INTRODUCTION
Colorectal cancer (CRC) is a prevalent and potentially life-threatening malignancy primarily affecting the colon and rectum[1,2]. Despite a 3% reduction in CRC mortality over the last decade, 30%-40% of CRC patients still develop metastases, and 50% succumb to CRC recurrence[3,4]. Active signaling through epidermal growth factor receptors (EGFR) plays a pivotal role in driving CRC progression by triggering downstream kinases and transcription factors[5,6]. Heightened EGFR expression has been linked to a poorer prognosis in patients with metastatic CRC[7]. Therefore, unraveling these cancer-promoting mechanisms may pave the way for the discovery of new drugs and innovative therapies, ultimately improving the survival of CRC patients.
Interleukin-22 (IL-22) is a cytokine with intricate implications in the context of CRC[8-10]. Belonging to the IL-10 cytokine family, IL-22 is primarily synthesized by immune cells like T cells and innate lymphoid cells in response to diverse stimuli, including bacterial infections and inflammation[11,12]. Accumulating evidence links IL-22 to a variety of human diseases, including autoimmunity and inflammation[13], infectious diseases[14], and cancers[15]. Elevated IL-22 Levels have been linked to several malignancies, including lung, liver, breast, colon, and pancreatic cancers[15-19]. In different cancer, the role of IL-22 is multifaceted and context-dependent. Certain studies suggest that IL-22 might exert a protective influence by promoting liver tissue repair and suppressing inflammation[18]. Conversely, in other instances, IL-22 has been associated with the promotion of CRC growth and advancement[20]. The up-regulation of IL-22 facilitates tumor proliferation through the protein kinase B (AKT) signaling pathway and triggers resistance to chemotherapy in human lung cancers[16]. However, the role of IL-22 in CRC progression and its potential for inducing resistance involving EGFR/extracellular signal-regulated kinase (ERK) pathways remain uncharted territory.
To investigate the role of IL-22 in EGFR/ERK resistance, we assessed how IL-22 affects cytotoxicity and induction of apoptosis in CRC cells in the absence and presence of oxaliplatin (L-OHP). In addition, we performed cell viability assays and Western blot experiments to gain insight into potential signaling pathways.
MATERIALS AND METHODS
Reagents
Human IL-22 was obtained from R&D Systems (Minneapolis, MN, United States). L-OHP was purchased from MedChemExpress. Osimertinib (Osi) (HY-15772) was purchased from MedChemExpress (NJ, United States). The cell counting kit-8 (CCK-8) was obtained from KeyGen Biotech Co., Ltd. (Nanjing, China).
Cell line and cell culture
CRC cell line HCT116 was obtained from Cell Bank of Chinese Academy of Sciences. HCT116 cells were cultured at 37 °C and 5% CO2 in RPMI-1640 medium (Gibco; Waltham, MA, United States) with 10% FBS (HyClone; Thermo Fisher Scientific) and 100 μg/mL streptomycin, and 100 U/mL penicillin (Beyotime Institute of Biotechnology, Haimen, China).
CCK-8 assay
The CCK-8 assay was used to evaluate cell viability. Before experiments, the cells were collected and adjusted to 1 × 105/mL, then 100 µL of cells were grown in 96-well plates for overnight. Then the cells were exposed to two doses of IL-22 (5 ng/mL and 50 ng/mL) based on the previous report[10,17,21] with or without L-OHP (0, 5, 10, 15, 20, 25 μg/mL) according to previous report[22] for 48 hours. On the next day, 10 μL of CCK-8 reagent was added to each well and incubated for another 1 hour in an incubator. The absorbance was then measured at a wavelength of 450 nm using a MultiskanTM GO plate reader (Thermo Fisher Scientific, Inc.). The values were corrected by subtracting the absorbance of control wells that did not contain cells. The concentration of drug that inhibited the growth of the tumor cells by 50% (IC50) was used to evaluate the drug effect.
Cell apoptosis assay
HCT116 cells were collected and detected for apoptosis by using FITC Annexin V Apoptosis Detection Kit (BD Biosciences, NJ, United States) according to the manufacture’s protocols. Briefly, cells were washed twice by cold PBS and resuspended in the binding buffer in a concentration of 5 × 105 cells/mL. Then, cells were stained with 5 mL of FITC Annexin V and 5 mL of Propidium Iodide and incubated for 15 minutes in dark at room temperature. Finally, cells apoptosis was performed by BD FACSVerse (BD Biosciences, NJ, United States).
Western blot
The total cellular proteins were extracted by the RIPA buffer (Cell Signal Technology, Inc., MA, United States) follow the principle of manufacturers’ protocols. Protein was quantified using the BCA assay (Thermo Scientific, Rockford, IL, United States). In brief, the protein lysates (20 µg) were isolated on 10% sodium dodecyl sulfate polyacrylamide gel, which was electrotransferred to polyvinylidene fluoride membranes. After transfer, we blocked the transferred membranes with 5% non-fat milk in TBS-T buffer for 30 minutes at room temperature, and then incubated with primary antibodies overnight at 4 °C. The next day, we incubated membranes with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000, Cell Signaling Technology, Beverly, MA, United States) for 1 hour at room temperature, and visualized the membranes through using Super Lumia ECL HRP substrate kit (Abbkine Inc., Wuhan, China) and photographed by the chemiluminescence imaging analysis system (Bio-Rad, United States). Densitometric analysis of band intensities was performed using ImageJ software (NIH, United States). The relative expression of each target protein was normalized to GAPDH. All Western blot experiments were conducted with a minimum of three independent biological replicates. The primary antibodies used in this study included SOX2 (SRY-box transcription factor 2, 1:1000, ab97959, Abcam), Oct4 (octamer-binding transcription factor 4, 1:1000, #2750, Cell Signaling), NANOG (1:2000, #4893, Cell Signaling), Bmi-1 (B-cell-specific Moloney leukemia virus insertion site 1, 1:5000, ab126783, Abcam), EGFR (1:5000, ab52894, Abcam), phosphorylated EGFR (p-EGFR, 1:500, sc57545, Santa Cruz), AKT (protein kinase B, 1:1000, #9272, Cell signaling), phosphorylated AKT (p-AKT, 1:1000, #9271, Cell signaling), ERK (1:1000, sc514302, Santa Cruz), phosphorylated ERK (p-ERK, 1:500, sc7383, Santa Cruz), and GAPDH (1:2000, sc47724, Santa Cruz) were used in this study.
Sphere forming assay
HCT116 cells were cultured in serum-free RPMI-1640 medium (Gibco; Waltham, MA, United States) supplemented with 5 ng/mL and 50 ng/mL IL-22 (R&D Systems, Minneapolis, MN, United States), and 100 μg/mL streptomycin, and 100 U/mL penicillin (Beyotime Institute of Biotechnology, Haimen, China). Cells (1 × 105 cells/mL) were cultured in ultra-low attachment six-well plates with IL-22 incubation for 7 days. Representative images of spheres were photographed and analyzed under a microscope (Nikon Corporation). Spheres up to 50 μm in diameter from one sample were counted.
Statistical analysis
Statistical analysis was performed using SPSS software (SPSS, Inc., Chicago, IL, United States). Data are presented as mean ± SE from at least three independent biological replicates. Comparisons among multiple groups were conducted using one-way analysis of variance followed by Tukey’s post hoc test. Statistical significance was determined as P < 0.05.
RESULTS
IL-22 treatment promotes CRC cell proliferation and enhances sphere forming
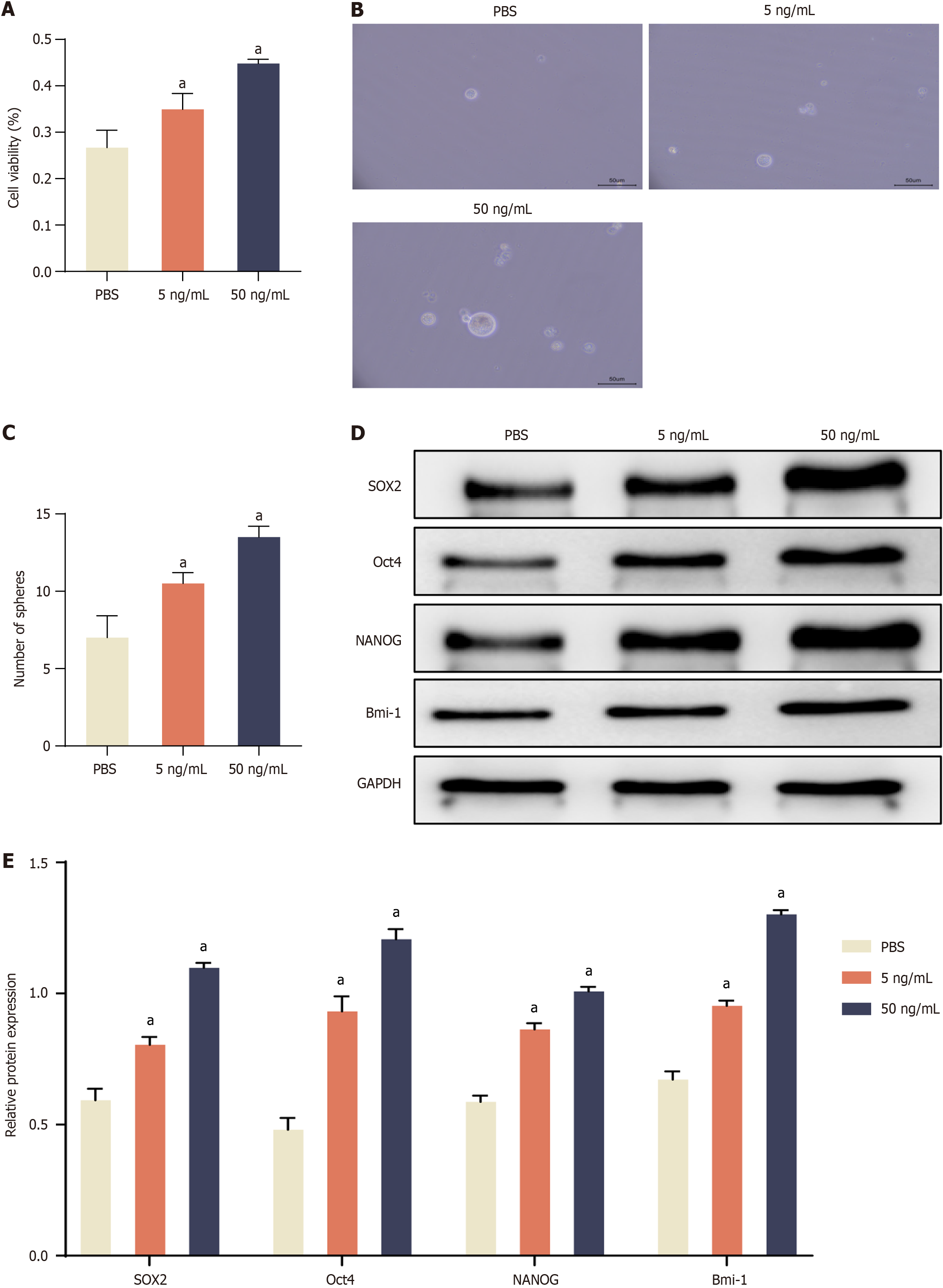
IL-22, produced by Th1, Th17, and NK cells, appears to serve as an immunomodulatory molecule, playing a role in maintaining the balance of colorectal tissue and influencing the development of colon tumors[23]. To investigate the effect of IL-22 on CRC cell proliferation and stemness, we initially assessed its effects on CRC cell proliferation using a CCK-8 assay. As depicted in Figure 1A, the results revealed that the proliferation of HCT116 cells was enhanced following IL-22 treatment at concentrations of 5 ng/mL and 50 ng/mL. Moreover, as the IL-22 concentration increased, there was a corresponding rise in the proliferation of CRC cells. Notably, there was a significant increase in proliferation in CRC cells cultured with 5 ng/mL or 50 ng/mL of IL-22 compared to those cultured without IL-22.
Figure 1 Interleukin-22 treatment promotes cell proliferation, enhances sphere forming, and activates stemness-related gene expression in colorectal cancer cells.
A: Cell availability assay in the treatment with Interleukin-22 (IL-22) (5 ng/mL and 50 ng/mL) in colorectal cancer (CRC) cells; B: Representative images of tumor spheres under IL-22 treatment; C: Quantification of tumor spheres; D: Representative protein bands of SOX2, Oct4, NANOG, and Bmi-1; E: Densitometric analysis of SOX2, Oct4, NANOG, and Bmi-1 normalized to GAPDH. Data are represented as mean ± SE (n = 3). Statistical analysis was performed using one-way analysis of variance followed by Tukey’s post hoc test. aP < 0.01 vs PBS group.
Previous studies have highlighted that cancer stem cells (CSCs) exhibit the ability to form tumor spheres under serum-free, non-adherent conditions in vitro[24,25]. Therefore, we examined the ability of HCT116 cells to form tumor spheres after treatment with different concentrations of IL-22 by using an in vitro tumor sphere formation assay. The results showed that there was a dramatic increase in the number of tumor spheres in IL-22 treated CRC cells compared to the control group (Figure 1B and C). Taken together, these results suggested that IL-22 significantly increased the growth of CRC cells and confers CSC-like characteristics of CRC cells.
IL-22 treatment activates stemness-related gene expression in CRC cells
CSCs play a pivotal role in tumor initiation, metastasis, and recurrence[26,27]. Consequently, they are regarded as the primary culprits behind the failure of traditional cancer treatments, mainly because of their inherent resistance to these therapies. CSC-related genes (i.e., SOX2, Oct4, NANOG, and Bmi-1) have frequently been used to identify CSC populations from various cancer cell lines[28,29]. In order to evaluate the effect of IL-22 treatment on stemness-related gene expression in CRC cells, we analyzed the levels of SOX2, Oct4, NANOG, and Bmi-1 using Western blot. As shown in Figure 1D and E, there was a substantial increase in the expression of stemness-associated genes (SOX2, Oct4, NANOG, and Bmi-1) observed in the IL-22 treated group in comparison to the control group (PBS-treated), indicating that IL-22 treatment significantly upregulated the expression of stemness-related genes in CRC cells.
IL-22 treatment attenuates the cytotoxic and apoptosis-inducing effects of L-OHP
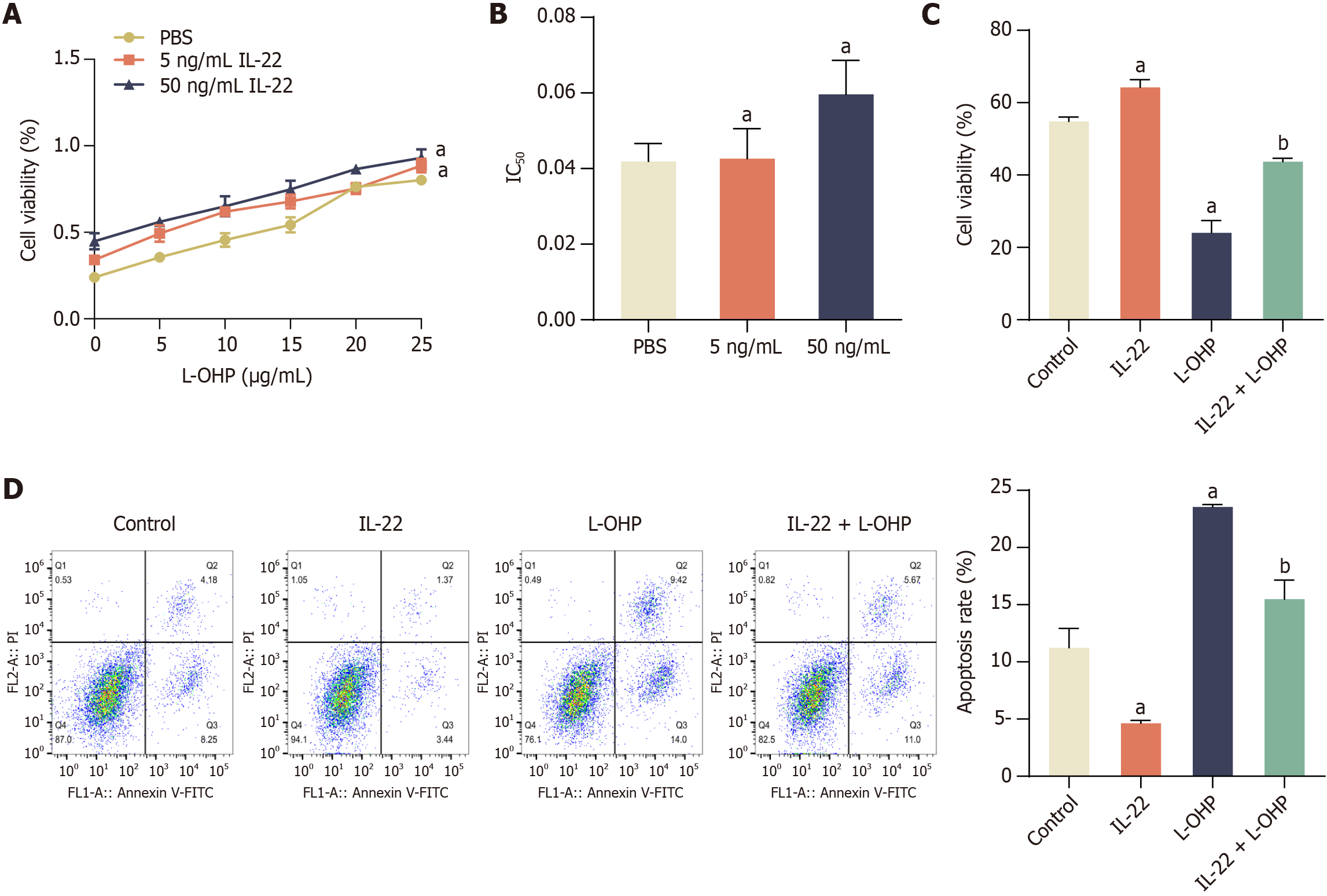
L-OHP has emerged as the most commonly utilized adjuvant chemotherapy regimen for CRC[30,31]. However, resistance to chemotherapy remains a major obstacle in the treatment of CRC[32,33]. Cell viability assay was performed to determine whether IL-22 can protect CRC cells from chemotherapy-induced cell death. Our results showed that, in comparison to the PBS-treated group, cell viability significantly increased in both the 5 ng/mL and 50 ng/mL IL-22 treatment groups, with the 50 ng/mL IL-22 group exhibiting even higher cell viability than the 5 ng/mL IL-22 group (Figure 2A). We also determined the half-maximal inhibitory concentration (IC50) of L-OHP in HCT116 cells treated with PBS, 5 ng/mL IL-22, or 50 ng/mL IL-22. Both IL-22 treatment groups exhibited significantly enhanced cell viability and higher IC50 values compared to the PBS group. Furthermore, the IC50 of the 50 ng/mL IL-22 group surpassed that of the 5 ng/mL IL-22 group (Figure 2B). When L-OHP (5 μg/mL) was applied to cells, the promoting effect of IL22 on cell activity was significantly reduced (Figure 2C). These results suggest that IL-22 attenuates the cytotoxic effect of chemotherapeutic drug L-OHP in CRC cells.
Figure 2 Interleukin-22 treatment attenuates the cytotoxic and apoptosis-inducing effects of oxaliplatin in colorectal cancer cells.
A: Cell viability under increasing concentrations of oxaliplatin (L-OHP) (0-25 μg/mL) in the presence of interleukin-22 (IL-22) (5 ng/mL and 50 ng/mL); B: Half maximal inhibitory concentration (IC50) of L-OHP with or without IL-22 treatment; C: Cell viability in control, IL-22, L-OHP, and IL-22 + L-OHP groups assessed by MTT assay; D: Flow cytometry analysis of apoptosis in the same four groups. Data are represented as mean ± SE (n = 3). Statistical analysis was performed using one-way analysis of variance followed by Tukey’s post hoc test. aP < 0.01 vs PBS or Control group; bP < 0.01 vs L-OHP group. L-OHP: Oxaliplatin; IL-22: Interleukin-22; IC50: Half maximal inhibitory concentration.
To assess whether IL-22 mitigated the apoptosis-inducing effects of L-OHP in CRC cells, we conducted an analysis of the apoptosis proportion utilizing flow cytometry. Our results showed that, in comparison to the control group, cell apoptosis was significantly reduced following treatment with IL-22, whereas cell apoptosis increased significantly in the L-OHP group (Figure 2D). However, after 48 hours of exposure to IL-22, the number of apoptotic cells induced by L-OHP was significantly reduced when compared to the L-OHP-only treated group (Figure 2D). This suggested that IL-22 effectively decreased the sensitivity of CRC cells to L-OHP-induced apoptosis.
IL-22 treatment activates EGFR/ERK signaling pathway in CRC cells
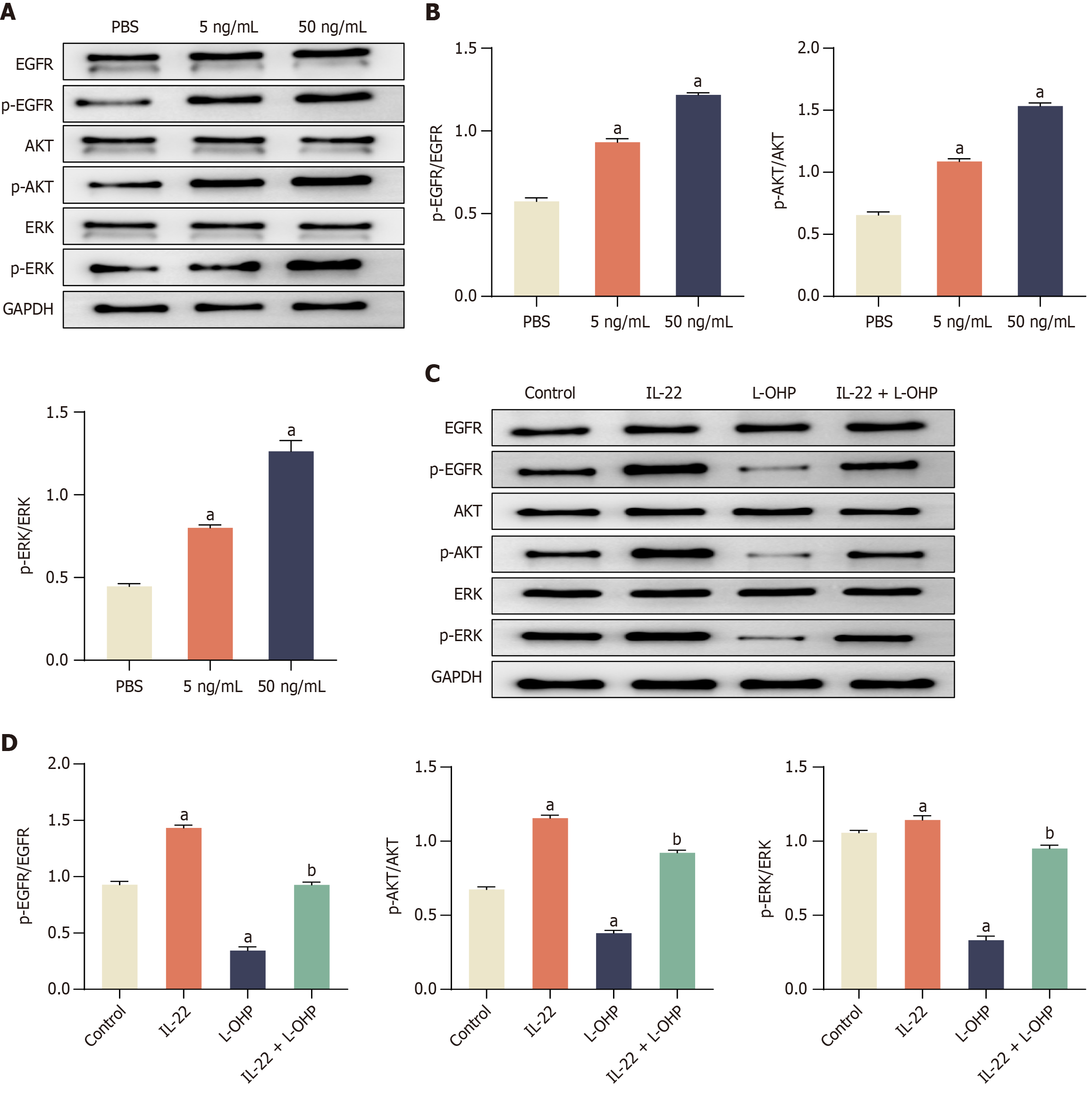
The EGFR signaling pathway plays a pivotal role in regulating various cellular processes such as proliferation, differentiation, and the survival of tumor cells[16,34,35]. It achieves this by activating EGFR's intrinsic kinases, subsequently leading to the activation of downstream signaling pathways including the extracellular related kinase (ERK) and AKT pathways[36]. Furthermore, EGFR signaling is essential for the self-renewal of rat embryonic stem cells and CSCs[37,38]. In light of these facts, we conducted a Western blot analysis to investigate whether IL-22 triggers the activation of the EGFR/ERK signaling pathway in CRC cells. Our observations revealed that treatment with IL-22 resulted in an increase in the phosphorylation of EGFR, AKT, and ERK. Importantly, the total levels of EGFR, AKT, and ERK remained unchanged (Figure 3A and B). These findings suggested that IL-22 induced the activation of the EGFR/ERK signaling pathway in CRC cells, which could have significant implications for the regulation of cell behavior and survival.
Figure 3 Interleukin-22 treatment attenuates oxaliplatin induced inhibition of epidermal growth factor receptors/extracellular signal-regulated kinase signaling pathway in colorectal cancer cells.
A: Western blot showing phosphorylation levels of epidermal growth factor receptors (EGFR), protein kinase B (AKT), and extracellular signal-regulated kinase (ERK) following interleukin-22 (IL-22) treatment; B: Quantification of p-EGFR/EGFR, p-AKT/AKT, and p-ERK/ERK ratios; C: Western blot showing phosphorylation changes in response to oxaliplatin (L-OHP) and IL-22 + L-OHP; D: Corresponding densitometric analysis. Data are represented as mean ± SE (n = 3). Statistical analysis was performed using one-way analysis of variance followed by Tukey’s post hoc test. aP < 0.01 vs PBS or Control group; bP < 0.01 vs L-OHP group. L-OHP: Oxaliplatin; IL-22: Interleukin-22; EGFR: Epidermal growth factor receptors; AKT: Protein kinase B; ERK: Extracellular signal-regulated kinase.
IL-22 treatment attenuates L-OHP induced inhibition of EGFR/ERK signaling pathway in CRC cells
Subsequently, we explored whether IL-22 treatment could attenuate the inhibitory effects of L-OHP on the phosphorylation of EGFR, AKT, and ERK in CRC cells. Upon treating CRC cells with L-OHP, we observed a decrease in the levels of phosphorylated EGFR, AKT, and ERK. However, when CRC cells were treated with IL-22 in conjunction with L-OHP (IL-22 + L-OHP), it mitigated the inhibitory effects of L-OHP on the phosphorylation of EGFR, AKT, and ERK, in contrast to L-OHP treatment alone (Figure 3C and D). Collectively, these findings suggested that IL-22 had the potential to attenuate the anticancer effects of L-OHP by activating the EGFR/ERK signaling pathway.
IL-22 promotes L-OHP resistance in CRC cells through activation of the EGFR/ERK signalling pathway
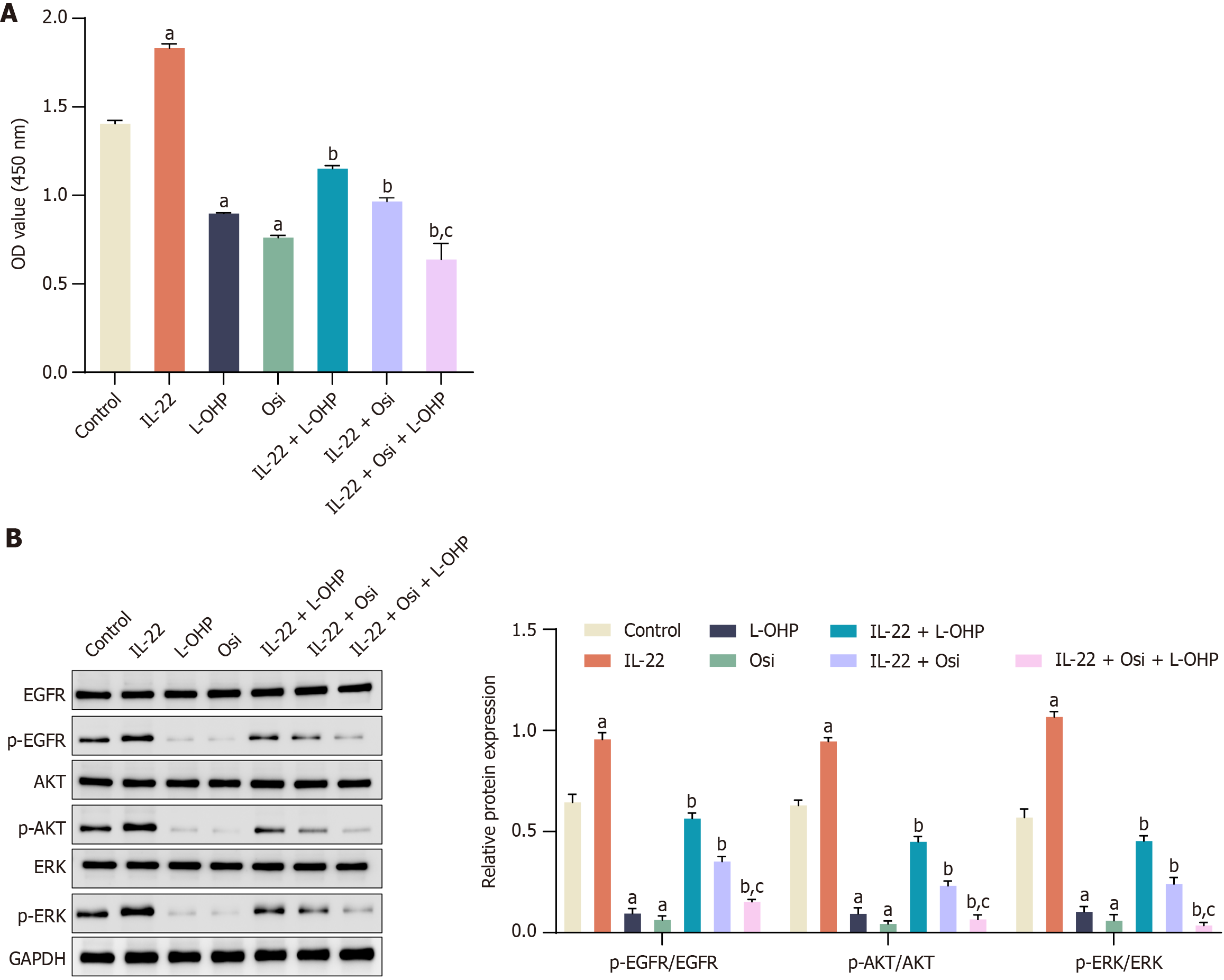
We supplemented our study with an EGFR inhibitor, Osi[39], and evaluated its impact on IL-22-induced chemoresistance. CCK8 results indicated a significant increase in cell proliferation with IL-22 treatment, which was mitigated by the addition of Osi (10 nM). Specifically, the combination of IL-22 and L-OHP significantly reduced cell viability compared to IL-22 treatment alone. Osi alone also reduced cell viability, and its combination with IL-22 + L-OHP further decreased viability (Figure 4A). The results of Figure 4B demonstrated that IL-22 treatment increased the phosphorylation of EGFR, AKT, and ERK, which are critical markers of pathway activation. Treatment with Osi reduced the phosphorylation levels of these proteins, counteracting the effects of IL-22. In the presence of L-OHP, IL-22 treatment mitigated the inhibition of phosphorylation, but the addition of Osi restored the inhibitory effects of L-OHP on these pathways.
Figure 4 Effect of extracellular signal-regulated kinase inhibition on interleukin-22-induced colorectal cancer cell proliferation and oxaliplatin chemoresistance.
A: Cell proliferation was assessed using a cell counting kit-8 assay across different treatment groups. Osimertinib (Osi), extracellular signal-regulated kinase (EGFR) inhibitor Osi, 10 nM; B: Western blot analysis showing the levels of epidermal growth factor receptors (EGFR), p-EGFR, protein kinase B (AKT), p-AKT, extracellular signal-regulated kinase (ERK), and p-ERK across different treatment groups. Data are represented as mean ± SE (n = 3). Statistical analysis was performed using one-way analysis of variance followed by Tukey’s post hoc test. aP < 0.01 vs Control group; bP < 0.01 vs Oxaliplatin (L-OHP) group; cP < 0.01 vs Interleukin-22 + L-OHP group. L-OHP: Oxaliplatin; IL-22: Interleukin-22; EGFR: Epidermal growth factor receptors; AKT: Protein kinase B; ERK: Extracellular signal-regulated kinase.
DISCUSSION
IL-22 plays a vital role in the development and advancement of various types of tumors, including lung, liver, gastric, colon, pancreatic and breast cancers[10,16-19]. Several studies have indicated that elevated levels of IL-22 are associated with tumor progression and poor survival rates[23,40]. In this study, we sought to assess the impact of IL-22 on stem cell-like characteristics and chemotherapy resistance in CRC cells. Our results provide compelling evidence that IL-22 promotes tumor growth and fosters resistance to chemotherapy in human CRC cells. This effect is achieved through the activation of the EGFR/ERK signaling pathways. These findings unveil a novel role for IL-22 in the context of CRC and underscore the significance of the IL-22-induced EGFR/ERK signaling axis in conferring resistance to chemotherapy in CRC. This research enhances our understanding of the complex interplay between cytokines like IL-22 and cancer progression, shedding light on potential avenues for therapeutic intervention in CRC.
EGFR is a crucial receptor tyrosine kinase that plays a pivotal role in the survival and proliferation of intestinal and colorectal epithelial cells[39]. The activation of EGFR is closely associated with the progression of CRC[40]. Targeting EGFR has proven to be an effective strategy for treating CRC patients. Therefore, the regulation of EGFR signaling holds great importance in understanding the pathology of CRC. Our results have shown that L-OHP has the capacity to inhibit the phosphorylation of EGFR, AKT, and ERK in CRC. However, the use of the EGFR inhibitor Osi significantly counteracted the chemoresistance induced by IL-22 in CRC cells. Both AKT and ERK are critical in bridging the connection between EGFR activation and the regulation of cell proliferation, survival, and invasion in CRC[41,42]. Phosphorylated AKT and ERK have been shown to enhance tumor cell growth, prevent apoptosis, and increase invasion abilities by upregulating the expression of cell cycle, survival, and invasion-related proteins in CRC[43,44]. In this study, we have demonstrated that IL-22 attenuates the cytotoxic and apoptosis-inducing effects of L-OHP through the EGFR/ERK signaling pathway. Previous research by Wang et al[17] has shown that IL-22 promotes migration and resistance to paclitaxel in triple-negative breast cancer cells via the JAK-STAT3/MAPKs/AKT signaling pathways. Additionally, Wu et al[10] demonstrated that the main downstream pathway activated by IL-22 is STAT3, which subsequently induces the expression of anti-apoptotic genes such as Bcl-2, Bcl-xl, and Mcl-1, thereby inhibiting apoptosis induced by chemotherapeutic agents. In our study, we provide evidence that IL-22 promotes the proliferation of CRC cells, enhances sphere formation through EGFR activation, and triggers the expression of stemness-related genes in CRC cells. These findings collectively emphasize the multifaceted role of IL-22 in the context of CRC, particularly in terms of its influence on signaling pathways that regulate cell survival, proliferation, and resistance to chemotherapy.
Our current work demonstrates that IL-22 has a potential role in conferring stem cell-like characteristics and chemotherapy resistance to CRC cells through the EGFR/ERK pathway, suggesting that IL-22 could potentially contribute to the progression of CRC and may offer a novel avenue for therapeutic intervention in the treatment of CRC. However, several limitations should be acknowledged. Firstly, our investigation focused solely on the chemotherapeutic drug L-OHP, whether IL-22 has similar effects on other chemotherapeutic drugs needs further study. Secondly, the current study utilized only the HCT116 cell line, which, while widely used and genetically well-characterized, does not capture the full heterogeneity of CRC. Future validation across multiple cell lines with distinct genetic backgrounds would strengthen the generalizability of our conclusions. Moreover, functional experiments involving IL-22 knockdown or inhibition are needed to further clarify its causal role in mediating chemoresistance. Lastly, the findings are based exclusively on in vitro models; therefore, additional validation in vivo models and clinical specimens will be essential to assess the translational relevance of IL-22 signaling in CRC.