected in peritoneal washing cytology have been demonstrated as significant and independent prognostic factor, influencing both recurrence free survival and overall survival of patients with gastric cancer. Therefore, peritoneal wash cytology is recommended in the Japanese Classification of Gastric Carcinoma from 1998[29]. Recently, the American Joint Committee on Cancer tumor node metastasis staging system classified the positive peritoneal cytology in gastric cancer as metastatic disease (M1) in the 7th edition[1].
However, the conventional cytology is often criticized for its relatively low sensitivity to detect intraperitoneal free cancer cells and to predict peritoneal metastasis[3,4,7,9]. Furthermore, previous studies reported that the conventional cytology in patient without any macroscopic peritoneal metastasis (P0) after curative resection had very low sensitivity (5%-15%)[3,4,7]. Although obtaining peritoneal cytology has been advocated by Japanese and Dutch investigators[30,31], this is not a uniform practice in other Western centers. Immunocytochemical methods that used a panel of monoclonal antibodies, directed to gastric cancer-associated antigens had improved detection of peritoneal cytology by providing more sensitive and may have a higher specificity than conventional cytology[32].
General principles: The high sensitivity of RT-PCR analysis has made it possible to detect micrometastasis on the cancer tissue specific messenger RNA (mRNA) expression in peripheral vein, bone marrow, lymph nodes, and peritoneal cavity[19,33-35]. Although RT-PCR analysis of peritoneal washes is a more sensitive than conventional cytology, the result variations in RT-PCR between different laboratories have been reported. Therefore standardization and quality control of the process of RT-PCR is very important.
For the detection of gastric cancer micrometastases in peritoneal washes, RT-PCR technique has been used from the twenty-first century[10-12]. The primer sequences, which are used in previous studies, for RT-PCR of peritoneal lavage in patients with gastric cancer are summarized in Table 1. The sensitivity of RT-PCR is higher than that of conventional cytology[10-12]. Based on several studies, There is strong correlation between the results of RT-PCR analysis from peritoneal washes and prognosis, including peritoneal recurrence rate, after curative resection in patients with advanced gastric cancer[6,14,16-20] (Table 2).
| Gene | Sequence of primer pair (5’→3’) | Tm (°C) | Amplicon length (bp) |
| CEA | F; TCTGGAACTTCTCCTGGTCTCTCAGCTGG | 69 | 160 |
| R; TGTAGCTGTTGCAAATGCTTTAAGGAAGAAGC | |||
| CK-20 | F; GGTCGCGACTACAGTGCATATTACA | 72 | 121 |
| R; CCTCAGCAGCCAGTTTAGCATTATC | |||
| Tripsinogen | F; ACCACCATGAATCCACTCCTG | 62 | |
| R; GCTTTAGCTATTGGCAGCTAT | |||
| MMP-7 | F; ATGTTAAACTCCCGCGTCATA | 72 | 418 |
| R; CAGCATACAGGAAGTTAATCC | |||
| DDC | F; AAGCACAGCCATCAGGATTCA | 70 | |
| R; TGGACATGCTTGCGGATATAAG | |||
| L3PP | F; GATGCTGTGTGTTTTGAT GTTGAC | 95 | |
| R; CTTGACTTGTTGCCTGATCACATT | |||
| MAGE A1-6 | F; CTGAAGGAGAAGATCTGCC | 60 | 855 |
| R; CTCCAGGTAGTTTTCCTGCAC |
| Ref. | Year | Method | Molecular marker | Outcome |
| Nakanishi et al[17] | 1997 | RT-PCR | CEA | |
| Fujimura et al[46] | 1998 | RT-PCR | Trypsinogen | |
| Kodera et al[20] | 2002 | RT-PCR | CEA | Peritoneal recurrence, survival |
| Nakanishi et al[17] | 2000 | Q-RT-PCR | CEA | Peritoneal recurrence, survival |
| Yonemura et al[6] | 2001 | RT-PCR | MMP-7 | |
| Sugita et al[14] | 2003 | Q-RT-PCR | CEA and CK-20 | Peritoneal recurrence, survival |
| Sakakura et al[62] | 2002 | Q-RT-PCR | Dopa decarboylase | |
| Shimomura et al[66] | 2004 | Q-RT-PCR | L3-PP | |
| Kodera et al[7] | 2006 | RT-PCR | CEA | Overall survival |
| Jeon et al[13] | 2010 | RT-PCR | MAGE | Overall survival |
| Jeon et al[71] | 2014 | RT-PCR | MAGE and CEA | 3 -yr survival |
Although many investigators have demonstrated that molecular techniques using RT-PCR may serve as a useful method for the detection of free cancer cells, there are still several problems. These are time-consuming, expensive, relatively laborious compared with conventional cytology, and the accuracy is widely variable between laboratories and the methods of processing the peritoneal washes is not yet standardized. Current experimental studies which aim to identify a rapid, accurate, and cost-effective detection method are proposed. The transcription-reverse transcription concerted reaction system and the LightCycler system have shown promise in intraperitoneal free cancer cell detection.
Molecular markers: (1) CEA: CEA is the most commonly studied tumor marker and is predominantly used clinically in patients with gastrointestinal cancer. Structurally, it is a glycoprotein with a molecular weight of 200 kd and is a component of the glycocalyx, located on the luminal side of the cell membrane of normal epithelial intestinal cells. Although its exact function is unknown, CEA has been shown to be involved in cell adhesion and is able to inhibit apoptosis induced by loss of anchorage to the extracellular membrane.
In 1997, Nakanishi et al[16] had proposed the first study that more sensitive detection of free cancer cells could be achieved through amplification of CEA mRNA by means of the RT-PCR, and reported a high sensitivity of the CEA RT-PCR assay for the detection of peritoneal micrometastasis and a 20% improvement in the positive rate, as compared with that of cytology alone. CEA is presented in all of gastric cancer cell lines examined irrespective of the differentiation degree, and it is absent in blood and mesothelium. That is indicating the specificity of CEA RT-PCR for detection of carcinoma cells in peritoneal lavage fluid.
Since this report, many investigators have been used CEA as the target gene of RT-PCR for the patients with gastric cancer. From 2001, many other studies have reported that quantitative CEA mRNA detection using RT-PCR is an accurate method for predicting the risk of peritoneal recurrence in patients with gastric cancer[36-42].
Many studies reported that the detection of CEA mRNA using RT-PCR of peritoneal washes was the most reliable prognostic marker for recurrence and peritoneal carcinomatosis in patient with gastric cancer, and more sensitive than conventional cytology[8,17]. Kodera et al[43] found that the use of RT PCR for CEA mRNA resulted in a detection rate of free cancer cells of 28% (41 of 148), with a 14% higher detection rate than for peritoneal wash cytology. However, the sensitivity of the CEA RT-PCR assay for the detection of peritoneal micrometastasis still remains low. Furthermore, false-positive results, caused by expression of CEA in non-cancer cells like mesothelial cells and lymphocytes, is remained as main problem of this technique[20].
(2) Trypsinogen-1: Trypsin is a member of the serine protease family composed of three trypsinogen genes (trypsinogen 1, 2 and 4) with a potential role in cancer invasion[44,45]. These family members are sharing same nucleotide structures above 90% with each other.
Trypsin has potent proteolytic activity, and its inappropriate activation may lead to peritoneal dissemination of infiltrative gastric cancer by defoliation of cancer cells from the surface of advanced primary tumor, attachment and invasion to extracellular matrix under the mesothelium[46].
(3) MMP-7: Recently, MMPs are gaining attention and attracted by many invastigators who are interested in new biomarkers for cancer spread and metastasis[47]. MMPs have key role in degrading extracellular matrix by proteolytic activity, as well as regulating other enzymes, chemokines and cell receptors[48,49]. MMP-7, also called Matrilysin, is a distinct family member in MMPs family because its highest proteolytic activity for wide range of molecules and pivotal role in activating other MMPs for cell degradation[50-53]. Another specific characteristic of matrilysin in contrast to other MMPs is that it is mainly expressed by tumor cells and not by stromal cells[54-56].
Yonemura et al[6] reported that MMP-7 mRNA was not expressed by fibroblasts, peripheral blood, mesothelial cells, normal gastric mucosa, or the peritoneal lavage fluid of patients with benign disease. In contrast, it was expressed by all of the examined specimens of peritoneal dissemination in gastric cancer. By combining cytology and MMP-7 RT-PCR, the sensitivity rate for the prediction of peritoneal dissemination was improved to 11% over the prediction by routine cytology.
(4) CK-20: The keratins are intermediate filament proteins responsible for the structural integrity of epithelial cells. Recently, many investigators identified CK-20 as one of the potential cancer-related biomarkers used for detecting peritoneal free cancer cells in gastric cancer[57].
CEA had been reported that there are strong positivity of mRNA expression in differentiated gastric carcinoma, but relatively weak or undetectable in poorly differentiated type[57]. Thus, CK-20 has been the use as option for multiple marker assay with CEA for the detection of nodal metastasis[58]. Also for the peritoneal recurrence, many studies reported the usefulness of multi-marker assay, employing both CEA and CK-20 RT-PCR[14,15,59,60].
(5) Dopa decarboxylase (DDC): DDC is an enzyme for the metabolism of dopamine, and also has a role for the synthesis of important neurotransmitters including serotonin. Although DCC is investigated as cancer-related marker with its high expression in lung cancer and neuroblastoma[61], the role of this marker in gastric cancer is still unclear.
Sakakura et al[62] have analyzed in their studies for the global gene expression of a gastric cancer cell line, and approximately 21168 genes were established from the tumor and metastatic site of gastric cancer patients. Among them, they found that 24 genes were upregulated and 17 genes downregulated, and DCC was one of these upregulated genes. DDC-specific RT–PCR is potentially a novel marker for peritoneal dissemination of gastric cancer and reliable and efficient for the prediction of peritoneal recurrence of gastric cancer.
(6) L-3 phosphoserine phosphatase heparanase (L3-PP): L3PP is one of the up-regulated genes and hundreds of protein phosphatases are known. It is an intermediary enzyme in both amino acid biosynthesis and gluconeogenesis, and is an enzyme that acts specifically in the synthesis of serine from 3-phosphoserine[63,64]. It has been reported that activity of the enzyme L3-PP increases when cell multiplication and frequency of mitosis increases[65].
Shimomura et al[66] had reported that L3-PP overexpression in gastric cancer cells from peritoneal metastasis by cDNA microarray as well as RT-PCR suggest that overexpression of L3-PP is probably involved in peritoneal metastasis of gastric cancers. When they had used combination RT-PCR of CEA and L3-PP to reduce false-negative result of CEA mRNA, the sensitivity of peritoneal dissemination was improved from 71.4% to 85.7%.
(7) Melanoma associated gene (MAGE): MAGE has been shown to be a cancer-specific marker that suppresses apoptosis and plays an important role in carcinogenesis[67]. MAGE gene expression in RT-PCR of gastric cancer is relatively higher than that of other markers[68,69]. Although the rate of expression is different according to their subtype, expression of MAGE-4, MAGE-6, MAGE-8, MAGE-9, MAGE-10, and MAGE-12 genes was very high as 82% in gastric cancer tissue[70]. Furthermore, previous studies reported that MAGE showed no expression in normal gastric tissue[69,70]. In this reason, MAGE has been attracted for the new target gene for predicting prognosis of gastric cancer, and expected as a marker for target therapy because of its specific expression[69,70].
Jeon et al[71] had reported, in their trial on the comparison of the 2 markers (CEA and MAGE) after long-term follow-up, that MAGE RT-PCR showed better specificity and more significant associations with peritoneal recurrence than CEA RT-PCR, and MAGE RT-PCR results was proved to be the most important prognostic factor for recurrence in patients with gastric cancer after curative resection.
Genetic detection in peritoneal washes with gastric carcinoma can be performed only at university hospitals and large volume cancer centers. Furthermore, there is no standard for processing of peritoneal fluid and the method of genetic detection. To make it a reliable diagnostic tool for detecting free cancer cells, the process and method of genetic detection with peritoneal washes should be standardized, and the development of simple diagnostic devices and easily available kits are necessary.
Also, effective modality of treatment for the patient with a positive peritoneal molecular diagnosis should be needed. If the molecular marker for peritoneal free cancer cell is used not only for the diagnosis but also for the therapeutic modality with the development of target therapy, it might be one of useful method for the treatment of advanced gastric cancer and the prevention of peritoneal recurrence.
The methods of detecting intraperitoneal free cancer cells represent are still area of evolution. In past, conventional cytology was regarded as the only method for detecting peritoneal free cancer cells from gastric cancer. However low sensitivity of this method had been criticized, and many studies about various method with peritoneal washes were performed for better prediction of peritoneal metastasis. In present, genetic detection using RT-PCR analysis has been used for improving the detection rate. It has been suggested that these tools have better sensitivity in detecting intraperitoneal free cancer cells with better correlation to peritoneal recurrence. But it can be performed only at university hospitals and large volume cancer centers. Furthermore, there is no standard for processing of peritoneal fluid and the method of genetic detection and effective modality of treatment for the patient with a positive peritoneal molecular diagnosis. In near future, standardization of diagnostic methods, combination of markers for improving detection rate, and development of molecular marker for target therapy could provide us with being relevant in clinical decision-making, detection methods need to be accurate, reliable, cost-effective and effective modality of treatment for the patient with a positive peritoneal diagnosis.
P- Reviewer: Huang CZ, Sun J S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 2. | Fujiwara Y, Doki Y, Taniguchi H, Sohma I, Takiguchi S, Miyata H, Yamasaki M, Monden M. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer. 2007;10:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T, Nishimura G, Miwa K. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 242] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Wang Z, Zhang X, Xu H, Zhou X, Jiang L, Lu C. Detection of peritoneal micrometastasis by reverse transcriptase-polymerase chain reaction for heparanase mRNA and cytology in peritoneal wash samples. J Surg Oncol. 2005;90:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Ryu CK, Park JI, Min JS, Jin SH, Bang HY, Chae GB, Lee JI. The clinical significance and detection of intraperitoneal micrometastases by ThinPrep® cytology with peritoneal lavage fluid in patients with advanced gastric cancer. J Korean Gastric Cancer Assoc. 2008;8:189-197. |
| 6. | Yonemura Y, Fujimura T, Ninomiya I, Kim BS, Bandou E, Sawa T, Kinoshita K, Endo Y, Sugiyama K, Sasaki T. Prediction of peritoneal micrometastasis by peritoneal lavaged cytology and reverse transcriptase-polymerase chain reaction for matrix metalloproteinase-7 mRNA. Clin Cancer Res. 2001;7:1647-1653. [PubMed] |
| 7. | Kodera Y, Nakanishi H, Ito S, Mochizuki Y, Ohashi N, Yamamura Y, Fujiwara M, Koike M, Tatematsu M, Nakao A. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: analysis of real time reverse transcriptase-polymerase chain reaction after 5 years of followup. J Am Coll Surg. 2006;202:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Wang JY, Lin SR, Lu CY, Chen CC, Wu DC, Chai CY, Chen FM, Hsieh JS, Huang TJ. Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus the combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Lett. 2005;223:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol. 2005;12:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Noura S, Yamamoto H, Ohnishi T, Masuda N, Matsumoto T, Takayama O, Fukunaga H, Miyake Y, Ikenaga M, Ikeda M. Comparative detection of lymph node micrometastases of stage II colorectal cancer by reverse transcriptase polymerase chain reaction and immunohistochemistry. J Clin Oncol. 2002;20:4232-4241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Yoshioka S, Fujiwara Y, Sugita Y, Okada Y, Yano M, Tamura S, Yasuda T, Takiguchi S, Shiozaki H, Monden M. Real-time rapid reverse transcriptase-polymerase chain reaction for intraoperative diagnosis of lymph node micrometastasis: clinical application for cervical lymph node dissection in esophageal cancers. Surgery. 2002;132:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Okada Y, Fujiwara Y, Yamamoto H, Sugita Y, Yasuda T, Doki Y, Tamura S, Yano M, Shiozaki H, Matsuura N. Genetic detection of lymph node micrometastases in patients with gastric carcinoma by multiple-marker reverse transcriptase-polymerase chain reaction assay. Cancer. 2001;92:2056-2064. [PubMed] |
| 13. | Jeon CH, Shin IH, Park JB, Chae HD. Prognostic significance of MAGE in peritoneal washes in gastric carcinoma patients without peritoneal metastasis: results of a 5-year follow-up study. J Clin Gastroenterol. 2010;44:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Sugita Y, Fujiwara Y, Taniguchi H, Mori T, Ishii T, Niwa H, Okada Y, Takiguchi S, Yasuda T, Yano M. Quantitative molecular diagnosis of peritoneal lavage fluid for prediction of peritoneal recurrence in gastric cancer. Int J Oncol. 2003;23:1419-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Kodera Y, Nakanishi H, Ito S, Yamamura Y, Fujiwara M, Koike M, Hibi K, Ito K, Tatematsu M, Nakao A. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer. 2005;8:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Nakanishi H, Kodera Y, Torii A, Hirai T, Yamamura Y, Kato T, Kito T, Tatematsu M. Detection of carcinoembryonic antigen-expressing free tumor cells in peritoneal washes from patients with gastric carcinoma by polymerase chain reaction. Jpn J Cancer Res. 1997;88:687-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Nakanishi H, Kodera Y, Yamamura Y, Ito S, Kato T, Ezaki T, Tatematsu M. Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric-cancer patients with real-time RT-PCR on the lightcycler. Int J Cancer. 2000;89:411-417. [PubMed] |
| 18. | Schmidt P, Thiele M, Rudroff C, Vaz A, Schilli M, Friedrich K, Scheele J. Detection of tumor cells in peritoneal lavages from patients with gastrointestinal cancer by multiplex reverse transcriptase PCR. Hepatogastroenterology. 2001;48:1675-1679. [PubMed] |
| 19. | Kodera Y, Nakanishi H, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. Prognostic value and clinical implications of disseminated cancer cells in the peritoneal cavity detected by reverse transcriptase-polymerase chain reaction and cytology. Int J Cancer. 1998;79:429-433. [PubMed] |
| 20. | Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Kato T, Tatematsu M. Quantitative detection of disseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reaction: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg. 2002;235:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Hirono M, Matsuki K, Nakagami K, Niimoto M, Hattori T. Comparative studies on cytological and histological evaluations of disseminating peritoneal metastasis in gastric cancer. Jpn J Surg. 1981;11:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Iitsuka Y, Shiota S, Matsui T, Murata Y, Kimura A, Koga S. Relationship between the cytologic characteristics of intraperitoneal free cancer cells and the prognosis in patients with gastric cancer. Acta Cytol. 1990;34:437-442. [PubMed] |
| 23. | Ikeguchi M, Oka A, Tsujitani S, Maeta M, Kaibara N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994;14:2131-2134. [PubMed] |
| 24. | Papanicolaou GN. Atlas of exfoliative cytology. Cambridge: Harvard University Press 1963; . |
| 25. | Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, Hioki K, Yamamoto M. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg. 1990;77:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Moriguchi S, Maehara Y, Korenaga D, Sugimachi K, Nose Y. Risk factors which predict pattern of recurrence after curative surgery for patients with advanced gastric cancer. Surg Oncol. 1992;1:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Ribeiro U, Gama-Rodrigues JJ, Bitelman B, Ibrahim RE, Safatle-Ribeiro AV, Laudanna AA, Pinotti HW. Value of peritoneal lavage cytology during laparoscopic staging of patients with gastric carcinoma. Surg Laparosc Endosc. 1998;8:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Burke EC, Karpeh MS, Conlon KC, Brennan MF. Peritoneal lavage cytology in gastric cancer: an independent predictor of outcome. Ann Surg Oncol. 1998;5:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 1942] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 30. | Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Motohashi H, Noguchi Y. Peritoneal cytology in patients with gastric cancer exposed to the serosa--a proposed new classification based on the local and distant cytology. Hepatogastroenterology. 2003;50:1183-1186. [PubMed] |
| 31. | Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ. Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg. 1996;83:672-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 168] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Benevolo M, Mottolese M, Cosimelli M, Tedesco M, Giannarelli D, Vasselli S, Carlini M, Garofalo A, Natali PG. Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J Clin Oncol. 1998;16:3406-3411. [PubMed] |
| 33. | Burchill SA, Bradbury MF, Pittman K, Southgate J, Smith B, Selby P. Detection of epithelial cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction. Br J Cancer. 1995;71:278-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 239] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Jauch KW, Heiss MM, Gruetzner U, Funke I, Pantel K, Babic R, Eissner HJ, Riethmueller G, Schildberg FW. Prognostic significance of bone marrow micrometastases in patients with gastric cancer. J Clin Oncol. 1996;14:1810-1817. [PubMed] |
| 35. | Mori M, Mimori K, Inoue H, Barnard GF, Tsuji K, Nanbara S, Ueo H, Akiyoshi T. Detection of cancer micrometastases in lymph nodes by reverse transcriptase-polymerase chain reaction. Cancer Res. 1995;55:3417-3420. [PubMed] |
| 36. | Yonemura Y, Endou Y, Fujimura T, Fushida S, Bandou E, Kinoshita K, Sugiyama K, Sawa T, Kim BS, Sasaki T. Diagnostic value of preoperative RT-PCR-based screening method to detect carcinoembryonic antigen-expressing free cancer cells in the peritoneal cavity from patients with gastric cancer. ANZ J Surg. 2001;71:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Fujii S, Kitayama J, Kaisaki S, Sasaki S, Seto Y, Tominaga O, Tsuno N, Umetani N, Yokota H, Kitamura K. Carcinoembryonic antigen mRNA in abdominal cavity as a useful predictor of peritoneal recurrence of gastric cancer with serosal exposure. J Exp Clin Cancer Res. 2002;21:547-553. [PubMed] |
| 38. | Marutsuka T, Shimada S, Shiomori K, Hayashi N, Yagi Y, Yamane T, Ogawa M. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res. 2003;9:678-685. [PubMed] |
| 39. | Tokuda K, Natsugoe S, Nakajo A, Miyazono F, Ishigami S, Hokita S, Takao S, Eizuru Y, Aikou T. Clinical significance of CEA-mRNA expression in peritoneal lavage fluid from patients with gastric cancer. Int J Mol Med. 2003;11:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Ueno H, Yoshida K, Hirai T, Kono F, Kambe M, Toge T. Quantitative detection of carcinoembryonic antigen messenger RNA in the peritoneal cavity of gastric cancer patients by real-time quantitative reverse transcription polymerase chain reaction. Anticancer Res. 2003;23:1701-1708. [PubMed] |
| 41. | Oyama K, Terashima M, Takagane A, Maesawa C. Prognostic significance of peritoneal minimal residual disease in gastric cancer detected by reverse transcription-polymerase chain reaction. Br J Surg. 2004;91:435-443. [PubMed] |
| 42. | Ito S, Nakanishi H, Kodera Y, Mochizuki Y, Tatematsu M, Yamamura Y. Prospective validation of quantitative CEA mRNA detection in peritoneal washes in gastric carcinoma patients. Br J Cancer. 2005;93:986-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Kodera Y, Isobe K, Yamauchi M, Satta T, Hasegawa T, Oikawa S, Kondoh K, Akiyama S, Itoh K, Nakashima I. Expression of carcinoembryonic antigen (CEA) and nonspecific crossreacting antigen (NCA) in gastrointestinal cancer; the correlation with degree of differentiation. Br J Cancer. 1993;68:130-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Tani T, Kawashima I, Mita K, Takiguchi Y. Nucleotide sequence of the human pancreatic trypsinogen III cDNA. Nucleic Acids Res. 1990;18:1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Minn A, Schubert M, Neiss WF, Müller-Hill B. Enhanced GFAP expression in astrocytes of transgenic mice expressing the human brain-specific trypsinogen IV. Glia. 1998;22:338-347. [PubMed] |
| 46. | Fujimura T, Ohta T, Kitagawa H, Fushida S, Nishimura GI, Yonemura Y, Elnemr A, Miwa K, Nakanuma Y. Trypsinogen expression and early detection for peritoneal dissemination in gastric cancer. J Surg Oncol. 1998;69:71-75. [PubMed] |
| 47. | Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013;34:2041-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 48. | Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 843] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 49. | Butler GS, Overall CM. Proteomic identification of multitasking proteins in unexpected locations complicates drug targeting. Nat Rev Drug Discov. 2009;8:935-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 959] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 51. | Miyazaki K, Hattori Y, Umenishi F, Yasumitsu H, Umeda M. Purification and characterization of extracellular matrix-degrading metalloproteinase, matrin (pump-1), secreted from human rectal carcinoma cell line. Cancer Res. 1990;50:7758-7764. [PubMed] |
| 52. | Parsons SL, Watson SA, Brown PD, Collins HM, Steele RJ. Matrix metalloproteinases. Br J Surg. 1997;84:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Imai K, Yokohama Y, Nakanishi I, Ohuchi E, Fujii Y, Nakai N, Okada Y. Matrix metalloproteinase 7 (matrilysin) from human rectal carcinoma cells. Activation of the precursor, interaction with other matrix metalloproteinases and enzymic properties. J Biol Chem. 1995;270:6691-6697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 200] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Adachi Y, Yamamoto H, Itoh F, Arimura Y, Nishi M, Endo T, Imai K. Clinicopathologic and prognostic significance of matrilysin expression at the invasive front in human colorectal cancers. Int J Cancer. 2001;95:290-294. [PubMed] |
| 55. | Pajouh MS, Nagle RB, Breathnach R, Finch JS, Brawer MK, Bowden GT. Expression of metalloproteinase genes in human prostate cancer. J Cancer Res Clin Oncol. 1991;117:144-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 148] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Yamamoto H, Adachi Y, Itoh F, Iku S, Matsuno K, Kusano M, Arimura Y, Endo T, Hinoda Y, Hosokawa M. Association of matrilysin expression with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Cancer Res. 1999;59:3313-3316. [PubMed] |
| 57. | Mori K, Aoyagi K, Ueda T, Danjoh I, Tsubosa Y, Yanagihara K, Matsuno Y, Sasako M, Sakamoto H, Mafune Ki. Highly specific marker genes for detecting minimal gastric cancer cells in cytology negative peritoneal washings. Biochem Biophys Res Commun. 2004;313:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Kubota K, Nakanishi H, Hiki N, Shimizu N, Tsuji E, Yamaguchi H, Mafune K, Tange T, Tatematsu M, Kaminishi M. Quantitative detection of micrometastases in the lymph nodes of gastric cancer patients with real-time RT-PCR: a comparative study with immunohistochemistry. Int J Cancer. 2003;105:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Tamura N, Iinuma H, Takada T. Prospective study of the quantitative carcinoembryonic antigen and cytokeratin 20 mRNA detection in peritoneal washes to predict peritoneal recurrence in gastric carcinoma patients. Oncol Rep. 2007;17:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 60. | Dalal KM, Woo Y, Kelly K, Galanis C, Gonen M, Fong Y, Coit DG. Detection of micrometastases in peritoneal washings of gastric cancer patients by the reverse transcriptase polymerase chain reaction. Gastric Cancer. 2008;11:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Gilbert J, Haber M, Bordow SB, Marshall GM, Norris MD. Use of tumor-specific gene expression for the differential diagnosis of neuroblastoma from other pediatric small round-cell malignancies. Am J Pathol. 1999;155:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Sakakura C, Hagiwara A, Nakanishi M, Shimomura K, Takagi T, Yasuoka R, Fujita Y, Abe T, Ichikawa Y, Takahashi S. Differential gene expression profiles of gastric cancer cells established from primary tumour and malignant ascites. Br J Cancer. 2002;87:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Koch GA, Eddy RL, Haley LL, Byers MG, McAvoy M, Shows TB. Assignment of the human phosphoserine phosphatase gene (PSP) to the pter leads to q22 region of chromosome 7. Cytogenet Cell Genet. 1983;35:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Collet JF, Gerin I, Rider MH, Veiga-da-Cunha M, Van Schaftingen E. Human L-3-phosphoserine phosphatase: sequence, expression and evidence for a phosphoenzyme intermediate. FEBS Lett. 1997;408:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Greengard O, Herzfeld A. The undifferentiated enzymic composition of human fetal lung and pulmonary tumors. Cancer Res. 1977;37:884-891. [PubMed] |
| 66. | Shimomura K, Sakakura C, Takemura M, Takagi T, Fukuda K, Kin S, Nakase Y, Miyagawa K, Ohgaki M, Fujiyama J. Combination of L-3-phosphoserine phosphatase and CEA using real-time RT-PCR improves accuracy in detection of peritoneal micrometastasis of gastric cancer. Anticancer Res. 2004;24:1113-1120. [PubMed] |
| 67. | Yang B, O’Herrin SM, Wu J, Reagan-Shaw S, Ma Y, Bhat KM, Gravekamp C, Setaluri V, Peters N, Hoffmann FM. MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res. 2007;67:9954-9962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 68. | Inoue H, Mori M, Honda M, Li J, Shibuta K, Mimori K, Ueo H, Akiyoshi T. The expression of tumor-rejection antigen “MAGE” genes in human gastric carcinoma. Gastroenterology. 1995;109:1522-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Kim YM, Lee YH, Shin SH, Kim EH, Choi YW, Lee KM, Park JH, Lee YU, Seel DJ, Kim MC. Expression of MAGE-1, -2, and -3 genes in gastric carcinomas and cancer cell lines derived from Korean patients. J Korean Med Sci. 2001;16:62-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Li J, Yang Y, Fujie T, Tanaka F, Mimori K, Haraguchi M, Ueo H, Mori M, Akiyoshi T. Expression of the MAGE gene family in human gastric carcinoma. Anticancer Res. 1997;17:3559-3563. [PubMed] |
| 71. | Jeon CH, Kim IH, Chae HD. Prognostic value of genetic detection using CEA and MAGE in peritoneal washes with gastric carcinoma after curative resection: result of a 3-year follow-up. Medicine (Baltimore). 2014;93:e83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |