Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.107380
Revised: April 17, 2025
Accepted: May 30, 2025
Published online: July 15, 2025
Processing time: 114 Days and 21.4 Hours
SHP2 is the first identified oncogenic tyrosine phosphatase that promotes colo
Core Tip: This paper summarizes the regulatory mechanisms of SHP2 in colorectal cancer (CRC) and emerging therapeutic strategies targeting SHP2. The findings demonstrated that SHP2 serves as a master oncogenic regulator in CRC pathogenesis by coordinating receptor tyrosine phosphatase-mediated signaling. Notably, SHP2 remodels the tumor immune microenvironment by modulating macrophage and T cell functions. Allosteric SHP2 inhibitors, which are characterized by high oral bioavailability and potent target specificity, are currently under evaluation in multicenter phase I/II trials. Although acquired resistance remains challenging, combination strategies, particularly immunotherapy-based treatments, have shown transformative potential, accelerating the transition of SHP2-targeted therapies and offering novel paradigms for personalized CRC treatment.
- Citation: Liu P, Chen J. Targeting SHP2: Dual breakthroughs in colorectal cancer therapy–from signaling pathway modulation to immune microenvironment remodeling. World J Gastrointest Oncol 2025; 17(7): 107380
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/107380.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.107380
Colorectal cancer (CRC) is a prevalent malignant tumor within the gastrointestinal tract and has emerged as a growing global health challenge[1,2]. With an increasing emphasis on physical examinations, the detection rate of CRC has risen annually. According to the latest epidemiological data, CRC accounts for 9.6% of incident malignancies (third most prevalent) and 9.3% of global cancer mortalities (second leading cause)[1,3]. Although recent therapeutic advancements have significantly improved survival outcomes in CRC, the 5-year survival rate for metastatic CRC is lower than 15%, underscoring the urgent need to elucidate its molecular mechanisms and identify novel therapeutic targets[4,5].
Protein tyrosine phosphorylation, a critical post-translational modification, regulates cellular signaling networks through the dynamic balance between protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs)[6-8]. Dysregulation of protein tyrosine phosphorylation is a key driver of oncogenesis across multiple cancer lineages[9]. There is a striking disparity in the current pharmacological landscape; namely, many PTK inhibitors have been approved for clinical tumor treatment, whereas PTP-targeted drugs remain in the exploratory phase[10,11]. Notably, SHP2, encoded by the PTPN11 gene, is the first validated proto-oncogenic phosphatase[12]. SHP2 manifests dual oncogenic functionality in colorectal carcinogenesis, as it drives tumor progression via classical pathways such as PI3K/AKT and RAS/MAPK signaling and plays a crucial role in immune microenvironment remodeling. This paper reviews the molecular mechanisms of SHP2 in CRC and its translational potential for clinical treatment.
SHP2 is a non-receptor protein tyrosine phosphatase (PTP) characterized by three domains: tandem N-terminal SH2 domains (N-SH2 and C-SH2) and a C-terminal catalytic (PTP) domain, as well as a regulatory tail containing tyrosine phosphorylation sites (Y542/Y580)[13,14]. In the resting state, SHP2 is auto-inhibited through interactions between N-SH2 and the PTP domain, which suppress the catalytic activity of SHP2[15]. Ligand-induced activation of RTKs triggers phosphorylation of specific intracellular tyrosine residues, which bind to the SH2 domain, inducing conformational changes to expose the catalytic site of SHP2. Importantly, phosphorylation at Y542/Y580 simultaneously releases the self-inhibitory state and activates the downstream signaling cascade by recruiting the adaptor protein Grb2[16,17].
Although early studies reported SHP2 downregulation in the cancer tissues of patients with CRC compared to normal adjacent tissues, recent large-scale cohort studies revealed that SHP2 expression is significantly higher in CRC tissues than in adjacent mucosal tissues, and its elevated expression is significantly associated with improved prognosis[18-20]. This dysregulation was also observed in sporadic colorectal adenomas, in which SHP2 was upregulated in hyperplastic epithelial compartments[21]. Significantly elevated SHP2 phosphorylation has also been observed in CRC tissues compared to non-neoplastic controls[22]. Clinicopathological correlation analysis demonstrated that decreased SHP2 expression is significantly associated with poor differentiation, lymph node metastasis, and advanced TNM stage, suggesting its potential as an independent prognostic biomarker of CRC[18,20].
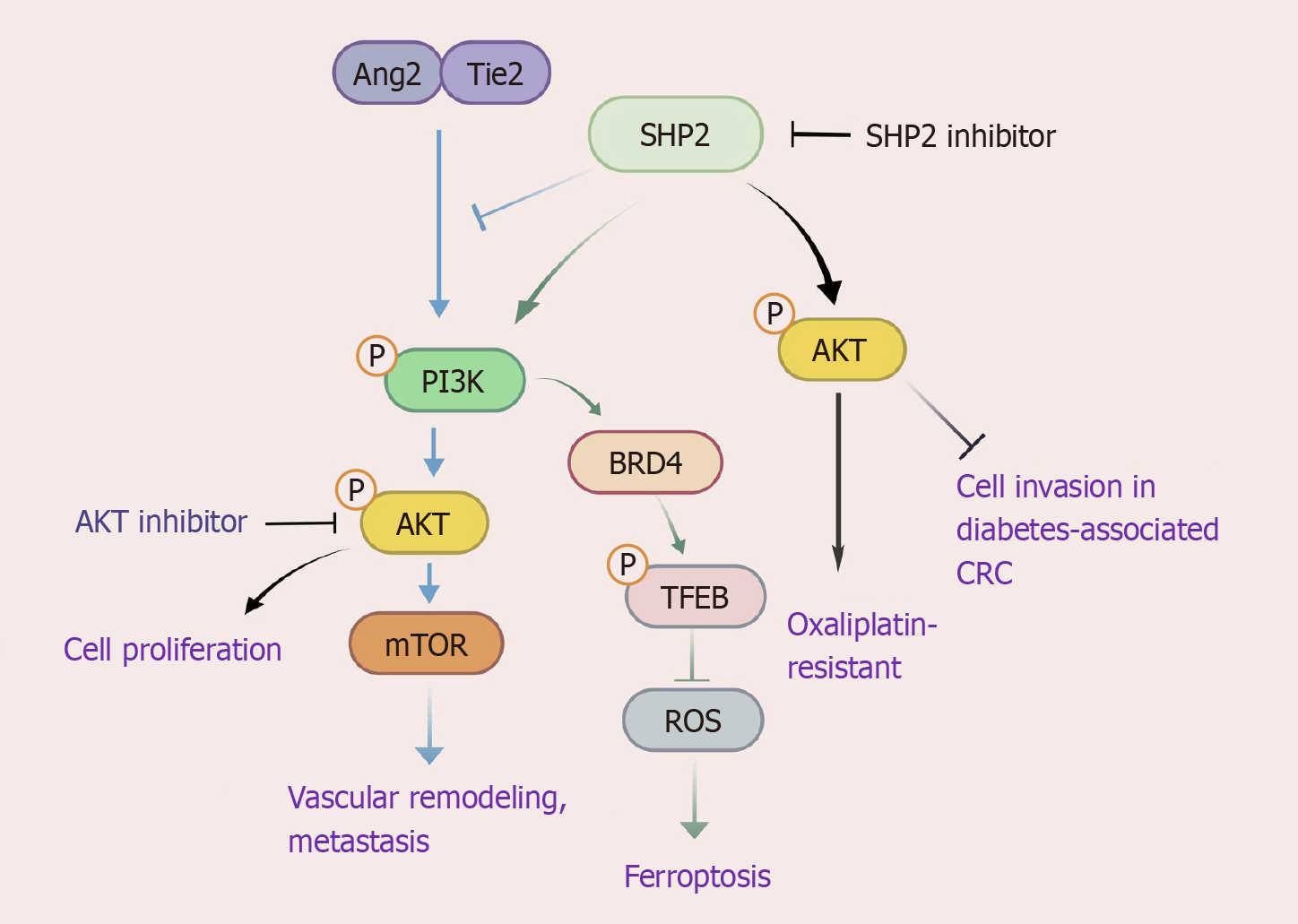
SHP2 plays a dual regulatory role in the PI3K/AKT pathway in CRC. SHP2 depletion in CRC cells demonstrates potent antitumor effects through dual suppression of cellular proliferation and induction of apoptosis[23]. It exerts its oncogenic activity via PI3K/AKT pathway activation; SHP2 is upregulated in oxaliplatin-resistant cells, where it drives chemoresistance via AKT hyperphosphorylation[23]. SHP2 plays a critical role in the ferroptosis regulatory network, as it orchestrates the PI3K/BRD4/TFEB axis to inhibit ferritinophagy, thereby attenuating ROS generation and blocking iron-dependent cell death mechanisms essential for tumor survival[24]. Concurrently, SHP2 promotes metastatic progression through Tie2-PI3K/AKT/mTOR-mediated vascular remodeling. Genetic ablation of SHP2 paradoxically amplifies Ang/Tie2-PI3K signaling, upregulating vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) to potentiate hepatic metastasis[25]. Intriguingly, the regulatory role of SHP2 in PI3K signaling exhibits pathological context-dependence: in diabetes-associated CRC, SHP2 knockdown attenuates PI3K/AKT phosphorylation but augments tumor cell invasiveness, implying metabolic reprogramming rewires SHP2-PI3K crosstalk[26]. Therapeutic challenges emerge from compensatory AKT reactivation through PDGFRβ-PI3K signaling during SHP2 monotherapy, which can be circumvented by SHP2 inhibitors and AKT/FAK inhibitors in combination to achieve synergistic pathway blockade[22]. Resistance mechanisms involve WWP1-mediated AKT resilience, effectively addressed through dual SHP2/WWP1 inhibition using agents like I3C[27]. Emerging therapeutic agents, such as metallocene-curcumin hybrid derivatives (e.g., compound 3f), show dual efficacy by directly suppressing PI3K-AKT signaling while modulating tumor immune microenvironment, highlighting the multifaceted potential of the SHP2-PI3K axis modulation in precision CRC therapeutics[28]. This bidirectional regulation highlights the therapeutic complexity of targeting SHP2 in CRC, especially in comorbid metabolic disorders (Figure 1).
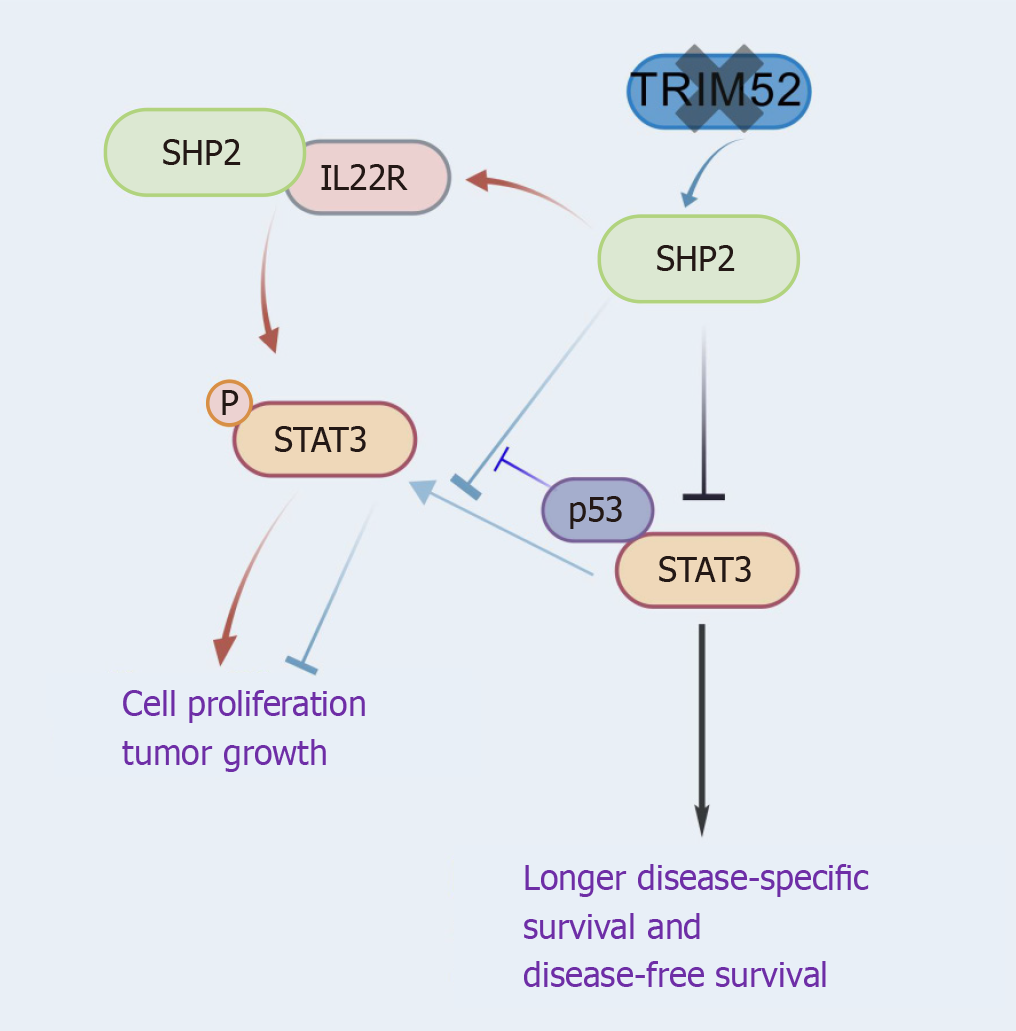
SHP2 expression exhibited a negative correlation with nuclear STAT3 expression in CRC, a marker of the JAK/STAT pathway. Patients with elevated SHP2 expression and diminished nuclear STAT3 levels experienced significantly longer disease-specific survival and disease-free survival[29]. TRIM52 deletion decreased STAT3 phosphorylation and increased SHP2 expression, thereby inhibiting cell proliferation and tumor growth[30]. SHP2 inhibited CRC cell proliferation by dephosphorylating STAT3 at Tyr705, whereas mutant-p53 reversed this suppression via competitive binding to STAT3[29-31]. Additionally, interactions between SHP2 and IL22R1 activate STAT3, thereby stimulating the JAK/STAT pathway and promoting cell proliferation[32]. Consequently, the regulation of STAT3 by SHP2 is pathway-specific (Figure 2).
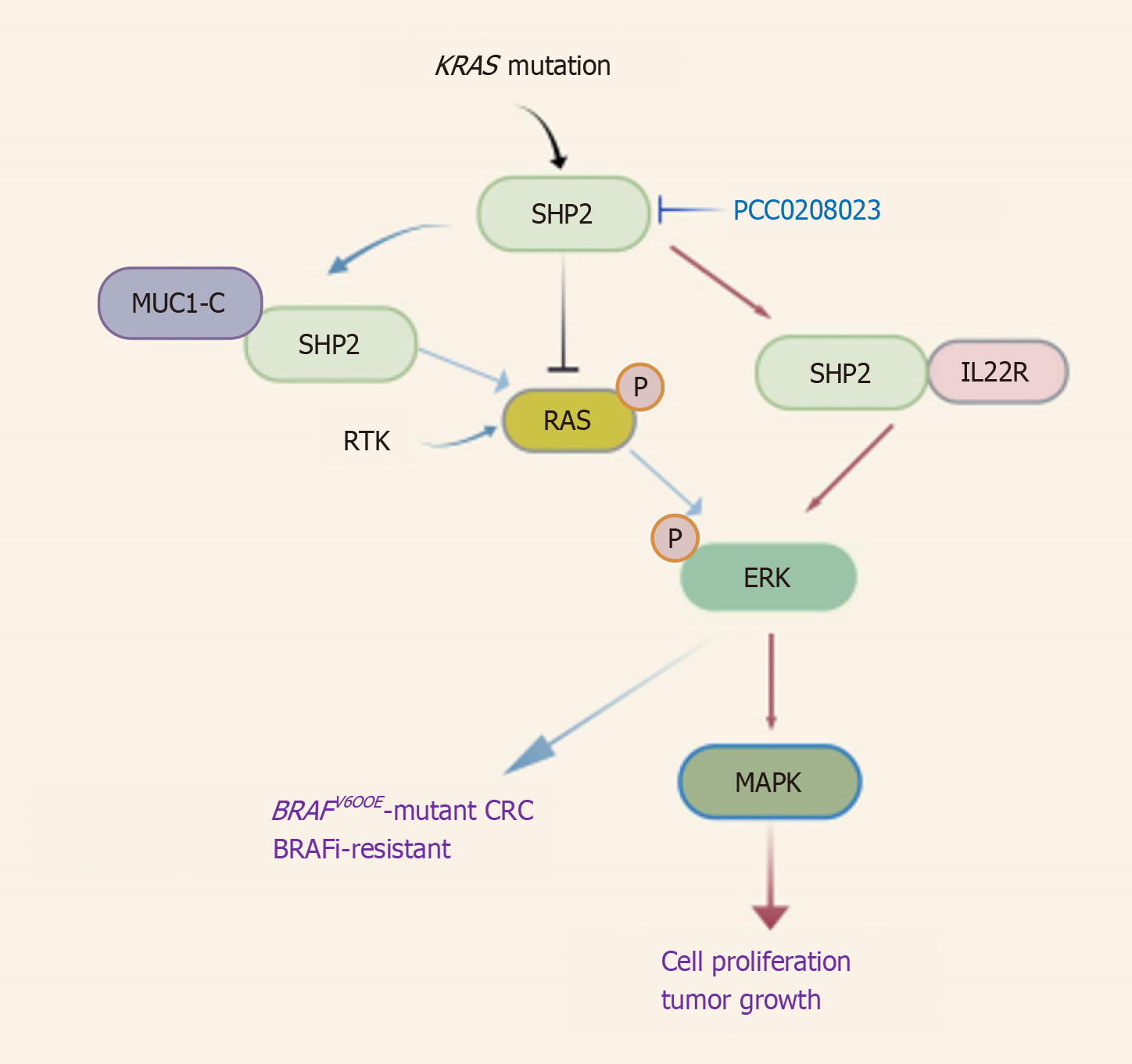
In the RAS/MAPK pathway, SHP2-IL-22R1 binding is essential for IL-22–mediated ERK activation, which drove downstream MAPK signaling to promote cell proliferation[32]. MUC1-C interacted with SHP2 to enhance RTK-mediated RAS/ERK signaling, making it a potential therapeutic target in BRAFV600E-mutant CRC[33]. Moreover, SHP2 deletion in KRAS-mutant CRC cells significantly reduced RAF/MEK/ERK phosphorylation, resulting in marked impairment of proliferation and invasive capacity[21]. In addition, treatment with the SHP2 allosteric inhibitor PCC0208023 suppressed KRAS-mutated CRC cell proliferation through RAS/MAPK pathway blockade and demonstrated potent antitumor efficacy in preclinical models[34] (Figure 3).
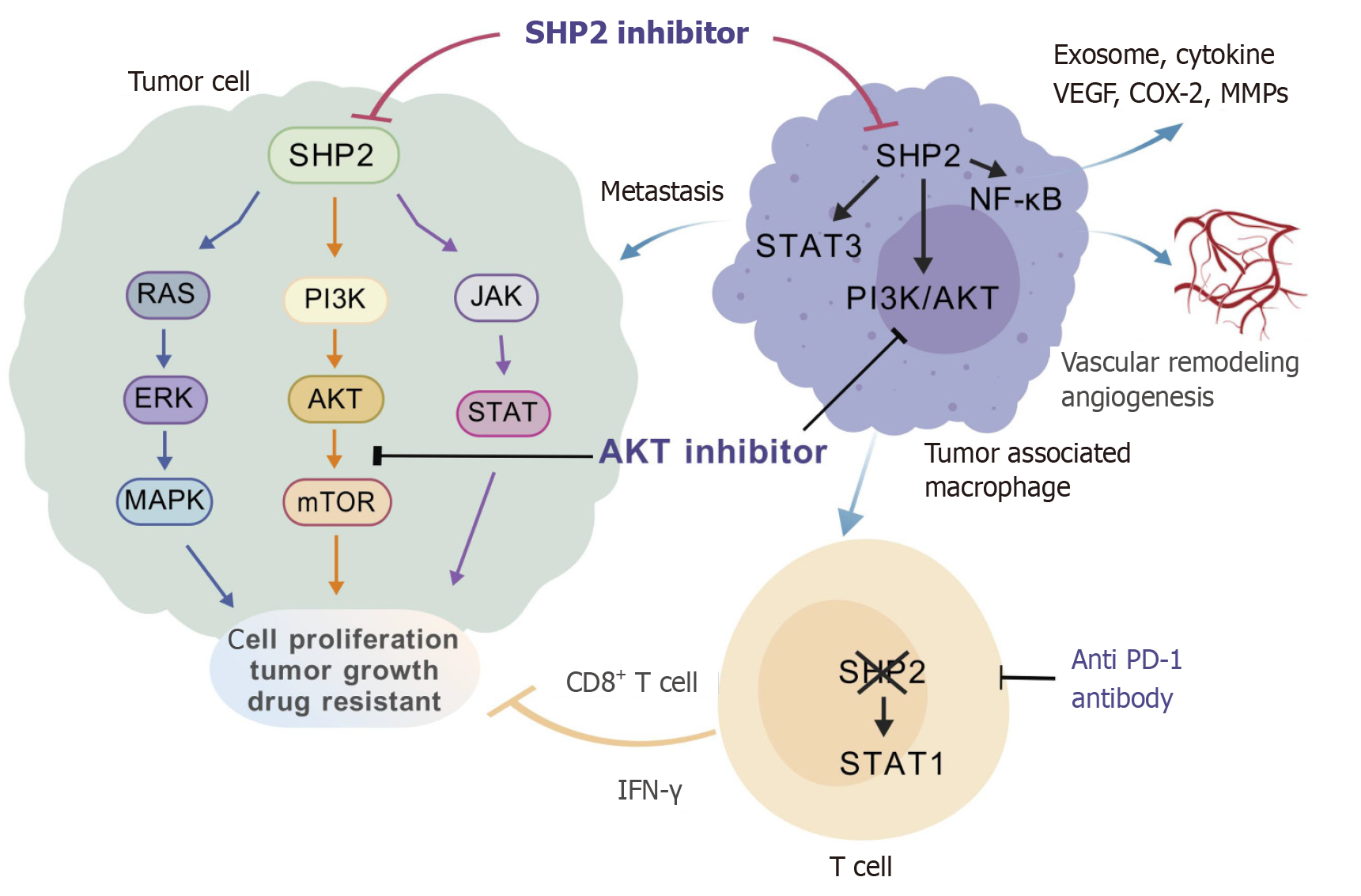
SHP2 has emerged as a critical regulator of the tumor microenvironment (TME) in CRC. In tumor-associated macrophages (TAMs), targeting SHP2 with PHPS1 attenuates its expression in macrophages, which activates the PI3K/AKT pathway to induce M2-polarized TAMs and exosome secretion, ultimately fostering CRC metastasis. Pharmacological blockade of PI3K by LY294002 reverses this pro-metastatic phenotype[35]. SHP2 deficiency in TAMs also biases macrophages toward M2 polarization through STAT3 activation and NF-κB inhibition, as evidenced by reduced pro-inflammatory cytokine secretion and elevated MMP levels[36]. Genetic ablation of SHP2 in macrophages exacerbates CRC hepatic metastasis through the Tie2-PI3K/AKT/mTOR signaling axis. This aberrant signaling cascade transcriptionally upregulates pro-metastatic mediators, including VEGF (angiogenesis), COX-2 (prostaglandin synthesis), and MMP2/MMP9 (extracellular matrix remodeling), collectively fostering a metastasis-permissive niche through dual modulation of angiogenic switching and stromal reorganization[25]. However, SHP2 deletion in TAMs was also reported to protect mice from colitis-associated colorectal carcinogenesis[37]. Strikingly, myeloid-specific SHP2 ablation activated the STING/TBK1/IRF3 pathway and enhanced type I interferon production, thus promoting CD8+ T cell infiltration and delaying tumor progression by reprogramming the immunosuppressive CRC TME[38].
CD4+ T cell-specific SHP2 deficiency resulted in a reduced tumor volume, accompanied by elevated IFN-γ expression and amplified cytotoxic CD8+ T cell activity[39]. Mice with T cell-specific deletion of SHP2 demonstrated slowed tumor growth. Moreover, inhibition of SHP2 using the allosteric inhibitor SHP099 potentiated antitumor immunity, as evidenced by STAT1 hyperphosphorylation, an elevated proportion of CD8+IFN-γ+ T cells and a marked reduction in tumor burden, underscoring its therapeutic potential in remodeling the CRC TME[40] (Figure 4).
Therapeutic targeting of SHP2 in CRC has entered a transformative phase, marked by a growing number of inhibitors progressing through preclinical development and clinical trials, including TNO155 (NCT03114319, NCT04000529), RMC-4630 (NCT03634982), ET0038(NCT05354843), BBP-398(NCT04528836), JAB-3312 (NCT04121286), and JAB-3068 (NCT03518554, NCT03565003; Table 1). These agents abolish downstream signaling of SHP2 by binding to the PTP or SH2 domain, thereby suppressing enzymatic activity or preventing substrate interactions[41,42]. Notably, TNO155 represents a new paradigm for allosteric inhibitors, as it demonstrated high selectivity for the allosteric pocket within the PTP domain. This mechanism effectively disrupts both the catalytic activity and substrate recruitment capacity of SHP2. Preclinical studies validated its antitumor efficacy and favorable pharmacokinetic data in tumor cell lines and mouse models[43-45]. Meanwhile, other inhibitors such as RMC-4630 exhibited similar antiproliferative effects in CRC preclinical models, underscoring the therapeutic potential of SHP2 inhibition in CRC.
| Trial name (NCT number) | Phase | Combination/therapeutic strategy | Target indication | Key populations/findings | Status/updates |
| NCT03634982 | Phase 1 | RMC-4630 | Advanced solid tumors (including CRC) | Monotherapy | Active |
| NCT04121286 | Phase 1 | JAB-3312 | Advanced solid tumors (including CRC) | Monotherapy | Recruiting |
| NCT03518554* | Phase 1 | JAB-3068 | Advanced solid tumors (including CRC) | Monotherapy | Complete |
| NCT03565003 | Phase 1/2 | JAB-3068 | Advanced solid tumors (including CRC) | Monotherapy | Complete |
| NCT05354843 | Phase 1 | ET0038 | Advanced solid tumors (including CRC) | Monotherapy | Recruiting |
| NCT04528836 | Phase 1 | BBP-398 | Advanced solid tumors (including CRC) | Monotherapy | Terminated in 2024 |
| NCT03114319 | Phase 1 | TNO155 + nazartinib | Advanced EGFR/KRAS-mutant solid tumors | Monotherapy or combination use with EGFR TKIs | Active |
| NCT04330664 | Phase 1 | TNO155 + MRTX849 | Advanced solid tumors with KRASG12C mutation | Combination therapy | Complete |
| NCT04294160 | Phase 1 | Dabrafenib + LTT462 + TNO155 Dabrafenib + trametinib + TNO155 | Advanced or metastatic BRAFV600E-mutated CRC | Combination therapy | Terminated in 2024 |
| NCT04000529 | Phase 1 | TNO155 + spartalizumab/ribociclib | Selected malignancies | Monotherapy or combination of TNO155 with spartalizumab or with ribociclib | Terminated in 2024 |
| NCT04699188 | Phase 1/2 | JDQ443 + TNO155 + tislelizumab | KRASG12C-mutant NSCLC, CRC | Monotherapy or with KRASG12C inhibitor, Dose Escalation Study | Active |
| NCT04916236 | Phase 1 | RMC-4630 + LY3214996 | Metastatic KRAS-mutant CRC, PDAC, and NSCLC | Combination therapy of RMC-4630 (SHP2 inhibitor) and LY3214996 (ERK inhibitor) | Terminated in 2024 |
| NCT04185883 | Phase 1 | Sotorasib + RMC-4630 | KRASG12C mutant advanced solid tumors | Combination therapy | Recruiting |
| NCT04670679 | Phase 1 | ERAS-601 + cetuximab/pembrolizumab | Advanced solid tumors (including CRC) | Monotherapy or combination treatment with cetuximab/pembrolizumab | Active |
| NCT04252339 | Phase 1 | RLY-1971 | Advanced or metastatic solid tumors | Monotherapy, dose escalation, and expansion study | Complete |
However, emerging clinical data also suggest the emergence of an acquired resistance mechanism in patients treated with SHP2 inhibitors. Certain SHP2 mutations (e.g., G503V) conferred resistance to SHP2 inhibitors[46]. Additionally, rapid feedback-induced re-activation of AKT signaling following SHP2 inhibition emerged as a dominant resistance mechanism in CRC[22]. Recent studies identified a phenyl urea compound as a novel SHP2 inhibitor, with the compound exerting dual therapeutic effects through potent antiproliferative effects against SHP099/TNO155-resistant tumor cells and by reversing PD-L1–mediated immunosuppression. This compound significantly suppressed tumor growth in murine models, offering new insights into SHP2-mediated therapeutic resistance[45].
Current combination strategies for CRC treatment are primarily classified into three categories: chemotherapy sensitization, targeted synergy, and immunotherapy. The combined use of SHP2 inhibitors with regorafenib or celastrol led to markedly enhanced therapeutic efficacy in CRC, as evidenced by reduced tumor size, decreased cell proliferation, increased apoptosis, and elevated antitumor immune responses in vivo[44,47]. Furthermore, the combined administration of TNO155 and CDK4/6 inhibitors resulted in superior tumor growth inhibition in patient-derived xenograft models of CRC[48]. Synergistic antitumor effects were also observed upon combining SHP2 inhibitors with EGFR/MEK inhibitors[49].
Notably, the combination of allosteric SHP2 inhibitors and immune checkpoint inhibitors re-sensitized immunotherapy-resistant CRC to immune checkpoint blockade. The combination of SHP099 and anti-PD-1 antibodies achieved significantly higher efficacy in suppressing tumor growth compared to monotherapy[40,46]. Moreover, neddylation-mediated SHP2 inactivation in macrophages increased phagocytosis, thereby substantially enhancing the outcomes of CRC immunotherapy[50].
Ongoing clinical trials are evaluating SHP2 inhibitor-based combination therapies, such as TNO155 (NCT04294160, NCT04330664, NCT04699188) and RMC-4630 (NCT04916236), in molecularly stratified patients, aiming to advance precision oncology strategies for CRC (Table 1).
SHP2 is emerging as an oncogenic driver across malignancies, such as lung cancer, CRC, breast cancer, liver cancer, melanoma, pancreatic cancer and acute myeloid leukemia[20,41,51]. As SHP2 inhibitors (e.g., RMC-4630, TNO155) have begun to show clinical efficacy in diverse malignancies, SHP2 represents the first phosphatase-targeted agent achieving pan-cancer clinical validation[41]. The incidence and mortality of CRC remains high among solid tumors, making it a major threat to human health[1]. Currently, Food and Drug Administration-approved drugs for CRC primarily include immunotherapy drugs and agents targeting specific gene mutations such as KRAS (Table 2).SHP2 functions as a pivotal signaling hub within the CRC signaling network, exerting pleiotropic effects on multiple oncogenic pathways. This unique characteristic provides novel opportunities for precision oncology and unlocks innovative avenues to overcome resistance to conventional therapies[20,22,23,29]. However, despite their therapeutic potential, SHP2 inhibitors carry some challenges such as off-target effects and pharmacokinetic limitations, which collectively limit their therapeutic efficacy in patients[44,45]. SHP2-targeted therapies in CRC demonstrate a "double-edged sword" effect, with therapeutic efficacy highly dependent on specific mutational subtypes. Combination strategies (e.g., immunotherapy) and precision stratification are critical for future breakthroughs[40,46]. Currently, multiple clinical trials are underway, with results eagerly anticipated. Overall, SHP2-targeted therapies could emerge as a cornerstone of multimodal CRC treatment frameworks, providing synergistic effects with existing therapies to address tumor heterogeneity and adaptive resistance.
| Drug name | Target | Approval year | Current clinical use | Developer/company |
| Cetuximab | HER1 (EGFR/ErbB1) | 2004 | First-line therapy for KRAS wild-type CRC combined with chemotherapy | Bristol-Myers Squibb |
| Panitumumab | HER1 (EGFR/ErbB1) | 2006 | Preferred in EU/US | Takeda/Amgen |
| Regorafenib | KIT/PDGFRβ/RAF/RET | 2012 | Third-line therapy for refractory CRC, OS extended by 2.5 months | Bayer |
| Aflibercept | VEGFA/B | 2012 | Combined with FOLFIRI for second-line therapy in EU/US | Sanofi |
| Ramucirumab | VEGFR2 | 2014 | Primarily used in gastric cancer; limited CRC application | Eli Lilly |
| Bevacizumab | VEGFR | 2004 | Cornerstone agent combined with chemotherapy across lines | Genentech |
| Encorafenib | BRAFV600E | 2020 | Core drug in triple therapy for BRAF-mutant CRC | Bristol-Myers Squibb |
| Pembrolizumab | PD-1 | 2017 | First-line immunotherapy for MSI-H/dMMR CRC | Merck & Co. |
| Ipilimumab | CTLA-4 | 2011 | Combined with PD-1 inhibitors for MSI-H CRC | Bristol-Myers Squibb |
| Fruquintinib (FRUZAQLA) | VEGFR1/2/3 | 2023 | Previously treated metastatic CRC, regardless of biomarker status | Takeda/HUTCHMED |
| Trifluridine/tipiracil+bevacizumab | Thymidine analog + TP inhibitor + VEGF | 2023 | Metastatic CRC progressing after prior chemotherapy and anti-VEGF/EGFR therapies | Taiho Oncology |
| Encorafenib+cetuximab+mFOLFOX6 | BRAFV600E + EGFR | 2024 | Metastatic CRC with BRAFV600E mutation (accelerated approval) | Pfizer/Array BioPharma |
| Sotorasib + panitumumab | KRASG12C + EGFR | 2025 | KRASG12C-mutated metastatic CRC | Amgen |
| Nivolumab | PD-1 | 2024 | MSI-H/dMMR metastatic CRC progressing after fluoropyrimidine, oxaliplatin, and irinotecan | Bristol Myers Squibb |
| Nivolumab+ipilimumab | PD-1 + CTLA-4 | 2025 | First-line treatment for unresectable or metastatic MSI-H/dMMR CRC | Bristol Myers Squibb |
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 4712] [Article Influence: 4712.0] [Reference Citation Analysis (3)] |
| 2. | Zhong H, Jiang J, Hussain M, Zhang H, Chen L, Guan R. The Encapsulation Strategies for Targeted Delivery of Probiotics in Preventing and Treating Colorectal Cancer: A Review. Adv Sci (Weinh). 2025;12:e2500304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8170] [Article Influence: 8170.0] [Reference Citation Analysis (2)] |
| 4. | Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44:222-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 287] [Reference Citation Analysis (0)] |
| 5. | Rumpold H, Niedersüß-Beke D, Heiler C, Falch D, Wundsam HV, Metz-Gercek S, Piringer G, Thaler J. Prediction of mortality in metastatic colorectal cancer in a real-life population: a multicenter explorative analysis. BMC Cancer. 2020;20:1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1259] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 7. | Hubbard MJ, Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993;18:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 667] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Hunter T. The genesis of tyrosine phosphorylation. Cold Spring Harb Perspect Biol. 2014;6:a020644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Chen YN, LaMarche MJ, Chan HM, Fekkes P, Garcia-Fortanet J, Acker MG, Antonakos B, Chen CH, Chen Z, Cooke VG, Dobson JR, Deng Z, Fei F, Firestone B, Fodor M, Fridrich C, Gao H, Grunenfelder D, Hao HX, Jacob J, Ho S, Hsiao K, Kang ZB, Karki R, Kato M, Larrow J, La Bonte LR, Lenoir F, Liu G, Liu S, Majumdar D, Meyer MJ, Palermo M, Perez L, Pu M, Price E, Quinn C, Shakya S, Shultz MD, Slisz J, Venkatesan K, Wang P, Warmuth M, Williams S, Yang G, Yuan J, Zhang JH, Zhu P, Ramsey T, Keen NJ, Sellers WR, Stams T, Fortin PD. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 673] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 10. | Frankson R, Yu ZH, Bai Y, Li Q, Zhang RY, Zhang ZY. Therapeutic Targeting of Oncogenic Tyrosine Phosphatases. Cancer Res. 2017;77:5701-5705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 11. | Kim M, Baek M, Kim DJ. Protein Tyrosine Signaling and its Potential Therapeutic Implications in Carcinogenesis. Curr Pharm Des. 2017;23:4226-4246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109:862-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 292] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 13. | Barford D, Neel BG. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure. 1998;6:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 248] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Huang WQ, Lin Q, Zhuang X, Cai LL, Ruan RS, Lu ZX, Tzeng CM. Structure, function, and pathogenesis of SHP2 in developmental disorders and tumorigenesis. Curr Cancer Drug Targets. 2014;14:567-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Neel BG, Gu H, Pao L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 982] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 16. | Zhang J, Zhang F, Niu R. Functions of Shp2 in cancer. J Cell Mol Med. 2015;19:2075-2083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 17. | Wang Q, Li H, Mao Y, Garg A, Park ES, Wu Y, Chow A, Peregrin J, Zhang X. Shc1 cooperates with Frs2 and Shp2 to recruit Grb2 in FGF-induced lens development. bioRxiv. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Cai P, Guo W, Yuan H, Li Q, Wang W, Sun Y, Li X, Gu Y. Expression and clinical significance of tyrosine phosphatase SHP-2 in colon cancer. Biomed Pharmacother. 2014;68:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Wang H, Wang W, Xue Y, Aweya JJ, Yang X, Zhu Z. Functional STR within PTPN11: a novel potential risk factor for colorectal cancer. Int J Clin Exp Pathol. 2017;10:11710-11716. [PubMed] |
| 20. | Liu X, Li M, Chen L, Wen F, Zheng S, Ge W. High expression of SHP2 predicts a promising prognosis in colorectal cancer. Indian J Pathol Microbiol. 2024;67:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Gagné-Sansfaçon J, Coulombe G, Langlois MJ, Langlois A, Paquet M, Carrier J, Feng GS, Qu CK, Rivard N. SHP-2 phosphatase contributes to KRAS-driven intestinal oncogenesis but prevents colitis-associated cancer development. Oncotarget. 2016;7:65676-65695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Li Y, Yuan Y, Zhang F, Guo A, Cao F, Song M, Fu Y, Xu X, Shen H, Zheng S, Pan Y, Chang W. Therapeutic Suppression of FAK-AKT Signaling Overcomes Resistance to SHP2 Inhibition in Colorectal Carcinoma. Front Pharmacol. 2021;12:739501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Yu M, Xu C, Zhang H, Lun J, Wang L, Zhang G, Fang J. The tyrosine phosphatase SHP2 promotes proliferation and oxaliplatin resistance of colon cancer cells through AKT and ERK. Biochem Biophys Res Commun. 2021;563:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Chen J, Li W, Zhang C, Wen D, Jiao C. Tyrosine phosphatase SHP2 promoted the progression of CRC via modulating the PI3K/BRD4/TFEB signaling induced ferroptosis. Discov Oncol. 2024;15:793. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Wu X, Guan S, Lu Y, Xue J, Yu X, Zhang QI, Wang X, Li T. Macrophage-derived SHP-2 inhibits the metastasis of colorectal cancer via Tie2-PI3K signals. Oncol Res. 2023;31:125-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Sun M, Han Z, Luo Z, Ge L, Zhang X, Feng K, Zhang G, Xu F, Zhou H, Han H, Jiang W. PTPN11 is a potential biomarker for type 2 diabetes mellitus complicated with colorectal cancer. Sci Rep. 2024;14:25155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Fan H, Hu X, Cao F, Zhou L, Wen R, Shen H, Fu Y, Zhu X, Jia H, Liu Z, Wang G, Yu G, Chang W, Zhang W. WWP1 inhibition increases SHP2 inhibitor efficacy in colorectal cancer. NPJ Precis Oncol. 2024;8:144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Zhang XZ, Li G, Hu GY, Wang CL, Fang YQ, Li Y, Qi XJ, Duan L. Ferrocenyl-Substituted Curcumin Derivatives as Potential SHP-2 Inhibitors for Anticolorectal Cancer: Design, Synthesis and In Vitro Evaluation. ACS Omega. 2024;9:51701-51718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Huang Y, Wang J, Cao F, Jiang H, Li A, Li J, Qiu L, Shen H, Chang W, Zhou C, Pan Y, Lu Y. SHP2 associates with nuclear localization of STAT3: significance in progression and prognosis of colorectal cancer. Sci Rep. 2017;7:17597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Pan S, Deng Y, Fu J, Zhang Y, Zhang Z, Ru X, Qin X. TRIM52 promotes colorectal cancer cell proliferation through the STAT3 signaling. Cancer Cell Int. 2019;19:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Schulz-Heddergott R, Stark N, Edmunds SJ, Li J, Conradi LC, Bohnenberger H, Ceteci F, Greten FR, Dobbelstein M, Moll UM. Therapeutic Ablation of Gain-of-Function Mutant p53 in Colorectal Cancer Inhibits Stat3-Mediated Tumor Growth and Invasion. Cancer Cell. 2018;34:298-314.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 183] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 32. | Meng S, Gui Q, Xu Q, Lu K, Jiao X, Fan J, Ge B, Ke Y, Zhang S, Wu J, Wang C. Association of Shp2 with phosphorylated IL-22R1 is required for interleukin-22-induced MAP kinase activation. J Mol Cell Biol. 2010;2:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Morimoto Y, Yamashita N, Hirose H, Fushimi A, Haratake N, Daimon T, Bhattacharya A, Ahmad R, Suzuki Y, Takahashi H, Kufe DW. MUC1-C is necessary for SHP2 activation and BRAF inhibitor resistance in BRAF(V600E) mutant colorectal cancer. Cancer Lett. 2023;559:216116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Chen X, Zou F, Hu Z, Du G, Yu P, Wang W, Wang H, Ye L, Tian J. PCC0208023, a potent SHP2 allosteric inhibitor, imparts an antitumor effect against KRAS mutant colorectal cancer. Toxicol Appl Pharmacol. 2020;398:115019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Li Z, Xi J, Li B, Liu Y, Wang G, Yu B, Ma H, Li Z, Zhang Z. SHP-2-induced M2 polarization of tumor associated macrophages via IL-4 regulate colorectal cancer progression. Front Oncol. 2023;13:1027575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Wang S, Yao Y, Li H, Zheng G, Lu S, Chen W. Tumor-associated macrophages (TAMs) depend on Shp2 for their anti-tumor roles in colorectal cancer. Am J Cancer Res. 2019;9:1957-1969. [PubMed] |
| 37. | Xiao P, Zhang H, Zhang Y, Zheng M, Liu R, Zhao Y, Zhang X, Cheng H, Cao Q, Ke Y. Phosphatase Shp2 exacerbates intestinal inflammation by disrupting macrophage responsiveness to interleukin-10. J Exp Med. 2019;216:337-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 38. | Gao J, Wu Z, Zhao M, Zhang R, Li M, Sun D, Cheng H, Qi X, Shen Y, Xu Q, Chen H, Chen D, Sun Y. Allosteric inhibition reveals SHP2-mediated tumor immunosuppression in colon cancer by single-cell transcriptomics. Acta Pharm Sin B. 2022;12:149-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 39. | Liu W, Guo W, Shen L, Chen Z, Luo Q, Luo X, Feng G, Shu Y, Gu Y, Xu Q, Sun Y. T lymphocyte SHP2-deficiency triggers anti-tumor immunity to inhibit colitis-associated cancer in mice. Oncotarget. 2017;8:7586-7597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Zhao M, Guo W, Wu Y, Yang C, Zhong L, Deng G, Zhu Y, Liu W, Gu Y, Lu Y, Kong L, Meng X, Xu Q, Sun Y. SHP2 inhibition triggers anti-tumor immunity and synergizes with PD-1 blockade. Acta Pharm Sin B. 2019;9:304-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 41. | Song Y, Zhao M, Zhang H, Yu B. Double-edged roles of protein tyrosine phosphatase SHP2 in cancer and its inhibitors in clinical trials. Pharmacol Ther. 2022;230:107966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 42. | Song Z, Wang M, Ge Y, Chen XP, Xu Z, Sun Y, Xiong XF. Tyrosine phosphatase SHP2 inhibitors in tumor-targeted therapies. Acta Pharm Sin B. 2021;11:13-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 43. | LaMarche MJ, Acker M, Argintaru A, Bauer D, Boisclair J, Chan H, Chen CH, Chen YN, Chen Z, Deng Z, Dore M, Dunstan D, Fan J, Fekkes P, Firestone B, Fodor M, Garcia-Fortanet J, Fortin PD, Fridrich C, Giraldes J, Glick M, Grunenfelder D, Hao HX, Hentemann M, Ho S, Jouk A, Kang ZB, Karki R, Kato M, Keen N, Koenig R, LaBonte LR, Larrow J, Liu G, Liu S, Majumdar D, Mathieu S, Meyer MJ, Mohseni M, Ntaganda R, Palermo M, Perez L, Pu M, Ramsey T, Reilly J, Sarver P, Sellers WR, Sendzik M, Shultz MD, Slisz J, Slocum K, Smith T, Spence S, Stams T, Straub C, Tamez V Jr, Toure BB, Towler C, Wang P, Wang H, Williams SL, Yang F, Yu B, Zhang JH, Zhu S. Identification of TNO155, an Allosteric SHP2 Inhibitor for the Treatment of Cancer. J Med Chem. 2020;63:13578-13594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 44. | Han X, Wang W, Wang R, Zhang W, Zhu L, Xu Q, Guo W, Gu Y. Allosteric SHP2 inhibition enhances regorafenib's effectiveness in colorectal cancer treatment. Biochem Biophys Res Commun. 2024;709:149812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 45. | Wang K, Zhang X, Hu Y, Guo J, Shen G, Zhang K, Jiang S, Wang T. Discovery of novel phenyl urea SHP2 inhibitors with anti-colon cancer and potential immunomodulatory effects. Eur J Med Chem. 2025;281:117036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Wang Y, Mohseni M, Grauel A, Diez JE, Guan W, Liang S, Choi JE, Pu M, Chen D, Laszewski T, Schwartz S, Gu J, Mansur L, Burks T, Brodeur L, Velazquez R, Kovats S, Pant B, Buruzula G, Deng E, Chen JT, Sari-Sarraf F, Dornelas C, Varadarajan M, Yu H, Liu C, Lim J, Hao HX, Jiang X, Malamas A, LaMarche MJ, Geyer FC, McLaughlin M, Costa C, Wagner J, Ruddy D, Jayaraman P, Kirkpatrick ND, Zhang P, Iartchouk O, Aardalen K, Cremasco V, Dranoff G, Engelman JA, Silver S, Wang H, Hastings WD, Goldoni S. SHP2 blockade enhances anti-tumor immunity via tumor cell intrinsic and extrinsic mechanisms. Sci Rep. 2021;11:1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | Zhang L, Hu X, Meng Q, Li Y, Shen H, Fu Y, Zhang F, Chen J, Zhang W, Chang W, Pan Y. SHP2 inhibition improves celastrol-induced growth suppression of colorectal cancer. Front Pharmacol. 2022;13:929087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Liu C, Lu H, Wang H, Loo A, Zhang X, Yang G, Kowal C, Delach S, Wang Y, Goldoni S, Hastings WD, Wong K, Gao H, Meyer MJ, Moody SE, LaMarche MJ, Engelman JA, Williams JA, Hammerman PS, Abrams TJ, Mohseni M, Caponigro G, Hao HX. Combinations with Allosteric SHP2 Inhibitor TNO155 to Block Receptor Tyrosine Kinase Signaling. Clin Cancer Res. 2021;27:342-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 49. | Ryan MB, Fece de la Cruz F, Phat S, Myers DT, Wong E, Shahzade HA, Hong CB, Corcoran RB. Vertical Pathway Inhibition Overcomes Adaptive Feedback Resistance to KRAS(G12C) Inhibition. Clin Cancer Res. 2020;26:1633-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 324] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 50. | Li Y, Zhou H, Liu P, Lv D, Shi Y, Tang B, Xu J, Zhong T, Xu W, Zhang J, Zhou J, Ying K, Zhao Y, Sun Y, Jiang Z, Cheng H, Zhang X, Ke Y. SHP2 deneddylation mediates tumor immunosuppression in colon cancer via the CD47/SIRPα axis. J Clin Invest. 2023;133:e162870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 51. | Wang Y, Qiao X, Zhu R, Zhou L, Zhang Q, Lu S, Chai Z. Computational Elucidation of a Monobody Targeting the Phosphatase Domain of SHP2. Biomolecules. 2025;15:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |