Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.109743
Revised: May 28, 2025
Accepted: June 23, 2025
Published online: August 15, 2025
Processing time: 85 Days and 21.8 Hours
Acute pancreatitis (AP) is a leading gastrointestinal cause of hospitalization worldwide. While gallstones, alcohol use, and hypertriglyceridemia account for most cases, pancreatic malignancy remains an underdiagnosed but critical etiology requiring prompt identification due to its significant prognostic implications.
To systematically evaluate the clinical characteristics of tumor-associated AP and identify risk factors influencing early diagnosis.
This retrospective cohort study analyzed 167 patients with pancreatic cancer-associated AP (2014-2023), stratified by diagnostic timing: Early-diagnosis (n = 75, identified during initial AP admission) vs delayed-diagnosis (n = 92, requiring ≥ 2 admissions). Multivariable logistic regression was performed to determine independent predictors of early cancer detection.
The early-diagnosis group demonstrated distinct clinical and biochemical signatures, with independent predictors including: Diabetes history [odds ratio (OR) = 2.69, 95% confidence interval (CI): 1.08-3.34], concurrent AP etiologies (OR = 4.77, 95%CI: 1.84-7.81), elevated carbohydrate antigen 19-9 (OR = 1.38, 95%CI: 1.03-1.84), hyperbilirubinemia (direct: OR = 2.36, 95%CI: 1.35-3.48; indirect: OR = 2.67, 95%CI: 1.38-4.62), and serum glucose (OR = 1.42, 95%CI: 1.08-2.55).
Key high-risk indicators for occult pancreatic malignancy in tumor- associated AP patients include: Advanced age, pre-existing diabetes mellitus, hyperbilirubinemia, and concurrent with conventional AP etiologies. These findings advocate for enhanced surveillance protocols incorporating serial tumor markers and multimodal imaging to earlier cancer detection.
Core Tip: Although pancreatic malignancies represent a relatively infrequent etiology of acute pancreatitis (AP), their association with adverse clinical outcomes necessitates prompt diagnostic evaluation. In this retrospective cohort study, significant differences in clinical characteristics, disease progression, and healthcare utilization were observed between the early and delayed diagnosis patients. Multivariable logistic analysis indicated that advanced age, pre-existing diabetes mellitus, hyperbilirubinemia, and concurrent with conventional AP etiologies are key high-risk indicators for occult pancreatic malignancy in tumor-associated AP patients.
- Citation: Xia CC, Ning LG, Xu Y, Xu GQ. Clinical characteristics and diagnostic factors of tumor-associated acute pancreatitis: A comparative analysis of early vs delayed diagnosis. World J Gastrointest Oncol 2025; 17(8): 109743
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/109743.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.109743
Acute pancreatitis (AP) represents one of the most common gastrointestinal disorders necessitating hospitalization, characterized by a complex and highly variable clinical course that poses significant challenges in early diagnosis and treatment[1]. While gallstone disease and alcohol abuse remain the most prevalent etiological factors, emerging evidence highlights the growing significance of metabolic disorders (e.g., hypertriglyceridemia, hypercalcemia) and genetic predispositions (e.g., hereditary pancreatitis) in AP pathogenesis[2]. Notably, structural abnormalities such as periampullary tumors, pancreatic head masses, and cystic lesions can induce pancreatic duct obstruction, thereby disrupting the physiological flow of pancreatic enzymes. This obstruction may trigger premature intra-pancreatic enzyme activation, culminating in pancreatitis. Given its increasing clinical relevance, particularly among elderly populations, pancreatic cancer has emerged as a critical and non-negligible contributor to AP pathogenesis[3]. Pancreatic ductal adenocarcinoma (PDAC) represents the most common and aggressive histological subtype of pancreatic cancer, exhibiting alarming global epidemiological trends: Reported cases surged from 196000 (1990) to 441000 (2017), reflecting a 125% increase[4,5]. Despite therapeutic advances, the 5-year survival rate remains dismal, improving marginally from < 5% in the 1990s to 9% in contemporary United States and European cohorts[6,7]. This poor prognosis is primarily attributable to delayed diagnosis, with only 20% of patients presenting surgically resectable disease at initial detection[8]. Such statistics underscore an urgent need for innovative diagnostic strategies to facilitate early intervention.
Despite advances in pancreatic cancer diagnostics, the pathophysiological mechanisms linking pancreatic malignancy to AP remain elusive. Crucially, the clinical trajectory of pancreatic cancer patients whose initial manifestation is AP remains poorly characterized. This knowledge gap carries significant clinical implications, as the overlapping symptomatology and imaging features between tumor-associated pancreatitis and conventional AP often lead to diagnostic overshadowing. In such cases, typical AP risk factors (e.g., biliary obstruction or alcohol use) may be erroneously implicated, while occult neoplasms evade detection[9].
Although contemporary imaging modalities particularly contrast-enhanced computed tomography (CE-CT) and magnetic resonance imaging (MRI) demonstrate > 90% sensitivity for pancreatic tumor identification, their ability to differentiate tumor-induced pancreatitis from primary AP during acute presentations remains limited[10]. This diagnostic ambiguity results in a non-negligible proportion of AP cases serving as the harbinger of an undiagnosed malignancy. The precise nature of the AP-pancreatic cancer association warrants systematic investigation. To address this, we conducted a comprehensive analysis of clinical data from tumor-associated AP cases, which aim to systematically analyze clinical characteristics and to identify key risk factors that may signal an underlying pancreatic tumor as the etiology of AP. Our findings provide actionable insights for early detection of tumor-associated AP and help improve patient management and outcomes.
This population-based retrospective study enrolled patients initially presenting with AP who were subsequently diagnosed with PDAC between January 2014 and December 2023 admitted at the First Affiliated Hospital, Zhejiang University School of Medicine. Clinical data, including demographic characteristics, laboratory findings, and imaging results, were extracted from institutional electronic medical records at the time of synchronous AP and tumor diagnosis.
Patients with tumor-associated AP were stratified into two groups based on diagnostic confirmation timelines: The early-diagnosis group: Patients who received definitive PDAC diagnosis as the underlying AP etiology during initial evaluation, and the delayed-diagnosis group: Patients meeting AP diagnostic criteria (Atlanta classification) without immediate PDAC identification, where malignancy was subsequently confirmed after ≥ 2 clinical encounters (minimum 14-day interval), based on progressive imaging findings or histopathological confirmation.
Exclusion criteria were as follows: (1) Prior diagnosis of pancreatic cancer or other malignancies preceding AP onset; (2) Insufficient pathological or radiographic evidence confirming pancreatic malignancy; (3) Incomplete medical records; (4) Patients who received a pancreatic tumor diagnosis with a latency period exceeding 12 months following their initial episode of pancreatitis; (5) Additionally, we excluded patients with acute-on-chronic pancreatitis given the established malignant transformation risk in chronic pancreatitis; and (6) Patients were excluded if comprehensive evaluation confirmed non-PDAC neoplasms, including: Pancreatic cystic neoplasms, pancreatic neuroendocrine tumors, metastatic carcinomas or other rare subtypes.
The diagnosis of AP was rigorously established according to the revised Atlanta classification criteria, requiring fulfillment of at least two of the following three diagnostic components: (1) Characteristic abdominal pain; (2) Elevated serum pancreatic enzymes (lipase or amylase) exceeding threefold the upper limit of normal; and (3) Typical imaging results of AP (for instance, MRI, CE-CT, or ultrasonography)[3]. Disease severity stratification was assessed at baseline using standardized criteria incorporating serum biomarkers, cross-sectional imaging features, and the presence of organ failure or pancreatic necrosis. For cases of tumor-associated AP, definitive etiological confirmation was achieved through histopathological verification using either surgically resected specimens, or endoscopic ultrasound-guided fine-needle aspiration/biopsy (EUS-FNA/B)[11].
A systematic retrospective review of clinical records was performed for all enrolled patients at the onset of AP. For cases in the delayed-diagnosis group, detailed clinical data were meticulously extracted, tracing back to the initial AP presentation. The dataset comprised baseline characteristics (age, sex, body mass index, presenting symptoms, and medical history), risk factors (lifestyle variables and biochemical profiles), and diagnostic and therapeutic data (radiological findings, treatment strategies, histopathology, hospitalization duration, and healthcare costs).
All clinical outcomes were rigorously assessed based on standardized diagnostic criteria, with data extracted from electronic medical records following a predefined protocol. The study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. IIT2025016B), ensuring compliance with the Declaration of Helsinki.
Demographic and clinical characteristics were summarized as frequencies (percentages) for categorical variables and as median interquartile range (IQR) or mean ± SD for continuous variables, based on their distribution. Pearson’s χ² test or Fisher’s exact test was employed for categorical comparisons, whereas Student’s t-test (for normally distributed data) or the Mann-Whitney U test (for nonparametric data) was used for continuous variables. Variables with a two-tailed P value < 0.05 in univariate analysis were entered into multivariable logistic regression models to identify independent predictors of tumor-associated AP. All statistical analyses were conducted using GraphPad Prism 9.0.
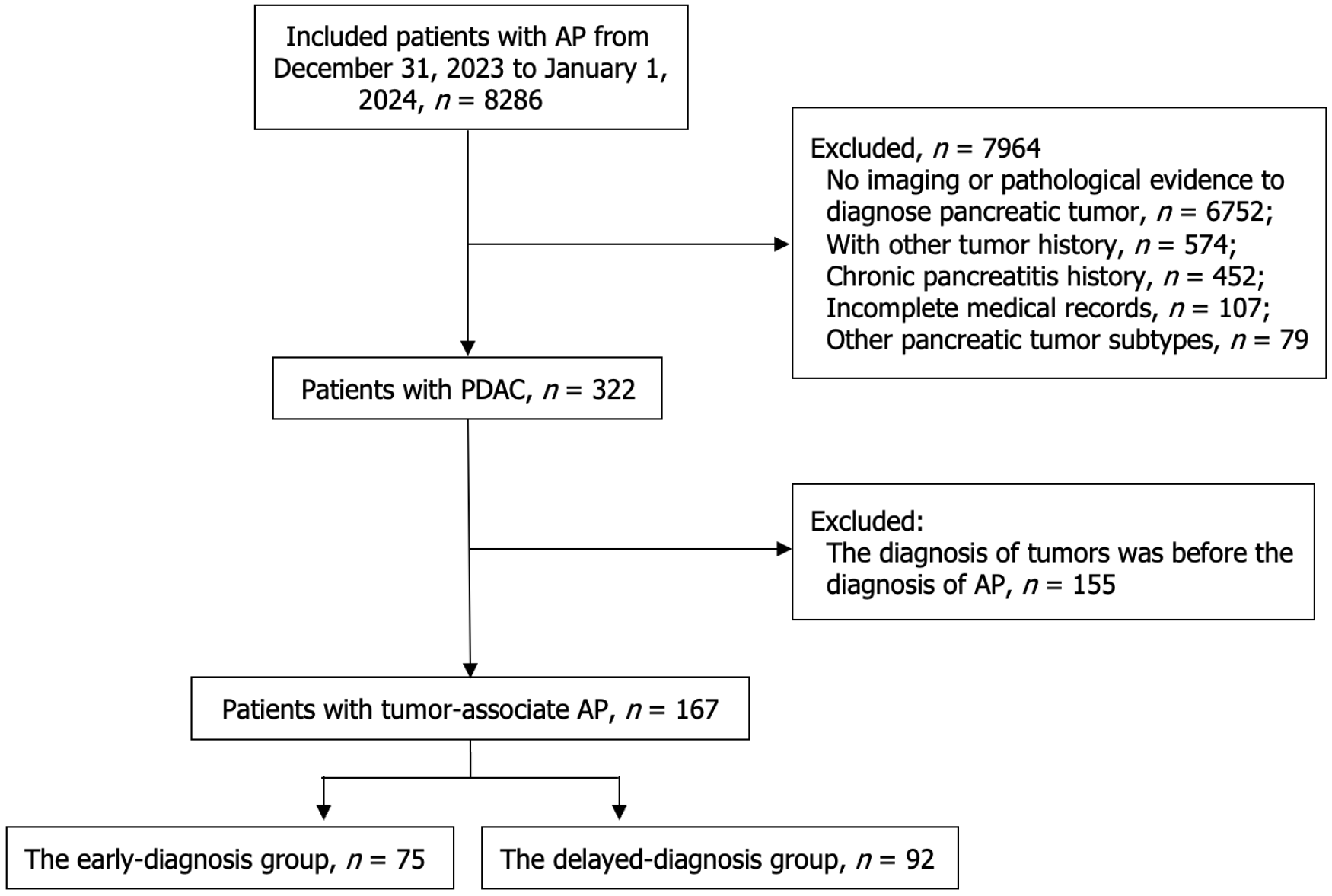
From January 2014 to December 2023, we retrospectively analyzed a cohort of 8286 patients with AP. After implementing rigorous exclusion criteria, 7964 patients (96.1%) were excluded. Among the 322 patients (3.9%) with confirmed PDAC, 155 patients were further excluded due to PDAC diagnosis preceding AP onset. The final cohort comprised 167 patients with tumor-associated AP, which were stratified into two groups based on diagnostic timing: 75 patients in the early-diagnosis group and 92 in the delayed-diagnosis group. Figure 1 illustrates the complete patient selection process using a standardized flowchart.
The baseline characteristics of the study cohort (n = 167) are summarized in Table 1. The population demonstrated a female predominance (58.1%, n = 97) with a median age of 63.9 years (IQR: 54.1-68.0). Notable lifestyle factors included current or former smokers (44.9%, n = 75) and regular alcohol consumers (47.9%, n = 80). Comorbidities were present in a substantial proportion, with diabetes mellitus documented in 31.7% (n = 53) of cases. A family history of pancreatic cancer was reported in 6.0% (n = 10) of patients. Regarding disease severity at presentation, the majority (58.1%, n = 97) exhibited mild AP, while 7.8% (n = 13) presented with severe disease. Emergency department admission was required for 28.4% (n = 47) of the cohort.
| Characteristics | Total (n = 167) | The early-diagnosis group (n = 75) | The delayed-diagnosis group (n = 92) | P value |
| Age, median (IQR), year | 63.9 (54.1-68.0) | 57.3 (54.5-62.5) | 68.2 (60.1-73.0) | 0.02a |
| Sex (F/M) | 97/70 | 41/34 | 56/36 | 0.59 |
| BMI, median (IQR) | 21.2 (20.3-22.4) | 21.1 (20.74-22.66) | 21.0 (20.3-22.1) | 0.20 |
| Smoking history | > 0.99 | |||
| Yes | 75 (44.9) | 32 (42.7) | 43 (46.7) | |
| No | 92 (55.1) | 43 (57.3) | 49 (53.4) | |
| Alcohol history | ||||
| Yes | 80 (47.9) | 15 (20.0) | 65 (70.7) | < 0.01a |
| No | 87 (52.1) | 60 (80.0) | 27 (29.3) | |
| Diabetes history | < 0.01a | |||
| Yes | 53 (31.7) | 6 (8.0) | 47 (51.1) | |
| No | 114 (68.3) | 69 (92.0) | 45 (48.9) | |
| Classification of AP | ||||
| MAP | 97 (58.1) | 40 (53.3) | 57 (70.0) | 0.33 |
| MSAP | 57(34.1) | 31(41.3) | 26(28.2) | |
| SAP | 13 (7.8) | 4 (5.4) | 9 (9.8) | |
| Family history of pancreatic cancer | > 0.99 | |||
| Yes | 10 (6.0) | 3 (4.0) | 7 (7.6) | |
| No | 157 (94.0) | 72 (96.0) | 85 (92.4) | |
| Admission way | 0.38 | |||
| Emergency admission | 47 (28.4) | 14 (18.7) | 33 (35.8) | |
| Outpatient admission | 120 (71.6) | 61 (81.3) | 59 (64.2) | |
No other clear etiology was found in 121 (72.5%) patients, while identified causes included gallstone obstruction (15.0%, n = 25), alcohol-induced pancreatitis (4.8%, n = 8), and hyperlipidemia-associated pancreatitis (7.7%, n = 13). Clinically, abdominal pain was the most prevalent symptom (79.0%, n = 132), followed by fever (47.9%, n = 80) and nausea/vomiting (28.1%, n = 47). Key laboratory findings revealed the following median values (IQR): Pancreatic enzymes: Lipase 475.6 U/L (221.7-834.6 U/L), amylase 72.5 U/mL (23.3-157.7 U/mL); Tumor markers: Carcinoembryonic antigen (CEA) 3.1 ng/mL (1.3-5.5 ng/mL), carbohydrate antigen (CA) 125 25.6 U/mL (11.3-87.5 U/mL), CA19-9 72.5 U/mL (23.2-157.7 U/mL); Hepatic function: Direct bilirubin 17.6 μmol/L (6.3-86.1 μmol/L), indirect bilirubin 5.3 μmol/L (3.8-17.6 μmol/L); Metabolic indices: Albumin 41.5 ± 4.3 g/L, serum glucose 6.3 mmol/L (4.5-10.9 mmol/L).
All patients underwent comprehensive abdominal imaging (CE-CT/MRI). Metastatic involvement was observed in 30.5% (liver, n = 72) and 33.0% (lymphatic, n = 55). Tumor distribution predominated in the pancreatic head (89.8%, n = 150), with fewer cases in the body (5.4%, n = 9) and tail (4.8%, n = 8). Diagnostic procedures included endoscopic retrograde cholangiopancreatography (ERCP) (34.1%, n = 37) and EUS-FNA/B (62.8%, n = 105).
Primary interventions comprised surgical resection (60.5%, n = 101) and systemic chemotherapy (53.3%, n = 113). The median hospitalization duration was 11 days (IQR: 8.0-20.5), with associated costs of 32054 Chinese yuan (IQR 16722-66529) (Table 2).
| Characteristics | Total (n = 167) | The early-diagnosis group (n = 75) | The delayed-diagnosis group (n = 92) | P value |
| Symptoms | 0.01a | |||
| Pain | 132 (79.0) | 51 (68.0) | 81 (88.0) | |
| Nausea or vomit | 47 (28.1) | 33 (44.0) | 14 (15.2) | |
| Fever | 80 (47.9) | 38 (50.7) | 42 (45.7) | |
| Amylase, median, IQR (U/L) | 247.3 (142.5-376.8) | 289.5 (187.3-453.2) | 231.1 (116.7-327.2) | 0.41 |
| Lipase, median, IQR (U/L) | 475.6 (221.7-834.6) | 411.5 (216.7-943.8) | 436.9 (203.6-1056.0) | 0.37 |
| CA19-9, median, IQR (U/mL) | 72.5 (23.2-157.7) | 57.4 (25.1-117.3) | 93.2 (37.9-168.2) | 0.03a |
| Elevated CA19-9 | 114 (79.0) | 56 (74.7) | 85 (92.4) | 0.04a |
| CEA, median, IQR (ng/mL) | 3.1 (1.3-5.5) | 2.2 (0.9-5.3) | 2.7 (1.4-5.7) | 0.76 |
| CA12-5, median, IQR (U/mL) | 25.6 (11.3-87.5) | 29.1(12.9-76.2) | 27.2(14.6-86.9) | > 0.99 |
| Direct bilirubin, median, IQR (μmol/L) | 17.6 (6.3-86.1) | 6.75 (3.9-91.8) | 27.4 (5.6-107.3) | 0.01a |
| Indirect bilirubin, median, IQR (μmol/L) | 5.3 (3.8-17.6) | 3.6 (2.7-9.6) | 7.50 (3.5-19.2) | 0.02a |
| Albumin, IQR (g/L) | 41.5 ± 4.3 | 40.2 ± 7.6 | 39.5 ± 3.2 | > 0.99 |
| Serum glucose, median, IQR (mmol/L) | 6.3 (4.5-10.9) | 5.1 (4.9-8.2) | 7.8 (5.3-13.2) | 0.03a |
| With abdominal CT or MRI | 167 (100) | 75 (100) | 92 (100) | > 0.99 |
| With ERCP | 37 (34.1) | 22 (29.3) | 15 (16.3) | 0.35 |
| With EUS-FNA/B | 105 (62.8) | 64 (85.3) | 41 (44.6) | 0.02a |
| Lymphatic metastasis | 55 (33.0) | 21 (28.0) | 34 (37.0) | > 0.99 |
| Liver metastasis | 51 (30.5) | 20 (26.7) | 31 (33.7) | 0.79 |
| Tumor location (H/B/T) | 150/9/8 (89.8/5.4/4.8) | 68/5/2 (90.6/6.7/2.7) | 82/4/6 (89.1/4.3/6.5) | 0.35 |
| With other causes | 0.03a | |||
| Gallstone obstruction | 25 (15.0) | 4 (5.3) | 21 (22.8) | |
| Heavy alcohol | 8 (4.8) | 2 (2.7) | 6 (6.5) | |
| Hyperlipidemia | 13 (7.7) | 1 (1.3) | 12 (13.0) | |
| None | 121 (72.5) | 68 (90.7) | 53 (57.7) | |
| With resectional surgery | 61 (36.5) | 34 (45.3) | 27 (29.3) | < 0.01a |
| With chemotherapy | 113 (67.7) | 42 (56.0) | 71 (77.2) | 0.03a |
| Hospital stays, median IQR, (days) | 11 (8-20.5) | 10 (7.5-14.25) | 17.1 (11.7-21.5) | < 0.01a |
| Cost, median IQR, (Chinese yuan) | 30241 (16722-66529) | 18255 (15042-26263) | 34889 (21074-61922) | < 0.01a |
The cohort was stratified into two groups based on diagnostic timing: The early-diagnosis group (n = 75) and the delayed-diagnosis group (n = 92). Comparative analyses of baseline and clinical characteristics between groups are presented in Tables 1 and 2.
Significant intergroup differences were observed in several parameters. The delayed-diagnosis group demonstrated: (1) Older median age (68.2 vs 57.3 years, P = 0.02); (2) Higher prevalence of alcohol use history (70.7% vs 20.0%, P < 0.001); (3) Greater diabetes mellitus incidence (51.1% vs 8.0%, P < 0.001); and (4) More frequent identification of alternative etiologies (42.3% vs 9.3%, P = 0.03). Symptom profiles also differed significantly between groups (P = 0.01). No statistically significant differences were observed in gender distribution, body mass index, smoking history, family history of pancreatic cancer, or admission route (all P > 0.05).
Laboratory investigations revealed significant differences between the early and delayed diagnosis groups (Table 2): The mean CA19-9 levels were significantly higher in the delayed-diagnosis group (93.2 vs 72.5 U/mL, P = 0.03), with a greater proportion exceeding the clinical threshold ( > 37 U/mL; 92.4% vs 74.7%, P = 0.04); No intergroup differences in CEA (median 4.2 vs 3.8 μg/L) or CA125 (28.6 vs 25.3 U/mL) levels (both P > 0.05); The delayed-diagnosis group demonstrated significantly elevated direct bilirubin (direct: 27.4 vs 6.75 μmol/L, P = 0.01; indirect: 7.5 vs 3.6 μmol/L, P = 0.02) and higher serum glucose levels (7.8 vs 5.1 mmol/L, P = 0.03).
Significant differences in diagnostic procedures, disease progression, and healthcare utilization were observed between the two groups (Table 2): The delayed-diagnosis group exhibited significantly lower utilization of EUS-FNA/B (44.6% vs 85.3%; P = 0.02); Comparable rates of liver metastases (26.7% vs 33.7%; P = 0.79) and lymph node involvement (28.0% vs 37.0%; P > 0.99); Radical resection rates were substantially reduced in the delayed-diagnosis group (40.2% vs 85.3%, P < 0.01); Prolonged hospitalization duration (17.1 vs 10.0 days; P < 0.01) and higher associated costs (Chinese yuan) (34889 vs 18255; P < 0.01) were observed in the delayed-diagnosis group.
Multivariate logistic regression analysis identified several independent predictors of early diagnosis in tumor-associated AP (Table 3). Specifically, a history of diabetes mellitus demonstrated a significant association [odds ratio (OR) = 2.69; 95% confidence interval (CI): 1.08-3.34; P = 0.04]. Other notable predictors included: With other etiologies (OR = 4.77; 95%CI: 1.84-7.81; P = 0.02), age (OR = 1.26; 95%CI: 1.04-1.89; P = 0.02), serum CA19-9 levels (OR = 1.38; 95%CI: 1.03-1.84; P = 0.04), increased direct bilirubin (OR = 2.36; 95%CI: 1.35-3.48; P = 0.031), elevated indirect bilirubin (OR = 2.67; 95%CI: 1.38-4.62; P = 0.027) and serum glucose levels(OR = 1.42; 95%CI: 1.08-2.55; P = 0.04) (Table 3).
Our large single-center study systematically characterizes the clinical characteristics and metabolic markers distinguishing early vs delayed pancreatic cancer diagnosis in AP patients. Our analysis identified significant intergroup differences across multiple clinical parameters. The findings reveal critical diagnostic challenges and highlight the factors in the early diagnosis of tumor-associated AP.
The delayed-diagnosis group exhibited distinct clinical features including advanced age and higher prevalence of alcohol use. Most notably, we identified diabetes mellitus as both a risk marker and potential etiological factor in pancreatic cancer-associated AP. This aligns with emerging evidence that new-onset diabetes may represent paraneoplastic β-cell dysfunction[12]. The observed hyperglycemia in delayed-diagnosis patients supports the hypothesis that inflammatory processes simultaneously drive both endocrine dysfunction and oncogenic transformation[13]. Meanwhile, a large international registry reported that a strong association between pre-existing diabetes and AP susceptibility[14]. Our multivariate analysis confirmed serum glucose as an independent predictor, reinforcing the need for cancer surveillance in diabetic AP patients, particularly elderly individuals.
The diagnostic complexity was further compounded by the higher prevalence of conventional AP etiologies (gallstones, alcohol, hyperlipidemia) in the delayed-diagnosis group. These competing diagnoses likely obscured neoplastic detection, creating critical windows for tumor progression. This finding underscores the importance of maintaining high clinical suspicion for malignancy even when typical AP triggers are identified.
Our cohort analysis revealed significantly higher CA19-9 levels in the delayed-diagnosis group compared to early diagnosis patients. As an established biomarker for PDAC, CA19-9 serves multiple clinical roles including screening high-risk populations, facilitating differential diagnosis, staging evaluation, and monitoring therapeutic response[15]. Some research indicated that CA19-9 has a 76.1% sensitivity for identifying pancreatic cancer in its early stages and an 80.1% sensitivity for identifying the disease in all stages[16]. Though it lacks substantial specificity, CA19-9 demonstrated the highest diagnostic ability for pancreatic cancer compared to other tumor markers. Notably, despite exhibiting superior diagnostic accuracy compared to alternative markers like CEA or CA125, CA19-9 failed to prompt timely cancer diagnosis in our delayed group. This diagnostic latency may be attributed to the confounding effects of obstructive jaundice or concurrent inflammatory conditions[17]. Due to insufficient clinical data, we were unable to assess the false-positive rate of CA19-9 in AP patients with underlying pancreatic cancer. Given that CA19-9 elevations can occur secondary to benign inflammatory processes (e.g., biliary obstruction, pancreatitis) rather than malignancy, this represents a critical knowledge gap in differentiating cancer-associated AP from non-neoplastic etiologies. For patients with pancreatitis suspected to be caused by tumors, CA19-9 monitoring after complete resolution of AP provides critical diagnostic value, irrespective of pre-morbid CA19-9 elevation status. However, current evidence lacks comparative studies analyzing CA19-9 dynamics in this specific population before and after AP episodes. In addition, our cohort size was relatively limited, restricting the statistical power to evaluate dynamic CA19-9 trends in pancreatic cancer-induced AP. Larger, prospective multicenter studies with serial biomarker measurements are needed to validate whether CA19-9 kinetics (e.g., persistent elevation despite resolution of inflammation) improve early cancer detection in this high-risk population.
Current international guidelines strongly recommend CT as the first-line imaging modality for evaluating suspected pancreatic cancer, given its established efficacy in tumor localization and staging[18]. While MRI demonstrates comparable diagnostic accuracy particularly for small lesions (< 2 cm) and cystic characterization[19] its clinical implementation may be constrained by practical considerations including prolonged acquisition times and resource limitations. The diagnostic paradigm has been transformed by EUS, which uniquely combines high-resolution imaging with tissue acquisition capability[20]. Our findings reveal a striking disparity in EUS-FNA/B utilization (85.3% vs 44.6% in early- vs delayed-diagnosis groups), highlighting a critical barrier to timely pathological confirmation. This is particularly consequential as EUS-FNA/B remains the diagnostic gold standard, offering superior safety and accuracy compared to ERCP-based techniques[21-23]. However, the diagnostic yield of EUS-FNA/B during AP may be compromised by pancreatic edema and inflammatory changes, suggesting the need for optimized timing or multimodal approaches in high-risk patients.
Notably, our analysis revealed comparable metastatic rates between groups, suggesting that diagnostic delay may not necessarily alter disease biology but significantly impacts clinical management. The delayed-diagnosis group experienced prolonged hospitalization (median increase of 7.1 days), elevated healthcare costs (Chinese yuan) (34889 vs 18255) and reduced surgical intervention rates (40.2% vs 85.3%). These findings underscore the substantial clinical and economic consequences of diagnostic delays in tumor-associated AP.
Three key factors contribute to diagnostic challenges in this population: (1) Imaging limitations: Acute inflammatory changes may obscure neoplastic masses on CT/MRI[24]; (2) Clinical attribution bias: Common AP etiologies may overshadow malignancy evaluation; and (3) Incomplete diagnostic workups: Relying solely on CT or MRI without complementary diagnostic modalities (e.g., endoscopic ultrasound or tumor markers) may be insufficient for accurate AP diagnosis in the context of malignancy. These diagnostic challenges highlight the need for heightened clinical suspicion and multimodal evaluation in high-risk patients[25,26]. The 2024 American College of Gastroenterology Guidelines for AP state that pancreatic tumors should be taken into consideration as a potential cause of AP in patients over 40 in whom the cause is unknown, and thus, a CE-CT scan or magnetic retrograde cholangiopancreatography was recommended in these patients[26]. Notably, our data highlight a critical diagnostic gap in high-risk aged patients, particularly those with biochemical abnormalities (elevated CA19-9, direct bilirubin, and/or indirect bilirubin levels), metabolic derangements (elevated serum glucose or pre-existing diabetes mellitus), or merging with other identifiable AP etiologies (gallstones, alcohol abuse or hyperlipidemia) emphasizing the need for enhanced malignancy surveillance in this population. For patients presenting with high-risk factors, a multimodal surveillance strategy incorporating tumor marker assessments (particularly following resolution of acute inflammatory processes) in conjunction with cross-sectional imaging modalities may enhance early tumor detection. The diagnostic yield can be further optimized through the integration of EUS. Future investigations will focus on prospective data collection to develop robust risk stratification algorithms and validate predictive diagnostic models through machine learning approaches.
The precise mechanisms underlying pancreatic cancer-induced AP remain incompletely understood. Current evidence suggests two predominant pathophysiological pathways: Tumor-mediated compression or occlusion of the main pancreatic duct may impair pancreatic fluid drainage, resulting in elevated intraductal pressure and subsequent acinar cell injury. This obstruction-reflux mechanism potentially initiates the enzymatic cascade characteristic of AP; Neoplastic growth may compromise pancreatic microcirculation through direct vascular compression by tumor mass or tumor cell embolism in pancreatic microvasculature. The resultant ischemia-reperfusion injury may initiate a cascade of pathological events, including oxidative stress-mediated cellular hypoxia, premature zymogen activation leading to acinar cell necrosis, and subsequent systemic inflammatory response[27]. However, no in vivo or in vitro studies have conclusively demonstrated the temporal sequence of these events or their relative contributions to AP pathogenesis in pancreatic cancer patients. This knowledge gap underscores the need for mechanistic studies employing animal models of tumor-associated pancreatitis or utilization of organoid co-culture systems to study tumor-inflammation interactions.
While this study provides clinically relevant insights into early tumor detection strategies, several methodological constraints warrant careful consideration: (1) Single-center generalizability: As a tertiary referral center serving severe/complex cases in Zhejiang Province, our cohort may not fully represent the broader patient spectrum. The “severity gradient” inherent to hierarchical healthcare systems likely excluded milder cases managed at primary/secondary hospitals, potentially biasing our phenotypic characterization. This selection phenomenon necessitates external validation across diverse healthcare tiers (e.g., community hospitals); (2) Retrospective design constraints: The inherent limitations of retrospective studies, including potential selection bias and unmeasured confounding variables, may influence the validity of our conclusions; (3) Pancreatitis-carcinoma transformation: Although chronic pancreatitis cases were excluded, the probability of pancreatitis-to-carcinoma cannot be avoided; and (4) Follow-up data limitations: The inherent challenges and unique complexities of longitudinal follow-up in cancer patients resulted in incomplete survival data, including overall survival, and progression-free survival, which limited meaningful prognostic comparisons between the early and delayed diagnosis groups. These limitations underscore the necessity for future multicenter prospective studies employing standardized diagnostic protocols and systematic follow-up schedules to validate our findings. Larger sample sizes would enhance statistical power and permit more robust subgroup analyses.
This retrospective cohort study provides critical insights into the clinical differentiation between early and delayed diagnosis of tumor-associated AP. Our analysis reveals significant differences in baseline characteristics, diagnostic findings, and treatment courses between the early diagnosis and the delayed-diagnosis group. Key high-risk indicators for occult pancreatic malignancy in AP patients include: Advanced age, pre-existing diabetes mellitus, hyperbilirubinemia, and concurrent with conventional AP etiologies. These results substantially advance the early detection strategy for tumor-associated AP. The identified predictive factors should be incorporated into existing diagnostic strategy to reduce time-to-diagnosis and improve oncologic outcomes in this vulnerable population. Future prospective studies should validate these predictive factors in multicenter settings and explore their integration with emerging artificial intelligence-based diagnostic platforms.
| 1. | Garg SK, Sarvepalli S, Campbell JP, Obaitan I, Singh D, Bazerbachi F, Singh R, Sanaka MR. Incidence, Admission Rates, and Predictors, and Economic Burden of Adult Emergency Visits for Acute Pancreatitis: Data From the National Emergency Department Sample, 2006 to 2012. J Clin Gastroenterol. 2019;53:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 579] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 3. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 487] [Article Influence: 121.8] [Reference Citation Analysis (1)] |
| 4. | GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 436] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 5. | Dietrich CF, Dong Y, Jenssen C, Ciaravino V, Hocke M, Wang WP, Burmester E, Moeller K, Atkinson NS, Capelli P, D'Onofrio M. Serous pancreatic neoplasia, data and review. World J Gastroenterol. 2017;23:5567-5578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4370] [Cited by in RCA: 4260] [Article Influence: 224.2] [Reference Citation Analysis (0)] |
| 7. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15301] [Article Influence: 3060.2] [Reference Citation Analysis (4)] |
| 8. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 689] [Article Influence: 172.3] [Reference Citation Analysis (0)] |
| 9. | Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, Zhang T, Dai M, Zhao Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 244] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 10. | Schima W, Böhm G, Rösch CS, Klaus A, Függer R, Kopf H. Mass-forming pancreatitis versus pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging. 2020;20:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2422] [Article Influence: 484.4] [Reference Citation Analysis (3)] |
| 12. | Yuan C, Babic A, Khalaf N, Nowak JA, Brais LK, Rubinson DA, Ng K, Aguirre AJ, Pandharipande PV, Fuchs CS, Giovannucci EL, Stampfer MJ, Rosenthal MH, Sander C, Kraft P, Wolpin BM. Diabetes, Weight Change, and Pancreatic Cancer Risk. JAMA Oncol. 2020;6:e202948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 13. | Fleming AK, Storz P. Protein kinase C isoforms in the normal pancreas and in pancreatic disease. Cell Signal. 2017;40:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Paragomi P, Papachristou GI, Jeong K, Hinton A, Pothoulakis I, Talukdar R, Kochhar R, Goenka MK, Gulla A, Gonzalez JA, Singh VK, Ferreira Bogado M, Stevens T, Barbu ST, Nawaz H, Gutierrez SC, Zarnescu N, Archibugi L, Easler JJ, Triantafyllou K, Peláez-Luna M, Thakkar S, Ocampo C, de-Madaria E, Cote GA, Wu BU, Lee PJ, Hart PA, Conwell DL, Toledo FGS, Yadav D. The relationship between pre-existing diabetes mellitus and the severity of acute pancreatitis: Report from a large international registry. Pancreatology. 2022;22:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Thomas C. Risk factors, biomarker and imaging techniques used for pancreatic cancer screening. Chin Clin Oncol. 2017;6:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Luo G, Guo M, Jin K, Liu Z, Liu C, Cheng H, Lu Y, Long J, Liu L, Xu J, Ni Q, Yu X. Optimize CA19-9 in detecting pancreatic cancer by Lewis and Secretor genotyping. Pancreatology. 2016;16:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, Qian Y, Huang Q, Ni Q, Liu C, Yu X. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer. 2021;1875:188409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (1)] |
| 18. | Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ, Merchant NB, Minter RM, Tamm EP, Sahani DV, Simeone DM. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology. 2014;146:291-304.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 19. | Treadwell JR, Zafar HM, Mitchell MD, Tipton K, Teitelbaum U, Jue J. Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcinoma: A Meta-Analysis. Pancreas. 2016;45:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 20. | Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 21. | Masuda S, Koizumi K, Shionoya K, Jinushi R, Makazu M, Nishino T, Kimura K, Sumida C, Kubota J, Ichita C, Sasaki A, Kobayashi M, Kako M, Haruki U. Comprehensive review on endoscopic ultrasound-guided tissue acquisition techniques for solid pancreatic tumor. World J Gastroenterol. 2023;29:1863-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Cho IR, Jeong SH, Kang H, Kim EJ, Kim YS, Cho JH. Comparison of contrast-enhanced versus conventional EUS-guided FNA/fine-needle biopsy in diagnosis of solid pancreatic lesions: a randomized controlled trial. Gastrointest Endosc. 2021;94:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | ASGE Standards of Practice Committee; Machicado JD, Sheth SG, Chalhoub JM, Forbes N, Desai M, Ngamruengphong S, Papachristou GI, Sahai V, Nassour I, Abidi W, Alipour O, Amateau SK, Coelho-Prabhu N, Cosgrove N, Elhanafi SE, Fujii-Lau LL, Kohli DR, Marya NB, Pawa S, Ruan W, Thiruvengadam NR, Thosani NC, Qumseya BJ; ASGE Standards of Practice Committee Chair. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in the diagnosis and management of solid pancreatic masses: summary and recommendations. Gastrointest Endosc. 2024;100:786-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 24. | Ahmed TM, Chu LC, Javed AA, Yasrab M, Blanco A, Hruban RH, Fishman EK, Kawamoto S. Hidden in plain sight: commonly missed early signs of pancreatic cancer on CT. Abdom Radiol (NY). 2024;49:3599-3614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Trikudanathan G, Yazici C, Evans Phillips A, Forsmark CE. Diagnosis and Management of Acute Pancreatitis. Gastroenterology. 2024;167:673-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 26. | Tenner S, Vege SS, Sheth SG, Sauer B, Yang A, Conwell DL, Yadlapati RH, Gardner TB. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am J Gastroenterol. 2024;119:419-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 27. | Gou A, Liu Z, Xiao Z, Li G, Xu Y, Song S, Guo K, Ma G. A narrative review of a type of pancreatitis worthy of attention: acute pancreatitis associated with pancreatic tumors-current problems and future thinking. Gland Surg. 2021;10:2304-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |