Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.104860
Revised: April 24, 2025
Accepted: June 6, 2025
Published online: July 15, 2025
Processing time: 191 Days and 22.3 Hours
Gastrointestinal cancers are among the most commonly diagnosed cancers globally. Traditional Chinese medicine (TCM) offers distinct advantages in preventing and treating these cancers.
To investigate the metabolic basis of a common TCM syndrome in gastrointestinal cancers, exploring underlying metabolic mechanisms and identifying potential biomarkers.
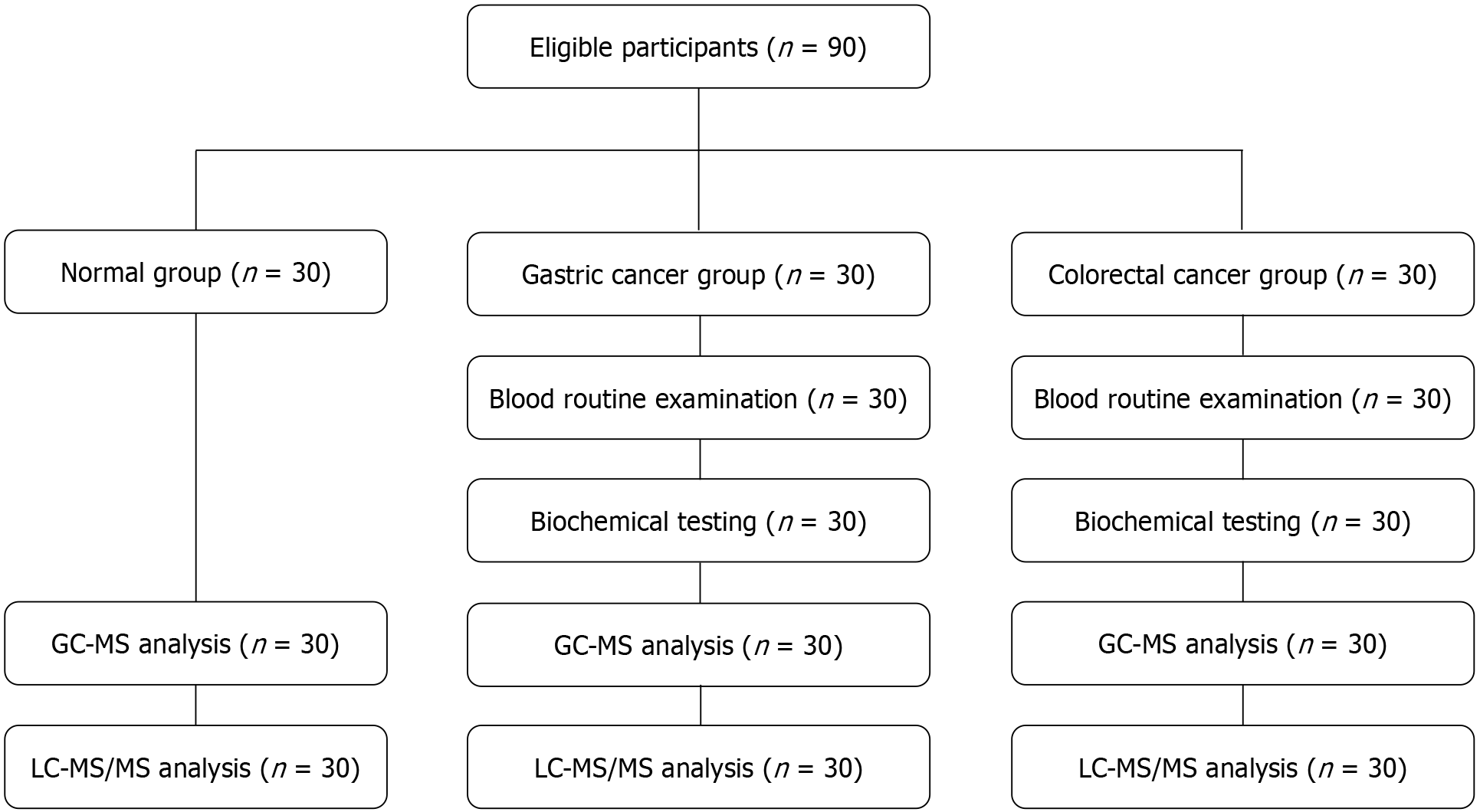
Thirty healthy controls (normal group), 30 patients with gastric cancer (GC), and 30 patients with colorectal cancer (CRC) were enrolled in 2023. Plasma metabolic profiles were detected using gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry, and pathway enrichment analysis was conducted using the Kyoto Encyclopedia of Genes and Genomes.
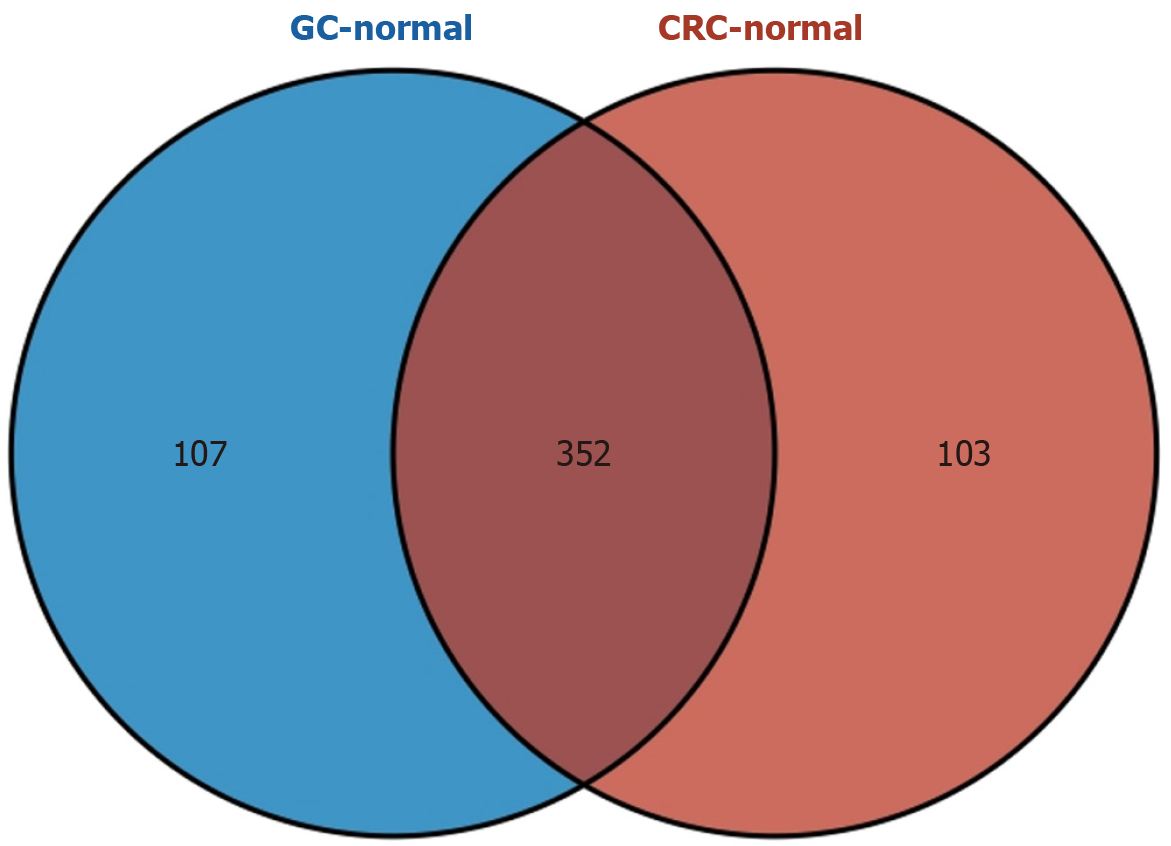
Metabolic profiling revealed distinct alterations in gastrointestinal cancers. CRC samples exhibited 455 differentially expressed metabolites (234 upregulated and 221 downregulated). Similarly, GC samples exhibited 459 differentially expressed metabolites (251 upregulated and 208 downregulated). Additionally, 352 shared metabolites were identified among gastrointestinal cancers. Enrichment analysis highlighted the involvement of these shared metabolites in 10 metabolic pathways.
To some extent, this study revealed the metabolomic characteristics of spleen deficiency and blood stasis toxin (PXYD) syndrome in gastrointestinal cancers. It provides the rationale for the "same treatment for different diseases" approach in PXYD syndrome of gastrointestinal cancers, and for identifying potential metabolomics-based biomarkers.
Core Tip: This study identified differentially expressed metabolites in colorectal cancer and gastric cancer, unraveling the metabolomic characteristics of spleen deficiency and blood stasis toxin (PXYD) syndrome in gastrointestinal cancers. It preliminarily identified 352 common metabolites and 10 metabolic pathways in gastrointestinal cancers, providing a theoretical basis for the "same treatment for different diseases" approach in PXYD syndrome of gastrointestinal cancers.
- Citation: Guo TH, Zhu WJ, Hui YF, Zhao SQ, Zhou TT, Wang XM, Zhang QC, Wang W, Li L, Shen WX, Wu XY, Cheng HB. Associations between blood metabolite levels and gastrointestinal cancer risk: A preliminary untargeted metabolomics study. World J Gastrointest Oncol 2025; 17(7): 104860
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/104860.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.104860
Gastrointestinal cancers are a leading type of cancer globally, with high incidence and mortality rates[1]. In 2020, more than one million new cases of gastric cancer (GC) and approximately 769000 GC-related deaths were recorded globally[1]. In the same year, over 1.9 million new cases of colorectal cancer (CRC) and 935000 CRC-related deaths were recorded, representing approximately 10% of all cancer cases and deaths[1]. The incidence rate of gastrointestinal cancers has progressively increased[2], particularly in China[3,4]. This trend poses a significant challenge for prevention and treatment, threatening patients' health and survival[5].
Traditional Chinese medicine (TCM) has demonstrated distinct benefits in cancer prevention and treatment[6], including gastrointestinal cancers, either as a standalone treatment[7] or in conjunction with Western therapies[8]. Its appeal among individuals of Chinese descent[9] lies in its potential to extend survival time without the side effects typically associated with Western medical therapies[10]. Treatment based on syndrome differentiation is a fundamental aspect of the TCM's theoretical framework[11]. A TCM syndrome captures the distinctive pathophysiological aspects, patterns, and characteristics of a disease at a specific stage, informing treatment strategies. Similar to modern precision medicine, this approach involves tailoring treatments to individual disease manifestations. The concept of "treating different conditions with the same method" is illustrated by pembrolizumab's Food and Drug Administration approval for treating MSI-H/dMMR solid tumors, regardless of their origin[12]. This parallels the TCM principle of "homotherapy for heteropathy", where different conditions receive similar treatment based on shared underlying patterns. The theory of cancerous toxins as a core concept in cancer pathogenesis is widely acknowledged by experts in TCM oncology and serves as a foundation for TCM-based cancer treatments[13]. The pathogenic characteristics of cancer toxins can explain the clinical features of tumors. According to this theory, spleen deficiency and blood stasis toxin (PXYD) syndrome is a common TCM syndrome in GC and CRC. Therefore, further study of the biological basis of this TCM syndrome is warranted.
Metabolomics has increasingly been used to explore the mechanisms underlying malignant tumors[14]. This approach allows for exploring the biological characteristics of different syndromes in gastrointestinal malignancies[15]. Previous studies have identified correlations between specific metabolites and TCM syndromes. For example, creatinine and aminomalonic acid alterations have been linked to dampness and heat syndrome (DHS), while D-tryptophan exhibited a potential association with spleen deficiency syndrome (SDS). Additionally, D-galactose and 1,2,3-propanetricarboxylic acid have been linked to liver and kidney Yin deficiency[16]. By analyzing metabolite variations, researchers can elucidate their relationships with physiology and pathology, identify biomarkers for TCM syndromes, and uncover the biological connotations of these syndromes.
This study aimed to identify differentially expressed metabolic products in GC and CRC. Additionally, it sought to investigate the underlying metabolic mechanisms of gastrointestinal cancers and explore the metabolic basis of the PXYD syndrome, a common TCM syndrome. To achieve this objective, untargeted metabolomics analysis was performed using gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS).
Given the challenges of sampling from clinical samples, the sample size for the preliminary untargeted metabolomics analysis was determined after consultation with a methodology expert and a technician from OE Biotech Co. Ltd. (Shanghai, China), and a thorough evaluation of various factors. The sample size estimation was based on previous research and statistical methods[14,17]. The study included 30 healthy controls (normal), 30 patients with GC, and 30 patients with CRC, with all subjects providing written informed consent before enrollment (Figure 1).
This study employed the criteria outlined in the World Health Organization's classification of digestive system tumors for diagnosing GC and CRC[18].
The diagnostic criteria for PXYD syndrome were based on the Guiding Principles for the Clinical Study of New Drugs for Use in TCM[19]. The criteria included primary symptoms such as abdominal pain, abdominal distension, and sloppy stool, as well as secondary symptoms like weakness, inappetence, a sallow or gloomy complexion, dark purple face and lips, and scaly dry skin. The tongue characteristics include pallor, dark purple color, or presence of petechiae, with a thin, white coating. The pulse characteristics include wiry, thready, or deep pulse.
A diagnosis of PXYD syndrome requires the presence of at least one primary symptom, one or more secondary symptoms, and consistent tongue and pulse conditions. The diagnosis was confirmed through consensus among three physicians-in-charge. In cases of uncertainty regarding syndrome differentiation, a consensus diagnosis was established through multidisciplinary review.
Patients with GC and CRC meeting the criteria of both Western medicine and TCM syndrome were eligible for inclusion in this study. The inclusion criteria were as follows: (1) Aged between 18 and 90 years; (2) Newly diagnosed with primary GC and CRC through histopathological examination; (3) Exhibited stable vital signs, clear consciousness, and adequate communication skills; and (4) Provided voluntary written informed consent before enrollment[17].
Patients were excluded if they had a history of malignant tumors in other organs, had received prior tumor-directed therapy such as chemotherapy or radiotherapy, or had severe dysfunction of vital organs like the heart, lungs, liver, or kidneys. This included conditions like heart failure, pulmonary insufficiency, or significantly elevated liver and kidney function indices [alanine aminotransferase (ALT) and aspartate aminotransferase (AST) > 2 × upper limit of normal, or blood creatinine and urea nitrogen > 1.5 × upper limit of normal][14].
For GC-MS analysis, serum samples from all 90 participants, stored at -80 °C, were passively thawed to room temperature. A 150 μL aliquot of each sample was mixed with 450 μL of a precooled methanol-acetonitrile solution (2:1, v/v) containing 2 μg/mL L-2-chlorophenylalanine (C2001, Shanghai Hengchuang Biotechnology) in a 1.5 mL Eppendorf tube. The methanol and acetonitrile used were sourced from Fisher (A452-4 and A998-4, respectively). After vortexing for 1 minute and ultrasonic extraction for 10 minutes in an ice-water bath, the samples were stored at -20 °C for 30 minutes. Centrifugation followed at 13780 × g (12000 rpm) for 10 minutes at 4 °C. The supernatant (150 μL) was then transferred to a glass vial and dried under vacuum at room temperature. Derivatization was carried out in two steps. First, 80 μL of methoxylamine hydrochloride in pyridine (15 mg/mL) was added, followed by incubation at 37 °C for 90 minutes after vigorous vortexing. Next, 50 μL of BSTFA (B0830-25 mL, TCI) containing 1% TMCS (T6381-10AMP, Sigma) and 20 μL of n-hexane (4.011518.0500, CNW) were added, followed by a 2-minute vortexing step and incubation at 70 °C for 1 hour. After 30 minutes at ambient temperature, the samples underwent GC-MS analysis. Pooled quality control (QC) samples were generated by combining equal aliquots of all individual samples.
For LC-MS analysis, samples stored at -80 °C were passively thawed at room temperature. Each 150 μL sample was mixed with 450 μL of a precooled methanol-acetonitrile solution (2:1, v/v) containing 2 μg/mL L-2-Chlorophenylalanine (C2001, Shanghai Hengchuang Biotechnology) in a 1.5 mL Eppendorf tube. Following a 1-minute vortexing step, samples were subjected to a 10-minute ultrasonic extraction in an ice-cold water bath. The samples were then briefly stored at -40 °C for 10 minutes. Next, the extracts underwent centrifugation at 13780 × g (12000 rpm) for 10 minutes at 4 °C. The supernatants (150 μL) were collected using crystal syringes, filtered through 0.22 μm microfilters, and transferred to LC vials for storage at -80 °C prior to further analysis. To prepare QC samples, equal aliquots from each individual sample were pooled.
Analysis of metabolomic data was outsourced to Shanghai Luming Biological Technology Co., Ltd. The analysis utilized an Agilent 7890B gas chromatography system coupled with an Agilent 5977A MSD system (7890B-5977A, Agilent Technologies Inc.). Separation of derivatives was achieved on a DB-5MS fused-silica capillary column (30 m × 0.25 mm × 0.25 μm; Agilent J & W Scientific) with helium (> 99.999%) as the carrier gas at a constant flow rate of 1 mL/minute. The injector temperature was maintained at 260 °C, and 1 μL of sample was injected in splitless mode. The oven temperature was initially set at 60 °C for 0.5 minute, then ramped to 125 °C at 8 °C/minute, to 210 °C at 8 °C/minute, to 270 °C at 15 °C/minute, and finally to 305 °C at 20 °C/minute, where it was held for 5 minutes. The MS quadrupole and ion source temperatures (electron impact) were set to 150 °C and 230 °C, respectively, with a 70 eV collision energy. Mass spectrometry data acquisition was performed in full-scan mode (m/z 50-500) with a solvent delay of 5 minutes.
The analysis utilized an ACQUITY UPLC I-Class Plus (Waters Corporation) coupled with a Q-Exactive MS equipped with a heated electrospray ionization source (Thermo Fisher Scientific, Inc.), operating in both positive and negative ion modes. Chromatographic separation was performed on an ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm, Waters Corporation) using a binary gradient elution system consisting of solution A [water with 0.1% formic acid (A117-50, Fisher), v/v] and solution B [acetonitrile (A998-4, Fisher) with 0.1% formic acid (A117-50, Fisher), v/v]. The gradient elution profile was as follows: 0-2 minutes, 5% B; 4 minutes, 30% B; 8 minutes, 50% B; 10 minutes, 80% B; 14-15 minutes, 100% B; 15.1-16 minutes, 5% B. Solution A was used for the rest of solution B. The analysis was performed at a flow rate of 0.35 mL/minutes and a column temperature of 45 °C, with samples kept at 4 °C. The injection volume was 3 μL.
Mass spectrometry settings included a mass range of m/z 100-1000, resolution of 70000 for full MS scans and 17500 for Higher Collisional Dissociation MS/MS scans, and collision energies of 10, 20, and 40 eV. The ionization settings included spray voltages of 3800 V (positive mode) and 3200 V (negative mode), with sheath and auxiliary gas flow rates set to 35 and 8 arbitrary units, respectively. Additionally, the capillary temperature was maintained at 320 °C, the auxiliary gas heater at 350 °C, and the S-lens RF level at 50.
Raw GC-MS data (.D format) were converted to .abf format using Analysis Base File Converter software 4.0.0 to facilitate quick data retrieval. The converted data were then analyzed with MS-DIAL software (version 4.24), which performed a range of preprocessing steps, including peak detection, identification, MS2Dec deconvolution, peak alignment, wave filtering, and imputation of missing values. Metabolite annotation was conducted using the LUG database, an untargeted GC-MS database developed by Lumingbio. The resulting data matrix included detailed information such as sample metadata, peak identification, retention times, retention indices, m/z ratios, and signal intensities. To ensure data quality, peak signal intensities were normalized using internal standards with stringent QC criteria (relative standard deviation < 0.1). Subsequently, redundant data points were removed, and peaks were merged to generate a refined data matrix suitable for further analysis.
Initial LC-MS data processing was performed using Progenesis QI version 2.3 (Nonlinear Dynamics), which involved baseline filtering, peak detection, integration, retention time correction, peak alignment, and normalization. Key parameters included a 5 ppm precursor tolerance, 10 ppm product tolerance, and a 5% product-ion threshold, with retention times set to ± 0.3. Compound annotation was achieved by matching m/z ratios, secondary fragments, and isotopic distributions against multiple databases, including the Human Metabolome Database (https://hmdb.ca/), Lipidmaps (version 2.3) (https://www.lipidmaps.org/), Metlin (https://metlin.scripps.edu/Landing_page.php?pgcontent=mainPage), and proprietary databases. The data refinement process consisted of three steps: Eliminating peaks with high rates of missing values (ion intensity = 0), imputing zero values with half of the smallest detected value, and applying a filter based on compound identification scores. A scoring system was applied, allocating 20 points each for accurate molecular weight matching in primary mass spectrometry, fragment matching in secondary mass spectrometry, isotope distribution matching, and retention time matching, with a maximum score of 80. Higher scores in qualitative analysis indicate greater accuracy. Fragment matching scores were calculated out of 100 based on the alignment of observed second-level fragments with theoretical fragments in the database. Compounds with a fragmentation score below 36 were deemed inaccurate and excluded from the analysis. A combined data matrix of positive and negative ion data was generated for further analysis.
Principal Component Analysis (PCA) was performed using R 3.6.2 to evaluate sample distribution and analytical stability. Partial Least Squares Discriminant Analysis (PLS-DA) and Orthogonal PLS-DA (OPLS-DA) were applied to identify metabolites that varied between groups, with model robustness assessed via 7-fold cross-validation and 200 Response Permutation Tests (RPT). Variable Importance of Projection (VIP) values from the OPLS-DA model ranked metabolites by their contribution to group differences.
Differentially expressed metabolites were defined as those with a VIP value > 1.0 and a statistically significant difference (P < 0.05) based on a two-tailed Student's t-test. To identify key metabolic pathways, differentially expressed metabolites were analyzed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/).
The study cohort included 30 GC patients with PXYD syndrome, 30 CRC patients with PXYD syndrome, and 30 healthy controls (normal group). Demographic and clinical characteristics are summarized in Table 1. There were no significant differences between the GC and CRC groups in terms of certain demographic characteristics (sex, age, height), blood indices (red blood cell count, hemoglobin, platelet count), liver and kidney function indices (AST, ALT, serum creatinine, blood urea nitrogen), and cancer markers [CA-199, α-fetoprotein (AFP), CA-125, and CA-153]. However, notable differences were observed in weight, body mass index, white blood cell count, neutrophil count, and carcinoembryonic antigen levels, potentially reflecting distinct characteristics of each cancer group.
| Parameters | GC (n = 30) | CRC (n = 30) | P value |
| Sex (M/F) | 25/5 | 18/12 | 0.084 |
| Age (year) | 65.667 ± 9.586 | 67.533 ± 10.569 | 0.477 |
| Height (cm) | 167.200 ± 8.109 | 165.300 ± 8.619 | 0.398 |
| Weight (kg) | 68.333 ± 10.516 | 62.983 ± 10.185 | 0.026 |
| BMI (kg/m2) | 24.398 ± 2.943 | 23.074 ± 3.465 | 0.049 |
| RBC (1012/L) | 4.102 ± 0.573 | 4.082 ± 0.572 | 0.895 |
| hemoglobin (g/L) | 119.367 ± 22.890 | 114.533 ± 27.860 | 0.466 |
| WBC (109/L) | 5.622 ± 1.448 | 6.548 ± 1.345 | 0.013 |
| NEU (109/L) | 3.351 ± 1.047 | 4.349 ± 1.278 | 0.001 |
| platelet (109/L) | 216.333 ± 63.992 | 253.900 ± 97.899 | 0.255 |
| AST (U/L) | 17.600 ± 4.394 | 19.400 ± 18.377 | 0.170 |
| ALT (U/L) | 15.867 ± 7.930 | 14.267 ± 9.089 | 0.139 |
| Scr (μmol/L) | 74.353 ± 14.962 | 104.697 ± 167.425 | 0.756 |
| BUN (mmol/L) | 5.452 ± 1.495 | 6.018 ± 2.399 | 0.595 |
| CEA (ng/mL) | 14.505 ± 48.041 | 30.936 ± 67.325 | 0.000 |
| CA-199 (U/mL) | 128.862 ± 337.614 | 45.717 ± 161.795 | 0.935 |
| AFP (ng/mL) | 11.253 ± 41.291 | 2.740 ± 1.061 | 0.234 |
| CA-125 (U/mL) | 12.204 ± 8.790 | 16.466 ± 16.454 | 0.511 |
| CA-153 (U/mL) | 7.557 ± 3.163 | 8.619 ± 5.258 | 0.610 |
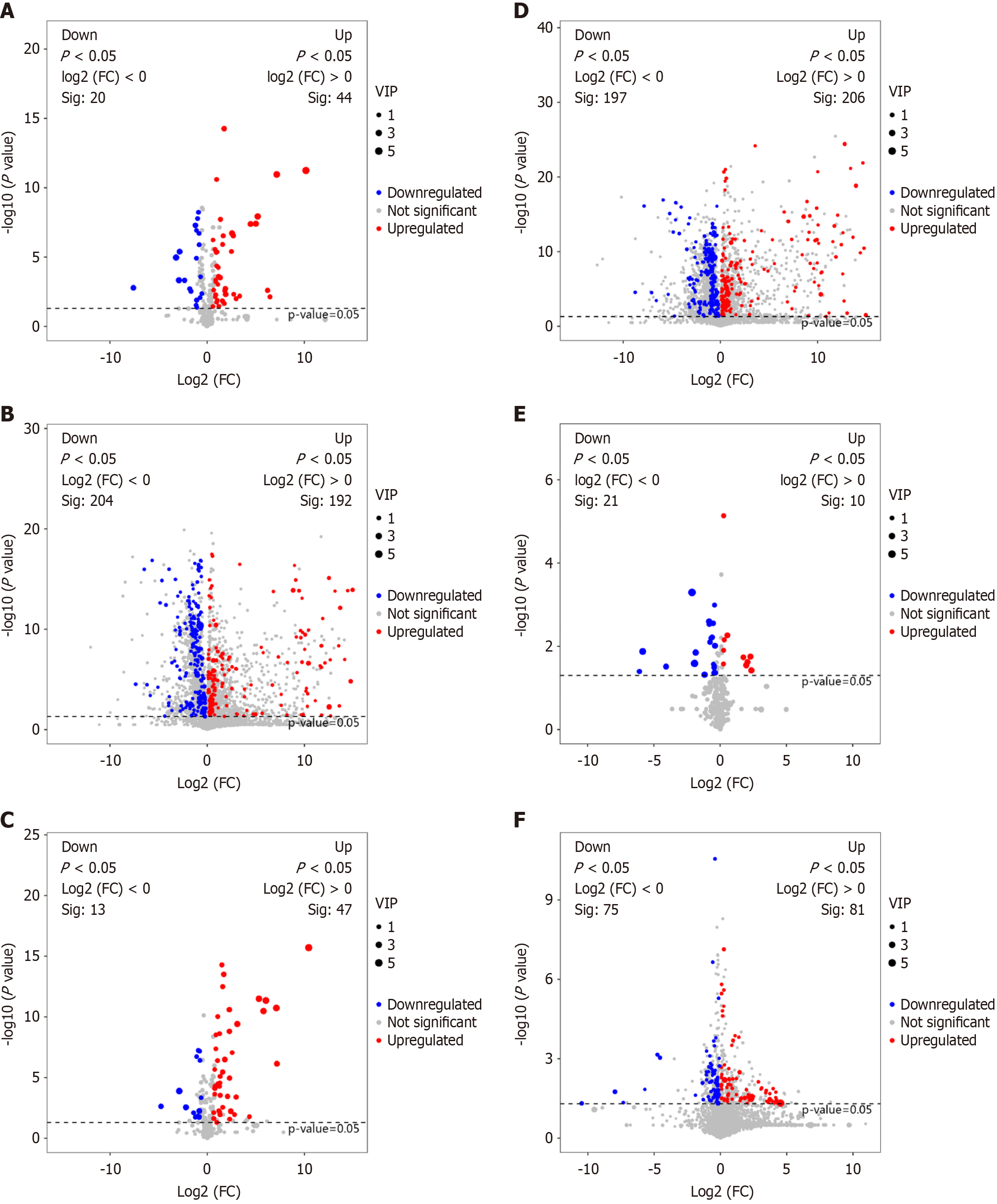
PCA and PLS-DA of the differentially expressed metabolites (P < 0.05) showed clear separations between CRC and normal samples, as well as between GC and normal samples (Supplementary Figure 1A-D). The models demonstrated good predictive performance, with Q2 (cum) and R2 (cum) values exceeding 0.5 (Supplementary Figure 2). Screening of differentially expressed metabolites in CRC, GC, and normal samples was performed (Figure 2), and the results are listed in Table 2.
| Groups | Differential metabolites number | Upregulated metabolites number | Downregulated metabolites number |
| A: CRC vs normal | 455 | 234 | 221 |
| B: GC vs normal | 459 | 251 | 208 |
| C: CRC vs GC | 187 | 91 | 96 |
CRC-associated metabolites included LysoPC [0:0/18:2 (9Z,12Z)], Floridin, PC [20:5 (6E,8Z,11Z,14Z,17Z)-OH(5)/P-18:1 (11Z)], Carissanol, LysoPC [20:4 (5Z,8Z,11Z,14Z)/0:0], L-Acetylcarnitine, Sphingosine 1-phosphate, L-tryptophan, D-galactose, and Polyglycerol esters of fatty acids, among others (Figure 3A and Supplementary Table 1). Previous studies have demonstrated a potential association of D-tryptophan, D-galactose, and fatty acid metabolites with TCM syndrome[16,20].
Notably, GC-associated metabolites included Cresol Glucuronide, Equilenin, Lactulose, 3-(Pyrazol-1-Yl)-L-Alanine, 2,6-Pyridinedicarboxylic Acid, Maltotriose, Cis-Aconitic Acid, Sphingosine 1-phosphate, L-tryptophan, and D-galactose (Figure 3B and Supplementary Table 1). D-tryptophan and D-galactose exhibited a potential association with TCM syndrome in a previous study[16].
Differentially expressed metabolites between CRC and GC included GM4 (d18:1/16:0), dihydrogen phosphate, 3,5-Dihydroxytetradecanoylcarnitine, Maltitol, and L-Erythrulose (Figure 3C and Supplementary Table 1).
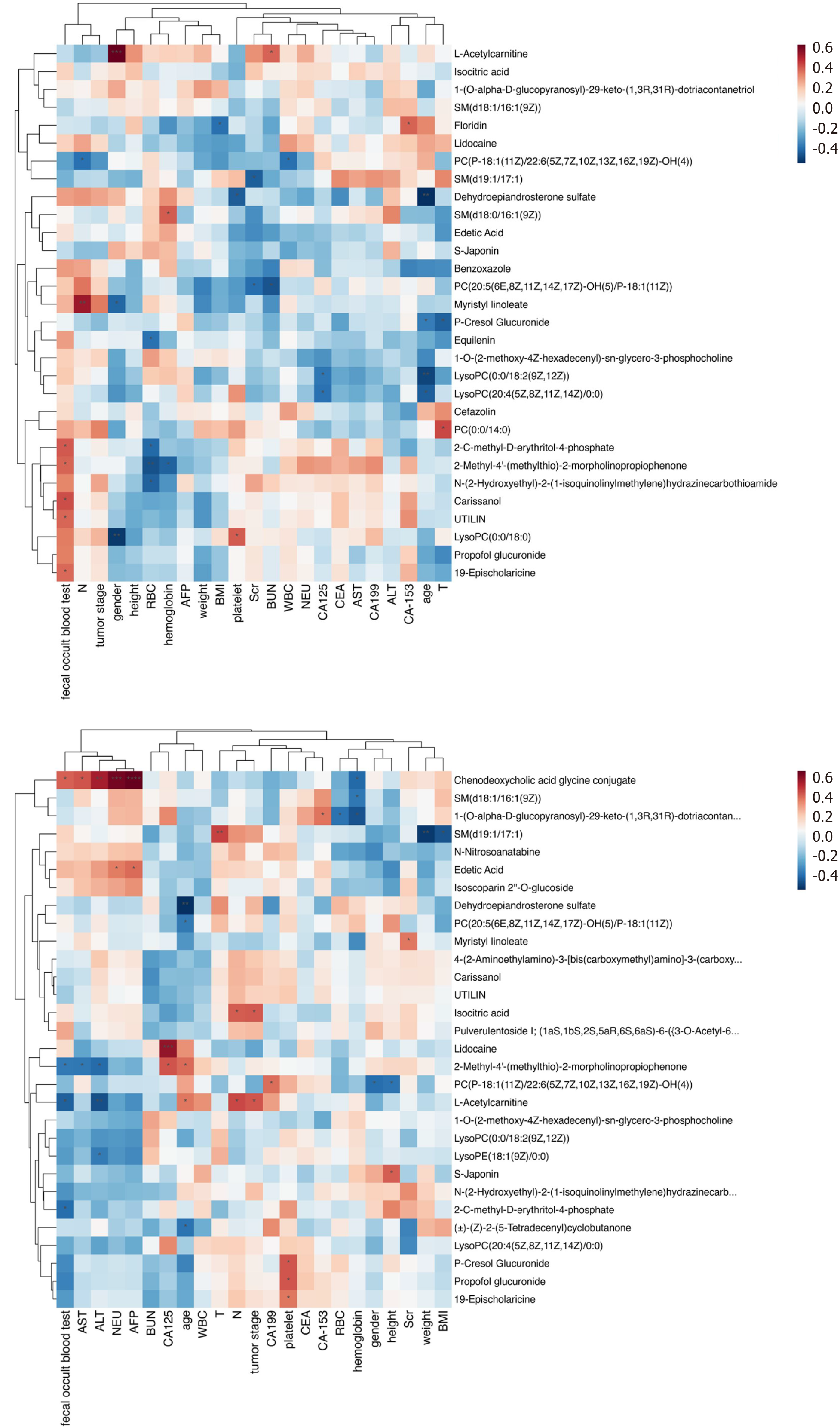
Correlation analysis revealed associations between differentially expressed metabolites and various clinical parameters in GC and CRC patients. These parameters included age, height, weight, body mass index, red blood cell, hemoglobin, white blood cell count, neutrophil count, platelet count, AST, ALT, serum creatinine, blood urea nitrogen, carcinoembryonic antigen, CA-199, AFP, CA-125, and CA-153 (Figure 4 and Supplementary Table 2).
Comparison of CRC and GC samples revealed no significant difference in metabolite expression profiles, as shown by PCA and PLS-DA analyses (Supplementary Figure 1E and F). VENN diagram analysis identified 352 common metabolites between the two cancer types, including P-Cresol Glucuronide, Equilenin, Lactulose, 3-(Pyrazol-1-Yl)-L-Alanine, 2,6-Pyridinedicarboxylic Acid, Maltotriose, Cis-Aconitic Acid, Lactitol, Sphingosine 1-phosphate, L-tryptophan, and D-galactose (Figure 5 and Supplementary Table 3). Notably, some of the shared metabolites, such as D-tryptophan and D-galactose, have been previously linked to TCM syndromes[16].
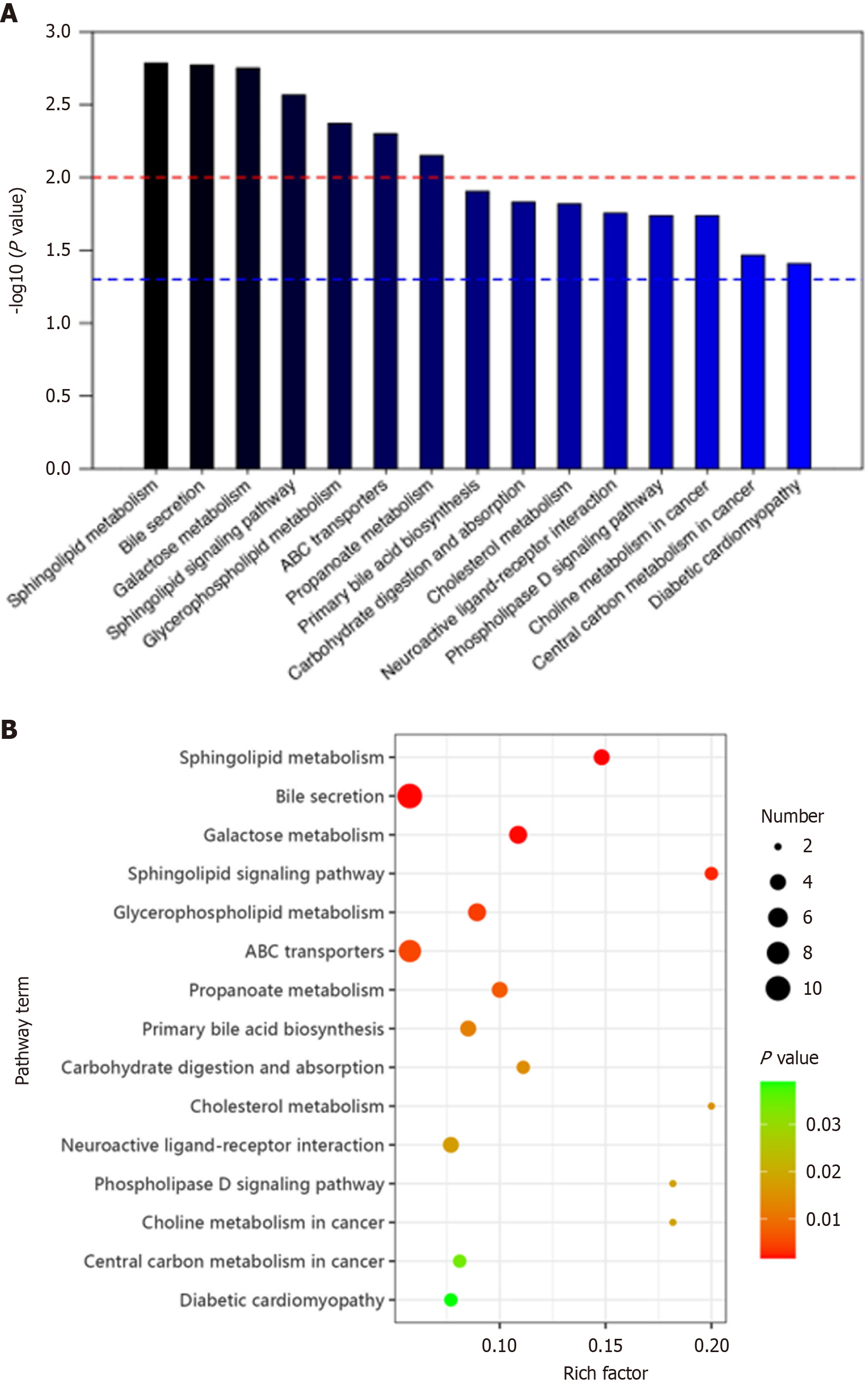
Differentially expressed metabolites between CRC and normal groups were involved in 15 metabolic pathways. These included sphingolipid metabolism, bile secretion, galactose metabolism, sphingolipid signaling pathway, glycerophospholipid metabolism, ABC transporters, propanoate metabolism, primary bile acid biosynthesis, carbohydrate digestion and absorption, cholesterol metabolism, neuroactive ligand-receptor interaction, phospholipase D signaling pathway, choline metabolism in cancer, central carbon metabolism in cancer, and diabetic cardiomyopathy (Figure 6).
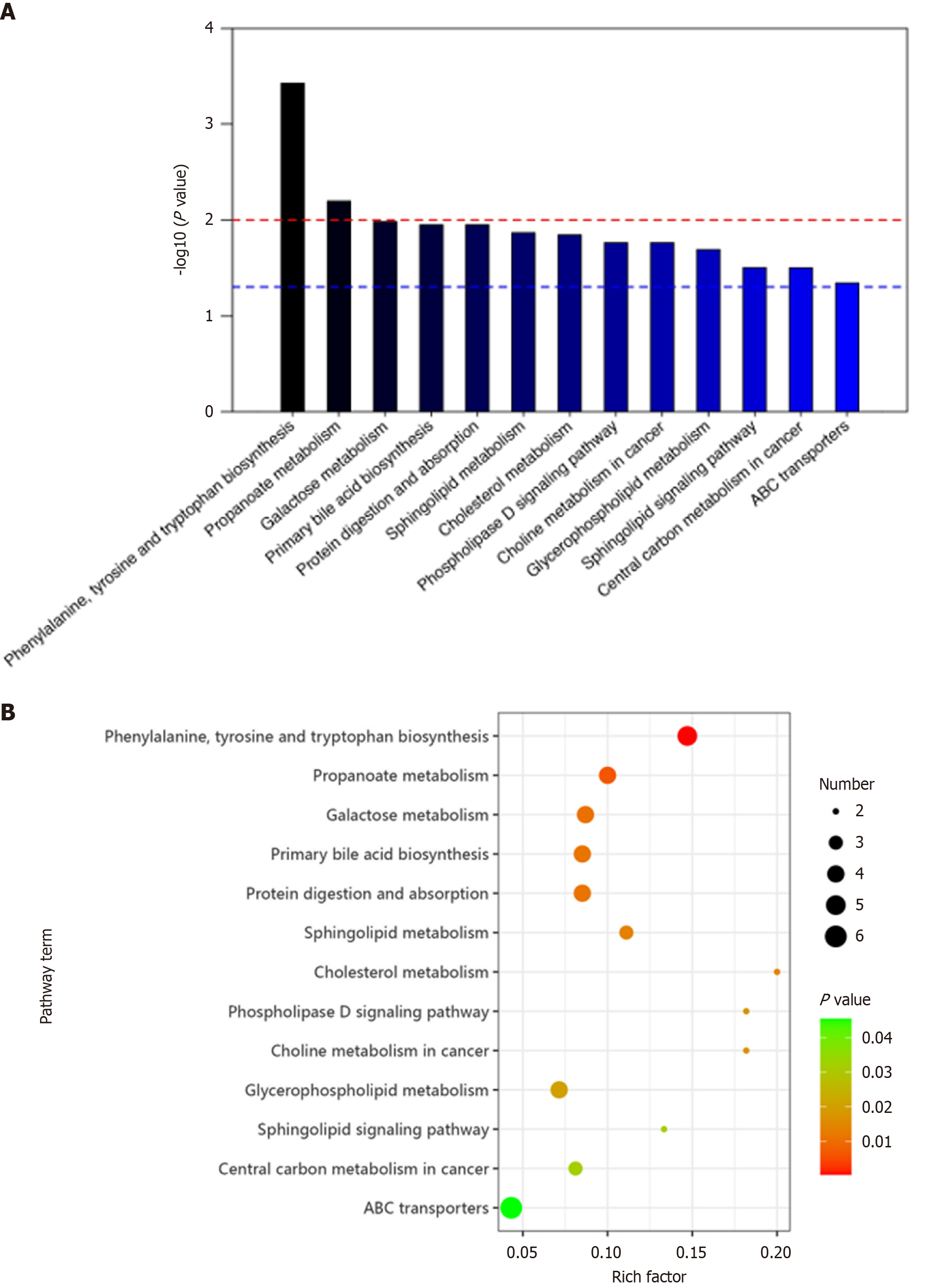
In GC vs normal groups, differentially expressed metabolites were involved in 13 metabolic pathways. These included amino acid biosynthesis (phenylalanine, tyrosine and tryptophan), propanoate metabolism, galactose metabolism, primary bile acid biosynthesis, protein digestion and absorption, sphingolipid metabolism, cholesterol metabolism, phospholipase D signaling pathway, choline metabolism in cancer, glycerophospholipid metabolism, sphingolipid signaling pathway, central carbon metabolism in cancer, and ABC transporters (Figure 7).
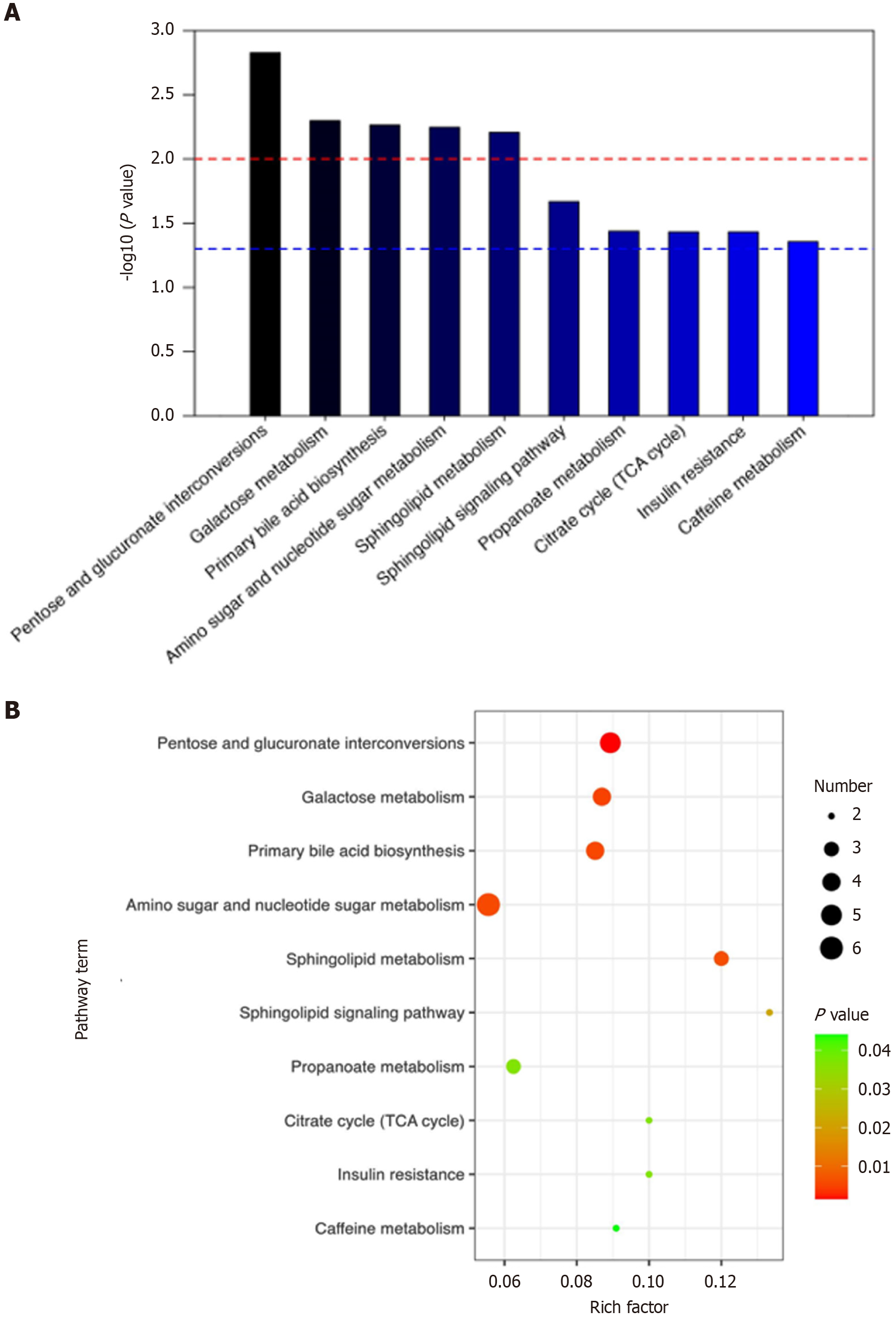
Pathway enrichment analysis of the 352 metabolites common to both CRC and GC revealed their involvement in 10 metabolic pathways: Pentose and glucuronate interconversion, galactose metabolism, primary bile acid biosynthesis, amino sugar and nucleotide sugar metabolism, sphingolipid metabolism, sphingolipid signaling pathway, propanoate metabolism, TCA cycle, insulin resistance, and caffeine metabolism (Figure 8).
Previous studies have demonstrated a potential association of galactose metabolism[16], sphingolipid metabolism[21], sphingolipid signaling pathway[22], and the TCA cycle[21] with TCM syndrome.
Gastrointestinal cancers are associated with alterations in the expression of various metabolites[23], including tryptophan[24], bile acids[24], choline metabolism[24], leucylalanine[25], serotonin[25], imidazole propionate[25], perfluorooctane sulfonate[25], glutamine[26], lipid metabolism[27], cholesterol[28], and urea[29]. Additionally, studies using Mendelian randomization have shown that patients with metastatic CRC with mutated-RAS/BRAF exhibit distinct metabolite profiles characterized by alterations in ascorbate and aldarate metabolism, pentose and glucuronate interconversion, steroid hormone biosynthesis, and bile secretion[30]. Recent studies have investigated the association of archaeal proteins involved in the biosynthesis of leucine and galactose metabolism in healthy individuals[31]. A retrospective single-center study found that pediatric patients with CRC exhibited enrichment of proteins associated with the lysine degradation pathway[32]. Bile acid metabolism has been implicated in CRC development, particularly after cholecystectomy[33]. A comparative analysis revealed distinct KEGG pathway profiles in CRC patients compared to younger and older healthy individuals, highlighting differences in metabolic processes such as ABC transporters, amino sugar metabolism, arginine and proline regulation, and aminoacyl-tRNA biosynthesis[34]. Multi-omics data identified a 3-sphingolipids metabolism gene signature that regulates GC progression by modulating cell cycle and apoptotic pathways, and its overexpression altered immune cell infiltration in the tumor microenvironment[35]. In distal GC, various metabolites are involved in multiple pathways, including sphingolipid signaling, arginine biosynthesis, and amino acid metabolism[36]. The MSC1 subtype of GC, which exhibits upregulated TCA cycle and lipid metabolism, along with frequent TP53 and RHOA mutations, is associated with a better prognosis[37]. Additionally, a prospective analysis of the Korean genome and epidemiology study implicated insulin resistance and lifestyle factors in the development of CRC[38].
A few studies have explored the metabolomic profiles associated with TCM syndromes related to gastrointestinal cancers. Specific alterations are observed in different TCM syndromes. For instance, DHS is associated with unique alterations in L-alanine, glycerol, glycine, creatinine, and palmitic acid[16], while SDS exhibits alterations in D-tryptophan. Similarly, liver and kidney Yin deficiency syndrome is characterized by alterations in L-proline, 1,2,3-propanetricarboxylic acid, D-galactose, and 2-indolecarboxylic acid[16]. Studies in CRC patients have also shown differences in serum metabolic profiles among Non-deficiency, Qi deficiency, and Yin deficiency syndromes[20], and impaired carbohydrate, fatty acid, and amino acid metabolism in Qi and Yin deficiency groups, suggesting potential biomarkers for diagnosis and prognostic assessment[20].
In the present study, non-targeted metabolomics analyses were used to identify differentially expressed metabolites in gastrointestinal cancer patients vs healthy individuals. Additionally, metabolites associated with gastrointestinal cancers and PXYD syndrome were identified. In CRC vs normal samples, 455 different metabolites were differentially expressed (234 upregulated and 221 downregulated). Similarly, in GC vs normal samples, 459 metabolites were differentially expressed (251 upregulated and 208 downregulated). Additionally, 352 common metabolites were identified in gastrointestinal cancer PXYD syndrome, including P-Cresol Glucuronide, Equilenin, Lactulose, 3-(Pyrazol-1-Yl)-L-Alanine, 2,6-Pyridinedicarboxylic Acid, Maltotriose, Cis-Aconitic Acid, Lactitol, Sphingosine 1-phosphate, L-tryptophan, and D-galactose. The shared metabolites were enriched in 10 metabolic pathways, i.e., pentose and glucuronate interconversions, galactose metabolism, lysine degradation, primary bile acid biosynthesis, amino sugar and nucleotide sugar metabolism, sphingolipid metabolism, sphingolipid signaling pathway, propanoate metabolism, TCA cycle, and insulin resistance. Many metabolic pathways have been implicated in the pathogenesis of gastrointestinal cancers, such as sphingolipid metabolism[39] and galactose metabolism[31]. Previous research has also linked various metabolic pathways to TCM syndromes in different diseases. For example, amino acid metabolism, sphingolipid metabolism, and the TCA cycle are associated with TCM syndromes in gastroesophageal reflux disease[21]. Similarly, glycerophospholipid metabolism and sphingolipid signaling are linked to phlegm-dampness syndrome in myocardial injury[22]. In type 2 diabetes, pathways such as ketone body synthesis and degradation, starch and sucrose metabolism, phenylalanine metabolism, arachidonic acid metabolism, butanoate metabolism, and TCA cycle are associated with Qi-deficiency[40].
Some limitations of our study should be considered while interpreting the findings, including its single-center scope, small sample size, and specific geographical constraints. The sample size is sufficient only for a preliminary investigation using untargeted metabolomics. The single-center design limits the generalizability of the findings, and the complex interplay of genetic, environmental, and lifestyle factors in CRC and GC may vary across regions and populations. Therefore, the results may not fully capture the diverse epidemiological patterns and disease manifestations encountered globally. The small sample size poses challenges in detecting subtle associations or identifying rare but clinically significant subgroups within the CRC and GC patient populations. The limited sample size also reduces statistical power and may amplify measurement errors. Single-center studies frequently exhibit limited demographic diversity-a constraint inherent to single-site patient recruitment-which can lead to overestimation of effect sizes. This limitation may obscure important correlations or prognostic indicators that may have been identified in a larger cohort. To address these limitations, a new multicenter cohort study with a larger sample size is underway, aiming to validate the present findings, explore clinical significance, and provide a more representative sample of CRC and GC patient populations.
In TCM, syndrome differentiation is the basis for treatment[11]. The effectiveness of TCM-based therapeutics can vary, potentially due to the specific biological basis of each TCM syndrome. Clinical trials have demonstrated promising results of some TCM treatments targeting specific syndromes, while other TCM treatments have been less effective, highlighting the need to understand the biological underpinnings of TCM syndromes to optimize treatment outcomes[41]. Multi-omics approaches, including genomics, transcriptomics, proteomics, metabolomics, and intestinal microbiota analysis, are being used to investigate the biological basis of TCM syndromes associated with gastrointestinal cancers[15]. For example, the classification of Excess and Deficiency syndromes may be associated with tumor heterogeneity and the microenvironment in CRC[42]. TCM offers distinct benefits in the treatment of respiratory viral illnesses by following principles like "different treatments for the same disease" and "same treatment for different diseases"[43]. Research on Astragalus membranaceus and its monomers demonstrates this concept, showing its potential in the treatment of peritoneal fibrosis and associated muscle atrophy via the AR/TGF-β1 pathway, exploring the TCM theory of "same treatment for different diseases", and providing evidence of the efficacy of Astragalus membranaceus in treating flaccidity syndrome[44]. Network pharmacological analysis suggests that Lingze tablets may effectively treat prostatitis and insomnia by immunomodulation, reducing inflammation, and achieving endocrine system homeostasis. These findings provide evidence for their potential therapeutic use in these conditions[45].
Our research group is working on establishing a biobank for gastrointestinal cancers and conducting both preclinical and clinical research. This research focuses on the biological basis of TCM syndromes associated with gastrointestinal cancers, guided by the theory of cancerous toxins[46]. The pathogenesis theory of cancerous toxins is a core concept of TCM-based treatments for cancer[15]. Future research will aim to classify gastrointestinal cancers according to TCM syndromes, uncover their biological underpinnings, and potentially inform diagnostic, prognostic, and therapeutic applications. This endeavor aims to provide evidence for TCM-based treatments, enhancing clinical efficacy. By identifying biomarkers and metabolic pathways associated with gastrointestinal cancers-guided by TCM syndrome classification frameworks-the study may lead to targeted therapies that boost antitumor immunity through improved immune modulation[47].
This study identified differentially expressed metabolites in CRC and GC, revealing metabolomic characteristics of PXYD syndrome in gastrointestinal cancers. It preliminarily identified 352 common metabolites and 10 metabolic pathways, providing a theoretical basis for the "same treatment for different diseases" approach in PXYD syndrome of gastrointestinal cancers.
We would like to thank Xue-Chun Dong and Feng-Yuan Zhen from OE Biotech Co., Ltd. (Shanghai, China) for the insightful suggestions and valuable technical support.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64340] [Article Influence: 16085.0] [Reference Citation Analysis (175)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11394] [Article Influence: 3798.0] [Reference Citation Analysis (4)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13202] [Article Influence: 1466.9] [Reference Citation Analysis (3)] |
| 4. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2182] [Article Influence: 727.3] [Reference Citation Analysis (1)] |
| 5. | Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, Siegel RL. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 1540] [Article Influence: 513.3] [Reference Citation Analysis (0)] |
| 6. | Ke G, Zhang J, Gao W, Chen J, Liu L, Wang S, Zhang H, Yan G. Application of advanced technology in traditional Chinese medicine for cancer therapy. Front Pharmacol. 2022;13:1038063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Mao Q, Min J, Zeng R, Liu H, Li H, Zhang C, Zheng A, Lin J, Liu X, Wu M. Self-assembled traditional Chinese nanomedicine modulating tumor immunosuppressive microenvironment for colorectal cancer immunotherapy. Theranostics. 2022;12:6088-6105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 8. | Zhang X, Wang K, Dai H, Cai J, Liu Y, Yin C, Wu J, Li X, Wu G, Lu A, Liu Q, Guan D. Quantification of promoting efficiency and reducing toxicity of Traditional Chinese Medicine: A case study of the combination of Tripterygium wilfordii hook. f. and Lysimachia christinae hance in the treatment of lung cancer. Front Pharmacol. 2022;13:1018273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Lou Y, Wang J, Yu C, Shen W. Research Status and Molecular Mechanism of the Traditional Chinese Medicine and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment. Front Immunol. 2020;11:609705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 10. | Cassileth B, Yeung KS. Supportive Cancer Care with Chinese Medicine. Focus Altern Complement Ther. 2010;15:261-262. [DOI] [Full Text] |
| 11. | Dou Z, Xia Y, Zhang J, Li Y, Zhang Y, Zhao L, Huang Z, Sun H, Wu L, Han D, Liu Y. Syndrome Differentiation and Treatment Regularity in Traditional Chinese Medicine for Type 2 Diabetes: A Text Mining Analysis. Front Endocrinol (Lausanne). 2021;12:728032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Messersmith WA. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J Natl Compr Canc Netw. 2019;17:599-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 13. | Guo T, Zhou H, Chen F, Gu Y, Li L, Cheng H. A review on the pathogenesis theory of cancerous toxin from the viewpoint of system theory. Sci Tradit Chin Med. 2024;2:187-193. [DOI] [Full Text] |
| 14. | Kaźmierczak-Siedlecka K, Muszyński D, Styburski D, Makarewicz J, Sobocki BK, Ulasiński P, Połom K, Stachowska E, Skonieczna-Żydecka K, Kalinowski L. Untargeted metabolomics in gastric and colorectal cancer patients - preliminary results. Front Cell Infect Microbiol. 2024;14:1394038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Guo T, Zhao S, Zhu W, Zhou H, Cheng H. Research progress on the biological basis of Traditional Chinese Medicine syndromes of gastrointestinal cancers. Heliyon. 2023;9:e20653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 16. | Hu XQ, Wei B, Song YN, Ji Q, Li Q, Luo YQ, Wang WH, Su SB. Plasma metabolic profiling on postoperative colorectal cancer patients with different traditional Chinese medicine syndromes. Complement Ther Med. 2018;36:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Liu S, Huang J, Liu Y, Lin J, Zhang H, Cheng L, Ye W, Liu X. Identification of serum N-glycans signatures in three major gastrointestinal cancers by high-throughput N-glycome profiling. Clin Proteomics. 2024;21:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2417] [Article Influence: 483.4] [Reference Citation Analysis (3)] |
| 19. | Hui YF, Zhao SQ, Ling TS, Li L, Zhang Y, Gu LM, Liao X, Cheng HB. [Guidelines for prevention and treatment of colorectal adenoma with integrated Chinese and western medicine]. Zhongguo Zhong Yao Za Zhi. 2023;48:6269-6277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Tao F, Lü P, Xu C, Zheng M, Liu W, Shen M, Ruan S. Metabolomics Analysis for Defining Serum Biochemical Markers in Colorectal Cancer Patients with Qi Deficiency Syndrome or Yin Deficiency Syndrome. Evid Based Complement Alternat Med. 2017;2017:7382752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Ye X, Wang X, Wang Y, Sun W, Chen Y, Wang D, Li Z, Li Z. A urine and serum metabolomics study of gastroesophageal reflux disease in TCM syndrome differentiation using UPLC-Q-TOF/MS. J Pharm Biomed Anal. 2021;206:114369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Lan T, Zeng Q, Jiang W, Liu T, Xu W, Yao P, Lu W. Metabolism disorder promotes isoproterenol-induced myocardial injury in mice with high temperature and high humidity and high-fat diet. BMC Cardiovasc Disord. 2022;22:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Cherkaoui S, Durot S, Bradley J, Critchlow S, Dubuis S, Masiero MM, Wegmann R, Snijder B, Othman A, Bendtsen C, Zamboni N. A functional analysis of 180 cancer cell lines reveals conserved intrinsic metabolic programs. Mol Syst Biol. 2022;18:e11033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Kong C, Liang L, Liu G, Du L, Yang Y, Liu J, Shi D, Li X, Ma Y. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut. 2023;72:1129-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 126] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 25. | Gao R, Wu C, Zhu Y, Kong C, Zhu Y, Gao Y, Zhang X, Yang R, Zhong H, Xiong X, Chen C, Xu Q, Qin H. Integrated Analysis of Colorectal Cancer Reveals Cross-Cohort Gut Microbial Signatures and Associated Serum Metabolites. Gastroenterology. 2022;163:1024-1037.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 26. | Shen Q, Wang R, Liu X, Song P, Zheng M, Ren X, Ma J, Lu Z, Li J. HSF1 Stimulates Glutamine Transport by Super-Enhancer-Driven lncRNA LINC00857 in Colorectal Cancer. Cancers (Basel). 2022;14:3855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 27. | Yang Y, Luo D, Shao Y, Shan Z, Liu Q, Weng J, He W, Zhang R, Li Q, Wang Z, Li X. circCAPRIN1 interacts with STAT2 to promote tumor progression and lipid synthesis via upregulating ACC1 expression in colorectal cancer. Cancer Commun (Lond). 2023;43:100-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 28. | Guo H, Zhuang K, Ding N, Hua R, Tang H, Wu Y, Yuan Z, Li T, He S. High-fat diet induced cyclophilin B enhances STAT3/lncRNA-PVT1 feedforward loop and promotes growth and metastasis in colorectal cancer. Cell Death Dis. 2022;13:883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 29. | Meng X, Peng J, Xie X, Yu F, Wang W, Pan Q, Jin H, Huang X, Yu H, Li S, Feng D, Liu Q, Fang L, Lee MH. Roles of lncRNA LVBU in regulating urea cycle/polyamine synthesis axis to promote colorectal carcinoma progression. Oncogene. 2022;41:4231-4243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 30. | Xiang Y, Zhang C, Wang J, Cheng Y, Wang K, Wang L, Tong Y, Yan D. Role of blood metabolites in mediating the effect of gut microbiome on the mutated-RAS/BRAF metastatic colorectal cancer-specific survival. Int J Colorectal Dis. 2024;39:116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Mathlouthi NEH, Belguith I, Yengui M, Oumarou Hama H, Lagier JC, Ammar Keskes L, Grine G, Gdoura R. The Archaeome's Role in Colorectal Cancer: Unveiling the DPANN Group and Investigating Archaeal Functional Signatures. Microorganisms. 2023;11:2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Busico A, Gasparini P, Rausa E, Cattaneo L, Bozzi F, Silvestri M, Capone I, Conca E, Tamborini E, Perrone F, Vitellaro M, Ricci MT, Casanova M, Chiaravalli S, Bergamaschi L, Massimino M, Milione M, Sozzi G, Pruneri G, Ferrari A, Signoroni S. Molecular profiling of pediatric and young adult colorectal cancer reveals a distinct genomic landscapes and potential therapeutic avenues. Sci Rep. 2024;14:13138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 33. | Pan SY, Zhou CB, Deng JW, Zhou YL, Liu ZH, Fang JY. The effects of pks(+) Escherichia coli and bile acid in colorectal tumorigenesis among people with cholelithiasis or cholecystectomy. J Gastroenterol Hepatol. 2024;39:868-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Zhang YK, Zhang Q, Wang YL, Zhang WY, Hu HQ, Wu HY, Sheng XZ, Luo KJ, Zhang H, Wang M, Huang R, Wang GY. A Comparison Study of Age and Colorectal Cancer-Related Gut Bacteria. Front Cell Infect Microbiol. 2021;11:606490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Yan J, Yu X, Li Q, Miao M, Shao Y. Machine learning to establish three sphingolipid metabolism genes signature to characterize the immune landscape and prognosis of patients with gastric cancer. BMC Genomics. 2024;25:319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Yang Y, Dai D, Jin W, Huang Y, Zhang Y, Chen Y, Wang W, Lin W, Chen X, Zhang J, Wang H, Zhang H, Teng L. Microbiota and metabolites alterations in proximal and distal gastric cancer patients. J Transl Med. 2022;20:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 37. | Chen H, Jing C, Shang L, Zhu X, Zhang R, Liu Y, Wang M, Xu K, Ma T, Jing H, Wang Z, Li X, Chong W, Li L. Molecular characterization and clinical relevance of metabolic signature subtypes in gastric cancer. Cell Rep. 2024;43:114424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 38. | Kityo A, Lee SA. Triglyceride-Glucose Index, Modifiable Lifestyle, and Risk of Colorectal Cancer: A Prospective Analysis of the Korean Genome and Epidemiology Study. J Epidemiol Glob Health. 2024;14:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 39. | Liu B, Zhou J, Jiang B, Tang B, Liu T, Lei P. The role of ACER2 in intestinal sphingolipid metabolism and gastrointestinal cancers. Front Immunol. 2024;15:1511283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Gao Y, Wu Y, Liu Z, Fu J, Zhang Y, Wu J, Liu S, Song F, Liu Z. Based on urine metabolomics to study the mechanism of Qi-deficiency affecting type 2 diabetes rats using ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1179:122850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 41. | Liu T, Qin M, Xiong X, Lai X, Gao Y. Multi-omics approaches for deciphering the complexity of traditional Chinese medicine syndromes in stroke: A systematic review. Front Pharmacol. 2022;13:980650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Wang P, Ding S, Sun L, Feng Y, Guo K, Zhu Y, Huang D, Ruan S. Characteristics and differences of gut microbiota in patients with different Traditional Chinese Medicine Syndromes of Colorectal Cancer and normal population. J Cancer. 2020;11:7357-7367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Bai Y, Liu T, Zhang S, Shi Y, Yang Y, Ding M, Yang X, Guo S, Xu X, Liu Q. Traditional Chinese Medicine for Viral Pneumonia Therapy: Pharmacological Basis and Mechanistic Insights. Int J Biol Sci. 2025;21:989-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | Sheng L, Sun J, Huang L, Yu M, Meng X, Shan Y, Dai H, Wang F, Shi J, Sheng M. Astragalus membranaceus and its monomers treat peritoneal fibrosis and related muscle atrophy through the AR/TGF-β1 pathway. Front Pharmacol. 2024;15:1418485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 45. | Luo Y, Zhang Y, Lan K, Zhang Y. The mechanism of lingze tablet in treating prostatitis and insomnia: Based on network pharmacology, bioinformatics and molecular docking. Asian J Surg. 2024;47:3185-3187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | Sang T, Qiu W, Li W, Zhou H, Chen H, Zhou H. The Relationship between Prevention and Treatment of Colorectal Cancer and Cancerous Toxin Pathogenesis Theory Basing on Gut Microbiota. Evid Based Complement Alternat Med. 2020;2020:7162545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Vijayan Y, James S, Viswanathan A, Aparna JS, Bindu A, Namitha NN, Anantharaman D, Babu Lankadasari M, Harikumar KB. Targeting acid ceramidase enhances antitumor immune response in colorectal cancer. J Adv Res. 2024;65:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |