Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.106688
Revised: March 26, 2025
Accepted: June 25, 2025
Published online: August 15, 2025
Processing time: 162 Days and 22.3 Hours
Esophageal cancer patients had the highest intensive care unit (ICU) admitted rate in cancer patients. But their prognosis and evaluation methods were rarely studied.
To depict the short-term mortality outcome and identify the potential prognostic factors of esophageal cancer patients admitted into ICU.
A multicenter cross-sectional study was performed from May 10, 2021 to July 10, 2021 at ICU departments of 37 cancer specialized hospitals in China. Patients aged ≥ 14 years with ICU duration ≥ 24 hours were included. Clinical records of patients with primary esophageal cancer diagnosis were reviewed. Patients were separated into groups according to the 90 days survival. Characteristics between groups were compared. Single and multi-variate regression tests were applied to analyze the correlated factors of ICU outcomes. Predictive values of disease severity scores were assessed using receiver operating characteristic curve analysis.
Total 180 esophageal cancer patients were included. The 90 days mortality was 22.2%. Patients with mortality outcome showed differences from those survived mostly in disease severity and unplanned transfer from clinical ward. The current evaluation tools, including Sequential Organ Failure Assessment and Acute Physiology and Chronic Health Evaluation II scores had low accuracy in prediction of short-term death. ICU admitted esophageal cancer patients have poor prognosis, especially those with acute illness.
The prognostic tools for these patients need to be further optimized.
Core Tip: Esophageal cancer patients have the highest intensive care unit admission rate among cancer types but limited prognostic data. This multicenter study in Chinese cancer-specialized hospitals found a 90-day mortality of 22.2%, especially high in patients with acute illness. Existing severity scores showed poor predictive performance, highlighting the need for optimized prognostic tools.
- Citation: Tang JF, Xia R, Xing XZ, Wang CS, Ma G, Wang HZ, Zhu B, Zhao JH, Zhou DM, Zhang L, Huang MG, Quan RX, Ye Y, Zhang GX, Jiang ZY, Huang B, Xu SL, Xiao Y, Zhang LL, Lin RY, Ma SL, Qiu YA, Zheng Z, Sun N, Xian LW, Li J, Zhang M, Guo ZJ, Tao Y, Zhou XZ, Chen W, Wang DX, Chi JY, Wang DH, Liu KZ. Intensive care unit outcomes and prognostic factors of esophageal cancer: A cross-sectional study in Chinese cancer-specialized hospitals. World J Gastrointest Oncol 2025; 17(8): 106688
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/106688.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.106688
Significant numbers of cancer patients may need intensive care unit (ICU) management due to adverse disease progression or treatment-associated side effects. It has been reported that cancer patients account for 15% of all ICU admissions, and 5% cancer patients would expect ICU admission[1,2]. The number of patients subjected to ICU care have increased significantly in recent years, mostly due to advances in therapeutic technologies that expanded care for terminally ill patients[3-5]. During ICU management, there have always been debates on standards of admission, especially for patients in advanced stages, concerning the potential benefit to risk and cost ratio. Therefore, prognosis evaluation is of great clinical importance, for both admission decision and treatment strategy[6,7]. Although various studies in characteristics and outcomes of ICU cancer patients been carried out, there still lack consensus on admission standard, and these studies rarely focus on specific cancer types[8-11].
Esophageal cancer is the eighth most commonly cancer and the sixth leading cause of cancer death worldwide[12,13]. The highest regional incidence rates are in Eastern Asia, and China has more than half of the total deaths worldwide[14]. It has been suggested that esophageal cancer patients had the highest ICU admission rate with 27.3% admitted to the ICU during the first 2 years after diagnosis[15]. Multicenter study in China reported it as one of the most common primary cancer diagnoses in ICUs, along with lung, colorectal, and gastric cancers[16]. The main reasons for ICU admission in esophageal cancer patients were sepsis/septic shock and acute respiratory failure[17]. Esophageal cancer patients in ICUs tended to experience more complications compared to other cancer types[16], as well as high mortality rates[17]. These findings highlight the need for careful consideration of ICU admission and treatment strategies for esophageal cancer patients. However, the characteristics, prognosis and related risk factors of ICU admitted esophageal patients were rarely studied[17,18]. Using the data from a multicenter study in 37 ICU departments of cancer specialized hospitals, an analysis focusing on characteristics and outcomes of esophageal patients was performed.
Patients admitted into ICU departments of 37 cancer specialized hospitals in China from May 10, 2021 to July 10, 2021 were screened for a cross-sectional analysis. Inclusion criteria were primary esophageal cancer diagnosis, age ≥ 14 years and ICU duration ≥ 24 hours. Patients with incomplete clinical records (lack of primary disease information, evaluation score at admission, or outcomes after discharge) were excluded. Patients were separated into two groups according to the 90 days mortality outcome. Differences between the groups in characteristics at baseline and during ICU management were analyzed to explore the predictors for short term mortalities. This study was approved by Ethic Committee of Tianjin Medical University Cancer Institute and Hospital (No. bc2021065), and written informed consent was obtained from participations and their parents. This study adheres to the Declaration of Helsinki.
Data extracted from clinical records included general demographic information [age, sex, body mass index (BMI)], clinical history (source of admission, primary diagnosis, and cancer treatment history), Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) score at admission, ICU critical condition diagnosis and treatment applied, ICU and in-hospital outcomes (delirium, ICU duration), as well as 90 days survival follow-up.
Shapiro-Wilk test was performed to check the normality of quantitative data distribution. Due to skewed distribution, median and interquartile range were used to describe quantitative data. Categorical data was described using frequency (n) and percentage (%). Quantitative data were compared among groups using the Mann-Whitney U test. Ordinal category data were tested with the Wilcoxon rank sum test. Categorical data were compared between groups using either the χ2 test or Fisher exact test. Single and multi-variate cox regression tests were applied to analyze the correlated factors of 90 days death. The independent variables of the multifactor regression model were selected by backward method of stepwise regression. The receiver operating characteristic (ROC) curve of the regression model was plotted using the R package timeROC was used to demonstrate predictive value, and the area under the curve (AUC) of the ROC was further calculated. The software R 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis, and all the tests were two-sided, with a test level of α = 0.05.
Total 180 patients with primary esophageal cancer diagnosis were admitted to ICUs during the study period. As shown in Table 1, at the 90 days after admission, 40 patients were decreased, resulting in a mortality rate of 22.2%. When compared between the survived and deceased patients, no significant differences were found in age and gender. Deceased patients were detected with lower BMI, higher percentage of immunotherapy history. More patients in the deceased group were unplanned transferred to ICU from clinical ward and patients survived were more likely to undergo elective surgery. At admission of ICU, decreased patients had higher severity scores and more significant myelosuppression parameters. These patients were also more likely to experience complications including sepsis, acute respiratory distress syndrome, acute kidney injury, shock and delirium. To confirm that the difference in delirium was not compromised by the small sample size (6 cases in the deceased group vs 5 cases in the survived group). Additional statistical indicators for the correlation analysis between delirium and 90-days mortality. At the setting of α = 0.05, an odds ratio of 4.281 (95% confidence interval: 1.237-14.817), absolute risk reduction of -0.327, (95% confidence interval: -0.627 to -0.026) were detected. In addition, the deceased patients tended to receive more anti-infection and sedation treatment, but less percentage received conventional oxygen therapy instead of mechanical ventilation. These results indicated that the esophageal patients admitted to ICU due to acute illness had significant higher risks of mortality. Patients with mortality outcome showed differences from those survived mostly in disease severity and ICU complications.
| Variables | All (n = 180) | Survived at 90 days (n = 140) | 90 days death (n = 40) | P value |
| Demographics | ||||
| Age (year), mean ± SD | 66.4 ± 7.9 | 66.0 ± 7.7 | 67.8 ± 8.5 | 0.207 |
| Gender | ||||
| Female | 45 (25.0) | 38 (27.1) | 7 (17.5) | 0.214 |
| Male | 135 (75.0) | 102 (72.9) | 33 (82.5) | |
| BMI (kg/m2), median (IQR) | 22.3 (19.5, 24.3) | 22.7 (20.5, 24.5) | 20.6 (18.3, 22.7) | 0.001a |
| BMI classification | 0.002a | |||
| Underweight (BMI < 18.5) | 31 (17.2) | 16 (11.7) | 15 (34.9) | |
| Normal (BMI 18.5-22.9) | 82 (45.6) | 63 (46.0) | 19 (44.2) | |
| Overweight (BMI 23-24.9) | 34 (18.9) | 29 (21.2) | 5 (11.6) | |
| Obese (BMI > 25) | 33 (18.3) | 29 (21.2) | 4 (9.3) | |
| Treatment history | ||||
| Target therapy | 6 (3.3) | 3 (2.1) | 3 (7.5) | 0.125 |
| Immunotherapy | 23 (12.8) | 14 (10.0) | 9 (22.5) | 0.037a |
| Chemotherapy | 53 (29.4) | 40 (28.6) | 13 (32.5) | 0.631 |
| Radiotherapy | 17 (9.4) | 10 (7.1) | 7 (17.5) | 0.064 |
| Transferring source | < 0.001a | |||
| Operation room | 77 (42.8) | 73 (52.1) | 4 (10.0) | |
| Emergency department | 3 (1.7) | 2 (1.4) | 1 (2.5) | |
| Clinical ward | 100 (55.6) | 65 (46.4) | 35 (87.5) | |
| Planned transfer | 101 (56.1) | 67 (47.9) | 34 (85.0) | < 0.001a |
| Elective or emergency surgery | < 0.001a | |||
| No surgery | 41 (22.8) | 16 (11.4) | 25 (62.5) | |
| Elective | 135 (75.0) | 121 (86.4) | 14 (35.0) | |
| Emergency | 4 (2.2) | 3 (2.1) | 1 (2.5) | |
| Severity scores | ||||
| SOFA, median (IQR) | 3.0 (2.0, 5.0) | 3.0 (2.0, 4.2) | 4.0 (2.8, 7.0) | 0.003a |
| APACHE II, median (IQR) | 11.5 (8.8, 16.0) | 10.5 (8.0, 14.0) | 16.0 (12.0, 21.0) | < 0.001a |
| Fourth degree myelosuppression | 17 (9.4) | 8 (5.7) | 9 (22.5) | 0.004a |
| ICU diagnosis | ||||
| Sepsis | 130 (72.2) | 94 (67.1) | 36 (90.0) | 0.004a |
| ARDS | 40 (22.2) | 25 (17.9) | 15 (37.5) | 0.008a |
| Respiratory failure | 102 (56.7) | 74 (52.9) | 28 (70.0) | 0.054a |
| AKI | 6 (3.3) | 1 (0.7) | 5 (12.5) | 0.002a |
| Shock | 47 (26.1) | 25 (17.9) | 22 (55.0) | < 0.001a |
| Delirium | 11 (6.1) | 5 (3.6) | 6 (1.0) | 0.014a |
| Anti-infection treatment | ||||
| Carbapenems | 68 (37.8) | 43 (30.7) | 25 (62.5) | < 0.001a |
| β-lactam | 83 (46.1) | 65 (46.4) | 18 (45.0) | 0.873 |
| Glycopeptides | 27 (15.0) | 19 (13.6) | 8 (20.0) | 0.315 |
| Tigecycline | 2 (1.1) | 1 (0.7) | 1 (2.5) | 0.396 |
| Echinocandins | 8 (4.4) | 3 (2.1) | 5 (12.5) | 0.014a |
| Triazoles | 16 (8.9) | 9 (6.4) | 7 (17.5) | 0.052 |
| Other treatment | ||||
| Mechanical ventilation | 103 (57.2) | 75 (53.6) | 28 (70.0) | 0.064 |
| Conventional oxygen therapy | 147 (81.7) | 129 (92.1) | 18 (45.0) | < 0.001a |
| Sedation treatment | 85 (47.2) | 57 (40.7) | 28 (70.0) | 0.001a |
| ICU duration (days), median (IQR) | 5 (3, 9) | 5 (3.5, 8.5) | 4 (3, 10) | 0.560 |
To further explore the potential predictor for short-term mortality of ICU admitted esophageal cancer patients, cox regression analysis was performed to detect the correlating factors. As shown in Table 2, in single-variate analysis, factors correlated with higher mortality risk included lower BMI, immunotherapy and radiotherapy history, higher severity scores and myelosuppression signs, more intense anti-infection treatment was associated with higher risk of mortality while sedation treatment appeared to be associated with lower risks.
| Variables, demographics | Univariate analysis1 | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (years) | 1.03 (0.99-1.07) | 0.213 | ||
| Gender | ||||
| Female | Ref | |||
| Male | 1.68 (0.74-3.80) | 0.213 | ||
| BMI (kg/m2) | 0.86 (0.77-0.95) | 0.002a | ||
| Treatment history | ||||
| Target therapy | 2.53 (0.78-8.22) | 0.122 | ||
| Immunotherapy | 2.22 (1.06-4.67) | 0.035a | ||
| Chemotherapy | 1.22 (0.63-2.37) | 0.554 | ||
| Radiotherapy | 2.38 (1.05-5.38) | 0.037a | ||
| Severity scores | ||||
| SOFA | 1.18 (1.09-1.27) | < 0.001a | 1.09 (0.98-1.20) | 0.0991 |
| APACHE II | 1.13 (1.08-1.17) | < 0.001a | 1.09 (1.03-1.16) | 0.0031 |
| Fourth degree myelosuppression | 3.84 (1.82-8.07) | < 0.001a | 3.37 (1.15-9.90) | 0.0271 |
| Anti-infection treatment | ||||
| Carbapenems | 3.19 (1.68-6.05) | < 0.001a | ||
| β-lactam | 0.94 (0.50-1.75) | 0.836 | ||
| Glycopeptides | 1.50 (0.69-3.26) | 0.302 | ||
| Tigecycline | 2.46 (0.34-17.95) | 0.374 | ||
| Echinocandins | 4.87 (1.90-12.47) | < 0.001a | ||
| Triazoles | 2.58 (1.14-5.84) | 0.023a | ||
| Other treatment | ||||
| Mechanical ventilation | 1.88 (0.95-3.69) | 0.068 | ||
| Conventional oxygen therapy | 4.14 (1.00-17.19) | 0.051 | ||
| Sedation treatment | 0.10 (0.05-0.19) | < 0.001a | ||
These results suggested that with current data, there lacks specific pathology related predictors. The most useful prognosis tools may still rely on the general functional status and disease severity evaluation. Therefore, multivariate analysis of correlations between 90 days death with SOFA, APACHE II and fourth degree myelosuppression were performed adjusted to age, gender, BMI, history of immunotherapy, history of radiotherapy, mechanical ventilation, sedation treatment, conventional oxygen therapy, use of carbapenems, echinocandins and triazoles. As shown in Table 2, both APACHE II and myelosuppression showed significant independent association with higher mortality risks, while for SOFA sore, only marginal association (P = 0.099) was detected.
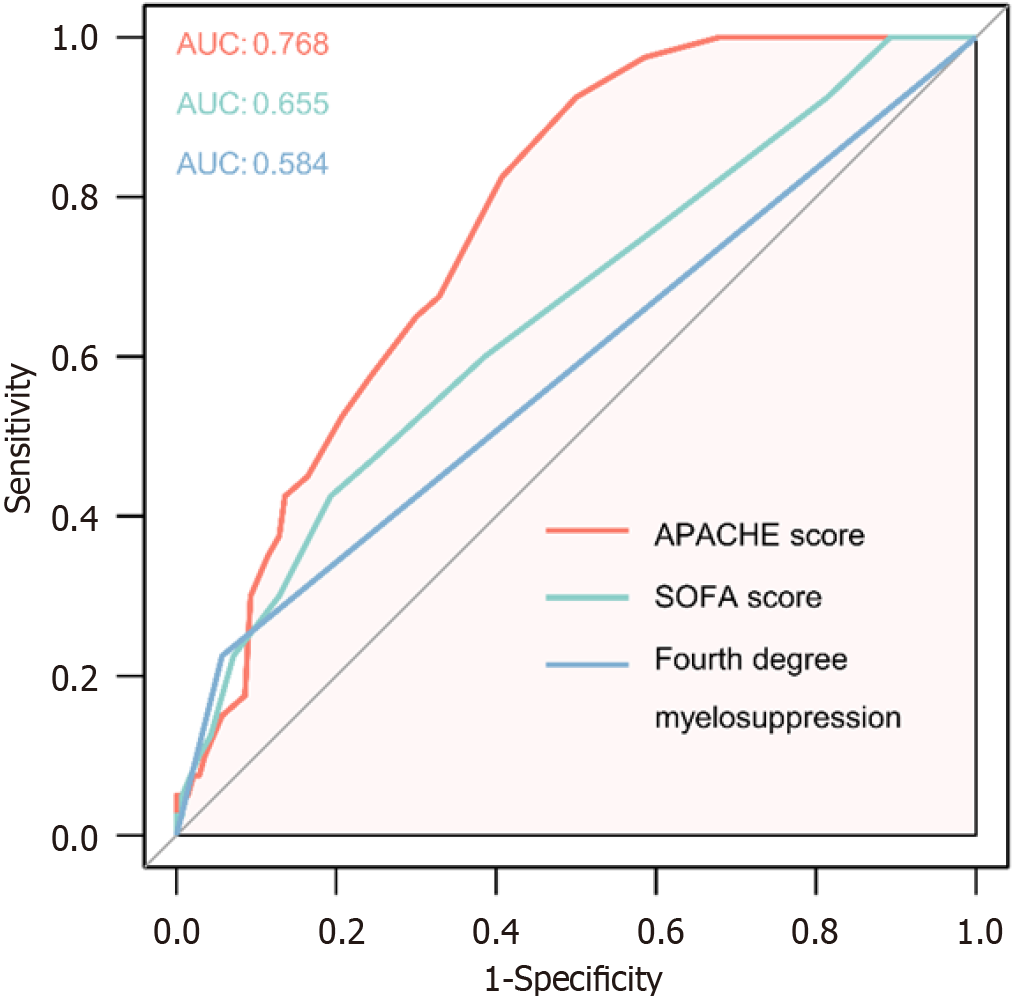
The predictive value of SOFA, APACHE II and fourth degree myelosuppression for short term mortality of ICU admitted esophageal cancer patients were further tested using ROC curve analysis. As shown in Figure 1, APACHE II showed moderate accuracy in prediction of short-term death, with an AUC of 0.768. SOFA score and myelosuppression showed mild and not significant predictive values (AUC = 0.655 and 0.584, respectively). These results indicated that these scores may not be sufficient in prognosis of ICU admitted esophageal cancer patients. Further exploration of predictors needs to be carried out in the future.
The data from 37 ICUs of cancer specialized hospitals showed that 180 esophageal cancer patients were admitted during a 2 months period. The 90 days mortality after ICU care was 22.2%. Compared to the survivors, the patients with 90 days death generally were with lower BMI and more severe conditions before admission, as well as more ICU complications and more intense treatment. Only APACHE II score showed moderate predictive values for short term mortality. These results indicated that ICU admitted esophageal cancer patients had relatively high mortality risk, but currently there still lacks dependable predictors for short-term mortality outcome.
Esophageal patients’ prognosis is generally poor, with 5 years overall survival around only 21%[12,19]. The inpatient mortality of esophageal cancer has been reported ranging from 9% to 13% in a large registry study[20]. While another single center study reported 58.0% in-hospital mortality for esophageal cancer patients with unplanned ICU admission[17]. The main reasons for ICU admissions of esophageal cancer patients include elective postoperative conditions or clinical complications, such as bronchial aspiration owing to dysphagia, esophageal perforation and mediastinitis[18,21]. There different in-hospital mortality results may indicate that esophageal patients admitted into ICU due to acute illness had high risks of death but those undergo perioperative management may have lower risks for ICU admission and mortality. In this study, we found that the 90 days mortality after ICU care of esophageal cancer patients was 22.2%, close to the average morality rate of solid tumors. More importantly, more than 87% of the deceased patients were transferred from clinical ward instead of operation room. These results again indicated that esophageal cancer patients underwent surgery management were relatively safer but those with acute complications had high mortality risks.
Weight loss is common in esophageal cancer patients due to a combination of cachexia and decreased caloric intake due to dysphagia, with 57% to 85% preoperative patients reported to have significant weight loss[22,23]. Weight loss, which indicating worse nutrition and health status, was also reported to be associated with worse prognosis of esophageal cancer[24]. Our results showed that decreased patients had significant lower BMI compared to those survived, which was consistent with previous findings. Nutrition during management, both pre- and in-ICU treatment, therefore may need to be paid more attention.
Immunotherapy and radiotherapy history, as well as myelosuppression, were identified as potential correlators of mortality. Radiotherapy is a crucial treatment for esophageal cancer, while combining radiotherapy with immunotherapy was suggested to improve outcomes for advanced esophageal cancer patients[25]. Therefore, they might be applied to patients with advanced cancer that can’t be treated surgically, or relapse after a previous treatment, or as adjuvant treatment option for resected esophageal cancer patients. The correlations between these therapy history with mortality might simply be due to more severe disease conditions. However, adverse effects and toxicities may also play a role. Immune related adverse effects have been noticed in various regimens including immunotherapy in cancer patients[26,27]. These adverse effects may cause damages in various organs, as well as increase the risk of immune dysregulation and infections[26,28,29]. It has been suggested that radiation-induced lymphopenia may impact tumor control and survival outcomes[30]. The esophagus is particularly susceptible to radiation-induced toxicity, with a crude incidence of symptomatic cardiac toxicity as high as 10.8%[31]. Interestingly, the immune response elicited by radiotherapy may play a crucial role in treatment efficacy, as well as linked to increased risks of acute hematologic and organ toxicities[32]. Radiotherapy many further increase the risks of adverse events of immunotherapy[33]. The association between these treatments, specifically the time relationship with ICU admission and outcomes, may be explored in future studies.
The APACHE II and SOFA scoring systems are widely used to predict outcomes in ICUs. Both scores show good predictive accuracy for ICU mortality, with APACHE II demonstrating better discrimination in some studies[34,35]. However, SOFA is often preferred for its simplicity and ease of use[34]. Our results also showed that APACHE II was more accurate in predicting death. Since SOFA was mainly purposed to assess organ dysfunction/failure while APACHE II was more detailed constructed to measure disease severity, these results were not surprising. Previous results have also suggested that the maximum SOFA score and the difference between maximum and initial SOFA scores are particularly effective in predicting mortality in elderly patients[36]. Therefore, it would be interesting to explore scoring at different times with more details in future studies.
The study was still limited by sample size and the completeness of available data. Moreover, with limited data source, we did not identify specific indicators for adverse prognosis of ICU admitted esophageal cancer patients. The correlators found were mostly indicators for disease severity, such as BMI and treatment history. More records of ICU complications and related treatment also may only reflect the seriousness of conditions at ICU admission. No specific predictor could be summarized from the current data. The prognosis evaluation may still depend on current available disease severity evaluation tools, although the predictive values of current tools were not sufficient neither. However, our results provided some further information on prognosis of ICU admitted esophageal patients, for whom studies were rarely available.
In conclusion, results from this study suggested ICU admitted esophageal cancer patients have poor prognosis, especially those with acute illness. The prognostic tools for these patients need to be further optimized.
| 1. | Martos-Benítez FD, Soler-Morejón CD, Lara-Ponce KX, Orama-Requejo V, Burgos-Aragüez D, Larrondo-Muguercia H, Lespoir RW. Critically ill patients with cancer: A clinical perspective. World J Clin Oncol. 2020;11:809-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 2. | Koutsoukou A. Admission of critically ill patients with cancer to the ICU: many uncertainties remain. ESMO Open. 2017;2:e000105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Puxty K, McLoone P, Quasim T, Sloan B, Kinsella J, Morrison DS. Risk of Critical Illness Among Patients With Solid Cancers: A Population-Based Observational Study. JAMA Oncol. 2015;1:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Azoulay E, Schellongowski P, Darmon M, Bauer PR, Benoit D, Depuydt P, Divatia JV, Lemiale V, van Vliet M, Meert AP, Mokart D, Pastores SM, Perner A, Pène F, Pickkers P, Puxty KA, Vincent F, Salluh J, Soubani AO, Antonelli M, Staudinger T, von Bergwelt-Baildon M, Soares M. The Intensive Care Medicine research agenda on critically ill oncology and hematology patients. Intensive Care Med. 2017;43:1366-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Halpern NA. In Between the Intensive Care Unit and the Ward. JAMA Intern Med. 2016;176:1499-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Valley TS, Schutz A, Miller J, Miles L, Lipman K, Eaton TL, Kinni H, Cooke CR, Iwashyna TJ. Hospital factors that influence ICU admission decision-making: a qualitative study of eight hospitals. Intensive Care Med. 2023;49:505-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Toffart AC, Gonzalez F, Hamidfar-Roy R, Darrason M. [ICU admission for cancer patients with respiratory failure: An ethical dilemma]. Rev Mal Respir. 2023;40:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Zampieri FG, Romano TG, Salluh JIF, Taniguchi LU, Mendes PV, Nassar AP Jr, Costa R, Viana WN, Maia MO, Lima MFA, Cappi SB, Carvalho AGR, De Marco FVC, Santino MS, Perecmanis E, Miranda FG, Ramos GV, Silva AR, Hoff PM, Bozza FA, Soares M. Trends in clinical profiles, organ support use and outcomes of patients with cancer requiring unplanned ICU admission: a multicenter cohort study. Intensive Care Med. 2021;47:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Epstein AS, Yang A, Colbert LE, Voigt LP, Meadows J, Goldberg JI, Saltz LB. Outcomes of ICU Admission of Patients With Progressive Metastatic Gastrointestinal Cancer. J Intensive Care Med. 2020;35:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Kemoun G, Weiss E, El Houari L, Bonny V, Goury A, Caliez O, Picard B, Rudler M, Rhaiem R, Rebours V, Mayaux J, Bachet JB, Belin L, Demoule A, Decavèle M. Clinical features and outcomes of patients with pancreatic cancer requiring unplanned medical ICU admission: A retrospective multicenter study. Dig Liver Dis. 2024;56:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Xu ZY, Hao XY, Wu D, Song QY, Wang XX. Prognostic value of 11-factor modified frailty index in postoperative adverse outcomes of elderly gastric cancer patients in China. World J Gastrointest Surg. 2023;15:1093-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 846] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 13. | GBD 2017 Oesophageal Cancer Collaborators. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:582-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 291] [Article Influence: 58.2] [Reference Citation Analysis (1)] |
| 14. | Arora J, Sehgal L, Satpathy H. Intensive Care Unit Management of a Patient with Tracheal Rent Repair Following Laryngopharyngoesophagectomy. Indian J Crit Care Med. 2020;24:77-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Bos MM, Verburg IW, Dumaij I, Stouthard J, Nortier JW, Richel D, van der Zwan EP, de Keizer NF, de Jonge E. Intensive care admission of cancer patients: a comparative analysis. Cancer Med. 2015;4:966-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Liu W, Zhou D, Zhang L, Huang M, Quan R, Xia R, Ye Y, Zhang G, Shen Z; Cancer Critical Care Medicine Committee of the Chinese Anti-Cancer Association. Characteristics and outcomes of cancer patients admitted to intensive care units in cancer specialized hospitals in China. J Cancer Res Clin Oncol. 2024;150:205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Freitas ICL, Assis DM, Amendola CP, Russo DDS, Moraes APP, Caruso P, Nassar Júnior AP. Characteristics and short-term outcomes of patients with esophageal cancer with unplanned intensive care unit admissions: a retrospective cohort study. Rev Bras Ter Intensiva. 2020;32:229-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Tong C, Cao H, Xu Y, Li D, Zhang H, Xu M, Luo Y, Wu J. Causes, Risk Factors and Outcomes of Patients Readmitted to the Intensive Care Unit After Esophageal Cancer Surgery: A Retrospective Cohort Study. World J Surg. 2021;45:2167-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Lundberg E, Lagergren P, Mattsson F, Lagergren J. Life Expectancy in Survivors of Esophageal Cancer Compared with the Background Population. Ann Surg Oncol. 2022;29:2805-2811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Sarvepalli S, Garg SK, Sarvepalli SS, Parikh MP, Wadhwa V, Jang S, Thota PN, Sanaka MR. Inpatient burden of esophageal cancer and analysis of factors affecting in-hospital mortality and length of stay. Dis Esophagus. 2018;31:doy022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Bissell L, Khan OA, Mercer SJ, Somers SS, Toh SK. Long term outcomes following emergency intensive care readmission after elective oesophagectomy. Acta Chir Belg. 2013;113:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Shen S, Araujo JL, Altorki NK, Sonett JR, Rodriguez A, Sungur-Stasik K, Spinelli CF, Neugut AI, Abrams JA. Variation by stage in the effects of prediagnosis weight loss on mortality in a prospective cohort of esophageal cancer patients. Dis Esophagus. 2017;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Zhang S, Tan Y, Cai X, Luo K, Wu Z, Lu J. Preoperative weight loss is associated with poorer prognosis in operable esophageal cancer patients: A single-center retrospective analysis of a large cohort of Chinese patients. J Cancer. 2020;11:1994-1999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Lyu JH, Li T, Han YT, Wu L, Peng L, Wang QF, Liang L. Effect of Weight Loss on Survival in Esophageal Cancer Patients undergoing Neoadjuvant Chemoradiotherapy and Surgery. J Nutr Oncol. 2020;5:137-146. [DOI] [Full Text] |
| 25. | Jiang M, Hu Y, Lin G, Chen C, Li H. Radiotherapy combined with immune checkpoint inhibitors in locally advanced/metastatic esophageal squamous cell carcinoma: clinical trials, efficacy and future directions. Front Immunol. 2023;14:1177085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Kichloo A, Albosta M, Dahiya D, Guidi JC, Aljadah M, Singh J, Shaka H, Wani F, Kumar A, Lekkala M. Systemic adverse effects and toxicities associated with immunotherapy: A review. World J Clin Oncol. 2021;12:150-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 27. | Joseph A, Lafarge A, Azoulay E, Zafrani L. Acute Kidney Injury in Cancer Immunotherapy Recipients. Cells. 2022;11:3991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 28. | Steinel NC, Lee EM, Viggiano D, Capasso A, Lee MW. The renal adverse effects of cancer immunotherapy. J Nephrol. 2020;33:467-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Joseph A, Lafarge A, Mabrouki A, Abdel-Nabey M, Binois Y, Younan R, Azoulay E. Severe infections in recipients of cancer immunotherapy: what intensivists need to know. Curr Opin Crit Care. 2022;28:540-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Wang X, Wang P, Zhao Z, Mao Q, Yu J, Li M. A review of radiation-induced lymphopenia in patients with esophageal cancer: an immunological perspective for radiotherapy. Ther Adv Med Oncol. 2020;12:1758835920926822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Beukema JC, van Luijk P, Widder J, Langendijk JA, Muijs CT. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol. 2015;114:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 32. | Ma JL, Jin L, Li YD, He CC, Guo XJ, Liu R, Yang YY, Han SX. The intensity of radiotherapy-elicited immune response is associated with esophageal cancer clearance. J Immunol Res. 2014;2014:794249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Anscher MS, Arora S, Weinstock C, Amatya A, Bandaru P, Tang C, Girvin AT, Fiero MH, Tang S, Lubitz R, Amiri-Kordestani L, Theoret MR, Pazdur R, Beaver JA. Association of Radiation Therapy With Risk of Adverse Events in Patients Receiving Immunotherapy: A Pooled Analysis of Trials in the US Food and Drug Administration Database. JAMA Oncol. 2022;8:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 34. | Hosseini M, Ramazani J. Evaluation of Acute Physiology and Chronic Health Evaluation II and sequential organ failure assessment scoring systems for prognostication of outcomes among Intensive Care Unit's patients. Saudi J Anaesth. 2016;10:168-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Shrestha GS, Gurung R, Amatya R. Comparison of Acute Physiology, Age, Chronic Health Evaluation III score with initial Sequential Organ Failure Assessment score to predict ICU mortality. Nepal Med Coll J. 2011;13:50-54. [PubMed] |
| 36. | Qiao Q, Lu G, Li M, Shen Y, Xu D. Prediction of outcome in critically ill elderly patients using APACHE II and SOFA scores. J Int Med Res. 2012;40:1114-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |