Published online Mar 15, 2010. doi: 10.4251/wjgo.v2.i3.165
Revised: October 26, 2009
Accepted: November 2, 2009
Published online: March 15, 2010
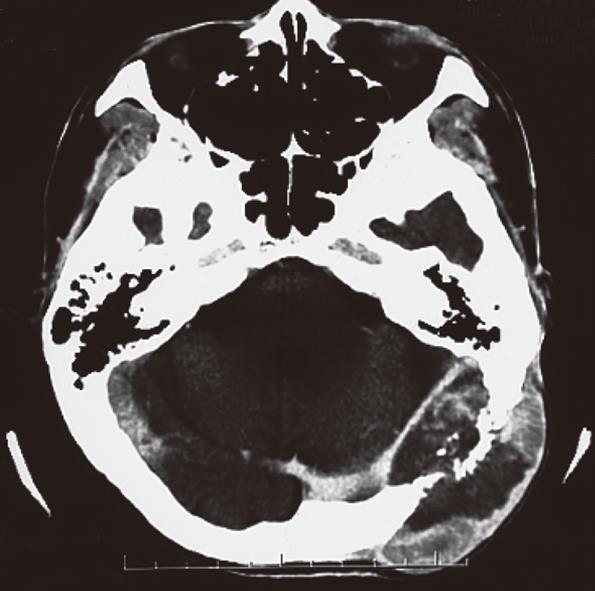
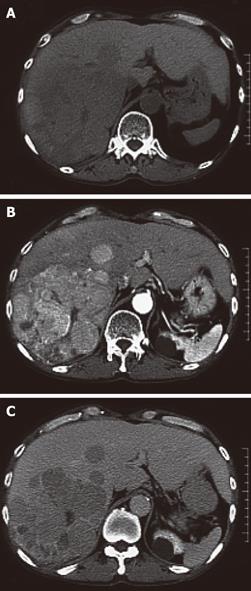
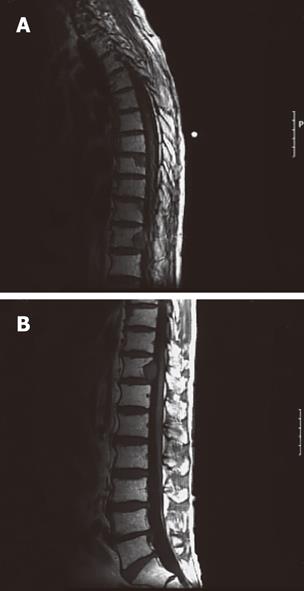
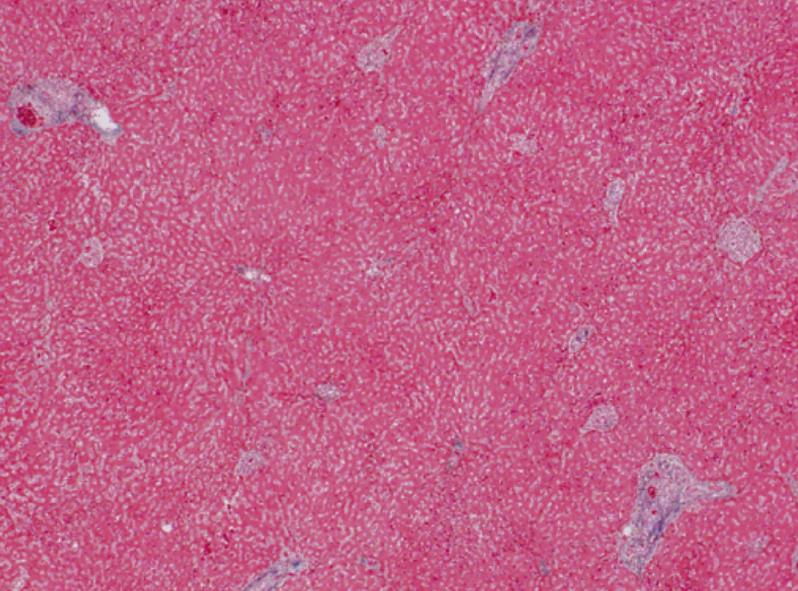
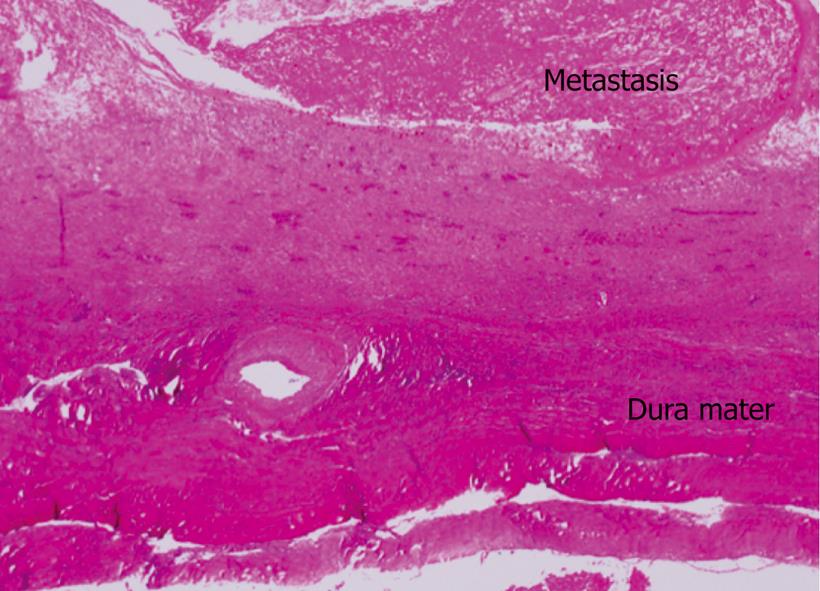
A 56-year-old male visited our hospital for evaluation of an occipital mass. Contrast computed tomography showed hypervascular enhancement with osteolytic change in the skull and a huge enhanced mass in the liver. Magnetic resonance imaging showed bone metastasis in the thoracic vertebrae. Assays for hepatitis B surface antigen and hepatitis B core antibody were positive and his liver condition was Child-Pugh grade A. Our diagnosis was hepatocellular carcinoma (HCC) with skull and vertebrae metastases on chronic hepatitis B. He was treated with radiation therapy for bone metastases and transcatheter arterial chemoembolization for HCC. But he developed acute respiratory failure because of aspiration pneumonia, congestion and oedema with haemorrhage of the lungs and died. Dissection showed HCC with multiple bone metastases. The liver tumor was categorized as well-differentiated HCC, Edmondson classification I, trabecular type and pseudoglandular type. In the liver mild infiltration of lymphocytes was seen in Glisson’s capsules which were significantly enlarged with well preserved limiting plates. Piecemeal necrosis was not obvious. No fibrosis was noted. An 8 cm × 7 cm × 3 cm metastatic lesion had formed in the left occipitotemporal part of the cranial bone. The lesion was osteolytic and showed invasion into the dura mater. Neither the subdural cavity nor the brain showed involvement from the metastatic tumor. However, skull metastasis from HCC is very rare and it affects the patient’s prognosis and the quality of life. Therefore, it is very important to make an early diagnosis and carry out proper management of skull metastasis from HCC.
- Citation: Goto T, Dohmen T, Miura K, Ohshima S, Yoneyama K, Shibuya T, Kataoka E, Segawa D, Sato W, Anezaki Y, Ishii H, Kon D, Yamada I, Kamada K, Ohnishi H. Skull metastasis from hepatocellular carcinoma with chronic hepatitis B. World J Gastrointest Oncol 2010; 2(3): 165-168
- URL: https://www.wjgnet.com/1948-5204/full/v2/i3/165.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v2.i3.165
Hepatocellular carcinoma (HCC) is one of the most common and principal cancers worldwide. The occurrence of HCC is primarily associated with chronic viral hepatitis type B and C which are common causes of hepatic cirrhosis. Despite advances in diagnosis and treatment modalities or techniques, the long-term prognosis of advanced HCC with metastasis is still poor[1].
HCC commonly metastasizes to the lung, regional lymph nodes, peritoneum and adrenal glands. The incidence of bone metastases from HCC is very low[2]. The most common sites are the vertebrae, followed by the pelvis and ribs. HCC very rarely metastasizes to the skull[3]. Here we report a case of skull metastasis from HCC with chronic hepatitis B.
A 56-year-old male visited our hospital for evaluation of an occipital mass. Contrast computed tomography (CT) showed hypervascular enhancement with osteolytic change in the skull (Figure 1) and a huge enhanced mass in the liver (Figure 2). Magnetic resonance imaging showed bone metastasis in the thoracic vertebrae (Figure 3). Indocyanine-green retention rate at 15 min (ICG R15) was 3.4% and his liver condition was Child-Pugh grade A. Assays for hepatitis B surface antigen and hepatitis B core antibody were positive, hepatitis B envelope antigen was negative and hepatitis B envelope antibody was positive. HBV-DNA was less than 3.7LGE/mL. Hepatitis C antibody was negative. The serum concentration of PIVKA II was 7719 mAU/mL (normal < 40) (Table 1). Our diagnosis was HCC with bone metastases with chronic hepatitis B. He was treated with radiation therapy (Total of 30 Gy) for bone metastases and transcatheter arterial chemoembolization (TACE) for HCC. The size of the skull metastasis decreased after radiation therapy but he developed acute respiratory failure because of aspiration pneumonia, congestion and edema with hemorrhage of the lungs and died. Dissection showed HCC with multiple bone metastases. The liver tumor was categorized as well-differentiated HCC, Edmondson classification I, trabecular type and pseudoglandular type. In the liver, mild infiltration of lymphocytes was seen in Glisson’s capsules which were significantly enlarged with well preserved limiting plates. Piecemeal necrosis was not obvious. No fibrosis was noted (Figure 4). An 8 cm × 7 cm × 3 cm metastatic lesion had formed in the left occipitotemporal part of the cranial bone. The lesion was osteolytic and showed invasion into the dura mater. Neither the subdural cavity nor the brain showed involvement from the metastatic tumor (Figure 5).
| Hematology | Blood chemistry | Serological test |
| WBC 5900/&mgr;L | AST 36 IU/L | CEA 4.1 ng/ mL |
| RBC 256 × 104/&mgr;L | ALT 17 IU/L | CA 19-9 23.9 U/mL |
| Hb 8.8 g/dL | ALP 638 IU/L | AFP 3.2 ng/mL |
| Ht 28.0% | LDH 175 IU/L | PIVKA II 7719 mAU/mL |
| Plate 44.2 × 104/&mgr;L | G-GT 241 IU/L | |
| T.BiL 0.2 mg/dL | ICG R15 3.4% | |
| Coagulation tests | D.BiL 0.1 mg/dL | |
| PT time 11.3 s | ChE 126 IU/L | HBs Ag (+) |
| PT % 90.2% | TP 6.0 g/dL | HBe Ag (-) |
| Alb 3.1 g/dL | HBe Ab (+) | |
| BUN 12.3 mg/dL | HBc Ab (+) × 200 93.4 | |
| Cre 0.43 mg/dL | HBV DNA (TMA) < 3.7 LGE/mL | |
| T.Chol 128 mg/dL | HCV Ab (-) | |
| Na 143 mEq/L | ||
| K 4.6 mEq/L | ||
| Cl 107 mEq/L | ||
| CRP 3.84 mg/dL |
HCC is one of the most common cancers worldwide with the highest incidence in regions with high prevalence of chronic viral hepatitis infection, especially hepatitis B and C infection. In HCC cases with cirrhosis, HCV infection was identified in 27%-73%, HBV infection in 12%-55%, heavy alcohol intake in 4%-38% and hemochromatosis and other causes in 2%-6% of cases, leaving 4%-6% of the total number of cases without an identified cause. Among persons with HCC but without underlying cirrhosis, HCV infection accounted for 3%-54%, HBV infection for 4%-29% and heavy alcohol intake for 0%-28% of cases[4]. In the Japan Society of Hepatology’s guide, HCC incidence was found to be 0.5-0.8 per 100 person-years in chronic hepatitis B without cirrhosis. In our case, mild infiltration of lymphocytes was seen in Glisson’s capsules which were significantly enlarged with well preserved limiting plates. Piecemeal necrosis was not obvious and no fibrosis was noted. His liver condition was Child-Pugh grade A based on the laboratory data. His diagnosis was HCC with bone metastases of the skull and vertebrae in chronic hepatitis B without cirrhosis.
The incidence of HCC metastases has been reported to be less than 5% with the most common sites being the regional lymph nodes and lung. The incidence of bone metastases from HCC is approximately 1.6%-16% with the most common sites being the vertebrae, followed by the pelvis and ribs[3,5]. HCC rarely metastasizes to the skull. Yanase et al[6] reviewed 4140 autopsy reports on Japanese patients with HCC and found only 17 (6.1%) skull metastases among 278 cases of bone involvement which further supports the view that skull metastasis from HCC is a rare event. Our case showed bone metastasis in the thoracic vertebrae and skull. Two metastatic pathways were hypothesized: a hematogenous route via the lungs to the brain parenchyma and an osseous route via Batson’s venous plexus to the skull[4].
Hsieh et al[7] summarized 68 patients with cranial metastasis from HCC. Their ages ranged from 13 years to 85 years old with a mean age of 57 years. The sex ratio was male: female 54:14. Six patients (8.8%) had multiple skull metastases involving the calvarium and skull base. The most frequent presentation was with a subcutaneous mass with occasional painful sensation which was reported in 40 cases (58.8%). Ten cases (14.7%) presented with headache, seven cases (10.3%) presented with weakness of limbs and two cases (2.9%) presented with seizures. Cranial nerve deficits, which were seen in 28 cases (41.2%) and included visual disturbance, dysphagia, deafness and facial numbness, were associated with the cranial sites where the tumor was involved, especially in the skull base. Skull X-ray films revealed osteolytic lesions in all cases. Twenty cases (29.4%) presented with high-density on CT scans of the skull and all cases showed enhancement on post-contrast CT scan. Survival time of 41 patients ranged from 6 d to 108 mo with mean survival time of 8.9 mo. Most of their deaths were linked to the primary disease. Our case had no symptoms except for the fact that he noticed an occipital mass. CT showed hypervascular enhancement with osteolytic change in the skull. Our radiological examination yielded a typical result. His survival time was about 7 mo after noticing the tumor of the head.
The treatment for skull metastasis, which includes radiotherapy, chemotherapy, surgery and palliative care, has been performed to relieve pain and reduce the risks of neurological sequelae, thus improving or maintaining the quality of life or even prolonging life[3,5,8]. Trans-arterial embolization or ethanol injection before surgery has been carried out before surgical intervention[9-12]. A single calvarial metastasis can be treated surgically[10]. Our case was treated with radiation therapy for the skull and vertebrae metastases and the size of the skull and vertebrae metastases decreased after radiation therapy. To prevent tumor rupture, HCC was treated with TACE.
However, skull metastasis from HCC is very rare and it affects the patient’s prognosis and quality of life. Therefore, it is very important to make an early diagnosis and carry out proper management of skull metastasis from HCC.
Peer reviewer: Takuya Watanabe, MD, PhD, Department of Internal Medicine and Gastroenterology, Medical Hospital, the Nippon Dental University School of Life Dentistry, 1-8 Hamauracho, Chu-o-ku, Niigata, 951-8580, Japan
S- Editor Li LF L- Editor Roemmele A E- Editor Yang C
| 1. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. |
| 2. | Attili VS, Babu KG, Lokanatha D, Bapsy PP, Ramachandra C, Rajshekar H. Bone metastasis in hepatocellular carcinoma: need for reappraisal of treatment. J Cancer Res Ther. 2008;4:93-94. |
| 3. | Fukutomi M, Yokota M, Chuman H, Harada H, Zaitsu Y, Funakoshi A, Wakasugi H, Iguchi H. Increased incidence of bone metastases in hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2001;13:1083-1088. |
| 4. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. |
| 5. | McIver JI, Scheithauer BW, Rydberg CH, Atkinson JL. Metastatic hepatocellular carcinoma presenting as epidural hematoma: case report. Neurosurgery. 2001;49:447-449. |
| 6. | Yanase Y, Sano K, Hama H. Case report of a primary hepatoma with metastasis to the femur and statistical review. J Kansai Denryoku Hosp. 1972;4:94–99. |
| 7. | Hsieh CT, Sun JM, Tsai WC, Tsai TH, Chiang YH, Liu MY. Skull metastasis from hepatocellular carcinoma. Acta Neurochir (Wien). 2007;149:185-190. |
| 8. | Chan CH, Trost N, McKelvie P, Rophael JA, Murphy MA. Unusual case of skull metastasis from hepatocellular carcinoma. ANZ J Surg. 2004;74:710-713. |
| 9. | Miura T, Hirabuki N, Kozuka T. Cranial metastasis from hepatocellular carcinoma. Clin Radiol. 1990;42:445-446. |
| 10. | Murakami R, Korogi Y, Sakamoto Y, Takhashi M, Okuda T, Yasunaga T, Nishimura R, Yoshimatsu S. Skull metastasis from hepatocellular carcinoma. CT, MR and angiographic findings. Acta Radiol. 1995;36:597-602. |
| 11. | Shibukawa M, Inagawa T, Katoh Y, Tokuda Y, Ohbayashi N, Yoshioka Y. [A case of cranial metastasis of hepatocellular carcinoma]. No To Shinkei. 1995;47:1087-1091. |
| 12. | Isaka T, Yoshimine T, Fujimoto K, Masuzawa M, Maruno M, Hayakawa T, Nakatani S. Direct ethanol injection for skull metastasis from hepatocellular carcinoma. The techniques and consequences of a therapeutic trial. Neurol Res. 1998;20:737-741. |