Published online Jul 15, 2014. doi: 10.4251/wjgo.v6.i7.244
Revised: January 17, 2014
Accepted: April 16, 2014
Published online: July 15, 2014
Processing time: 239 Days and 14.4 Hours
AIM: To determine if other molecules reported to modulate AMP-dependent protein kinase (AMPK) activity would have effects resembling those of metformin and phenformin on colon cancer cell proliferation and metabolism.
METHODS: Studies were performed with four human colon cancer cell lines, Caco-2, HCT116, HT29 and SW1116. The compounds that were studied included A-769662, 5-aminoimidazole-4-carboxamide-1-ribofuranoside, butyrate, (-)-epigallocatechin gallate (EGCG), KU-55933, quercetin, resveratrol and salicylates. The parameters that were measured were cell proliferation and viability, glucose uptake, lactate production and acidification of the incubation medium.
RESULTS: Investigations with several molecules that have been reported to be associated with AMPK activation (A-769662, 5-aminoimidazole-4-carboxamide-1-b-D-ribofuranoside, EGCG, KU-55933, quercetin, resveratrol and salicylates) or AMPK inhibition (compound C) failed to reveal increased medium acidification and increased glucose uptake in colon cancer cells as previously established with metformin and phenformin. The only exception was 5-aminosalicylic acid with which there were apparently lower glucose levels in the medium after incubation for 72 h. Further study in the absence of cells revealed that the effect was an artifact due to inhibition of the enzyme-linked glucose assay. The compounds were studied at concentrations that inhibited cell proliferation.
CONCLUSION: It was concluded that treatment with several agents that can affect AMPK activity resulted in the inhibition of the proliferation of colon cancer cells under conditions in which glucose metabolism is not enhanced, in contrast to the effect of biguanides.
Core tip: Treatment with several agents that can affect AMP-dependent protein kinase activity resulted in the inhibition of the proliferation of colon cancer cells under conditions in which glucose metabolism is not enhanced, in contrast to the effect of biguanides.
- Citation: Lea MA, Pourat J, Patel R, desBordes C. Growth inhibition of colon cancer cells by compounds affecting AMPK activity. World J Gastrointest Oncol 2014; 6(7): 244-252
- URL: https://www.wjgnet.com/1948-5204/full/v6/i7/244.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i7.244
In previous publications we reported that the biguanides, metformin and phenformin, inhibited proliferation of colon cancer cells under conditions in which glucose uptake was increased and there was increased glycolysis as judged by acidification of the incubation medium[1,2]. This is an unusual combination of effects and raises the question of whether other molecules might have similar action. Although the biguanides have a long history in the treatment of Type II diabetes there has been uncertainty regarding their mechanism of action[3,4]. Interest in mechanisms has been further stimulated by observations that metformin may exert a cancer chemopreventive effect and this has led to ongoing clinical trials against different types of cancer[5,6]. The most commonly suggested mechanisms for the action of biguanides include a stimulation of AMP-dependent protein kinase (AMPK) and inhibition of complex I in the mitochondrial electron transport chain. The hypothesis to be tested in the present work was that other molecules reported to modulate AMPK activity would have effects on colon cancer cell proliferation and metabolism resembling those of biguanides. We chose to examine the action of a variety of compounds that have been reported to activate or inhibit AMPK. Activators included A-769662[7], 5-aminoimidazole-4-carboxamide-1-b-D-ribofuranoside (AICAR)[8], (-)-epigallocatechin gallate (EGCG)[9], KU-55933[10], quercetin[11], resveratrol[12] and salicylates[13]. The most widely studied inhibitor of AMPK is compound C and that compound has been shown to affect proliferation of colon cancer cells[1]. Butyrate has been most commonly considered as an inhibitor of histone deacetylase activity but activation of AMPK by butyrate has been reported. In a previous study we observed that the induction of alkaline phosphatase by butyrate in colon cancer cells was not significantly affected by coincubation with A-769662[1]. However, in the present work some additive effects on metabolism and cell proliferation have been seen after coincubation of butyrate and A-76992 with colon cancer cells.
SW1116, HCT116, HT29, and Caco-2 human colon cancer cells were obtained from the American Type Culture Collection, Rockville, MD, United States, and were incubated at 37 °C in RPMI-1640 medium with 5% fetal calf serum. Of these cell lines, the HCT116 cells exhibited the most rapid proliferation, and the slowest growth was seen with the SW1116 cells. Cell proliferation was generally monitored by the increase in protein. In studies with 96-well plates, the procedure involved staining with sulforhodamine B essentially as described by Vichai et al[14]. Cells were routinely allowed to attach to tissue culture dishes or 96-well plates for 24 h before changing the medium. The cells were then incubated for a further 72 h before determining the impact of the compounds under study on medium pH, glucose concentration, and cell proliferation as judged by protein mass. Cell viability was monitored using the Presto Blue Viability Reagent from Life Technologies Corporation, Carlsbad, CA, United States.
A-769662 was purchased from LC Laboratories, Woburn, MA, United States. AICAR, butyrate, (-)-epigallocatechin gallate, metformin, phenformin, quercetin, resveratrol, salicylic acid, acetylsalicylic acid, 4-aminosalicylic acid and 5-aminosalicylic acid were obtained from Sigma-Aldrich, St. Louis, MO, United States. KU-55933 was purchased from Selleck Chemical, Houston, TX, United States.
pH determination with an electrode was found previously to correlate well with changes in the light absorbance at 560 nm reflecting changes in the pH indicator, phenol red, where a higher absorbance reflects a higher pH[1]. The latter method was found particularly convenient for work with 96 well plates and was used routinely in the present work.
Glucose was assayed in the cell culture medium using GAGO-20 Kit from Sigma-Aldrich. This is a colorimetric procedure in which the oxidation of glucose is coupled with glucose oxidase and peroxidase to the oxidation of dianisidine.
Lactate in the medium was determined using the assay kit obtained from Eton Bioscience, San Diego, CA, United States.
Data are presented as means and standard deviations. Statistical significance of the results was determined by a two-tailed Student’s t test or by Dunnett’s test for multiple comparisons using the Instat program from GraphPad Software, Inc., La Jolla, CA, United States. A probability of less than 5% was considered significant and differences compared to the control are shown.
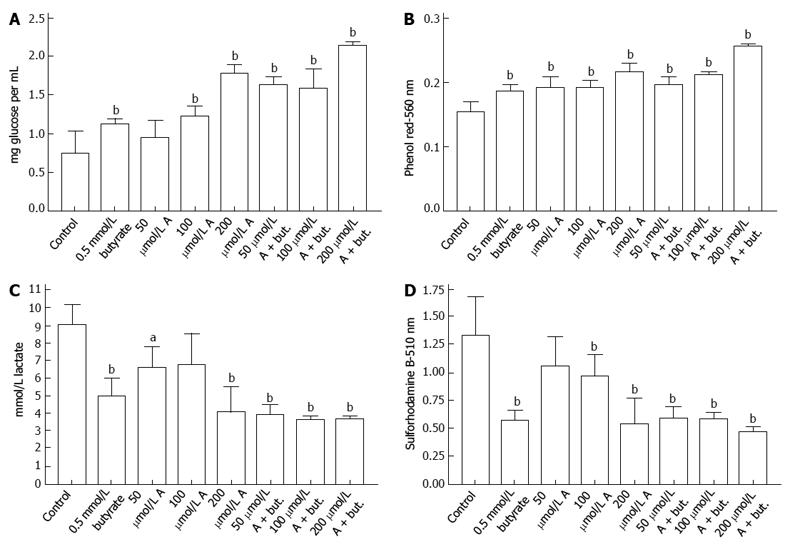
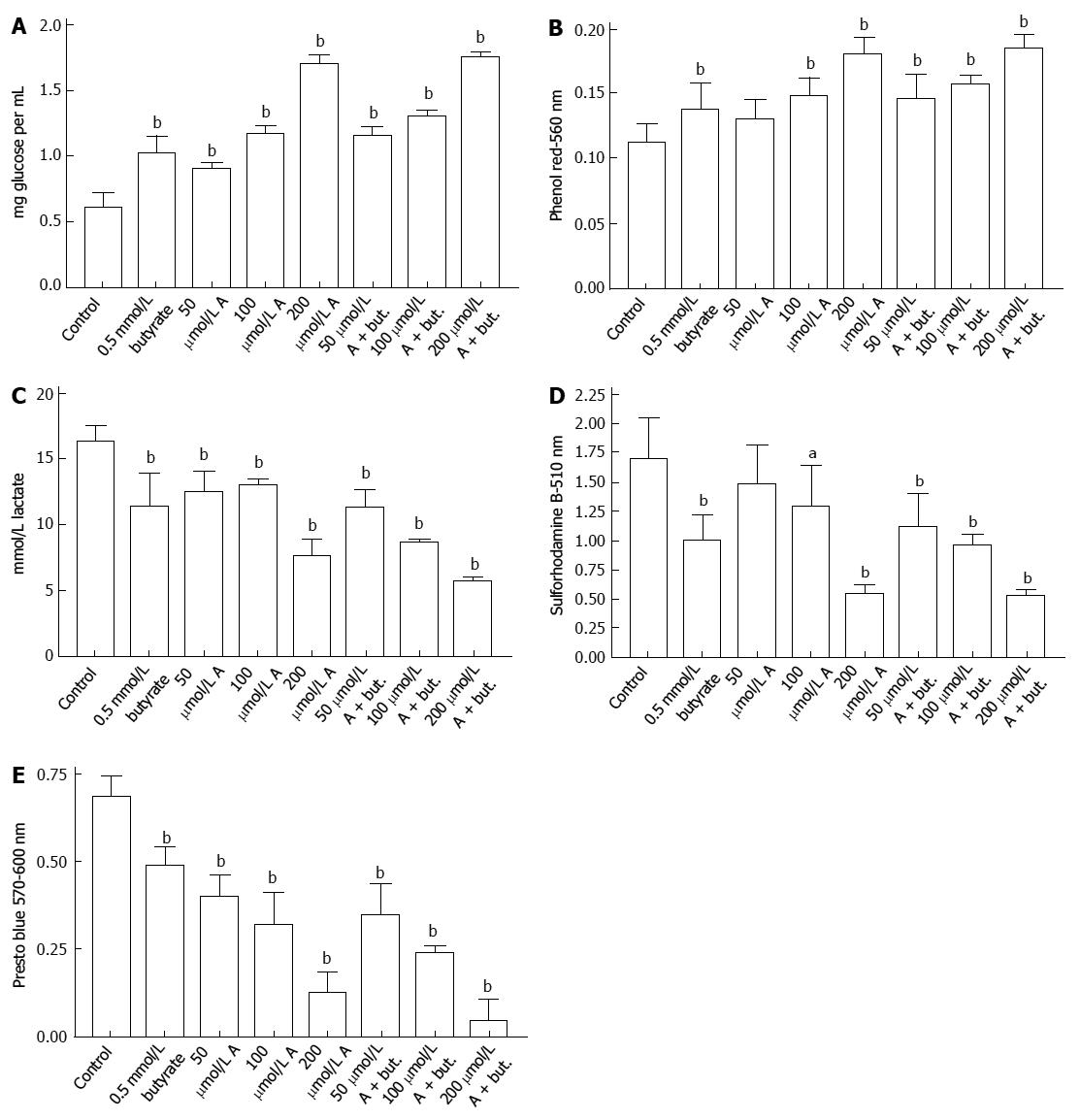
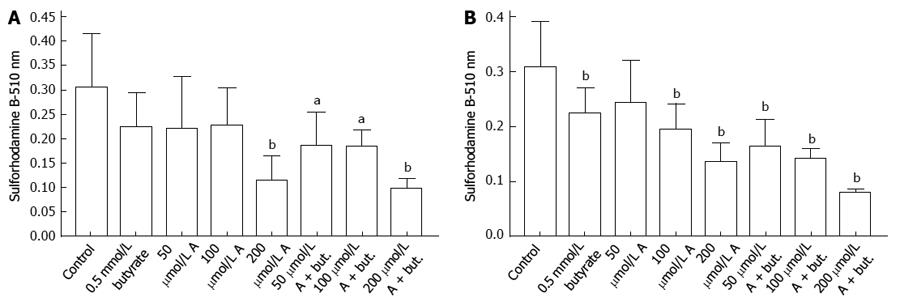
The uptake of glucose by HCT116 colon cancer cells was inhibited by incubation with butyrate or A-769662 for 72 h. This is shown in Figure 1A where the final glucose concentrations in the medium are shown after an initial glucose concentration of 2 mg/mL. Decreased glucose uptake paralleled decreased acidification of the incubation medium (Figure 1B) and decreased lactate production (Figure 1C). The data in Figure 1D indicate inhibitory effects of butyrate and A-769662 on proliferation of HCT116 cells as judged by staining with sulforhodamine B. The data in Figure 2A-D show similar responses in HT29 cells to those seen with HCT116 cells. Measurement of metabolic activity in HT29 cells as reflected in the reduction of Presto Blue show a similar profile to that seen with sulforhodamine B staining and suggest some additive action when there is coincubation with butyrate and A-769662 (Figure 2E). Effects on metabolism in the more slowly growing Caco-2 and SW1116 cells were not as marked as in the more rapidly growing HT29 and HCT1116 cells but the results in Figure 3 show some degree of additive effect of butyrate and A76992 on the inhibition of cell proliferation.
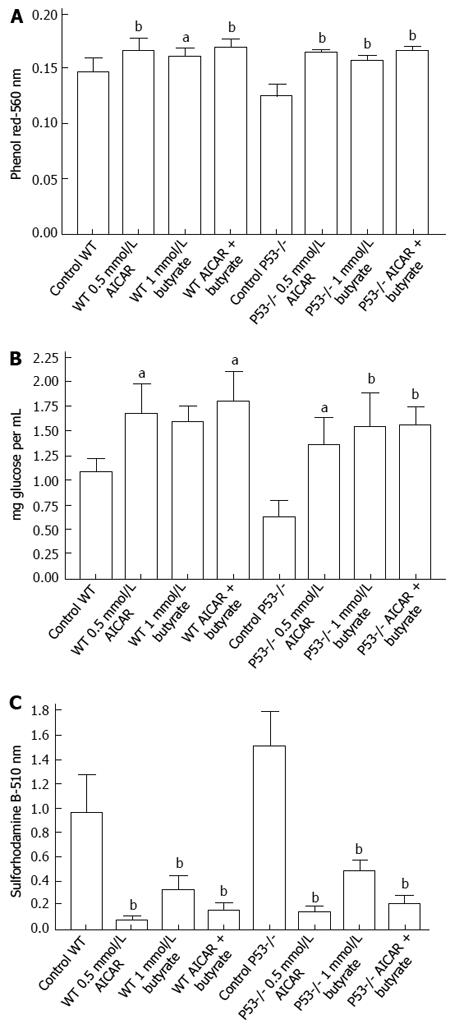
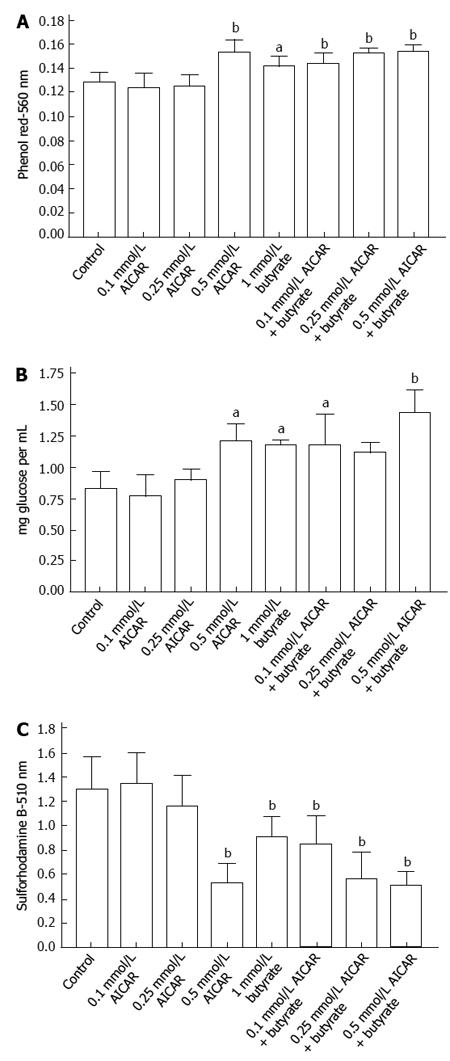
Significant effects on glucose uptake were not seen when Caco-2 cells were incubated for 72 h with 0.5 and 1 mm AICAR but as shown in Figure 4A there were increases in medium pH suggesting less glycolysis and this was accompanied by decreased proliferation (Figure 4B). With the more rapidly dividing HCT116 wild type or p53 null cells there were significant decreases in medium acidification when cells were incubated with 0.5 mmol/L AICAR (Figure 5A) together with decreased glucose uptake (Figure 5B) and decreased cell proliferation (Figure 5C). Similarly with HT29 cells, AICAR at 0.1, 0.25 and 0.5 mmol/L caused the same trends (Figure 6).
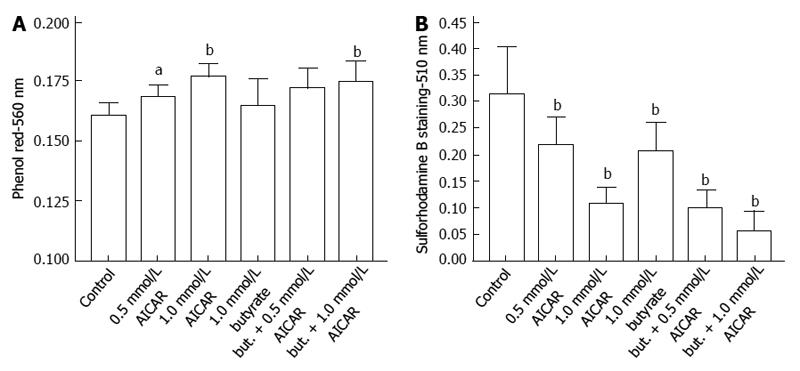
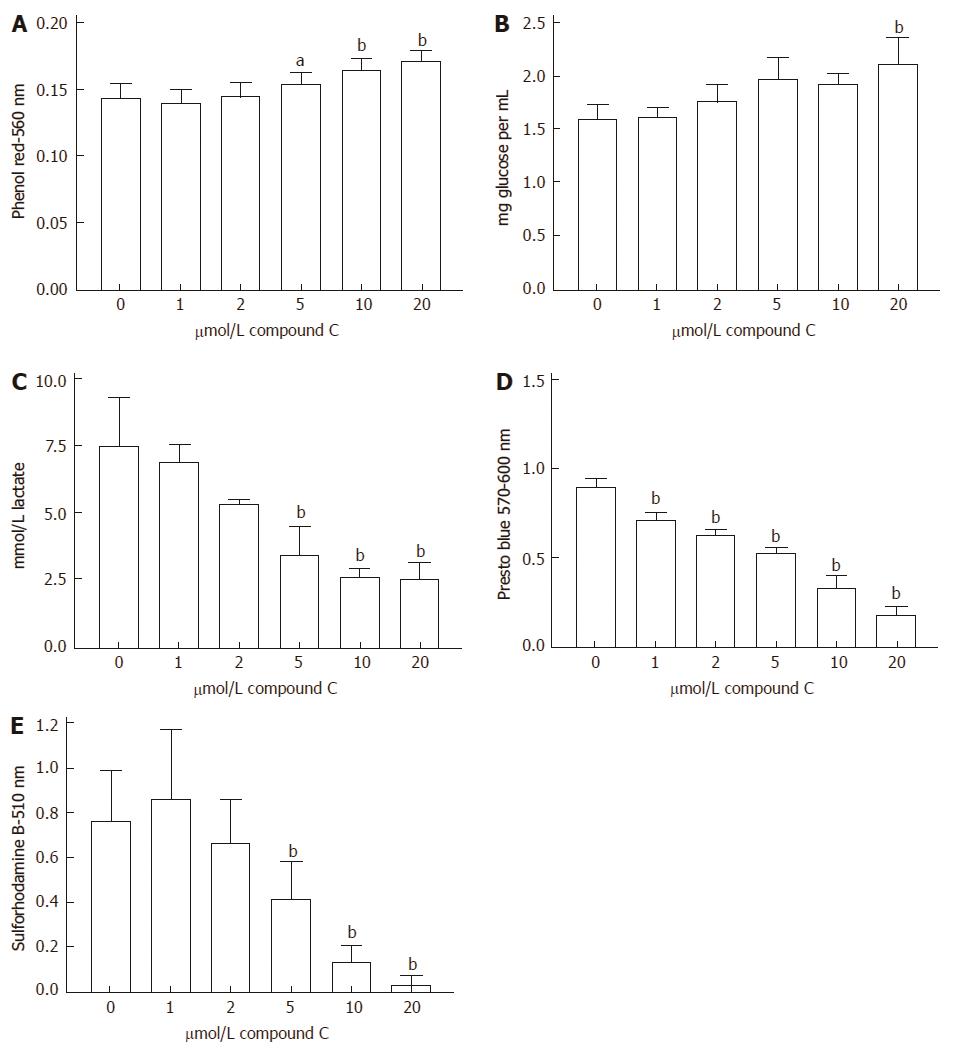
In addition to effects of AMPK activators, inhibitory effects on medium acidification, glucose uptake, lactate production, reduction of Presto Blue and cell proliferation were also seen when the AMPK inhibitor, compound C, was incubated for 72 h with HCT116 cells (Figure 7).
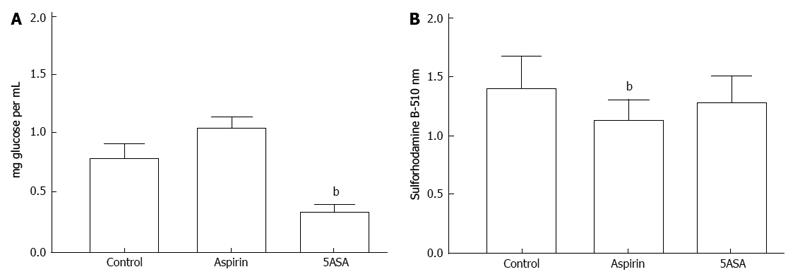
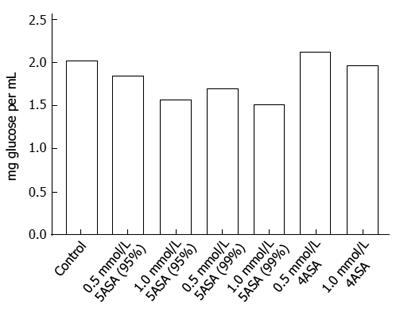
Studies with several molecules that have been reported to be associated with AMPK activation (salicylates, EGCG, KU-55933, quercetin and resveratrol) failed to reveal increased medium acidification and increased glucose uptake in colon cancer cells as previously established with metformin and phenformin[1]. The only exception was 5-aminosalicylic acid with which there were apparently lower glucose levels in the medium after incubation for 72 h (Figure 8A). This was surprising because the effect was not associated with increased medium acidification as seen with the biguanides and was seen at a concentration that did not result in significant inhibition of cell proliferation (Figure 8B). Further examination in the absence of cells revealed that the effect was an artifact due to inhibition of the enzyme-linked glucose assay. There was specificity for the effect because it was seen with 5-aminosalicylic acid but not with 4-aminosalicylic acid (Figure 9). The effect was seen with two samples of 5-aminosalicylic acid from Sigma-Aldrich, one containing 95% and the other containing 98% of the compound.
Our previous studies on the effects of metformin and phenformin on colon cancer cells revealed an unusual combination of effects[1]. These were an inhibition of cell proliferation despite an increase in glucose uptake and an increase in lactate production as monitored by acidification of the medium. Information in the literature suggests that biguanides may inhibit complex 1 of the mitochondrial transport chain and may result in activation of AMPK. The latter effect may not be direct and may be a consequence of increased levels of AMP and ADP or may be mediated through an upstream kinase, LKB1. We chose to examine the significance of AMPK activation on the metabolism and proliferation of colon cancer cells by studying the action of a variety of compounds reported to affect AMPK activity. The best characterized of these are A-769662[15] and AICAR[16]. These two compounds were found to be potential inhibitors of colon cancer cell proliferation but we observed neither an increase in glucose uptake nor increased medium acidification. To the contrary, decreased glucose uptake and decreased medium acidification was seen particularly with the more rapidly proliferating HT29 and HCT116 colon cancer cells.
Some of the compounds that were studied have been reported to be activators or inhibitors in different systems. Thus, quercetin has been reported to be an activator of AMPK[11] but Kim et al[17] found inhibition of AMPK by quercetin in HCT116 cells. Activation of AMPK by resveratrol has been reported[12,18] but Skrobuk et al[19] reported a situation in skeletal muscle where AMPK was inhibited by resveratrol. Compound C has consistently been found to be an inhibitor of AMPK. Although Compound C is a cell-permeable pyrrazolopyrimidine compound that can act as a reversible and ATP-competitive inhibitor of AMPK, actions on other target molecules have been reported[20]. We have extended our earlier studies with compound C and found that at concentrations frequently used to inhibit AMPK (1-10 μmol/L) it can be a potent inhibitor of colon cancer cell proliferation, most notably with HCT116 cells. Under those circumstances there was decreased glucose uptake and decreased acidification of the medium.
Potential chemopreventive action against colon cancer has been noted for some salicylates including acetylsalicylate[21] and 5-aminosalicylate[22]. At a concentration of 1 mmol/L we found that acetylsalicylic acid was a more potent inhibitor of colon cancer cell proliferation than 5-aminosalicylic acid. However, only with 5-aminosalicylic acid was there an apparent increase in glucose uptake. Further studies in the absence of cells indicated that this effect was due to interference with the enzyme-linked assay procedure for glucose. The assay uses a combination of glucose oxidase and peroxidase. It remains to be established whether one of these enzymes was more sensitive to the action of 5-aminosalicylic acid.
The tendency of cancer cells to show increased rates of glucose uptake and glycolysis even under aerobic conditions has become known as the Warburg effect. There is a paradox in that biguanides are of interest for their preventive or therapeutic action against cancer despite the observation that they seem to enhance the Warburg effect. The degree to which activation of AMPK relates to anti-cancer actions of biguanides remains an area of uncertainty[23-25]. It may be concluded from the present study that treatment with several agents that can affect AMPK activity results in the inhibition of the proliferation of colon cancer cells under conditions in which glucose metabolism is not enhanced.
Although there is a long history of the use of biguanides such as metformin in the treatment of type 2 diabetes, there is recent interest in the potential value of biguanides in the prevention and therapy of cancer. Rationale use of biguanides will be aided by comparison of their action with other compounds that can also affect AMP-dependent protein kinase (AMPK) activity.
Ongoing studies are investigating whether actions of biguanides on cancer cells relate to modulation of AMPK activity, effects mediated through inhibition of mitochondrial electron transport or changes in circulating insulin levels or combinations of these actions.
The observations described here emphasize that while biguanides and other compounds that modulate AMPK activity can affect the proliferation of cancer cells, there appears to be a unique pattern in the effects of metformin and phenformin that is also associated with increased glucose uptake and acidification of the extracellular environment.
The present work adds to the authors’ knowledge of combined action of biguanides with other agents that may guide future combination therapies for the treatment of colon cancer. The results emphasize the need to better characterize actions of biguanides that relate to mechanisms other than modulation of AMPK activity.
AMPK regulates metabolism so as to increase ATP production and limit ATP utilization. One potential mechanism of action for biguanides is to cause the upregulation of AMPK.
The authors have investigated the inhibition of growth of colon cancer cells by compounds that affect AMPK activity but have divergent effect on metabolism. They have managed to show that treatment with several agents that can affect AMPK activity results in the inhibition of the proliferation of colon cancer cells under conditions in which glucose metabolism is not enhanced.
P- Reviewers: Lee KY, Koukourakis GV, Wang XS S- Editor: Gou SX L- Editor: A E- Editor: Liu SQ
| 1. | Lea MA, Chacko J, Bolikal S, Hong JY, Chung R, Ortega A, desBordes C. Addition of 2-deoxyglucose enhances growth inhibition but reverses acidification in colon cancer cells treated with phenformin. Anticancer Res. 2011;31:421-426. [PubMed] |
| 2. | Lea MA, Qureshi MS, Buxhoeveden M, Gengel N, Kleinschmit J, desBordes C. Regulation of the proliferation of colon cancer cells by compounds that affect glycolysis, including 3-bromopyruvate, 2-deoxyglucose and biguanides. Anticancer Res. 2013;33:401-407. [PubMed] |
| 3. | Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62:2164-2172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 358] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 4. | Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link - ten years after. BMC Biol. 2013;11:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 5. | Pollak M. Potential applications for biguanides in oncology. J Clin Invest. 2013;123:3693-3700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Göransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282:32549-32560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 353] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 8. | Sakamoto K, Göransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310-E317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Park SY, Lee YK, Kim YM, Park OJ and Shin JI. Control of AMPK-activated protein kinase, Akt, and mTOR in EGCG-treated HT-29 colon cancer cells. Food Sci Biotech. 2013;22:147-151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Zakikhani M, Bazile M, Hashemi S, Javeshghani S, Avizonis D, St Pierre J, Pollak MN. Alterations in cellular energy metabolism associated with the antiproliferative effects of the ATM inhibitor KU-55933 and with metformin. PLoS One. 2012;7:e49513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93:891S-8916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 12. | Hayakawa N, Shiozaki M, Shibata M, Koike M, Uchiyama Y, Matsuura N, Gotow T. Resveratrol affects undifferentiated and differentiated PC12 cells differently, particularly with respect to possible differences in mitochondrial and autophagic functions. Eur J Cell Biol. 2013;92:30-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 588] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 14. | Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2223] [Cited by in RCA: 2580] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 15. | Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 736] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 16. | Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 955] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 17. | Kim HS, Wannatung T, Lee S, Yang WK, Chung SH, Lim JS, Choe W, Kang I, Kim SS, Ha J. Quercetin enhances hypoxia-mediated apoptosis via direct inhibition of AMPK activity in HCT116 colon cancer. Apoptosis. 2012;17:938-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Kim MY, Lim JH, Youn HH, Hong YA, Yang KS, Park HS, Chung S, Ko SH, Shin SJ, Choi BS. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia. 2013;56:204-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 19. | Skrobuk P, von Kraemer S, Semenova MM, Zitting A, Koistinen HA. Acute exposure to resveratrol inhibits AMPK activity in human skeletal muscle cells. Diabetologia. 2012;55:3051-3060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45:276-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 316] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 21. | Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, Alessi DR, Dunlop MG. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504-1505.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 334] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 22. | Munding J, Ziebarth W, Pox CP, Ladigan S, Reiser M, Hüppe D, Brand L, Schmiegel W, Tannapfel A, Reinacher-Schick AC. The influence of 5-aminosalicylic acid on the progression of colorectal adenomas via the β-catenin signaling pathway. Carcinogenesis. 2012;33:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol. 2012;29:1314-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 24. | Rizos CV, Elisaf MS. Metformin and cancer. Eur J Pharmacol. 2013;705:96-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Russo GL, Russo M, Ungaro P. AMP-activated protein kinase: a target for old drugs against diabetes and cancer. Biochem Pharmacol. 2013;86:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |