Published online May 15, 2010. doi: 10.4251/wjgo.v2.i5.247
Revised: December 21, 2009
Accepted: December 28, 2009
Published online: May 15, 2010
A 31-year-old female complained of upper abdominal and back pain. Laboratory tests showed elevated levels of aspartate aminotransferase, alanine aminotransferase and α-fetoprotein. Computed tomography revealed that the tumor, measuring 14.5 cm × 10.4 cm, occupied the anterior and medial segments of the liver and consisted of multicystic and solid lesions. The preoperative diagnosis was a hepatic cystadenocarcinoma. The operation was performed urgently because of tumor rupture. Histopathologically, spindle and asteroid cells were found to have proliferated diffusely. There were no neoplastic epithelial tumor cells. Tumor cells had periodic acid-Schiff-positive hyalin globules. At the periphery, trapped normal bile duct cells were observed. The final diagnosis was embryonal sarcoma of the liver (ESL). Interestingly, irregular islands of chondrosarcoma-like lesions were found in the tumor and the tumor-associated vascular endothelium showed immunoreactivity for KIT. Two months after the operation, the tumor recurred. At 6 mo follow-up, the patient is alive with the disease and undergoing chemotherapy. This is the first report of ESL with chondroid differentiation.
- Citation: Kinjo S, Sakurai S, Hirato J, Sunose Y. Embryonal sarcoma of the liver with chondroid differentiation. World J Gastrointest Oncol 2010; 2(5): 247-250
- URL: https://www.wjgnet.com/1948-5204/full/v2/i5/247.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v2.i5.247
Primary malignant mesenchymal tumors of the liver are rarer than epithelial neoplasms and account for no more than 2% of all primary hepatic tumors[1]. Embryonal sarcoma of the liver (ESL) is a rare pediatric malignant tumor and only 16% of the primary hepatic sarcoma[1]. It was first documented by Stocker and Ishak in 1978[2]. Although more than half of patients with ESL are between 6 and 10 years of age, ESL does occur in adults. ESL has generally been considered an aggressive neoplasm with an unfavorable prognosis. However, recent reports have demonstrated that multimodal treatment of ESL can improve survival and potentially cure ESL in adults[1,3,4]. ESL is a primitive neoplasm of mesenchymal origin. ESL tumors have frequently been defined as undifferentiated sarcoma but have also been referred to as primary sarcoma and malignant mesenchymoma because some cases show divergent characteristics. Another tumor showing similar pathological features to ESL is anaplastic sarcoma of the kidney (ASK). Vujanić et al[5] reported 20 cases of this unusual renal neoplasm. This tumor is also composed of spindle cells proliferation with anaplastic change. A point of difference is that chondroid differentiation is seen frequently in ASK but not in ESL. In the present case, we found chondrosarcoma-like lesions in the tumor. To the best of our knowledge, this is the first report of ESL with chondroid differentiation.
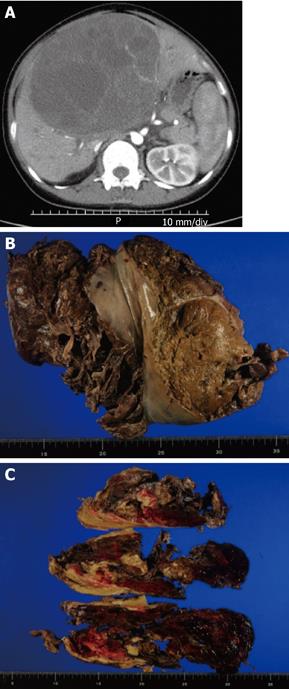
A 31-year-old female was admitted to our hospital because of upper abdominal and back pain. Laboratory tests showed elevated levels of aspartate aminotransferase (AST 125 IU/L) and alanine aminotransferase (ALT 199 IU/L). Total bilirubin level was in the normal range. The level of the tumor marker α-fetoprotein (AFP) was high (42.24 ng/L) and that of carcinoembryonic antigen (CEA) was within the normal range. Abdominal computed tomography (CT) showed a huge mass measuring 14.5 cm × 10.4 cm which occupied the anterior and medial segments of the liver. The mass consisted of multicystic and solid lesions (Figure 1A). The preoperative diagnosis was a hepatic cystadenocarcinoma. Two months after the first symptom, a bisectionectomy (liver segments IV-VIII) was urgently performed because the mass was rapidly increasing in size, measuring 16 cm × 13 cm × 17 cm in diameter, and bleeding. During the operation, there was a lot of hemorrhagic ascites and hematoma which came from the ruptured tumor. The tumor was soft and well-circumscribed by pseudocapsule. The tumor, inclusive of pseudocapsule, was resected. The postoperative course was uncomplicated and the patient discharged. Two months after the operation, the patient experienced back pain again. A CT scan revealed multiple metastases in the left lobe of the liver and peritoneal dissemination. Six months after the operation, the patient is alive with the disease and is undergoing chemotherapy with doxorubicin and ifosfamid.
Macroscopic findings: The liver was ruptured (Figure 1B). The gross appearance of the tumor was friable and hemorrhagic. Cutting of the surface revealed a soft white mass with multilocular appearance and fibrous pseudocapsules (Figure 1C).
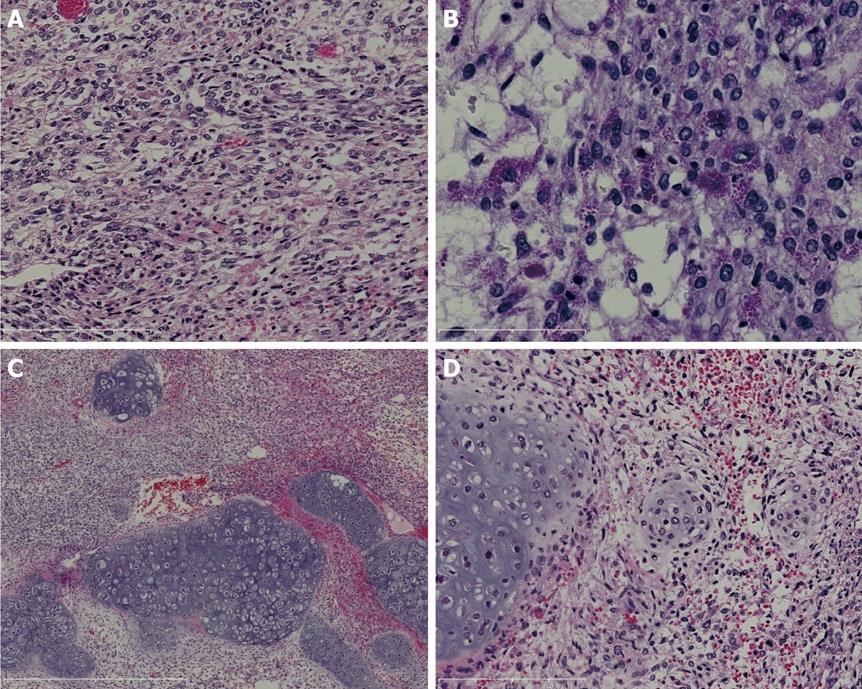
Microscopic findings: Upon microscopic examination, spindle-shaped cells were found to have proliferated diffusely. There were no epithelial tumor cells. Tumor cells were compactly or loosely arranged, with myxoid matrix or edema (Figure 2A). They showed marked anisonucleosis with hyperchromasia and some cells were found to be giant, multinuclear cells. Mitotic cells were abundant (19/10 high-power field). There were eosinophilic, PAS-positive and diastase-resistant globules in the cytoplasm of the tumor cells (Figure 2B), stroma and apoptotic bodies. The peripheral area contained entrapped benign-appearing bile ducts which were dilated. In the focal area, we observed hyaline cartilage formation with lacunae containing irregular angular cells with one or more unusual nuclei, some of which were mitotic and resembled chondrosarcoma (Figure 2C). In some parts of this lesion, as the cartilaginous matrix was deposited around the spindle tumor cells, the tumor cells were seen to change to chondrosarcoma (Figure 2D).
Immunohistochemically, most tumor cells were strongly reactive with vimentin. Some spindle or asteroid tumor cells showed cytoplasmic positivity for desmin and smooth muscle actin (SMA), whereas tests for keratin, S100P, h-caldesmon, hepatocyte-specific antigen, AFP, HMB-45 and p53 were negative for tumor cells.
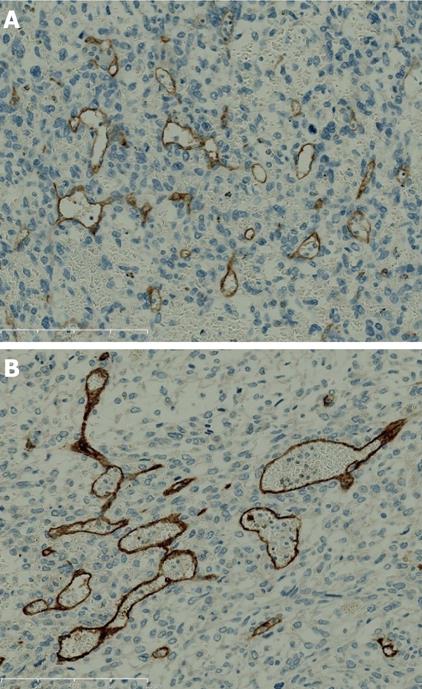
KIT and CD34 were not expressed in tumor cells but endothelial cells of tumor-associated vascularity expressed both KIT (Figure 3A) and CD34 (Figure 3B). The tumor was diagnosed as undifferentiated (embryonal) sarcoma of the liver. The resected margin was free of tumor infiltration.
Although several synonyms have been used in the past, ESL was clearly differentiated from other sarcomas and defined as a distinct entity by Stocker and Ishak in 1978[2]. Usually ESL is composed of medium to large spindle or asteroid cells with marked nuclear pleomorphism. In addition, there are prominent eosinophilic, PAS-positive and diastase-resistant globules in the cytoplasm of the tumor cells.
The different diagnoses of ESL in adults include hepatocellular carcinoma, hepatoblastoma, malignant fibrous histiocytoma, leiomyosarcoma, liposarcoma, angiomyolipoma, angiosarcoma and metastasis of mesenchymal tumor, such as gastrointestinal stromal tumors (GISTs). In the present case, a chondrosarcoma-like lesion is exhibited. A mesenchymal cartilage component is also found in hepatoblastoma but there are no epithelial hepatoblastoma cells in this case.
Immunohistochemical investigation may be necessary to distinguish ESL from other sarcomas or sarcomatoid variants of hepatocellular carcinoma. The tumor cells may be reactive to antibodies to α-1-antitrypsin[6,7], α-1-antichymotrypsin[3,6-8] and vimentin[3,8]. Occasionally, tumors express desmin[3,6,7], α-SMA[7], muscle-specific actin[7] and CD68[6-8]. In some studies, tumor cells showed negative results for keratin[4], HMB-45[3,4,8], CD34[3,4,8] and KIT[4]. In the present case, ESL appears positive for SMA and desmin but negative for h-caldesmon. These results suggest that immunohistochemically some of the tumor cells in the present case show a myofibroblast phenotype.
In the present case the histopathological findings are typical, with the exception of the chondroid differentiation. Although the exact histogenesis of ESL is still unknown, it is considered that ESL may develop from multipotential mesenchymal stem cells, which have been isolated from bone marrow, adipose tissue and also from liver tissue[9].
In our case, KIT was negative for the tumor cells as in a previous report, but we identified KIT expression in the endothelial cells within the tumor nodule. However, KIT was not expressed in endothelial cells in non-neoplastic regions.
KIT is a stem cell factor receptor and expressed during various stages of certain cell lineages, including germ cells, mast cells, stellate cells and some subsets of cerebellar neuron cells[10]. In mesenchymal tumors, KIT expression has been reported in GISTs, seminoma and some sarcomas such as angiosarcoma and Ewing’s sarcoma[10]. There have been no reports of KIT expression in vascular endothelium and the role of the KIT expression in tumor-associated vascularity in the present case is still unclear. However, Tallini et al[11] reported that KIT-positive cells can differentiate into smooth muscle and endothelium of the heart. KIT expression might be involved in the angiogenesis of ESL in the present case and molecular targeted drug against KIT might be effective.
In summary, we report on the first case of ESL with chondroid differentiation. The histogenesis of ESL is still unclear and further study is needed to clarify the origin of ESL.
Peer reviewers: Antonio Russo, MD, PhD, Associate Professor, Genetic and Molecular Oncology Unit, Interdepartmental Center of Research in Clinical Oncology, School of Medicine, University of Palermo, Via del Vespro 127-90127 Palermo, Italy; Lars Mueller, MD, Department of General and Thoracic Surgery, University Hospital Schleswig-Holstein, Campus Kiel, Arnold-Heller-Str. 3, Kiel 24105, Germany
S- Editor Li LF L- Editor Roemmele A E- Editor Yang C
| 1. | Ishak KG, Goodman ZD, Stocker JT. Tumors of the liver and intrahepatic bile ducts: Atlas of tumor pathology. 3rd series, fascicle 31. Washington DC: Armed Forces Institute of Pathology 2001; 313-320. |
| 2. | Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978;42:336-348. |
| 3. | Lenze F, Birkfellner T, Lenz P, Hussein K, Länger F, Kreipe H, Domschke W. Undifferentiated embryonal sarcoma of the liver in adults. Cancer. 2008;112:2274-2282. |
| 4. | Pachera S, Nishio H, Takahashi Y, Yokoyama Y, Oda K, Ebata T, Igami T, Nagino M. Undifferentiated embryonal sarcoma of the liver: case report and literature survey. J Hepatobiliary Pancreat Surg. 2008;15:536-544. |
| 5. | Vujanić GM, Kelsey A, Perlman EJ, Sandstedt B, Beckwith JB. Anaplastic sarcoma of the kidney: a clinicopathologic study of 20 cases of a new entity with polyphenotypic features. Am J Surg Pathol. 2007;31:1459-1468. |
| 6. | Aoyama C, Hachitanda Y, Sato JK, Said JW, Shimada H. Undifferentiated (embryonal) sarcoma of the liver. A tumor of uncertain histogenesis showing divergent differentiation. Am J Surg Pathol. 1991;15:615-624. |
| 7. | Nishio J, Iwasaki H, Sakashita N, Haraoka S, Isayama T, Naito M, Miyayama H, Yamashita Y, Kikuchi M. Undifferentiated (embryonal) sarcoma of the liver in middle-aged adults: smooth muscle differentiation determined by immunohistochemistry and electron microscopy. Hum Pathol. 2003;34:246-252. |
| 8. | Ma L, Liu YP, Geng CZ, Tian ZH, Wu GX, Wang XL. Undifferentiated embryonal sarcoma of liver in an old female: case report and review of the literature. World J Gastroenterol. 2008;14:7267-7270. |
| 9. | Tarnowski M, Koryciak-Komarska H, Czekaj P, Sebesta R, Czekaj TM, Urbanek K, Likus W, Malinowska-Kolodziej I, Plewka D, Nowaczyk-Dura G. The comparison of multipotential for differentiation of progenitor mesenchymal-like stem cells obtained from livers of young and old rats. Folia Histochem Cytobiol. 2007;45:245-254. |
| 10. | Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205-220. |
| 11. | Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA. 2009;106:1808-1813. |