Published online Jun 15, 2014. doi: 10.4251/wjgo.v6.i6.170
Revised: May 1, 2014
Accepted: May 14, 2014
Published online: June 15, 2014
Processing time: 223 Days and 3.7 Hours
In an attempt to improve upon the end results obtained in treating colorectal cancer it was apparent that the earlier the diagnosis that could be obtained, the better the chance for obtaining desired results. In the case of more advanced tumors typified by later stage colorectal cancer, surgical debulking is an important part of the treatment strategy. Here the use of additional therapeutic modalities including chemotherapy and present day immunotherapy has failed to accomplish the desired improvements that have been sought after. Adjuvant therapy, has offered little to the overall survival. The concept of early detection is now recognized as the initial step in reaching proper end results and can readily be demonstrated from colorectal cancer studies. Here survival has been found to be a reflection of the stage at which the tumor is first identified and treated. When specific monoclonals targeting colorectal cancer are employed diagnostically, we have been able to demonstrate detection of colorectal cancer at its inception as a premalignant lesion, such that genotypic features can be identified before the phenotypic appearance of cancer can be noted.
Core tip: The ideal monoclonal antibody to be employed in cancer management is one targeting an immunogenic protein expressed in a specific cancer system. Those presently employed in cancer management, target a growth factor or carbohydrate antigen seen in both cancer and normal tissue. Their value as such is limited. The monoclonals described herein are directed against colon cancer tumor associated antigen and have value in both diagnostic and therapeutic uses for controlling this disease.
- Citation: Arlen M, Arlen P, Coppa G, Crawford J, Wang X, Saric O, Dubeykovskiy A, Molmenti E. Monoclonal antibodies that target the immunogenic proteins expressed in colorectal cancer. World J Gastrointest Oncol 2014; 6(6): 170-176
- URL: https://www.wjgnet.com/1948-5204/full/v6/i6/170.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i6.170
For most malignancies such as colorectal cancer, the earlier the diagnosis the better the chance for offering the patient the opportunity to be cured[1-5]. The addition of additional methods to help improve survival, especially in the post operative period, have offered little to achieve a better response[6-9]. In order to define methods for earlier intervention, we began to look at behavioral patterns seen in various stages of colorectal cancer, attempting to define those patterns related to tumor antigen expression. We were able as such, to identify and characterize a unique group of immunogenic proteins that appeared to be expressed in all colorectal carcinomas that were examined. These tumor (associated) antigens were found to be present in all stages of colorectal tumor development, from inception to metastasis. Following the separation of these proteins from pooled tumor cell membranes, monoclonal antibodies targeting these proteins were developed. Hybridomas were produced by injection of BALBc mice with the antigens/proteins so obtained.
By employing those monoclonal antibodies derived against the tumor proteins, it appeared that the antigen, noted to be expressed in the earliest stages of tumor development, continued to be present throughout later stages of progression of tumor growth. As a result, we were able to define the appearance of genetic alterations occurring in normal appearing cells that first characterized the transformation process. This initial pattern of cellular transformation was typified by the expression of immunogenic tumor proteins in the earliest stages of genotypic transformation when phenotypic features still appeared normal by standard HE. As with the invasive cell which sheds its antigen into the serum, the premalignant cell similarly sheds antigen into the stool which can easily be identified by a stool enzyme-linked immunosorbent assay (ELISA). Tissue biopsies studied by immunohistochemistry to define cells expressing tumor antigen and examination of stool for the presence of tumor antigen can now offer the asymptomatic patient the opportunity for proper screening. As a result one can now offer a practical process for early detection of a developing malignancy when optimum results can almost always be anticipated.
We now believe that it is possible to define the presence or absence of colon cancer during the screening process of the asymptomatic patient. If validated by our studies, the need to employ colonoscopy would be markedly reduced and relegated to those patients where there is a high likely hood for defining an early malignancy or when biopsy is required for confirmation of as well as staging of the disease process.
As noted above, the early premalignant cells undergoing transformation, as well as polypoid tumors and larger malignancies do shed tumor antigen into the stool where they can be detected by stool ELISA using our colon tumor monoclonals. This procedure can be used as a confirmatory measure to determine whether colonoscopy is or is not indicated as a follow up in post op patients in order to detect early developing lesions as well as possible anastomotic recurrences.
These same antibodies, used for detecting colon specific tumor associated antigens, also have therapeutic efficacy. Should the clinical work up of a malignant lesion demonstrate spread of tumor, the monoclonals that were employed for diagnosis of the tumor marker, can now be delivered intravenously to target those cells producing tumor antigen and destroy them through the process of antibody dependent cell cytotoxicity (ADCC).
At the present time, most of the tumor markers employed commercially for tumor detection and diagnosis are non specific. Those clinically available, best serve to monitor the response to the therapy being employed rather than to detect and diagnose the presence of a lesion. Those markers that appear in the serum are mostly derived from carbohydrate antigens that are shed into the serum. They are not only expressed by the tumor, but also by adjacent normal tissue that may have been effected by an ongoing inflammatory process[10-12].
In order to detect the presence of colon tumors at the ideal time, it is important to be able to define a specific marker or family of markers on the tumor when clinical symptoms were minimal if not totally absent. Such markers have been shown to best be represented by one or several immunogenic proteins or glycoproteins expressed on the cell surface membrane and found to shed into the serum as well as surrounding tissue. Those immunogenic proteins that characterize colon cancer have been isolated and characterized by our group at Precision Biologics. Pooled allogeneic specimens of colon cancer were used to retrieve tumor membrane proteins, separate them by molecular weight and then skin test the patient to define that specific group of proteins producing delayed cutaneous sensitivity. Further separation by isoelectrophoresis yielded three distinct glycoproteins that proved to represent oncofetal proteins first expressed in the fetus and later in a mutated form, representing specific colon cancer proteins that help induce a mild immune response. The failure to achieve a full immune response proved to be due to minimal expression of antigen in the tumor that was necessary to induce a proper immune response.
Using monoclonal antibodies developed against these immunogens, a serum ELISA was also developed that is capable of identifying shed markers with a high degree of sensitivity and specificity[13]. The monoclonal antibodies that specifically target these tumor proteins, have demonstrated that these proteins serve both as diagnostic markers and a therapeutic targets[14].
It is well known that of the many methods being developed to control the more aggressive colon lesions, not only does one rely on newer chemotherapeutic agents, but additionally through enhancement of the immune system. This can be accomplished by combining chemotherapy with a monoclonal antibody such as the one directed against the epidermal growth factor 1[15]. The process of adding an immunotherapeutic agent to standard chemotherapeutic drugs does rely on the nature of the antigen expressed by the tumor. This of course can be accomplished by immunohistochemical analysis of the tumor. The same effective monoclonal antibody that detected the presence of the tumor antigen/marker in the biopsy specimen can then be used intravenously along with chemotherapy, to attack the marker as a therapeutic target. In such combinations, the chemotherapeutic agent may serve to minimize the presence of any shed blocking material from the tumor to secondarily enhancing the immune response. Such enhancement in immune reactivity frequently helps the host defense mechanisms to control disease progression[16-19].
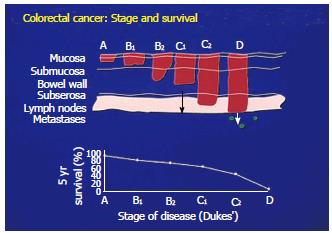
When a primary colon tumor is confined to the mucosa of the bowel, cure is just about guaranteed by surgical removal. However, when the tumor is found to penetrate into the muscular layers of the bowel, or invades the serosal surface with regional nodes possibly being involved, the opportunity for cure diminishes (Figure 1). Here additional modalities of therapy are essential if improvement in survival is to be accomplished.
The size of a tumor mass becomes part of the overall picture of how the lesion is viewed regarding its management. A greater host immune response is required in the more advanced cases as typified by bulky disease. This almost always necessitates surgical debulking to eliminate the larger number of tumor cells that are required to be brought under control. The presence of bulky tumor is in addition, frequently associated with a source of inhibitory surface molecules. When shed from the tumor cell membrane into the serum, these molecules function to inhibit those immunosurveillance mechanisms needed for helping to eliminate existing tumor cells that may have remained in the region of surgical resection or among those cells having entered the circulation[20]. As a consequence, a greater host immune response is required in the more advanced cases which is usually typified by bulky disease. There is little disagreement as such, that the ability to achieve an improved cure rate depends on early diagnosis and when possible, complete removal of the existing tumor.
The concept for achieving the early diagnosis of a malignant lesion was espoused by Lee Hartwell of the Fred Hutchinson Cancer Center, who evaluated procedures for achieving such early diagnosis as the more effective way of curing cancer. He looked at later stages of disease in solid tumor malignancies, where chemotherapy was employed to help improve survival. In such situations he found that this approach rarely resulted in cure, especially when the primary lesion had undergone the process of metastasis[21].
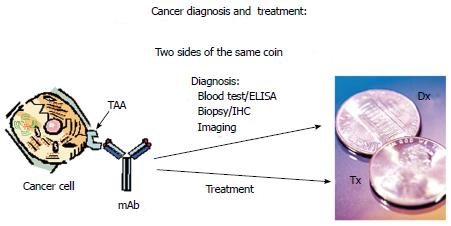
Hartwell stressed the need for finding a tumor protein expressed early in the onset of disease, functioning in a manner that the Pap smear had accomplished for cervix cancer. When such a tumor protein, functioning as a marker, could be detected by a monoclonal antibody, the clinical course of the disease would be altered in favor of an almost guaranteed cure. Larry Norton of the Sloan Kettering Cancer Center emphasized that if the tumor markers that Hartwell was hoping to find were immunogenic, then the monoclonal antibody that could determine the presence of the malignant lesion would be the same monoclonal that when delivered intravenously, would hunt, seek and destroy any cell in the metastatic setting that presented with such a marker. Essentially the presence of immunogenic tumor associated antigens (TAA’s) on the cell surface membrane serve to illustrate the tumor in the form of a coin displaying two sides. On the reverse side of the coin, the proper monoclonal can detect the tumor antigen as a diagnostic marker. The antigen on the opposite (head) side of the coin would now act as a therapeutic target for tumor destruction by utilizing the same monoclonal antibody delivered intravenously (Figure 2).
Such tumor immunogenic proteins (TAA’s) were isolated from a number of different malignancies including colon cancer and later characterized at Precision Biologics. The monoclonals that were derived from colon cancer antigen and later used to immunize BALBc mice for hybridoma production, are presently being tested clinically for both diagnostic and therapeutic efficacy. They have been found to be capable of detecting the earliest lesion in a manner illustrated by Figure 2. These colon tumor specific monoclonals are capable of functioning to diagnose the presence of the colon malignancy by both immuno-histochemistry of the resected specimen as well as serum ELISA. Should the tumor have invaded the blood stream, the metastatic lesions resulting from such invasion can now be effectively targeted. Extrapolating from animal studies with colon cancer transplants, metastatic foci from of these tumors can now be approached thru intravenous infusion of the monoclonal antibody with doses of the IgG1 delivered IV at 4-5 mg/kg. Phase IIB studies are now in progress with these antibodies.
When Ariel Hollinshead (1985)[22] employed pooled allogeneic tumor membrane antigen for treating a variety of malignant lesions, it became apparent that when the antigen was delivered at threshold levels and specifically for the malignancy expressing suboptimal levels of innate antigen, that the immune system could be shifted from one of performing immune-surveillance to that of providing a therapeutic mechanism for attacking and destroying the tumor, resulting in improvement in survival[23,24].
Clinical studies employing pooled allogeneic tumor antigen in the form of a vaccine, defined by its ability to turn on both cell and humoral immunity, resulted in improved survival over those where patients underwent surgery alone. In order to achieve an optimum response, the antigen had to be delivered at doses of between 750 and 1000 μg in 3 divided doses, given along with an oil based adjuvant. This allowed the now homogenized antigen to remain at the site of delivery for an extended period of time.
To define the nature of the tumor protein or proteins capable of inducing enhancement in tumor recognition, monoclonals were developed in BALBc mice. Three of the antibodies obtained from the fusion and subsequent hybridoma development showed specificity for colon cancer. There was minimal if any evidence of cross reactivity of these antibodies to the surrounding normal colonic tissue. When employed for therapy, first chimeric and then the humanized or human version of the antibodies were produced.
In reviewing the nature of the clinical response obtained following the initial trials employing pooled colon cancer antigen, all patients immunized had a strong delayed cutaneous hypersensitivity response as previously noted. This was response was associated with enhancement in cellular immunity as well a strong humoral response in most patients, with resulting high serum titers of an IgG1 targeting the antigen expressed on the tumor cells[25]. Those among the 10%-20% of patients showing signs of recurrent disease after immunization, were found to be unable to mount the needed humoral response needed to control the tumor. The cell lmediated immunity almost appeared to function in a bystander manner. The monoclonals described above that were developed from the original Hollinshead tumor antigen were then specifically produced GMP for initiation of food and drug administration (FDA) clinical trails. The IgG1 format developed for the trials was found to function in the same manner as those antibodies found in the host circulation in response to administration of the tumor vaccine.
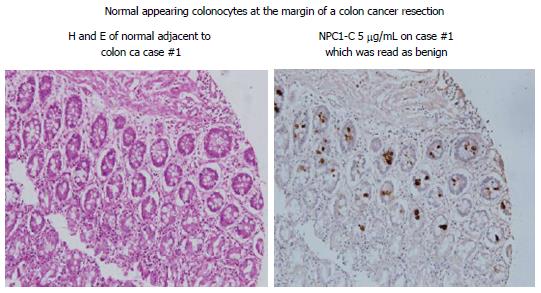
A detailed analysis of the monoclonals so produced against the colon antigen revealed each to be capable of inducing a strong ADCC response. Similarly, these mAbs showed effectiveness in a serum ELISA with a high degree of sensitivity and specificity. Using Immunohistochemistry (IHC) to define expression of antigen in the tissue under examination, cells that have undergone the initial genotypic changes can now be clearly defined even though the phenotypic features of cancer are not yet available for recognition by the pathologist. Studies to date have suggested that the colonocytes adjacent to a malignant lesion, have for the most part undergone genotypic transformation (Figure 3). It appears that this process of malignant transformation occurs several months before phenotypic features of cancer can be detected[26]. Obviously during resection of a primary colon lesion by colectomy, it is essential for the pathologist to guarantee that transformed colonocytes not be left behind in the margins of resection that are to be re-anastomosed. This appears to be best achieved by employing IHC with the monoclonal antibodies targeting colon tumor antigen. Along with the standard HE protocol. We plan to have antibody kits available in the OR so that frozen sections taken from margins of bowel following colectomy can be obtained for IHC.
Tumor antigen structure was analyzed, defined and characterized following immunoprecipitation of the pooled allogeneic colon cancer membrane material that had been used as a vaccine. Mass spectroscopy indicated that there were three separate antigens, seen alone and in combination in various colon cancers, each representing an oncofetal protein needed in the development of the human GI tract. These proteins were usually turned off as the fetus matured by re: methylation of the gene. In the adult, the onset of malignant transformation of the cell occurs via an oncogenic mutation. This appears to result in a modification of the protein structure through a mutation in the synthetic pathway or possibly thru a post translational modification of the oncofetal protein. The resulting tumor protein was found then to be immunogenic and serves to characterize the tumor system in which it is expressed. The immunogenic proteins that we identified were shown to be related to MUC5ac, A33, and CEAcam 5,6. While our monoclonals clearly define these proteins on Immunohistochemistry, commercial monoclonals used to define the known non modified antigens (oncofetal proteins) failed to recognize expression of the modified antigen in the malignant system.
All of the monoclonals that we have developed fit into a unique class of IgG’s that are both diagnostic as well as therapeutic in solid tumor malignancies. Mutated MUC5c antigen is defined by monoclonal Neo-101 and its newer version Neo 102, CEAcam5,6 by monoclonal 16C3/Neo 201 and altered A33 by monoclonal 31.1. To date no other anti-tumor IgG monoclonals have been found capable of performing in a similar fashion. The epidermal antibodies targeting epidermal growth factor I and II all have corresponding targets in normal tissue.
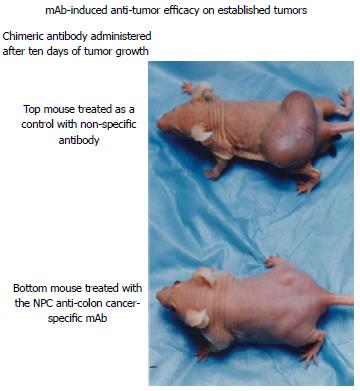
Knowing that the targeted antigen in colorectal cancer can result in tumor destruction, animal studies prior to initiation of clinical trials using therapeutic monoclonals, were devised to demonstrate in vivo tumor destruction Figure 4.
The ADCC response for most of the Precision monoclonals, range from 50%-70% tumor destruction in a 6-8 h. period of time, at an effector to tumor (E:T) ratio of 80-100:1 to over 90% with monoclonal 31.1. When these antibodies are delivered intraperitoneally in the animal model following establishment of tumor growth 10 d after subcutaneous administration of 10-20 million tumor cells in the thigh of nude mice, more than 50% of the animals were found to have a marked reduction in size the tumor mass. This can be seen at 10-15 d after immunization. The dosage of intraperitoneal IgG delivered along with human effector cells to assure an optimum ADCC response, was found to require approximately 400 μg in the animal model or an equivalent of approximately 400 mg in a 70 kg patient, this represents about 4-5 mg/kg of monoclonal antibody delivered at about 1 mg/min.
Considering the lack of toxicity following IV administration of our monoclonals in phase I FDA therapeutic trial, we began phase II studies. One of the problems encountered in the original GMP antibody preparation for FDA was that NEO-101 mAb was expressed at low levels and therefore not suitable for commercial production. Using a newer expression system, we are now able to produce the new monoclonal at a significantly higher level. Of interest was that while the sequence of the newly produced antibody, NEO-102 was virtually unchanged, we did see an approximate a definite improvement in ADCC as well as improvement in the quality of staining where background staining was virtually eliminated. This new version of the mAb, NEO-102 is being utilized in phase II and is being tested in escalating doses. Phase IIb has been designed to test the optimum dose of NEO-102 in combination with chemotherapy[27].
As mentioned above, the antibodies developed at Precision Biologics have their clinical efficacy in their capability of defining the tumor marker expressed in the tumor cell as a target for tumor detection as well as destruction. In tracing the pattern of expression of these markers, it became readily apparent that they were expressed not only in the later stages of tumor development where they could serve as an ideal therapeutic target, but at a time when genotypic changes were taking place in the normal but transforming cell, as noted above, and where the features of malignancy could not be readily recognized by the pathologist. We are now looking at the issue of Field Effect with regard to the genetic alterations occurring at the time of tumor induction. As such we are attempting to define the extent of premalignant alterations surrounding the primary lesion[28].
In terms of colon cancer, the mechanism for tumor induction whether by virus or carcinogen, probably effects an area in the bowel resulting in a pattern of genotypically altered colonocytes expressing tumor antigen, the so called Field Effect as noted above. Within this Field, further mutations lead to the eventual appearance of the early polypoid changes that may suppress the genotypically altered surrounding colonocytes. This polypoid lesion then continues with further mutational changes leading to the eventual appearance of an infiltrating colonocytic lesion. Resection of the polypoid lesion, leaving the altered colonocytic field intact, could then result in further progression of cellular changes in the premalignant cells. Such a concept, if proven correct as per an ongoing study at North Shore University Hospital and Precision Biologics will assist the pathologist, at the time of bowel resection, to define the extent of the Field Effect by immuno histochemistry.
In our ongoing therapeutic trials, phase IIb is in the process of initiation with the addition of chemotherapy to the therapeutic monoclonals being employed. It is generally agreed upon that Immunochemotherapy can be more effective than either chemotherapy or immunotherapy when employed alone. In general chemotherapy can diminish the immune inhibitory effect derived from the tumor and enhances the overall therapeutic response[29,30]. Finally we have prepared an alpha particle labeled NEO-102 monoclonal antibody to be introduced at a later date as part of the overall therapeutic approach to tumor control.
The availability of monoclonals targeting an immunogenic protein expressed in all phases of colon cancer development should be useful for both diagnosis and therapy and should have a major impact on how colon cancer is treated and the outcome that can be expected.
P- Reviewers: Kannen V, Li YY S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3126] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 2. | Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, Zauber AG, Jiang R, Ahnen DJ, Bond JH. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 427] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 3. | Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 4. | Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | Higaki S, Hashimoto S, Harada K, Nohara H, Saito Y, Gondo T, Okita K. Long-term follow-up of large flat colorectal tumors resected endoscopically. Endoscopy. 2003;35:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1644] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 7. | Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020. [RCA] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 971] [Article Influence: 53.9] [Reference Citation Analysis (1)] |
| 8. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4463] [Article Influence: 212.5] [Reference Citation Analysis (1)] |
| 9. | Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, Nitti D, Torri V, Elias D, O’Callaghan C. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906-4911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 10. | Bigbee W, Herberman RB. Tumor markers and immunodiagnosis. Kufe DW, Pollock RE, et al., editors. Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc 2003; . |
| 11. | Walsh JM, Terdiman JP. Colorectal cancer screening: scientific review. JAMA. 2003;289:1288–1296. [RCA] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 293] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Carpelan-Holmström M, Louhimo J, Stenman UH, Alfthan H, Järvinen H, Haglund C. Estimating the probability of cancer with several tumor markers in patients with colorectal disease. Oncology. 2004;66:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Arlen M, Arlen P, Wang X, Saric O, Martin DA, Deutsch G, Sathyanaryyana SA. The clinical detection of Pancreatic Carcinoma: A comparison of the Standard Biomarkers to that of a Newer Class of Biomarkers used for both diagnosis and Therapy. Pancreatic Disorders and Therapy. 2013;10:4172-4178. |
| 14. | Arlen M, Tsang KY, Bartal A, Wolf J, Saric O. Monoclonal Antibodies to Immunoreactive Tumor Associated Antigen (TAA) from Human Colon Cancer. Antibody Immunoconjugates and Radiopharmaceuticals. 1991;4:2. |
| 15. | Patel SP, Bristol A, Saric O, Wang XP, Dubeykovskiy A, Arlen PM, Morse MA. Anti-tumor activity of a novel monoclonal antibody, NPC-1C, optimized for recognition of tumor antigen MUC5AC variant in preclinical models. Cancer Immunol Immunother. 2013;62:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Nimmerjahn F, Ravetch JV. Translating basic mechanisms of IgG effector activity into next generation cancer therapies. Cancer Immun. 2012;12:13. [PubMed] |
| 17. | Lim SH, Beers SA, French RR, Johnson PW, Glennie MJ, Cragg MS. Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica. 2010;95:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Desjarlais JR, Lazar GA. Modulation of antibody effector function. Exp Cell Res. 2011;317:1278-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Arlen M. Escape Mechanisms employed by tumor cells to allow for Growth, Invasion and Metastasis In Chemoimmunosensitization of Resistant Tumor Cells to Cell Death by Apoptosis. Bonavida. 2006;243-261. |
| 22. | Hollinshead A, Elias EG, Arlen M, Buda B, Mosley M, Scherrer J. Specific active immunotherapy in patients with adenocarcinoma of the colon utilizing tumor-associated antigens (TAA). A phase I clinical trial. Cancer. 1985;56:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Arlen M, Arlen P, Tsang A, Wang XP. The Therapeutic Value of Monoclonal Antibodies Directed Against Immunogenie Tumor Glycoproteins J. Cancer. 2010;1:209-222. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Bartal A, Tsang KY, Saric O, Wooding M. Monoclonal Antibody defining an Antigen present within a purified Tumor Membrane Vaccine. Proc. Am. Assoc. Cancer Res. 1990;1539. |
| 25. | Arlen M, Tsang KY. Monoclonal antibodies and their role in modulation of the immune response. J Surg Oncol. 1993;54:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Arlen M, Saric O, Wang X, Dubeykovskiy A, Arlen P. Nanocytology vs. Immunohistochemistry of Intestinal Colonocytes to Assess the Risk of Colon Cancer based on Field Cancerization - A Preliminary Report. J Cancer. 2013;4:165-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Arlen M, Wang X, Luka J, Gupta R, Saric O, Arlen PM. The use of specific monoclonal antibodies to target immunogenic tumor membrane proteins in patients with recurrent pancreatic and colon cancer. Curr Drug Deliv. 2012;9:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Arlen M, Arlen P. Optimizing the immune system to achieve control of the metastatic malignant lesion. J Cancer. 2013;4:427-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102:115-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 30. | Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 378] [Article Influence: 25.2] [Reference Citation Analysis (0)] |