INTRODUCTION
Multimodal treatment of patients with pancreatic cancer remains one of the largest challenges in gastrointestinal oncology. Surgery is the cornerstone of curative intent treatment[1], however only 10%-20% of the patients are deemed resectable at presentation while 30%-40% already suffer from locally-advanced, irresectable disease and the remaining group shows distant metastases[2]. Given a median survival of approximately 24 mo and a 5-year overall survival rate of roughly 10%-20% even in the most favourable group with primarily resectable, locally confined disease, pancreatic cancer remains a disease with one of the most dismal prognosis in oncology[3].
While neoadjuvant strategies are already part of the standard approaches in most other gastrointestinal tumors (e.g., rectal cancer, esophageal cancer)[4,5], surgery followed by adjuvant treatment still represents the standard of care for resectable pancreatic cancer. Adjuvant chemotherapy seems the preferred approach in Europe based on the Conko-001 trial[6], while adjuvant chemoradiation is frequently used in the US based on the GITSG trial[7] and several non-randomized single-center studies with excellent results[8-10]. In primarily non-resectable locoregionally confined tumors, mainly definitive-palliative strategies have been used so far, which either consist of systemic therapy alone, combined chemoradiation or various combinations of both[11-13]. However, the mentioned strategies show limited success in terms of overall prognosis. On the other hand, the high rates of microscopically incomplete resections[14] with consecutively significant local recurrence rates[15] and the high frequency of locoregionally confined but primarily non-resectable tumors in combination with the clear advantages of neoadjuvant treatment strategies shown in other gastrointestinal tumor diseases despite clearly more favourable resectability, may form a strong rationale for the use of neoadjuvant strategies both for locally-advanced non-resectable as well as for primarily resectable pancreatic cancer patients.
Such strategies have different aims and include different possible advantages dependent on the resectability of the primary lesion: (1) in primarily non-resectable locoregionally confined pancreatic cancer, the main aim of neoadjuvant chemoradiation consists of tumor shrinkage including a drawback of the tumor from the major vessels to achieve secondary resectability; and (2) in primarily resectable cases, the main aim consists in enhanced local control either by increased probability of microscopic complete resection (R0) due to tumor shrinkage or by sterilization of microscopic tumor remnants in case of a microscopically incomplete (R1) resection. Substantial potential benefits further exist independent of the resection margin: (1) neoadjuvant treatment allows a local response evaluation which may reflect the overall disease prognosis; (2) neoadjuvant chemoradiation usually requires several weeks, which enables a stratification of patients with response or stable disease vs patients with rapid systemic progress. This may allow a potential omission of major surgery in those patients who are unlikely to benefit. The radiation therapy component thereby prevents patients without rapid systemic progression from a worsened overall prognosis due to local progression caused by locally insufficient effects of systemic therapy alone; (3) efficacy of radiation is enhanced in the neoadjuvant setting in comparison to postoperative radiotherapy because of the increased oxygenation of the untreated tissue; (4) the probability that additional therapy must be cancelled due to postoperative complications is reduced; and (5) target volume definition is simplified, resulting in smaller safety margins with consecutively lower dose to organs at risk and reduced toxicities.
Due to the complexity of the disease and the different aims in distinct stages, a variety of neoadjuvant concepts exist. They include chemotherapy, radiation therapy, chemoradiation or combinations like induction-chemotherapy followed by chemoradiation. This review focuses mainly on neoadjuvant radiotherapeutic strategies (radiation alone, radiation with concurrent chemotherapy) and advances in radiation technique rather than neoadjuvant concepts using chemotherapy alone or induction chemotherapy.
NEOADJUVANT RADIOTHERAPEUTIC TECHNIQUES AND CONCEPTS
3D-conformal radiation therapy
3D-conformal radiation therapy has been the standard radiation technique for the treatment of pancreatic cancer for the last two decades. This technique includes three-dimensional treatment planning based on computed tomography as first step. In the neoadjuvant setting, the target volume includes the primary tumor and the regional lymph nodes with a safety margin for daily repositioning error and tumor motion. If and to what extent the regional lymph node areas have to be included into the target volume is indeed part of an ongoing discussion. Multiple radiation fields are arranged in a way to ensure sufficient coverage of the target volume with best possible sparing of organs at risk at the same time (so called forward treatment planning) with small bowel and kidneys representing the main dose-limiting structures. Usually total doses of 45-54 Gy are applied in conventional fractionation (5-6 wk overall treatment time) in combination with simultaneous 5-FU or gemcitabine based chemotherapy.
Intensity-modulated radiation therapy
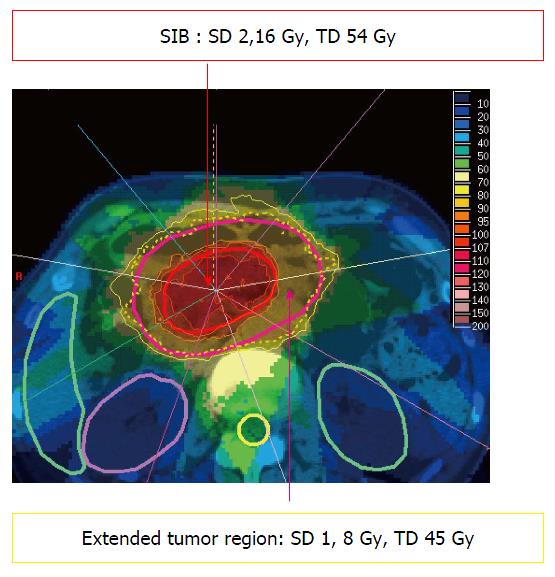
Treatment of irregularly shaped target volumes directly adjacent to radiation sensitive organs at risk can generally be improved by the use of so called “complex” photon irradiation techniques like intensity-modulated radiation therapy (IMRT). In contrast to 3D-conformal therapy, IMRT allows the delivery of different doses to certain segments of the same radiation field, creating a so called “fluence matrix” for every beam. By addition of multiple segments within several beams, superior coverage of irregularly shaped target volumes can be achieved. At the same time, steep dose gradients are possible, allowing improved sparing of directly adjacent organs at risk remain possible. Treatment planning is called “inverse” planning, because in contrast to 3D-conformal therapy the field geometry is usually not directly adjusted by the planner. Instead, doses are prescribed to the target volume(s) and to the outlined organs at risk (so called “dose constraints”) with a prespecified arrangement of beams. The treatment plan is then generated by an iterative computer-aided process by adjusting the fluence matrix and/or the constraints. This technique further allows the treatment of regions inside the target volume (for example gross tumor) with a higher dose, and other regions (for example elective nodal areas) with a lower dose within the same fraction. This enables dose escalation in certain areas within an unchanged number of fractions (so called “SIB = simultaneously integrated boost”, Figure 1).
Figure 1 Example for a 9-beam intensity modulated radiation therapy treatment plan in a pancreatic cancer patient with simultaneously integrated boost.
SD: Single dose; TD: Total dose in 25 fractions; SIB: Simultaneously integrated boost.
Numerous dosimetric studies showed clear advantages for intensity-modulated techniques compared to 3D-conformal treatments. In particular, lower doses in small bowel and liver could be achieved[16] and the possibility of a dose escalation up to 65 Gy was suggested[17]. Further on, several clinical studies have clearly confirmed, that these dosimetric advantages translate into reductions of acute and late side effects[18].
Image-guided radiation therapy
In general, several sources of uncertainty must be addressed in external beam radiation therapy regarding the coverage of target volumes with the prescribed dose. Intrafractional motion is mainly caused by respiration. On the other hand interfractional variations are the result of a combination of different factors. One major source is the displacement of the target by different filling of adjacent distensible structures like stomach or small bowel. Another source is the so-called “set-up error”, i.e., the uncertainty due to variation in daily positioning of the patient. All these variations must be compensated by safety margins. However, if directly adjacent organs at risk are present, every increase of safety margins consequently leads to increased side effects, which builds up the rationale for image-guided radiation therapy (IGRT). In doing so, three-dimensional datasets in treatment position are generated with imaging devices directly mounted on the linear accelerators (so called “on-board imaging”). These allow a comparison of the actual situation with the one during treatment planning and a real time correction of the position prior to irradiation. The increased precision of treatment application consequently allows a reduction of the safety margin. Several analyses have shown, that the safety margins needed to compensate for set-up error in the upper abdominal region can be reduced from 10 to 5 mm if IGRT is used[19]. In a tumor with 5 cm diameter, this margin reduction would lead to a 30% decrease of irradiated volume[18] with a significant dose reduction in small bowel, kidneys and liver[20].
Intrafractional respiratory motion differs from patient to patient, but can reach several centimeters[21]. Different strategies have been used to reduce the safety margins needed to account for such large variations. First, the individual respiratory motion can be measured for example by 4-dimensional computed tomography and allow definition of individualized anisotropic margins. This strategy can result in a mean reduction of the target volume by one third compared to the use conventional margins[20]. Some modern linear accelerators also allow gating, i.e., on board detection of tumor motion and application of radiation only at distinct positions of the tumor in its motion cycle. Another technique supported by some accelerators is a continuous adjustment of the beams to the particular tumor position (so-called “tracking”). Especially for these methods, the implantation of fiducials into the tumor may further increase the precision of dose application[22].
Adaptive radiation therapy strategies
In contrast to the mentioned techniques, adaptive strategies use regular imaging to adapt the radiation treatment plan semi-automatically to anatomical changes during the radiation therapy series, for example due to tumor shrinkage. Although the models for routine use of these techniques are currently still under development, theoretical studies suggest marked reductions in dose to several organs at risk, for example duodenum[23].
Intraoperative radiation therapy
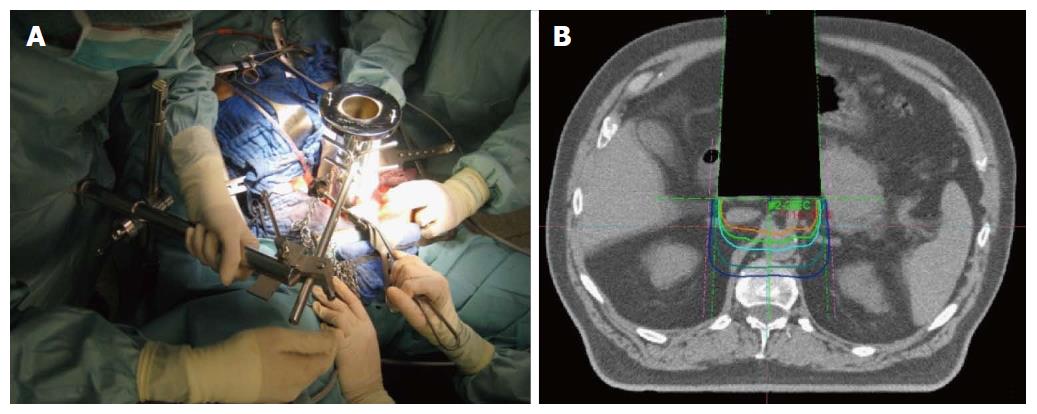
Although modern radiation techniques allow an improved sparing of surrounding organs at risk, dose-limitations in external beam therapy still exist, mainly due to directly adjacent structures with low radiation tolerance. Intraoperative electron radiation therapy (IOERT) offers an elegant possibility to overcome these dose-limitations after neoadjuvant radio (chemo)therapy. Due to its unique opportunity to guide a high single dose directly to the tumor bed or residual tumor during surgery, while adjacent organs at risk can be manually removed, IOERT can effectively prevents adjacent organs at risk from radiation exposure. Further advantages of IOERT in comparison to an external beam boost include at least theoretically smaller field sizes (because safety margins for daily positioning errors can be omitted) and the higher biological effectiveness of a single dose compared to the same amount of fractionated radiation therapy[24-29]. If typical dose concepts for the combination are used (10-15 Gy IOERT + 45-54 Gy EBRT), total doses can be reached which are biologically equivalent to 70-90 Gy of conventionally fractionated external beam radiation therapy without markedly increased toxicity[24-29]. Practically, an applicator of appropriate size is placed inside the abdominal cavity under visual control to cover the tumor bed/residual tumor after performed/attempted resection and manual removal of adjacent structures at risk. After moving and adjusting the patient below the accelerator, the irradiation itself is performed inside the operation theater, lasting about 1-2 min. Adequate depth coverage is achieved by appropriate selection of the electron energy (Figure 2). Unfortunately, this technique is only available at a limited number of centers so far (although numbers are heavily increasing in the recent years). Efficacy of IOERT remains difficult to assess, since many series report only single-center experiences covering large observation periods. Regarding primarily resectable pancreatic cancer, several Italian series reported significantly decreased local recurrence rates with the addition of IOERT[30,31]. Reni et al[32] confirmed these results in a larger comparison vs surgery alone. Beside an increased local control rate, they also found a significantly improved median overall survival in the subgroup of patients with early stages without increased perioperative morbidity. A multi-institutional series from Japan described a local recurrence rate of only 15% in 210 patients after gross complete resection with IOERT[33] and a European pooled analysis reported a very encouraging median overall survival of 30 mo for the combination of neoadjuvant chemoradiation, surgery and IOERT in a series of 270 patients[34]. Regarding primarily unresectable pancreatic cancer, IOERT can be used for dose escalation after neoadjuvant chemoradiation both in case of achieved secondary resectability as well as in case of further irresectability, resulting not only in improved local control but in the achievement of durable pain control in 75%-90% of the patients[35]. The Mayo group reported a series of 115 patients with unresectable pancreatic cancer and found that the addition of IOERT during explorative laparotomy after neoadjuvant irradiation resulted in a significantly increased 1-year local control rate (48% vs 82%)[36]. Shibamoto et al[37] compared EBRT, EBRT + IOERT and IOERT alone in a cohort of 150 patients and described an improved survival for the combination in the subgroup of patients with an initial CA 19-9 < 1000. The MGH group reported a median overall survival of 12 mo for the combination of EBRT and IOERT in their series of 194 patients with irresectable pancreatic cancer[38]. If the combined local treatment was further enhanced by a systemic treatment component, several series consistently reported median overall survival times of 16-18 mo with 2-year local control rates around 70%[39,40]. Even in patients with isolated local pancreatic cancer recurrences, the combination of EBRT, surgery and IOERT resulted in high local control and encouraging overall survival rates[26].
Figure 2 Intraoperative electron radiation therapy.
A: Placement of the applicator after resection; B: Schematic dose distribution.
In summary, IOERT provides an elegant possibility to escalate dose allowing total doses which could not be achieved by EBRT alone even with the use of the most sophisticated techniques. In resectable pancreatic cancer, IOERT seems clearly to improve local control, while its influence on overall survival cannot be finally assessed at present. In primarily irresectable pancreatic cancer, it can be suggested based on large single-center experiences, that especially the combination of EBRT, IOERT and chemotherapy achieves improved quality of life due to durable pain control, high local control rates and encouraging overall survival rates compared to other treatment approaches, although no phase III data currently exists to confirm these results.
Stereotactic body (ablative) radiation therapy
Stereotactic radiation therapy was primarily developed to treat small intracranial tumor lesions (for example brain metastases) and was successfully used in these situations for several decades. Initially it was defined as a treatment with a high single dose, which was guided precisely to the target using a stereotactic frame as external coordinate system for target localisation and very rigid patient fixation systems to reduce safety margins to a minimum (also known as radiosurgery). After expansion of the technique to extracranial sites and introduction of image guided radiation therapy, the definition of stereotactic body radiation therapy (SBRT) has widened. Today it summarizes different methods, which consistently apply so called “ablative” doses (biologically equivalent doses far beyond those achievable by conventional fractionation) in one to few fractions with optimal precision aiming at durable control of macroscopic tumor lesions. This technique has for example emerged as the standard of care in medically inoperable patients with early stage non-small cell lung cancer[41]. Currently it is used also in pancreatic cancer, although due to the lower contrast to the surrounding tissue (compared with lung cancer) implantation of fiducials is usually needed to achieve a safe detection of tumor position and motion with simple imaging modalities. Fiducial based approaches can additionally be combined with motion compensating radiation techniques (gating or tracking) to reduce safety margins to a minimum.
The present clinical experience for SBRT in pancreatic cancer is based mainly on small series of patients with irresectable locally-advanced pancreatic cancer[42]. Although very different dose schemes (1 × 15-25 Gy, 3 × 8-15 Gy, 5 × 6.5 Gy) have been used[42], these series consistently report very high local control rates of 80%-100% with partially very encouraging overall survival especially if combined with sequential chemotherapy[43-45]. However, the therapeutic window of this technique is narrow and therefore dose to directly adjacent organs at risk (like duodenum) must be strictly limited to avoid major complications[46], as shown by the range of gastrointestinal grade 3 complications reaching from 14% to 79% in the major series, and depending mainly on target volume size and dose to the duodenum[45,47]. Adaptive dose prescriptions depending for example on the distance between tumor and duodenum seem to be beneficial[44].
Recently, SBRT has also been introduced into neoadjuvant treatment approaches. One series describes the use of SBRT in 73 patients of whom 56 were deemed borderline resectable[48]. Treatment consisted of 3 cycles induction-chemotherapy followed by SBRT which guided 35-50 Gy to the vessel-approaching tumor parts and 25-30 Gy to the remaining tumor parts in 5 fractions. Seventy-seven percent of the borderline resectable patients responded and were surgically explored. Resection was possible in 56% of the patients (97% R0), showing a significantly improved survival. Severe gastrointestinal toxicity (grade 3) was observed only in 5% of the patients[48].
In summary, SBRT yields high local control rates, which seem so be superior to the results of conventionally fractionated RT. However, SBRT in the upper abdomen remains a demanding technique with a narrow therapeutic window and has been so far investigated mainly in irresectable pancreatic cancer. Nevertheless, it seems to be a promising approach also in the neoadjuvant setting especially if combined with systemic therapy.
Particle therapy
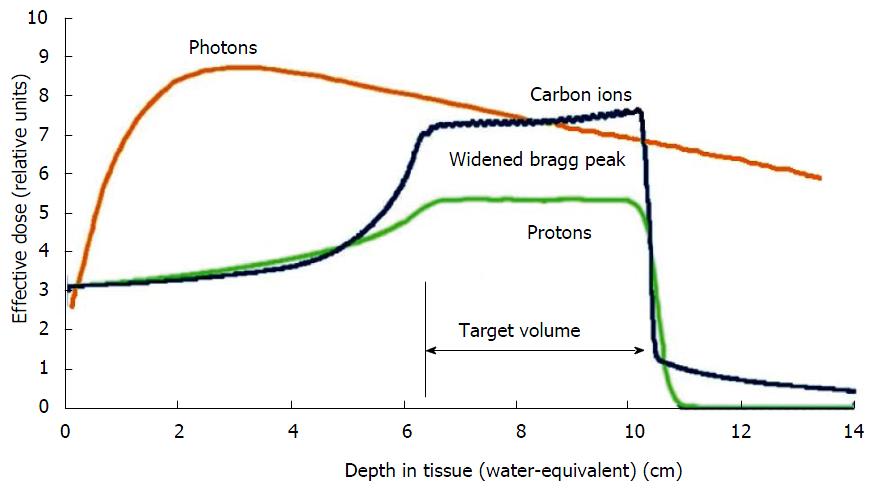
A least theoretically, more advantages could be exploited by the use of radiation qualities like protons or heavy ions. In contrast to photons, particle beams deposit most of the dose in a narrow range of tissue depth depending on the beam energy. This so-called “Bragg-peak” can be used to focus the dose very precisely to the target volume, while adjacent tissues can be safely spared (Figure 3). Especially heavy ions further show an enhanced biological effectiveness, because they generate a different pattern of DNA-damage in the tumor cells which is less easily repaired by cellular DNA-repair mechanisms in comparison to damages set by photon therapy. Some drawbacks remain in the upper abdomen due to difficulties to account for bowel gas movement during treatment planning. These can lead to large dosimetric uncertainties compared to photons[49]. Nevertheless several encouraging preliminary results have been reported by several centers. For example, the MGH group showed a very low severe gastrointestinal toxicity rate of 4% during chemoradiation in a phase I/II trial, where neoadjuvant proton radiotherapy with 5 × 5 Gy combined with simultaneous capecitabine and followed by resection and adjuvant gemcitabine was evaluated in primarily resectable pancreatic cancer. With a median follow-up of 38 mo, they reported a local recurrence rate of 16% and a median overall survival of 17 mo[50]. Investigators from Chiba (Japan) launched a phase I trial including 26 patients with resectable pancreatic cancer, treated with increasing doses of 30-36.8 Gy in 8 fractions with carbon ions of whom 81% proceeded to surgery. They reported a local control rate of 100% with 1- and 5-year survival rates of 89% and 52% in resected patients[51]. Irresectable pancreatic cancer patients were also included into a dose escalation trial with doses of 38.4-52.8 Gy in 12 fractions at the same center resulting in 81% local control and 60% overall survival after one year[52].
Figure 3 Schematic comparison of the depth dose curves of photons and particles.
Lower dose distribution in the radiation path before and after the target with particles by exploiting the Bragg-peak.
In summary, particle radiation therapy seems to be a promising modality with regard to high local control rates with low toxicity. However, the current knowledge is based only on a few studies with low patient numbers and short follow-up and has therefore to be regarded as preliminary. Due to the known uncertainties in dose calculation because of bowel gas movement, patients with pancreatic cancer should be treated only in prospective studies at experienced centers.
Current status of neoadjuvant radio (chemo) therapy in locally-advanced primarily non-resectable pancreatic cancer
The interpretation of the literature regarding the optimal therapy for locally-advanced pancreatic cancer is difficult for different reasons. First of all, very different treatment strategies exist, ranging from aggressive approaches with curative intent such as multimodal neoadjuvant treatment including regular surgical exploration to purely palliative systemic treatment approaches, with all conceivable steps in between. Further on, even if only series using very similar neoadjuvant approaches aiming at secondary resectability are assessed, they differ extensively in terms of patient selection. The distinction between resectable and irresectable lesions is flawed by a certain subjectivity, which clearly correlates with the experience of surgeon and center. Even if a lesion is deemed primarily irresectable, different sub-terms are in use. In most of the US literature, patients are sub-divided in borderline-resectable and unresectable depending on the extent of vessel involvement, while this differentiation is not commonly used in most parts of Europe and Asia. This results in the inclusion of very different advanced lesions in neoadjuvant approaches compromising reasonable comparisons. The primary aim of neoadjuvant approaches in patients with locally-advanced irresectable pancreatic cancer is the induction of tumor shrinkage and thereby the achievement of secondary resectability per se and the increase of the rate of microscopic negative (R0) resections. Secondary aims are response evaluation for further treatment stratification and improvement of quality of life by prevention of local symptoms in case of persistent irresectability. The impact of neoadjuvant radio(chemo)therapy has been evaluated in numerous retrospective and prospective studies. These show a wide range of results and therefore seem less reliable when taken individually[53]. However, Gillen et al[2] described some fundamental findings in an impressive metaanalysis including 111 studies with 4400 patients. They included trials evaluating primarily resectable and primarily irresectable patients but analyzed them separately. In the group deemed primarily resectable they found a final resection rate of 74% after neoadjuvant therapy which is very similar to the rate reported for surgery alone. In the group primarily deemed irresectable, they observed a final resection rate of 33% after neoadjuvant (mainly radiochemo-) therapy. Radiological response assessment after neoadjuvant therapy described complete and partial remission in 4% and 29% with a 21% progression rate. These rates were not different between resectable and irresectable patients. However, the most important finding was a median survival of 21 mo and a 2-year overall survival rate of 50% in the group of patients, who reached secondary resectability after neoadjuvant treatment. This equals the result in the primarily resectable group (median survival 24 mo, 2-year overall survival 47%), while patients in whom resection was not achieved showed a significantly worse overall survival (median 10 mo), independent of their initial resectability status. Morganti et al[53] performed another metaanalysis including 13 trials with 510 patients, which were deemed irresectable and had received neoadjuvant chemoradiation with at least 45 Gy. Interestingly, they reported similar results: final resection rate was 27% with 88% being complete (R0). Median survival after secondary resection (24 mo) was significantly improved in comparison to persistent irresectability (10 mo)[53]. One of the largest single center analyses from Heidelberg again showed similar results[54]. In 257 patients treated with neoadjuvant (mainly radiochemo-) therapy and surgical exploration, secondary gross total resection rate was 40% with a median survival of 25 mo after R0-resection[54]. Postoperative morbidity and mortality does not seem to be increased after neoadjuvant chemoradiation compared to surgery alone[55,56]. In summary, neoadjuvant radiochemotherapy for patients with primarily irresectable, locally-advanced pancreatic cancer results in secondary resectability in a substantial portion (30%-40%) of the patients, accompanied by a significantly improved prognosis in this subgroup. The median survival time (median approximately 24 mo) is similar to patients with primary resection and adjuvant chemotherapy. Even if secondary resectability is not achieved, the results after neoadjuvant chemoradiation are found at the upper end of the range reported for chemotherapy alone in the current literature, including the advantage of improved quality of life due to durable prevention of local complications by tumor progression.
Current status of neoadjuvant radio (chemo) therapy in primarily resectable pancreatic cancer
The rationale for neoadjuvant treatment approaches in resectable pancreatic cancer is based on several findings. First of all, locoregional progression is at least a component of disease progression in 50%-75% as shown by pattern of recurrence analyses after resection alone[57]. Even with the use of adjuvant chemotherapy, several studies reported local recurrence rates of 30%-60%. This suggests that eradication of locally persistent tumor cells by chemotherapy alone is not safely ensured[6,58]. As locoregional recurrences often result in local complications, the aim of achieving adequate local control seems justified with regard to quality of life.
Neoadjuvant strategies have replaced or at least supplemented sole adjuvant approaches as the standard of care in many other resectable gastrointestinal tumors, for example rectal cancer or esophageal cancer[5,59]. Unfortunately, no randomized data comparing neoadjuvant and adjuvant approaches in resectable pancreatic cancer have been published so far, although neoadjuvant approaches are investigated increasingly because of their potential benefits. Benefits include an improved local control rate for example due to an increased R0-resection rate, early initiation of at least a systemic therapy component to control potentially existing distant micrometastases, a simplified access to additional therapies and of course an optimal patient selection by exclusion of patients with early distant failure. The ongoing development will be illustrated exemplary by the work of the MDACC group, which designed and performed a number of consecutive phase II trials over nearly 2 decades. They started with conventionally-fractioned radiation therapy combined with 5-FU[60], went on with a shortened radiochemotherapy with additional IOERT[61] and ended up with paclitaxel[62] and finally gemcitabine-based preoperative chemoradiation[63]. The last concept was evaluated in a phase II trial with 86 patients, who received a shortened radiation therapy (10 × 3 Gy in 2 wk) in combination with weekly gemcitabine 400 mg/m2 over 7 wk. Resection was finally achieved in 74% of the patients. The median overall survival for the entire cohort was 23 mo with a 5-year survival rate of 27%. Resectable patients had a significantly improved median survival of 34 mo compared to 7 mo in irresectable patients. The same was true for the 5-year survival rate (36% vs 0%). The local control rate in resected patients was 89%[63]. The authors concluded, that neoadjuvant chemoradiation allows a good selection of patients, who probably will not profit from major surgery. They recommended further investigation of neoadjuvant gemcitabine-based chemoradiation in resectable patients based on the very encouraging overall survival results[63], especially because a parallel trial by the same group with additional induction chemotherapy showed no further benefit[64]. The consistency regarding definition of resectability, surgical treatment and histological examination further suggests a good comparability of the results in the different MDACC trials[65]. Gemcitabine-based radiochemotherapy resulted in improved response rates, improved R0-resection rates and longer median survival in comparison to combinations with 5-FU or paclitaxel[65]. A pooled analysis of the MDACC studies including 240 patients treated with surgery after neoadjuvant therapy finally revealed a median disease-free survival of 15 mo and a median overall survival of 34 mo[66]. The potential benefit of neoadjuvant radiation therapy for resectable pancreatic cancer is further supported by a SEER-analysis on more than 3800 patients, which described a significant improved median survival of 24 mo after neoadjuvant radiation therapy compared to 17 mo with adjuvant and 12 mo without radiation therapy[67].
In summary, neoadjuvant radio- or radiochemotherapy offers several potential benefits compared to adjuvant strategies, although no randomized data are currently available to support this assumption. Nevertheless, neoadjuvant radio(chemo)-therapy has shown outstanding results in terms of response, local control and overall survival at least in phase II trials. These results clearly justify further investigation of neoadjuvant radiation therapy approaches. In this context, further shortening of neoadjuvant radiation therapy schemes might be beneficial, as currently investigated in several prospective trials evaluating modern photon or proton techniques[28,50].
Future directions
As mentioned earlier, this article focuses on radiotherapeutic strategies including radiotherapy alone or combined with concurrent chemotherapy in the neoadjuvant setting. Within such approaches, chemotherapy is used mainly as a radiation sensitizer rather than as systemic treatment resulting in low doses and usually single drug treatment to keep combined toxicity acceptable. However, recently new chemotherapy agents and combinations like Gemcitabine/nab-Paclitaxel[68] or FOLFIRINOX[69] have been successfully introduced into the treatment of metastatic pancreatic cancer and resulted in improved response and overall survival. Therefore it seems reasonable to use these schemes also in the neoadjuvant setting either to target possible distant micrometastases as early as possible in patients with resectable disease or to induce tumor shrinkage in irresectable patients to achieve secondary resectability. Due to the increased toxicity profile of these potent combinations, concurrent application of radiation does not seem possible even with the most sophisticated radiation techniques. Therefore sequential applications for example induction chemotherapy with FOLFIRINOX followed by chemoradiation with 5-FU or gemcitabine seem to be very promising and are currently under investigation (for example in the german CONKO 007 study), with some groups already showing very encouraging preliminary results[70]. Therefore additional aims for future radiation research in pancreatic cancer should include the evaluation of optimal sequencing of systemic and radiotherapeutic approaches as well as the identification of biomarkers to predict the pattern of disease progression in the individual patient.
Biomarker for stratification
One of the main challenges in the treatment of pancreatic cancer remains the insufficient possibilities for an early prediction of disease progression. This compromises a reasonable stratification of patients in terms of treatment combinations. Established and new biomarkers could be helpful. This will be illustrated exemplary in the following with CA 19-9 serving as example for an established and SMAD for a new marker. Several groups established an association between increased pretreatment CA 19-9 levels and an unfavourable outcome[71], with very high values indicating an already disseminated disease. Kim et al[72] for example showed stage-dependent median CA 19-9 levels between 40 and 748 U/mL in stage IA-III compared to a median CA 19-9 level of 3239 U/mL in stage IV. However, two major disadvantages limit the value of pretreatment CA 19-9 levels for prediction of disease prognosis: 5%-10% of patients with pancreatic cancer show negative CA 19-9 levels due to a defect in the gen coding for Lewis enzyme[73] and CA 19-9 levels can be heavily influenced by other factors, for example cholestasis. Therefore increasing interest has been paid to new markers like SMAD4. The SMAD family of proteins plays a role in TGF-beta signaling, which is heavily involved in the regulation of cell proliferation, differentiation and apoptosis[74]. SMAD4 has been recently suggested as the most important candidate in regard to pancreatic cancer because it has been linked not only with tumor development but also with the pattern of disease progression[75]. In this context, the presence of intact SMAD4 seems to be associated with a rather locally-destructive growth, while loss of SMAD4 correlates with early distant metastasis[76]. These findings were supported by a trial performed by Crane et al[77], which found that 73% of the patients with intact SMAD4 showed locoregional progression while 74% of the patients with inactive SMAD4 developed distant failure. In summary, although current knowledge about biomarkers seems premature in regard to treatment stratification, this might be an encouraging opportunity to allow an improved allocation of patients to locally-aggressive vs systemic treatment approaches to strengthen personalized medicine also for pancreatic cancer in the future.
SUMMARY
In the absence of randomized data, published studies show consistently that neoadjuvant chemoradiation for locally-advanced, primarily non- or borderline resectable pancreas cancer results in secondary resectability in a substantial proportion of patients with consecutively markedly improved overall prognosis in this subgroup. Even if the goal of secondary resectability is not reached, radiation therapy may contribute to improved quality of life by the prevention of local complications. Neoadjuvant chemoradiation should therefore be considered as possible alternative in multidisciplinary pretreatment evaluations.
In resectable pancreatic cancer, outstanding results in terms of response, local control and overall survival have been observed with neoadjuvant radio- or radiochemotherapy in several phase I/II trials. These undoubtly justify further evaluation of this strategy. In this context, further shortening of the radiation therapy series to allow a simplified integration into multimodal concepts is evaluated in ongoing trials.
Further investigation of neoadjuvant chemoradiation strategies should be performed preferentially in randomized trials in order to improve comparability of the current results with other treatment modalities. This should include the evaluation of optimal sequencing with newer and more potent systemic induction therapy approaches. Advances in patient selection based on new molecular markers might be of crucial interest in this context.
Finally modern external beam radiation techniques (IMRT, IGRT, SBRT), new radiation qualities (protons, heavy ions) or combinations with alternative boosting techniques (IOERT) widen the therapeutic window and contribute to the reduction of toxicity by improving normal tissue sparing and/or increasing efficacy by dose escalation or enhanced biological effectiveness. These techniques offer innovative treatment strategies, which should be further evaluated in prospective controlled trials.