Published online Jul 15, 2013. doi: 10.4251/wjgo.v5.i7.115
Revised: June 5, 2013
Accepted: June 18, 2013
Published online: July 15, 2013
Processing time: 203 Days and 14.3 Hours
Hilar cholangiocarcinoma is a rare malignant tumor arising from the epithelium of the bile ducts. Surgery is still the only chance of potentially curative treatment in patients with perihilar cholangiocarcinoma. However, radical resection requires aggressive surgical strategies that should be tailored optimally according to the location, size and vascular invasion of the tumors. Accurate diagnosis and staging of these tumors is therefore critical for optimal treatment planning and for determining a prognosis. Multidetector computed tomography (MDCT), magnetic resonance imaging (MRI) and MR cholangiography are useful tools, both to diagnose and stage hilar cholangiocarcinoma. Modern imaging techniques allow accurate detection of the level of obstruction and the longitudinal and radial spread of the tumor. In addition, high-resolution MDCT and MR provide specific radiographic features to determine vascular involvement of anatomic structures, such as the hepatic artery or the portal vein, which are critical to decide the surgical strategy. Finally, radiological staging allows detection of patients with distant metastasis in the liver or peritoneum who will not benefit from a surgical approach.
Core tip: Hilar cholangiocarcinoma is a rare malignant tumor arising from the epithelium of the bile ducts. Surgery is still the only chance of potentially curative treatment in patients with perihilar cholangiocarcinoma. Multidetector computed tomography, magnetic resonance imaging and magnetic resonance cholangiography are useful tools, both to diagnose and stage hilar cholangiocarcinoma.
- Citation: Valls C, Ruiz S, Martinez L, Leiva D. Radiological diagnosis and staging of hilar cholangiocarcinoma. World J Gastrointest Oncol 2013; 5(7): 115-126
- URL: https://www.wjgnet.com/1948-5204/full/v5/i7/115.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v5.i7.115
Carcinomas of the extrahepatic biliary tree, commonly known as hilar cholangiocarcinoma (HCCA), Klatskin tumors or perihilar cholangiocarcinomas, are rare malignancies that account for up to 3% of all gastrointestinal cancers. With an incidence rate of 0.5-2.0 cases per 100000, it is estimated that there are between 2500 and 4000 new cases per year in the United States[1].
Although this tumor may potentially affect any location of the biliary tree, tumors involving the biliary confluence, left-hand drive (LHD) and right-hand drive (RHD) (true Klatskin tumors) are most common and account for 40%-60% of all cases. Around 2/3 of cholangiocarcinomas are hilar or extrahepatic and originate from the bile duct epithelium at the level of confluence of the right and left ducts, whereas 30% are distal or intrapancreatic and 5%-10% are intrahepatic[2]. Due to recent improvements in radiological techniques, there is a wide range of imaging tools available for diagnosis and staging HCCA, such as multidetector computed tomography (MDCT), magnetic resonance (MR), endoscopic ultrasonography, endoscopic retrograde cholangio pancreatography (ERCP) and angiography. However, to date, there is no consensus as to the best staging algorithm. The aim of this paper is to review the results in the preoperative staging of HCCA with modern non-invasive imaging techniques, including MDCT, magnetic resonance imaging (MRI) and MR cholangiopancreatography (MRCP).
Cholangiocarcinoma is a malignant tumor arising from bile duct epithelium of any portion of the biliary tree. It is the second most common primary liver cancer in the world, except for certain areas where it is more common than hepatocellular carcinoma (HCC). Traditionally, it has been classified as an intrahepatic and extrahepatic tumor according to its location with respect to the liver; this last category has been subdivided into upper, middle and distal by its location. However, recent literature classifies extrahepatic cholangiocarcinoma into perihilar and distal cancer, due to the relatively low incidence of middle bile duct cancer[2], and the correlation of these categories with anatomic distribution and preferable treatment. Perihilar cholangiocarcinoma, also called Klatskin tumors[3], can be defined as tumors originating on the right or left duct, near their junction or in close vicinity to the bile duct confluence, and represent two-thirds of cholangiocarcinomas[4].
Most cholangiocarcinomas are adenocarcinomas with variable differentiation grades and fibroplasia. According to the last World Health Organization (WHO) histological classification, different types of adenocarcinoma are considered, and precursor lesions such as biliary intraepithelial neoplasia and intraductal papillary neoplasms are also included[5]. Macroscopically, carcinomas of the extrahepatic bile ducts have been divided into polypoid, nodular, scirrhous constricting and diffusely infiltrating types. These categories can provide a guide to the operative procedure, extent of resection and prognosis. However, except for the polypoid type, this division is rarely possible due to overlapping on gross features. However, the nodular and scirrhous types which tend to coexist are prone to infiltrate surrounding tissues, while the diffusely infiltrating type tends to spread linearly along the ducts.
Cholangiocarcinoma occurs with a highly varying frequency in different areas of the world[6]. There are several recognized risk factors for cholangiocarcinoma, such as primary sclerosing cholangitis (PSC), although the vast majority of cancers are seen in patients with no readily identifiable predisposing condition. PSC is an autoimmune condition that causes bile duct inflammation resulting in scarring and fibrosis. This chronic inflammation is thought to lead to dysplastic and ultimately neoplastic changes in the bile ducts. The risk of cholangiocarcinoma in patients with PSC is thought to be over a 1000-fold higher than in the general population and is thought to be 0.5%-1.0% per year or 10%-40% lifetime risk[7]. While both intrahepatic and extrahepatic cholangiocarcinoma are well-known complications of PSC in Western countries, hepatolithiasis, infections due to liver flukes and bile stasis are risk factors strongly associated with cholangiocarcinoma in Eastern Asia. However, approximately 90% of patients diagnosed with cholangiocarcinoma do not have a recognized risk factor in Western countries[8]. Other risk factors include an abnormal choledochopancreatic junction, choledocal cyst, ulcerative colitis, cirrhosis, alcoholic liver disease, type II diabetes, thyrotoxicosis, pancreatitis and possibly duodenal ulcer disease[9].
Obstructive jaundice is the main clinical feature and can appear relatively early, even with small neoplasms. It may progress rapidly or fluctuate. Other symptoms may include weight loss, pruritus, right-upper quadrant pain or fever and chills if cholangitis develops.
The majority of patients with HCCA present with severe painless jaundice as the initial clinical presentation. However, not every patient with jaundice and biliary obstruction at the hepatic hilus will eventually be confirmed as HCCA. In fact, almost 25% of cases turn out to have other benign conditions (such as lymphoplasmacytic cholangitis or Mirizzi syndrome) or other malignant disease (such as gallbladder cancer or nodal metastasis) that has obstructed the hepatic confluence[10].
Initial radiological assessment is performed with sonography in most patients with perihilar biliary tract malignancies. Ultrasound rarely allows direct demonstration of perihilar biliary cancer, although indirect signs such as isolated intrahepatic dilatation can be useful in suggesting the diagnosis. However, in most cases, additional diagnostic procedures are necessary to confirm the diagnosis. The main goals of imaging in that setting will be to differentiate HCCA from other conditions leading to obstructive jaundice with intrahepatic dilatation and to perform an accurate preoperative evaluation of tumor resectability, focusing on vascular and biliary invasion as well as invasion and distant metastasis to the liver and lymph nodes. In the past, direct cholangiography combined with angiography was used to assess tumor extension. More recently, the advent of MDCT and MRCP has dramatically changed the imaging evaluation of patients with hilar cholangiocarcinoma.
The role of cholangiography in the evaluation of hilar cholangiocarcinoma is two-fold: to assess tumoral extension to identify potentially resectable patients and to help in planning palliative biliary drainage in non-resectable patients. In this clinical setting MRCP has a number of potential advantages over ERCP and transhepatic cholangiography (THC). MRCP allows rapid non-invasive evaluation of the biliary tract without instrumentation and therefore without the risk of inducing sepsis in patients with ductal obstruction. Additionally, MRCP depicts tumoral extension along the intrahepatic branches of the biliary tree more consistently than ERCP and THC, especially in high grade stenosis[11]. In Fulcher’s series[12], MRCP allowed a more detailed visualization of the bile ducts than direct cholangiography in 3 of 4 patients who underwent both techniques. In another study, Holzknecht et al[13] performed a prospective comparison of MRCP and ERCP in 61 patients who underwent ERCP for a variety of clinical indications. ERCP demonstrated 36 stenosis in 33 patients (15 malignant and 21 benign). MRCP diagnosis was correct in 89% of the cases (32/36) and there were 4 false negative findings. However, MRCP was correct in all 15 patients with high-grade malignant stenosis. On a patient-by-patient basis, statistical analysis to compare the grade of stenosis indicated that differences between MRCP and ERCP were not significant. In a recent study by Yeh et al[14], the efficacy of MRCP and ERCP in 40 patients with malignant perihilar obstruction, including 26 patients with Klatskin tumor, were compared. ERCP was unsuccessful in 2 patients. In this series, MRCP was superior to ERCP in delineating the anatomic extent of the tumoral lesions: 34/40 vs 24/38. Therefore, direct cholangiography, either endoscopic or percutaneous, has been definitively abandoned as a diagnostic tool and substituted by MRCP.
MDCT allows for faster scanning with thinner collimation and results in an improved diagnosis and staging hilar cholangiocarcinoma. CT is usually performed to assess the level of biliary obstruction (longitudinal extension) as well as involvement of liver parenchyma and vascular hilar structures (radial extension). High resolution CT allows for accurate depiction of a thickened bile duct wall and tumor spread into liver parenchyma or hilar vessels and therefore plays a key role in the diagnosis and staging of HCCA. Previous results of conventional CT in the diagnosis and staging of hilar cholangiocarcinoma have been limited. The advent of helical technology has dramatically improved the results of CT in the detection and diagnosis of Klatskin tumors, although its ability to perform an accurate staging is still limited. In the series by Tillich et al[15], HCT correctly detected all hilar cholangiocarcinomas using a biphasic technique. However, its overall accuracy for predicting resectability was only 60%. In Feydy’s series[16], HCT correctly detected and localized the mass in 91% of the cases.
Diagnostic features of HCCA include intrahepatic segmental biliary dilatation, periductal thickening, endoluminal lesions and direct tumor spread to the liver or adjacent vessels. Biliary dilatation is usually intrahepatic and often segmental and located proximally to an ill-defined biliary mass near the hepatic hilus. The transition between dilated and non dilated bile ducts is usually abrupt and this is a key feature for diagnosis. Therefore, these patients should not be drained before an adequate imaging study is performed.
On the basis of the Japanese Liver Cancer Group[17] classification, cholangiocarcinomas are classified into three types: mass-forming, intraductal growing and periductal infiltrating. The latter is the most prevalent in the hilar portion of the biliary tree and forms the majority of perihilar cholangiocarcinomas.
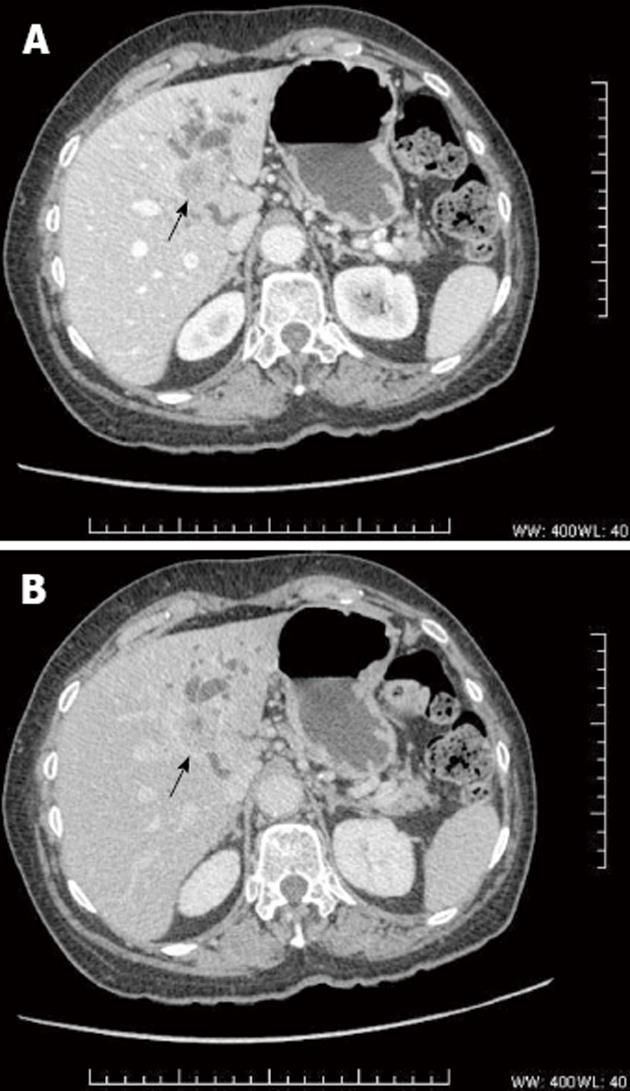
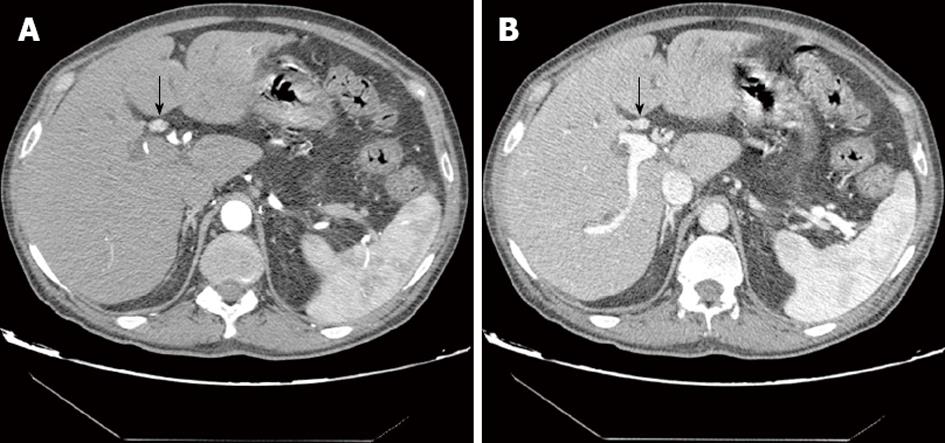
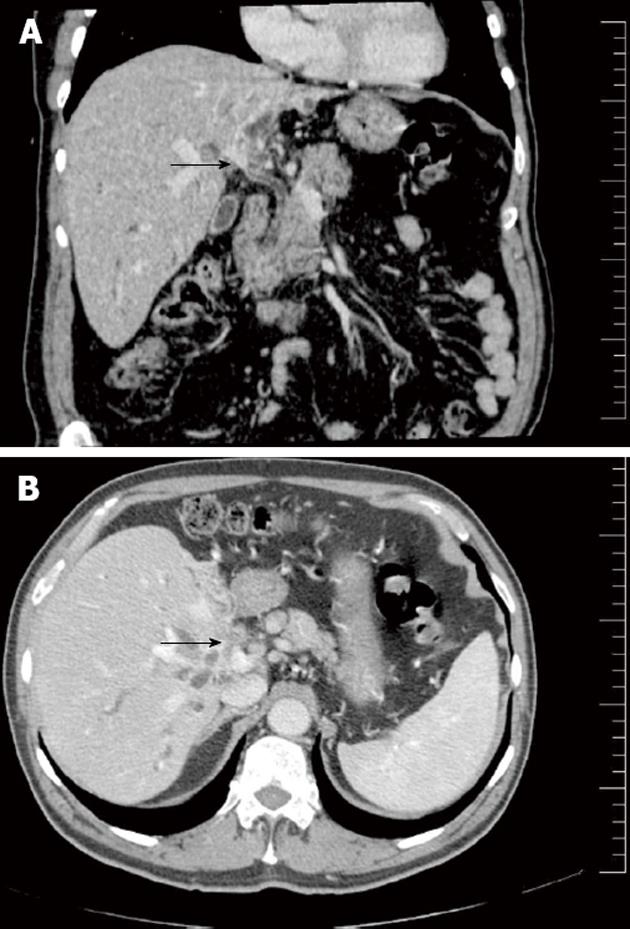
Mass-forming cholangiocarcinoma: In some cases, HCCA presents as a mass-forming lesion (Figure 1). This pattern of presentation includes periductal thickening and a solid tumor lesion involving the adjacent liver parenchyma[18]. Mass-forming intrahepatic cholangiocarcinoma is usually a bulky lesion with infiltrating features around the adjacent peripheral branches of the portal vein. On CT and MRI, mass-forming HCCA are usually heterogeneous hypovascular masses with rim-like peripheral enhancement in the arterial and portal phase and delayed enhancement in the equilibrium phase. These enhancement features reflect the nature of the tumor, which is mainly desmoplastic. The bile ducts peripheral to the tumor are usually dilated because of obstruction by the tumor.
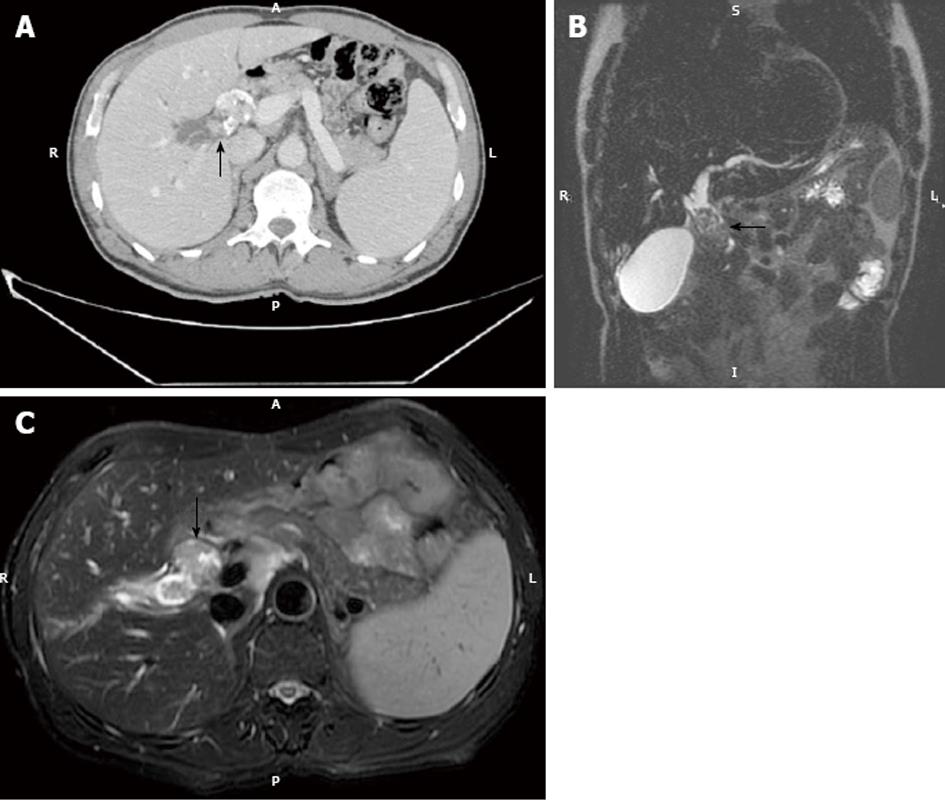
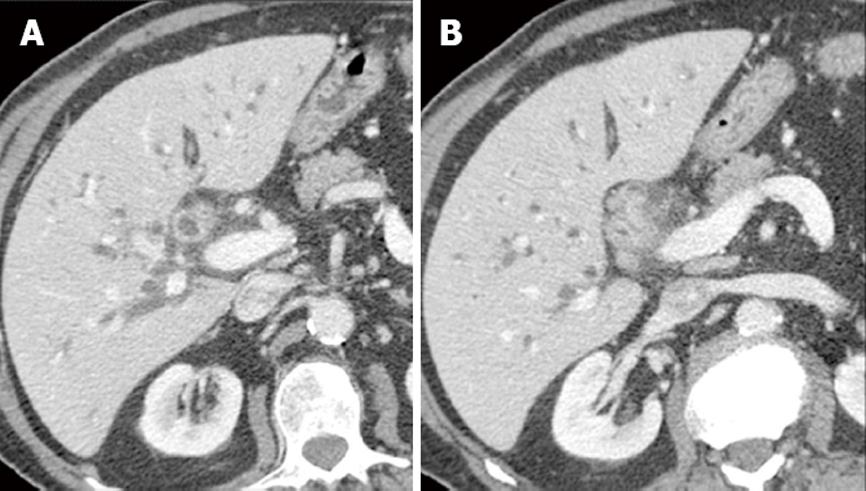
Intraductal growing cholangiocarcinoma: In intraductal growing cholangiocarcinoma, bile ducts can be dilated because of tumoral obstruction, increased mucin secretion or sloughed tumor debris. This pattern of cholangiocarcinoma is frequently found in papillary cholangiocarcinomas (Figure 2). On imaging, the involved bile ducts are asymmetrically dilated. Typically, an endoluminal mass is found in the segment with more intense dilatation. Intraductal cholangiocarcinoma is confined in the lumen of the dilated bile duct without direct tumoral extension to the surrounding liver parenchyma. This pattern of spread is radically different from mass-forming or periductal infiltrating cholangiocarcinoma that typically shows marked infiltration. This is related to the different histological patterns of the tumors. Intraductal growing cholangiocarcinoma appears as a nodular, well defined mass on CT or MR and typically shows intense enhancement after contrast injection. The mass is confined within the bile ducts and therefore there is preservation of the bile duct wall[18].
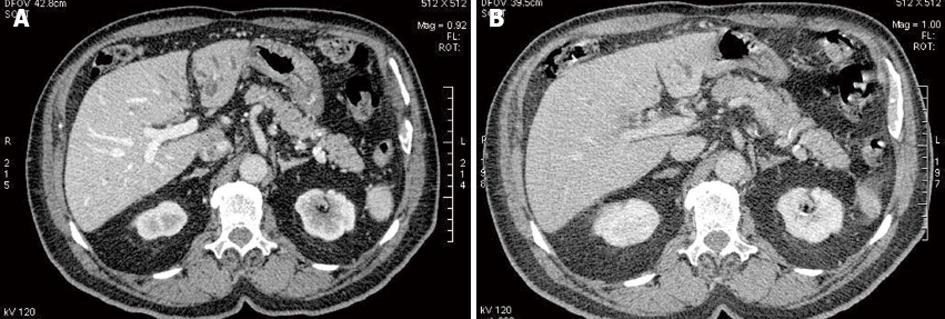
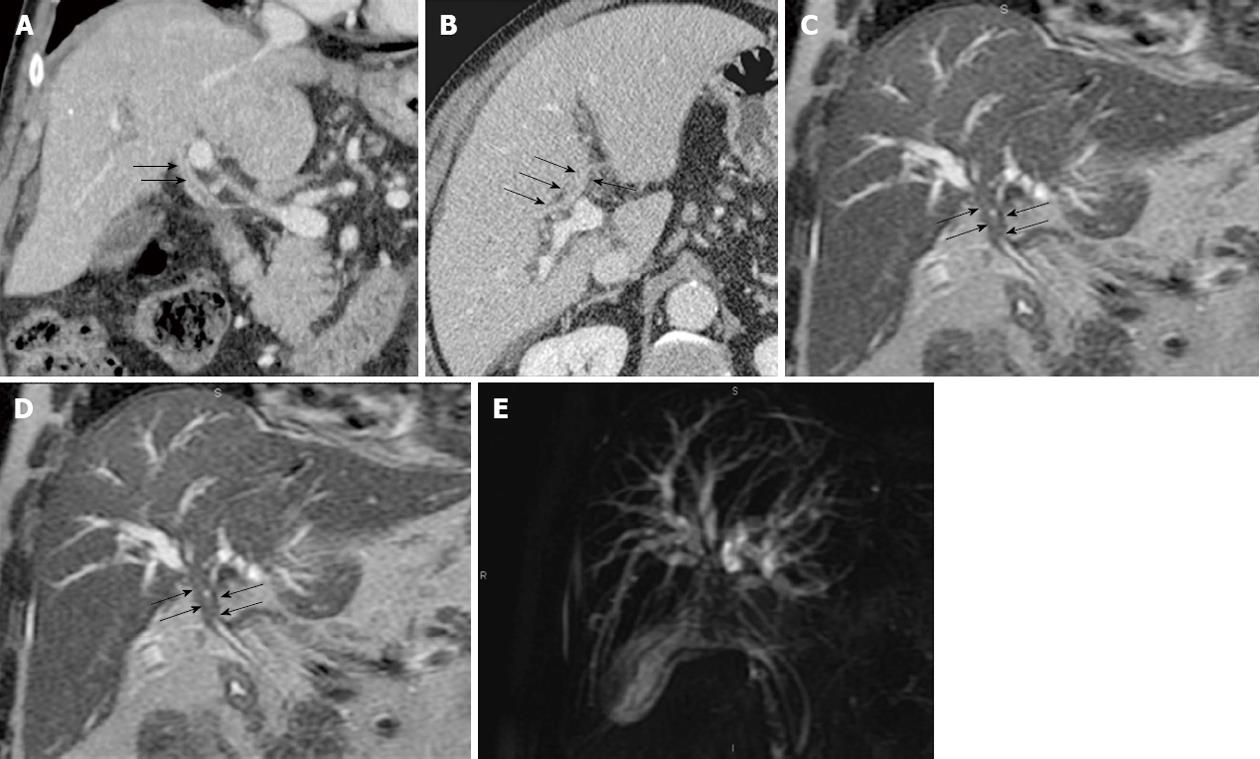
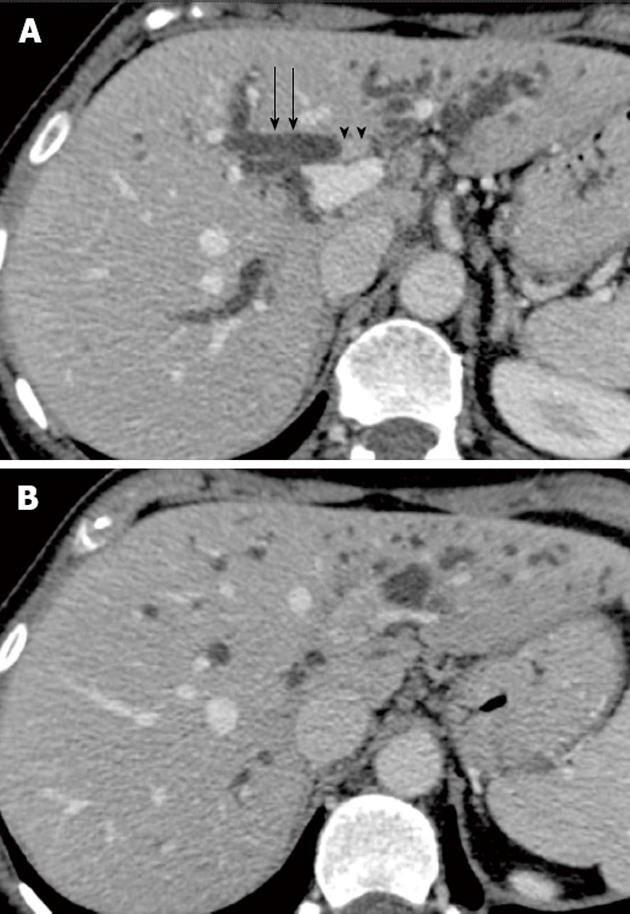
Periductal infiltrating cholangiocarcinoma: This pattern of cholangiocarcinoma is frequently found in perihilar cholangiocarcinoma. Periductal infiltrating cholangiocarcinoma typically shows marked dilatation on imaging of the biliary tree proximal to the tumoral lesion. On CT, the involved bile ducts are diffusely narrowed or obliterated. With conventional helical or incremental CT it was extremely difficult or impossible to depict the tumor mass on imaging studies. However, with the advent of MDCT, thin-collimation tumoral periductal involvement is almost always detectable. High-resolution CT shows tumoral involvement as an irregular periductal thickening completely obstructing or narrowing a short segment of the biliary tree around the biliary bifurcation or common hepatic duct (Figure 3).
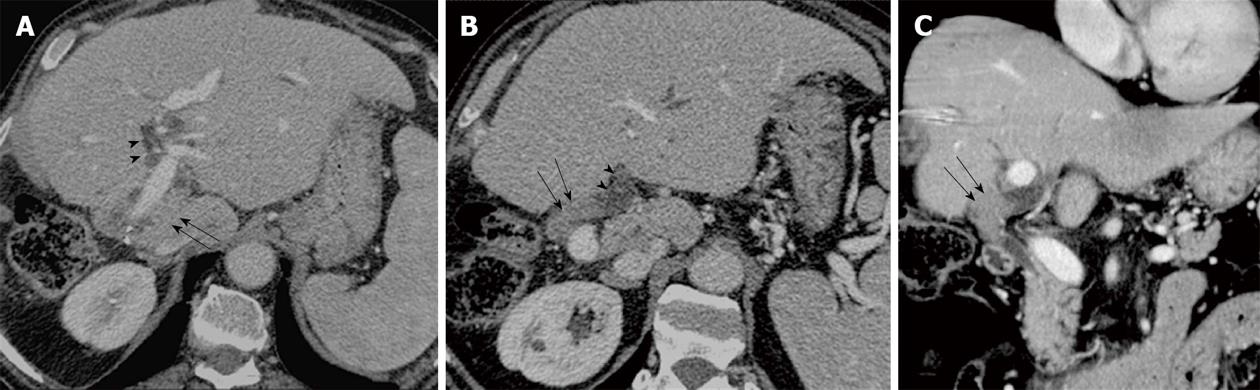
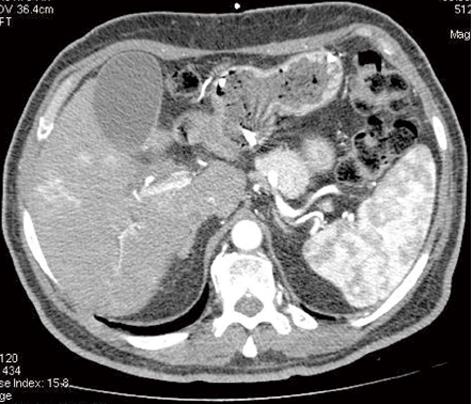
Periductal thickening is usually iso-hypo enhancing in arterial and portal phases and shows marked enhancement on delayed phase imaging (Figure 4). Occasionally periductal thickening may show brisk hyperenhancement in the arterial phase, mimicking endobiliary HCC (Figure 5). On MRCP, non union of the right and left hepatic ducts is a typical finding of infiltrating hilar cholangiocarcinoma.
There is a wide variety of benign and neoplastic lesions at the liver hilum that may cause biliary stricture. In particular, inflammatory lesions may present with the same radiological features as those from malignant tumoral causes.
The most frequent benign lesions that may mimic cholangiocarcinoma include lymphoplasmacytic cholangiopathy (IgG4 sclerosing cholangitis), endobiliary metastases, endobiliary HCC and Mirizzi Syndrome.
Several studies have noted that approximately 14% to 25% of resected patients for cholangiocarcinoma (HCCA) prove to have a benign lesion at histopathology[19,20]. Differentiation between malignant and benign strictures in patients with suspicion of cholangiocarcinoma is often impossible before laparotomy due to overlapping of radiological and clinical features. In addition, there are no specific radiological or laboratory tests that may distinguish HCCA from benign proximal bile duct lesions.
Malignant stricture is characterized by bile-duct wall thickening greater than 1.5 mm, arterial and venous hyperenhancement of the stricture, and greater extent of proximal dilatation compared with benign lesions[21]. Different imaging studies have shown that only one feature, vascular involvement, was significantly different in benign and malignant lesions[22].
IgG4-sclerosing cholangitis is considered part of IgG4 systemic-related diseases and is commonly associated with autoimmune pancreatitis[23]. The occasional absence of pancreatic involvement in these patients has been previously reported. No strict diagnostic criteria have been described to date and diagnosis relies on a combination of clinical and histopathological findings. A typical diagnostic feature of IgG4-sclerosing cholangitis is elevation of serum immunoglobulin G4. IgG4-sclerosing cholangitis shows clinical response to steroid therapy. Prompt initiation of steroid treatment in these patients could greatly improve outcomes, at least in part by avoiding unnecessary surgery. Both the intrahepatic and extrahepatic segments can be involved by lymphoplasmacytic infiltration characterized by transmural fibrosis which may extend to the periportal area of the liver, causing biliary stricture[24].
A stricture of the distal CBD is the most common abnormality of IgG4 sclerosing disease, reported in 54%-79% cases[25,26]. Associated imaging findings of autoimmune pancreatitis, such as focal or diffuse pancreatic enlargement with a peripheral ring of low attenuation and a diffusely narrowed pancreatic duct, are useful features in pointing the diagnosis of autoimmune cholangitis[26].
However, accurate preoperative diagnosis of lymphoplasmacytic cholangitis and differentiating this condition from hilar cholangiocarcinoma is still very difficult because of the overlapping imaging features of both conditions (Figures 6 and 7). Both HCCA and LC have a similar gross macroscopic appearance related to their fibrous nature.
Endobiliary metastases: Neoplastic intraductal filling defects are usually considered primary intraductal biliary neoplasms until they are proved otherwise pathologically. Nevertheless, it is also well-known that extrabiliary malignant tumors, such as lung, breast, gallbladder, colon, testis, prostate, pancreas, melanoma and lymphoma, can metastasize to the bile duct and manifest as an intraductal mass too. When an intraductal lesion is found in a patient with extrabiliary malignancy, the presence of a contiguous parenchymal mass, an expansible nature of the intraductal lesion, and a history of colorectal cancer may suggest the presence of intraductal metastasis rather than double primary intraductal cholangiocarcinoma[27].
However, occasionally endobiliary metastases can be isolated without associated liver metastases and only the clinical setting may help in the differential diagnosis (Figure 8).
HCC with endobiliary growth: Obstructive jaundice as the main clinical feature is uncommon in patients with HCC. Only 1%-12% of HCC patients present with obstructive jaundice as the initial complaint. HCC may involve the biliary tract in several different ways: tumor thrombosis, hemobilia or endoluminal tumor extension. Jaundice in patients with HCC is usually related to diffuse tumoral intrahepatic extension with hepatic insufficiency. Filling defects in the biliary tree in a patient with HCC may be related to blood clots, sludge or intrabiliary tumoral growth. In the absence of gallbladder stones, the most frequent cause of intrabiliary filling defects is HCC.
Intrabiliary growth of HCC shows the same enhancement pattern as the primary tumor and is hyperenhancing in the arterial phase with wash-out in portal and delayed phases. Occasionally, endobiliary growth of HCC may be found in patients without a definite hepatic mass[28]. In that setting, the differential with intraductal growing cholangiocarcinoma may be very difficult with imaging, although a clinical setting of chronic liver disease may suggest the diagnosis.
Mirizzi syndrome: Mirizzi syndrome is a form of obstructive jaundice caused by a bile duct stone impacted in the neck of the gallbladder or in the cystic duct. The stone and surrounding inflammation compress the common hepatic duct and results in dilation of the bile ducts upstream[29]. This is a rare complication of cholelithiasis. An accurate diagnosis is essential for proper management of a patient. From a pathophysiological point of view, Mirizzi syndrome may have two causes. In Mirizzi type I there is chronic inflammation of the gallbladder with marked contraction of the fibrous gallbladder wall that adheres to the common bile duct, resulting in fibrous stenosis of the biliary lumen. In Mirizzi type II there is a direct cholecysto-biliary fistula secondary to direct compression of large stones on the wall of the common hepatic duct. Imaging reveals dilated intrahepatic bile ducts with a normal common bile duct. The gallbladder is usually collapsed. The diagnosis may be suspected on CT when calcified bile stones are detected. However, in most cases stones are radiolucent and not detectable with CT. MRCP is the most efficient imaging technique in this setting, allowing detection of impacted stones at the gallbladder neck with compression of the hepatic duct and upstream biliary dilatation. The treatment of Mirizzi’s syndrome may be a simple cholecystectomy with or without hepaticojejunostomy in case of fistula.
Cholangiocarcinoma is one of the most difficult tumors, both to stage and treat. Surgery remains the only curative treatment for HCCA. However, surgical treatment of this malignant tumor is impaired by the lack of an effective adjuvant treatment. In addition, the specific anatomic location of these tumors usually involving critical vascular structures makes complete resection extremely challenging. This is a critical point because long-term survival in patients with HCCA depends on complete tumor resection. Due to the infiltrative growth pattern of HCCA, thorough preoperative evaluation of the extent of tumor along the biliary tree and major vessels at the hepatic hilum is needed in order to plan adequate surgical treatment. Initial reports on surgical resection of cholangiocarcinoma involved only patients treated with biliary resection with hepaticojejunostomy with poor survival benefits[30]. Experience over recent years has shown a dramatic increase of radical surgical procedures with extended right or left hemi-hepatectomies, reporting 5-year survival rates between 25% and 40%[31]. In addition, development of sophisticated preoperative imaging techniques with multiplanar, biliary and vascular reconstructions has improved the depiction of both direct radial hepatic invasion and longitudinal intraductal extension of cholangiocarcinoma. The main problem until now has been the poor results of preoperative staging methods in order to separate potentially resectable from non-resectable patients and to avoid unnecessary surgical procedures, because benefits in terms of survival are only achieved if the tumor is completely resected.
The cornerstone of oncological resection in the liver is to resect the whole tumor with free margins but still leave enough liver to maintain hepatic function. This is particularly difficult in HCCA due to its infiltrating nature. Preoperative staging should focus on biliary, vascular, hepatic, lymph node and extrahepatic extension.
Unresectability criteria generally include liver metastasis, distant lymph node metastasis, bilateral arterial or portal invasion, unilateral vascular invasion and contralateral lobar atrophy and distant metastases. Parenchymal invasion is not considered an unresectability criterion because a right or left hepatectomy can be performed. Bismuth stage IV is also not always considered an unresectability criterion because with an extra-Glissonian approach and hepatectomy some lesions can be safely resected.
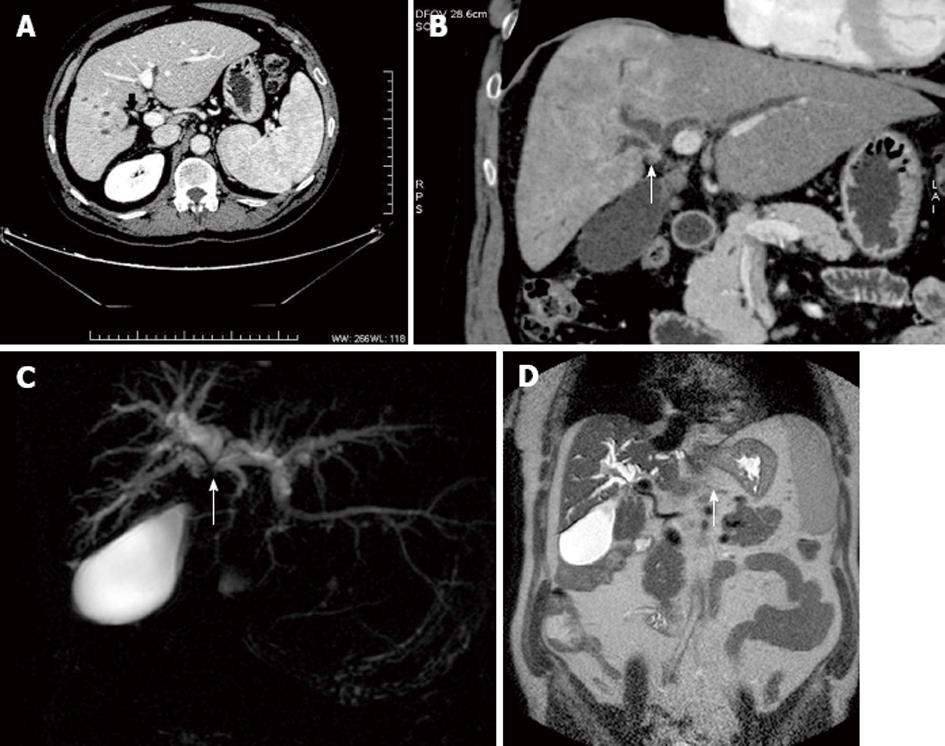
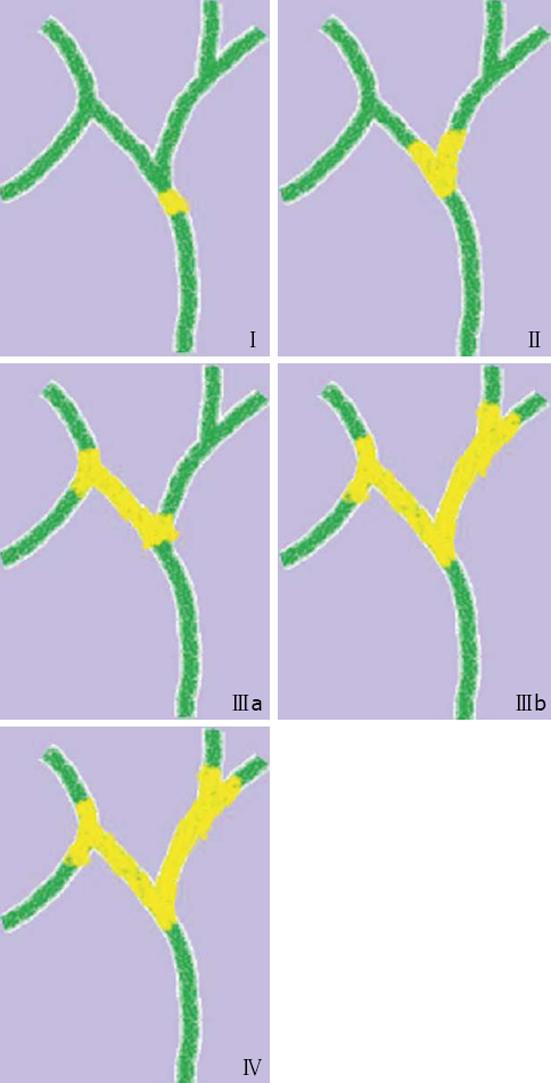
Biliary extension is usually assessed with MRCP. MRCP can confidently classify cholangiocarcinoma according to the Bismuth-Corlette Classification in stagesI, II, IIIa, IIIb and IV (Figure 9). StageI includes tumor confined to the common hepatic duct. Stage II includes tumor involving the confluence of the right and left hepatic ducts without proximal biliary involvement. Stage IIIa includes tumor involving the confluence of the right and left hepatic ducts with proximal extension to the confluence of the anterior and posterior right segmental bile ducts. Stage IIIb includes tumor involving the confluence of the right and left hepatic ducts with proximal extension to the confluence of bile ducts for segments IV and II-III.
Bismuth stage IV includes tumor involving the confluence of the right and left hepatic ducts with proximal extension to both secondary confluences of the right and left hepatic ducts.
In the paper by Maselli et al[32], fifteen patients with hilar cholangiocarcinoma underwent radical surgery and preoperative MR imaging. MRCP and gadolinium-enhanced dynamic T1-W images were correlated with surgical findings in the evaluation of the extent of biliary infiltration according to the Bismuth-Corlette classification.
Radiological-pathological correlation disclosed that the assessment of the bile duct stenosis by MRCP alone was correct in 80% of the patients (12/15). MRCP underestimated tumor spread in two patients who were considered preoperatively type II and type IIIA and turned out to be type IIIA and IV respectively at surgical exploration.
MRCP overestimated the neoplastic biliary extent in another patient who was considered type IIIa preoperatively and was proven to be type II at histology. Overall accuracy for MRCP to classify tumor according to the Bismuth Classification was 80%. In the series by Lee et al[33], longitudinal biliary involvement was accurately predicted with MDCT in 84% of the patients.
Vascular extension is either assessed with MDCT or gadolinium-enhanced dynamic MRI. Accurate preoperative assessment of HCCA requires thorough evaluation of the hepatic artery (right, left and common hepatic artery) and portal veins (right, left and main portal vein) (Figures 10 and 11).
MDCT and MRCP are useful techniques to delineate the extent of the tumor and to rule out vascular invasion and locoregional lymph node extension. Helical CT should be performed to rule out liver and lymph node metastasis as well as vascular encasement but proximal tumor extent along bile ducts is often underestimated.
On the other hand, helical CT also plays a key role in the management and staging of cholangiocarcinoma. HCT allows assessment of tumor spread to the liver or regional lymph nodes, as well as arterial or portal involvement[15]. Previous results of conventional CT in the diagnosis and staging of hilar cholangiocarcinoma have been limited. The advent of helical technology has dramatically improved the results of CT in the detection and diagnosis of Klatskin tumors, although its ability to perform an accurate staging is still limited. In the series by Tillich et al[15], HCT correctly detected all hilar cholangiocarcinomas using a biphasic technique. However, its overall accuracy for predicting resectability was only 60%. In a study by Cha et al[34], 21 patients with perihilar cholangiocarcinoma were studied with MDCT. CT correctly detected that the tumor was unresectable in 8 cases (PPV: 100%), due to vascular involvement in 8 cases and distant lymph-node metastases in 1 case. However, in 12 patients with suspected resectable disease by CT, 6 cases turned out to be unresectable (NPV: 50%), although none of them was related to vascular invasion (lymphadenopathy, metastasis, intraductal extension and anatomic variants).
In Maselli’s series[32], MRI correctly predicted vascular involvement in 73% of the cases. MR correctly showed arterial involvement in 88% of the cases (8/9) and had false positive findings in 12% (1/9). Portal invasion was correctly assessed in 73% of the cases (11/15) and underestimated in 20% of the cases (3/15). In 7% of the patients (1/15), portal vein invasion was overestimated and not confirmed by histology.
In the series by Lee et al[33], the accuracy of MDCT in the prediction of portal vein and hepatic artery invasion was 85.5% and 92.7% respectively. Overall accuracy of resectability was 74.5%.
In Chryssou’s series[35], preoperative dynamic MRI with gadolinium was fully concordant with surgical data in the assessment of tumoral invasion in 77% of the veins studied (63/82) and in 58% (43/74) arteries. Concordance was better concerning the assessment of venous invasion but several cases with discordance were reported in that series. MRI showed no tumoral involvement in 5 cases but surgical exploration suggested tumoral infiltration. These veins were resected but final histological study showed no tumor infiltration.
In addition, only 60% of the veins with suspected tumor infiltration on MR and survival exploration showed definite microscopic wall invasion at histological study. However, those lesions would probably not have been resectable without vascular resection.
Some surgical studies[36] have reported that tumoral involvement of portal veins may be overestimated by surgical exploration compared with histology because there was no adventitial tumoral invasion. These studies reflect the challenges of vascular staging of hilar cholangiocarcinoma. In most cases, there is close contact between the tumor and the venous or arterial structure and it is difficult to determine if it is invaded or not. In our experience, only complete tumor encasement (tumor contact with the vessel of more than 50%) is an adequate sign to predict tumor invasion. Despite the fact that in some cases the wall of the vein is actually not invaded microscopically by tumor, the fact is that the tumor cannot be resected without resecting the vessel. In cases of minor tumoral abutment, the patient should not be considered unresectable and surgery should be performed.
In Chryssou’s series[35], 80% of non-stented patients with tumor-to-portal vein contact of more than 90% but without stenosis on MR had invasion of the adventitia confirmed histologically. This may be a useful criterion for predicting invasion of the portal vein or its segmental branches in non-stented patients. In the same series[35], the excellent correlation between MRI and surgery was associated with a weaker correlation between MRI and pathology. These results reflect that both MRI and surgery have limitations in differentiating true microscopic invasion from adherent perivascular fibrosis. This kind of fibrosis is especially frequent in patients with previous biliary drainage because at the hepatic hilus there is close anatomic contiguity between the vessels and the biliary tree. Therefore, in patients with suspected perihilar cholangiocarcinoma it is critical that biliary intervention should be avoided before an adequate preoperative staging is performed. Invasion of adjacent liver parenchyma is also critical in order to assess resectability of cholangiocarcinoma. In mass forming cholangiocarcinoma, parenchymal infiltration is readily seen because tumor has an eccentrical location related to bile ducts. However, in periductal cholangiocarcinoma, parenchymal infiltration may be subtle and high-resolution thin collimation scanning is needed. Imaging with MDCT or MRI shows a hypovascular infiltrating tumoral mass growing beyond the duct and invading the adjacent liver parenchyma. In Masseli’s series[32], MRI accurately showed a local parenchymal invasion in 80% of the patients (Figure 12).
Different imaging techniques are currently used for the preoperative staging of hilar cholangiocarcinoma. However, accurate staging remains a challenge. Distant staging is usually performed with CT or MR at the same time as doing local vascular and biliary staging, yet most evidence is available from CT. Although the quality of imaging has improved in recent years, a substantial proportion of tumors are still found to be unresectable during laparotomy despite extensive pre-operative work and only 50% of the tumors surgically explored are eventually resectable[31].
Recently, with the advent of functional imaging such as positron emission tomography computed tomography (PET-CT) that allows the study of the whole body, the need for additional imaging in patients with cholangiocarcinoma has arisen, in order to rule out distant metastases before radical treatment. The utility of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET scan) in the diagnosis and staging of HCCA is uncertain. Anderson et al[37] showed that PET can accurately detect nodular cholangiocarcinomas as small as 1 cm with a sensitivity of 85%. However, infiltrating tumors which are more frequent are only detected in 18% of the cases. The addition of PET-CT changed the initial management in 30% of the cases by detecting unexpected distant metastasis. This study is relatively old and the results should be interpreted cautiously, especially in patients with PSC or biliary stents in place.
In a recent meta-analysis by Ruys et al[38], data of the literature concerning diagnosis and staging of hilar cholangiocarcinoma during the period 1966-2011 were reviewed. The initial search yielded 766 articles but only 16 papers, which included 448 patients, met the inclusion criteria and were actually analyzed. Most data concerned CT as the primary diagnostic tool and therefore comparison with MRI and PET-CT was not possible. Only data on CT were sufficient for pooling the findings. Pooled accuracy of CT for assessment of ductal extent of the tumor was 86%, whereas pooled sensitivity and specificity for assessment of portal vein involvement was 89% and 92% respectively. Pooled sensitivity and specificity in the assessment of hepatic artery tumoral involvement was 84% and 93% respectively. Concerning regional lymph node metastasis, pooled sensitivity and specificity were 61% and 88%. Only one study that met the inclusion criteria reported the results of PET-CT for the assessment of lymph node metastasis with a sensitivity and specificity of 42% and 80%, respectively[39].
Concerning distant metastases, the results of the meta-analysis are somewhat limited because only one study reported on the sensitivity and specificity of incremental non helical CT on metastasis (67% and 94% respectively). The same study reported a sensitivity and specificity of 56% and 88%, respectively for PET/CT in the detection of distant metastasis. The series by Ruys et al[40] evaluated retrospectively the additional value of FDG-PET/CT in staging of hilar cholangiocarcinoma after extensive conventional preoperative work-up with CT or MR. The primary tumor was 18F-FDG-positive in 88% of patients. However, all benign lesions in the series were also FDG-positive. Sensitivity and specificity for the detection of regional lymph node metastases and distant metastases were 67% and 68%, and 33% and 96%, respectively.
To summarize, the data in the literature concerning accuracy of imaging techniques in the diagnosis and staging of hilar cholangiocarcinoma are still very limited and most studies have important methodological limitations precluding direct head-to-head comparison of different imaging modalities. The only evidence available concerns staging with CT, which has acceptable accuracy for both longitudinal and radial staging, with sensitivity and specificity ranging between 84% and 90%.
However, no solid evidence is available concerning distant staging. The sensitivity of CT regarding lymph node involvement seems to be low (61%) but this is probably not very relevant in the planning of surgical resection since lymphadenectomy is systematically performed in these patients. There are still no solid data concerning the results of the different imaging modalities in the distant staging of hilar cholangiocarcinoma.
P- Reviewers Tougeron D, Sperti C S- Editor Huang XZ L- Editor Roemmele A E- Editor Yan JL
| 1. | Strom BL, Soloway RD, Rios-Dalenz JL, Rodriguez-Martinez HA, West SL, Kinman JL, Polansky M, Berlin JA. Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer. 1995;76:1747-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-473; discussion 473-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 865] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 3. | Klatskin G. Adenocarcinoma of the Hepatic Duct at Its Bifurcation Within the Porta Hepatis. An Unusual Tumor with Distinctive Clinical and Pathological Features. Am J Med. 1965;38:241-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 499] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 1017] [Article Influence: 56.5] [Reference Citation Analysis (1)] |
| 5. | Bosman FT, Carneiro F, Hruban RH, Theise N. WHO classification of tumours of the digestive system. 4th ed. Switzerland: WHO Press 2012; . |
| 6. | Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 7. | Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 366] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 8. | Ben-Menachem T. Risk factors for cholangiocarcinoma. Eur J Gastroenterol Hepatol. 2007;19:615-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 10. | Wetter LA, Ring EJ, Pellegrini CA, Way LW. Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg. 1991;161:57-62; discussion 62-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Soto JA, Barish MA, Yucel EK, Siegenberg D, Ferrucci JT, Chuttani R. Magnetic resonance cholangiography: comparison with endoscopic retrograde cholangiopancreatography. Gastroenterology. 1996;110:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 180] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Fulcher AS, Turner MA. HASTE MR cholangiography in the evaluation of hilar cholangiocarcinoma. AJR Am J Roentgenol. 1997;169:1501-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Holzknecht N, Gauger J, Sackmann M, Thoeni RF, Schurig J, Holl J, Weinzierl M, Helmberger T, Paumgartner G, Reiser M. Breath-hold MR cholangiography with snapshot techniques: prospective comparison with endoscopic retrograde cholangiography. Radiology. 1998;206:657-664. [PubMed] |
| 14. | Yeh TS, Jan YY, Tseng JH, Chiu CT, Chen TC, Hwang TL, Chen MF. Malignant perihilar biliary obstruction: magnetic resonance cholangiopancreatographic findings. Am J Gastroenterol. 2000;95:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Tillich M, Mischinger HJ, Preisegger KH, Rabl H, Szolar DH. Multiphasic helical CT in diagnosis and staging of hilar cholangiocarcinoma. AJR Am J Roentgenol. 1998;171:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Feydy A, Vilgrain V, Denys A, Sibert A, Belghiti J, Vullierme MP, Menu Y. Helical CT assessment in hilar cholangiocarcinoma: correlation with surgical and pathologic findings. AJR Am J Roentgenol. 1999;172:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Nakanuma Y, Minato H, Kida T, Terada T. Pathology of cholangiocellular carcinoma. Primary liver cancer in Japan. 1994;39-50. |
| 18. | Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol. 2003;181:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Nakayama A, Imamura H, Shimada R, Miyagawa S, Makuuchi M, Kawasaki S. Proximal bile duct stricture disguised as malignant neoplasm. Surgery. 1999;125:514-521. [PubMed] |
| 20. | Binkley CE, Eckhauser FE, Colletti LM. Unusual causes of benign biliary strictures with cholangiographic features of cholangiocarcinoma. J Gastrointest Surg. 2002;6:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Shanbhogue AK, Tirumani SH, Prasad SR, Fasih N, McInnes M. Benign biliary strictures: a current comprehensive clinical and imaging review. AJR Am J Roentgenol. 2011;197:W295-W306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Kloek JJ, van Delden OM, Erdogan D, ten Kate FJ, Rauws EA, Busch OR, Gouma DJ, van Gulik TM. Differentiation of malignant and benign proximal bile duct strictures: the diagnostic dilemma. World J Gastroenterol. 2008;14:5032-5038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Novotný I, Dítě P, Trna J, Lata J, Husová L, Geryk E. Immunoglobulin G4-related cholangitis: a variant of IgG4-related systemic disease. Dig Dis. 2012;30:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Horger M, Lamprecht HG, Bares R, Spira D, Schmalzing M, Claussen CD, Adam P. Systemic IgG4-related sclerosing disease: spectrum of imaging findings and differential diagnosis. AJR Am J Roentgenol. 2012;199:W276-W282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, Miyabe K, Yoshida M, Sano H. Clinical significance of extrapancreatic lesions in autoimmune pancreatitis. Pancreas. 2010;39:e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Kamisawa T, Takuma K, Egawa N, Tsuruta K, Sasaki T. Autoimmune pancreatitis and IgG4-related sclerosing disease. Nat Rev Gastroenterol Hepatol. 2010;7:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Lee YJ, Kim SH, Lee JY, Kim MA, Lee JM, Han JK, Choi BI. Differential CT features of intraductal biliary metastasis and double primary intraductal polypoid cholangiocarcinoma in patients with a history of extrabiliary malignancy. AJR Am J Roentgenol. 2009;193:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Diaz-Ruiz MJ, Falcó J, Martin J, Bella RM, Carrasco M, Tortajada L. Hepatocellular carcinoma presenting as portal thrombosis with intrabiliary growth: US and MR findings. Abdom Imaging. 2000;25:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 239] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 32. | Masselli G, Manfredi R, Vecchioli A, Gualdi G. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Eur Radiol. 2008;18:2213-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Lee HY, Kim SH, Lee JM, Kim SW, Jang JY, Han JK, Choi BI. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology. 2006;239:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Cha JH, Han JK, Kim TK, Kim AY, Park SJ, Choi BI, Suh KS, Kim SW, Han MC. Preoperative evaluation of Klatskin tumor: accuracy of spiral CT in determining vascular invasion as a sign of unresectability. Abdom Imaging. 2000;25:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Chryssou E, Guthrie JA, Ward J, Robinson PJ. Hilar cholangiocarcinoma: MR correlation with surgical and histological findings. Clin Radiol. 2010;65:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 37. | Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Ruys AT, van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012;85:1255-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Li J, Kuehl H, Grabellus F, Müller SP, Radunz S, Antoch G, Nadalin S, Broelsch CE, Gerken G, Paul A. Preoperative assessment of hilar cholangiocarcinoma by dual-modality PET/CT. J Surg Oncol. 2008;98:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Ruys AT, Bennink RJ, van Westreenen HL, Engelbrecht MR, Busch OR, Gouma DJ, van Gulik TM. FDG-positron emission tomography/computed tomography and standardized uptake value in the primary diagnosis and staging of hilar cholangiocarcinoma. HPB (Oxford. ). 2011;13:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |